Abstract
The liability of the H2-receptor antagonist nizatidine (NZ) to nitrosation in simulated gastric juice (SGJ) and under WHO-suggested conditions was investigated for the first time. For monitoring the nitrosatability of NZ, a hydrophilic interaction liquid chromatography (HILIC) method was optimized and validated according to FDA guidance. A Cosmosil HILIC® column and a mobile phase composed of acetonitrile: 0.04 M acetate buffer pH 6.0 (92:8, v/v) were used for the separation of NZ and its N-nitroso derivative (NZ-NO) within 6 min with LODs of 0.02 and 0.1 μg/mL, respectively. NZ was found highly susceptible to nitrosation in SGJ reaching 100% nitrosation in 10 min, while only 18% nitrosation was observed after 160 min under the WHO-suggested conditions. The chemical structure of NZ-NO was clarified by ESI+/MS. In silico toxicology study confirmed the mutagenicity and toxicity of NZ-NO. Experiments evidenced that ascorbic acid strongly suppresses the nitrosation of NZ suggesting their co-administration for protection from potential risks. In addition, the impacts of the HILIC method on safety, health, and environment were favorably evaluated by three green analytical chemistry metrics and it was proved that, unlike the popular impression, HILIC methods could be green to the environment.
Keywords: HILIC, In silico toxicity, Nizatidine, Nitrosation, Simulated gastric juice
1. Introduction
N-nitroso compounds (NOCs) have been recognized for decades as genotoxic and carcinogenic in a wide variety of animals as well as in humans [1]. Human exposure to NOCs results from exogenous sources such as diet, water, tobacco, occupational exposure, and some minor sources comprising pharmaceuticals and cosmetics. In this context, in 2018, the Food and Drug Administration (FDA) [2] announced recalling many batches of valsartan-containing products (anti-hypertension) from the global market due to the presence of a significant level of N-nitrosodimethylamine (NDMA) impurity which is a known human carcinogen. Such impurity resulted from changing the way of manufacturing of valsartan by the parent Chinese company. This issue made a strong worldwide alert concerning the health hazards of NOCs.
In addition to the exogenous sources, about 45–75% of human exposure to NOCs is a result of endogenous formation [3]. Endogenous NOCs formation occurs mostly in the stomach, where its acidity favors the nitrosation reactions. The source of gastric nitrite may be diet or water, however, enzymes frequently reduce nitrate in saliva or in gastric fluid to nitrite [1]. It is worth noting that many drugs are supposed to be liable to nitrosation due to the presence of nitrosatable groups such as amine, amide, cyanamide, guanidine, hydroxylamine, amidine, hydrazine, or hydrazide, which form NOCs by the reaction with nitrite. From this perspective, a “Nitrosation Assay Procedure” (NAP test) has been suggested by the World Health Organization (WHO) experts board as a standard test to in vitro study the nitrosation of drugs [4]. Since then, the NAP test has been applied to evaluate the susceptibility of many drugs to nitrosation [5–7]. These facts are the standpoints that motivated us to start our current research project for evaluation of the nitrosatability of some widely administered drugs that have not been previously studied.
The subject of this investigation is the histamine H2-antagonist nizatidine (NZ) (1,1-ethenediamine, N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]-ethyl]-N′-methyl-2 nitro) that is frequently prescribed for the inhibition of gastric acid secretion in conditions like peptic ulcer, gastroesophageal reflux, persistent dyspepsia, and hypersecretory illness such as the Zollinger-Ellison syndrome [8]. NZ contains some functional groups which are expected to undergo N-nitrosation especially in the acidic medium of the stomach. As yet, no study was conducted to explore the behavior of NZ in any of these conditions although many methods have been applied for its determination in various matrices [9–11]. So, there is a strong necessity for a simple, sensitive, and rapid method to monitor the possibility of nitrosation of NZ under the NAP test and simulated gastric juice (SGJ) conditions.
Hydrophilic interaction liquid chromatography (HILIC) is a technique for resolution of compounds with a high polarity that avoids some of the drawbacks of reversed phase liquid chromatography (RPLC). The retention of polar analytes is improved by using HILIC and they are eluted according to increasing hydrophilicity. The separation process in HILIC is affected by different mechanisms: hydrophilic interaction, ion exchange, and reversed-phase retention by the siloxane on the silica surface [12]. Being a highly polar compound, with log P(Octanol/Water) of −0.43 [13], it seems very convenient to apply the HILIC approach to investigate the liability of NZ to nitrosation. Keeping in mind the large volume of organic solvent used in HILIC procedures, it was important to evaluate the probable influences of such method on safety, health, and environment. For this purpose, three analytical metrics were applied including; The Green Analytical Procedure Index (GAPI) [14], analytical eco-scale [15], and HPLC-Environment Assessment Tool (EAT) [16].
Since there is no report to address the possible health hazards of the potential N-nitroso derivative of NZ, we employed in silico-predictive toxicology approach that depends on computational resources to predict the toxicity/safety of drugs and chemicals. Currently, this technique has received a great attention due to its time and cost-efficiency as well as accuracy [17].
Thus, the goals of this investigation can be summarized as follows: (i) to identify the N-nitroso derivative of NZ (NZ-NO) by ESI+/MS, (ii) to develop and validate a HILIC method for the separation of NZ and its N-nitroso derivative, (iii) to apply this method for monitoring the possible formation of this derivative under SGJ and WHO-recommended conditions, (iv) to assess the greenness of the HILIC method by three green analytical metrics, (v) to conduct in silico toxicity study for the formed N-nitroso derivative for investigation of its safety/toxicity compared to NZ itself.
2. Methods
2.1. Chemicals and reagents
In the current study, chemicals of extra-pure analytical grade and organic solvents of HPLC grade were used. NZ pure standard (assay (HPLC) = 98.0%), lot No. ALR6837, was from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Acetonitrile (HPLC grade) was obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). Hydrochloric acid (35–37%), sodium carbonate, and sodium nitrite were obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Ammonium acetate, sodium chloride, and pepsin from porcine gastric mucosa (powder ≥250 units/mg solid) were products of Sigma–Aldrich (St. Louis, MO, USA). SGJ (pH 1.2) was prepared as per the United States Pharmacopoeia (USP) [9] by dissolving 2 g of sodium chloride and 3.2 g of pepsin in 7 mL HCl and sufficient water was added to make 1000 mL.
2.2. Instrumentations and HILIC conditions
A Hitachi HPLC instrument (Tokyo, Japan) composed of a 655A-11 liquid chromatograph, L-4000H UV detector (a high sensitivity series), D-2500 chromato-integrator, LC-organizer, and a Rheodyne injector valve with a 50 μL sample loop was used. The ESI+-MS spectra were recorded using a Quattro micro™ triple-quadrupole mass spectrometer (Waters Co., Milford, MA, USA) integrated with an electrospray ionization source. The following apparatuses were also used: SK-620 pH-meter (Sato Keiryoki MFG Co., Ltd, China), a BT-100 thermostatically controlled water bath (Yamato Scientific Co., Ltd., Tokyo), ultrasonic bath (As One Corp., Osaka), and a SB-1100 rotary evaporator (Eyela, Tokyo).
