Abstract
Objective
The objective of this study was to ascertain to what extent adults with migraine value an early onset of efficacy for preventive migraine treatments.
Background
In placebo‐controlled clinical trials, treatment with eptinezumab resulted in a lower proportion of adults with migraine on the first day following infusion (day 1; 14% point‐reduction for chronic migraine [CM] in PROMISE‐2 and 8% point‐reduction for episodic migraine [EM] in PROMISE‐1).
Methods
Adults with migraine completed an online preference‐elicitation thresholding exercise to ascertain to what extent they value not having a migraine on day 1 postdosing relative to a clinically relevant reduction in number of migraine days during the first month postdosing (≥2 migraine‐free days for CM and ≥1 migraine‐free days for EM).
Results
One hundred and one participants (mean age, 50.6 ± 12.4 years; 81 [80%] women) were included. In participants with CM, 29 of 50 (58%) considered the eptinezumab‐generated reduction in the likelihood of migraine on day 1 postdosing to be at least as important as a clinically relevant reduction in number of migraine days the first month postdosing, whereas 37 of 50 (74%) considered a clinically relevant reduction of migraine days the first month postdosing to have a value equivalent to the eptinezumab‐generated reduction in the likelihood of migraine on day 1 postdosing. In participants with EM, 18 of 35 (51%) considered the eptinezumab‐generated reduction in the likelihood of migraine on day 1 postdosing to be at least as important as a clinically relevant reduction in migraine days the first month postdosing, whereas 24 of 35 (69%) considered a clinically relevant reduction of migraine days the first month postdosing to have a value equivalent to the eptinezumab‐generated reduction in the likelihood of migraine on day 1 postdosing.
Conclusion
Most participants considered the reduction in the likelihood of migraine offered by eptinezumab on day 1 postdosing to be at least as important as a clinically relevant reduction in migraine days the first month postdosing.
Keywords: chronic migraine, episodic migraine, patient preference, preventive treatment
Abbreviations
- CI
confidence interval
- CM
chronic migraine
- EM
episodic migraine
- IQR
interquartile range
- MAB
minimum acceptable benefit
- MMDs
monthly migraine days
- MRS
marginal rate of substitution
INTRODUCTION
Newer targeted preventive treatments for migraine offer reduced frequency of migraine and the possibility of an early onset of migraine‐preventive efficacy. 1 , 2 Although improvement in the frequency of migraine is well‐established as a key outcome of preventive treatments 3 and is considered important to patients, 4 the extent to which patients value an early onset of migraine‐preventive efficacy is not well known.
With the rise of patient‐focused drug development, health technology assessors and regulators have become increasingly interested in patient preferences, especially to what extent they value different treatment attributes and are willing to make trade‐offs between them. 5 , 6 , 7 Quantitative information about patient preferences can help in shared decision making between patients and healthcare providers to select treatments that reflect patients' lifestyles, values, and treatment goals. 5 In addition, both the US Food and Drug Administration 8 , 9 and the European Medicines Agency 10 encourage using patient preference data to support regulatory decisions and health technology assessments.
Although preferences of patients with migraine for the characteristics of preventive medicines have been reported, 11 , 12 the time to onset of migraine‐preventive efficacy has not been included as an attribute in preference elicitation studies. In one study of patient preferences for preventive migraine medicines, a ranking exercise showed that “speed of onset” was the most important attribute for only 12% of participants, whereas “efficacy” was the most important for 72%. 13 The study, however, did not include “speed of onset” in a subsequent preference elicitation exercise or provide insight into what trade‐offs patients are willing to make between “speed of onset” and reduced frequency of migraine. Further, at the time the study was done, time to onset of efficacy was weeks or months rather than days.
To support clinicians, regulatory bodies, and health technology assessors as they make decisions about preventive migraine treatments, the current study ascertained to what extent adults with chronic migraine (CM) or episodic migraine (EM) value early onset of migraine‐preventive efficacy. This was done by determining what improvement in the onset of migraine‐preventive efficacy patients would consider to have equal value as previously established thresholds for a clinically relevant improvement in monthly migraine days (MMDs). 4 To align with the endpoint used in clinical trials of eptinezumab, 14 , 15 early onset of migraine‐preventive efficacy was defined as the likelihood that a migraine would be experienced on the first day after treatment.
