Abstract
An active role of neuroinflammation and the NLRP3 inflammasome in Alzheimer's disease and related tauopathies is increasingly identified, supporting NLRP3 as an interesting therapeutic target. However, its effect on tau‐associated neurodegeneration, a key‐process in tauopathies, remains unknown. While tau pathology and neurodegeneration are closely correlated, different tau forms may act as culprits in both characteristics and NLRP3‐dependent microglial processes may differently affect both processes, indicating the need to study the role of NLRP3 in both processes concomitantly. To study the role of NLRP3 on tau pathology, prion‐like propagation and tau‐associated neurodegeneration we generated crosses of NLRP3 deficient mice with tauP301S (PS19) transgenic mice. In this model we studied non‐seeded tau pathology and hippocampal atrophy, reminiscent characteristics of tauopathies. Tau pathology in hippocampus and cortex was significantly decreased in tau.NLRP3−/− versus tau.NLRP3+/+ mice. Importantly, tau.NLRP3−/− mice also displayed significantly decreased hippocampal atrophy, indicating a role of NLRP3 in neurodegeneration. We furthermore assessed the effect of NLRP3 deficiency on tau propagation and associated hippocampal atrophy. NLRP3 deficiency significantly decreased prion‐like seeding and propagation of tau pathology, reflected in decreased tau pathology in ipsi‐ and contralateral hippocampus and cortex in tau.NLRP3−/− following tau seeding. Most importantly, hippocampal atrophy was significantly less in tau‐seeded tau.NLRP3−/− mice at 8 months. We here demonstrate for the first time that NLRP3 activation affects tau‐associated neurodegeneration and seeded and non‐seeded tau pathology, hence affecting key molecular processes in tauopathies. Our data thereby provide key‐information in the validation of NLRP3 inflammasome as therapeutic target for AD and related tauopathies.
Keywords: Alzheimer's disease, inflammation, microglia, neurodegeneration, NLRP3 inflammasome, tau, tauopathy
Main Points
NLRP3 modulates amyloid pathology, while its effect on tau‐induced neurodegeneration is unknown.
We show that NLRP3 increases seeded and non‐seeded tau pathology (AT100) and tau‐associated neurodegeneration (NeuN), using NLRP3 deficient tau mice (T+N‐/‐).
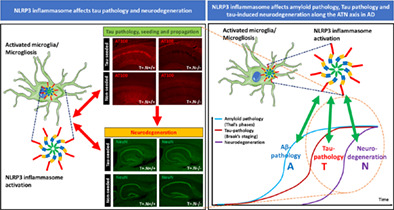
1. INTRODUCTION
Tauopathies are a family of neurodegenerative diseases characterized by the presence of tau‐aggregates (Dugger & Dickson, 2017), with Alzheimer's disease (AD) as the most prevalent one. Brains of AD patients are characterized by the presence of amyloid plaques, neurofibrillary tangles and neurodegeneration (Serrano‐Pozo et al., 2011), referred to as A/T/N pathology (Jack Jr. et al., 2016; Jack Jr. et al., 2018). AD patient brains are furthermore characterized by the presence of neuroinflammation (Serrano‐Pozo et al., 2011), including microgliosis, increasingly recognized as an active contributor to the disease process (Ransohoff, 2016). In AD, microgliosis is associated with both amyloid and tau pathology. An active modulatory role of microglia and the nucleotide‐binding oligomerization domain leucine‐rich repeat and pyrin domain containing receptor 3 (NLRP3) inflammasome on amyloid pathology was previously demonstrated, indicating NLRP3 as an interesting therapeutic target (Halle et al., 2008; Heneka et al., 2013; Venegas et al., 2017). However, microgliosis is also invariably detected in tauopathies, as demonstrated in postmortem tissue and in vivo imaging studies, indicating its association and potential role in tau pathology and tau‐induced neurodegeneration per se, independent of amyloid pathology (Bellucci et al., 2011; Gerhard et al., 2004; Gerhard et al., 2006; Ishizawa & Dickson, 2001).
An active modulatory role of microglia on tau‐pathology has also become increasingly evident over the last years. Microglia were found to contribute to tau pathology in in vivo tauopathy models (Gratuze et al., 2020; Ising et al., 2019; Mancuso et al., 2019; Maphis et al., 2015; Shi et al., 2017; Shi et al., 2019; Stancu et al., 2019; Yoshiyama et al., 2007) and following tau seeding resulting in prion‐like seeding and propagation of tau pathology (Asai et al., 2015; Brelstaff et al., 2018; Gratuze et al., 2020; Ising et al., 2019; Kunkle et al., 2019; Mancuso et al., 2019; Shi et al., 2017; St George‐Hyslop & Morris, 2008; Stancu et al., 2019). Crucial roles for ApoE in microglia‐dependent and astrocyte‐dependent modulation of tau pathology and tau‐induced neurodegeneration, were recently demonstrated (Shi et al., 2017; Shi et al., 2019; Wang et al., 2021). A critical role of microglia in tau pathology and tau‐induced neurodegeneration is further underscored by the observation that microglial elimination using colony stimulating factor 1 receptor inhibition in a tauopathy model decreased tau‐pathology and tau‐induced neurodegeneration (Mancuso et al., 2019). We and others have previously demonstrated that the NLRP3‐ASC (Apoptosis‐associated speck‐like protein containing a C‐terminal caspase recruitment domain—ASC) inflammasome is activated by tau and tau‐aggregates and contributes to tau pathology in non‐seeded as well as in tau‐seeded conditions (Ising et al., 2019; Stancu et al., 2019), further strengthening the potential of NLRP3 as therapeutic target in AD and tauopathies. However, the role of the NLRP3‐ASC inflammasome on neurodegeneration downstream of tau pathology remains unknown, while absolutely crucial to evaluate its therapeutic potential.
While tau aggregation is strongly associated with neurodegeneration and symptom progression in tauopathies, it is important to emphasize existing differences in the underlying processes and tau forms considered as culprits. In inducible preclinical models, a dissociation between fully mature tau aggregates and neurodegeneration has been demonstrated (Green et al., 2019; Van der Jeugd et al., 2012). In these models, tau pathology continued to accumulate or remain present while a neurodegenerative phenotype was reversed after switching off transgene expression. Furthermore, some models of tau‐seeded tau pathology display a strong correlation with neurodegeneration while others do not (Clavaguera et al., 2009; Iba et al., 2015; Lodder et al., 2021; Peeraer et al., 2015), indicating that different tau forms than fully mature tau aggregates may account for the induced neurodegeneration, as well as the overall concentrations of pathological tau (Lasagna‐Reeves et al., 2012; Lasagna‐Reeves, Castillo‐Carranza, Jackson, & Kayed, 2011; Lasagna‐Reeves, Castillo‐Carranza, Sengupta, et al., 2011). Along the same line some studies have indicated that neuronal populations with tau aggregates display neuronal dysfunction, while other studies indicated that neurons with tau aggregates only display minor or no changes (Kopeikina et al., 2012; Kuchibhotla et al., 2014; Marinkovic et al., 2019; Stancu et al., 2015). These data support the general concept that tau‐aggregation leading to neurofibrillary tangles and tau‐associated neurodegeneration/neuronal dysfunction may be caused by different entities of tau. In this respect oligomeric tau forms and smaller soluble tau forms are regarded as important culprits (Kopeikina et al., 2012; Lasagna‐Reeves et al., 2012; Lasagna‐Reeves, Castillo‐Carranza, Jackson, & Kayed, 2011; Lasagna‐Reeves, Castillo‐Carranza, Sengupta, et al., 2011). The mechanism of tau‐induced neurodegeneration remains however to be unequivocally identified. Tau‐induced neurodegeneration may be dependent on cell‐autonomous processes ranging from synaptic dysfunction, axonal transport dysfunction, oxidative stress, mitochondrial dysfunction, calcium dysregulation and excitotoxicity [reviewed in (Gendron & Petrucelli, 2009)]. While also non‐cell autonomous processes, and particularly microglia‐dependent processes have been identified to contribute to tau‐induced neurodegeneration, supported by the identification of microglial roles in neurodegeneration in tauopathy models (Bellucci et al., 2011; Brown & Neher, 2014; Gratuze et al., 2020; Lodder et al., 2021; Mancuso et al., 2019; Pampuscenko et al., 2020; Shi et al., 2017; Shi et al., 2019; Yoshiyama et al., 2007). Indeed, microglial elimination affects not only tau pathology but also tau‐induced neurodegeneration in models of tauopathy (Bellucci et al., 2011; Brown & Neher, 2014; Gratuze et al., 2020; Lodder et al., 2021; Mancuso et al., 2019; Pampuscenko et al., 2020; Shi et al., 2017; Shi et al., 2019; Yoshiyama et al., 2007). Furthermore, microglia were demonstrated to respond to eat‐me signals induced by tau pathology bearing neurons, induced by phosphatidyl serine (Benetatos et al., 2020; Brelstaff et al., 2018). And, microglia may contribute to neuronal death by cytokine production and by increasing oxidative stress (Heneka et al., 2015; Ransohoff, 2016). In view of the important role of microglia in modulating tau and tau pathology as well as in processes involved in neurodegeneration, analyzing the role of NLRP3 in tau‐associated neurodegeneration is absolutely required, to evaluate its potential as therapeutic target.
Here, we aimed to extend our previous work (Stancu et al., 2019) using genetically modified mice, to analyze the effects of NLRP3 deficiency on tau‐induced neurodegeneration in tauP301S mice (Stancu et al., 2015; Yoshiyama et al., 2007), as a critical remaining question for NLRP3 targeting. We here demonstrate that NLRP3 modulates (i) tau pathology and (ii) prion‐like seeding and propagation of tau pathology upstream of ASC, using a genetic approach, that is, NLRP3 knockout mice. Most importantly, we here demonstrate for the first time that (iii) NLRP3 also modulates neurodegeneration downstream of tau pathology, both in the context of exogenously seeded tau pathology and in non‐exogenously seeded tau pathology. We believe these data are important for evaluating the potential of NLRP3 as therapeutic target for tauopathies, demonstrating that the NLRP3 inflammasome not only contributes to tau pathology and propagation of tau‐pathology but also to tau‐induced neurodegeneration.
