Abstract
Different Viola species are known for their traditional use as analgesic, antitussive, febrifuge, hipnotic, analgesic and anti-inflammatory medicinal agents. Additionally, they are considered edible flowers in certain cultures. Thus, the aim of this work was to characterize the phenolic composition and to assess the neuroprotective properties of Viola cornuta and Viola x wittrockiana using in vitro and in vivo methodologies with Caenorhabditis elegans as model. The identification of the phenolic compounds was carried out with a LC-DAD-ESI/MSn. The antioxidant activity of the extracts was determined in vitro using Folin-Ciocalteu, DPPH and FRAP assays and in vivo with a juglone-induced oxidative stress in C. elegans. The neuroprotective properties were evaluated measuring the ability to inhibit CNS enzymes (MAO A, AChE), and the capability to avoid paralyzing the C. elegans CL4176, an Alzheimer disease model. The phenolic content was higher in V. x wittrockiana, being quercetin-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnoside the predominant compound in the extract, which also exhibited a stronger antioxidant capacity in vitro and a higher response to lethal oxidative stress on C. elegans than V. cornuta. Only V. x wittrockiana showed inhibitory effect on CNS enzymes, such as acetylcholinesterase and monoamine oxidase A, but both had protective effect against the paralysis of C. elegans. These findings suggest that the studied V. cornuta and V. x wittrockiana could be interesting candidates for age related neurodegenerative disorder associated with oxidative stress.
Keywords: Antioxidant, Caenorhabditis elegans, LC-DAD-ESI-MSn, Neuroprotective potential, Polyphenols, Viola
1. Introduction
Disorders of the Central Nervous System (CNS) such as Alzheimer’s and Parkinson’s disease are among the most prevalent pathologies in relation with oxidative stress and ageing. Despite the importance of medicines to improve the quality of life of these patients, most drugs just act as symptomatic treatments and the development of new drugs to treat or prevent them is limited [1].
In certain systems of traditional medicine, the use of various species of genus Viola has been reported as CNS agents [2,3], this fact is a good starting point to explore the potential of understudied species such as V cornuta L. (horned violet) and Viola x wittrockiana Gams (pansy) for neuroprotective activities, and related bioactivities with neurodegeneration such as antioxidant and anti-ageing properties. Previous studies about the chemical composition and antioxidant activity of V. x wittrockiana, have already been performed [4–6]. However, to the best of our knowledge, this is the first report about the phenolic composition and properties of V. cornuta.
New methods are currently being implemented and used for drug testing, particularly in the field of natural products. The use of mammals in experimentation is not only expensive and laborious, but also associated to ethical limitations. For these reasons, other models for screening and preliminary studies must be used. C. elegans, is, indeed, a good model because these organisms are small, easy handling and inexpensive. Moreover, signalling pathways and physiological processes relevant for humans are conserved in this species, and 60–80% of the human genes have homologues in C. elegans [7]. Additionally, the easy genetic manipulation of this nematode allows the existence of a wide range of mutant strains and the possibility of developing specific ones. Therefore, C. elegans is a powerful tool to test new bioactives, allowing researchers the chance to study their biological activity, and identifying primary or secondary targets, among other possibilities [7].
Thus, the goal of this research is the characterization of phenolic compounds and the assessment of antioxidant and neuroprotective properties of V. cornuta and V. x wittrockiana extracts, using both in vitro and in vivo assays.
2. Material and methods
2.1. Standards and reagents
Acetonitrile (99.9%) was of HPLC grade from Fisher Scientific (Lisbon, Portugal). Phenolic compound standards (apigenin-6-C-glucoside, quercetin-3-O-glucoside, quercetin-3-O-rutinoside, peonidin-3-O-glucoside) were from Extrasynthèse (Genay, France). Formic acid, DPPH· (2,2-diphenyl-1-picrylhydrazyl), TPTZ (2,4,6-tris(2-pyridyl)-s-triazine), ATCI (acetylthiocholine iodide), acetylcholinesterase (AChE), monoamine oxidase A (MAO A), clorgiline, tris–HCl and pyrogallol were purchased from Sigma–Aldrich (St. Louis, MO, USA). DNTB (5,5′-dithiobis (2-nitrobenzoic acid)) and juglone (5-hydroxy-1,4-naphthoquinone) were from Alfa Aesar (Ward Hill, MA, USA), Folin-Ciocalteu reagent was purchased from Chem-lab (Zeldelgem, Belgium). Water was treated in a Milli-Q water purification system (TGI Pure Water Systems, Greenville, SC, USA).
2.2. Plant material and preparation of extracts
Cultivated fresh flowers of V. cornuta and V. x wittrockiana were supplied and botanically identified by Dr Laura Carrera from Innoflower S.L. A voucher specimen was deposited in the herbarium of Universidad San Jorge with numbers 003–2014 (V. cornuta) and 004–2014 (V. x wittrockiana). Ethanolic extracts of flowers were obtained by percolation with a Soxhlet apparatus for 4 h. Solvent was removed from the extract with a rotary flash evaporator Buchi and the resulting extracts were preserved at −20°C in order to avoid degradation until its use.
2.3. Analysis of phenolic compounds
The phenolic compound profile (non-anthocyanin compounds and anthocyanin compounds) was determined through an LC-DAD-ESI/MSn system using a Dionex Ultimate 3000 UPLC (Thermo Scientific, San Jose, CA, USA).
Non-anthocyanin compounds
These compounds were separated and identified as previously described by Bessada et al. [8]. The obtained extracts were re-dissolved at a concentration of 10 mg/mL with ethanol. A double online detection was performed using a DAD (280, 330 and 370 nm as preferred wavelengths) and amass spectrometer (MS). The MS detection was performed in negative mode, using a Linear Ion Trap LTQ XL mass spectrometer (Thermo Finnigan, San Jose, CA, USA) equipped with an ESI source.
Anthocyanin compounds
These compounds were separated and identified as previously described by Gonçalves et al. [9]. The obtained extracts were re-dissolved at a concentration of 10 mg/mL with the acidified ethanol (0.05% TFA). For the double online detection, a 520 nm wavelength was used as preferred for the DAD and in a MS equipment described above, working in positive mode.