A Cosmosil® HILIC packed column (250 mm × 4.6 mm, 5-μm particle size) from Nacalai Tesque, Inc. was used with a mobile phase consisting of acetonitrile-acetate buffer (0.04 M, pH was adjusted to 6.0 with acetic acid) in the ratio of 92:8 (v/v). The flow rate of the mobile phase was 1 mL/min and the UV-detection was at 325 nm.
2.3. Software for In silico toxicity prediction and molecular docking
Toxicity parameters were calculated using ADMET Predictor™ 8.0 from Simulation Plus, Inc (CA, USA). Mutagenicity was further predicted by Molecular Operating Environment (MOE 2018.01) from Chemical Computing Group (Montreal, Canada). Ames mutagenicity was evaluated by OCHEM predictor [18] and blood-brain barrier (BBB) penetration was assessed by BBB predictor server [19]. The amino acid sequence of the human H2 receptor (hH2R) was retrieved from the Universal Protein Resource (https://www.uniprot.org/, accession number P25021). The crystal structure of turkey β1-adrenoceptor was extracted from the Protein Data Bank (PDB entry: 2VT4) and employed as the template [20]. Alignment of sequence, building, validation of homology model, and docking of NZ and NZ-NO into the generated model were performed using MOE 2018.01.
2.4. Identification of nizatidine nitrosation product
The nitroso derivative of NZ was prepared by amodification of the procedure of Foster et al. [21] for the synthesis of nitrosocimetidine. NZ (3 g, 9.0 mmol) was dissolved in 30 mL of 2 N HCl and cooled to 5 °C in a refrigerator. NaNO2 (3 g, 43.0 mmol) was dissolved in 6 mL of distilled water and added dropwise with stirring to NZ solution. The mixture was stirred for 15 min in an ice bath and then until reaching the room temperature. The solution was neutralized with Na2CO3 and complete nitrosation was confirmed by the disappearance of NZ peak from the HILIC chromatogram and appearance of NZ-NO peak. For structural elucidation of the formed compound, it was submitted for ESI+-MS.
2.5. Standard solutions
A standard solution of NZ (200 μg/mL) was prepared in acetonitrile and a standard solution of NZ-NO (150 μg/mL) was prepared in water. Stability of these solutions was conserved up to 7 days when kept in the refrigerator at 4 °C.
2.6. Procedure for calibration curves
Six calibration solutions of NZ and NZ-NO were separately prepared by proper dilution of the corresponding standard solutions with the mobile phase to achieve final concentration ranges of 0.1–2.0 and 0.5–15.0 μg/mL, respectively. Fifty μls were injected (triplicates) under the optimum chromatographic conditions. The linear dependence of the average peak areas on the final concentrations (μg/mL) was confirmed from the calibration curves and the regression
2.7. Conditions for the nitrosation study
To check the possible formation of NZ-NO as a result of the interaction of NZ with nitrite, the nitrosation reactions were carried out under the following conditions:
2.7.1. Condition A
The NAP test procedure [4] was carried out by incubation of NZ (10 mM) with sodium nitrite (40 mM) in 0.06% v/v acetic acid (pH 3.6).
2.7.2. Condition B
NZ (10 mM) was incubated with sodium nitrite (40 mM) in SGJ (pH 1.2).
2.7.3. Condition C
The nitrosation test was carried out using NZ (10 mM) and sodium nitrite (40 mM) in 0.06 M HCl (pH 1.2).
2.7.4. Condition D
The influence of sodium nitrite concentration was examined by incubating NZ (10 mM) with different concentrations of sodium nitrite (5, 10, and 40 mM) in SGJ (pH 1.2).
2.7.5. Condition E
The inhibitory effect of ascorbic acid on the nitrosation of NZ was examined by incubation of NZ (10 mM) with sodium nitrite (40 mM) in SGJ (pH 1.2) in the presence of ascorbic acid (40 mM).
2.7.6. Control
A control experiment was conducted by incubation of NZ (10 mM) in SGJ (pH 1.2) without sodium nitrite.
All incubation mixtures were kept at 37 °C in a thermostatically controlled water bath with stirring. Samples (5.0 μL) were taken at specific time intervals up to 4 h, neutralized with 0.03 M Na2CO3, made up to 10 mL with the mobile phase, well-mixed, and assayed by the developed HILIC method. The obtained chromatograms were examined and the concentrations of NZ remained unreacted and NZ-NO produced were calculated from their regression Then, the %nitrosation of NZ was calculated.
3. Results
3.1. Identification of NZ nitrosation product
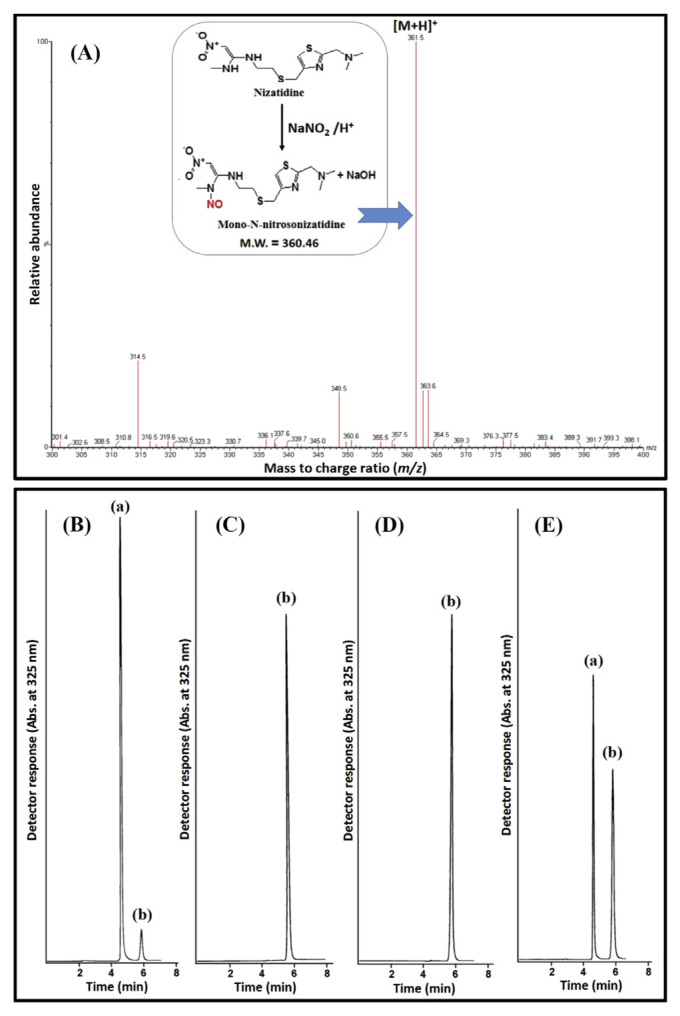
For the characterization of the structure of the nitrosation product of NZ, it was analyzed by ESI+-MS. The most abundant ion peak was at m/z 361.52 [M+H]+ (Fig. 1A) corresponding to the molecular formula of C12H20N6O3S2 (M.W = 360.46). Such a result approves the formation of a mono-N-nitroso derivative of NZ (NZ-NO). This agreed well with the reported literature for the nitrosation of the analogous compound cimetidine to mono-N-nitrosocimetidine [21]. Fig. 1A illustrates the nitrosation reaction of NZ and the ESI+-MS spectrum of NZ-NO.
Fig. 1.