2. MATERIALS AND METHODS
2.1. Animals
For this study, we have crossed the well‐characterized hemizygous tauP301S mice (PS19; B6;C3‐Tg[Prnp‐MAPT*P301S]PS19Vle/J; The Jackson Laboratory, stock no 021302), expressing human tauP301S (1N4R) driven by the mouse prion protein promoter (tau; T+) and homozygous NLR family, pyrin domain containing 3 (NLRP3) deficient mice (NLRP3−/−; B6.129S6‐Nlrp3tm1Bhk/J; The Jackson Laboratory, stock no 021302) to generate mice hemizygous for tauP301S and homozygous for NLRP3−/− (tau.NLRP3−/−; T+.N−/−) or NLRP3+/+ (tau.NLRP3+/+; T+.N+/+). Mice were genotyped by polymerase chain reaction (PCR) on the genomic DNA isolated from tail clips using the conditions indicated by The Jackson Laboratory (Bar Harbor, ME). Animals were maintained under standard animal housing conditions in a temperature‐controlled room (20 ± 3°C) on a 12‐h dark–light cycle with free access to food and water. All experiments were performed in compliance with the European Directive 2010/63/EU and were approved by the Ethics Committee for Animal Welfare of Hasselt University.
2.2. Tau seeding and stereotactic surgery
Tau seeds were generated as described previously (Lodder et al., 2021; Stancu et al., 2015; Stancu et al., 2019). Briefly, truncated human 4R tau containing the four‐repeat domain with the P301L mutation [K18; Q244‐E373], N‐terminally Myc tagged were produced in Escherichia coli. Tau‐seeds were obtained by incubation of monomeric K18 tau (66.7 μM) with low‐molecular weight heparin (MP Biomedicals, Santa Ana, CA, USA) in a ratio 1:2, for 5 days at 37°C. Following centrifugation (100,000 g, 1 h, 4°C) the pellet was resuspended in 100 mM ammonium acetate buffer (pH 7.0) without heparin, to a final concentration of 333 μM, aliquoted and stored at −80°C. Tau fibrilization was confirmed by Thioflavin T assay (Sigma‐Aldrich, St. Louis, MO, USA) and immunoblotting. For all experiments, tau seeds were sonicated (eight pulses of 30% amplitude) before use.
Mice were deeply anesthetized with an intraperitoneal injection of a mixture (2.5:4:12.5) of ketamine 10% wt/vol (Anesketin, Dechra), xylazine 2% wt/vol (Rompun, Bayer) and phosphate‐buffered saline (PBS), and placed in a stereotaxic frame (Kopf Instruments). Stereotactic injections of sonicated pre‐aggregated tau‐PFFs (5 μl; 333 μM) were performed unilaterally (right hemisphere) in the hippocampal CA1 region (A/P − 2.0 mm; L + 1.4 mm; D/V − 1.4 mm, relative to bregma) and frontal cortex (A/P + 2.0 mm; L + 1.4 mm; D/V − 1.0 mm, relative to bregma) of the 3.5 months old mice, using a 10 μl Hamilton syringe at a rate of 1 μl/min. The needle was kept in place for an additional 5 min after injection and withdrawn slowly. Following surgery, the animals were monitored until they recovered from the anesthesia and were housed individually for 48 h.
2.3. Immunohistological analysis
At 4.5 months after injection, the mice were transcardially perfused with ice cold PBS for 2 min. The brains were dissected and immersion fixed in 4% paraformaldehyde in PBS for 24 h at 4°C for histological analyses. Sagittal sections of 40 μm were cut on a vibrating HM650V microtome (Thermo Fisher Scientific, Waltham, MA, USA). Subsequent brain sections were selected based on the stereotaxic mouse brain atlas (Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates, 2012) for the various immunohistochemical staining starting from bregma lateral 0.84 mm. Immunohistochemistry was performed as described previously (Stancu et al., 2015, 2019). Briefly, the 40 μm free‐floating sections were blocked with 5% skim milk (Merk‐Millipore, Burlington, MA, USA) in PBS‐TritonX 0.05% (PBS‐T) for 1 h at RT followed by incubation with anti‐tau P‐S202/T205 (AT8; 1:200; Thermo Fisher Scientific), anti‐tau P‐T212/S214 (AT100; 1:800; Thermo Fisher Scientific) and anti‐neuronal nuclei (NeuN; 1:200; Merck‐Millipore) primary antibodies over night at 4°C or for 2 h at RT. Subsequently, after washes with PBS‐T, the brain slices were incubated with the appropriate AlexaFluor‐coupled secondary antibodies (1:500; Invitrogen) in 5% milk PBS‐T for 1 h at RT. Brain slices were mounted on glass slides using Fluoromount aqueous mounting medium (Sigma‐Aldrich) and cover slipped.
Images were acquired with a Leica DM400 B LED fluorescence microscope (Leica, Wetzlar, DE) equipped with a DFC450C camera. All images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) blinded to the genotype of the mice. All acquired images were subjected to the same computer subroutines to minimize investigator bias. For quantification of AT8‐ and AT100‐ positive area, automatic thresholding methods were applied throughout analysis, either using Triangle or MaxEntropy algorithms. Quantitative analysis of tau pathology was performed by measuring the stained area relative to the total image area of the brain regions of interest, using Image J software.
2.4. Hippocampal measurements
Hippocampal area measurement was performed on full hippocampus images, stained with NeuN, of well‐defined sagittal sections at 1.32 mm lateral from bregma. The contour of the hippocampus was done manually, blinded to the genotype of the mice, and the results were expressed as the percent of the area occupied by the hippocampus relative to the total image area, using Image J software.
The area and width of the NeuN stained neuronal layers of hippocampal CA1 region were measured on the NeuN stained brain sections, detailed above, using higher magnification images (×10). In the Image J program, the images were calibrated using the scale bar, the contour of the NeuN positive CA1 region was drawn manually, blinded to the genotype of the mice, and the area was calculated in mm2. Using the same settings, the width of the NeuN positive CA1 neuronal layers was measured at five different locations along the CA1 region (at a distance of approximatively 150–200 μm between them) and the average width was calculated and used for statistical analysis.
2.5. Brain homogenization and biochemical analysis
The hippocampi of the T+.N+/+ and T+.N−/− mice were homogenized with a potter‐type mechanical homogenizer (VOS 14S40, rate: 750 rpm; VWR International bv, Leuven, Belgium) in 10 weight‐volumes of ice‐cold Tris‐proteinase‐phosphatase‐inhibitor buffer (TPPI‐buffer), containing 25 mM Tris–HCl (pH 8.1), 150 mM sodium chloride (Sigma‐Aldrich), 1 mM ethylene diamine tetra‐acetic acid (Merck), 1 mM ethylene glycol tetra‐acetic acid (Sigma‐Aldrich), 5 mM sodium pyrophosphate (Sigma‐Aldrich), 5 mM sodium fluoride (Sigma‐Aldrich), 1 mM phenylmethylsulfonyl fluoride (Sigma‐Aldrich), 1 mM sodium vanadate (Sigma‐Aldrich), and a cocktail of proteinase inhibitors and phosphatase inhibitors (Roche, Sigma‐Aldrich). The hippocampal total homogenates were aliquoted and stored at −32°C until further used.
The levels of tau aggregates from the hippocampal total homogenates were measured using the homogeneous time resolved fluorescence (HTRF) tau aggregation kit (6FTAUPEG; Cisbio, Perkin‐Elmer, FR) following the manufacturer protocol. Briefly, for each sample, 10 μL of the hippocampal total homogenates was diluted 1/4 and 1/8, and added on a 384‐well plate (Cisbio). Subsequently, 5 μl of anti‐tau‐d2 antibody and 5 μl of anti‐tau‐tb antibody were mixed and added to each well. Positive control (synthetic aggregated human tau) and negative (tb) control were provided by the manufacturer (Cisbio). After incubation overnight at room temperature the HTRF signals were measured with a TECAN Safire 2 microplate reader (Tecan Group Ltd., CH). The levels of aggregated tau (Delta F%) were calculated using the ratios of the two emission signals (620 and 665 nm), according to the manufacturer formula.
2.6. Statistical analysis
Statistical analysis was performed in GraphPad Prism v9.2.0 (GraphPad Software, Inc., San Diego, USA). Data were analyzed with using unpaired Welch's t‐test. Results were presented as mean ± standard error of the mean (SEM). Statistical significance was considered for p value <.05 (*p < .05, **p < .01, ***p < .001, ****p < .0001).
3. RESULTS
3.1. NLRP3 modulates tau pathology and tau‐associated neurodegeneration in tauP301S mice, a tauopathy model
To analyze the role of the NLRP3 inflammasome in tau pathology and neurodegeneration, strongly correlating but not fully overlapping processes in tauopathies, we here generated crosses of tauP301S mice (PS19) with NLRP3 deficient mice. Tau transgenic mice develop tau pathology in the hippocampus, cortex and brainstem, followed by neurodegeneration, reflected in hippocampal atrophy at early stages at around 10–11 months of age and cortical atrophy in later stages (Stancu et al., 2015; Yoshiyama et al., 2007). Cross‐breeding was performed to obtain tau.NLRP3+/+ and tau.NLRP3−/− littermates as verified by PCR‐based genotyping (Figure S1). Tau pathology was assessed at 10 months of age, when tau pathology and the neurodegenerative phenotype develop in this model. We first assessed tau pathology by measuring phosphorylated tau (p‐tau) in hippocampus using immunostaining with AT100 (p‐tau Thr212/Ser214). This revealed significantly decreased hippocampal tau pathology in the absence of NLRP3 compared to the presence of NLRP3 in tauP301S mice (Figure 1b,c). Next, we assessed tau‐pathology using AT8 immunostaining (p‐tau Ser202/Thr205) demonstrating significantly decreased tau pathology in hippocampus in tau.NLRP3−/− mice compared to tau.NLRP3+/+ mice (Figure 1a,c). To further analyze whether NLRP3 modulates tau pathology in different brain regions, we measured AT100 stained tau pathology in frontal cortex (Figure S2a) and brainstem (Figure S2b), demonstrating significantly decreased tau pathology in the absence of NLRP3 (Figure S2c), albeit less strongly reduced compared to hippocampus. The latter may reflect regional or stage‐dependent differences in neuroinflammation and its effects on tau pathology, while tau pathology assessed by AT100 demonstrated significant differences in the absence of NLRP3 in all brain regions (Figure 1c, Figure S2c).
FIGURE 1.
