Abstract
A dried blood spot (DBS) sampling method was exploited to extract cardiovascular drugs using a small volume of whole blood of human and rodent. Thereafter, an analytical method using liquid chromatography with tandem mass spectrometry (LC-MS/MS) was developed and validated for the determination of 12 cardiovascular drugs. A 6 mm internal diameter disc containing 10 μL of blood was punched from a specifically designed card and analyzed by LC-MS/MS using a gradient elution method with a total run time of 16 min. For sample separation, a universal octadecyl-silica column was used with a flow rate of 0.2 mL/ min. The developed method was validated in terms of linearity, accuracy, and precision, which showed satisfactory results. In addition, the matrix effects were closely investigated to confirm the extraction efficiency. Additionally, the stability was tested by storing DBSs at room temperature; the results showed that these drugs were stable for at least 30 days. Accordingly, the proposed LC-MS/MS method is capable to analyze several cardiovascular drugs in a single analysis. It can be applied to therapeutic drug monitoring in patients as well as in the in vivo settings.
Keywords: Cardiovascular drugs, Dried blood spot, LC-MS/MS, Therapeutic drug monitoring
1. Introduction
Cardiovascular disease is defined as disorders related to the heart and blood vessels, such as angina, heart attack, hypertensive heart disease, and heart failure, and it is one of the leading causes of death worldwide [1–4]. There are several types of drugs to treat cardiovascular disease, which are classified by their mechanisms of action as follows: 1) Angiotensin-converting enzyme inhibitors; 2) Angiotensin II receptor blockers; 3) Beta-blockers; 4) Calcium channel blockers; 5) Diuretics; and 6) Statins (HMG-CoA reductase inhibitors). These drugs function as the blood pressure reducing or lipid-lowering agents [1,5–7]. Treatments using these medications can reduce the risk of cardiovascular diseases, and most patients take more than one medication for treatment [8]. Therefore, an efficient analytical method is needed to detect multiple classes of drugs simultaneously in a small amount of blood in a single analysis for a stable blood concentration monitoring or a medical emergency handling.
Generally, therapeutic drug monitoring (TDM) is performed through venous sampling. Previous studies have quantified several cardiovascular drugs for TDM purposes [9–11]. Dried blood spot (DBS) sampling is becoming popular for TDM due to its several advantages. DBS sampling was first conducted by Guthrie and Susi for newborn screening of phenylalanine to detect phenylketonuria using a simple heel pricking procedure [12]. DBS sampling can be performed by finger prick with a lancet in adults. These methods have several advantages compared to existing venous sampling methods: 1) patients can collect the sample at home without special techniques; 2) monitoring can be performed at any specified time; 3) compared to normal venous sampling, the methods are less painful and require only a small amount of blood, which places less of a burden on the patient; 4) since the samples are dried blood spots, there are no biohazard requirements, so the samples can be shipped by mail in sealed packages; and 5) sample stabilities are better for DBS than venous blood [13,14]. Due to these advantages, there are many reports using DBS sampling for drug analysis. Various immunosuppressants, antiretroviral drugs, antimicrobial drugs, antidiabetics, antiepileptics, and antidepressants have been studied [15–22]. Additionally, DBS studies on cardiovascular drugs have been reported, including assays to analyze the most-prescribed drugs in the UK [2,23]. However, it is still necessary to develop determination method of multiclass cardiovascular drugs using DBS for clinical studies.
In this study, we developed a quantification method for the simultaneous determination of 12 cardiovascular drugs using DBS sampling and high-performance liquid chromatography equipped with a triple quadrupole mass spectrometer (LC-MS/ MS). The method was validated for linearity, accuracy, precision, and matrix effects. To determine the stability of cardiovascular drugs, samples were stored in DBS form at room temperature for 30 days and were compared with samples analyzed immediately after sampling. Through this study, multiclass of cardiovascular drugs were analyzed simultaneously allowing a helpful method for the monitoring of multi-dose cardiovascular patients.
2. Materials and methods
2.1. Chemicals and materials
LC-MS grade acetonitrile, methanol, and water were purchased from J.T. Baker (Milwaukee, WI, USA). Amlodipine, atenolol, atorvastatin, digoxin, enalapril, losartan, propranolol, simvastatin, and sulfameter were purchased from Sigma–Aldrich (St. Louis, MO, USA). Fenofibrate, furosemide, nifedipine, and valsartan were purchased from Tokyo Chemical Industry (Tokyo, Japan). Whatman 903 blood sampling cards were purchased from GE Healthcare (Westborough, MA, USA). Human whole blood with EDTA was purchased from Innovative Research (Novi, MI, USA), which is a supplier of human blood samples. Experimental usage of human blood was approved by the Institutional Review Board of Seoul National University (IRB NO. E1605/003-003).