(A) ESI+-MS spectrum of the product of nitrosation reaction of NZ with sodium nitrite and the insert shows the mechanism of the nitrosation reaction. Representative chromatograms for NZ following the exposure to (B) condition A for 160 min, (C) condition B for 10 min, (D) condition C for 10 min, and (E) condition D for 10 min using 5 mM NaNO2, where: (a) is NZ and (b) is NZ-NO.
3.2. HILIC method optimization
The histamine H2-antagonist NZ is a basic and highly polar compound [13], so it is expedient to use the HILIC technique to separate it from its potential N-nitroso derivative (NZ-NO) and to monitor the possibility of its nitrosation under various conditions. A common feature of HILIC and normal phase chromatography (NPC) is the need for a non-polar mobile phase and a polar stationary phase. But, in HILIC the mobile phase must contain a certain amount of water to form an immobile aqueous layer on the surface of the column. The analytes are selectively partitioned into this layer. On the other hand, NPC employs pure non-polar solvents (e.g. hexane and methylene chloride). Moreover, the addition of a buffer to the HILIC mobile phase is important for controlling the ionization of analytes and stationary phase and regulating the acid-base equilibrium between them. Hence, it is common for HILIC separation to use a mobile phase containing not less than 5% aqueous buffer [22].
First, for the achievement of the best sensitivity that allowed monitoring of both NZ and NZ-NO, eluents were detected at different wavelengths (240, 250, 254, 280, 300, 325, 370, and 450 nm). Detection at 325 nm provided good sensitivity for both compounds. The sensitivity of the method for NZ is more than NZ-NO since the wavelength selected to allow their simultaneous determination, 325 nm, was the λmax of NZ while giving a considerable, but not the highest, absorbance of NZ-NO. Attempts to use the λmax of NZ-NO was not successful since NZ shows very weak absorbance at it.
As an initial step for the HILIC method optimization, we studied the influence of acetate buffer pH on the separation of NZ and NZ-NO over the range of 4.0–6.0. With acetate buffer of pH 4.0, a decreased retention of NZ-NO was observed, while that of NZ was not significantly affected by variation of the pH since its pKa = 6.8 [13], thus it is protonated in the whole studied pH range. Appropriate retention of both analytes with a considerable run-time was achieved using acetate buffer of pH 6.0.
The ratio of acetate buffer was also investigated (5–10%, v/v). With amobile phase containing 10% (v/v) buffer, decreased retention and poor resolution of NZ and NZ-NO was observed compared to the mobile phase containing smaller volumes. Such retention behavior is opposite to that in RPLC, which is a characteristic of HILIC. Good resolution and reasonable retention times were obtained using 8% (v/v) acetate buffer. Meanwhile, increasing the ionic strength of ammonium acetate (from 0.04M to 0.1 M) has a slight effect on the retention of both compounds.
Ultimately, a mobile phase containing acetonitrile and 0.04 M ammonium acetate with pH adjusted to 6.0 with acetic acid (92:8, v/v) provided good separation and resolution within a short run-time (about 6 min) (Fig. 1B–E).
3.3. Characteristics of the chromatographic peaks
Using the optimum HILIC conditions, NZ and NZ-NO were eluted at 4.70 ± 0.05 min and 5.82 ± 0.07 min (mean tR ± SD, n = 5), respectively, with a resolution factor (Rs) of 4.9 giving symmetrical, sharp, and narrow peaks having theoretical plate counts (N) of 8464 and 6022, respectively. These parameters were calculated according to the USP instructions [9].
3.4. Method validation
The method validation was done according to the regulations of FDA [23]. The calibration curves were constructed using six standard concentrations for each analyte. Linear correlations between the peak areas and concentrations were achieved over the ranges of 0.1–2.0 and 0.5–15.0 μg/mL for NZ and NZ-NO, respectively. In addition, limit of quantification (LOQ) and limit of detection (LOD) were calculated. Table 1 depicts a summary of the results.
Table 1.
Linearity, accuracy, and precision data for NZ and NZ-NO by the proposed method.
| Linearity results | |||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Results | ||||
|
|
|||||
| NZ | NZ-NO | ||||
| Concentration range (μg/mL) | 0.1–2.0 | 0.5–15 | |||
| Limit of detection (LOD) (μg/mL) | 0.02 | 0.1 | |||
| Limit of quantification (LOQ) (μg/mL) | 0.1 | 0.5 | |||
| Correlation coefficient (r) | 0.9998 | 0.9999 | |||
| Slope | 21.32 × 103 | 5.41 × 103 | |||
| Intercept | 3.38 × 103 | 1.63 × 103 | |||
| Standard deviation of the residuals (Sy/x) | 0.29 × 103 | 0.16 × 103 | |||
| Standard deviation of the intercept (Sa) | 0.14 × 103 | 0.05 × 103 | |||
| Standard deviation of the slope (Sb) | 0.17 × 103 | 0.01 × 103 | |||
| % RSD | 1.54 | 1.37 | |||
| % Error (% RSD/√n) | 0.61 | 0.52 | |||
|
| |||||
| Accuracy and precision results | |||||
|
| |||||
| Analyte | Conc. (μg/mL) | Intra-day (n = 5) | Inter-day (n = 5) | ||
|
|
|
||||
| Accuracy (%) | Precision (% RSD) | Accuracy (%) | Precision (%RSD) | ||
|
| |||||
| NZ | 0.1 | −1.06 | 2.36 | −3.61 | 3.91 |
| 0.6 | −2.35 | 2.13 | −3.04 | 2.50 | |
| 1.5 | +0.11 | 1.84 | +1.65 | 2.56 | |
| NZ-NO | 0.5 | +0.68 | 2.93 | +1.39 | 3.90 |
| 3.5 | +3.38 | 2.00 | +2.01 | 3.96 | |
| 15 | +1.52 | 1.45 | +3.71 | 2.20 | |
Selectivity of the developed method for NZ and NZ-NO in SGJ was proven by the analysis of a blank sample of SGJ and SGJ fortified with NaNO2 (40 mM) to inspect any potential interaction products from nitrite and SGJ components. No interference was detected with the assay of both analytes where the obtained chromatograms did not show any peaks at the retention times of NZ or NZ-NO. The high sensitivity of the developed method allowed high dilution factor of SGJ by the mobile phase (2000 times) which helps to obtain very clean chromatograms.
The accuracy of the developed method was also estimated by replicate determination of SGJ samples spiked with known concentrations of the analytes (five determinations per concentration). The deviation of the mean %recovery from the true value ranged from −3.61 to +3.71 confirming the accuracy of the method (Table 1).
The intra-day precision of the developed method was studied by the analysis of three concentrations of each analyte as five replicates/concentration in one day. Also, the inter-day precision was studied by the analysis of the two compounds at three concentration levels on five consecutive days. The %RSD of intra- and inter-day precision were −3.96 confirming the precision of the developed method (Table 1).
3.5. Investigation of nizatidine nitrosatability in SGJ and WHO-recommended conditions
Many researches explored the relationship between NOCs and human cancer. Additionally, the genotoxicity and ability of the N-nitroso derivatives of many drugs to produce DNA damage have been proven [1,24]. Despite the presence of some nitrosatable groups in NZ, its liability to form N-nitroso derivative has not been yet demonstrated by any investigation. In this study, we explored the nitrosatability of NZ under different conditions using the developed HILIC method.
