Abstract
Background
Biologic medications, specifically tumour necrosis factor‐α (TNF‐α) inhibitors, have become increasingly prevalent in the treatment of chronic inflammatory disease (CID) in pregnancy.
Objective
To determine pregnancy outcomes in women with CID exposed to biologics during pregnancy.
Search strategy
PubMed and EMBASE databases were searched through January 1998–July 2021.
Selection criteria
Peer‐reviewed, English‐language cohort, case–control, cross‐sectional studies, and case series that contained original data.
Data collection and analysis
Two authors independently conducted data extraction. A meta‐analysis of proportions using a random‐effects model was used to pool outcomes. Linear regression analysis was used to compare the mean of proportions of outcomes across exposure groups using the ‘treated’ group as the reference category. All studies were evaluated using an appropriate quality assessment tool. The GRADE approach was used to assess the overall certainty of evidence.
Main results
Thirty‐five studies, describing 11 172 pregnancies, were eligible for inclusion. Analysis showed pooled proportions for congenital malformations as follows: treated 0.04 (95% CI 0.03–0.04; I 2 = 77) versus disease‐matched 0.04 (95% CI 0.03–0.05. I 2 = 86; p = 0.238); preterm delivery treated 0.04 (95% CI 0.10–0.14; I 2 = 88) versus disease‐matched 0.10 (95% CI 0.09–0.12; I 2 = 87; p = 0.250); severe neonatal infection: treated 0.05 (95% CI 0.03–0.07; I 2 = 88) versus disease‐matched 0.05 (95% CI 0.02–0.07; I 2 = 94; p = 0.970); low birthweight: treated 0.10 (95% CI 0.07–0.12; I 2 = 93) versus disease‐matched 0.08 (95% CI 0.07–0.09; I 2 = 0; p = 0.241); pooled miscarriage: treated 0.13 (95% CI 0.10–0.15; I 2 = 77) versus disease‐matched 0.08 (95% CI 0.04–0.11; I 2 = 5; p = 0.078); pre‐eclampsia; treated 0.01 (95% CI 0.01–0.02; I 2 = 0) versus disease‐matched 0.01 (95% CI 0.00–0.01; I 2 = 0; p = 0.193). No statistical differences in proportions were observed. GRADE certainty of findings was low to very low.
Conclusion
We demonstrated comparable pregnancy outcomes in pregnancies exposed to biologics, disease‐matched controls and CID‐free pregnancies using the GRADE approach.
Keywords: medical disorders in pregnancy, systematic reviews
Tweetable abstract
Meta‐analysis of 11 172 pregnancies exposed to biologic medications shows no evidence of harm for the fetus or the mother.
Linked article: This article is commented on by Laurine L. van der Slink, pp. 1247 in this issue. To view this minicommentary visit https://doi.org/10.1111/1471-0528.17095.
1. INTRODUCTION
Chronic inflammatory diseases (CIDs) are a group of autoimmune diseases that affect between 5 and 7% of the population and include rheumatoid arthritis, psoriatic arthritis and inflammatory bowel disease (IBD). 1 , 2 Many CIDs have a female preponderance and are often associated with activity during reproductive years. 3 , 4 , 5 They share a similar pathophysiology centring on dysregulation of the systemic immune response mediated by cytokines including tumour necrosis factor (TNF), interleukin‐1 and interleukin‐6, which are known to affect pregnancy and embryogenesis. 6 , 7 Modulation of these cytokines with the introduction of biologic agents over two decades ago was a revolution in the care of these patients and biologics are increasingly used to manage chronic autoimmune diseases during pregnancy.
Activity of CID is inherently associated with an increased risk of a range of adverse pregnancy outcomes. 8 , 9 , 10 Women with active IBD during pregnancy have higher rates of miscarriage, preterm delivery, low birthweight, congenital anomalies and caesarean section compared with a general population. 8 Likewise, there is a strong correlation with activity in rheumatoid arthritis and adverse outcomes such as miscarriage, low birthweight, pre‐eclampsia and caesarean section. 9 Disease flares are associated with a greater magnitude of risk for both women and their pregnancy with balancing the risk of disease flare with fears regarding adverse effects of biologic medications. 10
Randomised control trials on biologic medications during pregnancy are lacking and the majority of data regarding safety in pregnancy arise from case series, population data review and cohort studies. As biologics are capable of passing through the placental interface, concerns regarding safety are at the forefront of patients’ and clinicians’ minds. 11 The most recent meta‐analysis by Tsao et al 12 focused solely on studies that had a disease‐matched control group, thus limiting their review to 24 studies including ten published as abstracts only. The other most recent meta‐analyses by Komaki and Nielsen and their colleagues compare outcomes in the treatment group with the general population only, with no disease‐matched cohort included. 13 , 14
The aim of this systematic review and meta‐analysis was to evaluate and pool the available evidence with regards to maternal and neonatal adverse pregnancy outcomes across three groups, pregnant women exposed to biologics for the management of underlying CID, a disease‐matched cohort and a disease‐free comparator group.
2. METHODS
This review was performed according to an a‐priori‐designed protocol recommended for systematic reviews and meta‐analyses. 15 Maternal and fetal systematic review protocols were prospectively registered on PROSPERO CRD4201707072 and CRD42020185926.
2.1. Search methodology
The search strategy was developed with librarian assistance and was carried out through PubMed and EMBASE search engines to identify peer‐reviewed published papers relating to the association between use of biologics for CID in pregnancy and the risk of maternal and neonatal outcomes. We also conducted a post hoc search of Web of Science. Searches were conducted in accordance with the PRISMA guidelines (Preferred Reporting Items for Systematic Review and Meta‐analysis). 16 PubMed and EMBASE searches were conducted through January 1998–April 2020 by two reviewers (LOB, SA) and updated July 2021. Full search terms can be found in Appendix S1. Bibliographies of included studies were also searched for additional studies eligible for inclusion. Titles and abstracts of studies retrieved from each database were stored and managed in endnote reference manager. Three review authors (LOB, SA, AOS) reviewed titles and abstracts, obtaining full text as required.
