Abstract
Implant therapy aims at providing the patient with a functional and esthetically pleasing rehabilitation in a long‐term perspective. The loss of an implant constitutes a major complication, which may have an impact on the treatment plan and/or jeopardize the longevity of the restoration. Implant loss may occur during the phase of osseointegration (early) or at a later time when the previously achieved osseointegration is lost (late). The present work evaluates the evidence on the occurrence of both events and discusses etiology, risk factors, and consequences.
Keywords: bone loss, complication, dental implant, etiology, loss, risk factors
1. INTRODUCTION
The use of dental implants as part of the rehabilitation of partially and fully edentulous patients constitutes a safe, accepted, and commonly applied treatment. 1 , 2 It was estimated that > 12 million implants are placed annually across the globe. 3 In Sweden, a national registry on dental status and treatment (SKaPa) was initiated in 2008. This registry includes > 4 million adults attending public dental healthcare settings and indicated that the proportion of patients with ≥ 1 dental implants increased from 1.7% to 2.8% from 2009/2010 to 2015/2016. 4
The success of dental restorative therapy supported by implants has traditionally been evaluated by the assessment of implant survival, indirectly reflecting implant loss. In fact, implant survival remains the dominant outcome measure in studies and has commonly been considered sufficient in the evaluation of implant therapy and has been reported at the implant level alone. Outcomes at the implant level, however, may be difficult to communicate in the clinical setting, where, in the eyes of the patient, the risk of experiencing any implant loss is probably of greater concern compared with the risk of losing one of multiple implants. Considering only the implant as the unit of analysis and disregarding clustering of data within patients may lead to an underestimation of complication rates 5 , 6 and may be of significance for meaningful data analysis. 7 , 8 Therefore, to improve understanding in the field, the 8th European Workshop on Periodontology encouraged future research on implant therapy to not only consider outcomes at implant as well as patient levels, but to also include additional outcome measures, such as the occurrence of biologic/technical complications and patient‐reported outcome measures. 9 It was argued that the mere presence or absence of a dental implant per se may not have any effect on oral health‐related quality of life.
Although implant loss as an outcome measure by itself is clearly not sufficient to properly evaluate implant dentistry as a therapy, it is quite obvious that survival of implants is an important parameter relevant to both the patient and the clinician. In terms of research, the assessment of implant loss has a distinct advantage over other outcome measures in that its outcome (loss or no loss) is definitive. The criteria used to define implant success or case definitions of peri‐implantitis, on the other hand, vary considerably between studies. 7 , 10 Hence, interpretation of such data is more demanding and complex, while implant loss as an outcome measure is easily assessed and communicated.
2. DEFINITIONS OF EARLY AND LATE IMPLANT LOSS
Implant loss may occur at different time points during therapy or follow‐up. The initial process of soft and hard tissue integration following implant installation typically requires several weeks. 11 , 12 , 13 , 14 From the biologic point of view, early implant loss constitutes a failure in achieving integration, while late implant loss may be described as a failure in maintaining osseointegration.
Traditionally, implants lost or removed prior to loading have been categorized as early losses. 15 , 16 This assessment was applied within established treatment protocols, which included healing periods of 3‐6 months before restorations were to be seated. 17 , 18 , 19 Hence, the majority of studies evaluating the occurrence of early implant loss have considered the period between implant installation and abutment connection/prosthetic loading to be of interest (Table 1). Only a few studies have included periods extending beyond the time point of delivery of the prosthetic restoration in their observations, mostly based on practical considerations. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 In recent years, immediate or early loading of implants following installation has been proposed and scientifically evaluated. 28 , 29 , 30 , 31 Studies on such treatment protocols require a fixed time frame for evaluation of early implant loss rather than clinical landmarks. Typically, the first 6 months after implant installation and simultaneous loading have been considered. 32 , 33
TABLE 1.
Studies describing early implant loss
| Study | Study design and definition of early implant loss | Sampling and sample size | Occurrence of early implant loss | Clinical features of early implant loss | Risk indicators for early implant loss | Consequences of early implant loss |
|---|---|---|---|---|---|---|
| Alsaadi et al 68 |
Retrospective Insertion – abutment connection |
Convenience 2004 subjects 6946 implants |
Patient level 8.9% Implant level 3.6% |
Fibrotic encapsulation |
Osteoporosis Crohn’s disease Smoking Region Implant diameter |
Not reported |
| Antoun et al 25 |
Retrospective Insertion – 1 y |
Convenience 1017 subjects 3082 implants |
Patient level 4.0% Implant level 1.6% |
Not reported |
Smoking Clinical experience Augmentation Immediate implant installation |
Not reported |
| Baqain et al 74 |
Prospective Insertion – abutment connection |
Convenience 169 subjects 399 implants |
Patient level 8.3% Implant level 3.8% |
Not reported |
Width of keratinized mucosa Suture material |
Not reported |
| Borba et al 92 |
Retrospective Insertion – prosthetic restoration |
Convenience 202 subjects 774 implants |
Patient level 7.4% Implant level 2.8% |
Not reported | Not reported | Not reported |
| Bornstein et al 67 |
Retrospective Insertion – abutment connection |
Convenience 1206 subjects 1817 implants |
Patient level 0.8% Implant level 0.7% |
Infection: 3 implants Implant mobility but no signs of infection: 10 implants |
Smoking (tendency) | Not reported |
| Brocard et al 100 |
Retrospective Insertion – prosthetic restoration |
Convenience 440 subjects 1022 implants |
Patient level Not reported Implant level 1.3% |
Not reported | Not reported | Not reported |
| Brügger et al 71 |
Retrospective Insertion – prosthetic restoration |
Convenience 1568 subjects 2279 implants |
Patient level 0.7% Implant level 0.6% |
Not reported | Not reported | Not reported |
| De Bruyn & Collaert 77 |
Retrospective Insertion – prosthetic restoration |
Convenience 117 subjects 452 implants |
Patient level 6.8% Implant level 2.4% |
Implant mobility | Smoking | Not reported |
| Buser et al 65 |
Prospective Insertion – prosthetic restoration |
Convenience 1003 subjects 2359 implants |
Patient level Not reported Implant level 0.6% |
Recurrent infection: 5 implants Implant mobility: 8 implants |
Not reported | Not reported |
| Cannizzaro et al 32 |
Prospective Insertion – 6 mo |
Convenience 80 subjects 160 implants |
Patient level 2.5% Implant level 1.3% |
Implant mobility | Not reported | Lost implants replaced, prostheses was remade |
| Carlsson et al 45 |
Prospective Insertion ‐ abutment connection |
Convenience 44 subjects 331 implants |
Patient level Not reported Implant level 1.3% |
Not reported | Not reported | Not reported |
| Cavalcanti et al 79 |
Retrospective Insertion – prosthetic restoration |
Convenience 1727 subjects 6720 implants |
Patient level Not reported Implant level 3.0% |
Not reported | Center effect | Not reported |
| Cecchinato et al 16 |
Randomized controlled trial Insertion – prosthetic restoration |
Convenience 84 subjects 324 implants |
Patient level Not reported Implant level 2.2% |
Reduced stability at installation for 5 out of 7 implants | Not reported | 4 out of 7 implants: new implant placement |
| Chrcanovic et al 42 |
Retrospective Insertion ‐ abutment connection |
Convenience 2670 subjects 10 096 implants |
Patient level 5.2% Implant level 1.7% |
Not reported |
Smoking Antidepressants |
Not reported |
| Cochran et al 20 |
Prospective Insertion – 1 y |
Convenience 135 subjects 439 implants |
Patient level Not reported Implant level 0.9% |
Not reported | Not reported | Not reported |
| Cochran et al 73 |
Prospective Insertion – prosthetic restoration |
Convenience 200 subjects 626 implants |
Patient level 0.5% Implant level 0.2% |
Not reported | Not reported | Not reported |
| Daubert et al 21 |
Retrospective Insertion – 1 y |
Convenience 96 subjects 225 implants |
Patient level Not reported Implant level 5.8% |
Not reported | Not reported | Not reported |
| Davarpanah et al 93 |
Prospective Insertion – prosthetic restoration |
Convenience 528 subjects 1583 implants |
Patient level Not reported Implant level 3.0% |
Not reported | Not reported | Not reported |
| DeLuca et al 75 |
Retrospective Insertion – prosthetic restoration |
Convenience 464 subjects 1852 implants |
Patient level 10.8% (based on subsample) Implant level 4.2% |
Not reported |
Smoking Implant length Jaw |
Not reported |
| Derks et al 5 |
Retrospective Insertion – prosthetic restoration |
Random selection 2765 subjects 11 311 implants |
Patient level 4.4% Implant level 1.4% |
Not reported |
Periodontitis Smoking Implant length Implant brand |
76 out of 121 patients: new implant placement 21 out of 121 patients: treatment plan adjusted 2 out of 121 patients: therapy discontinued 22 out of 121 patients: therapy completed without new implant placement or adjustments to the treatment plan |
| Esposito et al 58 |
Randomized controlled trial Insertion ‐ abutment connection |
Convenience/10 private dental clinics 506 subjects 972 implants |
Patient level 3.4% Implant level 2.1% |
Infection: 7 implants Pain: 1 implant Asymptomatic: 13 implants |
Immediate implant installation | Not reported |
| French et al 72 |
Retrospective Insertion – prosthetic restoration |
Convenience 2060 subjects 4591 implants |
Patient level 1.1% Implant level 0.5% |
Not reported | Not reported | Not reported |
| Friberg & Jemt 22 |
Retrospective Insertion – 1 y |
Convenience 76 subjects 380 implants |
Patient level 0% Implant level 0% |
No early implant loss | No early implant loss | No early implant loss |
| Friberg & Jemt 23 |
Retrospective Insertion – 1 y |
Convenience 259 subjects 1230 implants |
Patient level Not reported Implant level 1.3% |
Not reported | Not reported | Not reported |
| Han et al 64 |
Retrospective Insertion – not specified |
Convenience 879 subjects 2796 implants |
Patient level Not reported Implant level 3.1% |
Inflammation: 40 implants Failure of osseointegration: 23 implants Premature loading: 11 implants Host response: 7 implants Multiple: 2 implants Unknown: 3 implants |
Not reported | Not reported |
| He et al 84 |
Retrospective Insertion – prosthetic restoration |
Convenience 1377 subjects 2684 implants |
Patient level Not reported Implant level 0.7% |
Lack of osseointegration: 18 implants | Not reported | Not reported |
| Huynh‐Ba et al 101 |
Retrospective Insertion – prosthetic restoration |
Convenience 136 subjects 273 implants |
Patient level Not reported Implant level 2.9% |
Not reported | Not reported | Not reported |
| Lin et al 43 |
Retrospective Insertion – prosthetic restoration |
Random selection/2 hospitals 18 199 subjects 30 959 implants |
Patient level 1.0% Implant level 0.6% |
Not reported |
Age Gender Location Implant length |
Not reported |
| Jemt et al 24 |
Retrospective Insertion – 1 y |
Convenience 8528 subjects 39 077 implants |
Patient level 7.0% Implant level 2.0% |
Not reported |
Jaw Implant surface |
Not reported |
|
Jemt 27 Subsample from Jemt et al 24 |
Retrospective Insertion – 1 y |
Convenience 2848 subjects 9582 implants |
Patient level 2.7% Implant level 1.2% |
Not reported |
Implant site Number of implants Clinician |
Not reported |
| Jungner et al 90 |
Retrospective Insertion – prosthetic restoration |
Convenience 103 subjects 287 implants |
Patient level 1.9% Implant level 0.7% |
Not reported | Not reported | Not reported |
| Karaky et al 57 |
Randomized controlled trial Insertion – abutment connection |
Convenience 240 subjects 766 implants |
Patient level 15.0% Implant level 5.5% |
Not reported | Not reported | Not reported |
| Koldsland et al 15 |
Retrospective Insertion – prosthetic restoration |
Convenience 109 subjects 372 implants |
Patient level 9.2% Implant level 3.0% |