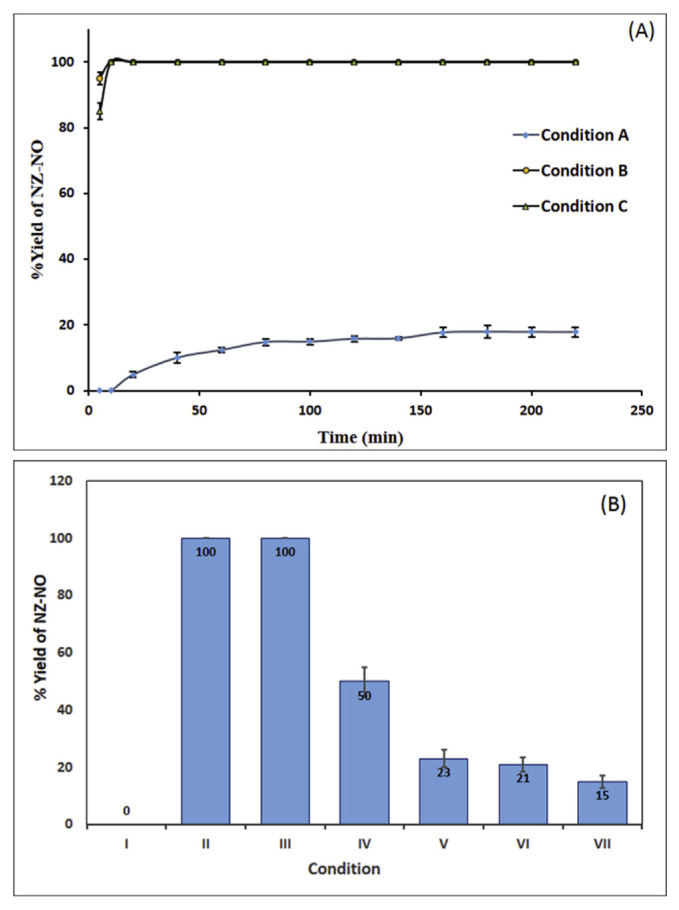
Firstly, the nitrosation reaction was conducted under the standard conditions of the NAP test [4] (Condition A). We found that NZ underwent nitrosation to NZ-NO gradually with a maximum and constant percentage nitrosation of ~18% after 160 min. Further standing time did not result in any increase in the nitrosation yield (Fig. 2A). Fig. 1B displayed a chromatogram obtained under this condition.
Fig. 2.
(A) Effect of time on the yield of NZ-NO under conditions A, B, and C. (B) Nitrosation of NZ under different conditions: (I) control experiment (SGJ contains 10 mM NZ without NaNO2), (II) condition B (NZ (10 mM) in SGJ containing 40 mM NaNO2), (III) NZ (10 mM) in SGJ containing 10mM NaNO2, (IV) NZ (10 mM) in SGJ containing 5 mM NaNO2 and (V) NZ (10 mM) in SGJ containing 40 mM NaNO2 and 40 mM ascorbic acid. Each result in these experiments is the mean of three separate determination and error bars represent the standard deviation (when applicable since 100% nitrosation is associated with complete disappearance of the peak in all experiments).
Despite diluted acetic acid (0.06%, v/v) was suggested to achieve the required acidity for the NAP test (pH 3.5) [7], it would be more convenient to simulate the biological conditions as possible. So, we repeated the experiment in SGJ of pH 1.2 [9] (Condition B). The use of the USP SGJ is recommended by the FDA [25] to conduct similar studies since obtaining this fluid from human is difficult and involves intubation and invasive treatment. Under Condition B, a substantial increase in the % nitrosation was observed by about 5.6 folds reaching 100% nitrosation after 10 min (Fig. 2A). A chromatogram represents this condition is shown in Fig. 1C.
To determine whether this increase of nitrosatability is a result of the change in the pH or due to some SGJ components (pepsin and/or NaCl) which may act as nitrosation enhancers [4], the experiment was repeated using 0.06 M HCl (pH 1.2) instead of SGJ (Condition C). The results were similar to those obtained in SGJ reaching 100% nitrosation after 10 min (Figs. 2A and 1D). Then, experiments were also conducted utilizing modified SGJs (pH 1.2) containing variable concentrations of NaCl (0.1–0.3%) and pepsin (0.16–0.50%). In all experiments, complete nitrosation was detected within 10 min. This indicates that neither pepsin nor NaCl has enhancing effect on the nitrosation of NZ, where the main factor controlling it is the pH. This pH-dependent nitrosation behavior agreed with that of cimetidine which increased by 6.8 folds at pH 1.0 relative to pH 3.0 [26].
We also studied the impact of NaNO2 level on the nitrosation of NZ in SGJ by reducing it from the concentration recommended for the NAP test (40 mM). When using 10 mM NaNO2, i.e. at an equal molar concentration to the drug, a 100% nitrosation yield was also obtained after 10 min. However, about 50% nitrosation was obtained upon using 5 mM NaNO2 which is a more physiologically-realistic concentration (Fig. 2B). The maximum yield of NZ-NO was obtained after 10 min, and more increase in the reaction time has no influence on the reaction yield. This result is an indicative of 1:1 stoichiometric reactivity of NZ and NaNO2 which is confirmed by the result of ESI+-MS. A chromatogram obtained for this condition is also shown in Fig. 1E.
Besides, a control experiment was performed using 10 mM of NZ in SGJ without the addition of NaNO2. No nitrosation takes place (Fig. 2B) and no peaks for NZ-NO was detected. Also, no degradation of NZ by the acidity of SGJ was observed as confirmed by its intact chromatographic peak and constant peak area.
Ascorbic acid was previously used to suppress the generation of NOCs by reduction of nitrite into NO with the formation of dehydroascorbic acid [4]. As a consequence, the influence of ascorbic acid on the nitrosation of NZ was examined by repeating the experiments using incubation mixture containing 40 mM NaNO2, 10 mM NZ, and 40 mM ascorbic acid in SGJ for different time intervals (10, 30, and 120 min). The mean yield of NZ-NO was about 22.5, 21.7, and 15%, respectively, of that produced using the same conditions but omitting ascorbic acid (Fig. 2B). Since the oral absorption time of NZ is about 1.5 h [13] and the normal half gastric emptying time ranged from 80 to 127 min [27], it is concluded that the inhibitory action of ascorbic acid occurs within the time frame of NZ residence in the stomach. These results strongly recommend the co-administration or co-formulation of ascorbic acid with NZ to decrease its vulnerability to nitrosation and reduce the risk of human exposure to NZ-NO.
3.6. In silico toxicity prediction and molecular docking of NZ-NO and comparison with NZ
The nitrosation behavior of NZ in SGJ is interesting and surprising to a high extent where it is completely nitrosated within a short time. This is essentially important for patients with healed duodenal ulcer, peptic ulcer, erosive esophagitis, and gastroesophageal reflux disease who received a daily maintenance therapy of 150 mg NZ for a long time [28,29], thus they are at a high risk of exposure to NZ-NO. Yet, it is important to emphasize that the NAP test [4] applied a high concentration of nitrite to promote the reaction and the resulted product yield is higher by far than the in vivo-reached level. Hence, the WHO protocol is usually applied to distinguish compounds with high affinity to nitrosation from compounds with low nitrosation vulnerability. Herein, the applied protocol shed light on the high nitrosation risk of NZ.