METHODS
Study design
An online preference‐elicitation thresholding exercise was conducted between December 2019 and January 2020 in adults in the United States diagnosed with CM or EM. The exercise aimed to understand the relative value that patients attach to the likelihood of the migraine treatment beginning to work on the first day following infusion (day 1; “When does the preventative treatment begin working?”) and the number of days with migraine in the first month of treatment (“Number of migraine days”). The thresholding exercise was tested by study team members prior to fielding and was tested during a qualitative pilot conducted with five adults with migraine to ensure that they could understand the selected attributes, would consider the attributes meaningful, and could provide meaningful responses to the elicitation questions. In the main study, the survey was emailed to eligible participants who completed the final preference‐elicitation thresholding exercise, assessments of health literacy 16 , 17 and numeracy, 18 and a questionnaire collecting sociodemographic and clinical characteristics (age at diagnosis, most recent migraine, medication history, comorbidities, gender, race/ethnicity, employment status, and education level). The study was approved by the Ethical and Independent Review Services (E&I Study Number 19181‐01A). All participants provided electronic informed consent. Participants in the qualitative pilot also provided consent to be audio‐recorded.
Participants
Adults in the United States (≥18 years of age) with a self‐reported diagnosis of CM (≥ 8 MMDs with ≥15 headache days/month) or EM (≥ 4 MMDs with <14 headache days/month) who had been diagnosed at less than or equal to 50 years of age were recruited via recruiter databases, patient associations, patient support groups, social media groups, online forums, and patient panels. Potentially eligible participants were invited to participate via email or advertisement and were followed up via telephone for screening. Participants had to self‐report having experienced migraine for greater than or equal to 12 months, greater than or equal to four MMDs for greater than or equal to 3 months, and less than or equal to 26 headache days per month. Individuals with confounding and clinically significant pain syndromes, such as fibromyalgia or with uncontrolled or untreated psychiatric conditions, were excluded. Up to 100 participants were to be recruited, of whom ~ 60% were to be patients with CM. There are no established procedures for estimating sample sizes for thresholding exercises. A thresholding exercise involves a descriptive analysis of the change in one attribute that gives the same value as a change in another attribute. As such, the feasible sample attainable in the scope of this study, n = 100, was acceptable for these purposes. Participants who took part in the qualitative pilot interviews were not eligible to take part in the main study. Participants were remunerated for completing the study in line with local regulations for fair market value.
Thresholding exercise
In a thresholding exercise, participants are presented with a choice between two treatment options, and the level of performance of one of the treatments for a single attribute is varied until the participant is indifferent between two treatments. 19 , 20 The thresholding exercises used in this study estimated the improvement in onset of efficacy of migraine prevention treatments that would leave patients indifferent between that improvement and a meaningful change during the first month following treatment. To align with the endpoint used in clinical trials of eptinezumab, 14 , 15 speed of onset of migraine‐preventive efficacy was defined as the likelihood that a migraine would be experienced on the first day after treatment.
Before beginning the thresholding exercises, participants completed two warm‐up tasks (multiple‐choice questions) intended to test comprehension of choice tasks.
In the thresholding exercises, participants were presented with two choice frames. In each choice frame, they were asked to select between two hypothetical migraine prevention medications that were defined by the likelihood of experiencing a migraine on day 1 postdosing and the number of migraine days the first month postdosing.
In choice frame 1, the number of migraine days during the first month postdosing was fixed at rates comparable to the average number of MMDs experienced by patients receiving eptinezumab and placebo in the PROMISE‐1 study 14 for EM and the PROMISE‐2 study 15 for CM. For patients with EM, this number was fixed at 5 days for treatment A and 6 days for treatment based on MMD frequencies of 4.7 for eptinezumab and 5.4 for placebo in the PROMISE‐1 study. 14 For patients with CM, this number was fixed at 10 days for treatment A and 12 days for treatment B based on MMD frequencies of 8.5 for eptinezumab and 10.5 for placebo in the PROMISE‐2 study. 15 The likelihood that a migraine would be experienced on the first day after infusion (i.e., day 1 postdosing) for treatment A was based on results for patients receiving placebo in PROMISE‐1 and PROMISE‐2 and converted to the number of people out of 100 on treatment who had a migraine on the first day postdosing (22% for EM based on 22.5% with placebo in PROMISE‐1, 42% for CM based on 42.3% with placebo in PROMISE‐2). The likelihood that a migraine would be experienced on day 1 postdosing for treatment B was varied.