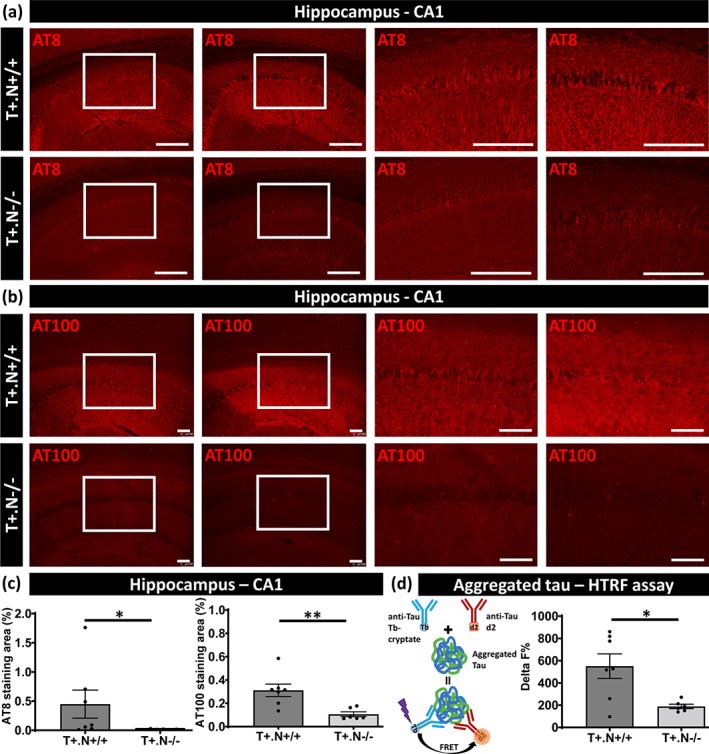
Decreased tau pathology in NLRP3‐deficient tau mice. Representative images of AT8 (anti‐tau P‐S202/T205) (a) and AT100 (anti‐tau P‐T212/S214) (b) immunostaining of the hippocampal CA1 region of tau.NLRP3−/− (T+.N−/−, lower panels) and tau.NLRP3+/+ (T+.N+/+, upper panels) mice. Higher magnifications of selected areas (white squared boxes) are shown on the right. (c) Quantitative analysis of immunostaining showed significantly decreased levels of tau phosphorylation in the CA1 region of the hippocampus of 10‐month‐old T+.N−/− (lower panels) versus T+.N+/+ (upper panels) mice. (d) Biochemical analysis of tau aggregation, by homogeneous time resolved fluorescence (HTRF) assay (left side), showed significantly decreased levels of tau aggregates in the NLRP3‐deficient tau (T+.N−/−) compared to wild‐type NLRP3 tau (T+.N+/+) mice (right side). Data are shown as mean ± SEM (T+.N+/+: N = 7; T+.N−/−: n = 6; *p < .05, **p < .01; unpaired Welch's t‐test; AT8, bar = 250 μm; AT100, bar = 100 μm)
To further assess tau alterations, we used a biochemical assay measuring aggregated tau, using a HTRF based assay, in hippocampal extracts. In this assay, tau aggregates are measured using a sandwich immunoassay with an anti‐tau monoclonal antibody as donor and acceptor. When donor and acceptor are in close proximity, the excitation of the HTRF donor will lead to an energy transfer (FRET) to the HTRF acceptor, generating a specific HTRF signal proportional to the amount of tau aggregates (Figure 1d). We have previously validated this technique in our cohort demonstrating a strong correlation between tau aggregation assessed with immunofluorescence based and HTRF based assays. Aggregated tau measured by HTRF was significantly decreased in hippocampal extracts of tau.NLRP3−/− compared to tau.NLRP3+/+ mice, confirming with a biochemical assay a role of NLRP3 in tau aggregation (Figure 1d).
In this work, we were particularly interested in the effect of NLRP3 deficiency on hippocampal atrophy and neurodegeneration, which is consistently detected in tauP301S mice (Stancu et al., 2014; Stancu et al., 2015; Yoshiyama et al., 2007). Previous studies have demonstrated that tauP301S mice develop a neurodegenerative phenotype starting at 10–11 months of age. This phenotype includes hippocampal atrophy and is followed by cortical atrophy in the final stages of a neurodegenerative phenotype (Stancu et al., 2014; Stancu et al., 2015; Yoshiyama et al., 2007). We measured total hippocampal area, the widths of NeuN positive CA1 neuronal layers and the NeuN positive CA1 area in well‐defined sagittal sections of tau.NLRP3+/+ compared to tau.NLRP3−/− mice. We found significantly decreased hippocampal atrophy assessed by quantitative analysis of hippocampal area, following NeuN staining, in tau.NLRP3−/− mice (Figure 2a,b). Moreover, we found significantly decreased NeuN positive CA1 neuronal layers widths and NeuN positive CA1 area in tau.NLRP3+/+ mice (Figure 2c,d). Taken together our data indicate that NLRP3 modulated tau pathology and tau‐induced hippocampal atrophy and neurodegeneration in tauP301S mice.
FIGURE 2.
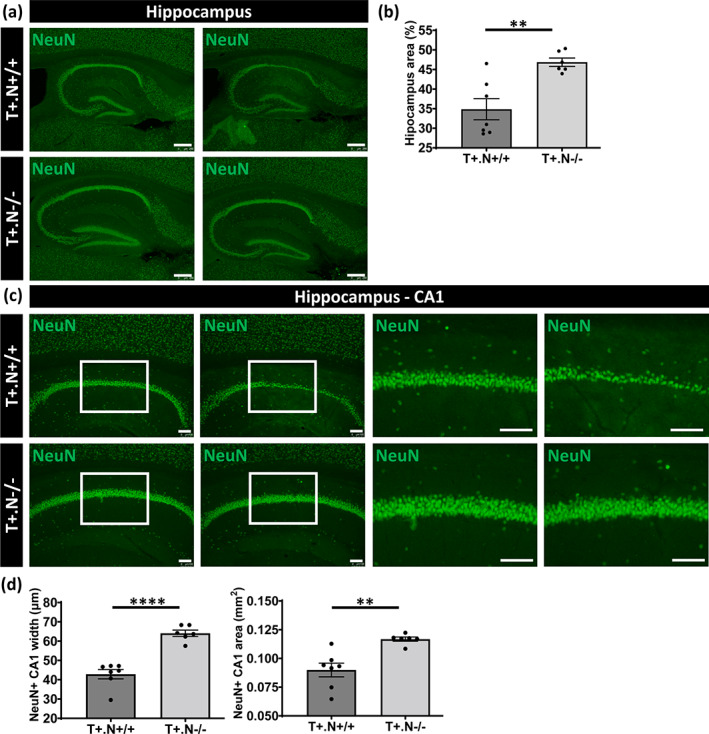
Hippocampal atrophy and neurodegeneration in tau.NLRP3 mice. (a) Representative images of NeuN (neuronal nuclear antigen) staining of the hippocampus in well‐defined brain slices of 10‐month‐old tau.NLRP3−/− (T+.N−/−, lower panels) and tau.NLRP3+/+ (T+.N+/+, upper panels), used for measuring the hippocampal area. (b) Quantitative analysis showed significantly decreased total hippocampal area in T+.N+/+ compared to T+.N−/− mice. (c) Representative images of NeuN immunostaining of the hippocampal CA1 region of T+.N−/− (lower panels) and T+.N+/+ (upper panels) mice. Higher magnifications of selected areas (white squared boxes) are shown on the right. (d) Quantitative analysis showed significantly decreased width of the NeuN positive (NeuN+) neuronal layers in the CA1 region (left side) and decreased NeuN+ CA1 area (right side). Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 6; *p < .05, **p < .01; unpaired Welch's t‐test; hippocampus, bar = 250 μm; CA1 region, bar = 100 μm)
3.2. NLRP3 modulates exogenously seeded tau pathology and prion‐like propagation in a tauopathy model
Prion‐like propagation of tau pathology has been unequivocally demonstrated in a variety of in vitro and in vivo models and is considered a compelling mechanism underlying characteristic spatio‐temporal progression of tau pathology in AD and related tauopathies (Clavaguera et al., 2009; Goedert, 2015, 2020; Guo et al., 2016; Guo & Lee, 2011; Jucker & Walker, 2018; Mudher et al., 2017; Walker & Jucker, 2015). We here assessed the effect of genetic deficiency of NLRP3 on tau‐seeded and prion‐like propagation of tau pathology in tauP301S transgenic mice, using a well‐characterized tau seeding model (Guo et al., 2016; Guo & Lee, 2011; Peeraer et al., 2015; Stancu et al., 2015). We performed tau seeding in tau.NLRP3−/− and tau.NLRP3+/+ mice at 3.5 months of age and analyzed their phenotype 4.5 months later, at 8 months of age (Figure 3a). Tau seeding was performed using intracerebral injection of pre‐aggregated tau seeds in hippocampus and cortex as previously published (Guo et al., 2016; Guo & Lee, 2011; Lodder et al., 2021; Peeraer et al., 2015; Stancu et al., 2015). In the current paradigm we used a tau seeding period of 4.5 months and dissection at 8 months, to obtain a strong induction of tau pathology and associated neurodegeneration (Figure 3a). This resulted in strong tau pathology and hippocampal atrophy before the development of hippocampal atrophy in the parental strains at 10–11 months of age.
FIGURE 3.