The identification of the phenolic compounds (non-anthocyanin and anthocyanin) was performed based on their chromatographic behaviour, and UV–vis and mass spectra by comparison with standard compounds, when available, and data reported in the literature giving a tentative identification. Data acquisition was carried out with Xcalibur® data system (Thermo Finnigan, San Jose, CA, USA). For quantitative analysis, a calibration curve for each available phenolic standard was constructed based on the UV–vis signal: apigenin-6-C-glucoside (y = 107025x + 61,531, R2 = 0.9989), quercetin-3-O-glucoside (y = 34843x – 160,173, R2 = 0.9998), and peonidin-3-O-glucoside (y = 166905x – 442,698, R2 = 0.9993). For the identified phenolic compounds for which a commercial standard was not available, the quantification was performed through the calibration curve of the most similar compound. Results were expressed as mg/g of extract.
2.4. In vitro cell-free antioxidant activity assays
2.4.1. Determination of Total Phenolic Content (TPC)
Total polyphenol content of flower extracts was measured using a modified Folin-Ciocalteu method in a 96 well-microplate [10]. Briefly, 9 μL of diluted extracts in ethanol were mixed with 201 μL of Folin-Ciocalteu reagent in a well. After 5 min, 90 μL of 15% sodium carbonate were added and the plate was incubated at room temperature in the darkness for 40 min. The absorbance was read at 750 nm and pyrogallol was used as standard. Results were expressed as mg of pyrogallol equivalent per g of extract (mg PE/g extract).
2.4.2. DPPH· radical scavenging activity assay
Free radical scavenging activity of the extracts was evaluated with the DPPH· assay following the method described by López et al. [11]. Flower extracts were serially diluted in ethanol (2.5–250 μg/mL) and 150 μL of these dilutions or ethanol were mixed with 150 μL of DPPH· (0.4 mg/mL, ethanol) in a plate. After 30 min in the dark at room temperature, the absorbance was measured at 517 nm. DPPH· radical-scavenging activity, % RSA, was calculated as:
| (1) |
where AS is the absorbance of solution containing the sample and ADPPH is the absorbance of the DPPH· ethanol blank solution.
2.4.3. Ferric reducing antioxidant power assay (FRAP)
This assay was performed according to Fernández-Moriano et al. with minor modifications [12]. The FRAP reagent was prepared daily and contained 2.5 mL of a TPTZ solution (10 mmol/L in 40 mmol/L HCl), 2.5 mL of FeCl3·6H2O (20 mmol/L) and 25 mL of 0.3 mol/L sodium acetate buffer (pH 3.6). Then, 900 μL of FRAP reagent (warmed at 37°C) was mixed with 90 μL of distilled water and 30 μL of flower extract (1 mg/mL in methanol) or methanol as reagent blank. Absorbance was measured after incubation at 37°C for 10 min at wavelength of 595 nm. The antioxidant capacity of the extracts was estimated by interpolating absorbance on a calibration curve of FeSO4·7H2O and expressed as μmol Fe+2 per g of extract (μmol Fe+2/g extract).
2.5. Neuroprotective potential
2.5.1. Acetylcholinesterase enzyme inhibitory activity
Inhibition of acetylcholinesterase enzyme (AChE) by the extracts was determined following the Ellman method described by Rhee et al. [13]. Briefly, 25 μL of ATCI (15 mM), 125 μL DNTB (3 mM in 50 mmol/L Tris–HCl buffer, pH = 8.3), 50 μL of Tris–HCl buffer, 25 μL of extracts sample (0.125–2 mg/mL in ethanol) or ethanol (control reaction) and 25 μL of AchE (0.22 U/mL) were mixed in each well of a microtiter plate. The absorbance was measured 5 times at 405 nm every 12 s. Percentage of inhibition was calculated as:
| (2) |
where VS is the reaction rate of the extract sample and VC is the rate of control.
2.5.2. Monoamine oxidase A enzyme inhibitory activity
Monoamine oxidase A (MAO A) inhibitory activity was measured by the peroxidase-linked assay described by Stafford et al. [14]. Each test well contained 50 μL of flower extract (0.002–0.250 mg/mL) or solvent, 50 μL of chromogenic solution (0.8 mM vanillic acid, 417 mM 4-aminoantipyrine and 4 U/mL horseradish peroxidase), 100 μL of 2.5 mM tyramine and 50 μL of 8 U/mL MAO-A. All components were dissolved in potassium phosphate buffer (0.2 M, pH 7.6). Absorbance was measured at 490 mn every 5 min over a period of 30 min. Percentage of enzymatic inhibition was calculated using equation 2.
2.6. In vivo biological activity - C. elegans assays
2.6.1. Worm strain, maintenance and treatment
N2 (wild type), CL4176 smg-1(cc546); dvls27, and SS104 glp-4 (bn2) C. elegans strains were used in this study and obtained from Caenorhabditis Genetics Center (CGC, USA) USA Worms. They were maintained on nematode growth medium (NGM) agar plates seeded with a lawn of E. coli OP50 (CGC, USA), according to standardized methods. N2 was propagated at 20°C, while transgenic CL4176 and SS104 were maintained at 16°C.
For the assay, the plates were prepared from a stock solution of extracts in water. The solutions were added directly to the agar during preparation of plates to the final indicated concentration.
2.6.2. Oxidative stress resistance assay
The effect of the extracts on the response of wild type C. elegans to a lethal oxidative stress was performed following Surco-Laos et al. method with some modifications [15]. The synchronized population were prepared by bleaching adults and incubated in M9 buffer until the eggs hatched. L1 larvae were incubated on NGM plates containing E. coli OP50 and different concentrations of flower extract (0, 50, 100, 250 μg/mL). Afterwards, adult worms were subjected to lethal oxidative-stress by means of juglone (150 μM) in a microtiter plate. After 24 h of incubation at 20°C, survival was measured by a touch-provoked movement. Worms that reacted to the mechanical stimulus were scored as alive, whereas non-responding worms were considered dead. Results were expressed as a percentage of survival rate and calculated as:
| (3) |
About 120 individuals per study group were evaluated in each assay.
2.6.3. Lifespan assay
Survival analysis was performed as described by Virk et al. with minor modifications [16]. Longevity of temperature-sensitive sterile C. elegans SS104 exposed to 4 different concentrations of flowers extracts were measured and compared to controls. Gravid adults were used to lay eggs onto fresh NGMOP50 plates at 15°C. Eggs were raised at 15°C till L4 stage, due to temperature sensitivity of mutant phenotypes. Once this phase was reached, animals were transferred into a 25°C environment. After 24 h at that temperature, 25 worms were put onto each of 5 replicate NGM fresh plates containing the extract or water as vehicle (control group). Animals were transferred to fresh plates after 7 and 14 days and scored for survival every 2–3 days. The scoring method was the same used as for the juglone oxidative stressed assays (2.6.2). Results were represented as Survival Rate %.