2.2. Study inclusion and exclusion criteria
The systematic review was based on the following PICO requirements – Population: pregnant women with a diagnosis of CID; Intervention/Exposure: treatment with biologic medication; Comparison: pregnant women with a diagnosis of CID without treatment with biologics and a CID‐free population; Outcomes: fetal outcomes included congenital malformations, preterm delivery (<37 weeks), severe neonatal infection requiring hospitalisation, low birthweight (<2.5 kg) and small for gestational age (<10th centile). Maternal outcomes included severe maternal infection requiring hospitalisation, miscarriage and pre‐eclampsia.
English language randomised controlled trials, cohort, case–control, cross‐sectional studies and case series with a minimum n = 30 were eligible for inclusion. Studies must have been peer‐reviewed and contain original data. A diagnosis of a CID requiring treatment with biologics had to be described for women treated during pregnancy/or the 3 months before pregnancy (as early cessation of these medications is common).
Ineligible studies were those that did not specify maternal underlying medical condition or a condition that was not a CID, e.g. neoplasia. Studies that were not published in English, those that did not address maternal or fetal outcomes, non‐peer reviewed studies, commentaries, reviews and conference abstracts were excluded.
Where multiple publications using the same data existed, we included the most recent study, provided the earlier publication did not contain reported information not available in the most recent study.
2.3. Data extraction
Three reviewers (LOB, SA, AOS) independently extracted data using a standardised collection form for all eligible studies, including study characteristics (study design, country of data collection, data source, type of biologic studies, exposure pre‐pregnancy, throughout pregnancy, gestation of cessation, inclusion and exclusion criteria, what confounders were adjusted for) and reported outcomes (congenital malformations, preterm delivery, neonatal infection, low birthweight, small for gestational age, maternal infection, miscarriage and pre‐eclampsia) for analysis. In studies in which adequate data were not reported, an effort was made to contact authors to provide us with additional information to allow us to compute effect estimates. Risk of bias assessment was undertaken by three reviewers (LOB, SA, AOS) using an appropriate quality assessment tool described by McDonald et al. 17 which examined six types of bias; selection, exposure, outcome, confounding, analytical and attrition. Studies were then rated as minimal, low, moderate or high levels of bias. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to rate the certainty of findings. In the GRADE approach, observational studies start as low‐quality evidence. Five factors (risk of bias, imprecision, inconsistency, indirectness and publication bias) may lead to rating down the quality of evidence and three factors (large effect, dose response and if residual confounding is likely to decrease rather than increase the magnitude of effect) may lead to rating up. 18
2.4. Statistical analysis
Where data permitted, stata 14 (StataCorp, College Station, TX, USA) was used to conduct meta‐analyses of proportions using a random‐effects model. We analysed the available data from three main population groups. The ‘treated’ group were women with CID who required treatment with biologic medications throughout their pregnancy. The ‘disease‐matched’ group were women with CID not prescribed biologics in pregnancy. The ‘disease‐free’ group was a group representative of the general population (i.e. women who were pregnant and did not have a diagnosis of CID). We modelled data using the program metaprop which augments the metan program. This allowed for computation of 95% CI 19 and pooling of proportions, presenting a weighted sub‐group and overall pooled estimates with inverse‐variance weights from a random effects model. The primary analysis was performed on all eligible studies, with subgroup analyses by biologic type and by chronic inflammatory disease subtype where appropriate. We used linear regression analysis to compare the mean of proportions of outcomes across exposure groups using the ‘treated’ group as the reference category. A sensitivity analysis by disease diagnosis, drug type, risk of bias and study design was performed where heterogeneity was high.
3. RESULTS
The search for fetal outcomes produced 1887 titles; after exclusion of duplicates and ineligible studies, 35 4 , 9 , 11 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 studies were eligible and 33 were included in the meta‐analysis (Figure S1). The initial search for maternal outcomes produced 2104 results; after exclusion for duplicates and ineligible studies, 34 were suitable for inclusion and 25 studies were included in the maternal outcome meta‐analysis (Figure S2).
Overall, there were 35 individual observational studies included in the meta‐analysis, 24 cohort studies and 11 case series fulfilling the inclusion criteria. This review contains a total of 35 studies with 11 172 pregnancies exposed to biologics, 17 studies with 39 290 disease‐matched controls and nine studies with 2 892 933 chronic inflammatory disease‐free pregnancies used for the meta‐analysis. The addition of the peer‐reviewed case series added 2653 women exposed to biologics in this meta‐analysis.
Risk of bias assessment was undertaken using an appropriate tool, 17 all studies included had a low to moderate grading (Table S3). Sensitivity analysis was performed across all outcomes in the meta‐analysis with available numbers by disease diagnosis of participants, drug type, risk of bias and study type. Overall, the heterogeneity is likely to be due to a combination of factors including disease diagnosis grouping, study type and drug type. The sensitivity analysis did not change the prevalence estimates among the subgroups. All results are included in Tables S4–S9. GRADE certainty of findings was low to very low (Table S10).
3.1. Congenital malformations
There were 28 eligible studies in the congenital malformation meta‐analysis. There were 1 288 762 infants in this cohort (7811 born to women using biologics in pregnancy, 29 171 infants born to women with CID who were not treated with biologics in pregnancy and 1 251 780 infants born to women who were CID free) (Table 1). The proportion of congenital malformations in the treated group was 0.04 (95% CI 0.03–0.04), in the disease‐matched group was 0.04 (95% CI 0.03–0.05) and in the disease‐free group was 0.04 (95% CI 0.03–0.05). No differences were observed in proportions between disease‐matched compared with disease‐treated women (p = 0.238), nor in disease‐free compared with disease‐treated women (p = 0.579) (Figure 1 and Table S1).
TABLE 1.
