Abstract
The triggering of gemcitabine (GEM) drug resistance in pancreatic cancer by the receptor for advanced glycation end products (RAGE) has been demonstrated. Hence, finding a safe and effective adjuvant for preventing pancreatic cancer progression is imperative. Quercetin is a flavonoid that is abundant in apples, grapes, red raspberry, and onions and has been reported to inhibit RAGE. This research aimed to investigate the mechanisms of quercetin in regulating cell death and enhancing drug effects through RAGE reduction, especially in GEM-resistant pancreatic cancer cells. Our results showed that silencing RAGE expression by RAGE-specific siRNA transfection significantly increased cell death by apoptosis, autophagy and GEM-induced cytotoxicity by suppressing the PI3K/AKT/mTOR axis in MIA Paca-2 and MIA Paca-2 GEMR cells (GEM-resistant cells). Notably, quercetin showed a dramatic effect similar to RAGE silencing that effectively attenuated RAGE expression to facilitate cell cycle arrest, autophagy, apoptosis, and GEM chemosensitivity in MIA Paca-2 GEMR cells, suggesting that an additional reaction occurred under combined quercetin and GEM treatment. In conclusion, the results demonstrated that the molecular mechanisms of quercetin in regulating apoptosis and autophagy-related pathways and increasing GEM chemosensitivity in pancreatic cancer cells involved inhibition of RAGE expression.
Keywords: Autophagy, Gemcitabine resistance, Pancreatic cancer, Quercetin, RAGE
1. Introduction
Pancreatic cancer is the 7th most frequent cause of cancer death in the world and presents the lowest five-year survival rate of 6%, with approximately 460,000 new cases in 2018. Despite the existence of many academic institutes devoted to improving the diagnosis and treatment of pancreatic cancer, it remains the most lethal cancer, can rarely be treated by surgical resection, and exhibits a poor prognosis and high mortality rate [1]. The poor prognosis of pancreatic cancer patients may mainly be attributed to its early metastasis and highly aggressive nature, which accelerate cancer progression and reduce the effects of treatment [2]. Currently, surgical resection is regarded as the only effective method for pancreatic cancer patient treatment [3]. Unfortunately, less than 20% of stage I or II pancreatic cancer patients are suitable for resection because most patients exhibit local vascular growth and distant metastatic disease that preclude curative surgery [4]. Thus, chemotherapy is the most common method for metastatic pancreatic cancer treatment.
Gemcitabine (GEM), an analog of deoxycytidine for DNA synthesis inhibition, is the first approach for pancreatic cancer treatment and is also used to treat head and neck cancers, breast cancer, melanoma, nonmuscle-invasive urothelial carcinoma, and nonsmall cell lung carcinoma [5–7]. Despite the wide use of GEM, intrinsic and acquired drug resistance frequently leads to treatment failure [8], and the underlying mechanism of chemoresistance is poorly understood.
The receptor for advanced glycation end products (RAGE) is expressed by cancer cells such as those of pancreatic, colonic, breast, prostate, liver, and lung cancers [9,10]. RAGE activation is responsible for increasing inflammation, promoting the development of a variety of cancers, causing the cancer metastasis phenomenon, and increasing the development of drug-resistant pancreatic cancer [11,12]. RAGE-initiated signals accelerate pancreatic tumor development; therefore, clarifying the cancer-related RAGE pathways may reduce the length of pancreatic cancer treatment and drug resistance.
In recent years, phytochemicals have been used as cancer chemopreventive agents [13,14]. Quercetin is a flavonoid found in many foods, including apples, grapes, red raspberry, vegetables, cabbage, broccoli, onions, shallots, tea, tomatoes, and nuts [15]. Research has indicated that quercetin exerts anti-cancer effects in different ways, such as anti-oxidation and anti-proliferation activities, cell cycle arrest, apoptosis induction, and metastasis inhibition [16,17]. A study on drug-resistant uterine sarcoma showed that quercetin inhibits multidrug resistance protein 1 (MDR1) expression, blocks drug efflux via P-glycoprotein (Pgp) transport proteins, and increases the activity of anti-cancer drugs in MES-SA cells, suggesting that quercetinmay act as a chemopreventive agent and plays an important role in modulating chemotherapy drug sensitivity [18].
However, the role of quercetin in the inhibition of RAGE-initiated pancreatic cancer progression and drug resistance remains unknown. Therefore, this study analyzed the impact of RAGE expression on cell viability and drug efflux in human pancreatic cancer cells. In addition, we investigated the role of quercetin in promoting cell death and reducing drug resistance via the RAGE-associated signaling pathway.
2. Materials and methods
2.1. Cell maintenance and GEM-resistant cell establishment
The human pancreatic cancer cell lines MIA Paca-2 (BCRC NO. 60139), BxPC-3 (BCRC NO. 60283), AsPC-1 (BCRC NO. 60494), HPAC (BCRC NO. 60495) and PANC-1 (BCRC NO. 60284) were purchased from the Bioresource Collection and Research Center (Hsin Chu, Taiwan). A stable GEM-resistant pancreas carcinoma cell line (designated MIA Paca-2 GEMR cells) was established from 0.05 μM GEM concentration and gradually increasing GEM concentrations for developing a cellular model tolerance of 0.5 μM GEM. All tumor cells were maintained according to ATCC guidelines and cultured in an incubator supplemented with 5% CO2 at 37 °C. The stimulants GEM and quercetin were purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.2. Transfection of small interfering RNA (siRNA)
Cells were seeded into six-well plates and transfected with 25 nM siRNA for 24 h. The procedure was performed according to the manufacturer’s instructions for the Dharmacon transfection kit (GE Healthcare, Lafayette, CO, USA).