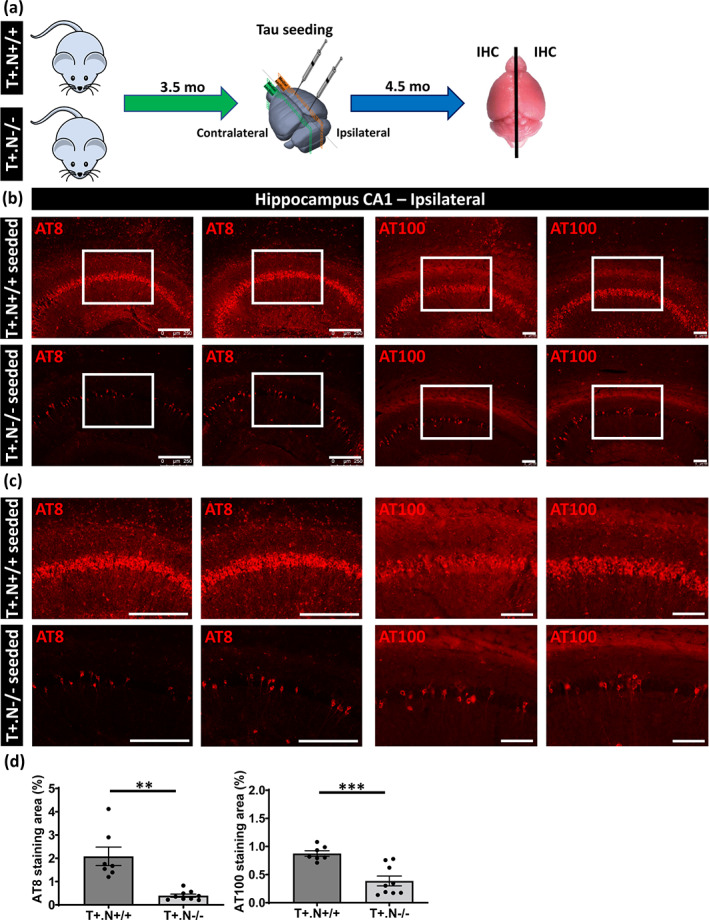
Decreased tau‐seeded tau pathology in absence of NLRP3 in tau mice. (a) Schematic representation of the in vivo tau seeding protocol. Tau seeds were injected unilaterally in the CA1 region of the hippocampus and frontal cortex of tau.NLRP3−/− (T+.N−/−) and tau.NLRP3+/+ (T+.N+/+) mice at 3.5 months of age and were sacrificed at 8 months of age (4.5 months post‐injection). (b) Representative images of AT8 (anti‐tau P‐S202/T205, left panels) and AT100 (anti‐tau P‐T212/S214, right panels) immunolabeling of the ipsilateral hippocampus showing significantly decreased levels of tau phosphorylation in the CA1 region of tau‐seeded T+.N−/− (lower panels) versus tau‐seeded T+.N+/+ (upper panels) mice. (c) Higher magnification of the CA1 region (corresponding to the white squared boxes in b) are shown. (d) Quantitative analysis showed significantly decreased AT8‐ and AT100‐stained area in tau‐seeded T+.N−/− versus tau‐seeded T+.N+/+ mice. Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 9; **p < .01, ***p < .001; unpaired Welch's t‐test: AT8, bar = 250 μm; AT100, bar = 100 μm)
We first assessed tau‐seeded tau pathology using immunostaining with AT100 (p‐tau Thr212/Ser214), at the ipsilateral site followed by quantitative analysis. Tau seeding induced a strong tau pathology in the ipsilateral hippocampus in tau.NLRP3+/+, similar as previously demonstrated (Figure 3b,c). Quantitative analysis demonstrated significantly less AT100 staining in the hippocampus of tau.NLRP3−/− compared to tau.NLRP3+/+ mice following tau seeding at the ipsilateral side (Figure 3d). Assessment of tau pathology by AT8 (p‐tau Ser202/Thr205) staining revealed significantly less tau pathology in the absence of NLRP3, in the ipsilateral hippocampal region (Figure 3b–d). Similarly, tau pathology following tau seeding in the frontal cortex was significantly less in tau.NLRP3−/− compared to tau.NLRP3+/+ mice at the ipsilateral side when assessed by AT100 or AT8 staining (Figure 4a–c). However, it must be noted that the effect of NLRP3 deficiency in cortex, similar to non‐exogenously seeded tau pathology, seemed less strong compared to hippocampus, indicating that neuroinflammatory processes in hippocampus and cortex may be differentially affected. Different microglia‐dependent and NLRP3‐dependent processes may contribute together to modulate tau pathology and may be differently regulated depending on the brain region, the inflammatory status, the region‐specific tau alterations, the microglial populations present and their activation state, resulting in regional differences of the NLRP3‐dependent effects.
FIGURE 4.
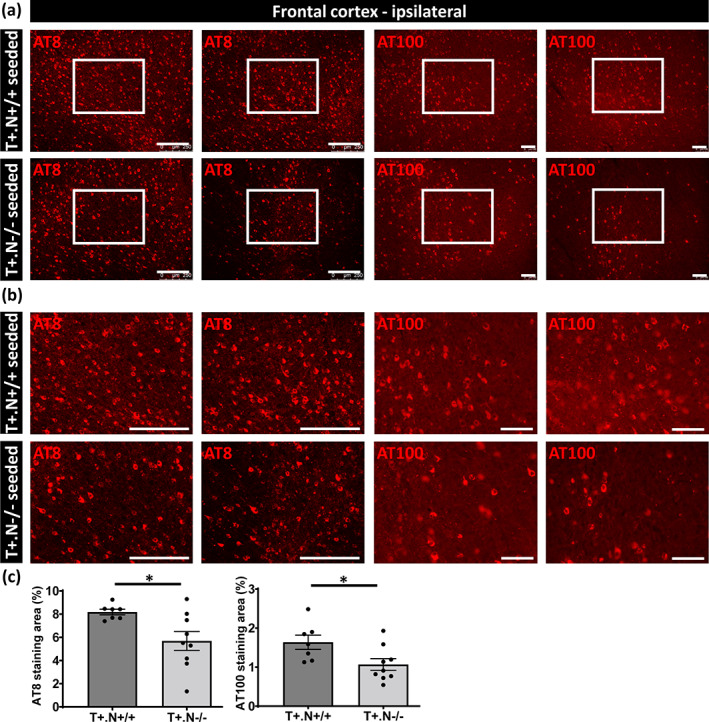
Decreased tau pathology in frontal cortex of tau‐seeded NLRP3‐deficient tau mice. (a) Representative images of AT8 (anti‐tau P‐S202/T205, left panels) and AT100 (anti‐tau P‐T212/S214, right panels) immunolabeling of the ipsilateral frontal cortex of tau.NLRP3−/− (T+.N−/−) and tau.NLRP3+/+ (T+.N+/+), showing decreased levels of tau phosphorylation in tau‐seeded T+.N−/− (lower panels) versus tau‐seeded T+.N+/+ (upper panels) mice. (b) Higher magnification of the ipsilateral frontal cortex region (corresponding to the white squared boxes in a) are shown. (c) Quantitative analysis showed significantly decreased AT8‐ and AT100‐positive area in the ipsilateral frontal cortex of tau‐seeded T+.N−/− versus tau‐seeded T+.N+/+ mice. Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 9; *p < .05; unpaired Welch's t‐test; AT8, bar = 250 μm; AT100, bar = 100 μm)
Next, NLRP3‐dependent effects on spreading of tau pathology were analyzed by measuring tau pathology (AT8 and AT100) in the hippocampus at the contralateral side (Figure 5a,b). Tau pathology in the contralateral hippocampus was significantly less in tau.NLRP3−/− mice compared to tau.NLRP3+/+ mice (Figure 5c). Furthermore, spreading of tau pathology to the contralateral side was significantly decreased in cortex assessed by AT8 and AT100 staining (Figure 6a–c). Staining for pathological tau using AT100 demonstrated significant decrease in NLRP3 deficient compared to wild type tauP301S mice in brainstem, reflecting effects on spreading to brain stem from the injection side (Figure S3). Taken together, our combined data indicate that propagation of tau‐seeded tau‐pathology was significantly decreased in the absence of NLRP3.
FIGURE 5.
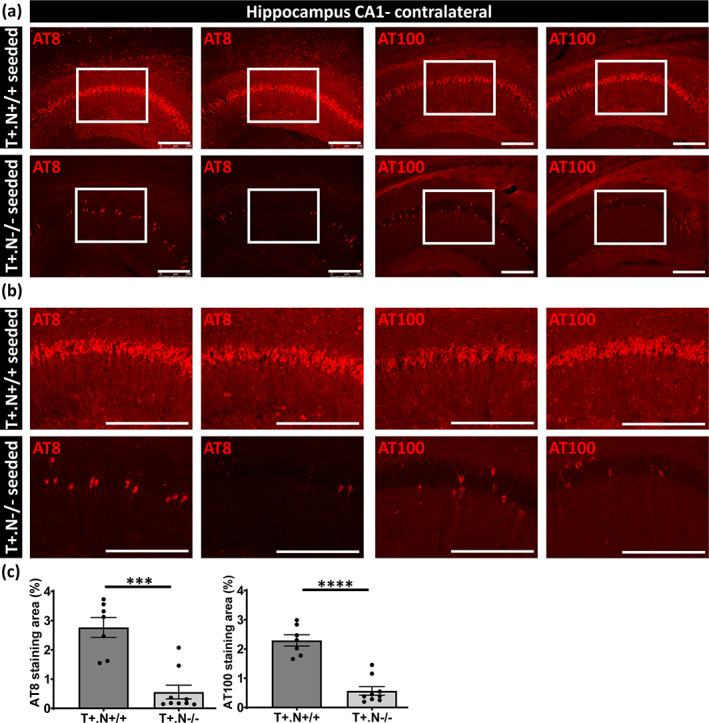
Decreased spreading of tau pathology in tau seeded NLRP3‐deficient tau mice. (a) Representative images of AT8 (anti‐tau P‐S202/T205, left panels) and AT100 (anti‐tau P‐T212/S214, right panels) immunolabeling of the contralateral CA1 hippocampal region of tau.NLRP3−/− (T+.N−/−) and tau.NLRP3+/+ (T+.N+/+), showing significantly decreased levels of tau phosphorylation of the tau seeded T+.N−/− (lower panels) versus tau seeded T+.N+/+ (upper panels) mice. (b) Higher magnification of the CA1 region (corresponding to the white squared boxes in a) are shown. (c) Quantitative analysis showed significantly decreased AT8‐ and AT100‐positive area in the contralateral CA1 region of the tau‐seeded T+.N−/− versus tau‐seeded T+.N+/+ mice. Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 9; **p < .01, ***p < .001; unpaired Welch's t‐test; bar = 250 μm)
FIGURE 6.