Not reported | Not reported | Not reported |
| Lambrecht et al 102 |
Retrospective Insertion – prosthetic restoration |
Convenience 191 subjects 468 implants |
Patient level Not reported Implant level 0.4% |
Loss of nerve sensibility: 1 implant Infection: 1 implant |
Not reported | Not reported |
| Levin et al 70 |
Retrospective Insertion – prosthetic restoration |
Convenience 717 subjects 2259 implants |
Patient level Not reported Implant level 1.9% |
Not reported | Not reported | Not reported |
| Malò et al 33 |
Prospective Insertion – 6 mo |
Convenience 103 subjects 380 implants |
Patient level 0.9% Implant level 0.3% |
Prosthesis fracture: 1 implant | Not reported | 1 patient: new implant placement |
| Noda et al 52 |
Retrospective Insertion – prosthetic restoration |
Convenience 296 subjects 721 implants |
Patient level 2.4% Implant level 1.5% |
Not reported | Smoking | Not reported |
| Noguerol et al 69 |
Retrospective Insertion – abutment connection |
Convenience 316 subjects 1084 implants |
Patient level Not reported Implant level 5.1% |
Not reported |
Age Smoking Bone type Primary implant stability |
Not reported |
| Olate et al 80 |
Retrospective Insertion – abutment connection |
Convenience 650 subjects 1649 implants |
Patient level Not reported Implant level 3.8% |
Not reported |
Implant length Region |
Not reported |
| Olmedo‐Gaya et al 66 |
Retrospective Insertion – prosthetic restoration |
Convenience 142 subjects 276 implants |
Patient level Not reported Implant level 5.8% |
Not reported |
Gender Periodontal disease Implant length Bone quality Surgical technique |
Not reported |
| Rasmusson et al 47 |
Prospective Insertion – abutment connection |
Convenience 36 subjects 199 implants |
Patient level 13.9% Implant level 3.0% |
Not reported | Not reported | Not reported |
| Roccuzzo et al 49 |
Prospective Insertion – prosthetic restoration |
Convenience 101 subjects 246 implants |
Patient level 0% Implant level 0% |
No early implant loss | No early implant loss | No early implant loss |
| Roccuzzo et al 48 |
Prospective Insertion – prosthetic restoration |
Convenience 123 subjects 252 implants |
Patient level 0% Implant level 0% |
No early implant loss | No early implant loss | No early implant loss |
| Roos‐Jansåker et al 51 |
Retrospective Insertion – prosthetic restoration |
Convenience 218 subjects 1057 implants |
Patient level 6.9% Implant level 2.7% |
Not reported | Not reported | Not reported |
| Rosenberg et al 59 |
Retrospective Insertion – prosthetic restoration |
Convenience 334 subjects 1511 implants |
Patient level Not reported Implant level 5.8% |
Not reported |
Implant surface Region |
Not reported |
| van Steenberghe et al 78 |
Prospective Insertion – abutment connection |
Convenience 399 subjects 1263 implants |
Patient level 5.3% Implant level 2.2% |
Not reported |
Bone quality Smoking Radiotherapy Chemotherapy Claustrophobia |
Not reported |
| Urban et al 61 |
Prospective Insertion – abutment connection |
Convenience 92 subjects 92 implants |
Patient level 16.3% Implant level 16.3% |
Not reported |
Smoking Dimensions of defect postextraction |
Not reported |
| Vervaeke et al 26 |
Retrospective Insertion – 6 mo |
Convenience 376 subjects 1320 implants |
Patient level 2.9% Implant level 0.8% |
Not reported | Not reported | Not reported |
| Wagenberg & Froum 86 |
Retrospective Insertion – prosthetic restoration |
Convenience 891 subjects 1925 implants |
Patient level Not reported Implant level 3.7% |
Not reported | Not reported | Not reported |
| Wennström et al 82 |
Randomized controlled trial Insertion – prosthetic restoration |
Convenience 51 subjects 149 implants |
Patient level 2.0% Implant level 0.7% |
Lack of osseointegration: 1 implant | None | 1 patient: no change in treatment plan |
By contrast, loss of implants occurring after the connection of abutments and/or restorations has, in the majority of reports, been termed late implant loss. 5 , 34 Studies using specified time points rather than clinical landmarks considered the time period of 6 or 12 months after prosthetic loading to be of interest for the assessment of late implant loss (Table 2).
TABLE 2.
Studies describing late implant loss
| Study | Study design and function time | Sampling and sample size | Occurrence of late implant loss | Etiology of late implant loss | Risk indicators for late implant loss | Consequences of late implant loss |
|---|---|---|---|---|---|---|
| Alsaadi et al 34 |
Retrospective 2 y |
Convenience 412 subjects 1514 implants |
Patient level 16.0% Implant level 6.7% |
Not reported |
Radiotherapy Implant location |
Not reported |
| Borba et al 92 |
Retrospective mean: 8.4 y |
Convenience 202 subjects 774 implants |
Patient level 1.5% Implant level 0.38% |
Not reported | Not reported | Not reported |
| Buser et al 65 |
Prospective 5 y |
Convenience 269 subjects 536 implants |
Patient level Not reported Implant level 1.4% |
Ongoing infection: 2 implants Implant mobility: 3 implants Implant fracture: 1 implant Progressive bone loss: 1 implant |
Not reported | Not reported |
| Cannizzaro et al 32 |
Prospective 5 y |
Convenience 80 subjects 160 implants |
Patient level 0% Implant level 0% |
No late implant loss | No late implant loss | No late implant loss |
| Carlsson et al 45 |
Prospective 15 y: mandibular implants 10.5 y: maxillary implants |
Convenience 44 subjects 331 implants |
Patient level 2.3% Implant level 0.4% |
Not reported | Not reported | Not reported |
| Chrcanovic et al 94 |
Retrospective mean: 7.9 y |
Convenience 999 subjects 3559 implants |
Patient level Not reported Implant level 3.7% |
Not reported | Not reported | Not reported |
|
Chrcanovic et al 87 Subsample from Chrcanovic et al 94 |
Retrospective 20‐36 y |
Convenience 227 subjects 1045 implants |
Patient level Not reported Implant level 10.7% |
Implant fracture: 15 implants Loss of integration: 97 implants |
Not reported | Not reported |
| Cochran et al 73 |
Prospective 5 y |
Convenience 174 subjects 542 implants |
Patient level Not reported Implant level 0.6% |
Not reported | Not reported | Not reported |
| Daubert et al 21 |
Retrospective mean: 10.9 y |
Convenience 96 subjects 225 implants |
Patient level Not reported Implant level 2.7% |
Implant fracture: 1 implant Peri‐implantitis: 5 implants |
Not reported | Not reported |
| Davarpanah et al 93 |
Prospective 1‐5 y |
Convenience 511 subjects 1535 implants |
Patient level Not reported Implant level 0.5% |
Not reported | Not reported | Not reported |
| DeLuca et al 75 |
Retrospective mean: 5.0 y |
Convenience 464 subjects 1852 implants |
Patient level 7.5% (based on subsample) Implant level 3.5% |
Not reported |
Smoking Implant length Jaw |
Not reported |
| Derks et al 5 |
Retrospective mean: 8.9 y |
Random 596 subjects 2367 implants |
Patient level 4.2% Implant level 2.0% |
Not reported | Implant brand |
New implant placement: 6 patients New supra‐construction: 8 patients Modified supra‐construction: 4 patients Supra‐construction lost and not replaced: 5 patients No impact on supra‐construction: 8 patients |
|
Donati et al 50 Continuation of Wennström et al 82 |
Prospective 20 y |
Convenience 51 subjects 148 implants |
Patient level 19.6% Implant level 12.2% |
Implant fracture: 17 implants Disintegration: 1 implant |
Not reported | Not reported |
| Dvorak et al 83 |
Retrospective 1‐24 y |
Convenience 177 subjects 828 implants |
Patient level 13.6% Implant level 8.3% |
Implants lost due to peri‐implantitis | History of periodontitis (protective) | Not reported |
| Ekelund et al 46 |
Prospective 20 y |
Convenience 30 subjects 179 implants |
Patient level 3.3% Implant level 0.6% |
Not reported | Not reported | Not reported |
| French et al 72 |
Retrospective 1‐11 y |
Convenience 2060 subjects 4591 implants |
Patient level 0.4% Implant level 0.2% |
Not reported | Not reported | Not reported |
| Friberg & Jemt 23 |
Retrospective 5 y |
Convenience 259 subjects 1230 implants |
Patient level Not reported Implant level 2.0% |
Not reported | Not reported | Not reported |
| Han et al 64 |
Retrospective 1‐19 y |
Convenience 879 subjects 2796 implants |
Patient level Not reported Implant level 1.0% |
Overloading: 34 implants Implant fracture: 11 implants Peri‐implantitis: 8 implants Host response: 4 implants Inflammation: 1 implant Unknown: 6 implants |
Not reported | Not reported |
| He et al 84 |
Retrospective 1‐8 y |
Convenience 1377 subjects 2684 implants |
Patient level Not reported Implant level 1.0% |
Overloading: 15 implants Peri‐implantitis: 5 implants Unknown: 7 implants |
Not reported | Not reported |
| Jemt et al 24 |
Retrospective 1‐28 y |
Convenience 8528 subjects 39 077 implants |
Patient level Not reported Implant level 2.2% |
Not reported |
Jaw Implant surface |
Not reported |
|
Jemt 95 Subsample from Jemt et al 24 |
Retrospective 4‐13 y |
Convenience 2848 subjects 9582 implants |
Patient level 1.7% Implant level 0.9% |
Not reported |
Bone resorption Number of implants Jaw |
Not reported |
| Jemt et al 89 |
Retrospective 4‐16 y |
Convenience 1017 subjects 3082 implants |
Patient level 2.8% Implant level Not reported |
Unknown: 6 implants Remaining implants: Inflammation or Peri‐implantitis |
Smoking Direct implant installation Implant type |
Not reported |
| Jungner et al 90 |
Retrospective 5‐7.8 y |
Convenience 103 subjects 287 implants |
Patient level 3.9% Implant level 2.1% |
Infection and marginal bone loss: 1 out of 6 lost implants | Not reported | Not reported |
| Koldsland et al 15 |
Retrospective 1‐16 y |
Convenience 109 subjects 372 implants |
Patient level 6.4% Implant level 1.9% |
Not reported | Not reported | Not reported |
| Lin et al 43 |
Retrospective 1‐6 y |
Random selection/2 hospitals 18 199 subjects 30 959 implants |
Patient level 1.1% Implant level 0.7% |
Not reported |
Age Gender Implant length Augmentation |
Not reported |
| Levin et al 70 |
Retrospective 1.5‐12 y |
Convenience 717 subjects 2259 implants |
Patient level Not reported Implant level 2.2% |
Not reported |
History of periodontitis (after > 50 mo of follow‐up) |
Not reported |
| Malò et al 33 |
Prospective 5 y |
Convenience 86 subjects 338 implants |
Patient level 1.2% Implant level 0.3% |
Peri‐implantitis: 1 implant | Not reported | New implant placement: 1 patient |
| Noda et al 52 |
Retrospective 0‐17 y |
Convenience 296 subjects 721 implants |
Patient level 2.8% Implant level 1.4% |
Not reported |
Jaw Region Number of teeth Opposing dentition Prosthetic retention |
Not reported |
| Romeo et al 85 |
Retrospective 1‐7 y |
Convenience 201 subjects 677 implants |
Patient level 10.5% Implant level 4.4% |
Overloading: 6 implants Implant fracture: 3 implants Peri‐implantitis: 21 implants |
Not reported | Not reported |
| Rasmusson et al 47 |
Prospective 10 y |
Convenience 36 subjects 199 implants |
Patient level 0% Implant level 0% |
No late implant loss | No late implant loss | No late implant loss |
| Roccuzzo et al 49 |
Prospective 10 y |
Convenience 101 subjects 246 implants |
Patient level 14.9% Implant level 7.3% |
All implants removed because of biologic complications | Supportive periodontal therapy | Not reported |
| Roccuzzo et al 48 |
Prospective 10 y |
Convenience 123 subjects 252 implants |
Patient level Not reported Implant level 2.4% |
All implants removed because of biologic complications | None identified | Not reported |
| Roos‐Jansåker et al 51 |
Retrospective 9‐14 y |
Convenience 218 subjects 1057 implants |
Patient level 4.6% Implant level 1.7% |
Not reported | Not reported | Not reported |
| Rosenberg et al 59 |
Retrospective 1‐13 y |
Convenience 334 subjects 1511 implants |
Patient level Not reported Implant level 2.3% |
Late implant loss was peri‐implantitis‐related | History of periodontitis | Not reported |
| Ueda et al 53 |
Retrospective 10‐24 y |
Convenience 101 subjects 202 implants |
Patient level 9.9% Implant level 6.4% |
Implant mobility: 11 implants Peri‐implantitis: 2 implants |
Not reported |
New implant placement: 6 patients Modified supra‐constructions: 3 patients Supra‐constructions lost and not replaced: 1 patient |
| Vervaeke et al 26 |
Retrospective 2‐5 y |
Convenience 376 subjects 1320 implants |
Patient level 2.1% Implant level 0.8% |
Not reported | Not reported | Not reported |
| Wagenberg & Froum 86 |
Retrospective 1‐16 y |
Convenience 891 subjects 1925 implants |
Patient level Not reported Implant level 0.3% |
Not reported | Not reported | Not reported |
It should be realized that the clinical distinction between early and late implant loss does not necessarily reflect the underlying biologic process, as no routine evaluation of the degree of osseointegration is possible. Hence, misclassification may be possible.