In choice frame 2, the number of migraine days for treatment B differed by an amount considered to be clinically relevant based on a targeted literature search and Dodick et al. 4 (1‐MMD reduction for EM and 2‐MMD reduction for CM). The likelihood that a migraine would be experienced on day 1 postdosing was fixed for treatment A so that the difference between treatments A and B represented the improvement observed in PROMISE‐1 for EM (13.9% for eptinezumab vs. 22.5% for placebo; change = 8.6%) 14 and PROMISE‐2 for CM (28.6% for eptinezumab vs. 42.3% for placebo; change = 13.7%). 15 The levels for migraine days the first month postdosing and the likelihood of migraine on day 1 postdosing for each treatment in each of the choice frames are summarized in Table 1. Participants were randomized to which choice frame they were presented with first. An example thresholding task is shown in Figure 1. Depending on the choice task, the participant population (CM or EM), and the participants’ answers, the choice task was repeated two to six times, each time changing the levels of performance of one attribute for one treatment. The changes were designed to move the participant toward being indifferent between the treatments and were made in accordance with predefined algorithms (see Supporting Methods).
TABLE 1.
Attributes and levels
| Attribute | Attribute description seen by participants | Participant group | Levels | |||
|---|---|---|---|---|---|---|
| Choice frame 1 | Choice frame 2 | |||||
| Treatment A | Treatment B | Treatment A | Treatment B | |||
| When does the preventative treatment begin working? | Treatments to prevent migraine may differ in when they begin working. While the preventative treatments you have taken may not have worked on day 1, some newer preventative treatments may begin to work on the first day of treatment. This is measured as the number of people out of 100 on the treatment who experience a migraine on the first day after receiving preventive treatment | Episodic migraine | Fixed 22% | Varied 0–22% | Fixed 14% | Fixed 22% |
| Chronic migraine | Fixed 42% | Varied 10–42% | Fixed 28% | Fixed 42% | ||
| Number of migraine days during the first month of treatment | Once preventive treatments start working, they may differ in how effective they are at preventing migraines. This is measured using the number of days per month that people on the preventive treatment experience a migraine. During this survey, please assume that the severity of migraines does not change. In the calendar below, the number of days represent the days with a migraine in the first month of treatment | Episodic migraine | Fixed 5 days | Fixed 6 days | Fixed 6 days | Varied 0–6 days |
| Chronic migraine | Fixed 10 days | Fixed 12 days | Fixed 12 days | Varied 0–12 days | ||
FIGURE 1.
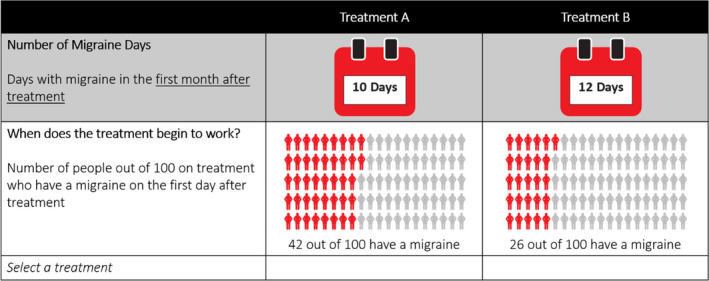
Example thresholding task [Color figure can be viewed at wileyonlinelibrary.com]
Evaluation of attendance
In the thresholding exercise, task 1 of choice frame 1 was repeated at the end of choice frame 1 to test stability of responses. Participants also completed a non‐experimental thresholding task to test dominance in which treatment option B was designed to be superior to treatment option A for all attributes. Participants who were considered non‐attenders (i.e., did not pay sufficient attention to the choice tasks) were excluded from the preference analysis. Participants were considered non‐attenders if they displayed multiple signs of non‐attendance, namely, (i) completing the thresholding exercise in less than 1 min or displaying lexicographic preferences (always choosing the treatment with the best performance on a single attribute); and (ii) failing multiple other validity assessments, including the two warm‐up tasks (i.e., providing incorrect responses to multichoice questions), the stability test (i.e., providing different responses to the repeated task), the dominance test (i.e., selecting the treatment option that performs better, or is no worse performing, on each attribute), and straight‐lining (i.e., always choosing either treatment option A or B).