2.2. Preparation of standard solutions
Twelve standard stock solutions were prepared in methanol at a concentration of 1 mg/mL. These stock solutions were then diluted with 0.9% NaCl to simulate blood conditions. The solution concentrations were determined depending on the sensitivity of each standard. Solutions were diluted to concentrations according to the calibration range. For the internal standard (IS), lovastatin was used to calculate the loss during the extraction process. Sulfameter was included as an IS to control for shifts in retention time, following previous studies by Dias et al. These standards were dissolved in methanol at 1 mg/mL and then further diluted to obtain 10 μg/mL internal standards (ISs) in the extraction solvent.
2.3. Optimization of sample extraction methods for DBS samples
Three types of sample extraction methods (protein precipitation, organic extraction, liquid–liquid extraction) were evaluated to optimize the extraction method. Protein precipitation is a two-step extraction method. First, 50 μL of water: methanol (9:1) was added as an aqueous solution to extract the blood spot into the aqueous phase. Then, 250 μL of methanol, was added to precipitate blood proteins from the DBSs. After the centrifugation, the upper layer was used for the analysis.
Organic extraction is a one-step extraction method that simply adds organic solvent directly to DBS samples. Usually, 100% methanol or acetonitrile is used, but the results can be altered by changing the proportion and volume of the organic solvent. For the comparison of extraction methods, 300 μL of 100% methanol was added for the extraction.
The third method is liquid–liquid extraction (LLE), which is a two-step extraction method. The aqueous buffer consists of 50mM ammonium acetate was added to DBS to dissolve blood samples into the aqueous phase. Next, 600 μL of hexane was added to the aqueous samples as a water-immiscible organic solvent. After shaking, the samples are divided into two phases. The organic layer is transferred and analyzed for the comparison of the extraction method.
For DBS samples, 20 μL of the standard solution was spiked into 980 μL of human blood to obtain blood samples. Then, 10 μL of a blood sample was spotted onto DBS card and dried at room temperature for 3 h before the extraction process. The extraction procedures were compared to identify the best method. All the samples were reconstituted with injection solvent (water:acetonitrile, 8:2) to compare with the consistent condition. After selecting the extraction method, the ratio and volume of extraction solvent were optimized.
2.4. Optimized DBS extraction
A 6mmdisc was punched from the DBS cards and transferred to a 2.0 mL microcentrifuge tube. The extraction solvent (500 μL of acetonitrile:methanol, 1:2) was then added to the tube. The tube was briefly vortexed and sonicated for 15 min and then centrifuged at 16,000 g for five minutes. After centrifugation, the supernatant was transferred to a new tube and evaporated to dryness with nitrogen gas. The dried sample was reconstituted with 100 μL of injection solvent (water:acetonitrile, 8:2).
2.5. LC-MS/MS analysis
The LC-MS/MS system consisted of an Agilent 1260 Infinity LC system connected with an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) with an electrospray ionization source. Cardiovascular drugs were separated using a Phenomenex Gemini C18 (50 × 2.00 mm, 3micron) column (Phenomenex, Torrance, CA, USA). The column oven temperature was set to 30 °C, and the sample injection volume was 5 μL. The sample was kept at 4 °C, and the injection needle was washed with water:-acetonitrile (1:1) solution after each injection. The mobile phases consisted of water containing 0.1% formic acid (mobile phase A) and acetonitrile containing 0.1% formic acid (mobile phase B) at a flow rate of 0.200 mL/min.
The mass spectrometer was operated in positive and negative multiple reaction monitoring (MRM) mode. The source conditions were set as follows: drying gas temperature: 300 °C; dry gas flow: 5 L/min; nebulizer: 45 psi; sheath gas temperature: 350 °C; sheath gas flow: 11 L/min. Optimized MRM transitions are listed in Table 1 with dwell time and cell accelerator voltage as 100 msec and 4 V, respectively.
Table 1.
Optimized MS/MS condition for target compounds.
| Compound | Precursor ion (m/z) | Product ion (m/z) | Fragmentor voltage | Collision energy | Polarity |
|---|---|---|---|---|---|
| Amlodipine | 409.1 | 237.8 | 135 | 3 | + |
| Atenolol | 267.1 | 190.1 | 135 | 13 | + |
| Atorvastatin | 559.6 | 440.2 | 175 | 17 | + |
| Digoxin | 651.3 | 131 | 160 | 24 | + |
| Enalapril | 377.1 | 234.1 | 115 | 24 | + |
| Fenofibrate | 361.0 | 233.0 | 115 | 11 | − |
| Furosemide | 329.0 | 284.9 | 110 | 10 | + |
| Losartan | 423.1 | 207.1 | 105 | 20 | + |
| Nifedipine | 347.1 | 315.1 | 90 | 3 | + |
| Propranolol | 260.4 | 116.1 | 145 | 14 | + |
| Simvastatin | 419.2 | 199.1 | 100 | 9 | + |
| Valsartan | 436.2 | 291.0 | 80 | 14 | + |
MassHunter Workstation Acquisition software B.07.00 (Agilent Technologies, Santa Clara, CA, USA) was used to operate the LC/MS systems, and the data were processed using Qualitative Analysis B.06.00 (Agilent Technologies, Santa Clara, CA, USA).