Fetal outcomes with the use of biologics in pregnancy
| Fetal outcomes | Number of studies | Number of patients | Proportion of event | 95% CI | I 2% |
|---|---|---|---|---|---|
| Congenital malformations | |||||
| Treated group | 28 | 7811 | 0.04 | 0.03–0.04 | 77.3 |
| Disease‐matched | 10 | 29 171 | 0.04 | 0.03–0.05 | 86.8 |
| Disease‐free | 5 | 1 251 780 | 0.04 | 0.03–0.05 | 46.3 |
| Preterm delivery | |||||
| Treated group | 26 | 7728 | 0.12 | 0.10–0.14 | 88.4 |
| Disease‐matched | 11 | 18 574 | 0.10 | 0.09–0.12 | 87.3 |
| Disease‐free | 6 | 1 626 254 | 0.06 | 0.04–0.07 | 85.9 |
| General population WHO | – | – | 9% | 0.09–0.09 | – |
| Neonatal infection | |||||
| Treated group | 9 | 3554 | 0.05 | 0.03–0.07 | 88.3 |
| Disease‐matched | 6 | 4015 | 0.05 | 0.02–0.07 | 94.0 |
| Disease‐free | 2 | 14 799 | 0.02 | 0.02–0.02 | 0.0 |
| Low birthweight | |||||
| Treated group | 17 | 5112 | 0.10 | 0.07–0.12 | 92.5 |
| Disease‐matched | 5 | 3046 | 0.08 | 0.07–0.09 | 0.0 |
| Disease‐free | 4 | 3316 | 0.04 | 0.01–0.08 | 95.4 |
| Small for gestational age | |||||
| Treated group | 8 | 3342 | 0.06 | 0.02–0.10 | 96.1 |
| Disease‐matched | 4 | 10 720 | 0.07 | 0.02–0.11 | 97.3 |
| Disease‐free | 4 | 1 624 533 | 0.10 | 0.10–0.10 | 0.00 |
FIGURE 1.

Metaprop proportions for congenital malformations
Sub‐analysis by chronic inflammatory disease type did not significantly change results. Pregnancies in women with IBD treated with biologics had a proportion of congenital malformations of 0.04 (95% CI 0.02–0.05) compared with CID overall having 0.04 (95% CI 0.02–0.06) and rheumatoid arthritis alone 0.04 (95% CI 0.02–0.05) (Figure S5).
3.2. Preterm delivery
There were 26 studies included in the preterm birth (PTB) meta‐analysis. This included 7728 pregnancies exposed to biologics and 18 574 disease‐matched controls (Table 1). The proportion of PTB was 0.12 (95% CI 0.10–0.14) in the treated group, 0.10 (95% CI 0.09–0.012) in the disease‐matched group and 0.06 (95% CI 0.04–0.07) in the disease‐free group (Figure S4). There was no statistical difference in the treated group versus the disease‐matched group (p = 0.250), but there was a statistical difference when comparing disease‐free with disease‐treated women (p = 0.008 Table S1).
Subgroup analysis examining anti‐TNF‐α only revealed a change in the PTB rate for the treated group to 0.11 (95% CI 0.09–0.13) (Figure 2). Subgroup analysis by disease classification showed anti‐TNF‐α‐treated IBD had a PTB rate of 0.09 (95% CI 0.07–0.11) with the disease‐matched IBD group having a PTB rate of 0.09 (95% CI 0.08–0.010) (Figure S5). Only one study focused on anti‐TNF‐α use and rheumatoid arthritis 20 with a treated PTB rate of 0.18 (95% CI 0.14–0.24) and disease‐matched PTB rate of 0.14 (95% CI 0.12–0.15). The remaining studies focused on mixed CID with a preterm birth rate of 0.12 (95% CI 0.09–0.15).
FIGURE 2.
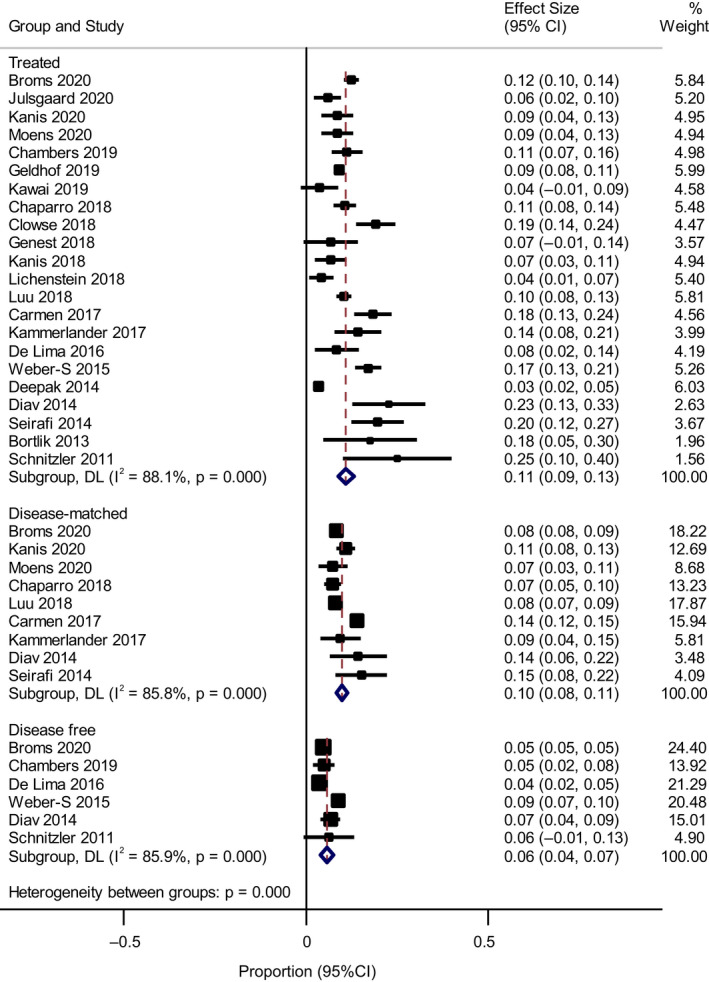
Metaprop proportions of preterm delivery with anti‐tumour necrosis factor‐α (TNF‐α) use only
3.3. Neonatal infection
The pooled data on severe neonatal infection included nine studies with 22 368 neonates. This was divided into 7569 neonates born to women with CID, 3554 neonates born to women requiring biologics treatment during pregnancy and 4015 disease‐matched controls (Table 1).