2.6.4. Paralysis assay
C. elegans CL4176 is a transgenic strain which expresses human β-amyloid peptide in muscle cells after up-shifting temperature from 16 to 25°C, rapidly causing a paralysis. CL4176 worms were egg-synchronized onto fresh NGM plates with or without flower extract at appropriate concentrations. When the animals grew to L3 larvae, the expression of human Aβ1–42 gene was induced by temperature upshifting from 15 to 25°C 24 h later, paralysis was scored at 2 h intervals until all worms were paralyzed [17]. Worms were considered as paralyzed if there was no response or they only moved their head when gently touched with a platinum wire. For each assay, at least 100 nematodes were used per studied condition.
2.7. Statistical analysis
Three independent experiments were performed for the cited assays. Statistical analysis was carried out using GraphPad Prism version 6.0c for Mac OS X (GraphPad Software, San Diego, CA, USA). Results were expressed as mean ± standard errors mean (SEM). The 50% inhibitory concentration (IC50) was estimated by means of a linear regression Comparisons and p value calculations were made between treated and control animal using one-way ANOVA and Tukey’s multiple comparison test. The treatment effect on lifespan and paralysis was determined using the Log-Rank and Wilcoxon tests of fitting to the Kaplan–Meier survival model. Differences with p ≤ 0.05 were considered statistically significant.
3. Results and discussion
3.1. Phenolic compounds of V. cornuta and V. x wittrockiana flower extracts
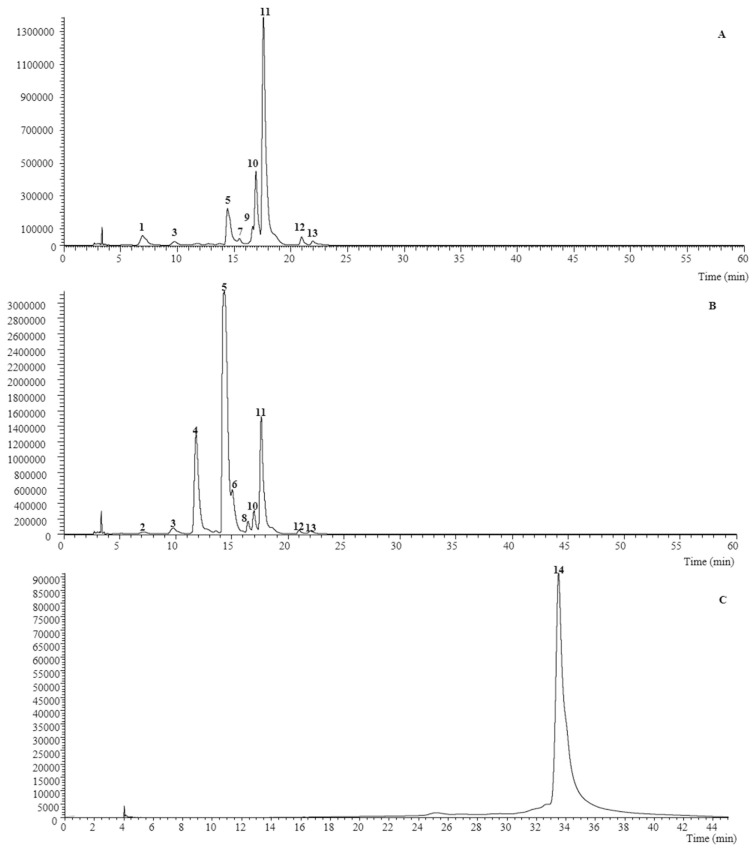
The obtained extracts had a yield of 4.83% and 5.65% (mass of extract/mass of fresh flowers) for V. cornuta and V. x wittrockiana, respectively, and were analysed by LC-DAD-ESI/MSn. Nine non-anthocyanin phenolic compounds were found in V. cornuta, while ten and one anthocyanin compound were detected in V. x wittrockiana (Table 1). Representative chromatograms are shown in Fig. 1.
Table 1.
Retention time (Rt), wavelengths of maximum absorption in the visible region (λmax), mass spectral data and tentative identification of the phenolic compounds present in V. cornuta and V. x wittrockiana.
| Peak | Rt (min) | λmax (nm) | Molecular ion [M–H]− (m/z) | MS2 (m/z) | Tentative identification | Reference | Quantification | t-Students test p-value | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| V. cornuta | V. x wittrockiana | ||||||||
| Non-anthocyanin phenolic compounds | |||||||||
| 1 | 6.82 | 263,294,348 | 609 | 519(24),489(100), 447(54),357(6),327(20) | Luteolin-O-hexoside-C-hexosideA | [19] | 1.23 ± 0.04 | nd | – |
| 2 | 7.30 | 265,293,347 | 609 | 489(100),399(6),369(5) | Luteolin-6,8-di-C-glucosideA | [32] | nd | 0.78 ± 0.001 | – |
| 3 | 9.77 | 334 | 593 | 503(29),473(100), 383(12),353(20),325(2) | Apigenin-6,8-di-C-glucosideA | [32] | 0.58 ± 0.01 | 2.40 ± 0.03 | <0.001 |
| 4 | 11.79 | 356 | 771 | 625(20),317(100) | Myricetin-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnosideB | DAD, ESI/MS | nd | 10.3 ± 0.2 | – |
| 5 | 14.31 | 355 | 755 | 609(32),301(100) | Quercetin-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnosideB | [5] | 1.27 ± 0.01 | 29.1 ± 0.4 | <0.001 |
| 6 | 15.03 | 350 | 625 | 317 (100) | Myricetin-3-O-rutinosideB | Standard | nd | 3.8 ± 0.1 | – |
| 7 | 15.44 | 336 | 577 | 473(100),457(28), 413(20),311(3) | Apigenin-C-hexosyl-C-deoxyhexosideA | [18] | 0.53 ± 0.01 | nd | – |
| 8 | 16.41 | 347 | 739 | 593(21),285(100) | Kaempferol-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnosideB | DAD, ESI/MS | nd | 1.26 ± 0.001 | – |
| 9 | 16.59 | 334 | 577 | 473(100),457(30), 413(10),353(30),295(2) | Apigenin-6-C-glucosyl-8-C-rhamnoside (violanthin)A | [5,6] | 1.05 ± 0.03 | nd | – |
| 10 | 16.91 | 268,335sh | 577 | 559(18),517(5), 487(49),457(100), 413(6),311(5) | Chrysin-6,8-di-C-glucosideA | [20] | 6.52 ± 0.04 | 4.80 ± 0.04 | <0.001 |
| 11 | 17.61 | 355 | 609 | 609 (100) | Quercetin-3-O-rutinosideB | Standard [5,6]; | 7.5 ± 0.1 | 10.1 ± 0.3 | <0.001 |
| 12 | 20.99 | 263,346 | 593 | 285 (100) | Kaempferol-3-O-rutinosideB | Standard | 0.71 ± 0.01 | 0.75 ± 0.01 | <0.001 |
| 13 | 22.93 | 354 | 623 | 315 (100) | Isorhamnetin-3-O-rutinosideB | Standard | 0.59 ± 0.01 | 0.75 ± 0.02 | <0.001 |
| Total non-anthocyanin phenolic compounds | – | 19.9 ± 0.01 | 64 ± 1 | <0.001 | |||||
|
| |||||||||
| Peak | Rt (min) | λ max (nm) | Molecular ion [M + H]+ (m/z) | MS 2 ( m/z ) | Tentative identification | Reference | V. cornuta | V. x wittrockiana | t- Students test p -value |
|
| |||||||||
| Anthocyanin phenolic compounds | |||||||||
| 14 | 33.48 | 530 | 625 | 479(20),317(100) | Petunidin-O-deoxyhexoside-hexosideC | DAD, ESI/MS | nd | 5.05 ± 0.04 | – |
nd-not detected. Standard calibration curves:
- apigenin-6-C-glucoside (y = 107025x + 61,531, R2 = 0.9989);
- quercetin-3-O-glucoside (y = 34843x – 160,173, R2 = 0.9998);
- peonidin-3-O-glucoside (y = 166905x – 442,698, R2 = 0.9993).