2.6. Validation
Validation of the developed method was performed following international guidelines [24]. Validation of the developed method was based on selectivity, linearity, lower limit of quantification, accuracy, precision, recovery, matrix effects, and stability.
2.6.1. Selectivity
Selectivity was validated by analyzing blank DBS samples and spiked drug samples. Chromatograms of blank DBSs were compared with those of samples with LLOQ level to check for overlapped peaks. The method was considered selective if there were no overlapped peaks between the blank and target compounds and the ISs.
2.6.2. Linearity and lower limit of quantification (LLOQ)
Standard solutions for linearity were prepared in three replicates on three days. Calibration plots were obtained by plotting the area of the target standards divided by the area of the ISs versus the concentration of the target compounds with 6 points. Linearity was evaluated based on the correlation coefficient using linear regression analysis. The lower limits of quantification (LLOQ) is the lowest concentration from the calibration curve which was validated by evaluating accuracy and precision with six replicates as low concentration.
2.6.3. Accuracy and precision
For accuracy and precision, three levels of concentration were selected. These levels were defined as low, medium, and high concentrations and these concentrations were determined from the calibration range.
These were prepared in six replicates for three days to validate the intraday/interday accuracy and precision. Accuracy was calculated based on the relative error, and precision was calculated based on the relative standard deviation (RSD). The results of three concentrations were regarded as acceptable if they were in the range of ≤15%, in accordance with international recommendations.
2.6.4. Recovery
Recovery was assessed by comparing the peak area ratios of compounds extracted from DBS samples and the peak area from compounds spiked into blank DBS extracts. Recovery was determined at three concentrations (low, medium, and high), using six replicates, as performed for accuracy and precision.
2.6.5. Matrix effects
To estimate the suppression or enhancement effects in DBS extracts, the matrix effects were tested. The target compounds were spiked into six replicates of blank DBS extracts at three concentrations and compared to standards that were spiked into reconstitution solvent.
2.6.6. Stability
DBS samples were stored in sealed plastic bags for 30 days at room temperature to determine the stability of the target compounds in DBS form. The purpose of the stability test was to estimate the storage time for the target compounds in the DBS state. Stability tests were performed on six replicates at three concentrations, and the accuracy and precision were calculated.
2.7. The application of method in in vivo experiment
The developed method was applied to quantify cardiovascular drugs in rat blood to confirm the applicability. Twelve drugs were administered to four different male Sprague Dawley rats, each with three different drugs. Rats were anesthetized under zoletil and cannulated with polyethylene tube into the femoral artery and vein. Cardiovascular drugs were dissolved in 30% DMSO and were administered into the femoral vein at a dose of 1 mg/kg with a volume of 500 μL. After 5 min, blood was collected and applied to the sample collection card to make DBS. Obtained blood spots were analyzed by the developed method and the drug concentration in rat whole blood was calculated. Animal studies were approved by the Animal Care and Use Committee of the College of Pharmacy, Seoul National University (approval number: SNU-170315-6).
3. Results and discussions
3.1. LC and MS conditions
The gradient conditions for LC analysis were optimized to effectively separate 12 drugs. The gradient started at 4% B, gradually increased to 98% B over 10 min, and held for 2 min. The gradient was returned to 4% B for 4 min for column re-equilibration. The fragmentor voltage, collision energy, and cell accelerator voltage were optimized by direct injection into the MS detector; the optimized parameters are listed in Table 1.
3.2. Extraction procedure optimization
To obtain the best extraction efficiencies, the extraction method, extraction solvent ratio, and volume were optimized. First, the extraction method was optimized. There are various possible sample extraction methods for DBS [25–27]. We tested three representative methods for DBS extraction, namely, protein precipitation, organic extraction, and liquid–liquid extraction.