Statistical analysis
This study consisted of a primary, a priori analysis conducted using Stata version 15 (Stata Corp., College Station, TX, USA). Descriptive statistics were calculated for categorical variables (number and percentage) and continuous variables (mean, standard deviation, median, and interquartile range [IQR]). Only fully completed surveys were included in the final analysis; partially completed surveys were excluded. Responses to the thresholding exercise were used to estimate minimum acceptable benefit (MAB). An MAB can be described as the minimum acceptable level of a single benefit endpoint in the context of a known change in a second endpoint. For choice frame 1, the MAB was the minimum change in the likelihood that a migraine would be experienced on day 1 that generated equivalent value as clinically relevant changes in migraine days. For choice frame 2, the MAB was the minimum change in migraine days that generated equivalent value as the improvement in the likelihood that a migraine would be experienced on day 1. To avoid burdening participants with too many choice tasks, the thresholding exercise identified the MAB as the mid‐point of the range of values as determined using the question flow algorithms (see Supporting Methods).
For choice frame 1, MABs were used to estimate the proportion of participants whose MAB was less than the change in likelihood that a migraine was experienced on day 1 between eptinezumab and placebo (8% point‐reduction for participants with EM, 14 14% point‐reduction for participants with CM 15 ). For choice frame 2, MABs were used to estimate the proportion of participants whose MAB was greater than the change in migraine days considered clinically relevant (1‐MMD reduction for participants with EM and 2‐MMD reduction for participants with CM). 4
Responses to both choice frames were used to estimate the marginal rate of substitution (MRS), which was the change in likelihood of a migraine after day 1 that had the equivalent value as a 1‐unit increase in migraine days. The MRS was calculated as the change in likelihood of a migraine after day 1 divided by the change in migraine days. A paired, two‐tailed t‐test was used to test if, for each individual, the difference in MRS between choice frame 1 and choice frame 2 differed from 0 (p < 0.05).
RESULTS
Participant characteristics
A total of 901 people responded to the initial study invite, with 126 being eligible for participation. The main reasons for ineligibility were reporting fewer than 4 MMDs per month over the previous 3 months (n = 221); reporting a diagnosis of fibromyalgia or no diagnosis of migraine (n = 186); not being interested in participating (n = 146); and failing to complete the screening questionnaire (n = 71). Of the 112 participants who consented to participate, 101 fully completed the survey (55 with CM, 46 with EM; Table 2). Overall, the study participants had an average age of 50.6 ± 12.4 years, 80% were women, 98% were non‐Hispanic Whites, and 59% had a university degree or higher. Age, sex, race, ethnicity, and level of education were similar between participants with CM and those with EM. Participants with CM had been experiencing migraine for an average of 25.1 years and had an average of 14.3 migraine days per month, whereas those with EM had been experiencing migraine for an average of 26.9 years and had an average of 7.1 migraine days per month. Mean age at diagnosis was similar for participants with CM (27.9 years) and EM (25.1 years). Triptans were being used to treat migraine by 56% of participants with CM and 37% of those with EM.
TABLE 2.