The proportion of severe neonatal infections requiring hospitalisations in the treated group was 0.05 (95% CI 0.03–0.07), the proportion in the disease‐matched group was 0.05 (95% CI 0.02–0.07) and in the disease‐free group was 0.02 (95% CI 0.02–0.02) (Figure S6). No differences were observed in proportions between disease‐matched and disease‐treated women (p = 0.970), nor in disease‐free compared with disease‐treated women (p = 0.225; Table S1). Subgroup analysis by disease diagnosis revealed no statistical difference between groups (results not shown).
3.4. Birthweight
In the low‐birthweight group there were 17 studies included with a total of 11 474 infants, 5112 exposed to maternal biologic use, 3046 disease‐matched and 3316 CID‐free pregnancies (Table 1).
The overall proportion of low birthweight in the treated group was 0.10 (95% CI 0.07–0.12), in the disease‐matched group was 0.08 (95% CI 0.07–0.09) and in the disease‐free group was 0.04 (95% CI 0.01–0.08) (Figure S7). No statistical differences were observed between disease‐matched and disease‐treated women (p = 0.241), nor in disease‐free women (p = 0.079; Table S1). Sub‐group analysis on anti‐TNF‐α use alone revealed no difference between group outcomes (results not shown).
The small‐for gestational‐age group included seven studies with 1 638 595 infants, 3342 born to women medicated with biologics during pregnancy and 10 720 disease‐matched controls (Table 1). All of the small‐for gestational‐age studies focused on anti‐TNF‐α biologics only. The proportion of small‐for gestational‐age infants in the treated group was 0.06 (95% CI 0.02–0.10), in the disease‐matched control group was 0.07 (95% CI 0.02–0.11) and in the disease‐free population was 0.10 (95% CI 0.10–0.10; Figure S8). No differences were observed in proportions between disease‐matched compared with disease‐treated women (p = 0.753), nor in comparison with disease‐free women (p = 0.170; Table S1).
3.5. Maternal infection
The data available on maternal serious infection requiring hospitalisation included two studies, both of which focused on anti‐TNF‐α use in pregnancy. There was a total of 1685 pregnant women in this cohort. 21 , 22 There were 916 women who were treated with biologics during their pregnancy (Table 2) and 453 disease‐matched control women. The studies included in this analysis were Chaparro et al., 21 who focused on anti‐TNF‐α use in IBD and pregnancy, and Clowse et al., 22 who investigated certolizumab pegol use in CID in pregnancy.
TABLE 2.
Maternal pregnancy outcomes with the use of biologics in pregnancy
| Maternal outcomes | Number of studies | Number of patients | Proportion of event | 95% CI | I 2 |
|---|---|---|---|---|---|
| Maternal severe infection | |||||
| Treated group | 2 | 916 | 0.04 | 0.03–0.05 | 0.0 |
| Disease‐matched | 1 | 453 | 0.01 | 0.00–0.02 | 0.0 |
| Disease‐free | 0 | – | – | – | |
| Miscarriage | |||||
| Treated group | 16 | 3348 | 0.13 | 0.010–0.15 | 76.9 |
| Disease‐matched | 4 | 3424 | 0.08 | 0.04–0.11 | 74.5 |
| Disease‐free | 4 | 3641 | 0.11 | 0.03–0.19 | 98.2 |
| Pre‐eclampsia | |||||
| Treated group | 5 | 1175 | 0.01 | 0.01–0.02 | 0.0 |
| Disease‐matched | 2 | 1017 | 0.01 | 0.00–0.01 | 0.0 |
| Disease‐free | 0 | – | – | – | |
Only two studies were included in this analysis with a statistical difference in proportions between disease‐matched compared with disease‐treated women (p < 0.001; Table S2). Overall, the pooled analysis showed that the treated group had a proportion of serious infection of 0.04 (95% CI 0.03–0.05), whereas the disease‐matched group had a proportion of serious infections of 0.01 (95% CI 0.00–0.02; Figure S9).
3.6. Miscarriage
The data available on miscarriage included 15 studies with 9368 pregnancies. There were 2708 pregnancies in women treated with biologics in pregnancy (Table 2). The proportion of miscarriage in the biologics‐treated group was 0.13 (95% CI 0.10–0.15), the proportion of miscarriage in the disease‐matched group was 0.08 (95% CI 0.04–0.11) and in the disease‐free group was 0.11 (95% CI 0.03–0.19) (Figure S10). There was no significant difference in proportions between disease‐matched compared with disease‐treated women (p = 0.078), or in disease‐free compared with disease‐treated women (p = 0.631; Table S2). One study was excluded from the analysis; it had no difference in miscarriage rate across the treated and untreated groups of 3%, but the rate was much lower than all other included studies. 11 . Subgroup analysis revealed no difference in groups when focusing on anti‐TNF‐α studies only (results not shown).
3.7. Pre‐eclampsia
There were five eligible studies for inclusion in the meta‐analysis for pre‐eclampsia. They included data on 1175 pregnant women treated with biologics and 1017 disease‐matched controls (Table 2). In the treated group the percentage of pre‐eclampsia was 0.01 (95% CI 0.01–0.02) and of disease‐matched controls was 0.01 (95% CI 0.00–0.01; Figure S11). No differences were observed in proportions between disease‐matched women and disease‐treated women (p = 0.193; Table S2).
4. DISCUSSION
4.1. Main findings
We have expanded on the previous reviews by increasing the pool of data to include peer‐reviewed cohort studies and case series 12 , 13 , 14 with statistical analysis of proportions rather than odds ratio. Another novel approach that we have taken was allowing comparisons across three populations: treated, disease‐matched and disease‐free. We also performed sub‐analysis by biologic type and disease type where appropriate.