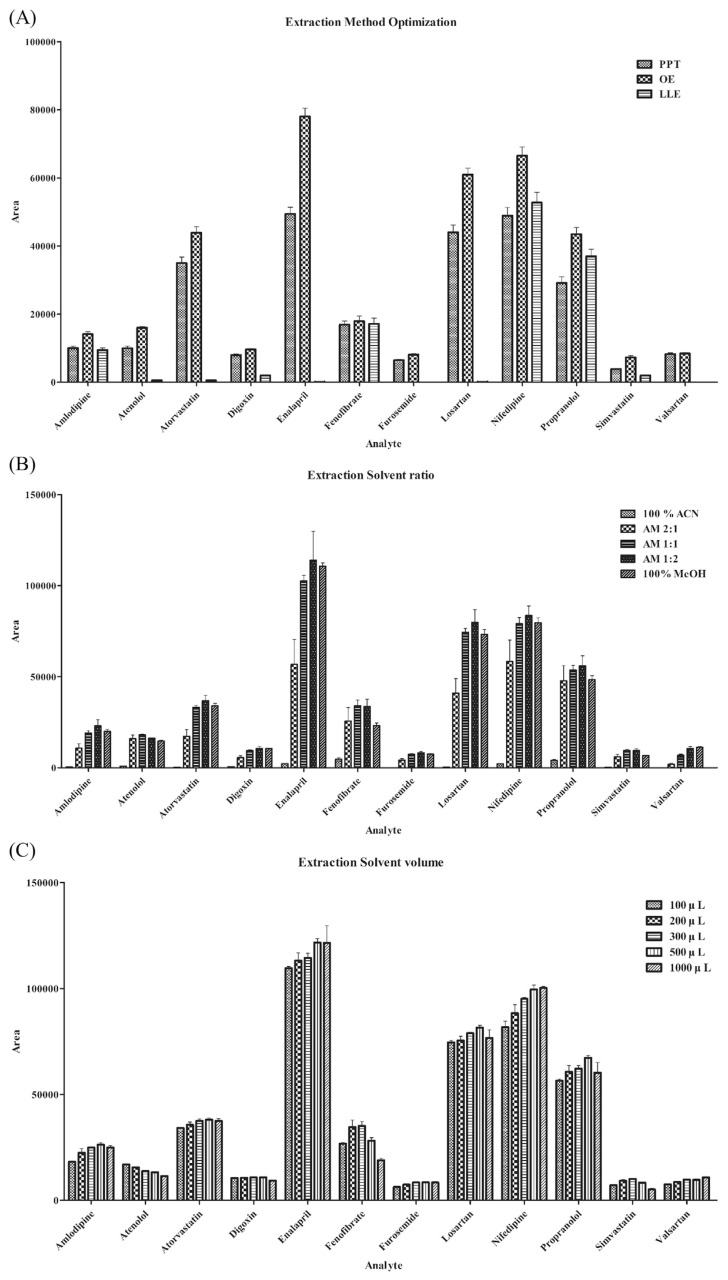
These three extraction methods were used to compare the extraction efficiency of the target compounds (Fig. 1A). Most of the compounds showed good efficiency in the organic extraction method compared to the other methods. Protein precipitation resulted in lower intensities than those of organic extraction. In the case of LLE, only some of the compounds could be detected. LLE uses an aqueous buffer, and the extraction efficiency can be affected by the pKa of the compounds. This makes it difficult to simultaneously extract multiple types of compounds using LLE. Therefore, organic extraction was selected as the extraction method. The next step was to determine the ratio and volume of the organic solvents. By adjusting the ratio of acetonitrile and methanol, the optimal composition of the organic solvent was determined (Fig. 1B). The solvent ratio of acetonitrile: methanol (1:2) produced the largest peak area. The volume was then optimized (Fig. 1C). Solvent volumes of 500 and 1000 μL produced similar peak areas, but the higher solvent volume could extract more blood constituents, which may result in matrix effects. Therefore, 500 μL was selected as the optimal solvent volume. As a result, 500 μL of acetonitrile:methanol (1:2) was selected for the extraction solvent, and validation was performed for the optimized method.
Fig. 1.
Optimization results of (A) extraction method. (PPT: Protein precipitation, OE: Organic extraction, LLE: Liquid–liquid extraction) (B) extraction solvent ratio with a total volume of 300 μL and (C) extraction solvent volume with an organic solvent ratio of acetonitrile: methanol (1:2).
3.3. Method validation
3.3.1. Selectivity
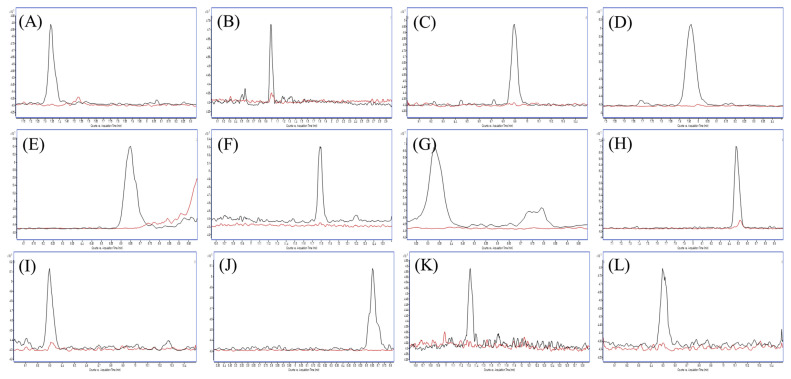
Selectivity was determined by comparing the chromatograms of blank DBS samples with those of the target compounds at LLOQ to check whether there were overlapped peaks (Fig. 2). This method demonstrated good selectivity since there were no interference peaks at the retention times of the twelve compounds in the blank DBS samples.