Fig. 1.
Phenolic profile of V. cornuta recorded at 370 nm (A), and V. x wittrockiana recorded at 370 nm (B) and 520 nm (C). Numbers 1 to 14 refers to peaks from Table 1.
In consideration of the chromatographic characteristic reported in Table 1, peaks 1–3, 7, 9, 10 were identified as flavone glycoside derivatives (luteolin, apigenin, and chrysin derivatives). All of the mentioned compounds presented a fragmentation pattern characteristic of C-glycoside and were all identified taking into account the hierarchical fragmentation pattern described by Ferreres et al. [18,19] thus peak 10 ([M–H]− at m/z 577), was identified taking into account the findings of Han et al. [20]. Moreover, compounds 7 and 9 ([M–H] − at m/z 577) have been previously described in Viola sp., being identified as apigenin-C-hexosyl-C-deoxyhexoside (peak 7), apigenin-6-C-glucosyl-8-C-rhamnoside (violanthin, peak 9) [5,6].
Otherwise compounds 6, 11, 12 and 13 were positively identified as myricetin-3-O-rutinoside quercetin-3-O-rutinoside (rutin), kaempferol-3-O-rutinoside, and isorhamnetin-3-O-rutinoside respectively, by comparison with authentic standards, as also by their MS fragmentation patterns, retention times and UV–vis characteristics. Rutin has also been previously described in V. x wittrockiana by other authors [5,6]. Compounds 4 ([M–H]− at m/z 771), 5 ([M–H]− at m/z 755), and 8 ([M–H]− at m/z 739) were identified as myricetin, quercetin and kaempferol derivatives owing to the product ion observed at m/z 317, 301 and 285, respectively, as also to their UV spectra (λmax around 347–356 nm). All the peaks MS2 fragments revealed the alternative loss of a deoxyhexosyl (−146 u) and deoxyhexosyl-hexoside (−308 u) residues, indicating location of each residue on different positions of the aglycone. González-Barrio et al. detected a similar compound to peak 5, being identified as quercetin-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnoside, thus this assumption was taken into account for this compounds identification [5]. For the remaining compounds (peak 4 and 8), similar reasoning’s were also considered, being these peaks identified as myricetin-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnoside and kaempferol-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnoside, respectively.
Peak 14 ([M–H]− at m/z 625) indicated that it corresponds to an anthocyanin derivative, such as a peonidin derivative, owing to the product ion observed at m/z 317. The observation of MS2 fragments at m/z 479 (−146 u) and 317 (−162 u), indicated the alternative loss of each of the deoxyhexosyl and hexosyl moieties, respectively, pointing to their location on different positions of the aglycone, thus being identified as petunidin-O-deoxyhexoside-hexoside. This compound was only found in the flower extract of V. x wittrockiana and, to the authors knowledge, has not been previously reported.
For V. cornuta, the three major components, ordered from the highest to the lowest concentration, were identified as quercetin-3-O-rutinoside (peak 11), chrysin-6,8-di-C-glucoside (peak 10) and quercetin-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnoside (peak 5), respectively; whereas for V. x wittrockiana extract, the major components were quercetin-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnoside (peak 5), myricetin-3-O-(6-O-rhamnosylglucoside)-7-O-rhamnoside (peak 4), and quercetin-3-O-rutinoside (peak 11). Other flavonoid glycosides derivatives were present in minor proportions, such as luteolin, apigenin, kaempferol, and isorhamnetin derivatives.
Anthocyanins compounds were not found in V. cornuta. As far as we know, the only research about its chemical composition was published by Farzad, Griesbach, & Weiss, (2002) [21], who studied V. cornuta (var. Yesterday Today and Tomorrow), which changed its colour from white to purple over 5–8 days. This work shows an increasing composition of anthocyanidins during colour change phases, a lack of them when petals were white and a maximum concentration when petals develop a purple colour. This is in concordance with the absence of anthocyanins in the extract, because their presence is related to violet and purple petal colours, being the sample studied of yellow petals. On the other hand, the polyphenol content of V. x wittrockiana flowers was higher than that described by González-Barrio et al. but the anthocyanin proportion was at the same level [5]. Authors reported flavonols as the main compounds, such as quercetin and myricetin, which were also the major phenolic compounds in the present study. Additionally, V. x wittrockiana flowers also showed a higher concentration of flavonoid when compared to the ones previously reported by Vukics et al. in violet V. x wittrockiana flowers [6]. The differences could be related to the type of extraction, as well as the origin and period of flower harvesting.
The high polyphenol content of both extracts suggests that the flowers of both species can be an interesting source of antioxidants and bioactive molecules.