Literature survey revealed insufficient information about the effect of NZ-NO on human or animals. Therefore, we decided to use in silico toxicity prediction to study the potential toxicity of NZ-NO since this tool allows rapid and accurate estimation of several properties of a chemical from its molecular structure [17]. Initially, we used the Physicochemical and Biopharmaceutical module of the ADMET predictor [30] to estimate the oral absorption of NZ-NO as well as NZ itself. The results demonstrated that both compounds have excellent oral absorption since their absorption risk values (Absn_Risk) are less than the typical score for orally-inactive compounds (3.5) [31]. The Absn_Risk of for NZ and NZ-NO are 0 and 0.5, respectively. Thereafter, we held a comparison between NZ and NZ-NO toxicity profiles (Table 2). At first, ADMET predictor toxicity module was used for carcinogenicity, mutagenicity and toxicity prediction [30]. The median toxic dose (TD50), defined as the dose of a substance given orally to rats or mice over the course of their lifetimes that results in the appearance of tumors in 50% of their population, was firstly estimated. Table 2 shows that the rat_TD50 and mouse_TD50 values of NZ-NO are around tenfold lower than NZ values. This emphasizes the high chronic carcinogenic risk of NZ-NO compared to NZ, where much smaller doses of NZ-NO is carcinogenic and could induce tumor. Furthermore, NZ-NO exhibited about the double acute toxicity of NZ towards the fathead minnow model (TOX_FHM) which is a toxicity model originates from a structure–activity relationship based on a large data base from the FDA Environmental Protection Agency. The result of TOX_FHM model is the amount of the substance (mg/L) that kill 50% of minnows (a species of freshwater fish) after exposure for 96 h [30].
Table 2.
In silico toxicology of NZ and NZ-NO using ADMET Predictor™.
| Parameter | NZ | NZ-NO |
|---|---|---|
| Rat_TD50 (mg/kg) | 12.857 | 0.966 |
| Mouse_TD50 (mg/kg) | 20.747 | 2.207 |
| TOX_FHM (mg/L) | 0.275 | 0.162 |
| Rat-acute LD50 (mg/kg) | 1382.32 | 1091.22 |
| Chrom_Aberr | Non-toxic | Toxic |
| Ser_AST | Normal | Elevated |
| Ser_ALT | Normal | Normal |
| MUT_Riska | 0.5 | 3 |
| TOX_Riskb | 0.95 | 3 |
Rat_TD50: median toxic dose in rat, Mouse_TD50: median toxic dose in mouse, TOX_FHM: fathead minnow model, Rat-acute LD50: median lethal dose rat acute toxicity, Ser_AST: serum aspartate aminotransferase, Ser_ALT: serum alanine aminotransferase, MUT_Risk: overall mutagenicity, and TOX_Risk: overall toxicity.
Desired value is <1.
Desired value is <3.
The acute rat toxicity model which evaluates the median lethal dose LD50 (the dose kills half the members of a rat population after a specified time) was also investigated. It is noted that NZ and NZ-NO have high values (Table 2), which means they do not have acute toxicity in rats. On the other hand, NZ-NO is recognized to have a hepatotoxic effect due to an elevation of serum aspartate aminotransferase (Ser_AST) which is indicative of liver cell damage. Meanwhile, the serum alanine aminotransferase (Ser_ALT) level is normal for both NZ and NZ-NO.
Another feature of correlated toxicity is the chromosomal aberrations which is configured in the ADMET predictor via a neural network ensemble model (Chrom_Aberr) that categorizes chemicals into toxic or non-toxic according to their ability to cause genotoxic effects [30]. According to Chrom_Aberr model, NZ-NO is genotoxic while NZ is non-toxic.
A qualitative estimate of the overall mutagenicity is expressed by a MUT_Risk score which is 0.5 for NZ and 3 for NZ-NO. A score exceeding 1 indicates a potential mutagenic activity. Overall toxicity is predicted by the TOX_Risk value that is 0.95 for NZ and 3 for NZ-NO (Table 2). This reveals the significant higher mutagenicity and toxicity of NZ-NO compared to its precursor, NZ.
To validate the former results, an assessment of NZ-NO toxicity and mutagenicity by a rule-based method [32] implemented in MOE, confirmed its mutagenicity and specified the nitrosaminemoiety as a toxophore group. In a similar vein, OCHEM predictor revealed that NZ-NO is predicted to be an active Ames mutagenic compound [33] and a potential carcinogenic as a consequence. More importantly, NZ-NO is predicted to pass BBB hence its harmful effects will include the brain.
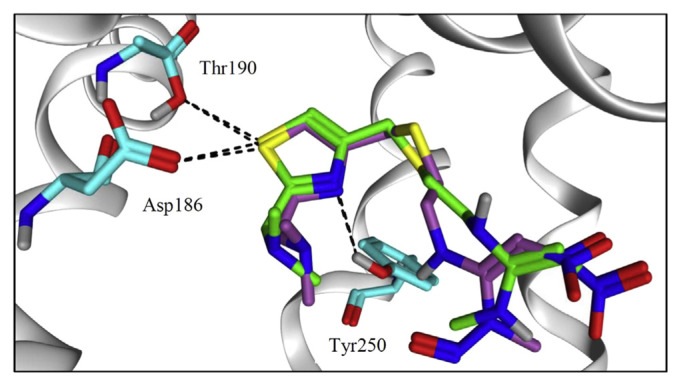
Furthermore, we utilized molecular docking tool to compare between NZ and NZ–NO binding affinity with human histamine 2 receptor (hH2R). hH2R is a G-protein-coupled receptors (GPCR), consists of seven transmembrane helices (TM1–TM7), that is yet to be crystallized. Therefore, homology model generation was performed as an alternative judicious approach [34]. To this end, we used the GPCR-turkey β1 receptor-as a template with an approximate of 38% sequence identity [35]. Ten models were generated and the most valid one was selected based on its Ramachandran plot (Supplementary material, Fig. S1) that showed the minimal number of outlier residues. Most of the residues are located in the appropriate environment. Earlier mutation studies on the receptor proved that Asp186 and Thr190 residues in TM5 and Asp98 residue in TM3 are crucial for histamine binding. Additionally, Tyr250 in TM6 may be essential for the binding of hH2R agonists through computational approaches [36,37]. Docking of NZ and NZ-NO showed that both molecules adopt an identical binding mode into themodel’s pocket (Fig. 3). Same interactions with key amino acids Asp186, Thr190 and Tyr250 were observed. We believe the two molecules may possess the same pharmacodynamics effect as they share a similar binding pose. Even the binding score is about −7 kcal/mol for each molecule.
Fig. 3.
Binding mode of NZ, shown in green sticks and NZ-NO, shown in purple sticks and colored by element, into hH2R model. Amino acid residues are depicted in cyan colored sticks. Settled intermolecular interactions were shown in black dashed lines. Both ligands share a similar binding mode into the model’s pocket.
3.7. Assessment of method agreement with green analytical chemistry conception
By virtue of the authors’ concern in green analytical chemistry field, it was important to evaluate the developed HILIC method regarding its impacts on the safety, health, and environment, especially with the use of such large volume of acetonitrile in the mobile phase. Thus, we used three analytical chemistry metric tools for evaluation of the developed method regarding its agreement with green chemistry rules [14–16,38]. The three used tools are complementary for a true evaluation of the method as possible.