Fig. 2.
MRM chromatograms of each cardiovascular drug (black line) at LLOQ overlayed with blank (red line)analyzed by optimized DBS samplingmethods: (A) amlodipine (B) atenolol (C) atorvastatin (D) digoxin (E) enalapril (F) fenofibrate (G) furosemide (H) losartan (I) nifedipine (J) propranolol (K) simvastatin and (L) valsartan.
3.3.2. Linearity and lower limit of quantification (LLOQ)
The linearity of twelve cardiovascular drugs was determined by drawing calibration plots using six points. The area of the target compound/area of IS was plotted against the concentration of the target compound, and linear regression was performed. The results are presented in Table 2. All drugs showed good linearity since every compound had an R2 value greater than 0.99. The LLOQ was used as the lowest calibration standard, as presented in Table 2.
Table 2.
Linearity, precision and accuracy for 12 compounds.
| Compound | Calibration curve range (ng/mL) | R2 | Precision (RSD %) | Accuracy (Error %) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Intraday | Interday | Intraday | Interday | |||
| Amlodipine | 15–7500 | 0.999 | 2.5–8.1 | 6.2–9.5 | 4.7–28.1 | 4.1–23.1 |
| Atenolol | 25–12500 | 0.999 | 4.3–5.9 | 7.8–9.1 | 6.0–13.2 | 1.2–17.8 |
| Atorvastatin | 1–500 | 0.991 | 2.2–6.3 | 3.7–13.1 | 8.7–18.3 | 5.1–15.3 |
| Digoxin | 10–5000 | 0.996 | 2.2–5.9 | 5.9–8.8 | 5.6–13.4 | 7.5–8.6 |
| Enalapril | 0.5–250 | 0.993 | 1.3–6.7 | 5.8–8.5 | 1.0–6.9 | 5.2–6.2 |
| Fenofibrate | 15–7500 | 0.992 | 1.1–9.7 | 10.2–13.0 | 8.5–15.7 | 5.7–12.1 |
| Furosemide | 100–50000 | 0.998 | 2.2–5.2 | 4.2–7.9 | 0.7–13.1 | 4.9–12.1 |
| Losartan | 1–500 | 0.996 | 2.0–5.0 | 4.5–9.2 | 7.3–17.1 | 4.6–12.4 |
| Nifedipine | 25–12500 | 0.998 | 2.7–4.7 | 6.8–7.7 | 1.2–9.5 | 6.3–7.6 |
| Propranolol | 1–500 | 0.996 | 5.4–9.0 | 6.7–9.2 | 1.8–4.3 | 2.5–6.1 |
| Simvastatin | 25–12500 | 0.992 | 3.9–6.9 | 4.7–6.9 | 2.5–9.5 | 5.2–7.4 |
| Valsartan | 5–2500 | 0.995 | 3.4–4.9 | 4.7–8.9 | 3.7–5.4 | 5.2–6.0 |
3.3.3. Accuracy and precision
Accuracy and precision were determined using intraday/ interday tests with six replicates at three concentrations. Low, medium, and high concentrations of each analyte were set as specific percentages of the calibration range. The accuracy was defined as the relative error, and precision was expressed as the RSD. Some results at low concentration exceeded limitation values, but most of the compounds were in the acceptable range of 15%, as recommended by international guidelines [24].
3.3.4. Recovery
Recovery was performed to check the extraction efficiency of the developed method. Samples prepared by the whole extraction process were compared with samples spiked directly into the injection solvent. The same low, medium, and high concentrations were used as in the accuracy test, and the overall results ranged from 85 to 106%. These results indicate that the whole extraction process is acceptable in the calibration range.
3.3.5. Matrix effects
To check the matrix effects of the DBS extracts, we compared standards spiked into blank DBS extracts and the injection solvent. The results are shown in Table 3; there were no compounds showing significant (<10%) enhancement or suppression matrix effects. According to these results, the blood matrix and DBS paper did not affect the extraction process and ionization via MS. The matrix had a relatively high suppression effect at the medium level of amlodipine and propranolol. To analyze these compounds, adding mobile phase additives could improve the analysis sensitivity. This factor should be considered when analyzing these compounds alone.
Table 3.