We detected no difference in the rates of congenital malformations, neonatal infection, low birthweight, small for gestational age, miscarriage or pre‐eclampsia with the use of biologics in pregnancy. The rates of preterm delivery were not statistically different between the treated and the disease‐matched groups but there was a statistical difference noted between the treated and disease‐free groups, possibly pointing towards the disease process being a factor. Too few studies examined serious maternal infection as an outcome to be concluded upon. These treatments are possibly safe, but their use in each woman must be individualised and include a thorough evaluation of the benefit–risk profile.
4.2. Strengths and limitations
Studies examining the use of biologics in pregnancy have been limited by observational studies with small sample size. To our knowledge, this is the largest cohort of pregnancies exposed to biologics collated to date. This review was based on a pre‐published protocol on PROSPERO and followed the PRISMA guidelines. This review allows areas of future research to be highlighted that are currently lacking, primarily elucidating the links with preterm birth and maternal infection risk, as well as exploring likely protective effects of these medications against uncontrolled disease activity. There are several limitations to this study; first, our search included English‐only literature, The full search strategy only included two databases; therefore, we conducted a post‐hoc search of Web of Science. However, no new studies were identified in the process. Second, disparities in the measurement criteria, e.g. birthweight, infection criteria and even the diagnostic criteria for congenital malformation, make meta‐analysis on this topic difficult with an already small sample being made smaller by misusing the appropriate definition and criteria. Studies included in this systematic review also contain some limitations. For example, although the majority of studies were classified as minimal or low risk of bias, GRADE certainty of findings was low to very low. Third, the recording of concomitant medications, particularly corticosteroids, dosing and pregnancy outcomes, which can differ between studies, is imprecise at best. Fourth, pregnancy outcomes in this population can be influenced by the activity of the underlying disease state peri‐conceptually and during pregnancy, which can be difficult to accurately collate in these observational studies and difficult to control for by using a’ ‘disease‐matched cohort’ when matched by diagnosis only. Finally, the available studies for inclusion in this systematic review were observational in nature (i.e cohort studies and case series studies only) as there are no randomised controlled trials examining the potential impact of biologics in pregnancy on fetal and maternal outcomes. Using proportions rather than measures of association such as odds ratios increased the total number of women substantially but may have introduced a higher risk of bias and residual confounding. Therefore, the results should be interpreted with caution. Furthermore, although our disease‐matched control group was drawn from a highly selected population of women with the same disease diagnosis as the treated group, confounding by indication is of concern.
4.3. Interpretation
We found no evidence to suggest that biologics, with the strongest evidence for the TNF‐α inhibitor class, increase the risk of congenital anomalies. This further expands on the evidence provided from previous meta‐analyses that found no difference in congenital anomalies such as reported by Tsao et al. 12 (OR 1.18, 95% CI 0.88–1.5), Komaki et al. 13 and Nielsen et al. 14 .This is the largest preterm birth meta‐analysis to date, with 26 studies included in the preterm birth meta‐analysis with 7728 pregnancies exposed to biologics. As expected, a higher rate of PTB was observed in the treated group (12%) compared with 6% in the disease‐free group (p = 0.008), with no difference in the rates observed between the treated and disease‐matched groups.
Comparing our findings with those of previous meta‐analyses, Tsao et al. 12 found the risk of PTB in treated versus disease‐matched women to be (adjusted hazard ratio 1.09, 95% CI 0.70–1.69), Komaki et al. 13 reported a 2.62‐fold higher rate of PTB in the treated group in comparison to the general population and Nielsen et al. 14 found the overall pooled prevalence of PTB to be 9% (95% CI 7–11%; I 2 = 89) with biologics use in pregnancy, comparable with a rate of preterm birth of 10% in a cohort of 1960 women with IBD including active disease and 11% in the background population.
The disease‐free group rates of 6% compare with WHO PTB rates for Europe and North America of 9% and 11%, respectively. 52 The lower rate of PTB in our disease‐free population likely reflects the inclusion of a large study by Bröms et al. 4 which encompasses population registry data from Denmark, Finland and Sweden.
One of the primary factors that may impact PTB rates is disease activity. Geldhof et al. concluded that women with active or flaring disease during pregnancy were at a much higher risk of complications irrespective of exposure to biologics with their exposure group having a PTB rate of 9.2% (n = 143) and their corticosteroid group a PTB rate of 14.7% (n = 36). 23 Of concern, maternal disease may flare when biologic agents are discontinued and this itself may impact on PTB rates; this was reported with discontinuation of biologics before 24 weeks of gestation and a higher incidence of preterm delivery in a number of studies included in this analysis. 4 , 23 , 24
Our meta‐analysis revealed no statistical difference in severe neonatal infection between groups. The only previous meta‐analysis to review neonatal infection was Tsao et al. 12 , there were four studies included (n = 1669) and no association between biologic exposure in utero and the risk of serious infections requiring hospitalisation in infants' first year of life (adjusted odds ratio 1.09, 95% CI 0.82–1.47).
This was similar to the findings of two large studies that were eligible but could not be included in the meta‐analysis, Tsao et al. (2019) 3 (because of a data‐sharing agreement) and Luu et al. 24 Tsao et al. in 2019 found no increased risk of serious infections in the first year postpartum for either the mothers or the neonates. 3 . Similarly, Luu et al. found no difference in community‐acquired or hospital‐acquired infections in the biologic‐treated group compared with a disease‐matched population. 24 Studies specifically examining biologic use during the third trimester found no alteration in the neonatal infection rate during the first 12 months of life for the neonate. 11 , 21 , 25 , 26 , 31 The only variable associated with an increased incidence of neonatal infection in multiple studies was PTB. 11 , 21 , 24
Our meta‐analysis found no statistical difference in birthweight with biologic use. Literature around this topic has been controversial with previous individual studies highlighting the greater prevalence of growth‐restricted/low‐birthweight babies in women with chronic inflammatory conditions. 4 , 20
Previous meta‐analysis by Nielsen et al. 14 found the overall pooled prevalence of low birthweight to be 8% (95% CI 5–10%) with biologics. Komaki et al. 13 found 5.95‐fold higher rate of low birthweight compared with the general population but no difference with users and non‐users 1.33 (p = 0.31, 95% CI 0.77–2.30). Tsao et al. 12 found the odds ratio for low birthweight was 1.68 (95% CI 1.21–2.31) in pregnancies exposed to a biologic compared with unexposed prgnancies.