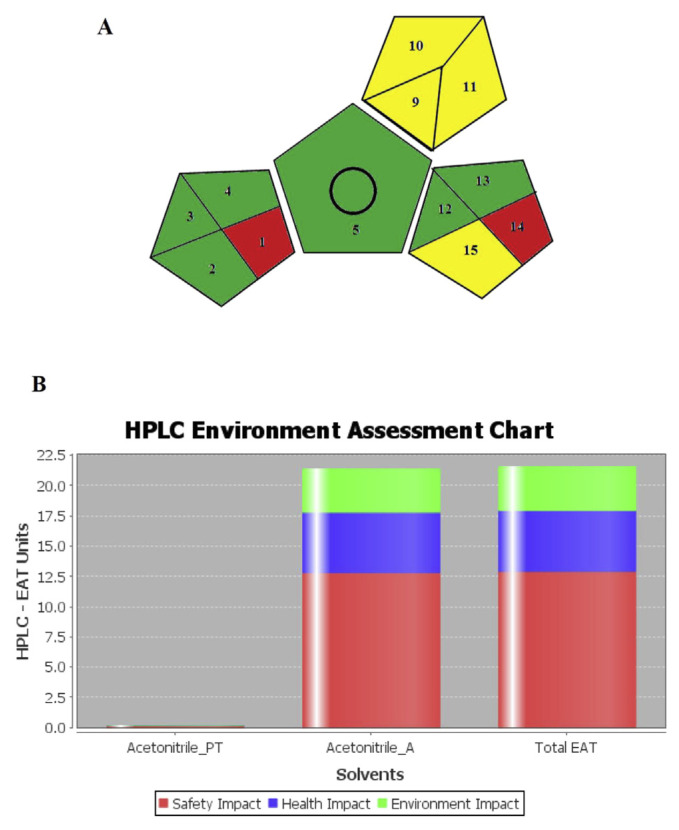
The Green Analytical Procedure Index (GAPI) is a new tool for greenness assessment which depends on five pentagrams illustration, with each pentagram subdivided into partitions representing different elements which contribute to the impacts of the analytical method on the environment. These partitions are colored red, yellow, or green for high, medium, or low environmental influences, respectively. GAPI is the most recent greenness assessment tool that gives much information on the reasons of environmental influences of the analytical methods. The application of this technique for evaluation of the HILIC method greenness shows a good greenness profile (Fig. 4A and Supplementary Material Table S1) [14].
Fig. 4.
(A) GAPI pictogram for the developed HILIC method (references to numbers shown in the pictogram are provided in Supplementary Material Tables S1) and (B) HPLC-EAT chart for the developed HILIC method (Acetonitrile_PT: acetonitrile in the standard solution, Acetonitrile_A: acetonitrile in the mobile phase).
The second assessment tool is the Analytical Eco-scale score proposed by Gałuszka et al. [15]. This tool considers many aspects such as the type and volume of reagent, instruments, energy consumption, waste, and occupational hazards. Every item of these is given penalty points then the summation of penalty points is subtracted from 100 (stands for the ideal green analysis) to calculate the analytical eco-scale score. For detailed calculation procedure, readers can refer to the publication of Gałuszka et al. [15]. Table 3 shows the results for penalty points and analytical eco-scale score calculations for the proposed method. The score of the developed method is 85 which is indicative of excellent greenness.
Table 3.
Calculation of penalty points and analytical eco-score for the proposed method.
| Reagents | |||
|---|---|---|---|
|
| |||
| Reagent, volume (mL) | Number of pictograms | Word sign | Penalty points |
| Acetonitrile, 5.5 | 2 | Danger | 4 |
| Ammonium acetate, 0.5 | 1 | Warning | 1 |
| Acetic acid, <1 | 2 | Danger | 4 |
|
| |||
| Instruments | |||
|
| |||
| Item | Penalty points | ||
|
| |||
| HPLC | 1 | ||
| Sonicator | 0 | ||
| Waste | 8 | ||
| Occupational hazards | 0 | ||
| Total penalty points | ∑18 | ||
| Analytical eco-score | 82 | ||
The third assessment tool is HPLC-EAT which has three main focuses: safety, health, and environmental effects (SHE) of the solvents used in the HPLC method. The HPLC-EAT score was calculated using a software provided by Gaber and co-workers (HPLC-EAT Version 20110505) [16]. Fig. 4B demonstrates the obtained results. The safety, health, and environmental impacts were found to be 12.892, 5.024, and 3.649, respectively, and the total EAT score was 21.565. The small value of the EAT score signifies the greenness of the analytical procedure [16].
The obtained results from the three green analytical chemistry metrics are well-matched and evidences for the satisfactory greenness and minor safety, health, and environmental impacts of the developed HILIC method.
4. Discussions
The development of a fully validated, simple, and sensitive HILIC method for efficient separation of NZ from its nitroso-derivative was very beneficial for investigating its behavior under the WHO-suggested NAP test [4] as well as in simulated gastric medium containing nitrite. The developed HILIC-UV method successfully separated NZ and NZ-NO yielding sharp and symmetrical peaks within 6 min (Fig. 1B–E) with LOD of 0.02 and 0.1 μg/mL, respectively. Evaluation of the congruence of the developed HPLC method with the green chemistry theory utilizing three metrics confirmed its greenness (Table 3 and Fig. 4). Taking in account all the advantages and benefits gained from the application of the developed method including rapidness, sensitivity, specificity, accuracy, precision, and greenness, it is considered a valuable tool for the analysis and monitoring of NZ gastric nitrosation.
NZ was completely nitrosated under SGJ condition within 10 min, while 18% nitrosation took place under the NAP test condition after 160 min. The product of the nitrosation reaction was mono-N-nitroso nizatidine as identified by ESI+-MS (Fig. 1A). In silico toxicity prediction utilizing ADMET® predictor evidenced the carcinogenicity, mutagenicity, hepatotoxicity, and chromosomal aberration potential of NZ-NO. As well, the results from OCHEM predictor and MOE strongly supported this outcome. The carcinogenicity of NZ-NO, like other NOCs, is probably a result of DNA alkylation by its ultimate carcinogenic form (diazonium ion) produced through metabolic activation by microsomal monooxygenase [39]. As well, reactive oxygen species and carbon-centered radicals are formed by NOCs and lead to abnormalities in the gene expression process [40].
Additionally, NZ-NO is expected to cross the BBB [19] spreading its hazards to the brain. It is well-established that nitrosamines are involved in neurodegenerative disorders such as Alzheimer’s disease [41]. Yet, we found that, the presence of ascorbic acid greatly inhibited the nitrosation of NZ by about 77.5–85%(Fig. 2B), a result that suggests their co-administration or co-formulation for protection of human from possible carcinogenic or genotoxic effects. The use of NZ as ascorbate salt offers also a good way to prevent these harmful effects [42]. Other antioxidants, like thiols and some phenolics, can also suppress the nitrosation reactions via the decomposition of the nitrosating agent ( ). However, ascorbic acid is superior since it can prevent the nitrosation over a wide pH range, while the inhibitory action of phenolics is reliant on many aspects such as their own structures, pH of the medium, the nature of substrate, and the comparative concentration of the substrate and nitrite. Additionally, some phenolics can be nitrosated producing C-nitroso derivatives which are strong nitrosating agent themselves. Similarly, thiols can also inhibit the nitrosation but S-nitrosothiols may be produced under certain conditions which can act as trans-nitrosating agent. Thus, in this study, we investigated ascorbic acid as a nitrosation suppressor by virtue of its efficiency, feasibility of administration, and wide applicability in similar experiments [43].