Sociodemographic and clinical characteristics of participants
| Characteristic | Overall sample (N = 101) | Chronic migraine (n = 55) | Episodic migraine (n = 46) |
|---|---|---|---|
| Age, mean (SD) | 50.6 (12.4) | 50.3 (14.0) | 50.9 (10.5) |
| Sex, n (%) | |||
| Female | 81 (80) | 43 (78) | 38 (83) |
| Male | 20 (20) | 12 (22) | 8 (17) |
| Racial background, n (%) | |||
| Hispanic/Latino | 5 (5) | 3 (6) | 2 (4) |
| Black/African American | 3 (3) | 2 (4) | 1 (2) |
| Asian | 1 (1) | 1 (2) | 0 (0) |
| Native American | 3 (3) | 2 (4) | 1 (2) |
| White, non‐Hispanic | 99 (98) | 54 (98) | 45 (98) |
| Education level, n (%) | |||
| Elementary/primary school | 1 (1) | 1 (2) | 0 (0) |
| High school | 11 (11) | 7 (13) | 4 (9) |
| Some college/university | 29 (29) | 15 (27) | 14 (30) |
| University degree | 34 (34) | 19 (35) | 15 (33) |
| Vocational degree/training | 9 (9) | 3 (6) | 6 (13) |
| Master’s degree | 13 (13) | 7 (13) | 6 (13) |
| Doctorate degree | 3 (3) | 2 (4) | 1 (2) |
| Others | 1 (1) | 1 (2) | 0 (0) |
| Age at diagnosis, mean (SD) | 26.6 (9.9) | 27.9 (10.3) | 25.1 (9.4) |
| Duration of migraine (years), mean (SD) | 25.9 (13.6) | 25.1 (14.6) | 26.9 (12.4) |
| Time since migraine diagnosis, n (%) | |||
| ≤1 year ago | 2 (2) | 1 (2) | 1 (2) |
| 1–3 years ago | 3 (3) | 2 (4) | 1 (2) |
| 3–6 years ago | 2 (2) | 1 (2) | 1 (2) |
| ≥6 years ago | 94 (93) | 51 (93) | 43 (94) |
| Days per month experiencing migraine, mean (SD) | 11.0 (5.1) | 14.3 (4.0) | 7.1 (3.1) |
| Currently using triptans, n (%) | 48 (48) | 31 (56) | 17 (37) |
Abbreviation: SD, standard deviation.
Internal validity and non‐attendance
Most of the 101 participants passed the dominance test (95% for CM and 91% for EM), passed the stability test (84% for CM and 76% for EM), answered the two warm‐up tasks correctly (warm‐up task 1: 93% for CM, 94% for EM; and warm‐up task 2: 98% for CM, 94% for EM), and spent adequate time (>1 min) on the choice tasks (91% CM and 83% EM; Table S1). None of the participants with CM and only 2% of the participants with EM were straight‐liners, meaning that they selected only one of the treatment options in both choice frames. A minority of participants with CM displayed lexicographic preferences (22% for choice frame 1 and 33% for choice frame 2), meaning that across the choice tasks, they always chose treatments that had a better performance for a single attribute, regardless of how much better. Lexicographic preferences were more common in participants with EM (46% for choice frame 1 and 61% for choice frame 2).
Of participants with CM, those with lexicographic preferences were less likely to perform poorly on the other validity tests (Table S2), suggesting that displaying lexicographic preferences alone did not necessarily indicate non‐attendance. Accordingly, for CM, only the five participants who completed the choice tasks in less than 1 min were excluded from the preference analysis. In contrast, for EM, the association was stronger between participants displaying lexicographic preferences and those failing the dominance test, the stability test, and the two warm‐up tasks, suggesting that lexicographic preferences were an indicator of non‐attendance for these patients. Thus, for EM, the eight participants who completed the choice tasks in less than 1 min and the three who failed multiple other validity tests were excluded from the preference analysis.
For participants with CM, MRS between the likelihood of experiencing a migraine on day 1 postdosing and the number of migraine days during the first month postdosing was consistent between choice frames (MRS = 6.81 ± 5.62 for choice task frame 1 and 7.63 ± 9.42 for choice task frame 2; p = 0.343 by paired t‐test; Table S3). Results were similar when participants completing the survey in less than 1 min were excluded. For participants with EM, the MRS was not consistent between the choice frames (MRS = 9.76 ± 8.05 for choice task frame 1 and 7.11 ± 6.38 for choice task frame 2; p = 0.003 by paired t‐test). The MRS was also not consistent between choice frames when the 11 participants with EM considered non‐attenders were excluded.
Preferences
Participants with CM
Based on responses to choice frame 1, 58% of participants (95% confidence interval [CI]: 44.3%–71.7%) with CM considered a 14% point‐reduction in the likelihood of having a migraine on day 1 postdosing to have equivalent or greater value as a reduction of 2 migraine days during the first month post‐dosing (Figure 2A). The median reduction of 9.0% (IQR, 13.0%) in the likelihood of a migraine on day 1 postdosing was considered to have the same value as a reduction of 2 migraine days the first month postdosing.