Of the eligible studies included in this meta‐analysis, Moens et al. had the largest treated group and found no difference between their treated and untreated groups. 27 Tsao et al. found an odds ratio for the association between biologic exposure and SGA was 0.91 (95% CI 0.46–1.78). 28 The PIANO trial found no difference in LBW in those exposed to biologics after controlling for PTB and disease activity. 11
Our analysis included only two studies examining severe maternal infection. Data from the recent large Canadian 10‐year retrospective cohort study by Tsao et al. could not be obtained. 3 However, in published work they reported the occurrence of serious maternal infections to be rare with an incidence of 0–5%, which is similar to the proportions found in our meta‐analysis. Luu et al. found no difference in infection rates (community or hospital treated) in women treated with biologic agents in their third trimester but echoing findings above, those who discontinued were more likely to have a flare of their inflammatory disease. 24 This topic was either not reported on in previous meta‐analyses or could not be analysed. 12 , 13 , 14
There was no greater likelihood of miscarriage for biologic‐treated patients compared with the comparator populations. This mirrors previous data reported, 22 , 29 , 30 , 31 which did not find an increased risk of miscarriage with biologics.
Assimilating the data on pre‐eclampsia, which is a less studied outcome of interest, we found no data to suggest that TNF inhibitors increase the risk. These data reflect information obtained from a small number of studies. Notably, no pre‐eclampsia cases were reported with certolizumab pegol use in Clowse et al. 22 Chaparro et al. found pre‐eclampsia equally distributed across both the biologics‐exposed group and the disease‐matched cohort. 21 Julsgaard et al. specifically found no difference in the pre‐eclampsia rates between women who ceased their medication before 30 weeks of gestation or continued. 32
5. CONCLUSION
The available data in the published literature show no increased risk with biologic use during pregnancy over disease‐matched cohorts with respect to congenital malformations, preterm delivery, neonatal infection, small for gestational age, low birthweight, miscarriage and pre‐eclampsia. There is a suggestion of an increased risk of maternal infections in the treated group but this is probably due to the lack of studies examining this outcome. It should be noted that the GRADE certainty of findings was low to very low.
With over 11 172 pregnancies exposed to biologic medications, our study shows no evidence of harm for the fetus or the mother. More evidence is required to prove the likely protective effects of these medications from unwanted outcomes that disease flare may cause in CID‐affected pregnancies. This is important for gastroenterologists, rheumatologists and obstetricians alike when reassuring women regarding continuation of treatment throughout pregnancy and for refractory disease during childbearing years.
Funding information
This project was funded via an education bursary awarded to Dr Grainne Murphy by UCB Pharma. The funder had no role in the design, analysis or interpretation of the research and manuscript and had no part in the research process following funding. Open access funding provided by IReL.
CONFLICT OF INTEREST
None declared. Completed disclosure of interests form available to view online as supporting information.
AUTHOR CONTRIBUTIONS
FMcC, AK and GMurphy conceived the study and FMcC, AK, GMurphy, SA, LOB and GMaher designed the protocol. LOB and SA performed the literature search. LOB, SA and AOS selected the studies and extracted the relevant information. LOB and GMaher synthesised the data. LOB wrote the first draft of the paper. FMcC, AK, GMurphy and GMaher critically revised successive drafts of the paper. LOB is the guarantor of the review.
Supporting information
Appendix S1
Data S2
Data S3
Data S4
Data S5
Data S6
Data S7
Data S8
O’Byrne LJ, Alqatari SG, Maher GM, O’Sullivan AM, Khashan AS, Murphy GP, McCarthy FP. (2022). Fetal and maternal outcomes after maternal biologic use during conception and pregnancy: A systematic review and meta‐analysis. BJOG: Int J Obstet Gy. 2022;129:1236–1246. 10.1111/1471-0528.17093
Linked article: This article is commented on by Laurine L. van der Slink, pp. 1247 in this issue. To view this minicommentary visit https://doi.org/10.1111/1471-0528.17095.
Grainne P. Murphy and Fergus P. McCarthy contributed equally.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article
REFERENCES
- 1. Maniadakis N, Toth E, Schiff M, Wang X, Nassim M, Szegvari B, et al. A targeted literature review examining biologic therapy compliance and persistence in chronic inflammatory diseases to identify the associated unmet needs, driving factors, and consequences. Adv Ther. 2018;35(9):1333–55. 10.1007/s12325-018-0759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacobs P, Bissonnette R, Guenther LC. Socioeconomic burden of immune‐mediated inflammatory diseases – focusing on work productivity and disability. J Rheumatol. 2011;38(Suppl 88):55–61. 10.3899/jrheum.110901. [DOI] [PubMed] [Google Scholar]
- 3. Tsao NW, Lynd LD, Sayre EC, Sadatsafavi M, Hanley G, De Vera MA. Use of biologics during pregnancy and risk of serious infections in the mother and baby: a Canadian population‐based cohort study. BMJ Open. 2020;29(3):316–27. 10.1136/bmjopen-2018-023714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bröms G, Kieler H, Ekbom A, Gissler M, Hellgren K, Lahesmaa‐Korpinen A‐M, et al. Anti‐TNF treatment during pregnancy and birth outcomes: a population‐based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29(3):316–27. 10.1002/pds.4930. [DOI] [PubMed] [Google Scholar]
- 5. Kvien TK, Uhlig T, Ødegård S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 2006;1069(1):212–22. 10.1196/annals.1351.019 [DOI] [PubMed] [Google Scholar]