3. STUDIES ON IMPLANT LOSS
3.1. Study samples
Scientific documentation of outcomes following the use of dental implants is predominantly based on assessments made in selected patient groups, so‐called “convenience samples”, 10 in which treatment was carried out by clinicians in specialist and/or university settings. Such studies evaluated efficacy (ie, the probability of an intervention being beneficial to patients treated under optimal conditions) rather than effectiveness (ie, the care provided to the general population under conditions found in practice). Regardless of study design, data from both clinical trials and observational research should aim at high internal and external validity. While internal validity relates to factors such as patient selection, measurement errors, and adequate statistical analysis, external validity describes the possibility of generalizing findings from a given study sample to the general population. Randomized controlled clinical trials, for instance, commonly enroll selected participants who might differ significantly from the overall population. For instance, participants in such trials have been shown to be healthier than the background populations studied. 35 , 36 Internal and external validity may be in conflict. 37 The trade‐off exists in that interventional research is usually superior to observational studies in terms of internal validity, while external validity may suffer. 38 , 39
Virtually all studies assessing early and late implant loss (Tables 1 and 2) included convenience samples, with treatment frequently provided at one clinical center. Only a few examples are available describing randomly selected patient samples. One such study is the nationwide survey from Sweden described by Derks et al, 5 in which the authors identified a cohort of 2765 individuals provided with 11 311 implants in a registry maintained by the Swedish Social Insurance Agency. Patients in this cohort represented different age categories, were treated by different categories of clinicians, and received various types of therapy under everyday conditions. Another study aiming at describing effectiveness of implant therapy used an implant registry in Finland to assess the occurrence of loss among 198,538 dental implants placed between 1994 and 2012, but not distinguishing between early and late loss. 40 In addition to information on implant loss, the registry included basic background information such as gender, jaw of treatment, implant brand, and implant length. In this context, it must be recognized that findings in the study by Antalainen et al 40 were based on events documented in the registry by clinicians on a voluntary basis, introducing a risk for underreporting. A different approach to sampling was presented by Seemann et al, 41 who considered market records. The authors evaluated sales data of almost 70 000 implants in Austria, focusing on return rates, and extrapolating from these data the incidence of implant loss over a 7‐year period.
Large study samples are not restricted to registry studies only. Several studies on conveniently selected populations of extensive size have been published. Two of the largest such reports also originate from Scandinavia. Jemt et al 24 included > 8500 patients treated at one clinical center and reported on early and late implant loss occurring over a 28‐year period. Chrcanovic et al 42 reported on early implant loss in > 2600 subjects treated in another specialist clinic. In this context, it should be noted that external validity is not only related to sample size, but rather reflects the variability among the population, treatment, and clinicians. In a study conducted by Lin et al, 43 the records of > 18 000 patients were analyzed with regard to implant loss. As a large number of subjects was identified in the databases of two major hospitals in Hangzhou, China, it may be speculated that such a patient cohort could, in fact, be representative of the city, region, and possibly even the country.
In summary, the majority of studies evaluating implant loss have the characteristics of efficacy studies. While data originating from such studies may be of high internal validity, the external validity of findings may be limited.
3.2. Study design
The incidence and occurrence of implant loss can be evaluated in longitudinal, observational research of prospective or retrospective design. Such studies, when a sufficient number of subjects are included, may also, through statistical analysis, explore potential risk indicators for the event. The majority of studies on early and particularly on late implant loss are retrospective and designed as case series 44 (Tables 1 and 2). However, some prospective, observational assessments of late implant loss with long‐term follow‐up periods are available. 45 , 46 , 47 , 48 , 49 Study design may have an impact on the level of implant loss observed. Thus, the highest loss rates relative to follow‐up were reported in prospective studies, 49 , 50 while retrospective reports generally indicated lower figures of late implant loss. 5 , 43 , 51 , 52 , 53
Associations between risk indicators and implant loss identified in observational research may be further analyzed in interventional studies with adequate controls. Typical examples of such trials evaluated the potential benefit of the adjunctive use of systemic antibiotics at the time of implant installation in terms of early implant loss. 54 , 55 , 56 , 57 , 58 These clinical trials included a short‐term follow‐up, not exceeding 5 months. An example regarding late implant loss assessed by interventional design is the long‐term follow‐up of a randomized controlled trial performed by Donati et al. 50 The authors compared implants with modified and nonmodified surfaces over a period of 20 years. However, a majority of evaluations of modifying factors for implant loss (eg, history of periodontitis, smoking, and implant brand) have been performed retrospectively, within a given patient cohort. 5 , 15 , 51 , 59 Another approach is the use of historical controls. Thus, Balshe et al 60 compared loss rates occurring during 1991‐1996 with outcomes from 2001‐2005. In the study performed by Jemt et al, 24 a similar approach was applied, considering the respective intervals 1986‐2002 and 2003‐2012. During the earlier time periods in both studies, clinicians used nonmodified implants, while modified surface implants were utilized during the latter periods.
Analysis of risk factors is, however, not only influenced by the study design. Heterogeneous definitions and assessments of exposures may result in biased evaluations. Also, the completeness and accuracy of anamnestic and therapeutic information is, regardless of design, a prerequisite for an adequate estimation. The challenge in terms of data collection may be illustrated by the report published by Lin et al. 43 As referred to above, the authors accessed patient files of > 18 000 patients provided with implants at two clinical centers in China. While the hospital files were accessible and information on implant therapy and implant loss could be retrieved, no data on systemic disorders, smoking, or periodontal history were available. Ideally, to maintain statistical power and generalizability, a careful balance between sample size and data quality needs to be considered.
4. EARLY IMPLANT LOSS
Relevant studies reporting on early implant loss are illustrated in Table 1. Study sizes varied from small case series with 36 subjects 47 to studies including > 8500 24 and > 18 000 individuals. 43 A retrospective study design was applied in 33 out of 50 identified studies, mostly designed as case series. Of the remaining reports, 13 were prospective and observational, while the other four included a controlled design. These randomized trials compared either the use of a one‐ vs two‐stage installation protocol or the adjunctive use of systemic antibiotics vs placebo. One trial evaluated the impact of implant surface characteristics.
In general, treatment procedures and protocols varied considerably among the studies focusing on early implant loss. This may be exemplified by the different selection of patients, ranging from fully edentulous individuals only 47 to single‐tooth replacement cases. 61 An additional example is the wide range of different surgical and prosthetic protocols considered.
In the studies on early implant loss, observation periods were typically limited to the first couple of months following implant installation, 16 , 42 sometimes extending up to 12 months. 20 , 21 , 22 , 23 , 24 , 27
4.1. Etiology
In the epidemiological context, the term etiology implies a causal association between an exposure and an outcome. In other words, for any event to occur, an etiological factor needs to be present. A risk factor, on the other hand, modifies the probability of the event but is not an absolute prerequisite. Criteria for scientific evidence supporting causation have been suggested and critically discussed. 62 , 63 Regardless of any specific criteria, it is obvious that research evaluating potential etiological factors needs to be longitudinal. This enables the distinction between exposed and nonexposed individuals or sites to be assessed or assigned prior to the event of interest. Any causal association needs to be confirmed in prospective and/or intervention studies. As the number of prospective studies on early implant loss is limited and restricted to a few potential factors, a clear etiological pathway has yet to be established. Any observed association between a condition or exposure and the event may, however, serve to identify potential etiological factors. In this context, one common feature described in some studies was the presence of clinical signs of inflammation at the affected implant site. Several authors reported up to 50% of early lost implants to demonstrate such features at the time of the event. Thus, in the study conducted by Han et al, 64 40 of the 86 early lost implants were associated with signs of inflammation. The corresponding proportions reported in the studies conducted by Buser et al, 65 Esposito et al, 58 and Olmedo‐Gaya et al 66 were five out of 13, seven out of 21, and six out of 16 lost implants, respectively. The lost implants not associated with inflammation were described as asymptomatic. In fact, the data reported by Bornstein et al 67 indicated that 10 out of 13 early lost implants exhibited mobility but showed no signs of inflammation. Pain in conjunction with early implant loss appears to be a rare event. 58 The establishment of a fibrotic encapsulation has been proposed as an explanation for a mobile implant free of any symptoms. 68 This soft tissue encapsulation, in turn, may be related to compromised primary stability of the installed implants. In the study conducted by Cecchinato et al, 16 seven implants were lost prior to prosthetic restoration. Of these, five presented with reduced primary stability at installation. Data reported by Noguerol et al 69 are in agreement. The authors found a significant association between reduced primary implant stability at installation, as assessed by resonance frequency analysis, and early implant loss. The effect of immediate or early prosthetic loading in this context is unclear.
In summary, no solid data revealing any clear and specific etiology of early implant loss are currently available. Early implant loss appears to present in two distinct clinical forms, one being completely asymptomatic, while the other is characterized by inflammation.