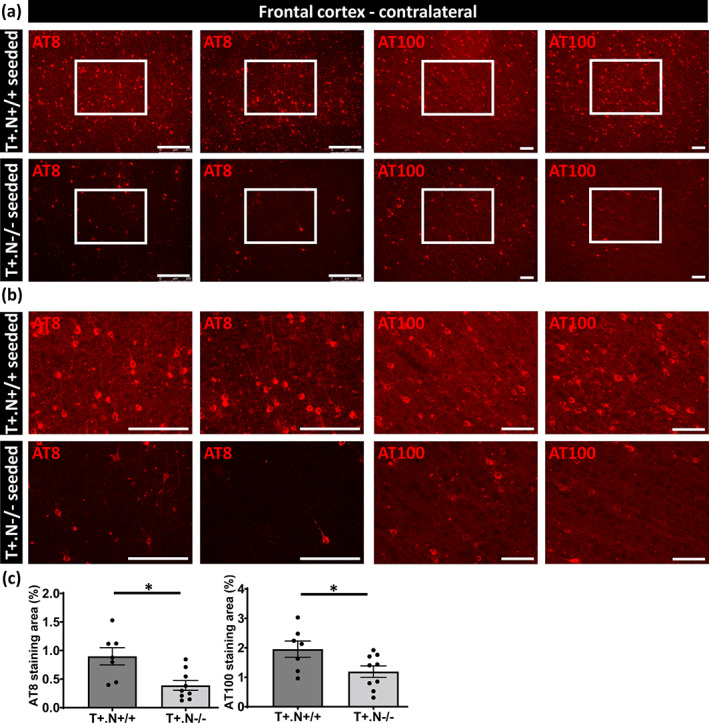
Decreased spreading of tau pathology in frontal cortex of tau‐seeded NLRP3‐deficient tau mice. (a) Representative images of AT8 (anti‐tau P‐S202/T205, left panels) and AT100 (anti‐tau P‐T212/S214, right panels) immunolabeling of the contralateral frontal cortex region of tau.NLRP3−/− (T+.N−/−) and tau.NLRP3+/+ (T+.N+/+), showing decreased levels of tau phosphorylation in the tau‐seeded T+.N−/− (lower panels) versus tau‐seeded T+.N+/+ (upper panels) mice. (b) Higher magnification of the contralateral frontal cortex region (corresponding to the white squared boxes in a) are shown. (c) Quantitative analysis showed significantly decreased AT8‐ and AT100‐positive area in the contralateral frontal cortex of the tau‐seeded T+.N−/− versus tau‐seeded T+.N+/+ mice. Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 9; *p < .05; unpaired Welch's t‐test; AT8, bar = 250 μm; AT100, bar = 100 μm)
Finally, we assessed whether NLRP3 modulated tau‐associated neurodegeneration, by measuring hippocampal atrophy, the widths of the NeuN positive CA1 neuronal layers and the NeuN positive CA1 area in tau‐seeded tau.NLRP3+/+ and tau.NLRP3−/− mice. Hippocampal area was measured following NeuN staining of the ipsilateral site (Figure 7a,c). This demonstrated a significantly increased in total hippocampal area in the absence of NLRP3 in tau‐seeded tauP301S mice at 8 months of age, 4.5 months post tau seeding (Figure 7c). Moreover, we found significantly decreased NeuN positive CA1 neuronal layers widths and decreased NeuN positive CA1 area in tau.NLRP3+/+ mice (Figure 7e,f). To assess whether NLRP3 deficiency affected hippocampal atrophy and neurodegeneration following propagation of tau pathology, we next measured total hippocampal area following NeuN staining at the contralateral side (Figure 7b,d). This demonstrated a significantly increased area of the contralateral hippocampus in the tau‐seeded tau.NLRP3−/− compared to tau.NLRP3+/+ mice (Figure 7d). Similarly, we found significantly increased NeuN positive CA1 neuronal layers widths and increased NeuN positive CA1 area in the contralateral hippocampus in tau‐seeded tau.NLRP3−/− compared to tau.NLRP3+/+ mice (Figure S4a,b). No significant atrophy was detected in cortical or other brain regions following tau‐seeding in presence or absence of NLRP3, precluding analysis of the modulatory effect of NLRP3 on neurodegeneration in these regions. Taken together our data indicate a modulatory role for NLRP3 in seeded tau pathology and its propagation. Furthermore, we here found that NLRP3 modulates early‐stage hippocampal atrophy following seeding and propagation of tau pathology in hippocampus.
FIGURE 7.
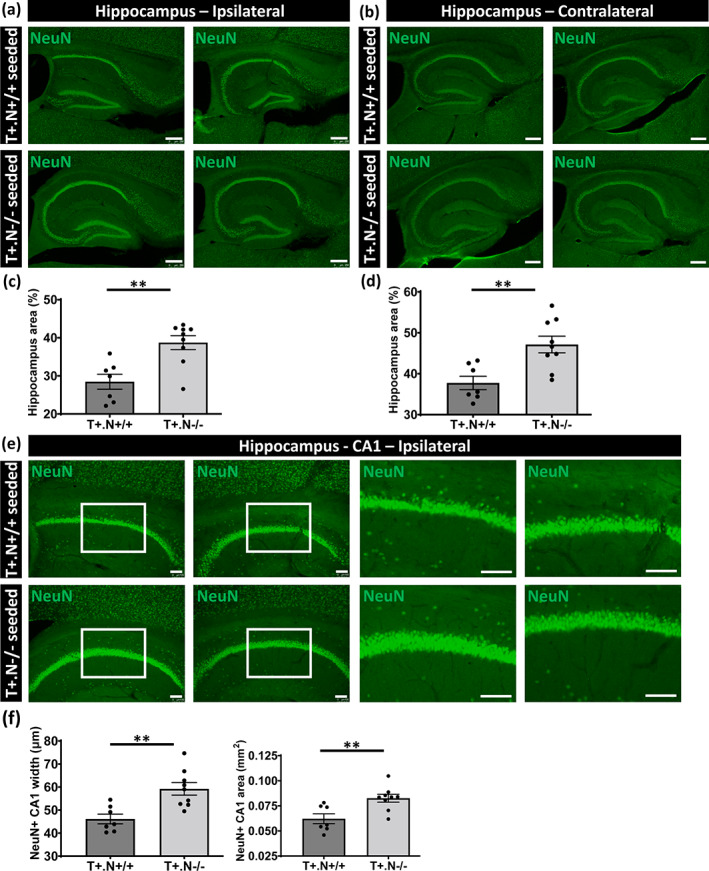
Hippocampal atrophy and neurodegeneration in tau‐seeded NLRP3‐deficient tau mice. (a, b) Representative images of NeuN (neuronal nuclear antigen) staining of the ipsi‐ (a) and contra‐ (b) lateral hippocampus in well‐defined brain slices of exogenously seeded tau in tau.NLRP3+/+ (T+.N+/+, upper panels) and tau.NLRP3−/− (T+.N−/−, lower panels) mice, used for measuring the total hippocampal area. (c, d) Quantitative analysis showed significantly decreased total hippocampal area, in both ipsi‐ (c) and contra‐ (d) lateral sides, in tau‐seeded T+.N+/+ versus T+.N−/− mice. (e) Representative images of NeuN immunostaining of the ipsilateral hippocampal CA1 region of T+.N+/+ (upper panels) and T+.N−/− (lower panels) mice. Higher magnifications of selected areas (white squared boxes) are shown on the right. (f) Quantitative analysis showed significantly decreased width of the NeuN positive (NeuN+) neuronal layers in the CA1 region (left side) and decreased NeuN+CA1 area (right side) in the ipsilateral side in exogenously seeded tau T+.N+/+ versus T+.N−/− mice . Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 9; **p < .01, ***p < .001; unpaired Welch's t‐test; hippocampus, bar = 250 μm; CA1 region, bar = 100 μm)
4. DISCUSSION
The contributory role of neuroinflammation in neurodegenerative diseases and in tauopathies specifically is increasingly demonstrated (Gerhard et al., 2004, 2006; Gratuze et al., 2020; Heneka et al., 2015; Ising et al., 2019; Leyns et al., 2019; Lodder et al., 2021; Mancuso et al., 2019; Pampuscenko et al., 2020; Ransohoff, 2016; Shi et al., 2017; Shi et al., 2019; Stancu et al., 2019; Venegas et al., 2017; Yoshiyama et al., 2007). Furthermore, NLRP3 presents as an interesting target for AD, as it modulates amyloid pathology (Halle et al., 2008; Heneka et al., 2013; Venegas et al., 2017). And a role of the NLRP3 inflammasome in tau pathology was previously identified (Ising et al., 2019; Stancu et al., 2019), further strengthening its role as therapeutic target in AD and related tauopathies. However, the modulatory role of NLRP3 on tau‐induced neurodegeneration remained unknown. Here we demonstrate that NLRP3 modulates non‐seeded and tau seeded tau pathology and its propagation, using NLRP3 knockout mice and we demonstrate for the first time that NLRP3 modulates tau induced neurodegeneration.
We and others have previously demonstrated that tau and pre‐aggregated tau activates the NLRP3‐ ASC inflammasome (Ising et al., 2019; Jiang et al., 2021; Stancu et al., 2019). We previously demonstrated a modulatory role of the NLRP3‐ASC inflammasome on seeded and non‐seeded tau pathology using ASC knockout and pharmacological NLRP3 inhibition in mice (Stancu et al., 2019). Independent demonstration of a modulatory role of the NLRP3 inflammasome on non‐seeded tau pathology was demonstrated by Ising and colleagues (Ising et al., 2019). Here, we aimed to (i) confirm the role of NLRP3 in tau pathology and (ii) in prion‐like seeding and propagation of tau pathology using NLRP3 deficient mice and ‐ most importantly‐ (iii) to assess the modulatory role of NLRP3 inflammasome activation on tau‐induced neurodegeneration in a model of tauopathy. We found that NLRP3 deficiency decreased tau pathology in hippocampus assessed by using AT8 and AT100. Our data thereby highlight a role of NLRP3 upstream of ASC, in line with previous data (Ising et al., 2019; Stancu et al., 2019). We next assessed the role of NLRP3 in a well‐characterized model of seeded tau pathology (Stancu et al., 2015, 2019). We found decreased tau‐seeded and propagated tau pathology in NLRP3 deficient tauP301S mice. This confirmed our previous findings using pharmacological inhibition of NLRP3, using chronic intracerebral delivery of MCC950, in a model of seeded tau pathology (Stancu et al., 2019), here using NLRP3 knockout in mice. It must be noted that although NLRP3 deficiency affected tau pathology in all brain regions examined, this was most profoundly the case in the hippocampus, highlighting brain region‐dependent processes. It must be considered that different microglia‐dependent processes—in addition to neuronal characteristics—may contribute to modulation of tau pathology. NLRP3 inflammasome activation may contribute to tau pathology by (i) secretion of cytokines (including IL1β) which may affect tau phosphorylation (Bhaskar et al., 2010; Ghosh et al., 2013; Li et al., 2003; Maphis et al., 2015), by (ii) inducing ASC speck formation (Venegas et al., 2017), (iii) by phagocytosing tau (Ising et al., 2019; Jiang et al., 2021; Stancu et al., 2019) and by (iv) modifying proliferation/cell death/differentiation of microglial populations which can differentially contribute to tau pathology. The overall modulation of NLRP3 on tau pathology will be defined by the resultant effects of these different microglia‐dependent processes, which are modulated by NLRP3. This overall effect of these microglia‐dependent processes may be defined by the brain region, the stage of inflammation, the type and concentration of tau seeds, the alterations of acceptor tau and the microglial populations present. The pleiotropic effects of microglia and the diverse microglial populations present in different brain regions and at different stages of pathology, add a layer of complexity to their study. A detailed study of their function is therefore of utmost importance to understand their role at different stages of pathology. Our data indicate the potential of NLRP3 as target for tauopathies in modulating tau pathology, and seeding and propagation of tau pathology.
