Abstract
Introduction
Understanding the impact of scoliosis surgery on lung function is important for counseling patients about risks and benefits of surgery. We prospectively compared the trends in lung function test (LFT) results before and after scoliosis surgery in children with neuromuscular diseases or dysmorphic syndromes. We hypothesized a stabilization.
Methods
We prospectively included children with neuromuscular or syndromic scoliosis able to perform LFTs. We studied (forced) vital capacity ([F]VC), ratio of forced expiratory volume in 1 s (FEV1) and FVC, and peak expiratory flow (PEF). Preoperative LFT results were compared with results 3–4 months after surgery. The mean monthly change in LFT results up to 2 years after surgery was compared with the preoperative natural history using linear mixed‐effects models.
Results
We included 43 patients. No significant change was observed in absolute values of (F)VC, FEV1/FVC, and PEF before and after surgery. In 23 neuromuscular patients median standardized VC, FVC, and PEF decreased significantly after surgery from 43% to 33%, 42% to 31%, and 51% to 40%, respectively. In 20 syndromic patients, median FVC decreased from 68% to 65%. The monthly rate of change in FVC did not change significantly in both groups with a mean difference of 0.18% (95% CI: −0.27, −0.61) and −0.44% (95% CI: −1.05, 0.16).
Conclusion
No stabilization of lung function 3–4 months after scoliosis surgery was observed in children with neuromuscular and syndromic scoliosis with restrictive lung function disease. The effect on the rate of lung function decline remains inconclusive.
Keywords: lung function, neuromuscular disease, scoliosis
1. INTRODUCTION
Scoliosis is a common complication in patients with neuromuscular diseases (NMDs) and genetic syndromes and scoliosis surgery may be necessary for a number of reasons. It improves ease of nursing care, reduces back pain, and allows the patient a much better sitting posture and balance, thereby improving the patient's overall quality of life and self‐image. 1 , 2
One of the more significant factors causing morbidity and mortality in patients with neuromuscular scoliosis is the deterioration in pulmonary function to which scoliosis progression may contribute. 1 By distorting thoracic anatomy and rib alignment, severe scoliosis decreases the compliance of the chest wall, thereby restricting vital capacity (VC), 2 while lordotic deformation of the thoracic spine may cause bronchial kinking or compression and bronchial obstruction. 3 Straightening the spine has the potential to improve the ability to elevate the ribs and thus expand the chest on inspiration. 2 On the other hand, children with preexisting respiratory compromise have an increased risk of developing postoperative complications. 4 Understanding the impact of neuromuscular or syndromic scoliosis surgery on lung function is important for counseling patients about risks and longer‐term benefits of scoliosis surgery on respiratory function. 2
Research on the impact of scoliosis surgery on lung function has yielded conflicting results.
In this prospective cohort study, we compared lung function test (LFT) results of children with probable restrictive lung function due to NMD or syndromic syndromes before and after scoliosis surgery in a single center.
We aimed to study the short‐term improvement of lung function after surgery and determine the mean change in postoperative compared to preoperative progression rate in lung function. We hypothesized that scoliosis surgery slows the rate of lung function decline.
2. MATERIALS AND METHODS
Eligibility was checked in pediatric patients planned for surgery for a neuromuscular or syndromic scoliosis at the preoperative clinic of the Pediatric Intensive Care Unit (PICU) of the Wilhelmina Children's Hospital Utrecht, the Netherlands in 2018 or 2019. Patients were included if they were able to perform LFTs. Only reproducible LFT results were included. We excluded patients with hereditary proximal spinal muscular atrophy (SMA) who had started treatment with genetic therapies during follow‐up, as this might provide an alternative explanation for improvement of LFT results. This study was approved by the local Medical Ethics Committee. Written informed consent was obtained from all participants and/or their parents. The reporting of this study conforms to the STROBE statement. 5
Spirometry data (Geratherm Spirostick®) were collected at the department of pulmonology before and 3 months after surgery. We included results of (forced) vital capacity ([F]VC), the ratio of forced expiratory volume in 1 s (FEV1) and FVC, and peak expiratory flow (PEF). All LFTs were measured in sitting position, without corsets or braces by a small team of professionals experienced in conducting these tests in children with NMDs and other comorbidities. All LFTs were measured and reported according to the European Respiratory Society guideline. 6 If patients were unable to stand to measure height, tape‐measured arm span was used as a surrogate measure. 7 , 8 We reported absolute and standardized LFT values, according to the Global Lung Initiative. 9
We compared values 0–3 months before and 3–4 months after scoliosis surgery. In patients with more than 3 months between the PICU clinic visit and surgery, LFTs were repeated on the day of admission. We chose this timing of 3–4 months after surgery, to combine it with regular orthopedic follow‐up and consequently minimize the burden for the patient.