Validation results of recovery and matrix effects.
| Compound | Nominal conc. (ng/mL) | Recovery (%) | Matrix effect% |
|---|---|---|---|
| Amlodipine | 15 | 102.69 | 2.32 |
| 150 | 100.75 | −8.53 | |
| 1500 | 92.08 | 3.11 | |
| Atenolol | 25 | 95.96 | 5.81 |
| 250 | 103.29 | −3.46 | |
| 2500 | 104.72 | 4.54 | |
| Atorvastatin | 1 | 106.50 | 1.68 |
| 10 | 103.46 | 3.39 | |
| 100 | 99.42 | 2.66 | |
| Digoxin | 10 | 98.08 | 0.78 |
| 100 | 97.61 | 5.71 | |
| 1000 | 91.04 | 0.58 | |
| Enalapril | 0.5 | 106.54 | 0.41 |
| 5 | 90.07 | −0.85 | |
| 50 | 96.13 | 3.20 | |
| Fenofibrate | 15 | 103.49 | 1.16 |
| 150 | 103.03 | −4.35 | |
| 1500 | 100.54 | −4.33 | |
| Furosemide | 100 | 85.63 | 6.47 |
| 1000 | 98.92 | 2.31 | |
| 10000 | 92.01 | −2.62 | |
| Losartan | 10 | 104.38 | 2.75 |
| 100 | 97.56 | −1.43 | |
| 1000 | 92.45 | 7.13 | |
| Nifedipine | 25 | 88.91 | 2.83 |
| 250 | 94.19 | 3.90 | |
| 2500 | 87.99 | 1.26 | |
| Propranolol | 1 | 93.68 | 3.16 |
| 10 | 91.56 | −7.05 | |
| 100 | 92.99 | −3.21 | |
| Simvastatin | 25 | 102.17 | 3.18 |
| 250 | 102.36 | −0.84 | |
| 2500 | 90.44 | 1.61 | |
| Valsartan | 50 | 87.20 | 2.04 |
| 500 | 91.54 | −1.99 | |
| 5000 | 85.46 | 4.23 |
3.3.6. Stability
Twelve cardiovascular drugs were spiked into DBS and stored at room temperature for 30 days to check the stability of the drugs. The precision result at low concentration was relatively higher than those at other concentrations, but all the results were acceptable. These results indicate that all tested cardiovascular drugs are stable for at least 30 days in DBSs stored at room temperature, which can be considered when creating blood sampling plans for cardiovascular patients staying at home (Table 4).
Table 4.
Stability of DBS after 30 days of storage.
| Compound | Nominal conc. (ng/mL) | Precision (RSD %) | Accuracy (Error %) |
|---|---|---|---|
| Amlodipine | 15 | 10.26 | 90.57 |
| 150 | 3.35 | 89.84 | |
| 1500 | 1.92 | 88.29 | |
| Atenolol | 25 | 6.61 | 88.42 |
| 250 | 3.11 | 97.69 | |
| 2500 | 1.92 | 88.29 | |
| Atorvastatin | 1 | 12.49 | 92.55 |
| 10 | 5.33 | 89.98 | |
| 100 | 4.88 | 102.16 | |
| Digoxin | 10 | 16.41 | 91.28 |
| 100 | 2.44 | 95.73 | |
| 1000 | 5.27 | 97.63 | |
| Enalapril | 0.5 | 14.43 | 93.24 |
| 5 | 1.02 | 102.53 | |
| 50 | 4.94 | 97.07 | |
| Fenofibrate | 15 | 3.47 | 97.54 |
| 150 | 6.09 | 104.70 | |
| 1500 | 3.65 | 98.65 | |
| Furosemide | 100 | 2.33 | 97.25 |
| 1000 | 2.12 | 106.16 | |
| 10000 | 3.32 | 95.46 | |
| Losartan | 10 | 5.76 | 88.07 |
| 100 | 3.48 | 99.34 | |
| 1000 | 6.88 | 91.38 | |
| Nifedipine | 25 | 5.00 | 97.34 |
| 250 | 5.57 | 98.34 | |
| 2500 | 2.53 | 102.62 | |
| Propranolol | 1 | 6.48 | 101.43 |
| 10 | 2.35 | 101.52 | |
| 100 | 5.97 | 92.96 | |
| Simvastatin | 25 | 5.83 | 94.49 |
| 250 | 10.32 | 92.82 | |
| 2500 | 5.83 | 94.49 | |
| Valsartan | 50 | 10.51 | 92.23 |
| 500 | 4.31 | 99.71 | |
| 5000 | 4.59 | 101.43 |
3.4. The application of the method in in vivo experiment
Since in vivo model is an importantmodel for drug metabolism monitoring, we hypothesized that our developed method for human blood could also be used with the whole blood samples from the rat. To this end, the developed method was applied to analyze whole blood samples collected from rats administered with 12 cardiovascular drugs. Twelve drugs were divided into four groups, each containing three drugs, and the divided groups were administered to rat separately by intravenous injection. The results showed that all of the target drugs in rat blood were detected and eight drugs were able to be quantified by the developed method (Table 5). With these result, it can be confirmed that the developed method can be successfully applied to quantify the concentration of cardiovascular drugs in the blood not only for the purpose of clinical studies but also be suitable in the in vivo experiments.
Table 5.
Level of cardiovascular drugs in rat whole blood (n = 3).
| Compound | Rat blood concentration (ng/mL) |
|---|---|
| Amlodipine | 24.94 ± 6.73 |
| Atenolol | <LOQ |
| Atorvastatin | 41.94 ± 4.04 |
| Digoxin | <LOQ |
| Enalapril | 7.67 ± 0.64 |
| Fenofibrate | 28.40 ± 6.10 |
| Furosemide | <LOQ |
| Losartan | 73.39 ± 7.95 |
| Nifedipine | <LOQ |
| Propranolol | 1.52 ± 0.29 |
| Simvastatin | 89.57 ± 3.26 |
| Valsartan | 13.06 ± 0.87 |
4. Conclusion
In this study, a rapid LC-MS method was developed for the determination of several classes of cardiovascular drug in DBS samples. The method was validated in terms of selectivity, linearity, precision, and accuracy. The DBS sampling method showed minimal matrix effects. The stability of cardiovascular drugs in DBS samples was evaluated after storage for 30 days at room temperature, and these drugs showed good stability in dried blood spots. Applicability of this method was confirmed by administering drugs to rats. The simplicity is the advantages of DBS sampling method and therefore blood from the patient can be collected without the restriction of site and time. It will help to perform therapeutic drug monitoring of cardiovascular drugs in a more convenient way and this will improve the process of cardiovascular treatment.
Acknowledgments
This work was supported by the Bio-Synergy Research Project of the Ministry of Science, ICT and Future Planning through the National Research Foundation (NRF-2012M3A9C4048796) and the National Research Foundation of Korea grant funded by the Korean government (MSIP) (NRF-2018R1A5A2024425).
Funding Statement
This work was supported by the Bio-Synergy Research Project of the Ministry of Science, ICT and Future Planning through the National Research Foundation (NRF-2012M3A9C4048796) and the National Research Foundation of Korea grant funded by the Korean government (MSIP) (NRF-2018R1A5A2024425).
REFERENCES
- 1. Gonzalez O, Iriarte G, Rico E, Ferreirós N, Maguregui MI, Alonso RM, et al. LC–MS/MS method for the determination of several drugs used in combined cardiovascular therapy in human plasma. J Chromatogr B. 2010;878:2685–92. doi: 10.1016/j.jchromb.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 2. Lawson G, Cocks E, Tanna S. Bisoprolol, ramipril and simvastatin determination in dried blood spot samples using LC–HRMS for assessing medication adherence. J Pharm Biomed Anal. 2013;81:99–107. doi: 10.1016/j.jpba.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 3. Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Leal J, Luengo-Fernandez R, et al. European cardiovascular disease statistics 2017. 2017 [Google Scholar]
- 4. Yang CS, Wang H, Sheridan ZP. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea. J Food Drug Anal. 2018;26:1–13. doi: 10.1016/j.jfda.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miao X-S, Metcalfe CD. Determination of cholesterollowering statin drugs in aqueous samples using liquid chromatography–electrospray ionization tandem mass spectrometry. J Chromatogr A. 2003;998:133–41. doi: 10.1016/s0021-9673(03)00645-9. [DOI] [PubMed] [Google Scholar]
- 6. Zha W. Transporter-mediated natural product–drug interactions for the treatment of cardiovascular diseases. J Food Drug Anal. 2018;26:S32–44. doi: 10.1016/j.jfda.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ki N-Y, Hur J, Kim BH, Kim KH, Moon BJ, Oh HB, et al. Rapid screening of sulfonamides in dietary supplements based on extracted common ion chromatogram and neutral loss scan by LC-Q/TOF-mass spectrometry. J Food Drug Anal. 2019;27:164–74. doi: 10.1016/j.jfda.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States a policy statement from the American heart association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 9. Patel B, Jangid AG, Suhagia B, Desai N. Challenges in simultaneous determination of hydrochlorothiazide and ramipril in human plasma: application to a bioequivalence study. J Chromatogr Sci. 2018;56:867–78. doi: 10.1093/chromsci/bmy055. [DOI] [PubMed] [Google Scholar]
- 10. Alhazmi H, Alnami A, Arishi M, Alameer R, Al Bratty M, Rehman Z, et al. A fast and validated reversed-phase HPLC method for simultaneous determination of simvastatin, atorvastatin, telmisartan and irbesartan in bulk drugs and tablet formulations. Sci Pharm. 2018;86:1. doi: 10.3390/scipharm86010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Babarahimi V, Talebpour Z, Haghighi F, Adib N, Vahidi H. Validated determination of losartan and valsartan in human plasma by stir bar sorptive extraction based on acrylate monolithic polymer, liquid chromatographic analysis and experimental design methodology. J Pharm Biomed Anal. 2018;153:204–13. doi: 10.1016/j.jpba.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 12. Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43. [PubMed] [Google Scholar]