4.2. Occurrence
Early implant loss as an outcome is routinely reported at the implant level (Table 1) and findings varied significantly between studies. Early implant loss at the implant level ranged from 0% 22 , 48 , 49 to values just below 6%. 21 , 57 , 59 , 66 The studies reporting no early losses were all limited in size and based on convenience samples. Thus, Roccuzzo et al included 101 49 and 123 patients, 48 respectively, while Friberg and Jemt 22 reported on 76 individuals. Studies with large patient cohorts, on the other hand, reported consistent levels of early implant loss. Thus, Jemt et al, 24 in a study involving 8528 subjects, found that 2.0% of all implants were lost within 1 year following installation. In a subsample of patients treated from 2003 to 2011, the corresponding proportion was 1.2%. 27 Levin et al, 70 Chrcanovic et al, 42 and Derks et al 5 reported results of 1.9%, 1.7%, and 1.4%, respectively. These three studies considered a time period from installation to prosthetic treatment only. In this context, the study conducted by Lin et al 43 is an outlier. The authors analyzed early implant loss in > 18 000 patients, also considering the time period from installation to prosthetic treatment. A relatively low incidence of 0.6% was reported.
The occurrence of early implant loss at the patient level (ie, the proportion of patients experiencing the event) was not consistently reported in all of the identified studies, but was generally higher compared with implant level data. Values ranged from 1% 33 , 43 , 67 , 71 , 72 , 73 to just below 10%. 15 , 68 , 74 Only four studies, describing particular scenarios, reported values exceeding 10%. Thus, DeLuca et al 75 evaluated a population with both a high number of implants per individual (mean: 4.0) and a high proportion of former or current smokers (49%). Karaky et al 57 used a flapless technique for the installation of the majority of implants, while Rasmusson et al 47 included only edentulous patients, resulting in a high number of implants per individual (mean: 5.5). Finally, Urban et al, 61 with the highest overall incidence of 16.3%, included only single implants that were installed immediately following extraction at molar sites.
Studies including large patient cohorts also reported consistent levels of early implant loss at the patient level. Jemt et al 24 noted an incidence of 7.0%, while Chrcanovic et al 42 and Derks et al 5 reported results of 5.2% and 4.4%, respectively. The large‐scale study conducted by Lin et al 43 was, again, an outlier, reporting a considerably lower figure of only 1.0%.
In summary, early loss is estimated to occur for 1.5% of implants (one in 67) and in approximately 4% of patients (one in 25). As suggested at the 8th European Workshop on Periodontology, patient level data are the clinically more relevant form of data presentation, which is also in line with today's patient‐centered approach to care. Early implant loss will potentially jeopardize the possibility of continuing with the restorative part of the treatment plan, thus negatively affecting the patient. Details regarding the consequences of early implant loss follow below.
4.3. Risk indicators for early implant loss
Prospective cohort studies, the study design for risk analysis with the lowest risk of bias, were rare. In this context, it should be noted that, because of ethical considerations, most risk factors cannot be studied in a prospective fashion. Nor can all types of exposure be randomly assigned. Typical examples in the field of implant dentistry are "smoking", "history of periodontitis", and "maintenance care". As patient care cannot be neglected and certain conditions may demand intervention, only retrospective and observational evaluations are feasible. Grimes and Schulz 76 addressed the risk of bias that such research entails. In a commentary on the limitations of observational epidemiology, the authors presented several historical examples of supposed associations that were later refuted by interventional research. Selection, information, and/or confounding bias were discussed as explanations for erroneously reported associations. 37 As a consequence, it was suggested that weak correlations demonstrated in observational research with odds ratios not exceeding 3 should be disregarded, irrespective of statistical significance. 76
4.3.1. Smoking
The majority of studies including analyses of risk indicators identified smoking as being associated with early implant loss. 25 , 52 , 61 , 67 , 68 , 69 , 75 , 77 , 78 The association was also supported by two of the largest investigations including > 2500 subjects. 5 , 42 A number of studies, however, failed to identify smoking as a predictor for early implant loss. 22 , 48 , 49 , 58 , 66 , 74 , 79 In three of these studies, 22 , 48 , 49 no early implant loss occurred. In the study conducted by Cavalcanti et al, 79 smoking was associated with overall implant loss and, although not statistically significant, Baqain et al 74 and Olmedo‐Gaya et al 66 described a 2‐ to 3‐fold proportion of early implant loss among smokers compared with nonsmokers. It may be speculated that the studies not identifying smoking as a relevant factor suffered from a lack of statistical power. Five studies 24 , 27 , 43 , 59 , 80 did not consider smoking as a factor in their risk analyses for early implant loss.
4.3.2. Systemic diseases/status
Convenient sampling tends to exclude individuals with systemic diseases. This is further highlighted by the fact that the majority of studies did not consider systemic disorders in their risk analysis of early implant loss. 24 , 27 , 43 , 48 , 49 , 52 , 58 , 59 , 61 , 69 , 79 , 80 Among studies including systemic diseases and/or status, several conditions have been associated with the outcome in single investigations yet to be confirmed by other evaluations. Thus, osteoporosis and Crohn's disease were highlighted in one study, 68 while another identified a history of radiotherapy and chemotherapy as well as claustrophobia to be of significance. 78 Furthermore, male patients were at higher risk compared with female patients, 43 , 66 while higher age was found to be protective in one study 69 but was identified as a risk indicator in another. 43 In the analysis performed by Chrcanovic et al, 42 intake of antidepressants was the only factor significantly associated with early implant loss. A number of additional studies considered patients' general health status but found no correlations. 5 , 22 , 25 , 67 , 74 , 75 , 77
4.3.3. History of periodontitis
Ten studies considered a history of periodontitis as a potential factor in their risk analyses. Only two studies 5 , 66 reported a higher risk for early implant loss in periodontally susceptible individuals, while the other eight found no such association. 25 , 42 , 48 , 49 , 59 , 61 , 69 , 78 In this context, it should be noted that the categorization in terms of history of periodontitis differed considerably among studies. Thus, Urban et al 61 considered the reason for extraction of the tooth to be replaced as the categorization criteria, while Roccuzzo et al 48 , 49 categorized the patients based on full‐mouth periodontal probing. Furthermore, the distinction in the study performed by Derks et al 5 was based solely on diagnoses reported in patient files. It may be speculated that a “history of periodontitis” is reflective of the presence of compromised sites at the time of implant installation. The bone loss caused by periodontal disease may therefore be the “true” predictor for early implant loss, rather than the underlying disease susceptibility.
4.3.4. Clinical experience/training
The level of training of clinicians has been discussed as an important factor for failure rates in implant dentistry. Albrektsson et al 81 stated that experienced and well‐trained dental professionals may achieve low rates of complications, including implant loss. The majority of screened studies, however, did not consider the level of experience/training of the surgeon in their risk analyses of early implant loss. In a number of reports, only one surgeon was involved, 48 , 49 , 59 , 61 , 66 , 78 making any analysis impossible. In three studies, an association between the clinician and early loss was observed. Thus, Antoun et al 25 noted less early implant loss with increasing clinical experience over time, while Cavalcanti et al 79 found a center effect, illustrated by a significantly higher number of early losses at one out of four clinics included in the study. And, finally, Jemt 27 described a significantly lower risk for early loss for patients treated by one specific surgeon out of a total of six clinicians. Three additional studies 5 , 58 , 75 considered clinical experience but failed to identify any association with the outcome. As it may be assumed that more complicated clinical situations are commonly handled by more experienced clinicians, it is possible that confounding may complicate this particular analysis. Adjusting for the inherent complexity of each individual case throughout any statistical analysis is probably unrealistic.
4.3.5. Treatment‐related factors
Almost all studies considered treatment‐related factors in their risk analyses on early implant loss. A number of studies identified implants placed in the maxilla, 24 , 75 or in the posterior region of the maxilla in particular, 59 , 68 , 80 to be at higher risk. Interestingly, the study with the largest cohort reported the highest risk for early loss for implants placed in the anterior region of the mandible. 43
Two studies 25 , 58 found immediate implant installation following tooth extraction to be associated with early loss, and one study 66 reported a higher risk for cases in which expansion techniques were used during implant osteotomy. Only one study found that the extent of therapy, as expressed by the number of inserted implants, was significantly associated with early loss. 27
A substantial number of studies failed to identify treatment‐related factors, such as area and extent of treatment, as well as the timing and technique of implant installation, as significant predictors for early implant loss. 5 , 16 , 22 , 42 , 48 , 49 , 52 , 61 , 67 , 69 , 74 , 77 , 78 It should be considered that the wide variation in therapeutic approaches evaluated in the different cohorts may have resulted in restricted numbers of any specific treatment, leading to a lack of statistical power.
4.3.6. Implant characteristics
All but seven studies considered implant characteristics in their risk analyses on early implant loss. Among these, several implant features have been associated with the outcome, but results across studies were inconsistent. Thus, implants with a narrow diameter were found to be at a higher risk by Alsaadi et al, 68 which was not confirmed by a number of other reports. 5 , 43 , 52 , 67 , 69 , 77 , 78 Implant length was found to be associated with early loss in five studies, 5 , 43 , 66 , 75 , 80 all reporting a higher risk for shorter implants. Again, these findings were not in line with data from several other reports, none of which found such a correlation. 52 , 61 , 67 , 69 , 77 , 78 It may be speculated that reduced implant dimensions (ie, narrow diameter and/or short implants) are commonly chosen at compromised sites, which may confound the analyses.
Three studies observed differences in rates of early implant loss according to implant brand and/or implant surface characteristics. 5 , 24 , 59 When evaluating the specific impact of implant surface characteristics, it should be recognized that implants by different brands may differ in ways other than implant surface characteristics (eg, in implant or thread geometry). Thus, while Rosenberg et al 59 and Derks et al 5 found higher rates of early implant loss for specific brands, Jemt et al 24 linked the modification of the implant surface to reduced loss rates. Data from several other studies are not in agreement as they failed to observe any differences for early loss explained by either surface characteristics, implant system, or implant brand. 25 , 42 , 43 , 68 , 77 , 78 Interestingly, a trial evaluating the impact of surface modifications in a randomized fashion also failed to identify its importance on the outcome. 82 The overall evidence on the potential impact of implant (surface) characteristics on the occurrence of early implant loss is limited.
4.3.7. Other risk indicators
A number of other potential risk indicators have been identified in single studies. Thus, Baqain et al 74 found that reduced dimensions of keratinized mucosa and the use of polyglactin sutures were associated with early implant loss. AlsoAUTHO, Noguerol et al 69 linked reduced primary implant stability, as assessed by resonance frequency analysis, to the event. And finally, Urban et al 61 found that the defect dimensions of extraction sites were indicative of early loss of immediately installed implants.
In summary, there is substantial evidence that smoking constitutes a risk indicator for early implant loss. While some data suggest that implants installed in the maxilla and implants of reduced length present a higher risk, evidence on additional putative risk indicators (eg, gender, age, systemic disorders, a history of periodontitis, clinical experience, and implant surface modifications) is inconclusive.