To study the progression rate of LFT results before and after surgery, we used LFT results obtained during regular follow‐up in the 2 years before and after surgery whenever available.
2.1. Statistical analyses
For baseline characteristics, we used descriptive statistics. For comparisons of LFT results 0–3 months before and 3–4 months after scoliosis surgery, a Wilcoxon‐signed rank test was used. To investigate the trend of function decline, linear mixed‐effects models were fitted, which included a random intercept and random slope for time per individual. We introduced a linear spline for time (both fixed as random) with one knot at the time of surgery. Subsequently, we determined the rate of progression before and after surgery. Confidence intervals were estimated using bootstrapping (n = 1000) and significance tests were based on the likelihood ratio test.
3. RESULTS
3.1. Patient characteristics
During 2018–2019, we assessed 111 patients before scoliosis surgery at the PICU clinic. In total, we included 43 patients. A large proportion of the patients referred to the preoperative PICU clinic (61%) was excluded mainly due to the inability to obtain LFT results due to developmental delay.
Baseline characteristics of patients and intra‐ and postoperative details are shown in Table 1.
Table 1.
Patients, intraoperative, and postoperative characteristics
| Baseline, n = 43 patients | |
| Age in years, median (IQR) | 11.7 (9.4–15.1) |
| Male gender, n (%) | 21 (49) |
| Underlying disease, n (%) | |
| Neuromuscular disease | 23 (53) |
| Spinal muscular atrophy | 11 (48) |
| Duchenne muscular dystrophy | 5 (22) |
| Congenital myopathy | 5 (22) |
| Arthrogryposis multiplex congenita | 1 (4) |
| Emery–Dreifuss dystrophy | 1 (4) |
| Other syndromes | 20 (47) |
| Skeletal dysplasia | 3 (15) |
| VACTERL | 2 (10) |
| Hurler syndrome | 3 (15) |
| Neurofibromatosis | 3 (15) |
| Spina bifida | 3 (15) |
| Miscellaneous | 6 (30) |
| Home mechanical ventilation, n (%) | 8 (19) |
| Cobbs angle, median (IQR) | 63 (54–76) |
| Intraoperative | |
| Number of vertebrae, median (IQR) | 11 (11.5–16) |
| Technique used, n (%) | |
| Growth friendly surgery | 31 (72) |
| Posterior minimal invasive surgery | 8 (19) |
| Posterior spinal fusion | 4 (9) |
| Adverse events, n | 1 |
| Loss of neuromonitoring signal | 1 |
| Postoperative | |
| Adverse events, n | 7 |
| Wound infection | 1 |
| Hemothorax | 1 |
| Pneumonia | 2 |
| Resuscitation (airway obstruction) | 1 |
| Hyperesthesia foot | 1 |
| PICU LOS, days median (range) | 1 (1–14) |
| >48 h invasive ventilation, n | 2 |
| Hospital LOS, days median (IQR) | 7 (6–9) |
| Cobbs angle, median (IQR) | 29 (20–39) |
Abbreviations: IQR, interquartile range; LOS, length of stay; PICU, pediatric intensive care unit.
Just over half of the patients suffered from an NMD, 48% of them were diagnosed with SMA type 2 (n = 10) and type 3 (n = 1). None of the patients initiated treatment with spinraza or were already using spinraza. None of the patients with Duchenne muscular dystrophy (DMD) initiated treatment with corticosteroids during follow‐up. The patients with DMD who used steroids used this for at least 2 years before surgery. Eight patients with NMDs used home mechanical ventilation, all started years before surgery. Diagnoses of the syndromic group are specified in Table 1. Miscellaneous were patients with a variety of syndromes (Fetal alcohol, Brain thyroid, Prader Willi, Goldenhar, Koolen de Vries, and chromosomal duplication syndrome). At baseline, 60% of patients suffered from moderate to severe restriction of lung function, that is, VC < 60%. Median standardized VC before surgery was 55% (IQR: 41%–76%). There was no obstructive lung disease with median FEV1/FVC of 91% before surgery. Majority of patients had growth‐friendly surgery, including traditional growing rod, magnetically controlled growing rods, and spring distraction systems.