3.2. In vitro cell-free antioxidant activity assays (TPC, DPPH· assay, FRAP)
Three different antioxidant methods were used to evaluate the reducing capacity for both flower extracts (results given in Table 2). Firstly, TPC was determined in comparison to pyrogallol standard, being significantly higher on V. x wittrockiana (p ≤ 0.001) than in V. cornuta, with mean values of 80.18 ± 0.007 and 54.92 ± 0.008 mg PE/g extract, respectively. These values differ from those obtained through the chromatographic analysis of phenolic compounds, being that the quantity in both species were overestimated. The difference could be attribute to the presence of other non-phenolic reducing compounds that can react with the Folin-Ciocalteu reagent, such as proteins, thiols or vitamins [22]. Due to non-specificity, TPC is currently used as a parameter of antioxidant-reducing activity. Our results of V. x wittrockiana are higher than those from the study by González-Barrio et al. who reported a TPC of 44.88 ± 1.43 mg GAE/g dry weight of extract obtained by maceration [5].
Table 2.
Antioxidant and enzyme inhibition activity of flower extracts. Results are represented as mean ± SEM of three independent replicates. Control substances were used in the assays (ascorbic acid for DPPH·, galantamine for AChE and clorgyline for MAO-A).
| TPC mg PE/g extract | DPPH· IC50 μg/mL | FRAP μmol Fe2+/g extract | AChE IC50 mg/mL | MAO A IC50 mg/mL | |
|---|---|---|---|---|---|
| V. cornuta | 54.92 ± 0.008 | 39 ± 2 | 25 ± 2 | ND | ND |
| V. wittrokiana | 80.18 ± 0.007 | 26.1 ± 0.8 | 35 ± 2 | 1.5 ± 0.1 | 0.3 ± 0.1 |
| t- Students test p -value | ≤0.001 | ≤0.01 | ≤0.05 | – | – |
| Control substance | – | 3.17 ± 0.03 | – | 6.3 × 10−4 ± 0.02 | 4.9 × 10−4 ± 0.01 |
ND, no detected activity. p-values were calculated to compared differences between the two extracts.
The free radical scavenging activity was determined by the DPPH· assay. As observed in Table 2, both studied ethanolic extracts inhibited around 85% of the DPPH· radicals at the maximum dose tested (250 μg/mL). The extract of V. x wittrockiana flowers showed a higher DPPH· radical-scavenging activity (IC50, 26.1 ± 0.8 μg/mL) in comparison to V. cornuta extract (IC50, 39 ± 2 μg/mL) and significant differences were found between them (p ≤ 0.01). Similar DPPH inhibition was found by González-Barrio et al. in a V. x wittrockiana flower extract obtained by maceration (71.20%) [5]. Besides, our results seem to be better to the data obtained for V. x wittrockiana carried out by Fernandes et al. [23,24].
The FRAP assay estimates the antioxidant activity related to the capacity of the samples to reduce Fe3+-TPTZ to Fe2+-TPTZ. Lower FRAP values were found for V. cornuta 24 ± 2 μmol Fe+2/g extract, while Viola wittrokiana revealed a slightly higher value 35 ± 2 μmol Fe+2/g extract (p ≤0.05). As far as we know, there is no previous data of reducing power for V. cornuta, however, for yellow flowers of V. x wittrockiana the reported value was 33.1 μmol Fe+2/g fresh weigh basis [4] and 2063.7 μmol Fe+2/g dry weight of sample [5]. Comparison is difficult due to the fact that results are expressed in relation to the mass of the dry plant material instead of the extracts.
The measurement of free radical scavenging activity and iron ion reduction ability shows a potent antioxidant effect in both species. This activity could be related to the presence of polyphenols.
3.3. Enzyme inhibition bioassays
The in vitro neuroprotective potential was measured through the MAO-A and AChE inhibitory activity, which are connected with neurodegenerative disorders like Alzheimer’s and Parkinson’s diseases. Results are summarized in Table 2. Interestingly, V. x wittrockiana extract showed inhibitory activity against both enzymes, while V. cornuta extract did not show any activity. Flower extract of V. x wittrockiana inhibited MAO A with a IC50 value of 0.3 ± 0.1 μg/mL. This inhibition could be due to quercetin and related flavonoids [25]. On the other hand, the IC50 value for AChE was 1.5 ± 0.1 mg/mL. This activity may also be due to the higher proportions of quercetin derivatives, which also have shown potential AChE inhibitory effect [26]. Thus, this is the first time the V. x wittrockiana has shown potential to inhibit CNS enzymes.
3.4. Flower extracts increases survival of C. elegans under lethal oxidative stress
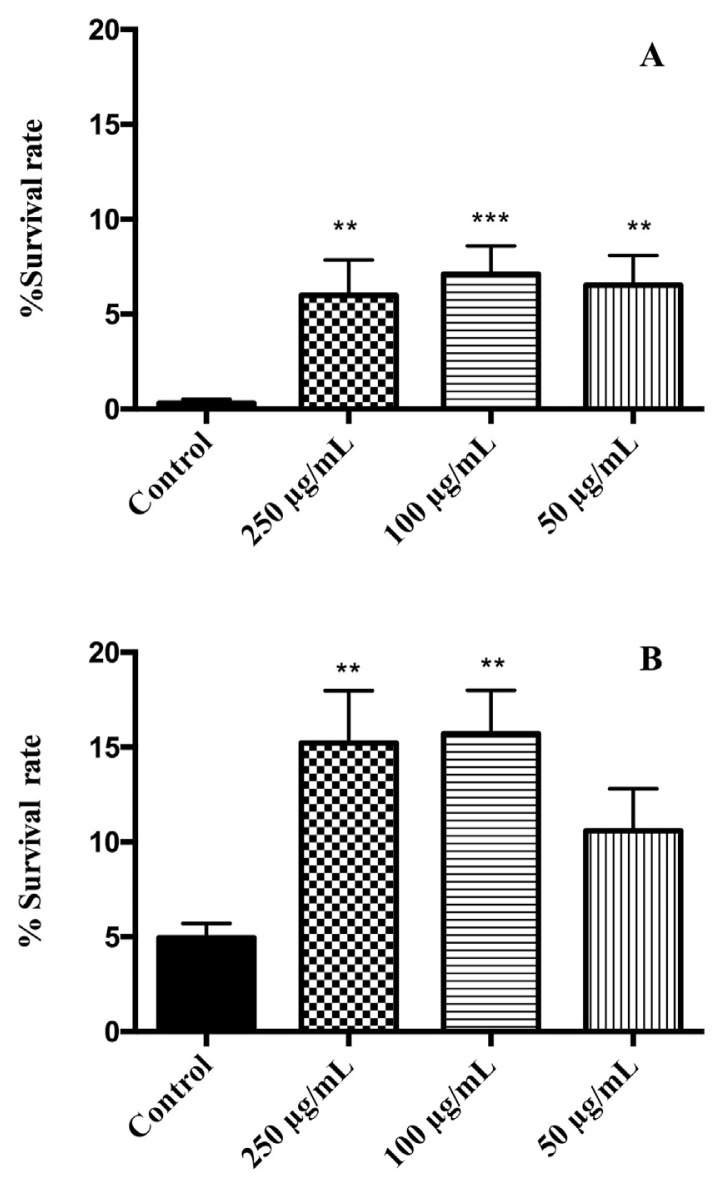
For the oxidative resistance assays, nematodes were pretreated with different doses of flower extracts and then exposed to juglone, a pro-oxidant agent. Both extracts reduced the toxicity of juglone increasing the survival rate with respect to the control group (Fig. 2). The best response to oxidative stress were found in the group treated with 100 μg/mL, showing an increase of 6.8% survival rate for V. cornuta (p ≤ 0.001) and 10.75% for V. x wittrokiana (p ≤ 0.01) with regards to the control group. For V. cornuta, all tested doses showed a significant increase of survival. On the other hand, the treatment with V. x wittrockiana had a noteworthy effect on survival rate at concentrations of 250 and 100 μg/mL (p ≤ 0.05). Our results report a better protective potential for V. x wittrokiana than for V. cornuta, which is in accordance with the phenolic content and the in vitro antioxidant activity previously described.