Molecular docking study showed that both NZ and NZ-NO have the same binding mode and affinity to hH2R receptors exhibiting the same interactions with key amino acids Asp186, Thr190 and Tyr250 (Fig. 3) with binding score of −7 kcal/mol for both compounds. Thus, it is expected that the two compounds have similar pharmacodynamics profile.
Although no experimental data are reported in the literature concerning the toxicity of NZ-NO, the results of the in silico toxicology of NZ compared very well with the published toxicity literature in this connection giving a proof on the accuracy of the in silico toxicology. The acute toxic dose of NZ is reportedly very high reaching many multiples of human daily dose. In addition, NZ has no carcinogenic, hepatotoxic, or mutagenic effects up on long term administration for about 2 years [44]. These results agreed completely with the results of in silico toxicology conducted in our study (Table 2). However, few case reports documented that NZ is involved in liver hepatotoxicity by unknown cause [45]. On the light of our results, this hepatotoxicity may result from NZ-NO. In addition, a research study by Brambilla et al. found that NZ has carcinogenicity in mice. Interestingly, these researchers suggested that this effect is attributed to the N-nitroso derivative of NZ [46]. Apparently, there is a marked agreement of our results with these epidemiological data. Additionally, our data are strongly concurred with the epidemiological studies inspected analogous H2-antagonists such as cimetidine and ranitidine. These studies found a high incidence level of cancer associated with the long-term administration of these H2-antagonists. This effect was attributed to the nitroso derivatives of these H2-antagonists formed in the stomach [47]. Being guanidine derivatives, these H2-antagonists are highly nitrosatable at low-pH medium [48], however, a considerable nitrosation is also occurred at higher pH that may be experienced in vivo during treatment with these H2-antagonists as demonstrated in our experiments at pH 3.5 and in the published literature [26]. It is worthy to note that some proton pump inhibitors such as omeprazole were associated with an increased risk of cancer, but, unlike H2-antagonists, this effect was attributed to elevation of the gastric pH to a level optimum for nitrosation of other endogenous precursors and proliferation of bacteria that catalyzes the nitrosation reaction, rather than undergoing nitrosation themselves [49].
Overall, the results of this study are of a special importance in cases of NZ long-term administration as a maintenance regimen in certain diseases such as healed duodenal ulcer, peptic ulcer, erosive esophagitis, and gastroesophageal reflux disease [28,29]. In such cases, we encourage the co-administration of ascorbic acid or ascorbic acid-rich diet to protect from potential harmful effects of the nitrosation product.
Acknowledgments
Rania El-Shaheny would like to express her sincere gratitude to the Takeda Science Foundation (Osaka, Japan) for the awarded fellowship in Nagasaki University, Japan. The authors would like to express their heartfelt gratitude towards Prof. Dr. Masami Otsuka from Kumamoto University and the founder of Science Farm Ltd., Japan for his support at performing the in silico calculations.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfda.2019.08.001.
Funding Statement
This work was supported in part by a Grant-in-Aid for Scientific Research No. 23590008, 26460124, and 18K06718 from the Japan Society for the Promotion of Science which is gratefully acknowledged.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research No. 23590008, 26460124, and 18K06718 from the Japan Society for the Promotion of Science which is gratefully acknowledged.
REFERENCES
- 1. Brambilla G, Martelli A. Genotoxic and carcinogenic risk to humans of drug-nitrite interaction products. Mutat Res Rev Mutat Res. 2007;635:17–52. doi: 10.1016/j.mrrev.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. FDA updates on valsartan recalls. 2018. [accessed July 30, 2018]. https://www.fda.gov/Drugs/DrugSafety/ucm613916.htm .
- 3. Tricker AR. N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. Eur J Cancer Prev. 1997;6:226–68. doi: 10.1097/00008469-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Some pharmaceutical drugs. Switzerland: 1980. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans; p. 24. [PubMed] [Google Scholar]
- 5. Regulska K, Murias M, Stanisz B, Regulski M. The mutagenicity analysis of imidapril hydrochloride and its degradant, diketopiperazine derivative, nitrosation mixtures by in vitro Ames test with two strains of Salmonella typhimurium. Rep Pract Oncol Radiother. 2014;19:412–9. doi: 10.1016/j.rpor.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pietraforte D, Brambilla G, Camerini S, Scorza G, Peri L, Loizzo A, et al. Formation of an adduct by clenbuterol, a β-adrenoceptor agonist drug, and serum albumin in human saliva at the acidic pH of the stomach: evidence for an aryl radical-based process. Free Radic Biol Med. 2008;45:124–35. doi: 10.1016/j.freeradbiomed.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 7. Gatehouse D, Wedd D. The bacterial mutagenicity of three naturally occurring indoles after reaction with nitrous acid. Mutat Res Toxicol. 1983;124:35–51. doi: 10.1016/0165-1218(83)90183-0. [DOI] [PubMed] [Google Scholar]
- 8.Sweetman SC. Martindale: the complete drug reference. 36th ed. London, England: The Pharmaceutical Press; 2009. [Google Scholar]
- 9.The United States Pharmacopeial Convention. The United States pharmacopeia. Vol. 35. Rockville, MD: 2012. NF 30. [Google Scholar]
- 10.British Pharmacopoeial Commission. The British Pharmacopoeia. London: Her Majesty Stationery Office; 2013. [Google Scholar]
- 11. Rathore SS, Navyasree GA, Doijode M, Sathyanarayana S. Analytical techniques for nizatidine: a review. Sep Sci Plus. 2019:1–14. doi: 10.1002/sscp.201900028. [DOI] [Google Scholar]
- 12. Naidong W. Bioanalytical liquid chromatography tandem mass spectrometry methods on underivatized silica columns with aqueous/organic mobile phases. J Chromatogr B. 2003;796:209–24. doi: 10.1016/j.jchromb.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Moffat AC, Osselton MD, Widdop B. Clarke’s analysis of drugs and poisons. Forth. UK: Pharmaceutical Press; 2011. [Google Scholar]
- 14. Płotka-Wasylka J. A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta. 2018;181:204–9. doi: 10.1016/j.talanta.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 15. Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal Chem. 2012;37:61–72. doi: 10.1016/j.trac.2012.03.013. [DOI] [Google Scholar]
- 16. Gaber Y, Törnvall U, Kumar MA, Ali Amin M, Hatti-Kaul R. HPLC-EAT (Environmental Assessment Tool): a tool for profiling safety{,} health and environmental impacts of liquid chromatography methods. Green Chem. 2011;13:2021–5. doi: 10.1039/C0GC00667J. [DOI] [Google Scholar]
- 17.Parthasarathi R, Dhawan A. Chapter 5 - in silico approaches for predictive toxicology. In: Dhawan A, editor. Kwon SBT-IVT. Academic Press; 2018. pp. 91–109. [DOI] [Google Scholar]
- 18. Sushko I, Novotarskyi S, Körner R, Pandey AK, Rupp M, Teetz W, et al. Online chemical modeling environment (OCHEM): web platform for data storage, model development and publishing of chemical information. J Comput Aided Mol Des. 2011;25:533–54. doi: 10.1007/s10822-011-9440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Wang L, Lv M, Pei R, Li P, Pei Z, et al. AlzPlatform: an Alzheimer’s disease domain-specific chemogenomics knowledgebase for polypharmacology and target identification research. J Chem Inf Model. 2014;54:1050–60. doi: 10.1021/ci500004h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, et al. Structure of a β1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–91. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foster AB, Jarman M, Manson D, Schulten H-R. Structure and reactivity of nitrosocimetidine. Cancer Lett. 1980;9:47–52. doi: 10.1016/0304-3835(80)90139-1. [DOI] [PubMed] [Google Scholar]
- 22.Olsen BA, Pack BW. Hydrophilic interaction chromatography: a guide for practitioners. Hoboken, New Jersey: John Wiley & Sons, Inc; 2013. [DOI] [Google Scholar]
- 23.Food and Drug Administration. Guidance for industry: bioanalytical method validation. 2018. [accessed July 28, 2018]. https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf .
- 24.Lijinsky W. Chemistry and biology of N-nitroso compounds. UK: Cambridge University Press; 2011. [Google Scholar]
- 25.Food and Drug Administration. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. 2017. [accessed March 18, 2019]. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm .
- 26. Montzka TA, Juby PF, Matiskella JD, Holava HM, Crenshaw RR. Comparative nitrosation of etintidine and cimetidine. Can J Chem. 1983;61:1771–7. [Google Scholar]
- 27. Hellmig S, Von Schoning F, Gadow C, Katsoulis S, Hedderich J, Folsch UR, et al. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol. 2006;21:1832–8. doi: 10.1111/j.1440-1746.2006.04449.x. [DOI] [PubMed] [Google Scholar]
- 28. Hamamoto N, Hashimoto T, Adachi K, Hirakawa K, Ishihara S, Inoue H, et al. Comparative study of nizatidine and famotidine for maintenance therapy of erosive esophagitis. J Gastroenterol Hepatol. 2005;20:281–6. doi: 10.1111/j.1440-1746.2004.03546.x. [DOI] [PubMed] [Google Scholar]
- 29. Lerebours E, Michel P, Hochain P, Berkelmans I. Nizatidine as maintenance treatment of duodenal ulcer. Clinical results. Scand J Gastroenterol Suppl. 1994;206:52–5. doi: 10.3109/00365529409091422. [DOI] [PubMed] [Google Scholar]
- 30. ADMET Predictor®. ADMET property prediction and QSAR model-building application. 2019 [Google Scholar]
- 31. El-Saadi MW, Williams-Hart T, Salvatore BA, Mahdavian E. Use of in-silico assays to characterize the ADMET profile and identify potential therapeutic targets of fusarochromanone, a novel anti-cancer agent. Silico Pharmacol. 2015;3:6. doi: 10.1186/s40203-015-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kazius J, McGuire R, Bursi R. Derivation and validation of toxicophores for mutagenicity prediction. J Med Chem. 2005;48:312–20. doi: 10.1021/jm040835a. [DOI] [PubMed] [Google Scholar]
- 33. Sushko I, Novotarskyi S, Körner R, Pandey AK, Cherkasov A, Li J, et al. Applicability domains for classification problems: benchmarking of distance to models for ames mutagenicity set. J Chem Inf Model. 2010;50:2094–111. doi: 10.1021/ci100253r. [DOI] [PubMed] [Google Scholar]
- 34. Ibrahim MA, El-Alfy AT, Ezel K, Radwan MO, Shilabin AG, Kochanowska-Karamyan AJ, et al. Marine inspired 2-(5-halo-1H-indol-3-yl)-N,N-dimethylethanamines as modulators of serotonin receptors: an example illustrating the power of bromine as part of the uniquely marine chemical space. Mar Drugs. 2017;15:428. doi: 10.3390/md15080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun X, Li Y, Li W, Xu Z, Tang Y. Computational investigation of interactions between human H2 receptor and its agonists. J Mol Graph Model. 2011;29:693–701. doi: 10.1016/j.jmgm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 36. Gantz I, DelValle J, Wang LD, Tashiro T, Munzert G, Guo YJ, et al. Molecular basis for the interaction of histamine with the histamine H2 receptor. J Biol Chem. 1992;267:20840–3. [PubMed] [Google Scholar]
- 37. Del Valle J, Gantz I. Novel insights into histamine H2 receptor biology. Am J Physiol. 1997;273:G987–96. doi: 10.1152/ajpgi.1997.273.5.G987. [DOI] [PubMed] [Google Scholar]
- 38. Tobiszewski M, Marć M, Gałuszka A, Namieśnik J. Green chemistry metrics with special reference to green analytical chemistry. Molecules. 2015;20:10928–46. doi: 10.3390/molecules200610928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duncan RH, Davies GS. Alkylation of DNA bases by carcinogenic N-nitrosamine metabolites: a theoretical study. Int J Quantum Chem. 1989;35:665–77. doi: 10.1002/qua.560350507. [DOI] [Google Scholar]
- 40. Hebels DGAJ, Briedé JJ, Khampang R, Kleinjans JCS, de Kok TMCM. Radical mechanisms in nitrosamine-and nitrosamide-induced whole-genome gene expression modulations in Caco-2 cells. Toxicol Sci. 2010;116:194–205. doi: 10.1093/toxsci/kfq121. [DOI] [PubMed] [Google Scholar]
- 41. de la Monte SM, Tong M. Mechanisms of nitrosamine-mediated neurodegeneration: potential relevance to sporadic Alzheimer’s disease. J Alzheimer’s Dis. 2009;17:817–25. doi: 10.3233/JAD-2009-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coll ALP, Nicolau FEP. New ascorbate salts of H2 receptors antagonists - e.g. cimetidine, preventing formation of potentially carcinogenic nitroso derivs. :1986BE905235A. [Google Scholar]
- 43. Bartsch H, Ohshima H, Pignatelli B. Inhibitors of endogenous nitrosation mechanisms and implications in human cancer prevention. Mutat Res Mol Mech Mutagen. 1988;202:307–24. doi: 10.1016/0027-5107(88)90194-7. [DOI] [PubMed] [Google Scholar]
- 44. Morton DM. Pharmacology and toxicology of nizatidine. Scand J Gastroenterol. 1987;22:1–8. doi: 10.3109/00365528709094479. [DOI] [PubMed] [Google Scholar]
- 45. Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology. 2016;63:590–603. doi: 10.1002/hep.28323. [DOI] [PubMed] [Google Scholar]
- 46. Brambilla G, Mattioli F, Martelli A. Genotoxic and carcinogenic effects of gastrointestinal drugs. Mutagenesis. 2010;25:315–26. doi: 10.1093/mutage/geq025. [DOI] [PubMed] [Google Scholar]
- 47. Zeng T, Mitch WA. Oral intake of ranitidine increases urinary excretion of N-nitrosodimethylamine. Carcinogenesis. 2016;37:625–34. doi: 10.1093/carcin/bgw034. [DOI] [PubMed] [Google Scholar]
- 48. Fernández I, Hervés P, Parajó M. Kinetic study of nitrosation of guanidines. J Phys Org Chem. 2008;21:713–7. doi: 10.1002/poc.1380. [DOI] [Google Scholar]
- 49. Mowat C, Williams C, Gillen D, Hossack M, Gilmour D, Carswell A, et al. Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation. Gastroenterology. 2000;119:339–47. doi: 10.1053/gast.2000.9367. [DOI] [PubMed] [Google Scholar]