Importantly, we here demonstrate for the first time a role of NLRP3 in tau‐associated hippocampal atrophy and neurodegeneration. This extends our findings to tau‐associated neurodegeneration, an important and relevant finding in the context of tauopathies, as halting of tau‐induced neurodegeneration presents a key target. Although the NLRP3 inflammasome was previously shown to affect tau pathology and tau aggregation (Ising et al., 2019; Stancu et al., 2019), it is important to assess its role in tau‐induced neurodegeneration. Different processes, including cell‐autonomous and non‐cell autonomous processes may contribute to tau associated neurodegeneration and add fuel to what constitutes neuronal vulnerability. Furthermore, different tau species may contribute to tau pathology and tau‐induced neurodegeneration, hence a dissociation between both processes may exist. Previously the role of microglia in tau associated neurodegeneration has been identified (Bellucci et al., 2011; Brown & Neher, 2014; Gratuze et al., 2020; Lodder et al., 2021; Mancuso et al., 2019; Pampuscenko et al., 2020; Shi et al., 2017; Shi et al., 2019; Yoshiyama et al., 2007). NLRP3 may affect tau‐associated neurodegeneration by modulating concentrations of tau forms intra‐ and extracellularly. Tau can be phagocytosed by microglia (Ising et al., 2019; Jiang et al., 2021; Stancu et al., 2019), but alternatively ASC specks similar as for Aβ may facilitate tau aggregation (Jiang et al., 2021; Venegas et al., 2017), and cytokines may increase phosphorylated tau forms intracellularly increasing toxic tau species (Bhaskar et al., 2010; Ghosh et al., 2013; Li et al., 2003; Maphis et al., 2015). Furthermore, microglial proliferation, differentiation and death can be modulated by NLRP3 activation induced by tau (Heneka et al., 2018), thereby shifting microglial functions and potentially their role in neurodegeneration. Our data indicate that NLRP3 dependent pathways are promising targets, meriting further analysis and validation for their therapeutic potential in tauopathies.
Taken together, the NLRP3 inflammasome is increasingly identified as therapeutic target in neurodegenerative diseases. We here demonstrate that the NLRP3 inflammasome modulates non‐seeded and tau‐seeded tau pathology and its propagation, using NLRP3 deficient mice. Most importantly, we demonstrate here for the first time that NLRP3 modulates tau‐induced neurodegeneration. This is a crucial finding in view of the fact that different tau forms and different microglial processes are involved in tau pathology, its propagation and tau‐induced neurodegeneration, and hence may be differently affected by NLRP3. These processes, and particularly neurodegeneration, present key‐targets for tauopathy therapies, indicating the necessity to analyze the role of NLRP3 in all these pathogenic processes. Our findings provide important missing information indicating that the NLRP3 inflammasome contributes to tau pathology, to prion‐like spreading of tau pathology but also to neurodegeneration downstream of tau pathology. Our work thereby contributes to crucial insights in the potential of targeting NLRP3‐dependent pathways as therapeutic targets in tauopathies.
CONFLICT OF INTEREST
The authors declare no conflict of interest. Astrid Bottelbergs is an employee of Janssen Pharmaceutica NV, Belgium.
AUTHOR CONTRIBUTIONS
I. Dewachter, A. Bottelbergs and I.C. Stancu contributed to conception, design and guidance of the study. I.C. Stancu, C. Lodder, P. Botella Lucena performed analysis of the seeded and non‐seeded tau.NLRP3 model. All authors, I.C. Stancu, C. Lodder, P. Botella Lucena, S. Vanherle, M. Gutiérrez de Ravé, D. Terwel, A. Bottelbergs and I. Dewachter participated in generating experimental data, data analysis and interpretation of data. I. Dewachter, I.C. Stancu, C. Lodder, P. Botella Lucena drafted the manuscript and generated the figures. I.C. Stancu, C. Lodder and P. Botella Lucena contributed equally to the manuscript. All authors read and approved the final manuscript.
Supporting information
Figure S1 Generation of the mice used in the study. Schematic representation of the cross‐breeding of tauP301S tg mice (T+) with NLRP3‐deficient (NLRP3−/−) mice to obtain NLRP3‐deficient (T+.NLRP3−/−) and NLRP3 wild‐type (T+.NLRP3+/+) tau mice (left). Representative gel of the genotyping performed by polymerase chain reaction (The Jackson Laboratory protocol) on the genomic DNA isolated from tail clips of the heterozygous tauP301S tg mice, which are respectively heterozygous for NLRP3 (T+.NLRP3+/−), homozygous for NLRP3−/− (T+.NLRP3−/−) and homozygous for NLRP3 wild‐type (T+.NLRP3+/+) on the right.
Figure S2 Decreased tau pathology in frontal cortex and brainstem of NLRP3‐deficient tau mice. (a, b) Representative images of AT100 (anti‐tau P‐T212/S214) immunostaining in frontal cortex (a) and brainstem (b) of tau.NLRP3−/− (T+.N−/−) and tau.NLRP3+/+ (T+.N+/+) mice showing decreased levels of tau phosphorylation in 10‐month‐old T+.N−/− (lower panels) versus T+.N+/+ (upper panels) mice. Higher magnifications of selected areas (white squared boxes) are shown on the right. (c) Quantitative analysis showed significantly decreased AT100‐positive area in the frontal cortex (left panel) and brainstem (right panel) regions of the T+.N−/− versus T+.N+/+ mice. Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 6; *p < 0.05; unpaired Welch's t‐test; bar = 100 μm).
Figure S3 Decreased spreading of tau pathology in the brainstem of tau‐seeded NLRP3‐deficient tau mice. (a) Representative images of AT100 (anti‐tau P‐T212/S214) immunolabeling of the brainstem of exogenously tau‐seeded tau.NLRP3+/+ (T+.N+/+) and tau.NLRP3−/− (T+.N−/−) mice, showing decreased levels of tau‐pathology in tau‐seeded T+.N−/− (lower panels) versus tau‐seeded T+.N+/+ (upper panels) mice. (b) Quantitative analysis showed significantly decreased AT100‐positive area in the brainstem of tauseeded T+.N−/− versus tau‐seeded T+.N+/+ mice. Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N‐ /−: n = 9; *p < 0.05; unpaired Welch's t‐test; AT8, bar = 250 μm; AT100, bar = 100 μm).
Figure S4 Hippocampal CA1 neurodegeneration in the contralateral side of tau‐seeded NLRP3‐ deficient tau mice. (a) Representative images of NeuN immunostaining of the contralateral hippocampal CA1 region of exogenously tau‐seeded tau.NLRP3+/+ (T+.N+/+, upper panels) and tau.NLRP3−/− (T+.N−/−, lower panels) mice. Higher magnifications of selected areas (white squared boxes) are shown on the right. (b) Quantitative analysis showed significantly decreased width of the NeuN positive (NeuN+) neuronal layers in the CA1 region (left side) and decreased NeuN+ CA1 area (right side) in the contralateral side in exogenously seeded tau T+.N+/+ versus T+.N−/− mice . Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 9; **p < 0.01, ***p < 0.001; unpaired Welch's t‐test; bar = 100 μm).
ACKNOWLEDGMENTS
This work was supported by Vlaams Agentschap Innoveren en Ondernemen (VLAIO)—Research project No. HBC.2017.0948, Stichting Alzheimer Onderzoek (SAO) and Fonds Wetenschappelijk Onderzoek ‐ Vlaanderen (FWO)—Research project No. G0C6819N and FWO Postdoc Fellowship No. 1259621N.
Stancu, I. C. , Lodder, C. , Botella Lucena, P. , Vanherle, S. , Gutiérrez de Ravé, M. , Terwel, D. , Bottelbergs, A. , & Dewachter, I. (2022). The NLRP3 inflammasome modulates tau pathology and neurodegeneration in a tauopathy model. Glia, 70(6), 1117–1132. 10.1002/glia.24160
Ilie Cosmin Stancu, Chritica Lodder and Pablo BotellaLucena contributed equally to this work.
Funding information Fonds Wetenschappelijk Onderzoek, Grant/Award Numbers: 1259621N, G0C6819N; Stichting Alzheimer Onderzoek (SAO), Belgium; Vlaams Agentschap Innoveren en Ondernemen (VLAIO), Grant/Award Number: HBC.2017.0948
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are included in the published and in the supplementary material of this article.