Fig. 2.
Effects of (A) V. cornuta and (B) V. x wittrockiana extracts on the response to a lethal oxidative stress induced by juglone (150 μM) on C. elegans. Three independent biological replicates were performed. Differences compared to control group were considered significant at p ≤ 0.05 (*), p ≤ 0.01 (**) and p ≤ 0.001 (***).
Other authors have demonstrated that compounds present in the extracts have revealed a protective effect against oxidative stress on C. elegans. Surco-Laos et al. reported an increased survival rate in worms pre-treated with quercetin exposed to juglone-induced oxidative stress [15], also myricetin treatment reduced oxidative damage of biomolecules [27].
3.5. V. cornuta flower extract extends the lifespan of C. elegans
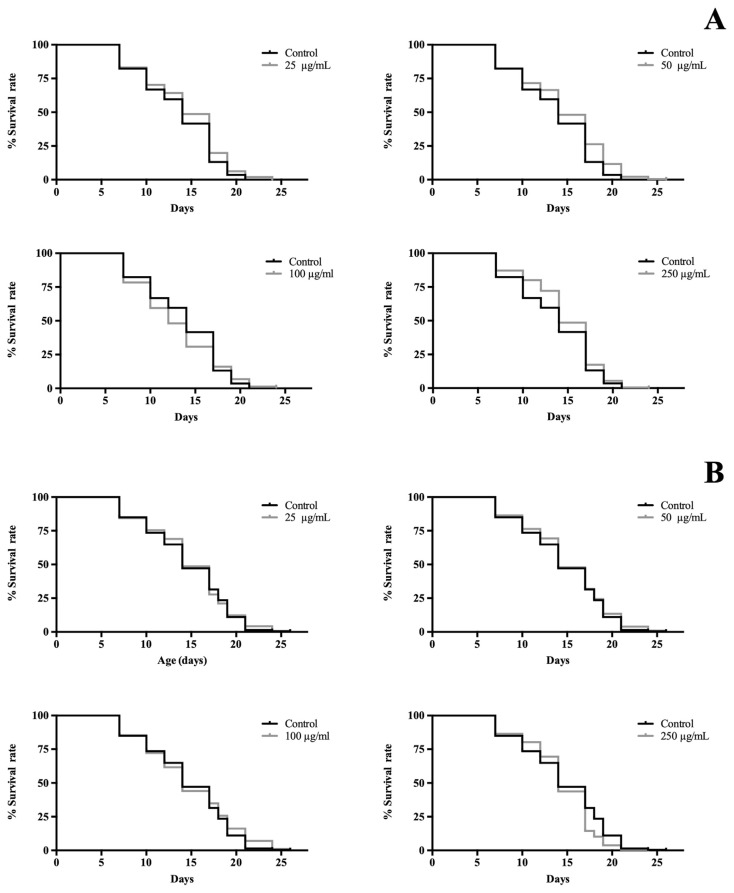
C. elegans is a widespread model to study aging because of its short lifespan and well conserved key mechanism of ageing [7]. As illustrated in Fig. 3, V. x wittrockiana showed no impact on lifespan extension, but at the highest dose tested (250 μg/mL) had a negative effect in the survival curve compared with control (p < 0.01). According to previous works, higher doses of quercetin, which is the major compound found in the tested extract, shorten worm lifespan being in vivo pro-oxidant and toxic effects responsible for this fact [28].
Fig. 3.
Effects of (A) V. cornuta and (B) V. x wittrockiana extracts on lifespan of C. elegans SS104. Three replicates were used per treatment. The mean lifespan was 14 days in all groups, except in nematodes treated with 100 μg/mL was 12 days. Results of lifespan experiments were analysed using Kaplan–Meier survival model and for significance by means of a long rank pairwise comparison test between the control and treatment groups. Differences in survival curves between treatment and control groups were found in:(A) 25 *, 50 ***, and 250** μg/mL; (B): 250 (**) μg/mL. Differences compared to control group were considered significant at p ≤ 0.05 (*), p ≤ 0.01 (**) and p ≤ 0.001 (***).
In the case of V. cornuta, all concentrations had a positive effect on lifespan, significant differences were found in the survival curves of the treatments with the control group (p ≤ 0.05). The present study does not allow us to conclude which mechanisms are involved in lifespan lengthening, but similar experiments were carried out with the majority of phytochemicals present in this extract such as quercetin and chrysin. The life-extending effect in wild type C. elegans exposed to 30 μg/mL of quercetin was observed by Grünz et al., who then repeated the assay using strain TK22, which has a hypersensitivity to oxidative stress, to assess if it was related to antioxidant properties, thus no effect in lifespan was found [27]. However, quercetin reduced mitochondrial ROS levels of nematodes. Furthermore, chrysin also increased lifespan maybe due to AMPK activation [29].