FIGURE 2.
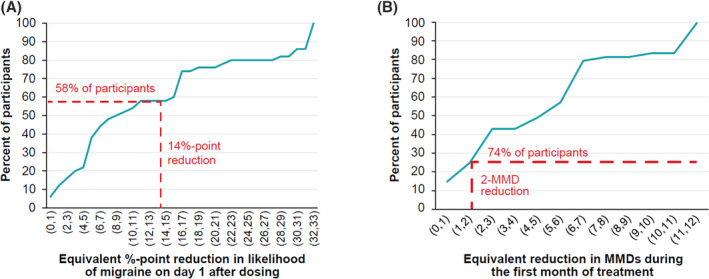
Results of thresholding exercise in participants with chronic migraine. (A) Reduction in likelihood of migraine days on day 1 that has the equivalent value as reduction of 2 migraine days during the first month of treatment. (B) Reduction in monthly migraine days that has the equivalent value as the 14% reduction in the likelihood of a migraine on day 1 observed between eptinezumab and placebo in the PROMISE‐2 trial. 15 MMD, monthly migraine day [Color figure can be viewed at wileyonlinelibrary.com]
Based on choice frame 2, 74% of participants (95% CI: 61.8%–86.2%) with CM thought that a 14% point‐reduction in the likelihood of a migraine on day 1 post‐dosing had the same value as a reduction of greater than or equal to 2 migraine days the first month post‐dosing (Figure 2B). A median reduction of 5 migraine days (IQR, 5.0) the first month postdosing was considered to have the same value as a 14% point‐reduction in the likelihood of a migraine on day 1 postdosing.
Participants with EM
Based on responses to choice frame 1, 51% of participants with EM (95% CI: 34.9%–68.0%) considered an 8% point‐reduction in the likelihood of migraine on day 1 postdosing to have an equivalent or greater value as a reduction of 1 migraine day the first month postdosing (Figure 3A). A median reduction of 6.5% (IQR, 18.0%) in the likelihood of a migraine on day 1 postdosing was considered to have the same value as a reduction of greater than or equal to 1 migraine day the first month postdosing.
FIGURE 3.
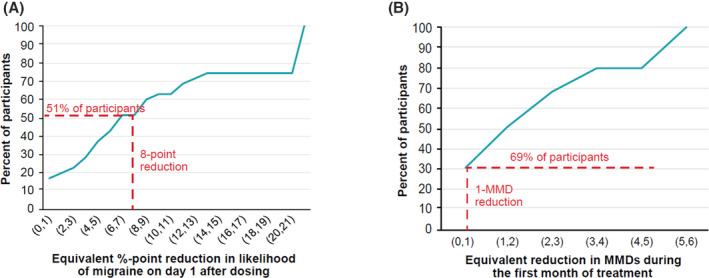
Results of thresholding exercise in participants with episodic migraine. (A) Reduction in likelihood of migraine on day 1 that has the equivalent value as reduction of 1 migraine days during the first month of treatment. (B) Reduction in monthly migraine days that has the equivalent value as the 8% reduction in the likelihood of a migraine on day 1 observed between eptinezumab and placebo in the PROMISE‐1 trial. 14 , 15 MMD, monthly migraine day [Color figure can be viewed at wileyonlinelibrary.com]
Based on choice frame 2, 69% of participants with EM (95% CI: 53.2%–84.0%) thought that an 8% point‐reduction in the likelihood of a migraine on day 1 postdosing had the same value as a reduction of greater than or equal to 1 migraine day the first month postdosing (Figure 3B). A median reduction of 1.5 days (IQR, 3.0) in the number of migraine days the first month postdosing was considered to have the same value as an 8% point‐reduction in the likelihood of a migraine on day 1 postdosing.
DISCUSSION
Previous studies have explored the willingness of patients with migraine to make trade‐offs between symptom severity during the headache and post‐headache phases of migraine 11 and between different attributes of migraine prevention medications, including MMD improvements, adverse events, and mode of administration. 12 Preferences of patients for early onset of migraine‐preventive efficacy have not been described but need to be better understood as treatments with the potential to improve it, like eptinezumab, become available.