- 13. Sharma A, Jaiswal S, Shukla M, Lal J. Dried blood spots: concepts, present status, and future perspectives in bioanalysis. Drug Test Anal. 2014;6:399–414. doi: 10.1002/dta.1646. [DOI] [PubMed] [Google Scholar]
- 14. Spooner N, Olatunji A, Webbley K. Investigation of the effect of blood hematocrit and lipid content on the blood volume deposited by a disposable dried blood spot collection device. J Pharm Biomed Anal. 2018;149:419–24. doi: 10.1016/j.jpba.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 15. Ramesh T, Rao PN, Rao RN. LC–MS/MS method for the determination of rosiglitazone on rat dried blood spots and rat urine: application to pharmacokinetics. J Pharm Biomed Anal. 2015;111:36–43. doi: 10.1016/j.jpba.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 16. Shah NM, Hawwa A, Millership J, Collier P, McElnay J. A simple bioanalytical method for the quantification of antiepileptic drugs in dried blood spots. J Chromatogr B. 2013;923:65–73. doi: 10.1016/j.jchromb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 17. Koster RA, Veenhof H, Botma R, Hoekstra AT, Berger SP, Bakker SJ, et al. Dried blood spot validation of five immunosuppressants, without hematocrit correction, on two LC–MS/MS systems. Bioanalysis. 2017;9:553–63. doi: 10.4155/bio-2016-0296. [DOI] [PubMed] [Google Scholar]
- 18. Duthaler U, Berger B, Erb S, Battegay M, Letang E, Gaugler S, et al. Automated high throughput analysis of antiretroviral drugs in dried blood spots. J Mass Spectrom. 2017;52:534–42. doi: 10.1002/jms.3952. [DOI] [PubMed] [Google Scholar]
- 19. Barco S, Castagnola E, Moscatelli A, Rudge J, Tripodi G, Cangemi G. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: method development, validation and comparison with dried blood spot. J Pharm Biomed Anal. 2017;145:704–10. doi: 10.1016/j.jpba.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 20. Schauer AP, Sykes C, Cottrell ML, Prince H, Kashuba AD. Validation of an LC–MS/MS assay to simultaneously monitor the intracellular active metabolites of tenofovir, emtricitabine, and lamivudine in dried blood spots. J Pharm Biomed Anal. 2018;149:40–5. doi: 10.1016/j.jpba.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ippolito MM, Huang L, Siame M, Thuma P, Shapiro TA, Aweeka FT. Semi-quantitative measurement of the antimalarial lumefantrine from untreated dried blood spots using LC–MS/MS. J Pharm Biomed Anal. 2018;155:241–6. doi: 10.1016/j.jpba.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber J, Oberfeld S, Bonse A, Telger K, Lingg R, Hempel G. Validation of a dried blood spot method for therapeutic drug monitoring of citalopram, mirtazapine and risperidone and its active metabolite 9-hydroxyrisperidone using HPLC–MS. J Pharm Biomed Anal. 2017;140:347–54. doi: 10.1016/j.jpba.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 23. Bernieh D, Lawson G, Tanna S. Quantitative LC–HRMS determination of selected cardiovascular drugs, in dried blood spots, as an indicator of adherence to medication. J Pharm Biomed Anal. 2017;142:232–43. doi: 10.1016/j.jpba.2017.04.045. [DOI] [PubMed] [Google Scholar]
- 24.US food and drug administration. Bioanalytical Method Validation. US food and drug administration. 2001. [Accessed 20 February 2019]. Available at: http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf.
- 25. Xie F, De Thaye E, Vermeulen A, Van Bocxlaer J, Colin P. A dried blood spot assay for paclitaxel and its metabolites. J Pharm Biomed Anal. 2018;148:307–15. doi: 10.1016/j.jpba.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 26. Rower JE, Anderson DJ, Sherwin CM, Reilly CA, Ballard PL, McEvoy CT, et al. Development and validation of an assay for quantifying budesonide in dried blood spots collected from extremely low gestational age neonates. J Pharm Biomed Anal. 2019;167:7–14. doi: 10.1016/j.jpba.2019.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Resende LA, da Silva PHR, Fernandes C. Quantitative determination of the antimalarials artemether and lumefantrine in biological samples: a review. J Pharm Biomed Anal. 2019;165:304–14. doi: 10.1016/j.jpba.2018.12.021. [DOI] [PubMed] [Google Scholar]