3.6. Neuroprotective effects of V. x wittrockiana extract in C. elegans
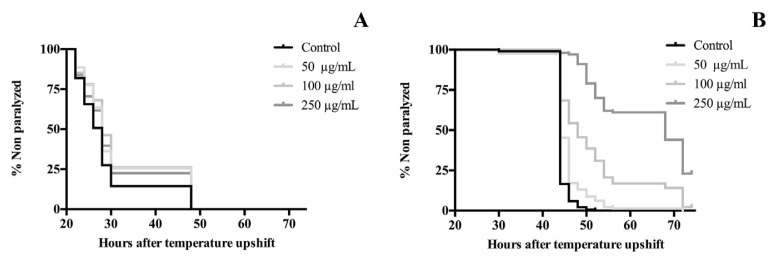
In order to determine the protective effect of the edible flowers against β-amyloid toxicity, C. elegans CL4176 were exposed to different concentrations of extracts, from egg stage until paralysis. The time to develop paralysis was analysed using survival curves. As shown in Fig. 4, paralysis was delayed with both extracts; apparently, treatments with V. x wittrockiana were better than with V. cornuta. The paralysis time for 50% of nematodes (PT50) was not different between treatment groups of V. cornuta and control with a value of 28 h, but all treatment concentrations caused a significant increased lifespan (p < 0.01). On the other hand, V. x wittrockiana extract delayed the onset of paralysis in a dose–response manner. Compared to the control group, V. x wittrockiana extract at 50 μg/mL showed the same value of PT50 which correspond to 44 h, however, this value was delayed up to 9.09% and 54.44% at 100 and 250 μg/mL, respectively, for treated groups (p < 0.001).
Fig. 4.
Effect of (A) V. cornuta and (B) V. x wittrockiana extracts on Aβ-induced paralysis in transgenic C. elegans CL4176. Three replicates were examined per treatment. Statistical significance difference between the curves was analysed by log-rank (Kaplan–Meier) statistical test which compares the survival distributions between the control and treatment groups. Differences in survival curves between treatment and control groups were found in:(A) 50 **, 100*** and 250* μg/mL; (B): 50, 100 and 250 (***)μg/mL. Differences compared to control group were considered significant at p ≤ 0.05 (*), p ≤ 0.01 (**) and p ≤ 0.001 (***).
Experimental and clinical evidence suggest that oxidative stress plays a crucial role in the pathogenesis of Alzheimer disease. This phenomenon also occurs in C. elegans CL4176, where oxidative stress precedes to β-amyloid accumulation [17]. The antioxidant potential observed in vitro as well as the improvement in response to lethal oxidative stress in C. elegans can explain the protective effect of V. cornuta extract in this neurodegenerative model. The results obtained from V. x wittrockiana possibly due to a synergy of inhibitory enzymatic and antioxidant activity, as well as the polyphenol content of the extract. The neuroprotective effects of the main polyphenols have never been evaluated in this model.
It is important to highlight that, in this nematode, there are key enzymes related to neurotransmitter metabolism, such as AChE and MAO [30], being acetylcholinesterase inhibitors one of the cornerstones of Alzheimer disease treatment. Flavonoids extracted from plants, such as quercetin, have been reported to possess an outstanding preclinical efficacy as AChE inhibitors with potential to be candidates for clinical applications such as treating Alzheimer’s disease and other cholinergic dysfunctions [31].
4. Conclusion
To summarize, our study reveals that both edible flowers, V. cornuta and V. x wittrokiana, are a good source of phenolic compounds. These two flowers have demonstrated in vitro and in vivo antioxidant activity but V. x wittrockiana shows higher levels of phenolic compounds and bioactivity. Moreover, the potential of these extracts as agents to prevent certain CNS diseases has been mainly established for V. x wittrokiana.
Acknowledgments
Universidad San Jorge is acknowledged for financial support and providing Cristina Moliner with a PhD scholarship. Innoflower S.L. is thanked for supplying fresh flowers. The authors are also grateful to the Foundation for Science and Technology (FCT, Portugal) and FEDER under Programme PT2020 for financial support to CIMO (UID/AGR/00690/2013) and L. Barros contract; to FEDER-Interreg España-Portugal programme for financial support through the project 0377_Iberphenol_6_E.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfda.2019.05.005.
Funding Statement
Universidad San Jorge is acknowledged for financial support and providing Cristina Moliner with a PhD scholarship.
Footnotes
Conflicts of interest
The authors declare that no conflict of interest exists.