The current study demonstrated that patients with EM or CM value the ability of preventive treatments to reduce the likelihood of a migraine the first day postdosing. Most participants considered the time to onset of migraine‐preventive efficacy offered by eptinezumab compared to placebo in the PROMISE‐1 and PROMISE‐2 trials 14 , 15 to be as or more important than clinically relevant reductions in the number of migraine days the first month postdosing. The relative value of reductions in number of migraine days the first month postdosing and improvements in time to onset, however, varied considerably between participants.
This finding has important implications for decisions about which migraine prevention treatment options to discuss with patients. In particular, a more rapid onset of migraine‐preventive efficacy has the potential to decrease the burden of migraine for patients and may help them regain control in a way that is meaningful to them. Further, when faced with treatment options that offer faster or slower onset of migraine‐preventive efficacy and different levels of reduction in the number of migraine days the first month postdosing, patients will have different views on the combination of these attributes that best meets their needs. This highlights the importance of shared decision making between clinicians and patients when selecting preventive treatments for migraine, which is part of a broader trend of focusing on the patient in regulatory, health technology, and clinical decisions. 5 , 6 , 7
This study used a thresholding methodology, which allows the minimum acceptable level of a single endpoint to be evaluated in the context of a known change in another endpoint. By focusing on preferences for changes in a single endpoint, this approach placed less cognitive burden on participants and required smaller sample sizes than other preference methods, such as discrete choice experiments. 6 Although thresholding is less common than these other preference methods, a recent review identified 43 examples in which it has been used in healthcare. 19 The review concluded that, as with other preference methods, thresholding may be sensitive to how the questions are framed. In part to address this, the current study included two choice frames to test the impact of framing on participants’ preferences. The way that participants with CM traded off attributes was consistent across the two choice frames but was less consistent for participants with EM. Straight‐lining (always choosing the same treatment option) and lexicographic preferences (always choosing the treatment with the best performance on a single attribute) can also occur in thresholding exercises, raising concerns about non‐attendance to elicitation tasks. 20 In the current study, straight‐lining was only observed in a few participants. Lexicographic preferences were more common, but because they could represent true preferences, several validity tests were used to determine whether they reflected non‐attendance or were more likely due to participants’ true preferences.
The results of this study should be interpreted in light of some potential limitations. First, the population was a small sample drawn from recruiter databases, patient associations, patient support groups, social media groups, online forums, and patient panels; eligibility was not clinically confirmed. Consequently, the study sample may not be representative of the broader population of patients with migraine. Second, stated preference methods may be subject to hypothetical bias, and the responses to hypothetical choice tasks in an experimental setting may not be replicated if patients were to face similar choices in the real world. Third, although the CM sample appeared to provide valid and consistent responses, the EM sample provided less consistent responses.
CONCLUSION
In conclusion, most participants with CM or EM considered the magnitude of the reduction in the likelihood of migraine on day 1 postdosing offered by eptinezumab to be at least as important as a clinically relevant reduction in migraine days the first month postdosing. This suggests that onset of migraine‐preventive efficacy should be included as a key outcome in clinical trials of migraine‐preventive treatments, considered in regulatory and reimbursement decisions about them, and used to inform shared decision making between clinicians and patients when selecting a treatment.
CONFLICT OF INTEREST
Agathe Le Lay, Martin Bøg, Anders Blædel Lassen, and Ann Hartry are employees of H. Lundbeck A/S. Thomas Brevig is an employee of and owns stock in H. Lundbeck A/S. Kevin Marsh and Katelyn Cutts are employees of Evidera, which was paid by Lundbeck for work related to this study.
AUTHOR CONTRIBUTIONS
Study concept and design: Kevin Marsh, Katelyn Cutts, Anders Blædel Lassen, Ann Hartry, Agathe Le Lay, and Thomas Brevig. Acquisition of data: Kevin Marsh and Katelyn Cutts. Drafting the manuscript: Kevin Marsh, Katelyn Cutts, and Ann Hartry. All authors contributed to analysis and interpretation of data and revising the manuscript for intellectual content. All authors approved the final version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Medical writing was provided by Ajinkya Nikam and Phillip S. Leventhal (Evidera) and paid for by Lundbeck.