4.4. Consequences of early implant loss
It is obvious that the consequences of a complication, rather than the diagnosis or event itself, may be the primary concern of the patient. It is therefore noteworthy that the consequences of early implant loss were reported in only five of the identified studies. Thus, Cecchinato et al 16 described the early loss of seven implants. At four of these sites, implant installation was repeated and then the original treatment plan was followed. Malò et al 33 reported one implant loss at 6 months following installation and immediate loading. After an additional 6 months of healing, a new implant was installed and a new supra‐construction was fabricated. Similar consequences were described by Cannizzaro et al 32 as the two early implant losses resulted in new implant installation and the fabrication of new prostheses. One of the five studies included a large patient cohort, 5 in which a total of 121 patients were affected. Early implant loss resulted in new implant placement in 76, in an adjusted treatment plan in 21, in discontinued therapy in two, and in the completion of therapy without new implant placement or adjustments in 22 individuals. The results are graphically illustrated in Figure 1. In the study conducted by Wennström et al, 82 the treatment plan of the one patient experiencing early implant loss was not affected.
FIGURE 1.
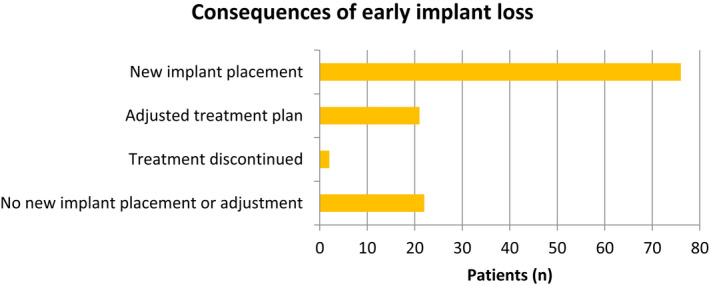
Consequences of early implant loss as reported by Derks et al, 5 with 121 patients affected by early implant loss
In summary, data indicate that early implant loss has significant consequences for the affected patient, commonly resulting in renewed implant installation and/or adjustment of the treatment plan.
5. LATE IMPLANT LOSS
Relevant studies reporting on late implant loss are illustrated in Table 2. Here also, study sizes varied from 36 subjects 47 to > 8500 24 and > 18 000 individuals. 43 Of the 37 identified studies, 26 were retrospective and 11 had a prospective follow‐up. The large majority of reports were observational, designed as case series or cohort studies. Only the study conducted by Donati et al 50 included an interventional approach (ie, a randomized assignment of subjects or sites into test and control groups). Among studies on late implant loss, treatment procedures, protocols, and extent of therapy varied considerably.
In order to make valid assessments of late implant loss and associated risk parameters, studies need to extend over clinically meaningful follow‐up periods. This is illustrated by the data presented by Levin et al. 70 While no effect of susceptibility to periodontitis on the rate of implant loss was observed for the first 50 months of follow‐up, data indicated an 8‐fold greater hazard for patients with severe periodontitis to lose an implant in the longer perspective (> 50 months). In prospective studies, where a protocol for follow‐up is established a priori, all patients reaching the end point of the study present with the same time of follow‐up. In retrospective studies, the follow‐up may be extensive but commonly varies considerably within the study sample. 24 , 43 , 53 , 83 In addition, some authors reported the mean follow‐up time, while others focused on the maximum time of observation (in the title and abstract). This is exemplified by one study including > 2000 patients, 72 in which the upper limit of the observation period reached 11 years. However, the dataset indicated that only two of the observed patients had, in fact, a follow‐up of ≥ 10 years. More than half of all patients were followed for ≤ 2 years, resulting in a mean observation period of < 3 years for the entire cohort. This type of retrospective analysis, including patients with minimal follow‐up time, is common. 15 , 24 , 43 , 52 , 59 , 64 , 70 , 84 , 85 , 86 An exception, in this regard, is the study conducted by Chrcanovic et al. 87 The authors identified 227 patients with a long‐term follow‐up ranging from a lower minimum of 20 years to a maximum of 36 years. As implant loss occurs and accumulates over time, it is expected that studies with longer follow‐up periods will also report higher rates of late loss, while the inclusion of recently treated patients will most likely result in underestimation.
5.1. Etiology
As discussed earlier, any causal association between an exposure (etiological factor) and an outcome (eg, late implant loss) needs to be confirmed in prospective and/or intervention studies. Similar to early implant loss, the number of prospective studies is limited. Observational, retrospective studies identified some potential etiological factors, which are listed below.
5.1.1. Peri‐implantitis
In the available literature, peri‐implantitis 88 has also been defined by descriptive terms such as "progressive bone loss", "biological complication", and "ongoing infection", and was the most commonly reported etiological factor for late implant loss. Thus, in five studies, all late loss occurred as a consequence of the disease, 33 , 48 , 49 , 59 , 83 and in three additional studies the majority of implants were lost as a result of peri‐implantitis. 21 , 85 , 89 The proportion of implant loss related to peri‐implantitis was ≤ 50% in only five studies 53 , 64 , 65 , 84 , 90 and 0% in one long‐term, prospective study. 50
5.1.2. Implant fracture
The second most common etiological factor reported was implant fracture. In fact, it was the most common reason for implant loss in the long‐term, prospective study performed by Donati et al, 50 explaining 17 of the 18 observed losses. Other studies, however, reported considerably lower rates of 1/6, 65 15/112, 87 1/6, 21 11/64, 64 and 3/30. 85 A number of studies found that none of the observed late implant losses were caused by fractures. 48 , 49 , 59 , 84 , 89 It is also conceivable that the two factors, "implant fracture" and "peri‐implantitis", may, in some cases, be related. A fracture of the implant may secondarily display the clinical and radiographic features associated with peri‐implantitis.
5.1.3. Overload/trauma resulting in loss of integration
In a preclinical study, Isidor 91 distinguished between the effects of occlusal overload and soft tissue inflammation on osseointegration. While overload/trauma led to the sudden loss of all osseointegration and the development of a gap between the implant surface and the surrounding mineralized tissues, the ligature‐induced soft tissue inflammation resulted in progressive, marginal bone loss. Thus, although both situations are technically characterized by peri‐implant bone loss and may result in late implant loss, the etiological factors are distinctly different (Figure 2A,B).
FIGURE 2.

A, Implant installed 12 y earlier. Clinical features: mobility and shallow peri‐implant probing. Note the peri‐implant radiolucency (gap) and seemingly maintained peri‐implant marginal bone levels. Etiological factor for late implant loss: overload/trauma resulting in loss of integration. B, Implant installed 8 y earlier. Clinical features: deep peri‐implant probing, signs of soft tissue inflammation, and absence of mobility. Note the crater‐like bone defect. Etiological factor for late implant loss: peri‐implantitis
Overload/trauma leading to loss of integration and late implant loss was clearly described in only one study. 50 The authors categorized one out of the 18 observed late losses accordingly. However, excessive occlusal load could also be implicated in implant fractures, resulting in the loss of the remaining 17 implants. Three studies considered a substantial proportion of the observed late losses to be attributable to "overloading" without specifying the associated features. Thus, Han et al, 64 He et al, 84 and Romeo et al 85 categorized 34 out of 64, 15 out of 27, and six out of 30 losses, respectively. None of the authors defined the status as "overloading".
In summary, data suggest that peri‐implantitis constitutes the most common etiological factor for late implant loss. Other causes of late losses are implant fractures and overload/trauma resulting in loss of integration. Some studies referred to "implant mobility" and "loss of integration" when reporting on the etiology of late implant loss. It may be argued that these terms describe symptoms related to the event rather than a cause.
5.2. Occurrence
Late implant loss was routinely reported at the implant level (Table 2) and findings varied significantly among studies. Outcomes at the implant level ranged from below 1% 26 , 32 , 33 , 43 , 45 , 46 , 47 , 72 , 73 , 86 , 89 , 92 , 93 to around 10%. 50 , 83 , 87 The significant variation in outcomes may, in part, be explained by the differences in observation periods. The two studies reporting the highest rates of late implant loss at the implant level 50 , 87 had follow‐up periods of 20‐36 and 20 years. Studies with low rates of late loss, on the other hand, were either small, 45 , 46 , 47 retrospective in design, 52 , 59 , 92 and/or included patients with minimal follow‐up. 43 , 52 , 64 , 72 , 84 , 86 , 93 Similar to the findings presented on early implant loss, studies with large patient cohorts followed over relevant time periods reported consistent results for late loss at the implant level. Thus, after a mean observation time of 8 and 5 years, Chrcanovic et al 94 and DeLuca et al 75 found a late loss rate of 3.7% and 3.5%, respectively. Levin et al 70 and Jemt et al 24 both reported a slightly lower figure of 2.2%. One study including a cohort of randomly selected patients followed for 9 years noted a late loss of 2.0% of all implants. 5 The study conducted by Lin et al 43 was the only study including a large patient cohort that indicated a substantially lower incidence of late implant loss (0.7%). It should be kept in mind, however, that the dataset covered a mean follow‐up time of only 3 years.
Data on late implant loss at the patient level, when reported, were higher compared with the implant level. Rates ranged from 1% 33 , 43 , 72 , 92 to 10% and higher. 34 , 49 , 53 , 83 , 85 In the 20‐year follow‐up described by Donati et al, 50 19.6% of all patients experienced late implant loss. In a description of a large Swedish population treated during 2003‐2011 including > 2800 individuals followed for 4‐13 years, 27 1.7% of patients were affected. This was somewhat lower than observed in two other Swedish cohorts. Thus, Roos‐Jansåker et al 51 and Derks et al, 5 after observation periods of 9‐14 and 9 years, respectively, reported that 4.6% and 4.2% of patients experienced late implant loss. The differences may, in part, be explained by the differences in follow‐up referred to. In particular, the inclusion of patients after periods as short as 0, 52 1, 24 , 43 , 64 , 72 , 84 , 86 , 93 2, 26 , 70 or 4 years 27 may dilute outcomes of late implant loss. In this context, the retrospective data presented by Alsaadi et al 34 are remarkable as a relatively high proportion of patients (16.0%) had already experienced late implant loss after a short follow‐up period of 2 years. The reasons for these findings are not fully understood. The authors, however, reported that patients with compromised systemic health were also routinely offered implant therapy.
In summary, data suggest that late loss occurs at 3% of implant sites (one of 33 implants) over observation periods of 10 years. In the longer perspective (20 years), figures of up to 10% (one in 10 implants) may be expected. Late implant loss affects approximately 4% of patients over 10 years (one in 25).
5.3. Risk indicators for late implant loss
5.3.1. Smoking
The majority of studies did not identify smoking as associated with late implant loss. 5 , 32 , 34 , 52 , 83 Only two studies demonstrated a higher risk for smokers. Thus, DeLuca et al, 75 in a population with a high proportion of former or current smokers (49%), found that a history of smoking was associated with late implant loss. The authors also noted that, in contrast to smoking history, smoking status at the time of implant installation was not a significant predictor of the outcome. Jemt et al, 89 on the other hand, reported that smoking at the time of implant surgery was associated with late loss over an observation period of 4‐16 years. A total of 19% of individuals were smokers. Former smokers were not considered in the analysis.
5.3.2. Systemic diseases/status
As was observed for the risk analyses on early implant loss, most studies on late loss did not include systemic disorders in their evaluation. Neither gender nor age was found to be significantly associated with late implant loss in three studies. 52 , 75 , 95 Lin et al, 43 on the other hand, noted an elevated risk for both older and male patients. Radiotherapy was found to be associated with implant loss in one study. 34 It should be noted, however, that out of 412 patients included, the number of subjects undergoing radiotherapy was two. The remaining studies failed to observe any correlation between patients' general health and late implant loss. 5 , 32 , 83 , 89
5.3.3. History of periodontitis
Seven studies considered a history of periodontitis as a potential predictor of late implant loss, but only two reported a positive association. Thus, Rosenberg et al 59 and Levin et al 70 observed a 5‐8 times higher incidence of late implant loss in periodontally compromised patients compared with healthy subjects. None of the other studies considering either a history of periodontitis or current periodontitis confirmed this finding. 5 , 48 , 49 , 83 , 89 As discussed earlier, the categorization in terms of susceptibility for periodontitis differed among studies.
5.3.4. Clinical experience/training
Only a minority of the identified papers studied the effect of experience or training of dental professionals on late implant loss and these analyses were limited to the surgical part of the therapy, 75 , 89 , 95 while only one report considered the level of clinical expertise with respect to prosthetic treatment and during supportive care. 5 None of the investigations identified any statistically significant association with late implant loss.
5.3.5. Maintenance therapy and infection control
Information on the quality and extent of maintenance therapy was rarely considered in the risk analyses for late implant loss, nor was the level of patient‐performed infection control. Only three studies included such data. Thus, Roccuzzo et al 49 followed 101 subjects prospectively over a 10‐year period. The authors found that the risk of late implant loss was greater in subjects not adhering to supportive periodontal therapy. This finding, however, was not confirmed in a later investigation by the same group. 48 In the observational study performed by Derks et al, 5 late implant loss during a follow‐up of 9 years was found not to be related to frequency of recall and/or maintenance visits. While adherence to a suggested maintenance protocol may be easily assessed, it is probably more difficult to adequately describe the quality of supportive therapy and the level of self‐performed home care. Thus, analyses as described above may suffer from inaccurate categorization of patient compliance.
5.3.6. Treatment‐related factors
As was the case for early implant loss, most studies evaluated the impact of treatment‐related factors on late implant loss. A total of five studies found implant location and/or jaw of treatment to be significant predictors for the outcome. Findings, however, were conflicting. While four of the studies reported a higher incidence for late loss for maxillary implants, 24 , 34 , 52 , 75 one study described a higher risk for implants placed in the mandible. 95 Four studies failed to identify any association in terms of jaw of treatment. 5 , 43 , 83 , 89 Several other significant factors were described only by single studies. Thus, one study 89 found that a two‐stage procedure used for implant installation was associated with late loss, while another study reported that the extent of therapy, as expressed by the number of inserted implants, was significantly related. 95 Furthermore, one study reported a higher incidence of late implant loss at augmented sites, 43 while one investigation identified a higher risk for implants supporting removable supra‐constructions and for implant‐supported reconstructions occluding with removable partial dentures. 52
5.3.7. Implant characteristics
Virtually all the identified studies considered implant characteristics in their risk analyses on late implant loss and different features were found to be of significance. The odds ratio for short (< 10 mm) relative to long (> 13 mm) implants was > 10 in one report, 43 not adjusting for other potential factors such as smoking and history of periodontitis. Two studies found significant differences between different implant systems/brands. Thus, Jemt et al 89 reported higher loss rates for one system within one brand, while Derks et al 5 observed lower loss rates for one particular implant brand. Two studies described differences in the incidence of late loss related to implant‐surface characteristics. 24 , 59 Only in the study by Jemt et al, 24 however, were the implants otherwise identical in terms of design and geometry. The authors observed a lower rate of late implant loss for surface‐modified as opposed to nonmodified (turned) implants. However, a randomized trial comparing surface‐modified and nonmodified implants of otherwise identical design failed to find differences in terms of implant loss over the long term. 50 , 82 Studies were in general agreement that implant diameter was not associated with late implant loss. 5 , 32 , 34 , 43 , 52 , 75 , 95
In summary, to date, no risk indicators for late implant loss have been consistently and clearly identified.
5.4. Consequences of late implant loss
The consequences of late implant loss were reported in only three studies. Thus, in the prospective 5‐year follow‐up by Malò et al, 33 one patient lost one implant. The authors reported that a new implant was installed but they did not elaborate upon whether the supra‐construction also had to be replaced. As the study included only patients provided with full‐jaw restorations supported by four implants, it may be assumed that, in addition to the implant, new prosthetic work was required. Ueda et al 53 reported late implant loss in 10 patients restored with mandibular overdentures on two implants. Out of all affected patients, six received new implants. In three patients, supra‐constructions were modified and, in one, the supra‐construction was lost and not replaced. Of the 25 patients experiencing late loss in the study performed by Derks et al, 5 six underwent new implant placement procedures. For eight patients, new supra‐constructions were produced. For four patients, supra‐constructions were modified, while for five subjects, the whole supra‐construction was lost and not replaced. For eight cases, late implant loss had no impact on the prosthetic rehabilitation (Figure 3).
FIGURE 3.

Consequences of late implant loss as reported by Derks et al, 5 with 25 patients affected by late implant loss. Note that patients could be included in multiple categories
In summary, data suggest that late implant loss commonly has significant consequences for the affected patient, resulting in new implant installation and/or new supra‐constructions.
6. TOTAL IMPLANT LOSS
From the patient's perspective, implant loss is a serious complication, regardless of the categorization into early or late. A number of studies described the total proportion of patients experiencing implant loss. Findings in four different investigations from Scandinavia were largely in agreement, ranging from 7.6% to 10.1% of affected patients. 5 , 15 , 24 , 51 Results from two studies performed in the USA reported by Wagenberg and Froum 86 and Balshe et al 60 were also in line, with 7.6% and 8.6% of patients losing at least one implant after 1‐16 and 2‐7 years, respectively. Somewhat by contrast, a registry study reported lower numbers of implant loss with 3.1% of patients affected, 40 which, as referred to above, may have been a result of incomplete reporting.
At implant level, clinical studies reported an overall loss rate of 3.0%, 5 4.1%, 70 and 4.3%. 24 Interestingly, data on implant loss originating from sales returns were in agreement with results from these clinical investigations. Thus, Seemann et al 41 found that, over a 7‐year period, 2.8% of almost 70 000 sold implants were returned because of loss.
In summary, data suggest that loss occurs for 4% of implants (one in 25) and in 8% of patients (one in 13).
7. CONCLUDING REMARKS
The majority of studies evaluating implant loss have the characteristics of efficacy studies. Evaluations in terms of effectiveness are rare.
No solid data revealing any clear and specific etiology of early implant loss are currently available. Early implant loss appears to present in two distinct clinical forms, one being completely asymptomatic, while the other is characterized by inflammation.
Early loss is estimated to occur for 1.5% of implants (one in 67) and in approximately 4% of patients (one in 25).
There is substantial evidence that smoking constitutes a risk indicator for early implant loss. While some data suggest that implants installed in the maxilla and implants of reduced length are at higher risk, evidence on additional putative risk indicators (eg, gender, age, systemic disorders, history of periodontitis, clinical experience, and implant surface modifications) is inconclusive.
Data indicate that early implant loss has significant consequences for the affected patient, commonly resulting in renewed implant installation and/or adjustment of the treatment plan.
Data suggest that peri‐implantitis constitutes the most common etiological factor for late implant loss. Other causes of late losses are implant fractures and overload/trauma resulting in loss of integration.
Data suggest that late loss occurs at 3% of implant sites (one in 33 implants) over observation periods of 10 years. Late implant loss affects approximately 4% of patients over 10 years (one in 25).
To date, risk indicators for late implant loss have been consistently and clearly identified.
Data suggest that late implant loss commonly has significant consequences for the affected patient, resulting in new implant installation and/or new supra‐constructions.
8. FUTURE PERSPECTIVE
Implant loss will continue to be an outcome variable of high interest in research on implant‐supported restorative therapy, which should also be evaluated at reconstruction and at patient level. Implant loss should, however, be part of a more comprehensive evaluation than has historically been the case. The consequences of the loss of the implant for the reconstruction and for the patient should be part of this analysis, also addressing cost/benefit issues (ie, the number of appointments needed to adjust/redo the affected reconstruction). In addition, studies should evaluate the incidence/prevalence of biologic and technical complications, as well as esthetic appreciation and overall patient satisfaction.
Peri‐implantitis is an area of particular interest as it probably presents the primary cause of late implant loss. Implant loss has also become a relevant outcome in studies on the management of peri‐implantitis (eg, surgical therapy), as these now include observation periods of ≥ 5 years. 96 , 97 , 98 , 99 The ultimate goal of such interventions is the preservation of implants/restorations. Generating data for evidence‐based decision making on whether to treat or remove (implant loss) affected implants will have to be a point of emphasis in future studies.
The limited number of evaluations of consequences and patient‐reported outcome measures related to implant loss is conspicuous. These parameters should also be considered in future research.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Tomasi C, Derks J. Etiology, occurrence, and consequences of implant loss. Periodontol 2000. 2022;88:13–35. doi: 10.1111/prd.12408
Funding information
The study is self‐funded.
REFERENCES
- 1. Jung RE, Zembic A, Pjetursson BE, Zwahlen M, Thoma DS. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow‐up of 5 years. Clin Oral Implants Res. 2012;23:2‐21. [DOI] [PubMed] [Google Scholar]
- 2. Pjetursson BE, Thoma D, Jung R, Zwahlen M, Zembic A. A systematic review of the survival and complication rates of implant‐supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin Oral Implants Res. 2012;23:22‐38. [DOI] [PubMed] [Google Scholar]
- 3. Albrektsson T, Dahlin C, Jemt T, Sennerby L, Turri A, Wennerberg A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin Implant Dent Relat Res. 2014;16:155‐165. [DOI] [PubMed] [Google Scholar]
- 4. Swedish Quality Registry for caries and periodontal disease. SKaPa ‐ Annual Report. 2016.
- 5. Derks J, Håkansson J, Wennström JL, Tomasi C, Larsson M, Berglundh T. Effectiveness of implant therapy analyzed in a Swedish population: early and late implant loss. J Dent Res. 2015;94:44S‐51S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri‐implantitis. J Dent Res. 2016;95:43‐49. [DOI] [PubMed] [Google Scholar]
- 7. Needleman I, Chin S, O'Brien T, Petrie A, Donos N. Systematic review of outcome measurements and reference group(s) to evaluate and compare implant success and failure. J Clin Periodontol. 2012;39:122‐132. [DOI] [PubMed] [Google Scholar]
- 8. Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002;29:197‐212. discussion 232–193. [DOI] [PubMed] [Google Scholar]
- 9. Tonetti M, Palmer R. Clinical research in implant dentistry: study design, reporting and outcome measurements: consensus report of Working Group 2 of the VIII European Workshop on Periodontology. J Clin Periodontol. 2012;39:73‐80. [DOI] [PubMed] [Google Scholar]
- 10. Tomasi C, Derks J. Clinical research of peri‐implant diseases–quality of reporting, case definitions and methods to study incidence, prevalence and risk factors of peri‐implant diseases. J Clin Periodontol. 2012;39:207‐223. [DOI] [PubMed] [Google Scholar]
- 11. Abrahamsson I, Berglundh T, Linder E, Lang NP, Lindhe J. Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clin Oral Implants Res. 2004;15:381‐392. [DOI] [PubMed] [Google Scholar]
- 12. Berglundh T, Abrahamsson I, Albouy JP, Lindhe J. Bone healing at implants with a fluoride‐modified surface: an experimental study in dogs. Clin Oral Implants Res. 2007;18:147‐152. [DOI] [PubMed] [Google Scholar]
- 13. Berglundh T, Abrahamsson I, Welander M, Lang NP, Lindhe J. Morphogenesis of the peri‐implant mucosa: an experimental study in dogs. Clin Oral Implants Res. 2007;18:1‐8. [DOI] [PubMed] [Google Scholar]
- 14. Tomasi C, Tessarolo F, Caola I, et al. Early healing of peri‐implant mucosa in man. J Clin Periodontol. 2016;43:816‐824. [DOI] [PubMed] [Google Scholar]
- 15. Koldsland OC, Scheie AA, Aass AM. Prevalence of implant loss and the influence of associated factors. J Periodontol. 2009;80:1069‐1075. [DOI] [PubMed] [Google Scholar]
- 16. Cecchinato D, Olsson C, Lindhe J. Submerged or non‐submerged healing of endosseous implants to be used in the rehabilitation of partially dentate patients. J Clin Periodontol. 2004;31:299‐308. [DOI] [PubMed] [Google Scholar]
- 17. Adell R, Lekholm U, Grondahl K, et al. Reconstruction of severely resorbed edentulous maxillae using osseointegrated fixtures in immediate autogenous bone grafts. Int J Oral Maxillofac Implants. 1990;5:233‐246. [PubMed] [Google Scholar]
- 18. Brånemark PI, Adell R, Albrektsson T, Lekholm U, Lundkvist S, Rockler B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials. 1983;4:25‐28. [DOI] [PubMed] [Google Scholar]
- 19. Zarb GA, Schmitt A. The longitudinal clinical effectiveness of osseointegrated dental implants: the Toronto study. Part I: surgical results. J Prosthet Dent. 1990;63:451‐457. [DOI] [PubMed] [Google Scholar]
- 20. Cochran DL, Jackson JM, Bernard JP, et al. A 5‐year prospective multicenter study of early loaded titanium implants with a sandblasted and acid‐etched surface. Int J Oral Maxillofac Implants. 2011;26:1324‐1332. [PubMed] [Google Scholar]
- 21. Daubert DM, Weinstein BF, Bordin S, Leroux BG, Flemming TF. Prevalence and predictive factors for peri‐implant disease and implant failure: a cross‐sectional analysis. J Periodontol. 2015;86:337‐347. [DOI] [PubMed] [Google Scholar]
- 22. Friberg B, Jemt T. Rehabilitation of edentulous mandibles by means of five TiUnite implants after one‐stage surgery: a 1‐year retrospective study of 90 patients. Clin Implant Dent Relat Res. 2008;10:47‐54. [DOI] [PubMed] [Google Scholar]
- 23. Friberg B, Jemt T. Rehabilitation of edentulous mandibles by means of osseointegrated implants: a 5‐year follow‐up study on one or two‐stage surgery, number of implants, implant surfaces, and age at surgery. Clin Implant Dent Relat Res. 2015;17:413‐424. [DOI] [PubMed] [Google Scholar]
- 24. Jemt T, Olsson M, Franke SV. Incidence of first implant failure: a retroprospective study of 27 years of implant operations at one specialist clinic. Clin Implant Dent Relat Res. 2015;17:e501‐510. [DOI] [PubMed] [Google Scholar]
- 25. Antoun H, Karouni M, Abitbol J, Zouiten O, Jemt T. A retrospective study on 1592 consecutively performed operations in one private referral clinic. Part I: Early inflammation and early implant failures. Clin Implant Dent Relat Res. 2017;19:404‐412. [DOI] [PubMed] [Google Scholar]
- 26. Vervaeke S, Collaert B, Cosyn J, Deschepper E, De Bruyn H. A multifactorial analysis to identify predictors of implant failure and peri‐implant bone loss. Clin Implant Dent Relat Res. 2015;17:e298‐307. [DOI] [PubMed] [Google Scholar]
- 27. Jemt T. A retro‐prospective effectiveness study on 3448 implant operations at one referral clinic: a multifactorial analysis. Part I: Clinical factors associated to early implant failures. Clin Implant Dent Relat Res. 2017;19:980‐988. [DOI] [PubMed] [Google Scholar]
- 28. Benic GI, Mir‐Mari J, Hammerle CH. Loading protocols for single‐implant crowns: a systematic review and meta‐analysis. Int J Oral Maxillofac Implants. 2014;29:222‐238. [DOI] [PubMed] [Google Scholar]
- 29. Papaspyridakos P, Chen CJ, Chuang SK, Weber HP. Implant loading protocols for edentulous patients with fixed prostheses: a systematic review and meta‐analysis. Int J Oral Maxillofac Implants. 2014;29:256‐270. [DOI] [PubMed] [Google Scholar]
- 30. Schimmel M, Srinivasan M, Herrmann FR, Muller F. Loading protocols for implant‐supported overdentures in the edentulous jaw: a systematic review and meta‐analysis. Int J Oral Maxillofac Implants. 2014;29:271‐286. [DOI] [PubMed] [Google Scholar]
- 31. Schrott A, Riggi‐Heiniger M, Maruo K, Gallucci GO. Implant loading protocols for partially edentulous patients with extended edentulous sites–a systematic review and meta‐analysis. Int J Oral Maxillofac Implants. 2014;29:239‐255. [DOI] [PubMed] [Google Scholar]
- 32. Cannizzaro G, Felice P, Lazzarini M, et al. Immediate loading of two flapless placed mandibular implants supporting cross‐arch fixed prostheses: a 5‐year follow‐up prospective single cohort study. Eur J Oral Implantol. 2016;9:165‐177. [PubMed] [Google Scholar]
- 33. Malò P, Nobre MDA, Lopes A, Ferro A, Gravito I. Immediate loading of implants placed in patients with untreated periodontal disease: a 5‐year prospective cohort study. Eur J Oral Implantol. 2014;7(3):295‐304. [PubMed] [Google Scholar]
- 34. Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of late oral implant loss. Clin Oral Implants Res. 2008;19:670‐676. [DOI] [PubMed] [Google Scholar]
- 35. Halbert JA, Silagy CA, Finucane P, Withers RT, Hamdorf PA. Recruitment of older adults for a randomized, controlled trial of exercise advice in a general practice setting. J Am Geriatr Soc. 1999;47:477‐481. [DOI] [PubMed] [Google Scholar]
- 36. Moinpour CM, Lovato LC, Thompson IM, et al. Profile of men randomized to the prostate cancer prevention trial: baseline health‐related quality of life, urinary and sexual functioning, and health behaviors. J Clin Oncol. 2000;18:1942‐1953. [DOI] [PubMed] [Google Scholar]
- 37. Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248‐252. [DOI] [PubMed] [Google Scholar]
- 38. Chalmers TC, Celano P, Sacks HS, Smith H. Bias in treatment assignment in controlled clinical trials. New Engl J Med. 1983;309:1358‐1361. [DOI] [PubMed] [Google Scholar]
- 39. Feinstein AR. Clinical Epidemiology. W.B. Saunders; 1985. [Google Scholar]
- 40. Antalainen A‐K, Helminen M, Forss H, Sándor GK, Wolff J. Assessment of removed dental implants in Finland from 1994 to 2012. Int J Oral Maxillofac Implants. 2013;28:1612‐1618. [DOI] [PubMed] [Google Scholar]
- 41. Seemann R, Jirku A, Wagner F, Wutzl A. What do sales data tell us about implant survival? PLoS One. 2017;12:e0171128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. Factors influencing early dental implant failures. J Dent Res. 2016;95:995‐1002. [DOI] [PubMed] [Google Scholar]
- 43. Lin G, Ye S, Liu F, He F. A retrospective study of 30,959 implants: risk factors associated with early and late implant loss. J Clin Periodontol. 2018;45:733‐743. [DOI] [PubMed] [Google Scholar]
- 44. Rocchietta I, Nisand D. A review assessing the quality of reporting of risk factor research in implant dentistry using smoking, diabetes and periodontitis and implant loss as an outcome: critical aspects in design and outcome assessment. J Clin Periodontol. 2012;39:114‐121. [DOI] [PubMed] [Google Scholar]
- 45. Carlsson GE, Lindquist LW, Jemt T. Long‐term marginal periimplant bone loss in edentulous patients. Int J Prosthodont. 2000;13:295‐302. [PubMed] [Google Scholar]
- 46. Ekelund J‐A, Lindquist LW, Carlsson GE, Jemt T. Implant treatment in the edentulous mandible: a prospective study on Brånemark system implants over more than 20 years. Int J Prosthodont. 2003;16:602‐608. [PubMed] [Google Scholar]
- 47. Rasmusson L, Roos J, Bystedt H. A 10‐year follow‐up study of titanium dioxide‐blasted implants. Clin Implant Dent Relat Res. 2005;7:36‐42. [DOI] [PubMed] [Google Scholar]
- 48. Roccuzzo M, Bonino L, Dalmasso P, Aglietta M. Long‐term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10‐year data around sandblasted and acid‐etched (SLA) surface. Clin Oral Implants Res. 2014;25:1105‐1112. [DOI] [PubMed] [Google Scholar]
- 49. Roccuzzo M, De Angelis N, Bonino L, Aglietta M. Ten‐year results of a three‐arm prospective cohort study on implants in periodontally compromised patients. Part 1: implant loss and radiographic bone loss. Clin Oral Implants Res. 2010;21:490‐496. [DOI] [PubMed] [Google Scholar]
- 50. Donati M, Ekestubbe A, Lindhe J, Wennström JL. Marginal bone loss at implants with different surface characteristics ‐ a 20‐year follow‐up of a randomized controlled clinical trial. Clin Oral Implants Res. 2018;29:197‐198. [DOI] [PubMed] [Google Scholar]
- 51. Roos‐Jansåker A‐M, Lindahl C, Renvert H, Renvert S. Nine‐ to fourteen‐year follow‐up of implant treatment. Part I: implant loss and associations to various factors. J Clin Periodontol. 2006;33:283‐289. [DOI] [PubMed] [Google Scholar]
- 52. Noda K, Arakawa H, Kimura‐Ono A, et al. A longitudinal retrospective study of the analysis of the risk factors of implant failure by the application of generalized estimating equations. J Prosthodont Res. 2015;59:178‐184. [DOI] [PubMed] [Google Scholar]
- 53. Ueda T, Kremer U, Katsoulis J, Mericske‐Stern R. Long‐term results of mandibular implants supporting an overdenture: implant survival, failures, and crestal bone level changes. Int J Oral Maxillofac Implants. 2011;26:365‐372. [PubMed] [Google Scholar]
- 54. Abu‐Ta'a M, Quirynen M, Teughels W, van Steenberghe D. Asepsis during periodontal surgery involving oral implants and the usefulness of peri‐operative antibiotics: a prospective, randomized, controlled clinical trial. J Clin Periodontol. 2008;35:58‐63. [DOI] [PubMed] [Google Scholar]
- 55. Anitua E, Aguirre JJ, Gorosabel A, et al. A multicentre placebo‐controlled randomised clinical trial of antibiotic prophylaxis for placement of single dental implants. Eur J Oral Implantol. 2009;2:283‐292. [PubMed] [Google Scholar]
- 56. Tan WC, Ong M, Han J, et al. Effect of systemic antibiotics on clinical and patient‐reported outcomes of implant therapy ‐ a multicenter randomized controlled clinical trial. Clin Oral Implants Res. 2013;25:185‐193. [DOI] [PubMed] [Google Scholar]
- 57. Karaky AE, Sawair FA, Al‐Karadsheh OA, et al. Antibiotic prophylaxis and early dental implant failure: a quasi‐random controlled clinical trial. Eur J Oral Implantol. 2011;4:31‐38. [PubMed] [Google Scholar]
- 58. Esposito M, Cannizzaro G, Bozzoli P, et al. Effectiveness of prophylactic antibiotics at placement of dental implants: a pragmatic multicentre placebo‐controlled randomised clinical trial. Eur J Oral Implantol. 2010;3:135‐143. [PubMed] [Google Scholar]
- 59. Rosenberg ES, Cho SC, Elian N, et al. A comparison of characteristics of implant failure and survival in periodontally compromised and periodontally healthy patients: a clinical report. Int J Oral Maxillofac Implants. 2004;19:873‐879. [PubMed] [Google Scholar]
- 60. Balshe AA, Assad DA, Eckert SE, Koka S, Weaver AL. A retrospective study of the survival of smooth‐ and rough‐surface dental implants. Int J Oral Maxillofac Implants. 2009;24:1113‐1118. [PubMed] [Google Scholar]
- 61. Urban T, Kostopoulos L, Wenzel A. Immediate implant placement in molar regions: risk factors for early failure. Clin Oral Implants Res. 2012;23:220‐227. [DOI] [PubMed] [Google Scholar]
- 62. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95:S144‐150. [DOI] [PubMed] [Google Scholar]
- 64. Han HJ, Kim S, Han DH. Multifactorial evaluation of implant failure: a 19‐year retrospective study. Int J Oral Maxillofac Implants. 2014;29:303‐310. [DOI] [PubMed] [Google Scholar]
- 65. Buser D, Mericske‐stern R, Pierre Bernard JP, et al. Long‐term evaluation of non‐submerged ITI implants. Part 1: 8‐year life table analysis of a prospective multi‐center study with 2359 implants. Clin Oral Implants Res. 1997;8:161‐172. [DOI] [PubMed] [Google Scholar]
- 66. Olmedo‐Gaya MV, Manzano‐Moreno FJ, Cañaveral‐Cavero E, de Dios Luna‐del Castillo J, Vallecillo‐Capilla M. Risk factors associated with early implant failure: a 5‐year retrospective clinical study. J Prosthet Dent. 2016;115:150‐155. [DOI] [PubMed] [Google Scholar]
- 67. Bornstein MM, Halbritter S, Harnisch H, Weber H‐P, Buser D. A retrospective analysis of patients referred for implant placement to a specialty clinic: indications, surgical procedures, and early failures. Int J Oral Maxillofac Implants. 2008;23:1109‐1116. [PubMed] [Google Scholar]
- 68. Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol. 2007;34:610‐617. [DOI] [PubMed] [Google Scholar]
- 69. Noguerol B, Munoz R, Mesa F, de Dios LJ, O'Valle F. Early implant failure. Prognostic capacity of Periotest: retrospective study of a large sample. Clin Oral Implants Res. 2006;17:459‐464. [DOI] [PubMed] [Google Scholar]
- 70. Levin L, Ofec R, Grossmann Y, Anner R. Periodontal disease as a risk for dental implant failure over time: a long‐term historical cohort study. J Clin Periodontol. 2011;38:732‐737. [DOI] [PubMed] [Google Scholar]
- 71. Brügger O, Bornstein M, Kuchler U, et al. Implant therapy in a surgical specialty clinic: an analysis of patients, indications, surgical procedures, risk factors, and early failures. Int J Oral Maxillofac Implants. 2015;30:151‐160. [DOI] [PubMed] [Google Scholar]
- 72. French D, Larjava H, Ofec R. Retrospective cohort study of 4591 Straumann implants in private practice setting, with up to 10‐year follow‐up. Part 1: multivariate survival analysis. Clin Oral Implants Res. 2015;26:1345‐1354. [DOI] [PubMed] [Google Scholar]
- 73. Cochran DL, Jackson JM, Jones AA, et al. A 5‐year prospective multicenter clinical trial of non‐submerged dental implants with a titanium plasma‐sprayed surface in 200 patients. J Periodontol. 2011;82:990‐999. [DOI] [PubMed] [Google Scholar]
- 74. Baqain ZH, Moqbel WY, Sawair FA. Early dental implant failure: risk factors. Br J Oral Maxillofac Surg. 2012;50:239‐243. [DOI] [PubMed] [Google Scholar]
- 75. DeLuca S, Habsha E, Zarb GA. The effect of smoking on osseointegrated dental implants. Part I: implant survival. Int J Prosthodont. 2006;19:491‐498. [PubMed] [Google Scholar]
- 76. Grimes DA, Schulz KF. False alarms and pseudo‐epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120:920‐927. [DOI] [PubMed] [Google Scholar]
- 77. De Bruyn H, Collaert B. The effect of smoking on early implant failure. Clin Oral Implants Res. 1994;5:260‐264. [DOI] [PubMed] [Google Scholar]
- 78. van Steenberghe D, Jacobs R, Desnyder M, Maffei G, Quirynen M. The relative impact of local and endogenous patient‐related factors on implant failure up to the abutment stage. Clin Oral Implants Res. 2002;13:617‐622. [DOI] [PubMed] [Google Scholar]
- 79. Cavalcanti R, Oreglia F, Manfredonia MF, Gianserra R, Esposito M. The influence of smoking on the survival of dental implants: a 5‐year pragmatic multicentre retrospective cohort study of 1727 patients. Eur J Oral Implantol. 2011;4:39‐45. [PubMed] [Google Scholar]
- 80. Olate S, Lyrio MC, de Moraes M, Mazzonetto R, Moreira RW. Influence of diameter and length of implant on early dental implant failure. J Oral Maxillofac Surg. 2010;68:414‐419. [DOI] [PubMed] [Google Scholar]
- 81. Albrektsson T, Buser D, Sennerby L. Crestal bone loss and oral implants. Clin Implant Dent Relat Res. 2012;14:783‐791. [DOI] [PubMed] [Google Scholar]
- 82. Wennström JL, Ekestubbe A, Gröndahl K, Karlsson S, Lindhe J. Oral rehabilitation with implant‐supported fixed partial dentures in periodontitis‐susceptible subjects. A 5‐year prospective study. J Clin Periodontol. 2004;31:713‐724. [DOI] [PubMed] [Google Scholar]
- 83. Dvorak G, Arnhart C, Heuberer S, Huber CD, Watzek G, Gruber R. Peri‐implantitis and late implant failures in postmenopausal women: a cross‐sectional study. J Clin Periodontol. 2011;38:950‐955. [DOI] [PubMed] [Google Scholar]
- 84. He J, Zhao B, Deng C, Shang D, Zhang C. Assessment of implant cumulative survival rates in sites with different bone density and related prognostic factors: an 8‐year retrospective study of 2,684 implants. Int J Oral Maxillofac Implants. 2015;30:360‐371. [DOI] [PubMed] [Google Scholar]
- 85. Romeo E, Lops D, Margutti E, et al. Long‐term survival and success of oral implants in the treatment of full and partial arches: a 7‐year prospective study with the ITI dental implant system. Int J Oral Maxillofac Implants. 2004;19:247‐259. [PubMed] [Google Scholar]
- 86. Wagenberg B, Froum SJ. A retrospective study of 1925 consecutively placed immediate implants from 1988 to 2004. Int J Oral Maxillofac Implants. 2006;21:71‐80. [PubMed] [Google Scholar]
- 87. Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. A retrospective study on clinical and radiological outcomes of oral implants in patients followed up for a minimum of 20 years. Clin Implant Dent Relat Res. 2018;20(2):199‐207. [DOI] [PubMed] [Google Scholar]
- 88. Berglundh T, Armitage G, Araujo MG, et al. Peri‐implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. J Clin Periodontol. 2018;45:S286‐S291. [DOI] [PubMed] [Google Scholar]
- 89. Jemt T, Karouni M, Abitbol J, Zouiten O, Antoun H. A retrospective study on 1592 consecutively performed operations in one private referral clinic. Part II: peri‐implantitis and implant failures. Clin Implant Dent Relat Res. 2017;19:413‐422. [DOI] [PubMed] [Google Scholar]
- 90. Jungner M, Lundqvist P, Lundgren S. A retrospective comparison of oxidized and turned implants with respect to implant survival, marginal bone level and peri‐implant soft tissue conditions after at least 5 years in function. Clin Implant Dent Relat Res. 2014;16:230‐237. [DOI] [PubMed] [Google Scholar]
- 91. Isidor F. Histological evaluation of peri‐implant bone at implants subjected to occlusal overload or plaque accumulation. Clin Oral Implants Res. 1997;8:1‐9. [DOI] [PubMed] [Google Scholar]
- 92. Borba M, Deluiz D, Lourenco EJV, Oliveira L, Tannure PN. Risk factors for implant failure: a retrospective study in an educational institution using GEE analyses. Braz Oral Res. 2017;31:e69. [DOI] [PubMed] [Google Scholar]
- 93. Davarpanah M, Martinez H, Etienne D, et al. A prospective multicenter evaluation of 1,583 3i implants: 1‐ to 5‐year data. Int J Oral Maxillofac Implants. 2002;17:820‐828. [PubMed] [Google Scholar]
- 94. Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. Intake of proton pump inhibitors is associated with an increased risk of dental implant failure. Int J Oral Maxillofac Implants. 2017;32:1097‐1102. [DOI] [PubMed] [Google Scholar]
- 95. Jemt T. A retro‐prospective effectiveness study on 3448 implant operations at one referral clinic: a multifactorial analysis. Part II: Clinical factors associated to peri‐implantitis surgery and late implant failures. Clin Implant Dent Relat Res. 2017;19:972‐979. [DOI] [PubMed] [Google Scholar]
- 96. Berglundh T, Wennström JL, Lindhe J. Long‐term outcome of surgical treatment of peri‐implantitis. A 2–11‐year retrospective study. Clin Oral Implants Res. 2018;29:404‐410. [DOI] [PubMed] [Google Scholar]
- 97. Heitz‐Mayfield LJA, Salvi GE, Mombelli A, et al. Supportive peri‐implant therapy following anti‐infective surgical peri‐implantitis treatment: 5‐year survival and success. Clin Oral Implants Res. 2018;29:1‐6. [DOI] [PubMed] [Google Scholar]
- 98. Roccuzzo M, Pittoni D, Roccuzzo A, Charrier L, Dalmasso P. Surgical treatment of peri‐implantitis intrabony lesions by means of deproteinized bovine bone mineral with 10% collagen: 7‐year‐results. Clin Oral Implants Res. 2017;28:1577‐1583. [DOI] [PubMed] [Google Scholar]
- 99. Schwarz F, John G, Schmucker A, Sahm N, Becker J. Combined surgical therapy of advanced peri‐implantitis evaluating two methods of surface decontamination: a 7‐year follow‐up observation. J Clin Periodontol. 2017;44:337‐342. [DOI] [PubMed] [Google Scholar]
- 100. Brocard D, Barthet P, Baysse E, et al. A multicenter report on 1,022 consecutively placed ITI implants: a 7‐year longitudinal study. Int J Oral Maxillofac Implants. 2000;15:691‐700. [PubMed] [Google Scholar]
- 101. Huynh‐Ba G, Friedberg JR, Vogiatzi D, Ioannidou E. Implant failure predictors in the posterior maxilla: a retrospective study of 273 consecutive implants. J Periodontol. 2008;79:2256‐2261. [DOI] [PubMed] [Google Scholar]
- 102. Lambrecht JT, Filippi A, Kunzel AR, Schiel HJ. Long‐term evaluation of submerged and nonsubmerged ITI solid‐screw titanium implants: a 10‐year life table analysis of 468 implants. Int J Oral Maxillofac Implants. 2003;18:826‐834. [PubMed] [Google Scholar]