- 6. De Lorenzo R, Ramirez GA, Punzo D, Lorioli L, Rovelli R, Canti V, et al. Neonatal outcomes of children born to mothers on biological agents during pregnancy: state of the art and perspectives. Pharmacol Res. 2020;152(SI):104583. 10.1016/j.phrs.2019.104583 [DOI] [PubMed] [Google Scholar]
- 7. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49(3):397–412. 10.1016/j.immuni.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornish J, Tan E, Teare J, Teoh TG, Rai R, Clark SK, et al. A meta‐analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007;56(6):830–7. 10.1136/gut.2006.108324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Genest G, Spitzer KA, Laskin CA. Maternal and fetal outcomes in a cohort of patients exposed to tumor necrosis factor inhibitors throughout pregnancy. J Rheumatol. 2018;45(8):1109–15. [DOI] [PubMed] [Google Scholar]
- 10. Selinger CP, Nelson‐Piercy C, Fraser A, Hall V, Limdi J, Smith L, et al. IBD in pregnancy: recent advances, practical management. Front Gastroenterol. 2020;12(3):1–11. 10.1136/flgastro-2019-101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahadevan U, Long MD, Kane SV, Roy A, Dubinsky MC, Sands BE, et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology. 2021;160(4):1131–9. 10.1053/j.gastro.2020.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsao NW, Rebic N, Lynd LD, De Vera MA. Maternal and neonatal outcomes associated with biologic exposure before and during pregnancy in women with inflammatory systemic diseases: a systematic review and meta‐analysis of observational studies. Rheumatology. 2020;59(8):1808–17. [DOI] [PubMed] [Google Scholar]
- 13. Komaki F, Komaki Y, Micic D, Ido A, Sakuraba A. Outcome of pregnancy and neonatal complications with anti‐tumor necrosis factor‐α use in females with immune mediated diseases; a systematic review and meta‐analysis. J Autoimmun. 2017;76:38–52. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen OH, Gubatan JM, Juhl CB, Streett SE, Maxwell C. Biologics for inflammatory bowel disease and their safety in pregnancy: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2021;20(1):74–87. [DOI] [PubMed] [Google Scholar]
- 15. NHS Centre for Reviews and Dissemination . Systematic reviews: CRD's guidance for undertaking reviews in health care. York, UK: University of York; 2009. [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta‐analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):138–48. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Thomas J, Chandler J. Cochrane Handbook for Systematic Reviews of Interventions.; 2019. 10.1002/9781119536604. [DOI] [Google Scholar]
- 19. Nyaga VN, Arbyn M, Aerts M. Metaprop: a stata command to perform meta‐analysis of binomial data. Arch Public Heal. 2014;72(1):1–10. 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carman WJ, Accortt NA, Anthony MS, Iles J, Enger C. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26(9):1109–18. 10.1002/pds.4261. [DOI] [PubMed] [Google Scholar]
- 21. Chaparro M, Verreth A, Lobaton T, Gravito‐Soares E, Julsgaard M, Savarino E, et al. Long‐term safety of in utero exposure to antiTNFα drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY study. Am J Gastroenterol. 2018;113(3):396–403. [DOI] [PubMed] [Google Scholar]
- 22. Clowse MEB, Scheuerle AE, Chambers C, Afzali A, Kimball AB, Cush JJ, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70(9):1399–407. 10.1002/art.40508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geldhof A, Slater J, Clark M, Chandran U, Coppola D. Exposure to in Infliximab during pregnancy: post‐marketing experience. Drug Saf. 2020;43:147–61. 10.1007/s40264-019-00881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luu M, Benzenine E, Doret M, Michiels C, Barkun A, Degand T, et al. Continuous Anti‐TNFα use throughout pregnancy: possible complications for the mother but not for the fetus. A retrospective cohort on the french national health insurance database (EVASION). Am J Gastroenterol. 2018;113(11):1669–77. [DOI] [PubMed] [Google Scholar]
- 25. Kanis SL, Modderman S, Escher JC, Erler N, Beukers R, de Boer N, et al. Health outcomes of 1000 children born to mothers with inflammatory bowel disease in their first 5 years of life. Gut. 2021;70(7):1266–74. 10.1136/gutjnl-2019-319129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Lima A, Zelinkova Z, van der Ent C, Steegers EAP, van der Woude CJ. Tailored anti‐TNF therapy during pregnancy in patients with IBD: Maternal and fetal safety. Gut. 2016;65(8):1261–8. [DOI] [PubMed] [Google Scholar]
- 27. Moens A, van der Woude CJ, Julsgaard M, Humblet E, Sheridan J, Baumgart DC, et al. Pregnancy outcomes in inflammatory bowel disease patients treated with vedolizumab, anti‐TNF or conventional therapy: results of the European CONCEIVE study. Aliment Pharmacol Ther. 2020;51(1):129–38. 10.1111/apt.15539 [DOI] [PubMed] [Google Scholar]
- 28. Tsao NW, Sayre EC, Hanley G, Sadatsafavi M, Lynd LD, Marra CA, et al. Risk of preterm delivery and small‐for‐gestational‐age births in women with autoimmune disease using biologics before or during pregnancy: a population‐based cohort study. Ann Rheum Dis. 2018;77(6):869–74. 10.1136/annrheumdis-2018-213023 [DOI] [PubMed] [Google Scholar]
- 29. Chambers CD, Johnson DL, Xu R, Luo Y, Lopez‐Jimenez J, Adam MP, et al. Birth outcomes in women who have taken adalimumab in pregnancy: a prospective cohort study. PLoS One. 2019;14(10):1–17. 10.1371/journal.pone.0223603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawai Y, Tsuchiya T, Aoki S. Pregnancy outcomes of patients exposed to Adalimumab in Japan. Dig Dis. 2019;37(2):123–30. 10.1159/000493462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lichtenstein GR, Feagan BG, Mahadevan U, Salzberg BA, Langholff W, Morgan JG, et al. Pregnancy outcomes reported during the 13‐ year TREAT registry: a descriptive report. Am J Gastroenterol. 2018;113(11):1678–88. 10.1038/s41395-018-0202-9. [DOI] [PubMed] [Google Scholar]