Ailani J, Winner P, Hartry A, et al. Patient preference for early onset of efficacy of preventive migraine treatments. Headache. 2022;62:374–382. doi: 10.1111/head.14255
Funding information
The study was funded by H. Lundbeck A/S
REFERENCES
- 1. Dodick DW, Gottschalk C, Cady R, Hirman J, Smith J, Snapinn S. Eptinezumab demonstrated efficacy in sustained prevention of episodic and chronic migraine beginning on day 1 after dosing. Headache. 2020;60:2220‐2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Detke HC, Millen BA, Zhang QI, et al. Rapid onset of effect of galcanezumab for the prevention of episodic migraine: analysis of the EVOLVE studies. Headache. 2020;60:348‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tassorelli C, Diener H‐C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815‐832. [DOI] [PubMed] [Google Scholar]
- 4. Dodick DW, Turkel CC, DeGryse RE, et al. Assessing clinically meaningful treatment effects in controlled trials: chronic migraine as an example. J Pain. 2015;16:164‐175. [DOI] [PubMed] [Google Scholar]
- 5. Janus SI, Weernink MG, van Til JA, Raisch DW, van Manen JG, IJzerman MJ. A systematic review to identify the use of preference elicitation methods in health care decision making. Value Health. 2014;17:A515‐A516. [DOI] [PubMed] [Google Scholar]
- 6. de Bekker‐Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145‐172. [DOI] [PubMed] [Google Scholar]
- 7. Marsh K, Lanitis T, Neasham D, Orfanos P, Caro J. Assessing the value of healthcare interventions using multi‐criteria decision analysis: a review of the literature. Pharmacoeconomics. 2014;32:345‐365. [DOI] [PubMed] [Google Scholar]
- 8. Food and Drug Administration . Patient Preference Information—Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling: Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health and Center for Biologics Evaluation and Research; 2016. [Google Scholar]
- 9. Food and Drug Administration . Factors to Consider When Making Benefit‐Risk Determinations in Medical Device Premarket Approval and De Novo Classifications. Guidance for Industry and Food and Drug Administration Staff (FDA‐2011‐D‐0577). 2019. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/factors‐consider‐when‐making‐benefit‐risk‐determinations‐medical‐device‐premarket‐approval‐and‐de [Google Scholar]
- 10. European Medicines Agency . EMA Regulatory Science to 2025. EMA; 2020. [Google Scholar]
- 11. Gonzalez JM, Johnson FR, Runken MC, Poulos CM. Evaluating migraineurs’ preferences for migraine treatment outcomes using a choice experiment. Headache. 2013;53:1635‐1650. [DOI] [PubMed] [Google Scholar]
- 12. Mansfield C, Gebben DJ, Sutphin J, et al. Patient preferences for preventive migraine treatments: a discrete‐choice experiment. Headache. 2019;59:715‐726. [DOI] [PubMed] [Google Scholar]
- 13. Peres MFP, Silberstein S, Moreira F, et al. Patients’ preference for migraine preventive therapy. Headache. 2007;47:540‐545. [DOI] [PubMed] [Google Scholar]
- 14. Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double‐blind, placebo‐controlled study (PROMISE‐1). Cephalalgia. 2020;40:241‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE‐2. Neurology. 2020;94:e1365‐e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588‐594. [PubMed] [Google Scholar]
- 17. Fransen MP, Van Schaik TM, Twickler TB, Essink‐Bot ML. Applicability of internationally available health literacy measures in the Netherlands. J Health Commun. 2011;16(Suppl 3):134‐149. [DOI] [PubMed] [Google Scholar]
- 18. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37‐44. [DOI] [PubMed] [Google Scholar]
- 19. Hauber B, Coulter J. Using the threshold technique to elicit patient preferences: an introduction to the method and an overview of existing empirical applications. Appl Health Econ Health Policy. 2020;18:31‐46. [DOI] [PubMed] [Google Scholar]
- 20. Sri Bhashyam S, Marsh K, Quartel A, et al. A benefit‐risk analysis of pegvaliase for the treatment of phenylketonuria: a study of patients’ preferences. Mol Genet Metab Rep. 2019;21:100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