REFERENCES
- 1. Pangalos MN, Schechter LE, Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov. 2007;6:521–32. doi: 10.1038/nrd2094. [DOI] [PubMed] [Google Scholar]
- 2. Feyzabadi Z, Jafari F, Kamali SH, Ashayeri H, Badiee Aval S, Esfahani MM, et al. Efficacy of Viola odorata in treatment of chronic insomnia. Iran Red Crescent Med J. 2014;16 doi: 10.5812/ircmj.17511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guzmán Gutiérrez SL, Reyes Chilpa R, Bonilla Jaime H. Medicinal plants for the treatment of “nervios”, anxiety, and depression in Mexican Traditional Medicine. Rev Bras Farmacogn. 2014;24:591–608. doi: 10.1016/j.bjp.2014.10.007. [DOI] [Google Scholar]
- 4. Benvenuti S, Bortolotti E, Maggini R. Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Sci Hortic (Amsterdam) 2016;199:170–7. doi: 10.1016/j.scienta.2015.12.052. [DOI] [Google Scholar]
- 5. González-Barrio R, Periago MJ, Luna-Recio C, Garcia-Alonso FJ, Navarro-González I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018;252:373–80. doi: 10.1016/j.foodchem.2018.01.102. [DOI] [PubMed] [Google Scholar]
- 6. Vukics V, Kery A, Guttman A. Analysis of polar antioxidants in heartsease (Viola tricolor L.) and garden pansy (Viola x wittrockiana Gams.) J Chromatogr Sci. 2008;46:823–7. doi: 10.1093/chromsci/46.9.823. [DOI] [PubMed] [Google Scholar]
- 7. Calvo DR, Martorell P, Genovés S, Gosálbez L. Development of novel functional ingredients: need for testing systems and solutions with Caenorhabditis elegans. Trends Food Sci Technol. 2016;54:197–203. doi: 10.1016/j.tifs.2016.05.006. [DOI] [Google Scholar]
- 8. Bessada SMF, Barreira JCM, Barros L, Ferreira ICFR, Oliveira MBPP. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb. f.: an underexploited and highly disseminated species. Ind Crops Prod. 2016;89:45–51. doi: 10.1016/j.indcrop.2016.04.065. [DOI] [Google Scholar]
- 9. Gonçalves GA, Soares AA, Correa RCG, Barros L, Haminiuk CWI, Peralta RM, et al. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J Funct Foods. 2017;33:408–18. doi: 10.1016/j.jff.2017.04.009. [DOI] [Google Scholar]
- 10. Les F, Prieto JM, Arbonés-Mainar JM, Valero MS, López V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015;6:2049–57. doi: 10.1039/c5fo00426h. [DOI] [PubMed] [Google Scholar]
- 11. López V, Akerreta S, Casanova E, García-Mina JM, Cavero RY, Calvo MI. In vitro antioxidant and anti-rhizopus activities of lamiaceae herbal extracts. Plant Foods Hum Nutr. 2007;62:151–5. doi: 10.1007/s11130-007-0056-6. [DOI] [PubMed] [Google Scholar]
- 12. Fernández-Moriano C, González-Burgos E, Divakar PK, Crespo A, Gómez-Serranillos MP. Evaluation of the antioxidant capacities and cytotoxic effects of ten parmeliaceae lichen species. Evidence-Based Complement Altern Med. 2016;201:11. doi: 10.1155/2016/3169751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhee IK, Meent M, Van De, Ingkaninan K, Verpoorte R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J Chromatogr A. 2001;915:217–23. doi: 10.1016/s0021-9673(01)00624-0. [DOI] [PubMed] [Google Scholar]
- 14. Stafford GI, Pedersen PD, Jäger AK, Van Staden J. Monoamine oxidase inhibition by southern African traditional medicinal plants. South Afr J Bot. 2007;73:384–90. doi: 10.1016/j.sajb.2007.03.001. [DOI] [Google Scholar]
- 15. Surco-Laos F, Cabello J, Gómez-Orte E, González-Manzano S, González-Paramás AM, Santos-Buelga C, et al. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011;2:445–56. doi: 10.1039/c1fo10049a. [DOI] [PubMed] [Google Scholar]
- 16. Virk B, Correia G, Dixon DP, Feyst I, Jia J, Oberleitner N, et al. Excessive folate synthesis limits lifespan in the C. elegans: E. coli aging model. BMC Biol. 2012;10:67. doi: 10.1186/1741-7007-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1 – 42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24:415–20. doi: 10.1016/S0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 18. Ferreres F, Sousa C, Valentão P, Andrade PB, Seabra RM, Gil-Izquierdo Á. New C-deoxyhexosyl flavones and antioxidant properties of Passiflora edulis leaf extract. J Agric Food Chem. 2007;55:10187–93. doi: 10.1021/jf072119y. [DOI] [PubMed] [Google Scholar]
- 19. Ferreres F, Gil-Izquierdo A, Andrade PB, Valentão P, Tomás-Barberán FA. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2007;1161:214–23. doi: 10.1016/j.chroma.2007.05.103. [DOI] [PubMed] [Google Scholar]
- 20. Han J, Ye M, Xu M, Sun J, Wang B, Guo D. Characterization of flavonoids in the traditional Chinese herbal medicine-Huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2007;848:355–62. doi: 10.1016/j.jchromb.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 21. Farzad M, Griesbach R, Weiss MR. Floral color change in Viola cornuta L. (Violaceae): a model system to study regulation of anthocyanin production. Plant Sci. 2002;162:225–31. doi: 10.1016/S0168-9452(01)00557-X. [DOI] [Google Scholar]
- 22. Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB. A thorough study of reactivity of various compound classes towards the Folin-Ciocalteu. J Agric Food Chem. 2014;58:8139–44. doi: 10.1021/jf1005935.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes L, Casal S, Pereira JA, Pereira EL, Saraiva JA, Ramalhosa E.Physicochemical, antioxidant and microbial properties of crystallized pansies (Viola x wittrockiana) during storage. Food Sci Technol Int. 2019. [DOI] [PubMed]
- 24. Fernandes L, Ramalhosa E, Baptista P, Pereira JA, Saraiva JA, Casal SIP. Nutritional and nutraceutical composition of pansies (Viola × wittrockiana) during flowering. J Food Sci. 2019;84:490–8. doi: 10.1111/1750-3841.14482. [DOI] [PubMed] [Google Scholar]
- 25. Chimenti F, Cottiglia F, Bonsignore L, Casu L, Casu M, Floris C, et al. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: extraction, biological analysis, and computational study. J Nat Prod. 2006;69:945–9. doi: 10.1021/np060015w. [DOI] [PubMed] [Google Scholar]
- 26. Katalinić M, Rusak G, Domaćinović Barović J, Šinko G, Jelić D, Antolović R, et al. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur J Med Chem. 2010;45:186–92. doi: 10.1016/j.ejmech.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 27. Grünz G, Haas K, Soukup S, Klingenspor M, Kulling SE, Daniel H, et al. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech Ageing Dev. 2012;133:1–10. doi: 10.1016/j.mad.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 28. Pallauf K, Duckstein N, Rimbach G. A literature review of flavonoids and lifespan in model organisms. Proc Nutr Soc. 2016;76:145–62. doi: 10.1017/S0029665116000720. [DOI] [PubMed] [Google Scholar]
- 29. Lashmanova E, Zemskaya N, Proshkina E, Kudryavtseva A, Volosnikova M, Marusich E, et al. The evaluation of geroprotective effects of selected flavonoids in Drosophila melanogaster and Caenorhabditis elegans. Front Pharmacol. 2017;8:884. doi: 10.3389/fphar.2017.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rand JB, Duerr JS, Frisby DL. Neurogenetics of vesicular transporters in C. elegans. FASEB J. 2000;14:2414–22. doi: 10.1096/fj.00-0313rev. [DOI] [PubMed] [Google Scholar]
- 31. Khan H, Marya Amin S, Kamal MA, Patel S. Flavonoids as acetylcholinesterase inhibitors: current therapeutic standing and future prospects. Biomed Pharmacother. 2018;101:860–70. doi: 10.1016/j.biopha.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 32. Ferreres F, Llorach R, Gil-Izquierdo A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2004;39:312–21. doi: 10.1002/jms.586. [DOI] [PubMed] [Google Scholar]