2.3. MTT assay
A total of 5 × 104 cells/well were seeded into 24-well plates, followed by incubation for 24 h. After cell attachment, the MTT solution (5 mg/mL) was added, followed by incubation for 2 h. Then, 500 μL of DMSO solution was added to each well to determine cell viability. The absorbance was read at 570 nm. As control 100%, % = [Abs (sample)/Abs (control)] x 100.
2.4. RNA preparation and PCR
Total RNA of MIA Paca-2 cell was extracted by TRIzol plus RNA purification kit (Thermo Fisher Scientific, Waltham, MA, USA) according the description of the manufacturer’s manual. Then, cDNA was synthesis and amplified by reverse transcription-PCR analysis to detect RAGE and 18s (as an internal standard) gene expression. The primers used for amplification were as follows:
RAGE (forward 5′CACCTTCTCCTGTAGCTTCA3′; reverse 5′TGCCACAAGATGACCCCAAT3′);
18s (forward 5′TTGGAGGGCAAGTCTGGTG3′; reverse 5′CCGCTCCCAAGATCCAACTA3′).
2.5. Western blotting analysis
Western blotting was performed according to our previous reported [17]. Antibodies of RAGE, phospho-mTOR (Millipore, Billerica, MA, USA), Bcl-2, Bax, Akt, phospho-Akt, PI3K, phospho-PI3K (Cell signaling technology, Danvers, MA, USA), β-actin, becline-1, ATG5, LC3 (Novus Biologicals, Littleton, Colorado, USA), and NF-κB p65 (Abcam, Cambridge, UK) were used in this study. The protein-relative intensity were quantified by VisionWorks LS 6.3.3 (UVP, Cambridge, UK) and normalized to the β-actin control.
2.6. Cell cycle measurement
The cells were treated with GEM and quercetin, respectively. Cell cycle was measured by propidium iodide (PI) staining assay according to our previous reported [19] by using FACS-can flow cytometry (FACScan®. BD Co). The distribution of sub-G1 was analyzed using CellQuest software.
2.7. Annexin V-FITC/PI staining
After treatment, the FITC Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) was used to measure apoptotic cells staining with Annexin-V and PI solution and then analyzed by Flow Cytometer.
2.8. Caspase 3 activity analysis
Caspase-3 activity was analyzed according to our previous report [19]. After treatment and preparation, cell lysates were measured by caspase-3/CPP32 fluorometric assay kit (Bio-Vision, Inc., Milpitas, CA, USA). Activity was calculated by FLUOstar glaxy fluorescence plated reader.
2.9. Calcein-AM measurement
Cells were plated into 24-well culture plates and added 500 μL (250 nM) calcein incubation at 37 °C for 30-min. After incubation, the fluorescence was measured by Flow Cytometer. The mean fluorescence intensity (MFI) values was represented as calcein accumulation.
2.10. Statistical analysis
Data analysis was performed by using the SPSS statistical program. Results are presented as the mean ± SD. Duncan’s test was used to assess differences between multiple groups by using one-way ANOVA. A P value < 0.05 was considered significant.
3. Results
3.1. Enhanced effect of RAGE-specific siRNA combined with GEM on inducing cell death and drug influx in MIA Paca-2 cells
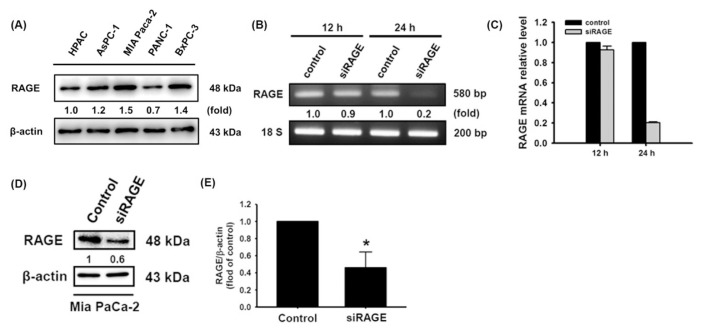
RAGE activation is responsible for increasing metastasis and the development of drug-resistant pancreatic cancer [12,20]. To assess the role of RAGE in pancreatic cancer cells, endogenous RAGE levels in HPAC, AsPC-1, MIA Paca-2, PANC-1, and BxPC-3 cells were initially measured (Fig. 1A). Furthermore, we established cells with low RAGE expression by siRNA transfection of MIA Paca-2 cells, which exhibited the highest level of endogenous RAGE expression in this study (Fig. 1B–E). After low RAGE-expressing MIA Paca-2 cells were established, the role of RAGE in cell growth was investigated.
Fig. 1.
Silencing RAGE mRNA expression in MIA Paca-2 cells. (A) Basal expression of RAGE in the HPAC, AsPC-3, MIA Paca-2, PANC-1 and BxPC-3 cell lines. (B–E) RAGE knockdown efficiency was measured by RT-PCR and Western blotting, respectively. Reported values are means ± SD (n = 3), *p < 0.05 versus the control.