- 32. Julsgaard M, Hvas CL, Gearry RB, Gibson PR, Fallingborg J, Sparrow MP, et al. Anti‐TNF therapy in pregnant women with inflammatory bowel disease: effects of therapeutic strategies on disease behavior and birth outcomes. Inflamm Bowel Dis. 2020;26(1):93–102. 10.1093/ibd/izz110 [DOI] [PubMed] [Google Scholar]
- 33. Kimball AB, Guenther L, Kalia S, de Jong EMGJ, Lafferty KP, Chen DY, et al. Pregnancy outcomes in women with moderate‐to‐severe psoriasis from the psoriasis longitudinal assessment and registry (PSOLAR). JAMA Dermatology. 2021;157(3):301. 10.1001/jamadermatol.2020.5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanis SL, de Lima‐Karagiannis A, van der Ent C, Rizopoulos D, van der Woude CJ. Anti‐TNF levels in cord blood at birth are associatedwith anti‐TNF type. J Crohns Colitis. 2018;12(8):939–47. 10.1093/ecco-jcc/jjy058 [DOI] [PubMed] [Google Scholar]
- 35. Vinet É, De Moura C, Pineau CA, Abrahamowicz M, Curtis JR, Bernatsky S. Serious infections in rheumatoid arthritis offspring exposed to tumor necrosis factor inhibitors: a cohort study. Arthritis Rheumatol. 2018;70(10):1565–71. 10.1002/art.40536 [DOI] [PubMed] [Google Scholar]
- 36. Burmester GR, Landewé R, Genovese MC, Friedman AW, Pfeifer ND, Varothai NA, et al. Adalimumab long‐term safety: infections, vaccination response and pregnancy outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(2):414–7. 10.1136/annrheumdis-2016-209322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bröms G, Granath F, Ekbom A, Hellgren K, Pedersen L, Sørensen HT, et al. Low risk of birth defects for infants whose mothers are treated with anti‐tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14(2):234–41. 10.1016/j.cgh.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 38. Clowse MEB, Feldman SR, Isaacs JD, Kimball AB, Strand V, Warren RB, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39(8):755–62. 10.1007/s40264-016-0431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoeltzenbein M, Beck E, Rajwanshi R, Gøtestam Skorpen C, Berber E, Schaefer C, et al. Tocilizumab use in pregnancy: analysis of a global safety database including data from clinical trials and postmarketing data. Semin Arthritis Rheum. 2016;46(2):238–45. 10.1016/j.semarthrit.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 40. Nakajima K, Watanabe O, Mochizuki M, Nakasone A, Ishizuka N, Murashima A. Pregnancy outcomes after exposure to tocilizumab: a retrospective analysis of 61 patients in Japan. Mod Rheumatol. 2016;26(5):667–71. 10.3109/14397595.2016.1147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bazzani C, Scrivo R, Andreoli L, et al. Prospectively‐followed pregnancies in patients with inflammatory arthritis taking biological drugs: an Italian multicentre study. Clin Exp Rheumatol. 2015;33(5):688–93. [PubMed] [Google Scholar]
- 42. Kumar M, Ray L, Vemuri S, Simon TA. Pregnancy outcomes following exposure to abatacept during pregnancy. Semin Arthritis Rheum. 2015;45(3):351–6. 10.1016/j.semarthrit.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 43. Weber‐Schoendorfer C, Oppermann M, Wacker E, Bernard N, Beghin D, Cuppers‐Maarschalkerweerd B, et al. Pregnancy outcome after TNF‐α inhibitor therapy during the first trimester: a prospective multicentre cohort study. Br J Clin Pharmacol. 2015;80(4):727–39. 10.1111/bcp.12642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cooper WO, Cheetham TC, Li DK, Stein CM, Callahan ST, Morgan TM, et al. Risk of adverse fetal outcomes associated with immunosuppressive medications for chronic immune‐mediated diseases in pregnancy. Arthritis Rheumatol. 2014;66(2):444–50. 10.1002/art.38262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deepak P, Stobaugh DJ. Maternal and foetal adverse events with tumour necrosis factor‐alpha inhibitors in inflammatory bowel disease. Aliment Pharmacol Ther. 2014;40(9):1035–43. 10.1111/apt.12936. [DOI] [PubMed] [Google Scholar]
- 46. Diav‐Citrin O, Otcheretianski‐Volodarsky A, Shechtman S, Ornoy A. Pregnancy outcome following gestational exposure to TNF‐alpha‐inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78–84. 10.1016/j.reprotox.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 47. Seirafi M, De Vroey B, Amiot A, Seksik P, Roblin X, Allez M, et al. Factors associated with pregnancy outcome in anti‐TNF treated women with inflammatory bowel disease. Aliment Pharmacol Ther. 2014;40(4):363–73. 10.1111/apt.12833 [DOI] [PubMed] [Google Scholar]
- 48. Bortlik M, Machkova N, Duricova D, Malickova K, Hrdlicka L, Lukas M, et al. Pregnancy and newborn outcome of mothers with inflammatory bowel diseases exposed to anti‐TNF‐α therapy during pregnancy: three‐center study. Scand J Gastroenterol. 2013;48(8):951–8. 10.3109/00365521.2013.812141. [DOI] [PubMed] [Google Scholar]
- 49. Schnitzler F, Fidder H, Ferrante M, Ballet V, Noman M, Van Assche G, et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm Bowel Dis. 2011;17(9):1846–54. 10.1002/ibd.21583. [DOI] [PubMed] [Google Scholar]
- 50. Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn's disease and rheumatoid arthritis. Am J Gastroenterol. 2004;99(12):2385–92. 10.1111/j.1572-0241.2004.30186.x. [DOI] [PubMed] [Google Scholar]
- 51. Kammerlander H, Nielsen J, Knudsen T, Kjeldsen J, Friedman S, Nørgård BM. Anti–TNF‐α use during the third trimester of pregnancy in women with moderate–severe inflammatory bowel disease and the risk of preterm birth and low birth weight. Inflamm Bowel Dis. 2017;23(11):1916–23. 10.1097/MIB.0000000000001234. [DOI] [PubMed] [Google Scholar]
- 52. Chawanpaiboon S, Vogel JP, Moller A‐B, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Heal. 2019;7(1):e37–46. 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data S2
Data S3
Data S4
Data S5
Data S6
Data S7
Data S8
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article