REFERENCES
- Asai, H. , Ikezu, S. , Tsunoda, S. , Medalla, M. , Luebke, J. , Haydar, T. , Wolozin, B. , Butovsky, O. , Kügler, S. , & Ikezu, T. (2015). Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nature Neuroscience, 18(11), 1584–1593. 10.1038/nn.4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci, A. , Bugiani, O. , Ghetti, B. , & Spillantini, M. G. (2011). Presence of reactive microglia and neuroinflammatory mediators in a case of frontotemporal dementia with P301S mutation. Neurodegenerative Diseases, 8(4), 221–229. 10.1159/000322228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetatos, J. , Bennett, R. E. , Evans, H. T. , Ellis, S. A. , Hyman, B. T. , Bodea, L. G. , & Gotz, J. (2020). PTEN activation contributes to neuronal and synaptic engulfment by microglia in tauopathy. Acta Neuropathologica, 140(1), 7–24. 10.1007/s00401-020-02151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar, K. , Konerth, M. , Kokiko‐Cochran, O. N. , Cardona, A. , Ransohoff, R. M. , & Lamb, B. T. (2010). Regulation of tau pathology by the microglial fractalkine receptor. Neuron, 68(1), 19–31. 10.1016/j.neuron.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelstaff, J. , Tolkovsky, A. M. , Ghetti, B. , Goedert, M. , & Spillantini, M. G. (2018). Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Reports, 24(8), 1939–1948. 10.1016/j.celrep.2018.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, G. C. , & Neher, J. J. (2014). Microglial phagocytosis of live neurons. Nature Reviews Neuroscience, 15(4), 209–216. 10.1038/nrn3710 [DOI] [PubMed] [Google Scholar]
- Clavaguera, F. , Bolmont, T. , Crowther, R. A. , Abramowski, D. , Frank, S. , Probst, A. , Fraser, G. , Stalder, A. K. , Beibel, M. , Staufenbiel, M. , Jucker, M. , Goedert, M. , & Tolnay, M. (2009). Transmission and spreading of tauopathy in transgenic mouse brain. Nature Cell Biology, 11(7), 909–913. 10.1038/ncb1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger, B. N. , & Dickson, D. W. (2017). Pathology of Neurodegenerative Diseases. Cold Spring Harbor Perspectives in Biology, 9(7), a028035. 10.1101/cshperspect.a028035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron, T. F. , & Petrucelli, L. (2009). The role of tau in neurodegeneration. Molecular Neurodegeneration, 4, 13. 10.1186/1750-1326-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard, A. , Trender‐Gerhard, I. , Turkheimer, F. , Quinn, N. P. , Bhatia, K. P. , & Brooks, D. J. (2006). In vivo imaging of microglial activation with [11C](R)‐PK11195 PET in progressive supranuclear palsy. Movement Disorders, 21(1), 89–93. 10.1002/mds.20668 [DOI] [PubMed] [Google Scholar]
- Gerhard, A. , Watts, J. , Trender‐Gerhard, I. , Turkheimer, F. , Banati, R. B. , Bhatia, K. , & Brooks, D. J. (2004). In vivo imaging of microglial activation with [11C](R)‐PK11195 PET in corticobasal degeneration. Movement Disorders, 19(10), 1221–1226. 10.1002/mds.20162 [DOI] [PubMed] [Google Scholar]
- Ghosh, S. , Wu, M. D. , Shaftel, S. S. , Kyrkanides, S. , LaFerla, F. M. , Olschowka, J. A. , & O'Banion, M. K. (2013). Sustained interleukin‐1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. The Journal of Neuroscience, 33(11), 5053–5064. 10.1523/JNEUROSCI.4361-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert, M. (2015). NEURODEGENERATION. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Abeta, tau, and alpha‐synuclein. Science, 349(6248), 1255555. 10.1126/science.1255555 [DOI] [PubMed] [Google Scholar]
- Goedert, M. (2020). Tau proteinopathies and the prion concept. Progress in Molecular Biology and Translational Science, 175, 239–259. 10.1016/bs.pmbts.2020.08.003 [DOI] [PubMed] [Google Scholar]
- Gratuze, M. , Leyns, C. E. G. , Sauerbeck, A. D. , St‐Pierre, M.‐K. , Xiong, M. , Kim, N. , Serrano, J. R. , Tremblay, M.‐È. , Kummer, T. T. , Colonna, M. , Ulrich, J. D. , & Holtzman, D. M. (2020). Impact of TREM2R47H variant on tau pathology–induced gliosis and neurodegeneration. Journal of Clinical Investigation, 130(9), 4954–4968. 10.1172/jci138179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, C. , Sydow, A. , Vogel, S. , Anglada‐Huguet, M. , Wiedermann, D. , Mandelkow, E. , Mandelkow, E.‐M. , & Hoehn, M. (2019). Functional networks are impaired by elevated tau‐protein but reversible in a regulatable Alzheimer’s disease mouse model. Molecular Neurodegeneration, 14(1), 13. 10.1186/s13024-019-0316-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J. L. , & Lee, V. M. (2011). Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer‐like tangles. The Journal of Biological Chemistry, 286(17), 15317–15331. 10.1074/jbc.M110.209296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J. L. , Narasimhan, S. , Changolkar, L. , He, Z. , Stieber, A. , Zhang, B. , Gathagan, R. J. , Iba, M. , & McBride, J. D. , Trojanowski, J. Q. , & Lee, V. M.Y. (2016). Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. Journal of Experimental Medicine, 213(12), 2635–2654. 10.1084/jem.20160833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle, A. , Hornung, V. , Petzold, G. C. , Stewart, C. R. , Monks, B. G. , Reinheckel, T. , Fitzgerald, K. A. , Latz, E. , Moore, K. J. , & Golenbock, D. T. (2008). The NALP3 inflammasome is involved in the innate immune response to amyloid‐β. Nature Immunology, 9(8), 857–865. 10.1038/ni.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , Carson, M. J. , El Khoury, J. , Landreth, G. E. , Brosseron, F. , Feinstein, D. L. , Jacobs, A. H. , Wyss‐Coray, T. , Vitorica, J. , Ransohoff, R. M. , Herrup, K. , Frautschy, S. A. , Finsen, B. , Brown, G. C. , Verkhratsky, A. , Yamanaka, K. , Koistinaho, J. , Latz, E. , Halle, A. , … Kummer, M. P. (2015). Neuroinflammation in Alzheimer's disease. The Lancet Neurology, 14(4), 388–405. 10.1016/s1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira‐Saecker A., Griep A., Axt D., Remus A., Tzeng T.‐C., Gelpi E., Halle A., Korte M., Latz E., & Golenbock D. T. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature, 493(7434), 674–678. 10.1038/nature11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , McManus, R. M. , & Latz, E. (2018). Inflammasome signalling in brain function and neurodegenerative disease. Nature Reviews Neuroscience, 19(10), 610–621. 10.1038/s41583-018-0055-7 [DOI] [PubMed] [Google Scholar]
- Iba, M. , McBride, J. D. , Guo, J. L. , Zhang, B. , Trojanowski, J. Q. , & Lee, V. M. (2015). Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC's afferent and efferent connections. Acta Neuropathologica, 130(3), 349–362. 10.1007/s00401-015-1458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa, K. , & Dickson, D. W. (2001). Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. Journal of Neuropathology and Experimental Neurology, 60(6), 647–657. 10.1093/jnen/60.6.647 [DOI] [PubMed] [Google Scholar]
- Ising, C. , Venegas, C. , Zhang, S. , Scheiblich, H. , Schmidt, S. V. , Vieira‐Saecker, A. , Schwartz, S. , Albasset, S. , McManus, R. M. , Tejera, D. , Griep, A. , Santarelli, F. , Brosseron, F. , Opitz, S. , Stunden, J. , Merten, M. , Kayed, R. , Golenbock, D. T. , Blum, D. , … Heneka, M. T. (2019). NLRP3 inflammasome activation drives tau pathology. Nature, 575(7784), 669–673. 10.1038/s41586-019-1769-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R., Jr. , Bennett, D. A. , Blennow, K. , Carrillo, M. C. , Dunn, B. , Haeberlein, S. B. , Holtzman, D. M. , Jagust, W. , Jessen, F. , Karlawish, J. , Liu, E. , Molinuevo, J. L. , Montine, T. , Phelps, C. , Rankin, K. P. , Rowe, C. C. , Scheltens, P. , Siemers. E. , Snyder. H. M. , … Silverberg N., (2018). NIA‐AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia, 14(4), 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R., Jr. , Bennett, D. A. , Blennow, K. , Carrillo, M. C. , Feldman, H. H. , Frisoni, G. B. , Hampel, H. , Jagust, W. J. , Johnson, K. A. , Knopman, D. S. , Petersen, R. C. , Scheltens, P. , Sperling, R. A. , & Dubois, B. (2016). A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology, 87(5), 539–547. 10.1212/wnl.0000000000002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, S. , Maphis, N. M. , Binder, J. , Chisholm, D. , Weston, L. , Duran, W. , Peterson, C. , Zimmerman, A. , Mandell, M. A. , Jett, S. D. , Bigio, E. , Geula, C. , Mellios, N. , Weick, J. P. , Rosenberg, G. A. , Latz, E. , Heneka, M. T. , & Bhaskar, K. (2021). Proteopathic tau primes and activates interleukin‐1β via myeloid‐cell‐specific MyD88‐ and NLRP3‐ASC‐inflammasome pathway. Cell Reports, 36(12), 109720. 10.1016/j.celrep.2021.109720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker, M. , & Walker, L. C. (2018). Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nature Neuroscience, 21(10), 1341–1349. 10.1038/s41593-018-0238-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina, K. J. , Hyman, B. T. , & Spires‐Jones, T. L. (2012). Soluble forms of tau are toxic in Alzheimer's disease. Translational Neuroscience, 3(3), 223–233. 10.2478/s13380-012-0032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla, K. V. , Wegmann, S. , Kopeikina, K. J. , Hawkes, J. , Rudinskiy, N. , Andermann, M. L. , Spires‐Jones, T. L. , Bacskai, B. J. , & Hyman, B. T. (2014). Neurofibrillary tangle‐bearing neurons are functionally integrated in cortical circuits in vivo. Proceedings of the National Academy of Sciences, 111(1), 510–514. 10.1073/pnas.1318807111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle, B. W. , Grenier‐Boley, B. , Sims, R. , Bis, J. C. , Damotte, V. , Naj, A. C. , Boland, A. , Vronskaya, M. , van der Lee, S. J. , Amlie‐Wolf, A. , Bellenguez, C. , Frizatti A., Chouraki V., Martin E. R., Sleegers K., Badarinarayan, N. , Jakobsdottir, J. , Hamilton‐Nelson, K. L. , Moreno‐Grau, S. , … Pericak‐Vance, M. A. (2019). Genetic meta‐analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nature Genetics, 51(3), 414–430. 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna‐Reeves, C. A. , Castillo‐Carranza, D. L. , Jackson, G. R. , & Kayed, R. (2011). Tau oligomers as potential targets for immunotherapy for Alzheimer's disease and tauopathies. Current Alzheimer Research, 8(6), 659–665. 10.2174/156720511796717177 [DOI] [PubMed] [Google Scholar]