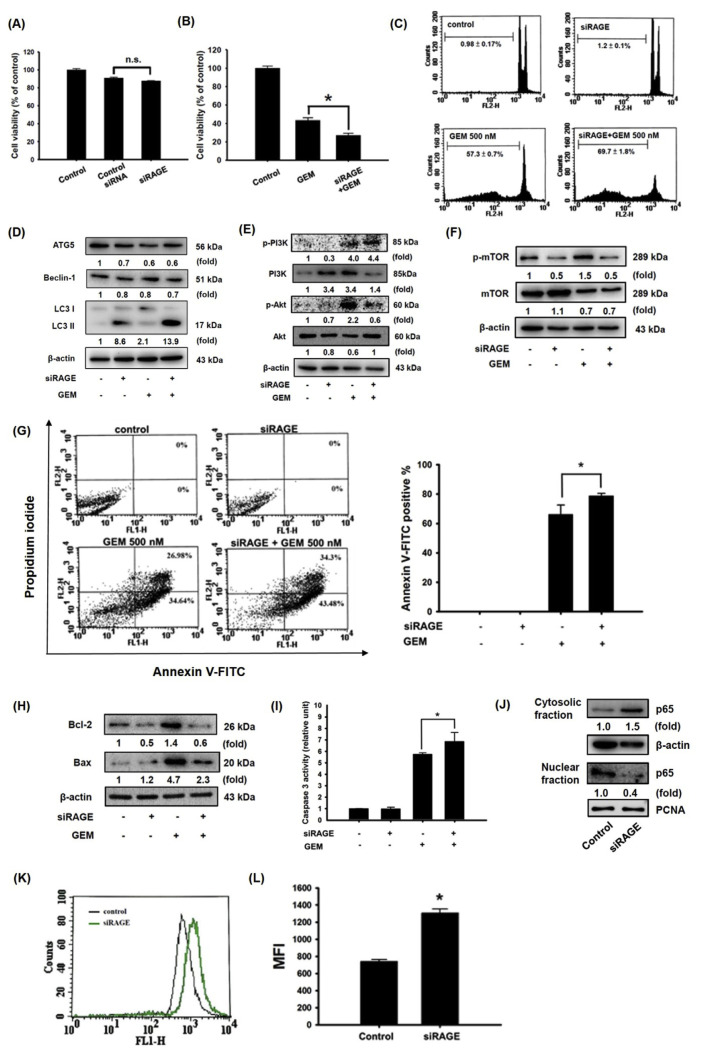
Knocking down RAGE expression did not affect cell viability but significantly increased GEM cytotoxicity in MIA Paca-2 cells (Fig. 2A and B). The same effect was also observed through cell cycle analysis. Treatment of low RAGE-expressing cells with 500 nM GEM for 72 h increased cell cycle arrested at the sub-G1 stage (69.7 ± 1.8%) compared with the control (0.98 ± 0.17%) (Fig. 2C). Previous evidence has shown that cell cycle arrest in an early phase can trigger autophagy through inhibition of the PI3K/AKT/mTOR axis [21,22]. Unexpectedly, autophagy-related 5 (ATG5) and Beclin-1 protein expression was not significantly changed by GEM treatment in RAGE knockdown cells (Fig. 2D). Nevertheless, the autophagy-related light chain 3 (LC3)-II protein level was dramatically increased in both cells subjected to RAGE silencing alone and GEM-treated RAGE-silenced cells (Fig. 2D). To examine the PI3K/AKT/mTOR axis in low RAGE-expressing MIA Paca-2 cells, the phosphorylation activities of PI3K, AKT, and mTOR were reduced in cells subjected to either RAGE silencing alone or RAGE silencing combined with GEM treatment (Fig. 2E and F).
Fig. 2.
Enhanced effect of RAGE-specific siRNA and GEM in inducing the cell death of MIA Paca-2 cells. Cells were transfected with RAGE-specific siRNA for 24 h and then treated with 500 nM GEM for 72 h. (A, B) Cell viability. (C) Sub-G1 phase. (D) Autophagy-associated ATG5, Beclin-1, LC3I, and LC3II protein expression. (E) Akt, PI3K, and (F) mTOR protein expression. (G) Apoptotic cells were stained with Annexin V-FITC positive/PI negative staining. (H) Bcl-2 and Bax protein expression. (I) Caspase 3 activity. (J) Protein levels of p65. (K, L) Effect of RAGE knockdown on the mean calcein fluorescence intensity in MIA Paca-2 cells. Reported values are means ± SD (n = 3), *p < 0.05 versus the control. (n.s. = Not significant). MFI: mean fluorescence intensity.
Some reports have demonstrated that mTOR-mediated autophagy precedes apoptosis and can induce the apoptosis process [23,24]. In this study, an increase apoptotic cells was observed in GEM-treated RAGE-silenced cells (Fig. 2G). Furthermore, increasing pro-apoptotic Bax protein expression and lower anti-apoptotic Bcl-2 levels were observed in cells subjected to either RAGE silencing alone or RAGE silencing combined with GEM treatment, respectively (Fig. 2H). Additionally, caspase 3 activity was significantly increased in GEM-treated RAGE-silenced cells compared to that in GEM-treated MIA Paca-2 cells (Fig. 2I). Previous studies have found that downregulation of the nuclear factor-kappa B p65 (NF-κB p65) subunit acts synergistically with GEM to restrict the growth of pancreas adenocarcinoma [25]. Knocking down RAGE expression greatly decreased NF-κB p65 nuclear protein production (Fig. 2J), suggesting that RAGE-specific siRNA exerts enhanced effect with GEM treatment in inducing cell death in MIA Paca-2 cells.
It has been well documented that Ca2+ influx and MDR1 upregulation are highly associated with GEM metabolism in human pancreatic carcinoma [26]. MDR1 principally regulates drug efflux. In addition, a previous study showed that NF-κB induces drug resistance through MDR1 expression [27]. In this study, RAGE-silenced cells exhibited a higher median fluorescence intensity (MFI) value, implying that a low level of MDR1 expression obstructed metabolization and increased the cytotoxicity effect (Fig. 2K,L). These results suggested that knocking down RAGE resulted in a decrease in NF-κB production, which may decrease MDR1 expression and concentrate GEM-induced intracellular cytotoxicity.