Median Cobbs angle was 63° preoperatively and 29° after surgery.
In four patients, severe adverse events occurred that were fortunately resolved.
3.2. Short‐term effects of scoliosis surgery on lung function
Wilcoxon signed‐rank test was used to compare LFT results before and after surgery in 43 patients. No significant change was observed of absolute values of (F)VC, FEV1/FVC, and PEF before surgery compared to 3–4 months after surgery in both the neuromuscular and the syndromic group, except from a significant decrease of PEF in a subgroup of patients with SMA (Table 2). Standardized values of (F)VC and PEF were significantly lower 3–4 months after surgery compared to results before surgery in the neuromuscular group. Standardized FVC was significantly lower after surgery in the syndromic group (Table 2).
Table 2.
Lung function before and 3 months after surgery
| Patients | Lung function parameter | Before surgery | 3 months after surgery | p‐value (Z‐statistic) |
|---|---|---|---|---|
| NMDs (n = 23) | VC (L), median (IQR) | 0.99 (0.68–1.27) | 0.86 (0.57–1.15) | 0.097 (−1.661) |
| VC stand (%), median (IQR) | 43 (29–67) | 33 (27–54) | 0.021* (−2.310) | |
| FVC (L), median (IQR) | 0.99 (0.62–1.30) | 0.79 (0.60–1.15) | 0.221 (−1.225) | |
| FVC stand (%), median (IQR) | 42 (19–55) | 31 (17–42) | 0.027* (−2.205) | |
| FEV1/FVC (%), median (IQR) | 93.0 (88.0–98.3) | 94.0 (82.5–98) | >0.5 (0.079) | |
| PEF (L/s), median (IQR) | 2.45 (1.41–2.99) | 2.03 (1.46–2.64) | 0.087 (−1.712) | |
| PEF stand (%), median (IQR) | 51 (35–58) | 40 (27–49) | 0.009* (−2.624) | |
| Subgroup: SMA (n = 11) | VC (L), median (IQR) | 1.11 (0.88, 1.46) | 0.93 (0.75, 1.45) | 0.108 (−1.609) |
| VC stand (%), median (IQR) | 49 (40, 70) | 34 (32, 61) | 0.024* (−2.254) | |
| FVC (L), median (IQR) | 1.06 (0.8, 1.30) | 0.85 (0.60, 1.15) | 0.168 (−1.380) | |
| FVC stand (%), median (IQR) | 50 (34, 64) | 32 (27, 51) | 0.028* (−2.193) | |
| FEV1/FVC (%), median (IQR) | 93 (88, 98) | 94 (86, 98) | >0.5 (−0.178) | |
| PEF (L/s), median (IQR) | 2.45 (1.85, 2.99) | 2.03 (1.37, 2.63) | 0.028* (−2.192) | |
| PEF stand (%), median (IQR) | 52 (44, 63) | 42 (27, 53) | 0.008* (−2.670) | |
| Syndromes (n = 20) | VC (L), median (IQR) | 1.56 (0.99–2.20) | 1.53 (1.04–2.22) | >0.5 (−0.315) |
| VC stand (%), median (IQR) | 65 (54–87) | 64 (54–80) | 0.490 (−0.691) | |
| FVC (L), median (IQR) | 1.49 (0.95–2.05) | 1.47 (1.03– 1.80) | >0.5 (−0.205) | |
| FVC stand (%), median (IQR) | 68 (59–90) | 65 (49–83) | 0.001* (−3.181) | |
| FEV1/FVC (%), median (IQR) | 89.0 (81.8–94.5) | 90.0 (80.5– 97.5) | >0.5 (−0.299) | |
| PEF (L/s), median (IQR) | 2.81 (2.18–4.90) | 3.10 (2.22– 4.20) | 0.305 (−1.027) | |
| PEF stand (%), median (IQR) | 73 (58–95) | 71 (53–86) | 0.107 (−1.611) |
Abbreviations: FVC, forced vital capacity; IQR, interquartile range; L, liter, NMDs, neuromuscular diseases; PEF, peak expiratory flow; s, second; SMA, spinal muscular atrophy; stand, standardized; VC, vital capacity.
Significant (p < 0.05).
3.3. Change in post versus preoperative progression rate
To study the longitudinal course before and after surgery, we analyzed 137 measurements of VC, 158 FVC measurements, and 157 PEF results in 43 patients.