- Lasagna‐Reeves, C. A. , Castillo‐Carranza, D. L. , Sengupta, U. , Clos, A. L. , Jackson, G. R. , & Kayed, R. (2011). Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild‐type mice. Molecular Neurodegeneration, 6, 39. 10.1186/1750-1326-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna‐Reeves, C. A. , Castillo‐Carranza, D. L. , Sengupta, U. , Sarmiento, J. , Troncoso, J. , Jackson, G. R. , & Kayed, R. (2012). Identification of oligomers at early stages of tau aggregation in Alzheimer's disease. The FASEB Journal, 26(5), 1946–1959. 10.1096/fj.11-199851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns, C. E. G. , Gratuze, M. , Narasimhan, S. , Jain, N. , Koscal, L. J. , Jiang, H. , Manis, M. , Colonna, M. , Lee, V. M. Y. , Ulrich, J. D. , & Holtzman, D. M. (2019). TREM2 function impedes tau seeding in neuritic plaques. Nature Neuroscience, 22(8), 1217–1222. 10.1038/s41593-019-0433-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Liu, L. , Barger, S. W. , & Griffin, W. S. (2003). Interleukin‐1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38‐MAPK pathway. The Journal of Neuroscience, 23(5), 1605–1611. 10.1523/JNEUROSCI.23-05-01605.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder, C. , Scheyltjens, I. , Stancu, I. C. , Botella Lucena, P. , Gutiérrez de Ravé, M. , Vanherle, S. , Vanmierlo, T. , Cremers, N. , Vanrusselt, H. , Brône, B. , Hanseeuw, B. , Octave, J.‐N. , Bottelbergs, A. , Movahedi, K. , & Dewachter, I. (2021). CSF1R inhibition rescues tau pathology and neurodegeneration in an A/T/N model with combined AD pathologies, while preserving plaque associated microglia. Acta Neuropathologica Communications, 9(1), 108. 10.1186/s40478-021-01204-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso, R. , Fryatt, G. , Cleal, M. , Obst, J. , Pipi, E. , Monzón‐Sandoval, J. , Ribe, E. , Winchester, L. , Webber, C. , Nevado, A. , Jacobs, T. , Austin, N. , Theunis, C. , Grauwen, K. , Daniela Ruiz, E. , Mudher, A. , Vicente‐Rodriguez, M. , Parker, C. A. , Simmons C., & Perry V. H. (2019). CSF1R inhibitor JNJ‐40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain, 142(10), 3243–3264. 10.1093/brain/awz241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maphis, N. , Xu, G. , Kokiko‐Cochran, O. N. , Jiang, S. , Cardona, A. , Ransohoff, R. M. , Lamb, B. T. , & Bhaskar, K. (2015). Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain, 138(6), 1738–1755. 10.1093/brain/awv081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic, P. , Blumenstock, S. , Goltstein, P. M. , Korzhova, V. , Peters, F. , Knebl, A. , & Herms, J. (2019). In vivo imaging reveals reduced activity of neuronal circuits in a mouse tauopathy model. Brain, 142(4), 1051–1062. 10.1093/brain/awz035 [DOI] [PubMed] [Google Scholar]
- Mudher, A. , Colin, M. , Dujardin, S. , Medina, M. , Dewachter, I. , Alavi Naini, S. M. , Mandelkow, E.‐M. , Mandelkow, E. , Buée, L. , Goedert, M. , & Brion, J.‐P. (2017). What is the evidence that tau pathology spreads through prion‐like propagation?. Acta Neuropathologica Communications, 5(1), 99. 10.1186/s40478-017-0488-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampuscenko, K. , Morkuniene, R. , Sneideris, T. , Smirnovas, V. , Budvytyte, R. , Valincius, G. , Brown, G. C. , & Borutaite, V. (2020). Extracellular tau induces microglial phagocytosis of living neurons in cell cultures. Journal of Neurochemistry, 154(3), 316–329. 10.1111/jnc.14940 [DOI] [PubMed] [Google Scholar]
- Peeraer, E. , Bottelbergs, A. , Van Kolen, K. , Stancu, I.‐C. , Vasconcelos, B. , Mahieu, M. , Duytschaever, H. , Ver Donck, L. , Torremans, A. , Sluydts, E. , Van Acker, N. , Kemp, J. A. , Mercken, M. , Brunden, K. R. , Trojanowski, J. Q. , Dewachter, I. , Lee, V. M.Y. , & Moechars, D. (2015). Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiology of Disease, 73, 83–95. 10.1016/j.nbd.2014.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff, R. M. (2016). How neuroinflammation contributes to neurodegeneration. Science, 353(6301), 777–783. 10.1126/science.aag2590 [DOI] [PubMed] [Google Scholar]
- Serrano‐Pozo, A. , Frosch, M. P. , Masliah, E. , & Hyman, B. T. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 1(1), a006189. 10.1101/cshperspect.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Manis, M. , Long, J. , Wang, K. , Sullivan, P. M. , Remolina Serrano, J. , Hoyle, R. , & Holtzman, D. M. (2019). Microglia drive APOE‐dependent neurodegeneration in a tauopathy mouse model. Journal of Experimental Medicine, 216(11), 2546–2561. 10.1084/jem.20190980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Yamada, K. , Liddelow, S. A. , Smith, S. T. , Zhao, L. , Luo, W. , Tsai, R. M. , Spina, S. , Grinberg, L. T. , Rojas, J. C. , Gallardo, G. , Wang, K. , Roh, J. , Robinson, G. , Finn, M. B. , Jiang, H. , Sullivan, P. M. , Baufeld, C. , Wood, M. W. , … Holtzman, D. M. (2017). ApoE4 markedly exacerbates tau‐mediated neurodegeneration in a mouse model of tauopathy. Nature, 549(7673), 523 527. 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George‐Hyslop, P. H. , & Morris, J. C. (2008). Will anti‐amyloid therapies work for Alzheimer's disease? Lancet, 372(9634), 180–182. 10.1016/S0140-6736(08)61047-8 [DOI] [PubMed] [Google Scholar]
- Stancu I.‐C., Cremers N., Vanrusselt H., Couturier J., Vanoosthuyse A., Kessels S., Lodder C., Brône B., Huaux F., Octave J.‐N., Terwel D., & Dewachter I. (2019). Aggregated Tau activates NLRP3–ASC inflammasome exacerbating exogenously seeded and non‐exogenously seeded Tau pathology in vivo. Acta Neuropathologica, 137(4), 599–617. 10.1007/s00401-018-01957-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancu I.‐C., Ris L., Vasconcelos B., Marinangeli C., Goeminne L., Laporte V., Haylani L. E., Couturier J., Schakman O., Gailly P., Pierrot N., Kienlen‐Campard P., Octave J.‐N., & Dewachter I. (2014). Tauopathy contributes to synaptic and cognitive deficits in a murine model for Alzheimer's disease. The FASEB Journal, 28(6), 2620–2631. 10.1096/fj.13-246702 [DOI] [PubMed] [Google Scholar]
- Stancu, I.‐C. , Vasconcelos, B. , Ris, L. , Wang, P. , Villers, A. , Peeraer, E. , Buist, A. , Terwel, D. , Baatsen, P. , Oyelami, T. , Pierrot, N. , Casteels, C. , Bormans, G. , Kienlen‐Campard, P. , Octave, J.‐N. , Moechars, D. , & Dewachter, I. (2015). Templated misfolding of Tau by prion‐like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in Tau transgenic mice. Acta Neuropathologica, 129(6), 875–894. 10.1007/s00401-015-1413-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Jeugd, A. , Hochgräfe, K. , Ahmed, T. , Decker, J. M. , Sydow, A. , Hofmann, A. , Wu, D. , Messing, L. , Balschun, D. , D’Hooge, R. , & Mandelkow, E.‐M. (2012). Cognitive defects are reversible in inducible mice expressing pro‐aggregant full‐length human Tau. Acta Neuropathologica, 123(6), 787–805. 10.1007/s00401-012-0987-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas, C. , Kumar, S. , Franklin, B. S. , Dierkes, T. , Brinkschulte, R. , Tejera, D. , Vieira‐Saecker, A. , Schwartz, S. , Santarelli, F. , Kummer, M. P. , Griep, A. , Gelpi, E. , Beilharz, M. , Riedel, D. , Golenbock, D. T. , Geyer, M. , Walter, J. , Latz, E. , & Heneka, M. T. (2017). Microglia‐derived ASC specks cross‐seed amyloid‐β in Alzheimer’s disease. Nature, 552(7685), 355–361. 10.1038/nature25158 [DOI] [PubMed] [Google Scholar]
- Walker, L. C. , & Jucker, M. (2015). Neurodegenerative diseases: Expanding the prion concept. Annual Review of Neuroscience, 38, 87–103. 10.1146/annurev-neuro-071714-033828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Xiong, M. , Gratuze, M. , Bao, X. , Shi, Y. , Andhey, P. S. , Manis, M. , Schroeder, C. , Yin, Z. , Madore, C. , Butovsky, O. , Artyomov, M. , Ulrich, J. D. , & Holtzman, D. M. (2021). Selective removal of astrocytic APOE4 strongly protects against tau‐mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron, 109(10), 1657–1674. 10.1016/j.neuron.2021.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama, Y. , Higuchi, M. , Zhang, B. , Huang, S.‐M. , Iwata, N. , Saido, T. C. , Maeda, J. , Suhara, T. , Trojanowski, J. Q. , & Lee, V. M.‐Y. (2007). Synapse Loss and Microglial Activation Precede Tangles in a P301S Tauopathy Mouse Model. Neuron, 53(3), 337–351. 10.1016/j.neuron.2007.01.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Generation of the mice used in the study. Schematic representation of the cross‐breeding of tauP301S tg mice (T+) with NLRP3‐deficient (NLRP3−/−) mice to obtain NLRP3‐deficient (T+.NLRP3−/−) and NLRP3 wild‐type (T+.NLRP3+/+) tau mice (left). Representative gel of the genotyping performed by polymerase chain reaction (The Jackson Laboratory protocol) on the genomic DNA isolated from tail clips of the heterozygous tauP301S tg mice, which are respectively heterozygous for NLRP3 (T+.NLRP3+/−), homozygous for NLRP3−/− (T+.NLRP3−/−) and homozygous for NLRP3 wild‐type (T+.NLRP3+/+) on the right.
Figure S2 Decreased tau pathology in frontal cortex and brainstem of NLRP3‐deficient tau mice. (a, b) Representative images of AT100 (anti‐tau P‐T212/S214) immunostaining in frontal cortex (a) and brainstem (b) of tau.NLRP3−/− (T+.N−/−) and tau.NLRP3+/+ (T+.N+/+) mice showing decreased levels of tau phosphorylation in 10‐month‐old T+.N−/− (lower panels) versus T+.N+/+ (upper panels) mice. Higher magnifications of selected areas (white squared boxes) are shown on the right. (c) Quantitative analysis showed significantly decreased AT100‐positive area in the frontal cortex (left panel) and brainstem (right panel) regions of the T+.N−/− versus T+.N+/+ mice. Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 6; *p < 0.05; unpaired Welch's t‐test; bar = 100 μm).
Figure S3 Decreased spreading of tau pathology in the brainstem of tau‐seeded NLRP3‐deficient tau mice. (a) Representative images of AT100 (anti‐tau P‐T212/S214) immunolabeling of the brainstem of exogenously tau‐seeded tau.NLRP3+/+ (T+.N+/+) and tau.NLRP3−/− (T+.N−/−) mice, showing decreased levels of tau‐pathology in tau‐seeded T+.N−/− (lower panels) versus tau‐seeded T+.N+/+ (upper panels) mice. (b) Quantitative analysis showed significantly decreased AT100‐positive area in the brainstem of tauseeded T+.N−/− versus tau‐seeded T+.N+/+ mice. Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N‐ /−: n = 9; *p < 0.05; unpaired Welch's t‐test; AT8, bar = 250 μm; AT100, bar = 100 μm).
Figure S4 Hippocampal CA1 neurodegeneration in the contralateral side of tau‐seeded NLRP3‐ deficient tau mice. (a) Representative images of NeuN immunostaining of the contralateral hippocampal CA1 region of exogenously tau‐seeded tau.NLRP3+/+ (T+.N+/+, upper panels) and tau.NLRP3−/− (T+.N−/−, lower panels) mice. Higher magnifications of selected areas (white squared boxes) are shown on the right. (b) Quantitative analysis showed significantly decreased width of the NeuN positive (NeuN+) neuronal layers in the CA1 region (left side) and decreased NeuN+ CA1 area (right side) in the contralateral side in exogenously seeded tau T+.N+/+ versus T+.N−/− mice . Data are shown as mean ± SEM (T+.N+/+: n = 7; T+.N−/−: n = 9; **p < 0.01, ***p < 0.001; unpaired Welch's t‐test; bar = 100 μm).
Data Availability Statement
The data that supports the findings of this study are included in the published and in the supplementary material of this article.