3.2. Quercetin promoted GEM-induced cytotoxicity by suppressing RAGE expression in MIA Paca-2 cells
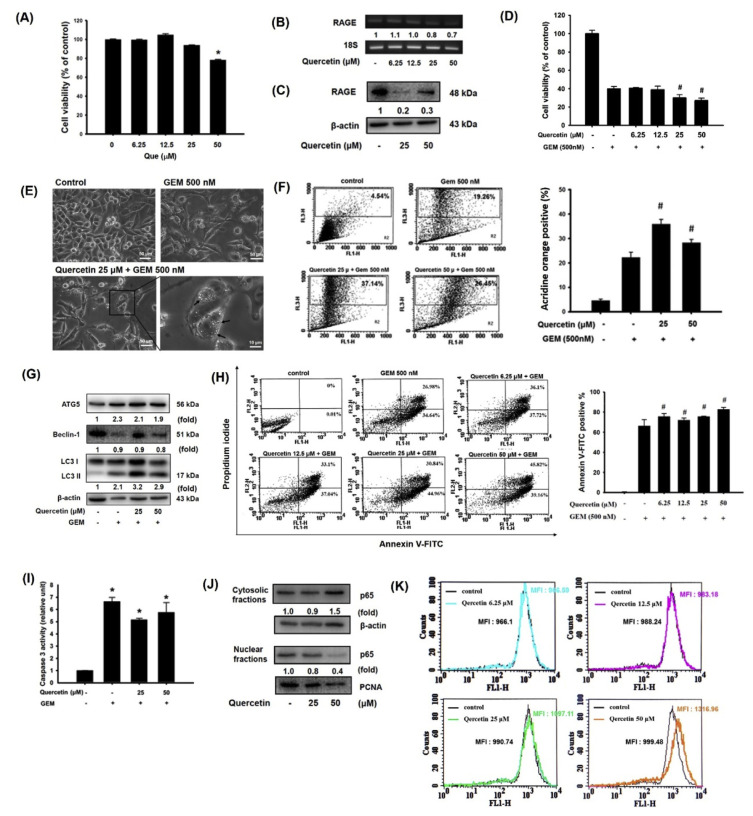
Research has shown that quercetin protects against amnesic injury through inactivation of the RAGE-mediated ERK/CREB/ BDNF pathway [28]. Our previous results also demonstrated that quercetin inhibits RAGE-mediated oxidative stress and inflammatory responses in THP-1 cells [29]. Here, we first noted that RAGE mRNA and protein expression was significantly suppressed by quercetin treatment in MIA Paca-2 cells, accompanied by cell growth inhibition (Fig. 3A–C). To determine the effect of quercetin on GEM sensitivity in MIA Paca-2 cells, cells were treated with quercetin at various concentrations (6.25, 12.5, 25, and 50 μM) for 24 h and then cultured with 500 nM GEM for 72 h. An additional reaction was observed that reduced the viability level under both 25 and 50 μM quercetin treatment during the GEM incubation period (Fig. 3D). Moreover, autophagy-associated vacuolation was observed in MIA Paca-2 cells treated with quercetin combined with GEM (Fig. 3E). Autophagic cells were further detected by acridine orange staining, and acridine orange-positive cells were increased by 25 or 50 μM quercetin treatment during the GEM incubation period (Fig. 3F).
Fig. 3.
Quercetin promoted GEM-induced cytotoxicity by suppressing RAGE expression in MIA Paca-2 cells. Effect of quercetin on (A) cytotoxicity, (B) RAGE mRNA expression, and (C) RAGE protein levels in Mia Paca-2 cells. (D) Cells were pretreated with various concentrations (6.25, 12.5, 25 and 50 μM) of quercetin for 24 h and then treated with 500 nM GEM for 72 h. Cell viability was measured via the MTT assay. (E) Autophagy-associated vacuolation in cells. (F) Percentages of autophagosomes. (G) Autophagy-associated ATG5, Beclin-1, LC3I, and LC3II protein expression. (H) Apoptotic cells were stained with Annexin V-FITC positive/PI-negative staining. (I) Caspase 3 activity. (J) Protein levels of p65. (K) Effect of quercetin on mean calcein fluorescence intensity in MIA Paca-2 cells. Reported values are means ± SD (n = 3), *p < 0.05 versus the control. MFI: mean fluorescence intensity.
Moreover, ATG5 and LC3-II protein expression was increased in both cells subjected to quercetin-treated alone and quercetin combined with GEM-treated cells compared with untreated control cells (Fig. 3G). Quercetin treatment of MIA Paca-2 cells not only induced autophagy but also increased apoptosis-associated caspase 3 activity and NF-κB p65 expression (Fig. 3H–J). Interestingly, the MFI value of calcein was increased in a dose-dependent manner by quercetin treatment in MIA Paca-2 cells (Fig. 3K), implying that a low level of MDR1 expression succeeded in impeding GEM efflux and increasing cytotoxicity.