There was no significant change in the rate of decline of VC, FVC, and PEF in both patients with NMDs and patients with syndromes (Table 3).
Table 3.
Change in monthly lung function test results 2 years before compared to 2 years after surgery
| Group | Lung function parameter | Before surgery | After surgery | Change |
|---|---|---|---|---|
| Slope (95% CI) | Slope (95% CI) | Mean diff (95% CI) | ||
| NMDs | FVC (%) | −0.40 (−0.70, −0.08) | −0.22 (−0.47, 0.03) | 0.18 (−0.27, −0.61) |
| FVC (ml) | 0.95 (−8.47, 10.90) | −1.42 (−9.74, 6.51) | −2.37 (−15.57, 10.3) | |
| PEF (%) | −0.42 (−0.91, 0.05) | −0.34 (−0.72, 0.02) | 0.08 (−0.54, 0.70) | |
| Syndromes | FVC (%) | 0.14 (−0.33, 0.59) | −0.30 (−0.64, 0.03) | −0.44 (−1.05, 0.16) |
| FVC (ml) | 12.07 (−4.07, 26.94) | 4.28 (−7.66, 15.96) | −7.79 (−27.2, 11.67) | |
| PEF (%) | 0.03 (−0.49, 0.52) | −0.14 (−0.56, 0.31) | −0.17 (−0.90, 0.55) |
Abbreviations: CI, confidence interval; diff, difference; FVC, forced vital capacity; ml, milliliter; NMDs, neuromuscular diseases; PEF, peak expiratory flow.
Analysis of 74 FVC measurements in patients with NMDs showed a monthly change of FVC before surgery of −0.40% (95% CI: −0.70 to −0.08) compared to −0.22% (95% CI: −0.47 to 0.03), resulting in a mean difference in slope of 0.18% (95% CI: −0.27 to −0.61) (Figure 1). In the syndromic group, a monthly change of FVC before surgery of 0.14% (95%CI: −0.33 to 0.59) compared to −0.30% (95% CI: −0.64 to 0.03) was observed, resulting in a mean difference in slope of −0.44% (95% CI: −1.05 to 0.16) (Figure 2).
Figure 1.
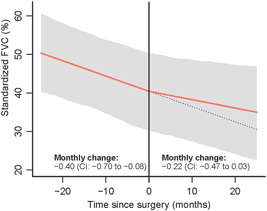
Standardized forced vital capacity (FVC) months before and after surgery in patients with neuromuscular diseases. CI, confidence interval [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.

Standardized forced vital capacity (FVC) months before and after surgery in syndromic patients. CI, confidence interval [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This prospective cohort study on LFT results in children with neuromuscular and syndromic scoliosis with restrictive lung function disease shows no stabilization of lung function 3–4 months after surgery. The effect of surgery on the rate of lung function decline remains inconclusive in this study with 43 patients.
Research on the impact of scoliosis surgery on LFT results has yielded conflicting results.
Previous studies have reported that scoliosis correction has beneficial effect on respiratory function, 1 , 10 , 11 while other studies, including a recent Cochrane review in patients with DMD, 12 have demonstrated no obvious benefit in terms of respiratory function. 13 , 14 , 15
In a very recent retrospective study, Farber reported decreased VC after scoliosis surgery in 14 out of 20 patients with SMA and muscular dystrophies. They were not able to conclude on changes in rate of VC decline. 2 All patients with SMA in this study had a decrease in VC after surgery. A large proportion of patients in our study were patients with SMA. We compared prospectively collected LFT results before and after surgery, both in a predefined limited time range. We observed a significant decrease of PEF 3–4 months after surgery in this subgroup of SMA patients.
Our results may be explained by the fact that 3–4 months after surgery, the majority of patients did not fully recover to their preoperative functional level. 16 For this reason, we also studied the course of LFT results over a longer period in a larger cohort than Farber. 2
Contrary to our study, two recent studies did show a positive effect in terms of a decreased rate of decline in FVC after scoliosis surgery. 1 , 11 This may be explained by the retrospective nature 1 and inclusion of patients with less severe scoliosis. 11
We did not observe obstructive lung disease before surgery, in contrast to a study by McPhail. This study showed obstructive lung disease probably caused by mainstem airway compression from spine rotation in 33% of children with congenital scoliosis or syndromic scoliosis, compared to a population prevalence of 2% to 5%. 17
Our work has several strengths. First, we only included patients with (the risk of) restrictive lung disease and excluded idiopathic scoliosis. Even though we studied a heterogeneous group of patients, the majority of patients had a standardized VC < 60% before scoliosis surgery, which a recent study found to be the most sensitive LFT for predicting prolonged postoperative mechanical ventilation. 4 Stabilization of lung function has major impact in these patients, as it probably results in a delayed onset of chronic respiratory failure and reduced incidence of respiratory tract infections.
Due to the prospective nature of the study, confounding was limited. None of the included patients with SMA or DMD initiated new therapies, like Spinraza or steroids during data collection. In addition, none of the patients initiated NIV during data collection. Previously published studies were observational 10 , 11 , 14 or retrospective, 1 , 13 , 15 increasing the risk of bias.
We acknowledge also several limitations of our work. The rate of decline in LFT results had broad confidence intervals due to small sample, which possibly explains the nonsignificant mean change in progression rate. However, compared to other studies we included more patients. 1 , 2 , 10 , 13 , 14 , 15 , 18 The follow‐up time of our study was limited. Introduction of new therapies, like genetic therapies for SMA, or other confounding factors may complicate interpretation of observational studies with longer follow up. Accurate predicted values of LFTs are difficult to obtain, due to errors introduced by methods of height estimation. 2 For this reason, we reported both absolute as well as standardized LFT results and used arm span as a well‐established alternative. 7 , 8
We included a heterogeneous group of patients. The surgical technique varied, although in majority of patients growth‐friendly surgery was used.
Unfortunately, we did not prospectively study the effect on Maximal Inspiratory and Expiratory Pressures (PImax and PEmax). Saito retrospectively reviewed lung function and respiratory muscle strength preoperatively, 1 month and 6 months postoperatively in 16 patients with DMD. Although no significant difference was observed in FVC and VC, mean values of PImax and PEmax significantly improved postoperatively. 18
The question remains if the positive effect on PImax and PEmax in this retrospective study is the result of surgery. Also, other factors such as training effect, chest wall configuration, and stabilization may contribute to the results of Saito, as large variations of PImax and PEmax are also observed in healthy children. 19
To increase the power, study the effect on individual NMDs and possibly compare different surgical techniques, a multicenter study is needed to include a large enough number of patients.
A Cochrane review concluded uncertainty of benefits and potential risks of scoliosis surgery in patients with DMD and stated that RCTs are needed to investigate effects of scoliosis surgery on respiratory function in patients with DMD. 12 Although our study cannot conclude that pulmonary function is expected to benefit from surgical treatment, this is not a reason to withhold this treatment for progressive scoliosis in neuromuscular patients since untreated patients develop severe deformities that cause pain and inability to sit straight and severe difficulties in daily nursing care.
Systematic data collection however should be standard to allow high‐quality comparative cohort studies.
5. CONCLUSION
This prospective cohort study on LFT results in children with neuromuscular and syndromic scoliosis with restrictive lung function disease shows no short‐term beneficial effect of surgery on lung function. The effect of surgery on the rate of lung function decline remains inconclusive.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
Esther S. Veldhoen: Conceptualization‐Lead, Data curation‐Lead, Formal analysis‐Lead, Methodology‐Lead, Writing – original draft‐Lead, Writing – review & editing‐Lead. Anneloes de Vries: Data curation‐Lead, Formal analysis‐Equal, Writing – original draft‐Equal, Writing – review & editing‐Equal. Tom P. C. Schlosser: Conceptualization‐Equal, Data curation‐Equal, Writing – review & editing‐Equal. Moyo C. Kruyt: Conceptualization‐Equal, Data curation‐Equal, Writing – review & editing‐Equal. Ruben P. A. van Eijk: Formal analysis‐Equal, Methodology‐Equal, Writing – review & editing‐Equal. Joyce M. Tersmette: Data curation‐Equal, Investigation‐Equal, Writing – review & editing‐Equal. Erik H. Hulzebos: Conceptualization‐Equal, Data curation‐Equal, Writing – review & editing‐Equal. Ludo W. van der Pol: Conceptualization‐Equal, Methodology‐Equal, Writing – review & editing‐Equal. Roelie M. Wösten‐van Asperen: Data curation‐Equal, Methodology‐Equal, Writing – review & editing‐Equal. Cornelis K. van der Ent: Data curation‐Equal, Methodology‐Equal, Supervision‐Equal, Writing – review & editing‐Equal.