3.3. Enhanced effect of RAGE-specific siRNA combined with GEM on inducing chemosensitivity in MIA Paca-2 GEMR cells
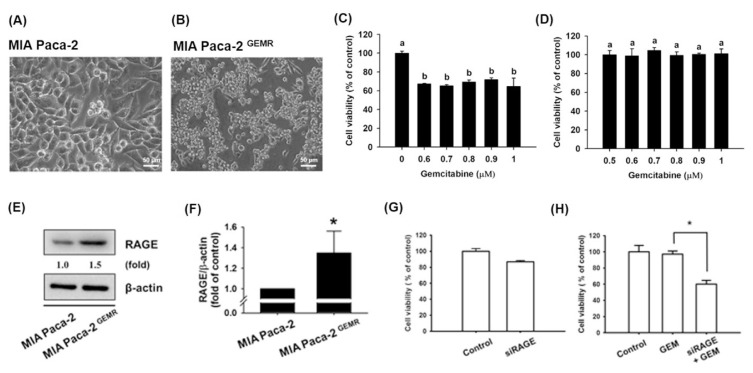
To assess whether quercetin treatment is effective against GEM-chemoresistant pancreatic cancer cell growth, we first established a GEM-resistant MIA Paca-2 human pancreatic cancer cell line (designated MIA Paca-2 GEMR cells) and assessed cell viability. As shown in Fig. 4A–D, the resistance to the pharmacologic cytotoxicity of GEM was noteworthy in MIA Paca-2 GEMR cells compared with parental cells. Not surprisingly, a high RAGE expression level was noted in MIA Paca-2 GEMR cells compared with MIA Paca-2 cells (Fig. 4E and F). Neither silencing RAGE gene expression nor GEM treatment reduced cell viability in MIA Paca-2 GEMR cells. However, an enhanced effect on increasing GEM chemosensitivity and reducing cell viability was observed under combined treatment with RAGE small interfering RNA and 500 nM GEM in MIA Paca-2 GEMR cells (Fig. 4G and H).
Fig. 4.
High RAGE protein levels in GEM-resistant MIA Paca-2 cells. Cell morphology of (A) MIA Paca-2 and (B) GEM-resistant MIA Paca-2 cells (denoted as MIA Paca-2 GEMR). Effect of GEM on the cell viability of (C) MIA Paca-2 cells and (D) MIA Paca-2 GEMR cells. (E, F) Protein levels of RAGE in both cell types. (G, H) MIA Paca-2 GEMR cells were transfected with RAGE-specific siRNA for 24 h and then treated with 500 nM GEM for 72 h. Cell viability was measured via the MTT assay. Reported values are means ± SD (n = 3), *p < 0.05 versus the control.
3.4. Quercetin induced autophagy and apoptosis by suppressing RAGE expression in MIA Paca-2 GEMR cells
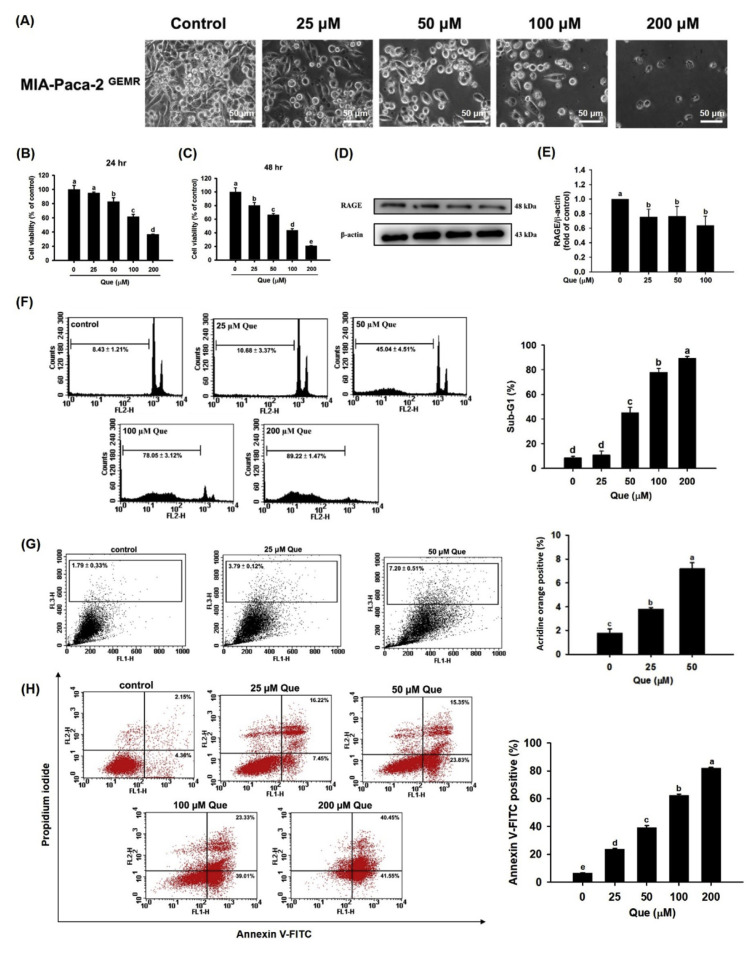
Cell death was exacerbated in a dose-dependent manner by quercetin treatment of MIA Paca-2 GEMR cells at 24 h or 48 h (Fig. 5A–C). The level of RAGE protein expression was decreased by quercetin treatment not only in MIA Paca-2 cells but also in MIA Paca-2 GEMR cells (Fig. 5D and E). Remarkably, the percentages of sub-G1 cell cycle arrest, autophagy, and apoptotic cells were increased by a dose-response effect upon quercetin treatment of MIA Paca-2 GEMR cells (Fig. 5F–H).
Fig. 5.
Quercetin induced cell death by inhibiting RAGE protein expression in MIA Paca-2 GEMR cells. (A) Effect of quercetin on the morphology of MIA Paca-2 GEMR cells. Cell viability of MIA Paca-2 GEMR cells after quercetin treatment for (B) 24 h and (C) 48 h. (D, E) Effect of quercetin on RAGE protein expression. (F) Cells were treated with various concentrations (25, 50, 100, and 200 μM) of quercetin for 48 h. The cell cycle was assessed by PI staining. (G) Percentages of autophagosomes in quercetin-treated MIA Paca-2 GEMR cells. (H) Quercetin-induced apoptotic cells were stained with Annexin V-FITC positive/PI negative staining. The data are expressed as the mean ± SD of three independent measurements. Data from the same time point with different letters (a, b, c, d) show significant differences (p < 0.05) by Duncan’s test.
3.5. Additional effect of quercetin pretreatment coupled with GEM treatment in inducing cell death and increasing the MFI in MIA Paca-2 GEMR cells
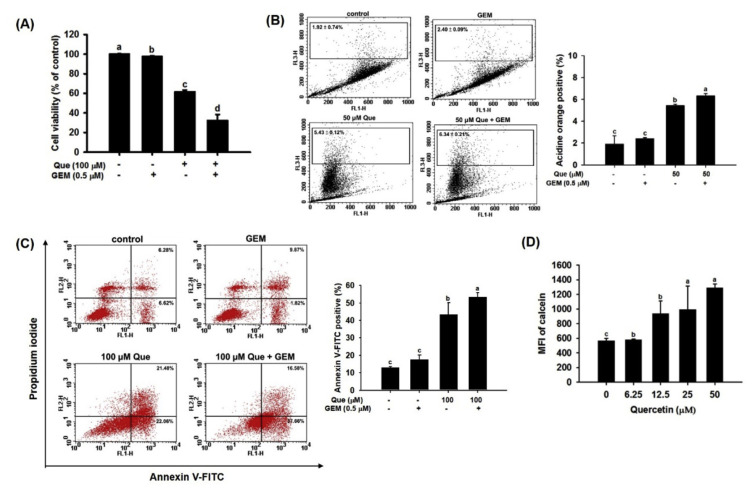
Cell viability was significantly decreased by quercetin treatment compared with GEM treatment in MIA Paca-2 GEMR cells (Fig. 6A). Interestingly, quercetin pretreatment combined with GEM treatment remarkably inhibited cell growth in MIA Paca-2 GEMR cells (Fig. 6A). Indeed, cell death via both autophagy and apoptosis was elicited by quercetin pretreatment coupled with GEM treatment in MIA Paca-2 GEMR cells (Fig. 6B and C). Moreover, MFI values were increased in a concentration-dependent manner by quercetin treatment in MIA Paca-2 GEMR cells (Fig. 6D), implying a reduction in drug elimination and extension of cytotoxicity effects under quercetin treatment.
Fig. 6.
Chemoprotective effect of quercetin in MIA Paca-2 GEMR cells. (A) Cells were pretreated with 100 μM Que for 24 h and then treated with 0.5 μM GEM for 72 h. Cell viability was measured via the MTT assay. (B) Percentages of autophagosomes in quercetin-treated MIA Paca-2 GEMR cells. (C) Percentage of apoptotic cells in quercetin-treated MIA Paca-2 GEMR cells. (D) Mean fluorescence intensity of calcein in MIA Paca-2 GEMR cells treated with quercetin. The data are expressed as the mean ± SD of three independent measurements. Data from the same time point with different letters (a, b, c, d) show significant differences (p < 0.05) by Duncan’s test. MFI: mean fluorescence intensity.
4. Discussion
The difficulty of early diagnosis of pancreatic cancer and resistance to chemotherapy result in a high mortality rate for pancreatic cancer patients. Accumulating evidence has shown that RAGE plays an important role in mediating pancreatic cancer tumorigenesis through distinct signaling cascades [9,30]. Hence, we knocked down RAGE expression in MIA Paca-2 and MIA Paca-2 GEMR cells to investigate the effects of RAGE on cell death and GEM metabolism. Silencing RAGE gene expression significantly reduced cell viability through cell cycle arrest, autophagy, and apoptosis in both cell lines. Furthermore, suppression of MDR1 levels was observed in RAGE knockdown cells, indicating that GEM efflux was prevented by RAGE inhibition. Interestingly, quercetin treatment significantly inhibited RAGE protein expression in both types of cells. The dramatic additive reaction effectively induced cell death and MDR1 expression in a dose-dependent manner in MIA Paca-2 and MIA Paca-2 GEMR cells.
RAGE has been shown to increase tumorigenesis and cancer processes in early pancreatic neoplasia [31]. Knocking down RAGE increases apoptosis and decreases inflammatory responses in mice with 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin cancer [32]. Similarly, RAGE is a potential factor involved in autophagy and apoptosis regulation in pancreatic cancer [33]. Accordingly, our results demonstrated that silencing RAGE gene expression significantly reduced cell viability by increasing autophagy and apoptosis in MIA Paca-2 and MIA Paca-2 GEMR cells, respectively (Figs. 2 and 4). Suppression of RAGE gene expression resulted in reduced PI3k/ Akt/mTOR signaling protein expression and increased autophagy by increasing LC3 II protein levels in MIA Paca-2 cells (Fig. 2D–F). Notably, increased PI3k/AKT/mTOR signaling and suppression of autophagy are observed in macrophages isolated from rats with streptozotocin (STZ)-induced diabetic encephalopathy (DE) [34]. In addition, Rottlerin elicits autophagocytosis, which causes apoptosis by inhibition of the PI3K/Akt/mTOR cascade in human pancreatic cancer stem cells [35]. Accordingly, our results showed that autophagy was negatively regulated by the RAGE/PI3K/Akt/mTOR axis.
LC3-II, a hallmark of autophagy, is tightly associated with autophagosomes and essential for autophagy [36]. Although other autophagy-associated proteins were not significantly altered by GEM treatment in RAGE knockdown cells, LC3-II protein expression was dramatically increased in RAGE-silenced cells and GEM-treated RAGE-silenced cells (Fig. 2D). In addition, a previous report showed that abnormal induction of autophagy by 6-OHDA-induced ROS accumulation could promote cytochrome c release and ultimately accelerate caspase-3-dependent apoptosis [37]. Moreover, increasing the ratio of Bax to Bcl-2 may activate caspase-3-modulated apoptosis [38]. Here, we found that the induction of autophagy by RAGE suppression led to an increase in the Bax/Bcl-2 ratio and a decrease in NF-κB p65 expression, causing caspase-3-dependent apoptosis in MIA Paca-2 cells (Fig. 2H–J). Convincing evidence has established that NF-κB can induce drug resistance through MDR1 expression [27,39]. In agreement with previous reports, our results showed that down-regulation of NF-κB suppressed MDR1 expression in GEM-treated MIA Paca-2 cells (Fig. 2I-L). As mentioned above, our findings demonstrated enhanced effect of RAGE-specific siRNA combined with GEM treatment in inducing cell death via autophagy and apoptosis and reducing GEM efflux in MIA Paca-2 cells.
Quercetin is a flavonoids with outstanding anti-cancer potential due to its anti-proliferation effect and roles in cell cycle arrest, the induction of apoptosis, and the inhibition of cell invasion [16,40]. Our previous results demonstrated that quercetin inhibited RAGE-mediated oxidative stress and inflammatory responses in THP-1 cells [17]. Previous findings have shown that quercetin inhibitsMDR1 expression, prevents drug efflux via Pgp transport proteins, and increases the activity of anti-cancer drugs in MES-SA cells [18]. However, the underlying mechanism of quercetin in reducing MDR1 expression is unknown. As anticipated, treatment with quercetin significantly inhibited RAGE expression in MIA Paca-2 cells (Fig. 3B and C). Similar to what was observed in RAGE siRNA knockdown cells, autophagy, apoptosis, and increased MFI values were observed to promote GEM-induced cytotoxicity under quercetin treatment in MIA Paca-2 cells (Fig. 3).
Importantly, a higher RAGE expression level was observed in MIA Paca-2 GEMR cells compared with MIA Paca-2 cells (Fig. 4E and F). Quercetin dramatically reduced cell viability, increased autophagy, and promoted apoptosis in a dose-dependent manner by suppressing RAGE expression in MIA Paca-2 GEMR cells (Fig. 5). Furthermore, autophagy, apoptosis, and chemosensitivity were greatly increased by the additional effect of quercetin combined GEM treatment in MIA Paca-2 GEMR cells (Fig. 6).
Collectively, these results indicate that silencing RAGE significantly increases cell death and GEM-induced cytotoxicity through the PI3K/AKT/mTOR axis in MIA Paca-2 and MIA Paca-2 GEMR cells. Notably, quercetin treatment (similar to RAGE silencing) effectively attenuated RAGE expression, resulting in cell cycle arrest, autophagy, apoptosis, and GEM chemosensitivity in MIA Paca-2 GEMR cells, suggesting that an additional reaction occurred under combined quercetin and GEM treatment. This study provides direct evidence for revealing the molecular mechanisms of quercetin in increasing autophagy, apoptosis, and chemosensitivity in pancreatic cancer.
Acknowledgments
This research work was supported in part by the grant MOST 107-2320-B-005-009- from the Ministry of Science and Technology, Taiwan.
Abbreviations
- Atg
autophagy gene
- Bax
Bcl2-associated X Protein
- Bcl-2
B-cell lymphoma 2
- GEM
gemcitabine
- LC3
microtubule-associated protein light chain 3
- MDR1
multidrug resistance protein 1
- MFI
median fluorescence intensity
- NF-κB
nuclear factor κB
- Pgp
P-glycoprotein
- RAGE
receptor for advanced glycation end products
- siRNA
small interfering RNA.
Funding Statement
This research work was supported in part by the grant MOST 107-2320-B-005-009- from the Ministry of Science and Technology, Taiwan.
Footnotes
Conflicts of interest
The authors declare no conflict of interests.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Ca - Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2. Kikuyama M, Kamisawa T, Kuruma S, Chiba K, Kawaguchi S, Terada S, et al. Early diagnosis to improve the poor prognosis of pancreatic cancer. Cancers (Basel) 2018:10. doi: 10.3390/cancers10020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eberlin LS, Margulis K, Planell-Mendez I, Zare RN, Tibshirani R, Longacre TA, et al. Pancreatic cancer surgical resection margins: molecular assessment by mass spectrometry imaging. PLoS Med. 2016;13:e1002108. doi: 10.1371/journal.pmed.1002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kindler HL. A glimmer of hope for pancreatic cancer. N Engl J Med. 2018;379:2463–4. doi: 10.1056/NEJMe1813684. [DOI] [PubMed] [Google Scholar]
- 5. Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan3682. [DOI] [PubMed] [Google Scholar]
- 6. Stone L. Gemcitabine reduces recurrence. Nat Rev Urol. 2018;15:466. doi: 10.1038/s41585-018-0033-x. [DOI] [PubMed] [Google Scholar]
- 7. Sidaway P. Head and neck cancer: gemcitabine improves patient survival. Nat Rev Clin Oncol. 2016;13:654. doi: 10.1038/nrclinonc.2016.149. [DOI] [PubMed] [Google Scholar]
- 8. Binenbaum Y, Na’ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updates. 2015;23:55–68. doi: 10.1016/j.drup.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 9. Shahab U, Ahmad MK, Mahdi AA, Waseem M, Arif B, Moinuddin Ahmad S. The receptor for advanced glycation end products: a fuel to pancreatic cancer. Semin Cancer Biol. 2018;49:37–43. doi: 10.1016/j.semcancer.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 10. Ahmad S, Khan H, Siddiqui Z, Khan MY, Rehman S, Shahab U, et al. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin Cancer Biol. 2018;49:44–55. doi: 10.1016/j.semcancer.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 11. Hudson BI, Lippman ME. Targeting RAGE signaling in inflammatory disease. Annu Rev Med. 2018;69:349–64. doi: 10.1146/annurev-med-041316-085215. [DOI] [PubMed] [Google Scholar]
- 12. Huang CY, Chiang SF, Chen WT, Ke TW, Chen TW, You YS, et al. HMGB1 promotes ERK-mediated mitochondrial Drp1 phosphorylation for chemoresistance through RAGE in colorectal cancer. Cell Death Dis. 2018;9:1004. doi: 10.1038/s41419-018-1019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weng CJ, Yen GC. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 14. Shankar E, Kanwal R, Candamo M, Gupta S. Dietary phytochemicals as epigenetic modifiers in cancer: promise and challenges. Semin Cancer Biol. 2016;40–41:82–99. doi: 10.1016/j.semcancer.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peluso I, Miglio C, Morabito G, Ioannone F, Serafini M. Flavonoids and immune function in human: a systematic review. Crit Rev Food Sci Nutr. 2015;55:383–95. doi: 10.1080/10408398.2012.656770. [DOI] [PubMed] [Google Scholar]
- 16. Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, et al. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6:24049. doi: 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang SL, Hsu CL, Yen GC. Growth inhibitory effect of quercetin on SW 872 human liposarcoma cells. Life Sci. 2006;79:203–9. doi: 10.1016/j.lfs.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 18. Kim MK, Choo H, Chong Y. Water-soluble and cleavable quercetin-amino acid conjugates as safe modulators for P-glycoprotein-based multidrug resistance. J Med Chem. 2014;57:7216–33. doi: 10.1021/jm500290c. [DOI] [PubMed] [Google Scholar]
- 19. Hsu CL, Yen GC. Induction of cell apoptosis in 3T3-L1 pre-adipocytes by flavonoids is associated with their antioxidant activity. Mol Nutr Food Res. 2006;50:1072–9. doi: 10.1002/mnfr.200600040. [DOI] [PubMed] [Google Scholar]
- 20. Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–76. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J, et al. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res. 2018;37:63. doi: 10.1186/s13046-018-0731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Zhu M, Wang Q, Hou Y, Li L, Weng H, et al. Publisher Correction: alpha-fetoprotein inhibits autophagy to promote malignant behaviour in hepatocellular carcinoma cells by activating PI3K/AKT/mTOR signalling. Cell Death Dis. 2019;10:214. doi: 10.1038/s41419-018-1292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park A, Koh HC. NF-kappaB/mTOR-mediated autophagy can regulate diquat-induced apoptosis. Arch Toxicol. 2019;93:1239–53. doi: 10.1007/s00204-019-02424-7. [DOI] [PubMed] [Google Scholar]
- 24. Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kong R, Sun B, Jiang H, Pan S, Chen H, Wang S, et al. Downregulation of nuclear factor-kappaB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett. 2010;291:90–8. doi: 10.1016/j.canlet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 26. Monteith GR, Prevarskaya N, Roberts-Thomson SJ. The calcium-cancer signalling nexus. Nat Rev Cancer. 2017;17:367–80. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- 27. Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–7. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 28. Liu R, Zhang TT, Zhou D, Bai XY, Zhou WL, Huang C, et al. Quercetin protects against the Abeta(25–35)-induced amnesic injury: involvement of inactivation of rage-mediated pathway and conservation of the NVU. Neuropharmacology. 2013;67:419–31. doi: 10.1016/j.neuropharm.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 29. Huang SM, Wu CH, Yen GC. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol Nutr Food Res. 2006;50:1129–39. doi: 10.1002/mnfr.200600075. [DOI] [PubMed] [Google Scholar]
- 30. Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, et al. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33:567–77. doi: 10.1038/onc.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, et al. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci U S A. 2012;109:7031–6. doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–85. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang R, Tang D, Lotze MT, Zeh HJ., 3rd RAGE regulates autophagy and apoptosis following oxidative injury. Autophagy. 2011;7:442–4. doi: 10.4161/auto.7.4.14681. [DOI] [PubMed] [Google Scholar]
- 34. Wang B, Zhong Y, Li Q, Cui L, Huang G. Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy. Aging (Albany NY) 2018;10:2772–82. doi: 10.18632/aging.101586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh BN, Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem Pharmacol. 2012;84:1154–63. doi: 10.1016/j.bcp.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 36. Wilkinson DS, Jariwala JS, Anderson E, Mitra K, Meisenhelder J, Chang JT, et al. Phosphorylation of LC3 by the Hippo kinases STK3/STK4 is essential for autophagy. Mol Cell. 2015;57:55–68. doi: 10.1016/j.molcel.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung Y, Lee J, Jung S, Lee Y, Cho JW, Oh YJ. Dysregulated autophagy contributes to caspase-dependent neuronal apoptosis. Cell Death Dis. 2018;9:1189. doi: 10.1038/s41419-018-1229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang B, Johnson TS, Thomas GL, Watson PF, Wagner B, Furness PN, et al. A shift in the Bax/Bcl-2 balance may activate caspase-3 and modulate apoptosis in experimental glomerulonephritis. Kidney Int. 2002;62:1301–13. doi: 10.1111/j.1523-1755.2002.kid587.x. [DOI] [PubMed] [Google Scholar]
- 39. Skrypek N, Duchene B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2013;32:1714–23. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russo M, Spagnuolo C, Volpe S, Mupo A, Tedesco I, Russo GL. Quercetin induced apoptosis in association with death receptors and fludarabine in cells isolated from chronic lymphocytic leukaemia patients. Br J Canc. 2010;103:642–8. doi: 10.1038/sj.bjc.6605794. [DOI] [PMC free article] [PubMed] [Google Scholar]