ACKNOWLEDGMENTS
We would like to thank all the patients taking part in this study. No financial support or grants.
Veldhoen ES, de Vries A, Schlosser TPC, et al. Short‐term effect and effect on rate of lung function decline after surgery for neuromuscular or syndromic scoliosis. Pediatric Pulmonology. 2022;57:1303‐1309. 10.1002/ppul.25857
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions
REFERENCES
- 1. Chua K, Tan CY, Chen Z, et al. Long‐term follow‐up of pulmonary function and scoliosis in patients with Duchenne's muscular dystrophy and spinal muscular atrophy. J Pediatr Orthop. 2016;36(1):63‐69. [DOI] [PubMed] [Google Scholar]
- 2. Farber HJ, Phillips WA, Kocab KL, et al. Impact of scoliosis surgery on pulmonary function in patients with muscular dystrophies and spinal muscular atrophy. Pediatr Pulmonol. 2020;55(4):1037‐1042. [DOI] [PubMed] [Google Scholar]
- 3. Farrell J, Garrido E. Effect of idiopathic thoracic scoliosis on the tracheobronchial tree. BMJ Open Respir Res. 2018;5:e000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan N, Skaggs DL, Dorey F, Keens TG. Preoperative predictors of prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol. 2005;40:414‐419. [DOI] [PubMed] [Google Scholar]
- 5. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344‐349. [DOI] [PubMed] [Google Scholar]
- 6. Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 7. Hibbert ME, Lanigan A, Raven J, Phelan PD. Relation of armspan to height and the prediction of lung function. Thorax. 1988;43(8):657‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wijngaarde CA, Veldhoen ES, Van Eijk RPA, et al. Natural history of lung function in spinal muscular atrophy. Orphanet J Rare Dis. 2020;15(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3‐95‐yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40(6):1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoon WW, Sedra F, Shah S, Wallis C, Muntoni F, Noordeen H. Improvement of pulmonary function in children with early‐onset scoliosis using magnetic growth rods. Spine (Phila Pa 1976). 2014;39(15):1196‐1202. [DOI] [PubMed] [Google Scholar]
- 11. Velasco MV, Colin AA, Zurakowski D, Darras BT, Shapiro F. Posterior spinal fusion for scoliosis in Duchenne muscular dystrophy diminishes the rate of respiratory decline. Spine (Phila Pa 1976). 2007;32(4):459‐465. [DOI] [PubMed] [Google Scholar]
- 12. Cheuk DKL, Wong V, Wraige E, Baxter P, Cole A. Surgery for scoliosis in Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2015;10:CD005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chng SY, Wong YQ, Hui JH, Wong HK, Ong HT, Goh DY. Pulmonary function and scoliosis in children with spinal muscular atrophy types II and III. J Paediatr Child Health. 2003;39:673‐676. [DOI] [PubMed] [Google Scholar]
- 14. Alexander WM, Smith M, Freeman BJC, Sutherland LM, Kennedy JD, Cundy PJ. The effect of posterior spinal fusion on respiratory function in Duchenne muscular dystrophy. Eur Spine J. 2013;22:411‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robinson D, Galasko CSB, Delaney C, Williamson JB, Barrie JL. Scoliosis and lung function in spinal muscular atrophy. Eur Spine J. 1995;4:268‐273. [DOI] [PubMed] [Google Scholar]
- 16. Izatt MT, Harvey JR, Adam CJ, Fender D, Labrom RD, Askin GN. Recovery of pulmonary function following endoscopic anterior scoliosis correction: Evaluation at 3, 6, 12, and 24 months after surgery. Spine (Phila Pa 1976). 2006;31(21):2469‐2477. [DOI] [PubMed] [Google Scholar]
- 17. McPhail GL, Howells SA, Boesch RP, et al. Obstructive lung disease is common in children with syndromic and congenital scoliosis: A preliminary study. J Pediatr Orthop. 2013;33:781‐785. [DOI] [PubMed] [Google Scholar]
- 18. Saito W, Mizuno K, Inoue G, et al. Perioperative evaluation of respiratory muscle strength after scoliosis correction in patients with Duchenne muscular dystrophy. Asian. Spine J. 2017;11(5):787‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fauroux B, Aubertin G, Cohen E, Clément A, Lofaso F. Sniff nasal inspiratory pressure in children with muscular, chest wall or lung disease. Eur Respir J. 2009;33(1):113‐117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions


