Abstract
Subtidal marine sediments are one of the planet's primary carbon stores and strongly influence the oceanic sink for atmospheric CO2. By far the most widespread human activity occurring on the seabed is bottom trawling/dredging for fish and shellfish. A global first‐order estimate suggested mobile demersal fishing activities may cause 0.16–0.4 Gt of organic carbon (OC) to be remineralized annually from seabed sediment carbon stores (Sala et al., 2021). There are, however, many uncertainties in this calculation. Here, we discuss the potential drivers of change in seabed sediment OC stores due to mobile demersal fishing activities and conduct a literature review, synthesizing studies where this interaction has been directly investigated. Under certain environmental settings, we hypothesize that mobile demersal fishing would reduce OC in seabed stores due to lower production of flora and fauna, the loss of fine flocculent material, increased sediment resuspension, mixing and transport and increased oxygen exposure. Reductions would be offset to varying extents by reduced faunal bioturbation and community respiration, increased off‐shelf transport and increases in primary production from the resuspension of nutrients. Studies which directly investigated the impact of demersal fishing on OC stocks had mixed results. A finding of no significant effect was reported in 61% of 49 investigations; 29% reported lower OC due to fishing activities, with 10% reporting higher OC. In relation to remineralization rates within the seabed, four investigations reported that demersal fishing activities decreased remineralization, with three reporting higher remineralization rates. Patterns in the environmental and experimental characteristics between different outcomes were largely indistinct. More evidence is urgently needed to accurately quantify the impact of anthropogenic physical disturbance on seabed carbon in different environmental settings and to incorporate full evidence‐based carbon considerations into global seabed management.
Keywords: blue carbon, carbon, carbon storage, dredging, fishing, marine, sediment, trawling
Subtidal marine sediments are one of the planet's primary carbon stores and strongly influence the oceanic sink for atmospheric CO2. Considering basic principles, we hypothesize that under certain environmental settings, trawling/dredging for fish and shellfish would reduce seabed sediment carbon storage; however, this may be offset by positive feedback mechanisms in some circumstances. A review of studies which directly investigated this impact in situ also highlighted mixed results. More evidence is urgently needed to accurately quantify this impact in different environmental settings, and incorporate full evidence‐based carbon considerations into global seabed management.

1. INTRODUCTION
Through a mixture of physical, chemical and biological processes, the ocean has absorbed ~40% of anthropogenic CO2 emissions since the industrial revolution (Gruber et al., 2019; Sabine & Tanhua, 2010). The term ‘blue carbon’ describes the ability of marine ecosystems to absorb CO2 from the atmosphere or water column, assimilate this inorganic carbon (IC) into organic compounds and isolate it from remineralization for centennial to millennial timescales (Nellemann et al., 2009). This process of carbon capture is key to maintaining the ecological functioning of the ocean (Bauer et al., 2013) and is beneficial as a sink for anthropogenic CO2 (Gruber et al., 2019; Khatiwala et al., 2009; Watson et al., 2020).
Research on blue carbon initially focused on the coastal vegetated habitats of mangroves, seagrass and saltmarsh, due to their ability to fix CO2 directly, trap external organic and inorganic materials, store high concentrations of organic carbon (OC) in situ within underlying sediments and accrete at high rates (Duarte et al., 2013; McLeod et al., 2011). Although these habitats have among the highest carbon sequestration rates on the planet per unit area (Duarte et al., 2013), with rates considerably higher than forests on land (McLeod et al., 2011), their limited spatial scale of approximately 1 million km2 or ~0.2% of the ocean's surface means they only contain a small proportion of the ocean's total OC stock (Atwood et al., 2020; Duarte, 2017; Duarte et al., 2013; Howard et al., 2017; Macreadie et al., 2021; Nellemann et al., 2009).
Primary production by phytoplankton is at par with terrestrial primary production (Field et al., 1998) and constitutes a major flux between the oceanic IC pool, hence the atmosphere, and the oceanic OC pool (Sarmiento & Gruber, 2006). A variable fraction of this newly produced OC is exported to depths below 1000 m, where this pelagic OC may become isolated from atmospheric exchange processes for centennial timescales (Caldeira et al., 2002; Krause‐Jensen & Duarte, 2016; Nellemann et al., 2009; Siegel et al., 2021). Whether this pelagic OC stock can be better protected or enhanced, and the duration of time for which it remains isolated from atmospheric exchange under different environmental settings is still uncertain. Pelagic OC is therefore infrequently used in the quantification of marine carbon storage and rarely classified as blue carbon (Caldeira et al., 2002; Lovelock & Duarte, 2019; Siegel et al., 2021). That withstanding subtidal marine sediments contain the ocean's biggest OC store, estimated to hold ~87 Gt of OC (1 Gt = 1 Pg = 1015 g) in the upper 5 cm (Lee et al., 2019) or ~2300 Gt of OC in the top 1 m (Atwood et al., 2020). Quantification of annual burial rates in these sediments is poorly constrained; however, they have been estimated globally at approximately 0.12–0.35 Gt OC year−1 (Berner, 1982; Burdige, 2007; Keil, 2017; Lee et al., 2019; Seiter et al., 2004).
Seabed sediments are subjected to a wide range of direct physical impacts from human pressures, namely, shipping, mineral extraction, fishing, energy developments, deployment of cables and pipelines, coastal development, dredging of shipping access channels and disposal of dredge spoil (Halpern et al., 2019; O’Hara et al., 2021). By far the most widespread source of disturbance is bottom trawling and dredging for fish and shellfish (Amoroso et al., 2018; Eigaard et al., 2017; Kroodsma et al., 2018; O’Hara et al., 2021; Oberle, Storlazzi, & Hanebuth, 2016). These pressures are pervasive and long‐lasting, with improved technologies over the last two centuries, and in particular since the 1950s, increasing the spread of mobile fishing gears to deeper waters and much of the global ocean (Kroodsma et al., 2018; Morato et al., 2006; Roberts, 2007; Watson & Morato, 2013). Compared to many other types of stressors, in intensively fished areas, trawling and dredging can also occur on the same area of seabed numerous times in a year (Eigaard et al., 2017; Hinz et al., 2009; Oberle, Storlazzi, & Hanebuth, 2016; Tillin et al., 2006).
Globally, fishing pressure with mobile demersal gear is concentrated in subtidal areas in coastal habitats and offshore on continental shelves and slopes at depths above 1000 m (Amoroso et al., 2018; Kroodsma et al., 2018). In total, these areas cover around 9% of the global seabed, yet they store an estimated 360 Gt OC in their top 1 m of sediment (Atwood et al., 2020) and are estimated to account for up to 86% of all OC that is buried annually in global subtidal sediments (Atwood et al., 2020; Berner, 1982; Seiter et al., 2004).
Mobile demersal fishing activity significantly alters seabed faunal communities (Hiddink et al., 2017; Kaiser et al., 2006; Sciberras et al., 2016), restructures the top layers of benthic sediments (Eigaard et al., 2016; Oberle, Swarzenski, et al., 2016; Puig et al., 2012; Trimmer et al., 2005) and resuspends large volumes of sediment into the water column (de Madron et al., 2005; Jones, 1992; Martín, Puig, Palanques, & Giamportone, 2014; Palanques et al., 2014; Ruffin, 1998; Thrush & Dayton, 2002). However, the net effect of this disturbance on OC stores is poorly resolved. Through mixing, resuspension and oxidation of surface sediments, along with the disturbance of benthic communities, fishing may generate a source of ‘underwater carbon dioxide emissions’ via increased remineralization of OC and may also limit future OC burial by inhibiting long‐term sediment settlement and consolidation (De Borger et al., 2021; Keil, 2017; Luisetti et al., 2019; Martín, Puig, Palanques, & Giamportone, 2014; Sala et al., 2021). This disturbance may increase IC concentrations in the ocean, lower its buffering capacity and via this, slow the rate of CO2 uptake from the atmosphere, while contributing to ocean acidification and potentially leading to increased release of oceanic CO2 to the atmosphere (Bauer et al., 2013; Keil, 2017; Khatiwala et al., 2009; LaRowe et al., 2020; Lovelock et al., 2017; Luisetti et al., 2019; Pendleton et al., 2012; Sala et al., 2021). However, to place the effect of mobile demersal fishing in full context, it is important to better quantify the impacts of different pressures on OC storage and to understand how these compare with natural hydrological disturbances to seabed sediments in different environmental settings (Arndt et al., 2013; Pusceddu et al., 2005; Rühl et al., 2020; Winterwerp & Kranenburg, 2002).
The cycling and storage of OC at the seabed is highly complex and is influenced by sediment fauna, flora and microbes; seabed lithology and granulometry; and the chemistry, hydrology and biology of the surrounding water column (Bauer et al., 2013; Burdige, 2007; Keil, 2017; LaRowe et al., 2020; Middelburg, 2018; Rühl et al., 2020; Snelgrove et al., 2018). With all of these factors affected by many positive and negative feedback mechanisms, it is challenging to definitively identify the impact of trawling and dredging on net OC storage (Keil, 2017; LaRowe et al., 2020; Rühl et al., 2020; Snelgrove et al., 2018). In this review, we discuss the potential drivers of change in sediment OC due to mobile demersal fishing activities and summarize empirical evidence where their effects on sediment OC have been directly investigated. We also discuss recent peer‐reviewed publications which aim to quantify the impact of mobile demersal fishing at global, regional and national scales, and highlight why the results must be viewed with concern and caution (Luisetti et al., 2019; Paradis et al., 2021; Sala et al., 2021). If seabed sediments were to be recognized as a quantifiable and manageable blue carbon resource, it could unlock huge climate change mitigation potential and carbon financing opportunities (Avelar et al., 2017; Seddon et al., 2019).
2. LINKS BETWEEN SEABED SEDIMENT OC AND MOBILE DEMERSAL FISHING
2.1. Production of benthic micro‐ and macroalgae
Seabed sediment OC is mostly allochthonous, with much of it originating from terrestrial run‐off and primary production in surface waters from phytoplankton, macroalgae and wetland vegetation (Bauer et al., 2013; Krause‐Jensen & Duarte, 2016; LaRowe et al., 2020; Legge et al., 2020; Turner, 2015). Much of this OC will be consumed, repackaged, excreted or remineralized before a small remaining proportion of OC reaches the seabed (Keil, 2017; Middelburg, 2018; Turner, 2015). On sediments in the euphotic zone, some OC is autochthonous—that is, produced in situ by microphytobenthos, and by macroalgae found on more stable sediments, hard substrate or attached to biogenic material (Gattuso et al., 2006; MacIntyre et al., 1996).
While the impact of mobile demersal fishing on benthic algae is little studied, it is known that benthic macroalgae are easily damaged by physical disturbance, and the structure and abundance of microphytobenthos are highly dependent on both natural and anthropogenic perturbation (Fragkopoulou et al., 2021; Larson & Sundbäck, 2012; MacIntyre et al., 1996). At least in the short term, mobile demersal fishing can reduce algal cover and sediment surface chlorophyll a concentration (Figure 1a) (Fragkopoulou et al., 2021; MacIntyre et al., 1996; Mayer et al., 1991; Tiano et al., 2019; Watling et al., 2001). For example, scallop dredging at depths of 8–15 m in the Damariscotta River Estuary of the Northwest Atlantic led to clear visual disturbance of diatom mats and caused a significant reduction in chlorophyll a concentration (Mayer et al., 1991; Watling et al., 2001). However, there are mixed results in some longer term studies (Brylinsky et al., 1994; Pusceddu et al., 2014).
FIGURE 1.
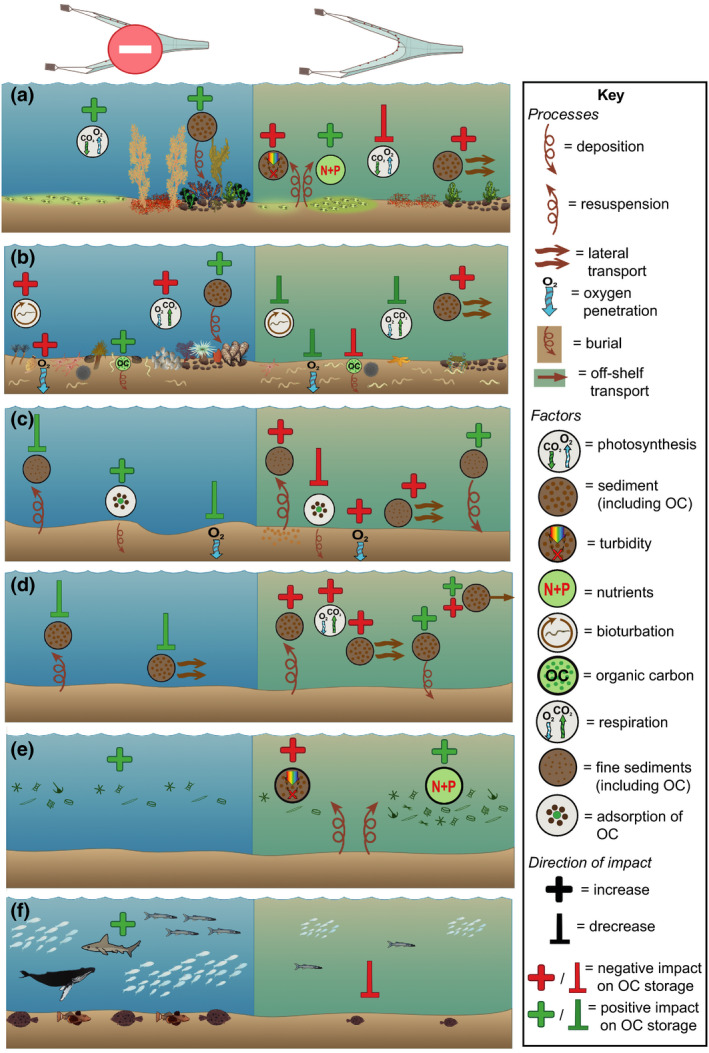
Potential impact of mobile demersal fishing on processes that affect seabed sediment OC (organic carbon) storage. The effects of mobile demersal fishing activity (right) and absence of demersal fishing activity (left) are shown on: (a) benthic algae, (b) benthic infauna and epifauna, (c) sediment characteristics, (d) sediment dynamics, (e) pelagic primary production, (f) vertebrate fauna and how each of these changes may impact OC storage. Addition symbols indicate when a factor/process would be expected to increase in the presence/absence of fishing whereby inhibitory arrows indicate when a factor/process would be expected to decrease. The colour of the addition/inhibition symbols indicates whether this change is predicted to impact OC sequestration and storage either positively (green) or negatively (red). Symbols courtesy of Integration and Application Network (ian.umces.edu/media‐library)
Among algae, kelp and coralline algae can require years and decades, respectively, to recover following disturbance (e.g. Dayton et al., 1992; Fragkopoulou et al., 2021). By contrast, ephemeral macroalgae and microphytobenthos can recover quickly, especially from less chronic disturbance (MacIntyre et al., 1996; Ordines et al., 2017). For example, in the Pesquera Rica trawling grounds of the Balearic Islands, red algae beds of Peyssonneliaceae and Corallinophycidae persist within trawled areas, although their biomass is around 39%–47% lower compared to untrawled areas (Ordines et al., 2017).
As well as physically impacting the benthos, disturbance from mobile demersal fishing also releases nutrients from subsurface sediments which may promote primary production in benthic algae, especially in oligotrophic environments (Figure 1a) (Dounas et al., 2007; Falcão et al., 2003; Fanning et al., 1982). Dependent on the environmental setting, this may offset some of the losses from direct physical disturbance. However, counteracting this, sediment suspended by fishing can increase turbidity (Capuzzo et al., 2015; Palanques et al., 2001; Ruffin, 1998) which reduces light penetration and thus photosynthetic rates (Figure 1a) (MacIntyre et al., 1996).
In many settings, high‐frequency mobile demersal fishing would be expected to reduce the abundance of benthic flora on euphotic sediments and is therefore predicted to limit the OC supply and quantity stored directly, and via secondary production (Figure 1a) (Mandal et al., 2021; Middelburg, 2018; Miller et al., 1996). Additionally, benthic micro‐ and macroalgae are known to increase the stability and accumulation rate of seabed sediments (Miller et al., 1996; Montserrat et al., 2008; Yallop et al., 1994), a primary driver of OC burial and storage (LaRowe et al., 2020; Middelburg, 2018). This represents a further mechanism through which the disturbance of benthic algae from mobile demersal fishing could limit the potential burial rate of OC within sedimentary seabed habitats (Figure 1a).
2.2. Benthic faunal production and processing of OC
The impact of mobile demersal fishing gears on benthic fauna has been widely studied. It depends on the intensity, depth and frequency of demersal fishing; legacy of prior perturbations; type of fishing gear; intensity and frequency of natural disturbances; sediment type; and benthic community composition; and is consequently site‐specific (Collie et al., 2000; Hiddink et al., 2017; Kaiser et al., 2002, 2006; Sciberras et al., 2018; Thrush & Dayton, 2002). Gears which penetrate most deeply into sediment, such as dredges and hydraulic gears, tend to have greater impact than gears with less penetration, such as demersal seines and otter trawls (Collie et al., 2000; Hiddink et al., 2017; Kaiser et al., 2006; Sciberras et al., 2018), although habitat type also has an influence (Rijnsdorp et al., 2020). The largest impacts follow initial experimental trawling events or are seen when comparisons are made to an area of long‐standing protection (Cook et al., 2013; Thrush & Dayton, 2002). Many studies may underestimate the damage done by mobile fishing gears and overestimate the speed of recovery because they measure the recovery of areas already impacted (Collie et al., 2000; Cook et al., 2013; Hiddink et al., 2017; Hinz et al., 2009; Kaiser et al., 2002, 2006; Sciberras et al., 2018).
To a greater or lesser extent, bottom trawling and dredging reduce total benthic biomass and production of benthic macrofauna, and cause loss in abundance and diversity of sessile epifauna and long‐lived shallow burrowing infauna (Jennings et al., 2001, 2002; Kaiser et al., 2002; Queirós et al., 2006; Sciberras et al., 2018; Tiano et al., 2020; Tillin et al., 2006). Long‐term fishing with mobile gears leads to the preponderance of small‐bodied, opportunistic, motile infauna, and larger, highly vagrant, scavenging macrofauna (Jennings et al., 2001, 2002; Kaiser et al., 2002, 2006; Thrush & Dayton, 2002; Tillin et al., 2006). Mobile demersal fishing can also directly affect the diversity and community structure within the largely resistant opportunistic meiofauna (Pusceddu et al., 2014; Schratzberger et al., 2009).
Benthic fauna are strong drivers of biogeochemical cycling in sediments (LaRowe et al., 2020; Middelburg, 2018; Rühl et al., 2020; Snelgrove et al., 2018). For example, in a well‐studied area off the coast of Vancouver Island, taxonomic and functional richness of benthic fauna explained a similar proportion of variance in pelagic–benthic nutrient flux (~20%) when compared to a suite of environmental variables (Belley & Snelgrove, 2016, 2017). OC that reaches the seabed is directly consumed by deposit and suspension feeding fauna, and is thereafter incorporated into biomass, expelled as faeces and pseudofaeces or metabolized and remineralized through respiration (Arndt et al., 2013; Keil, 2017; Middelburg, 2018; Rühl et al., 2020; Snelgrove et al., 2018). While respiration reduces the total amount of OC available for burial and storage, the preferential utilization of labile OC by benthic communities may result in the accumulation of refractory compounds that are more resistant to microbial decomposition (Figure 1b) (Arndt et al., 2013; LaRowe et al., 2020; Middelburg, 2018).
Bioturbation and bio‐irrigation are, respectively, the reworking of sediment particles and solutes by fauna (Ekdale et al., 1984; Meysman et al., 2006); this can impact OC remineralization in two ways. First, bio‐irrigation activities enhance the oxygenation of surface sediments and cause an increase in the concentration of other electron acceptors such as nitrate, metal oxides and sulphate, therefore promoting microbial degradation of OC (Figure 1b) (Arndt et al., 2013; Hulthe et al., 1998; Keil, 2017; LaRowe et al., 2020; Meysman et al., 2006; Snelgrove et al., 2018). Second, particle mixing transports labile OC from the surface to deeper sediment layers. On the one hand, this potentially increases their chance of burial and long‐term storage (Figure 1b) (De Borger et al., 2021; Middelburg, 2018; Rühl et al., 2020; Snelgrove et al., 2018; van der Molen et al., 2012). On the other hand, the transfer of high‐quality OC from the surface to deeper layers may prime microbial communities and in this way stimulate degradation of more refractory OC found in deeper sediment layers (Middelburg, 2018; van Nugteren et al., 2009). This can lead to significantly increased total OC remineralization rates, although the process is known to vary between environmental settings (Bengtsson et al., 2018; Riekenberg et al., 2020; van Nugteren et al., 2009).
The composition and abundance of benthic fauna can also influence the stability and accumulation rates of sediment, which are key drivers of OC burial and storage (LaRowe et al., 2020; Middelburg, 2018). While increased bioturbation activity generally has a destabilizing effect, burrowing fauna can increase the stability and accumulation rate of sediment if there is an increase in biogenic materials such as worm tubes or mucus production, or an increase in structural complexity at the sediment surface from the presence of sedentary and sessile epifauna and biogenic habitat (Figure 1b) (Borsje et al., 2014; Ekdale et al., 1984; Roberts, 2007; Rühl et al., 2020; Thrush & Dayton, 2002). For example, in fine sands and muds of the Northeast Atlantic, the presence of the tube building polychaete Lanice conchilega can lead to increased sediment accretion rates due to changes in flow dynamics around the worm tubes, with impacts on sedimentation dynamics beyond the biogenic structure and over a longer duration than the lifetime of an individual worm (Borsje et al., 2014). As species that build biogenic structures are particularly vulnerable to damage from mobile demersal fishing (Fariñas‐Franco et al., 2018; Kaiser et al., 2002), their abundance and distribution could be greatly altered by the widespread nature of this pressure.
Faunal biomass and production are some of the main contributors of OC in seabed sediments; therefore, in many environmental settings, the impact of mobile demersal fishing on benthic fauna is hypothesized to cause a reduction in seabed OC storage. However, this effect would be offset to varying extents by reduced bioturbation, OC consumption and respiration causing lower remineralization rates. The balance depends on the many complex interactions discussed above, which are site‐specific.
2.3. Alteration to sediment composition
Mobile demersal fishing gears can alter the granulometry, topography and vertical structuring of seabed sediments (Depestele et al., 2019; Martín, Puig, Palanques, & Giamportone, 2014; Oberle, Storlazzi, & Hanebuth, 2016; Oberle, Swarzenski et al., 2016; Puig et al., 2012; Trimmer et al., 2005), with extent of change influenced by gear used, sediment type, local hydrology and frequency of fishing (Kaiser et al., 2002; Martín, Puig, Palanques, & Giamportone, 2014; Oberle, Swarzenski et al., 2016; O'Neill & Summerbell, 2016; O'Neill et al., 2018; Trimmer et al., 2005). Gears that penetrate more deeply into sediment and have a larger footprint cause most impact (Depestele et al., 2015, 2019; Eigaard et al., 2016; Kaiser et al., 2002; Martín, Puig, Palanques, & Giamportone, 2014). In highly mobile habitats, for example, ones with abundant shallow sand, the structure and composition of sediment may not be greatly altered by mobile demersal fishing due to strong natural forcing mechanisms, while those found in less hydrologically active environments could be highly affected (Kaiser et al., 2002; Martín, Puig, Palanques, & Giamportone, 2014; Oberle, Swarzenski et al., 2016; Trimmer et al., 2005). However, greater sediment mobility may itself be a consequence of long‐term use of mobile fishing gears, due to loss of fauna and flora that stabilize sediments (Roberts, 2007).
Topographic alterations from mobile fishing gears can consist of visible trawl/dredge tracks and homogenization in large‐scale seabed topography (Depestele et al., 2015, 2019; Eigaard et al., 2016; Kaiser et al., 2002; Martín, Puig, Palanques, & Giamportone, 2014; Oberle, Storlazzi, & Hanebuth, 2016; Oberle, Swarzenski, et al., 2016; O'Neill & Summerbell, 2016; Palanques et al., 2014; Tiano et al., 2020). For example, multibeam surveys have shown that chronic trawling on the continental slopes of the Palamós canyon in the Northwest Mediterranean has had drastic flattening effects on soft sediments (Puig et al., 2012). Mobile demersal fishing also mixes and overturns the top layer of seabed, generally causing a homogenization of the sediment structure and an increase in density of surface sediments (Depestele et al., 2019; Martín, Puig, Masque, et al., 2014; Oberle, Swarzenski, et al., 2016; Paradis et al., 2021; Pusceddu et al., 2014). The sediment's vertical profile can also be altered, with an increase in coarse material towards the surface, caused by winnowing, resuspension and loss of fine material (Figure 1c) (Martín, Puig, Masque, et al., 2014; Martín, Puig, Palanques, & Giamportone, 2014; Mengual et al., 2016; Oberle, Swarzenski, 2016; Palanques et al., 2014; Paradis et al., 2021; Pusceddu et al., 2014). If the local hydrology favours deposition, the sediment may be overlain by a surface layer of fine material from the redeposition of fine sediment which has been resuspended from deeper layers (Oberle, Swarzenski, et al., 2016; Palanques et al., 2014; Tiano et al., 2020). On the Northwest Iberian shelf, all these processes and impacts were identified within a study across different trawling intensities and environmental settings, highlighting the complexity in predicting fine‐scale effects of mobile demersal fishing on sediment structure (Oberle, Swarzenski, et al., 2016).
The physical mixing of surface sediments can cause an increase in oxygen penetration and/or sediment oxygen concentrations (Allen & Clarke, 2007; De Borger et al., 2021; Tiano et al., 2019). Although oxygen penetration depths usually rapidly reestablish after perturbations, they do not necessarily return to a predisturbed state (Allen & Clarke, 2007; De Borger et al., 2021; Tiano et al., 2019). Increased sediment oxygenation can increase microbial respiration and remineralization of OC (Figure 1c) (Dauwe et al., 2001; Keil, 2017; Kristensen et al., 1995; van de Velde et al., 2018). Mixing of sediments by mobile demersal fishing may also transport OC from the surface to deeper sediment layers (Mayer et al., 1991). As with faunal‐mediated sediment reworking, this may increase the chance of burial and long‐term storage, but may also stimulate the degradation of more refractory OC that was already present in deeper sediment layers (Duplisea et al., 2001; Mayer et al., 1991; Middelburg, 2018; van Nugteren et al., 2009).
The loss of fine, flocculent material and OC–mineral interactions is another mechanism by which OC storage could be reduced (Figure 1c) (Martín, Puig, Palanques, & Giamportone, 2014; Oberle, Storlazzi, & Hanebuth, 2016; Pusceddu et al., 2014). The process of physical encapsulation of OC by sediment particles and the resultant protection from remineralization is seen as a key process in long‐term OC storage (Arndt et al., 2013; Burdige, 2007; Estes et al., 2019; Hemingway et al., 2019; LaRowe et al., 2020). For example, in sediment samples from the Northeast Pacific coasts of Mexico and Washington state, 50% of the oldest OC stores were sorbed to mineral surfaces (Arnarson & Keil, 2007). Fine‐grained sediments such as silts and clays with large specific surface areas and often reduced redox potentials typically have higher OC contents compared to habitats dominated by sand and coarse sediment (Burdige, 2007; Paradis et al., 2021; Smeaton et al., 2021). As mobile demersal fishing generally exposes or suspends fine material, this may reduce overall OC storage through loss of OC–mineral interactions and remineralization (Figure 1c) (Arnarson & Keil, 2007; Estes et al., 2019). However, if disturbed OC associated with fine sediments is largely refractory in nature and is not remineralized but reallocated to surface sediments in situ or transported laterally to different locations, this disturbance may increase seabed surface OC in certain environmental settings (Figure 1c) (LaRowe et al., 2020; Oberle, Swarzenski, et al., 2016; Palanques et al., 2014; Tiano et al., 2021).
2.4. Sediment resuspension and transport
Large volumes of seabed sediments can be moved laterally and vertically, and become resuspended in the water column by tides, waves and storms (Ferré et al., 2008; Soulsby, 1997; Winterwerp & Kranenburg, 2002). Mobile demersal fishing activities are a large contributor to the quantities of sediment displaced by these natural forcing mechanisms (Depestele et al., 2015; Ferré et al., 2008; Jones, 1992; Martín, Puig, Palanques, & Giamportone, 2014; Mengual et al., 2016; Oberle, Storlazzi, & Hanebuth, 2016; O'Neill & Summerbell, 2016; Paradis et al., 2018; Pusceddu et al., 2005, 2015). Magnitudes involved are highly dependent on depth, gear and sediment type, with deeper penetrating gears and finer sediments causing larger dispersed volumes (Churchill, 1989; de Madron et al., 2005; Ferré et al., 2008; Martín, Puig, Palanques, & Giamportone, 2014; Mengual et al., 2016; Oberle, Storlazzi, & Hanebuth, 2016; O'Neill & Ivanović, 2015; O'Neill & Summerbell, 2016; Palanques et al., 2014; Pusceddu et al., 2005; Ruffin, 1998). Depending on local hydrographic conditions, sediment may remain in suspension for extended periods of time, and can be transported across large vertical and lateral distances (de Madron et al., 2005; Ferré et al., 2008; Martín et al., 2006, 2008, 2014; Oberle, Storlazzi, & Hanebuth, 2016; Palanques et al., 2006, 2014; Pusceddu et al., 2015). In the Northern Mediterranean, otter trawling resulted in average suspended sediment concentrations ranging between 6 and 50 mg/L, depending on the study site (de Madron et al., 2005; Palanques et al., 2001). Sediment within the water column was found to persist for up to 5 days (Palanques et al., 2001), while off‐shelf transport was 1.4–9 times higher when compared to sediment volumes without trawling (Ferré et al., 2008; Palanques et al., 2014). The loss of seabed topography, as discussed above (Martín, Puig, Palanques, & Giamportone, 2014; Oberle, Swarzenski, et al., 2016; Puig et al., 2012), may also alter local‐scale hydrographic conditions, increasing sediment boundary water flows and the magnitude of sediment resuspension (Smith & McLean, 1977; Soulsby, 1997).
Natural sediment disturbance during storms is known to stimulate increased water column microbial production (Cotner et al., 2000) and OC remineralization rates (Pusceddu et al., 2005; Wainright & Hopkinson Jr, 1997). The resuspension and transport of sediment from mobile demersal fishing is hypothesized to lead to a reduction in OC concentration (Martín, Puig, Palanques, & Giamportone, 2014), largely due to increased oxygen exposure times and shifts between anoxic and oxic states, which can increase remineralization rates (Figure 1d) (Dauwe et al., 2001; Hulthe et al., 1998; Keil, 2017; Kristensen et al., 1995). Fishing‐induced disturbance may further promote remineralization, as sediment which is deposited under oxic conditions, then buried under anoxia and re‐exposed to oxygen can stimulate OC degradation rates (Hulthe et al., 1998). This has been identified in the biochemical signature of suspended particulate OC within trawling grounds of the North Mediterranean, with a significant shift from labile to refractory OC compounds (Pusceddu et al., 2005, 2005, 2015).
Previous studies have shown that it is challenging to fully quantify the amount of OC that will be remineralized after disturbance, rather than simply being moved elsewhere (Lovelock et al., 2017; Martín et al., 2006, 2008; Pusceddu et al., 2005; Wainright & Hopkinson Jr, 1997). There is also the potential that sediment resuspension from mobile demersal fishing could increase OC storage in adjacent areas (Figure 1d). This could occur from higher sedimentation rates near to fishing grounds leading to increased burial of OC which is already present within the seabed, or burial of benthic algae and sessile fauna (Churchill, 1989; Jones, 1992; Oberle, Storlazzi, & Hanebuth, 2016; Sciberras et al., 2016). It could also lead to the transportation of OC‐rich shelf and slope sediments (Atwood et al., 2020) to deeper waters which may be isolated from atmospheric exchange for centennial timeframes (Figure 1d) (Caldeira et al., 2002; Ferré et al., 2008; Martín et al., 2006, 2008; Paradis et al., 2018; Siegel et al., 2021). Such off‐shelf induced transport of sediment and OC has been recorded as deep as 1750 m in continental slope trawling grounds of the Palamós canyon in the Northwest Mediterranean (Martín et al., 2006, 2008; Palanques et al., 2006). Any OC transport from shelf to deeper waters that are isolated from the atmosphere for centennial timeframes could be considered a sink for atmospheric carbon dioxide, irrespective of whether the carbon accumulates as OC in sediments or is respired to carbon dioxide.
Increased sediment resuspension from mobile demersal fishing is predicted to reduce the current store of OC in seabed sediments due to the disturbance of accumulations and increased oxygen exposure times (Keil, 2017; Luisetti et al., 2019; Martín, Puig, Palanques, & Giamportone, 2014; Sala et al., 2021). Future burial may also be limited as newly settled organic material would be kept in suspension, precluding it from burial and storage (Churchill, 1989; Martín, Puig, Palanques, & Giamportone, 2014; Oberle, Storlazzi, & Hanebuth, 2016; Ruffin, 1998). However, reductions in OC could be offset to varying extents by trawl‐induced burial of OC through sediment mixing, redeposition and increased off‐shelf transport of OC (Martín et al., 2008; Mayer et al., 1991). The site‐specific nature of this impact will be largely based on the vulnerability of OC to remineralization (Arndt et al., 2013; Middelburg, 2018) and local hydrography, which will primarily determine the fate of resuspended OC (Ferré et al., 2008; Keil, 2017; LaRowe et al., 2020; Wainright & Hopkinson Jr, 1997). In highly dynamic environments and where sediment OC is highly refractory, the additional impact of fishing‐related disturbance on sediment OC may be limited.
2.5. Alteration in pelagic primary production
As most seabed OC is allochthonous, the total amount which reaches seabed sediments is strongly driven by the level of primary production in the overlying water column (Atwood et al., 2020; Seiter et al., 2004; Turner, 2015). Sediment disturbance by mobile fishing gears, or natural forces, can lead to a decrease or increase in primary production. Resuspension of particles can lead to stronger attenuation of light conditions with the consequence that primary production decreases (Figure 1e) (Adriano et al., 2005; Capuzzo et al., 2015; Cloern et al., 2014; Palanques et al., 2001; Ruffin, 1998). However, sediment disturbance can release significant concentrations of nutrients into the water column (de Madron et al., 2005; Falcão et al., 2003; Fanning et al., 1982; Polymenakou et al., 2005; Pusceddu et al., 2015). In shallower areas, released nutrients will likely enter into or remain in the euphotic zone, where their fertilization effect can increase phytoplankton primary production (Figure 1e) (Dounas et al., 2007; Fanning et al., 1982; Palanques et al., 2014). For example, modelling predictions from trawling experiments in the Eastern Mediterranean at Heraklion Bay led to estimates that nutrient upwelling from bottom trawling could increase net annual primary production by 15% (Dounas et al., 2007) with subsequent settlement raising OC in seabed sediments (Falcão et al., 2003; Palanques et al., 2014; Polymenakou et al., 2005; Turner, 2015). The net effect of demersal fishing activity on pelagic primary production is therefore expected to differ in systems where primary producers are limited by either light or nutrients.
2.6. The contribution of vertebrate fauna to OC storage
Although not a focus of this review, the removal of vertebrate species by benthic and pelagic fisheries could influence the mass of OC stored in seabed sediments (Atwood et al., 2015; Mariani et al., 2020; Pershing et al., 2010). The emerging field of ‘fish carbon’ describes the contribution of vertebrate fauna to OC storage within seabed sediments from defecation, pelagic mixing, bioturbation, trophic interactions and deadfall (Saba et al., 2021; Trueman et al., 2014; Turner, 2015). Although the magnitudes of effect are poorly resolved, the reduction in population size and average body size of marine vertebrates that can occur from their exploitation (Britten et al., 2021; Hatton et al., 2021; Pacoureau et al., 2021) is predicted to reduce the amount of carbon exported to the seabed (Figure 1f) (Atwood et al., 2015; Bianchi et al., 2021; Mariani et al., 2020; Pershing et al., 2010; Trueman et al., 2014). For example, since 1950, the combined catch of Tuna, Mackerel, Shark and Billfish is estimated to have prevented approximately 0.02 Gt of OC being stored in seabed sediments (Mariani et al., 2020). The removal of predatory vertebrates will also cause trophic cascades, potentially leading to alterations in benthic faunal communities, triggering the feedback mechanisms on OC discussed above (Atwood et al., 2015). Further research which uses fingerprinting techniques to identify the provenance of OC in seabed sediments under different environmental settings may allow the magnitude of contribution by vertebrate fauna to be better resolved (Geraldi et al., 2019; Larsen et al., 2013); however, processes such as pelagic mixing, bioturbation and trophic interactions would remain challenging to fully quantify.
2.7. Interactions and feedback mechanisms
Although largely outside of the scope of this review, the six main factors discussed here interact in a variety of positive and negative feedback loops which will add further complexity to outcomes on seabed sediment OC. For example, the alterations in sediment structure that may occur due to mobile demersal fishing activities will, in itself, influence the community structure of benthic flora and fauna even if the biota are not significantly impacted by the physical disturbance itself (McArthur et al., 2010). A second example could derive from fishing disturbance induced changes in pelagic primary production, in and of itself, influencing the community structure or abundance of vertebrate fauna which could affect the seabed OC downstream (Brown et al., 2010). Empirical studies which take a whole system approach to seabed OC may allow some of these interactions to be better understood in the future.
3. EXPERIMENTAL RESULTS
From a literature review (see Supporting Information), 38 peer‐reviewed studies were identified which investigated the impact of mobile demersal fishing on the seabed, and directly measured OC or organic matter (OM) and/or remineralization rates in seabed sediments (Table 1). The 38 studies covered 12 oceanic realms with greatest representation from the Northeast Atlantic (37%), Mediterranean (24%), and Northwest Atlantic (16%) (Table 1). The majority of studies (60%) investigated impacts of commercial fishing activities not under the direct control of the investigators. The remainder either used experimental trawling/dredging methods (34%) or a mixture of experimental trawling and monitoring of commercial fishing (5%) (Table 1). Studies which used experimental fishing methods generally considered acute disturbance events which were conducted by the investigators for periods ranging from 1 day to 15 months, but mostly lasting only a single day (Table S1). Commercial fishing studies generally consider more chronic impacts, comparing areas with different levels of fishing intensities or areas closed to mobile demersal fishing for periods of months to multiple years (Table S1). As evidence to support the different treatment levels, commercial fishing studies rely on information such as vessel monitoring data, fishing legislation or local environmental knowledge (Table S1).
TABLE 1.
Summary of studies which investigated the impact of mobile demersal fishing on the seabed and directly measured organic carbon (OC) or organic matter (OM), and/or remineralization rates of OC/OM in the sediment. The last two columns indicate whether the presence or increase in demersal fishing activity was reported to cause lower (red), higher (green), no significant effect (orange) or mixed effects (grey) in the concentration or content of OC/OM (‘OC/OM’), or organic carbon remineralization rate (‘Remin’ rate’), within seabed sediments
| Reference | Oceanic region | Sediment | Depth (m BCD) | Gear | Study type | Impact type | Sediment depth | Investigations | OC/OM | Remin' rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Adriano et al. (2005) | N Mediterranean | Sandy‐mud | ~1 | Clam dredge | BA | Commercial fishing | Homog' surface | 1 | ||
| Atkinson et al. (2011) | SE Atlantic | Muddy‐sand | 346–459 | Otter‐trawl | LH | Commercial fishing | Homog' surface | 1 | ||
| Bhagirathan et al. (2010) | N Indian | Mud | 15–40 | Otter‐trawl | BA | Experimental | Homog' surface | 1 | ||
| Brown et al. (2005) | NE Pacific | Muddy‐sand | 25–35 | Otter‐trawl |
BACI IC |
Experimental Commercial fishing |
0–5 cm | 2 | ||
| Dolmer et al. (2001) | NE Atlantic | Muddy‐sand | 7 | Mussel dredge | IC | Experimental | Homog’ surface | 1 | ||
| Eleftheriou and Robertson (1992) | NE Atlantic | Sand | ~7 | Scallop dredge | BA | Experimental | 0–6 cm | 1 | ||
| Ferguson et al. (2020) | SW Pacific | Muddy‐sand | 4 | Otter trawl | BACI | Experimental | 0.5 cm | 1 | ||
| Fiordelmondo et al. (2003) | N Mediterranean | Sand | ~2 | Clam dredge | IC | Experimental | 1 cm | 1 | ||
| Goldberg et al. (2014) | NW Atlantic | Fine sand | 3–5 | Hydraulic dredge | IC | Experimental | ~0–20 cm | 1 | ||
| Hale et al. (2017) | NE Atlantic | Mud & Sand | 19–29 | Otter trawl & Scallop dredge | LH | Commercial fishing | 1 cm | 2 | ||
| Lamarque et al. (2021) | NE Atlantic | Sandy‐mud | 33–78 | Mixed trawls | LH | Commercial fishing | 0–1 cm | 1 | * | |
| Lindeboom and de Groot (1998) | NE Atlantic | Mud & Sand | 30–75 | Mixed trawls |
BACI IC |
Experimental Commercial fishing |
Homog’ surface/0–10 cm | 3 | ||
| Liu et al. (2011) | W Pacific | Sandy‐mud | 20 | Mixed trawls | IC | Commercial fishing | Homog’ surface | 1 | ||
| Martín, Puig, Masque, et al. (2014) | NW Mediterranean | Mud | 453–591 | Otter trawl | IC | Commercial fishing | 0–50 cm | 1 | ||
| Mayer et al. (1991) | NW Atlantic | Mud & Mixed | 8–20 | Otter trawl & Scallop dredge | IC | Experimental | 0–12 cm | 2 | ||
| McLaverty et al. (2020) | NE Atlantic | Sandy‐mud | 3–11 | Mussel dredge | LH | Commercial fishing | Homog’ surface | 4 | * | |
| Mercaldo‐Allen et al. (2016) | NW Atlantic | Fine sand | 3–5 | Hydraulic dredge | IC | Experimental | Homog’ surface | 1 | ||
| Meseck et al. (2014) | NW Atlantic | Fine sand | 5–6 | Hydraulic dredge | BACI | Experimental | ~0–20 cm | 1 | ||
| Morys et al. (2021) | Baltic | Muddy‐sand | 12 | Benthic Dredge | IC | Experimental | 0–15 cm | 1 | ||
| Palanques et al. (2014) | NW Mediterranean | Mud | 40–70 | Otter trawl | IC | Commercial fishing | 0–30 cm | 1 | ||
| Paradis et al. (2019) | SW Mediterranean | Mud | 550 | Otter trawl | IC | Commercial fishing | 0–35 cm | 1 | ||
| Paradis et al. (2021) | NW Mediterranean | Mud | 425–494 | Otter trawl | IC | Commercial fishing | 0–10 cm | 1 | ||
| Polymenakou et al. (2005) | NE Mediterranean | Sandy‐mud | 30–51 | Otter trawl | BA | Commercial fishing | 0–1 cm | 1 | ||
| Pusceddu et al. (2005a) | NE Mediterranean | Sandy‐mud | 30–80 | Otter trawl | BA | Commercial fishing | 0–10 cm | 1 | ||
| Pusceddu et al. (2014) | NW Mediterranean | Mud | 454–556 | Otter trawl | IC | Commercial fishing | 0–10 cm | 1 | ||
| Rajesh et al. (2019) | N Indian | Sand | 5–35 | Beam trawl | BA | Experimental | Homog’ surface | 2 | ||
| Ramalho et al. (2018) | NE Atlantic | Muddy‐sand | 285–550 | Otter trawl | IC | Commercial fishing | Homog’ surface | 1 | ||
| Ramalho et al. (2020) | NE Atlantic | Muddy‐sand | 285–550 | Otter trawl | LH | Commercial fishing | 0–5 cm | 1 | ||
| Rosli et al. (2016) | SW Pacific | Sandy‐mud | 670–1561 | Otter trawl | LH | Commercial fishing | 0–1 cm | 2 | * | |
| Sciberras et al. (2016) | NE Atlantic | Mud & Sand | 20–43 | Otter trawl & Scallop dredge | LH | Commercial fishing | Homog’ surface | 2 | ||
| Serpetti et al. (2013) | NE Atlantic | Muddy‐sand | 769–823 | Mixed trawls | IC | Commercial fishing | 0–10 cm | 1 | ||
| Sheridan and Doerr (2005) | NW Atlantic | Mud & Sand | 5–20 | Otter trawl | IC | Commercial fishing | 0–5 cm | 1 | ||
| Smith (2000) | NE Mediterranean | Sandy‐mud | ~200 | Otter trawl | BACI | Commercial fishing | 0–4 cm | 1 | ||
| Tiano et al. (2019) | NE Atlantic | Muddy‐sand | 34 | Mixed trawls | BA | Experimental | 0–2.5 cm | 2 | ||
| Trimmer et al. (2005) | NE Atlantic | Muddy‐sand | ~20–80 | Beam trawl | LH | Commercial fishing | 0–10 cm | 2 | * | |
| van de Velde et al. (2018) | NE Atlantic | Mud | ~7 | Unknown | BA | Commercial fishing | 0–30 cm | 1 | ||
| Wang et al. (2021) | W Pacific | Mud & Sand | 1–28 | Mixed trawls | Recovery | Commercial fishing | Homog’ surface | 1 | ||
| Watling et al. (2001) | NW Atlantic | Muddy‐sand | 15 | Scallop dredge | BA | Experimental | 0–15 cm | 1 | * |
For ‘Study type’: BA = Before–after fishing impact, IC = Impact–control site comparison, LH = low to high impacted sites, BACI = before–after control–impact, ‘Recovery’ = change after removal of commercial fishing. ‘Investigations’ = the number of individual investigations conducted in each study. ‘Homog’ surface’ = A homogenized sample of surface sediment was measured (often taken from a grab sample). ‘BCD’ = Below chart datum. For ‘OC/OM’, those with an asterisk (*) indicate where further analysis was needed—see Supporting Information. The ‘OC/OM’ column is empty for Polymenakou et al. (2005) as the result was based on the same data which are reported in Pusceddu et al. (2005a).
A variety of experimental set‐ups were employed including impact–control site comparisons (39%), before–after fishing impact (24%) and low–high impact contrasts which lacked controls (21%). Additionally, 13% of studies used a before–after control–impact design either alone or in combination with an impact–control experiment, and one investigated the recovery of seabed sediment OC after a long‐term closure to mobile demersal fishing (Table 1). It should be noted that for many of these studies, in areas considered ‘control sites’, there is the potential for them to still be affected by mobile demersal fishing activities. This often occurs due to insufficient monitoring (e.g. no vessel monitoring system data on smaller vessels), lack of enforcement (i.e. within a supposed closed area) or lack of recovery time since cessation of fishing given the long timescales of recovery for many habitats (Roberts, 2007).
Of the 38 studies identified, 10 investigated the effect of mobile demersal fishing across multiple sites, habitat types or gear types and made inferences for each investigation separately (Table 1), producing a total of 51 individual investigations (Table S1). Most of these considered the impact of demersal trawling gears (65%) with the remainder assessing the impact from types of dredge fisheries (35%) (Table S1). The majority of investigations only investigated the impact of mobile demersal fishing on OC in homogenized surface samples (35%) or in sediment depths to a maximum of 5 cm (29%) (Table S1). Of the remaining investigations, most considered sediment depths up to a maximum of 20 cm (28% of all investigations). Only 6% of investigations measured impacts up to 35 cm and only one up to 50 cm (Table S1). The depth of the seabed under investigation ranged from 1 to 1561 m BCD (below chart datum); however, the majority of investigations (67%) were conducted below 50 m (Table S1).
There were only seven inferences regarding the impact of mobile demersal fishing pressure on in situ seabed sediment carbon remineralization rates. Of these, four reported that demersal fishing activity decreased remineralization rate in seabed sediments, with three concluding the opposite (Table S1). Although no clear trend was identified between studies, one hypothesis is that the direction of effects may be dependent on local hydrographic conditions. For example, in more depositional environments, mobile demersal fishing may cause oxygenation of sediments and redeposition of recently expulsed organic material back to the seabed, leading to an increase in remineralization rate (Duplisea et al., 2001; Polymenakou et al., 2005; van de Velde et al., 2018). In more hydrologically active environments, resuspension and lateral/vertical transport of sediments may be expected to reduce OC in surface sediments which, along with removal of fauna, could limit the rate of remineralization (De Borger et al., 2021; Morys et al., 2021; Pusceddu et al., 2014; Tiano et al., 2019).
Of the 51 individual investigations, 49 measured changes in OC/OM concentration/content. A finding of no significant effect was reported in 61% of investigations; 29% reported lower OC in fished sites compared to unfished control sites or in areas with higher fishing intensities; with the remaining 10% of investigations reporting higher OC (Table S1). Patterns in the environmental and experimental characteristics between different outcomes were largely indistinct (Figure 2). The median depth at which the research was conducted was relatively similar between different experimental outcomes, although those that reported an increase in OC were generally conducted at shallower depths (Figure 2b, Table S1). Median depths for the experimental outcomes were 22, 31 and 20, and ranged between 2–591, 4–1561 and 1–55 m for studies which reported a decrease, no significant effect and an increase in OC respectively (Table S1). Those investigations which reported no significant effect of demersal fishing on OC had some distinguishing features: They were more likely to be undertaken on sand, measure OC to shallower sediment depths and use a study design comparing sites with different levels of fishing intensities but lacking controls (Figure 2). All these factors may make it more challenging to detect impact signatures from mobile demersal fishing, especially as sandy sediments are generally characterized by low quantities of OC, higher levels of natural disturbance, higher oxygen penetration depths and faster remineralization rates when compared to muds (Burdige, 2007; Huettel et al., 2014).
FIGURE 2.
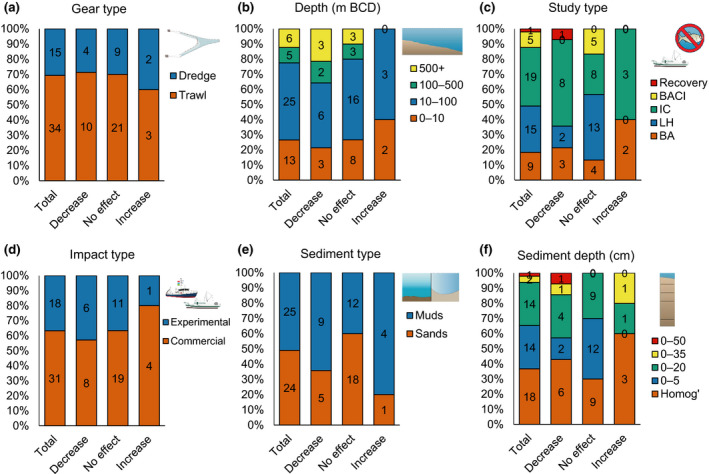
Environmental and experimental characteristics of investigations assessing the effect of mobile demersal fishing on organic carbon (OC). Bar charts represent the proportion of investigations for each category, with inset numbers indicating frequency. Data are shown for all investigations which directly measured changes of OC/OM (organic carbon/organic matter) concentration/content in seabed sediments (‘Total’, n = 49), those which reported a decrease in OC/OM due to mobile demersal fishing (‘Decrease’, n = 14), investigations with no significant effect (‘No effect’, n = 30) and those which found an increase in OC/OM due to mobile demersal fishing (‘Increase’, n = 5). ‘Homog’ = homogenized surface sediment. BCD = Below chart datum. Symbols courtesy of Integration and Application Network (ian.umces.edu/media‐library)
While this literature review gives a summary of empirical research and an indication into patterns and drivers of experimental outcomes, it is not exhaustive so cannot be considered fully systematic. For example, it only considered peer‐reviewed primary literature obtainable from two bibliographic databases and ignored grey literature. Additionally, we made no attempt to critically assess the quality or validity of each study before its inclusion, and the data extracted were largely qualitative or semiquantitative in nature. A more thorough systematic review and meta‐analysis may provide further evidence and/or varying results.
4. FUTURE RESEARCH
As highlighted by the varied experimental results, there is a clear need for further research into the potential impact of mobile demersal fishing on OC burial and long‐term storage in seabed sediments under different environmental settings. Recent first‐order estimates have suggested that globally, mobile demersal fishing could remineralize between 0.16 and 0.4 Gt of OC from marine sediment stores annually (Sala et al., 2021). It has also been suggested that historical trawling on global continental slopes could have removed ~0.06 Gt of OC from the uppermost centimetre of sediment alone (Paradis et al., 2021). In addition, it has been estimated that ~0.002 Gt of OC is remineralized from UK shelf sediments each year by mobile demersal fishing (Luisetti et al., 2019). Although these estimates contain large uncertainties, their scale reveals the large potential for mobile demersal fishing to reduce carbon stores.
Following disturbance and/or resuspension by mobile demersal fishing, a proportion of OC may become remineralized in the seabed or in the water column due to the processes discussed above; however, some will simply remain in situ and be reburied, and a further proportion will be transported over a range of distances eventually being consumed or reburied (Lovelock et al., 2017; Pendleton et al., 2012). A key research gap is the tracking and quantification of OC that follows each of these processes in different environmental settings, in areas with different sediment carbon characteristics and under different types of fishing impact. Sala et al. (2021) only account for remineralization of disturbed OC which remains in situ or resettles within 1 km2, as they consider the fate of sediment which stays in suspension as unknown. In their paper, Sala et al. (2021) consider that 87% of disturbed OC remains in situ or resettles uniformly across global fishing effort, and of this anything between 1 and 69.3% will be remineralized. Their estimate is based on a simple model including (1) an estimate of the proportion of OC which is labile and (2) an average first‐order reaction constant. For both model parameters, they used basin‐scale average values for the incoming OC flux rather than values representative for the sedimentary stock which are much lower (Arndt et al., 2013; Soetaert et al., 1996), with the result that the impact of fishing may have been overestimated. In their regional study, Luisetti et al. (2019) use an upper estimate that 100% of the OC resuspended by mobile demersal fishing will be remineralized, but do not consider the fate of OC that is disturbed but remains in situ.
Although the studies by Sala et al. (2021) and Luisetti et al. (2019) give a representation to the scale of OC which may be lost, improved quantification of these metrics is clearly needed before accurate measures of OC lost, or inorganic carbon produced, can be quantified. OC in seabed sediments is not naturally inert and only a small percentage of OC that reaches the seabed is stored with the remainder passing through a range of aerobic and anaerobic remineralization pathways to varying sediment depths (Arndt et al., 2013; Burdige, 2007; Middelburg, 2018). Additionally, the characteristics of OC in seabed sediments are highly heterogeneous, with numerous chemical compounds and both abiotic and biotic environmental settings all influencing OC reactivity (Arndt et al., 2013; LaRowe et al., 2020; Middelburg, 2018). Thus, more consideration is needed to understand the influence of natural remineralization rates and the vulnerability of seabed OC to remineralization under different environmental settings, and therefore how to quantify the additional effect of mobile demersal fishing in each area. In seabed sediment habitats with high hydrodynamic activity, low deposition rates, large oxygen penetration depths and highly refractory OC, the effect of disturbance by demersal fishing on OC may be limited.
The cumulative or finite nature of disturbance by demersal mobile fishing on OC stores must also be considered, and currently, it is not clear how much of the estimated 360 Gt of OC in the top 1 m of sediment is actually threated by the activity (Atwood et al., 2020). While mobile demersal fishing can only penetrate between around 2 and 20 cm into the sediment (Hiddink et al., 2017), repeated chronic impacts may continue to disturb and displace sediment more deeply (Sala et al., 2021). It is also possible that in chronically fished areas, significant further loss of OC stores will not occur due to historic depletion in surface OC stocks (Sala et al., 2021). By contrast, if new fishing grounds emerge (Gogarty et al., 2020; e.g. Morato et al., 2006), this could lead to large OC stocks potentially becoming vulnerable to remineralization.
The scale of penetration and legacy of disturbance must also be considered alongside differing sediment depth OC profiles (Martín, Puig, Masque, et al., 2014; Middelburg, 2018; Paradis et al., 2019). In the study by Sala et al. (2021), it is assumed that carbon stocks are equally distributed in the top metre of sediment; however in the vast majority of cases, it is known that this does not occur (Berner, 1982; Burdige, 2007). In stable accreting sediments, OC concentrations are generally highest at the surface and reduce with depth until a steady‐state burial rate is reached (Arndt et al., 2013; Burdige, 2007). Sediments which are frequently mixed either through natural or anthropogenic disturbances may have more uniform OC depth profiles (Dauwe & Middelburg, 1998; Martín, Puig, Masque, et al., 2014; Middelburg, 2018; Paradis et al., 2019); however, historical signatures of OC accumulation, disturbance or deposits may also be identified under surface layers of accreting or mixed sediments dependent on the depth limit of investigations and the geology of the site (de Haas et al., 2002; Martín et al., 2008; Palanques et al., 2014). The majority of studies identified in this review investigated the impact of OC in homogenized surface sediments or to sediment layers only up to 5 cm (Table S1). It is likely that in certain environmental settings, disturbance signatures could be identified much deeper within the sediment and should be better considered (Martín, Puig, Masque, et al., 2014). As the characteristics and reactivity of OC can also alter with sediment depth (Arndt et al., 2013; LaRowe et al., 2020; Middelburg, 2018), it is paramount that future studies consider the effects of mobile demersal fishing on a range of sediment layers. The scale and direction of effects of mobile demersal fishing disturbance on surface sediment OC may differ from that in deeper layers.
There is also a need to identify a clear baseline from which changes in OC can be measured. Standing stock of OC in global seabed sediments is relatively well resolved at a number of spatial scales (Atwood et al., 2020; Diesing et al., 2017, 2021; Lee et al., 2019; Legge et al., 2020; Luisetti et al., 2019; e.g. Seiter et al., 2004; Smeaton et al., 2021). However, precise estimates of OC remineralization, accumulation and burial rates are generally lacking (Berner, 1982; Burdige, 2007; Diesing et al., 2021; Keil, 2017; Legge et al., 2020; Luisetti et al., 2019; Wilkinson et al., 2018). For robust conclusions to be drawn studies which aim to quantify the impact of demersal fishing on carbon storage must therefore quantify both before and after scenarios.
On land, retrospective analyses of changes in human use and vegetation cover have been critical to estimating how people have altered the planetary carbon cycle. It is vital that this historical context is also considered when further investigating the potential impact of mobile demersal fishing on global seabed OC storage, and the opportunities for recovery if this pressure is removed. Due to the extended timeframes needed for some seabed habitats to fully recover, true long‐term protection and monitoring of OC are needed to fully deduce carbon storage potential. Without considering areas of seabed that have experienced genuine long‐term protection, it is not possible to gain an accurate baseline from which impacts can be compared (Pinnegar & Engelhard, 2008). Within this review, we identified only one study which looked at the direct recovery of OC in seabed sediments following the medium‐ to long‐term removal of fishing pressure (Wang et al., 2021). Gaining further evidence of this nature is vital to understand how much OC can accumulate when mobile demersal fishing is removed, and how this may change over the course of recovery.
It is important that future research into the impact of mobile demersal fishing on carbon storage is focused in areas which are expected to contain significant stocks of OC or have large future burial potential, based on their geography (Atwood et al., 2020), sediment characteristics (Smeaton et al., 2021) and local hydrology (Lee et al., 2019). Research should also focus on areas that overlap with significant mobile demersal fishing pressure (Amoroso et al., 2018; Kroodsma et al., 2018; Sala et al., 2021), and where this can be compared to areas that could be considered truly ‘unfished’, either from well‐enforced protected areas or specific environmental settings.
5. CONCLUDING REMARKS
Seabed sediments are one of the planet's primary OC stores and strongly influence the oceanic sink for atmospheric CO2 (Atwood et al., 2020; Gruber et al., 2019; Sala et al., 2021; Watson et al., 2020). It is an urgent priority to better understand the effect of mobile fishing gear use on seabed OC storage, and to incorporate clear blue carbon considerations into global seabed management. As only around 2%–3% of the world's seabed is currently closed to trawling and dredging (Marine Conservation Institute, 2021; Roberts et al., 2017), increasing the scale of protection could offer huge climate change mitigation potential and bring corresponding gains in biodiversity (Roberts et al., 2017, 2020; Sala et al., 2021; Seddon et al., 2019). Across the world, mobile demersal fisheries are highly fuel inefficient and produce most of the fishing industry's direct greenhouse gas emissions (Parker et al., 2018). A shift to less damaging fishing methods could provide major net benefits for increasing natural carbon storage in the seabed, whilst significantly reducing emissions of CO2.
The results of recent regional and global scale publications which have calculated first‐order estimates of CO2 produced from disturbance to seabed sediments by mobile demersal fishing must be viewed with both concern and caution (Luisetti et al., 2019; Paradis et al., 2021; Sala et al., 2021). As identified in this review, demersal fishing by trawling and dredging is in some cases likely to limit the storage of OC, but to draw firm conclusions more experimental and modelling studies that cover a wide range of environmental settings, habitat types and fishing pressures are required to address the large number of unknowns and site‐specific drivers associated with the status of OC on the seabed.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
GE prepared the original draft and undertook the literature review. CR & CN contributed to conceptualization and planning. All authors contributed to writing, reviewing and editing.
Supporting information
Table S1
Table S2
ACKNOWLEDGEMENTS
We would like to thank four anonymous reviewers for their detailed, thorough and constructive comments which greatly improved the quality of this manuscript.
Epstein, G. , Middelburg, J. J. , Hawkins, J. P. , Norris, C. R. , & Roberts, C. M. (2022). The impact of mobile demersal fishing on carbon storage in seabed sediments. Global Change Biology, 28, 2875–2894. 10.1111/gcb.16105
Funding information
This work was funded by BLUE Marine Foundation through the Barclays Ocean Climate Impact grant.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article or are openly available in figshare at https://doi.org/10.6084/m9.figshare.16776250.
REFERENCES
- Adriano, S. , Massimiliano, F. , Sonia, C. , Chiara, F. , & Marcomini, A. (2005). Organic carbon changes in the surface sediments of the Venice lagoon. Environment International, 31(7), 1002–1010. 10.1016/j.envint.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Allen, J. , & Clarke, K. (2007). Effects of demersal trawling on ecosystem functioning in the North Sea: A modelling study. Marine Ecology Progress Series, 336, 63–75. 10.3354/meps336063 [DOI] [Google Scholar]
- Amoroso, R. O. , Pitcher, C. R. , Rijnsdorp, A. D. , McConnaughey, R. A. , Parma, A. M. , Suuronen, P. , Eigaard, O. R. , Bastardie, F. , Hintzen, N. T. , Althaus, F. , Baird, S. J. , Black, J. , Buhl‐Mortensen, L. , Campbell, A. B. , Catarino, R. , Collie, J. , Cowan, J. H. , Durholtz, D. , Engstrom, N. , … Jennings, S. (2018). Bottom trawl fishing footprints on the world's continental shelves. Proceedings of the National Academy of Sciences of the United States of America, 115(43), E10275–E10282. 10.1073/pnas.1802379115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnarson, T. S. , & Keil, R. G. (2007). Changes in organic matter–mineral interactions for marine sediments with varying oxygen exposure times. Geochimica et Cosmochimica Acta, 71(14), 3545–3556. 10.1016/j.gca.2007.04.027 [DOI] [Google Scholar]
- Arndt, S. , Jørgensen, B. B. , LaRowe, D. E. , Middelburg, J. J. , Pancost, R. D. , & Regnier, P. (2013). Quantifying the degradation of organic matter in marine sediments: A review and synthesis. Earth‐Science Reviews, 123, 53–86. 10.1016/j.earscirev.2013.02.008 [DOI] [Google Scholar]
- Atkinson, L. J. , Field, J. G. , & Hutchings, L. (2011). Effects of demersal trawling along the west coast of southern Africa: Multivariate analysis of benthic assemblages. Marine Ecology Progress Series, 430, 241–255. 10.3354/meps08956 [DOI] [Google Scholar]
- Atwood, T. B. , Connolly, R. M. , Ritchie, E. G. , Lovelock, C. E. , Heithaus, M. R. , Hays, G. C. , Fourqurean, J. W. , & Macreadie, P. I. (2015). Predators help protect carbon stocks in blue carbon ecosystems. Nature Climate Change, 5(12), 1038–1045. 10.1038/nclimate2763 [DOI] [Google Scholar]
- Atwood, T. B. , Witt, A. , Mayorga, J. , Hammill, E. , & Sala, E. (2020). Global patterns in marine sediment carbon stocks. Frontiers in Marine Science, 7, 165. 10.3389/fmars.2020.00165 [DOI] [Google Scholar]
- Avelar, S. , van der Voort, T. S. , & Eglinton, T. I. (2017). Relevance of carbon stocks of marine sediments for national greenhouse gas inventories of maritime nations. Carbon Balance and Management, 12(1), 10. 10.1186/s13021-017-0077-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, J. E. , Cai, W.‐J. , Raymond, P. A. , Bianchi, T. S. , Hopkinson, C. S. , & Regnier, P. A. G. (2013). The changing carbon cycle of the coastal ocean. Nature, 504(7478), 61–70. 10.1038/nature12857 [DOI] [PubMed] [Google Scholar]
- Belley, R. , & Snelgrove, P. V. R. (2016). Relative contributions of biodiversity and environment to benthic ecosystem functioning. Frontiers in Marine Science, 3, 242. 10.3389/fmars.2016.00242 [DOI] [Google Scholar]
- Belley, R. , & Snelgrove, P. V. R. (2017). The role of infaunal functional and species diversity in short‐term response of contrasting benthic communities to an experimental food pulse. Journal of Experimental Marine Biology and Ecology, 491, 38–50. 10.1016/j.jembe.2017.03.005 [DOI] [Google Scholar]
- Bengtsson, M. M. , Attermeyer, K. , & Catalán, N. (2018). Interactive effects on organic matter processing from soils to the ocean: Are priming effects relevant in aquatic ecosystems? Hydrobiologia, 822(1), 1–17. 10.1007/s10750-018-3672-2 [DOI] [Google Scholar]
- Berner, R. A. (1982). Burial of organic carbon and pyrite sulfur in the modern ocean; Its geochemical and environmental significance. American Journal of Science, 282(4), 451–473. 10.2475/ajs.282.4.451 [DOI] [Google Scholar]
- Bhagirathan, U. , Meenakumari, B. , Jayalakshmy, K. V. , Panda, S. K. , Madhu, V. R. , & Vaghela, D. T. (2010). Impact of bottom trawling on sediment characteristics—A study along inshore waters off Veraval coast, India. Environmental Monitoring and Assessment, 160(1–4), 355–369. 10.1007/s10661-008-0700-0 [DOI] [PubMed] [Google Scholar]
- Bianchi, D. , Carozza, D. A. , Galbraith, E. D. , Guiet, J. , & DeVries, T. (2021). Estimating global biomass and biogeochemical cycling of marine fish with and without fishing. Science Advances, 7(41), eabd7554. 10.1126/sciadv.abd7554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsje, B. W. , Bouma, T. J. , Rabaut, M. , Herman, P. M. J. , & Hulscher, S. J. M. H. (2014). Formation and erosion of biogeomorphological structures: A model study on the tube‐building polychaete Lanice conchilega . Limnology and Oceanography, 59(4), 1297–1309. 10.4319/lo.2014.59.4.1297 [DOI] [Google Scholar]
- Britten, G. L. , Duarte, C. M. , & Worm, B. (2021). Recovery of assessed global fish stocks remains uncertain. Proceedings of the National Academy of Sciences of the United States of America, 118(31), e2108532118. 10.1073/pnas.2108532118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, E. J. , Finney, B. , Dommisse, M. , & Hills, S. (2005). Effects of commercial otter trawling on the physical environment of the southeastern Bering Sea. Continental Shelf Research, 25(10), 1281–1301. 10.1016/j.csr.2004.12.005 [DOI] [Google Scholar]
- Brown, C. J. , Fulton, E. A. , Hobday, A. J. , Matear, R. J. , Possingham, H. P. , Bulman, C. , Christensen, V. , Forrest, R. E. , Gehrke, P. C. , Gribble, N. A. , Griffiths, S. P. , Lozano‐montes, H. , Martin, J. M. , Metcalf, S. , Okey, T. A. , Watson, R. , & Richardson, A. J. (2010). Effects of climate‐driven primary production change on marine food webs: Implications for fisheries and conservation. Global Change Biology, 16(4), 1194–1212. 10.1111/j.1365-2486.2009.02046.x [DOI] [Google Scholar]
- Brylinsky, M. , Gibson, J. , & Gordon Jr, D. C. (1994). Impacts of flounder trawls on the intertidal habitat and community of the Minas Basin, Bay of Fundy. Canadian Journal of Fisheries and Aquatic Sciences, 51(3), 650–661. 10.1139/f94-066 [DOI] [Google Scholar]
- Burdige, D. J. (2007). Preservation of organic matter in marine sediments: Controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chemical Reviews, 107(2), 467–485. 10.1021/cr050347q [DOI] [PubMed] [Google Scholar]
- Caldeira, K. , Wickett, M. E. , & Duffy, P. B. (2002). Depth, radiocarbon, and the effectiveness of direct CO2 injection as an ocean carbon sequestration strategy. Geophysical Research Letters, 29(16), 13‐11–13‐14. 10.1029/2001GL014234 [DOI] [Google Scholar]
- Capuzzo, E. , Stephens, D. , Silva, T. , Barry, J. , & Forster, R. M. (2015). Decrease in water clarity of the southern and central North Sea during the 20th century. Global Change Biology, 21(6), 2206–2214. 10.1111/gcb.12854 [DOI] [PubMed] [Google Scholar]
- Churchill, J. H. (1989). The effect of commercial trawling on sediment resuspension and transport over the Middle Atlantic Bight continental shelf. Continental Shelf Research, 9(9), 841–865. 10.1016/0278-4343(89)90016-2 [DOI] [Google Scholar]
- Cloern, J. E. , Foster, S. Q. , & Kleckner, A. E. (2014). Phytoplankton primary production in the world's estuarine‐coastal ecosystems. Biogeosciences, 11(9), 2477–2501. 10.5194/bg-11-2477-2014 [DOI] [Google Scholar]
- Collie, J. S. , Hall, S. J. , Kaiser, M. J. , & Poiner, I. R. (2000). A quantitative analysis of fishing impacts on shelf‐sea benthos. Journal of Animal Ecology, 69(5), 785–798. 10.1046/j.1365-2656.2000.00434.x [DOI] [PubMed] [Google Scholar]
- Cook, R. , Fariñas‐Franco, J. M. , Gell, F. R. , Holt, R. H. F. , Holt, T. , Lindenbaum, C. , Porter, J. S. , Seed, R. , Skates, L. R. , Stringell, T. B. , & Sanderson, W. G. (2013). The substantial first impact of bottom fishing on rare biodiversity hotspots: A dilemma for evidence‐based conservation. PLoS One, 8(8), e69904. 10.1371/journal.pone.0069904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotner, J. B. , Johengen, T. H. , & Biddanda, B. A. (2000). Intense winter heterotrophic production stimulated by benthic resuspension. Limnology and Oceanography, 45(7), 1672–1676. 10.4319/lo.2000.45.7.1672 [DOI] [Google Scholar]
- Dauwe, B. , & Middelburg, J. J. (1998). Amino acids and hexosamines as indicators of organic matter degradation state in North Sea sediments. Limnology and Oceanography, 43(5), 782–798. [Google Scholar]
- Dauwe, B. , Middelburg, J. J. , & Herman, P. M. J. (2001). Effect of oxygen on the degradability of organic matter in subtidal and intertidal sediments of the North Sea area. Marine Ecology Progress Series, 215, 13–22. 10.3354/meps215013 [DOI] [Google Scholar]
- Dayton, P. K. , Tegner, M. J. , Parnell, P. E. , & Edwards, P. B. (1992). Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecological Monographs, 62(3), 421–445. 10.2307/2937118 [DOI] [Google Scholar]
- De Borger, E. , Tiano, J. , Braeckman, U. , Rijnsdorp, A. D. , & Soetaert, K. (2021). Impact of bottom trawling on sediment biogeochemistry: A modelling approach. Biogeosciences, 18, 2539–2557. 10.5194/bg-2020-328 [DOI] [Google Scholar]
- de Haas, H. , van Weering, T. C. E. , & de Stigter, H. (2002). Organic carbon in shelf seas sinks or sources, processes and products. Continental Shelf Research, 22, 691–717. 10.1016/S0278-4343(01)00093-0 [DOI] [Google Scholar]
- Depestele, J. , Degrendele, K. , Esmaeili, M. , Ivanović, A. , Kröger, S. , O’Neill, F. G. , Parker, R. , Polet, H. , Roche, M. , Teal, L. R. , Vanelslander, B. , & Rijnsdorp, A. D. (2019). Comparison of mechanical disturbance in soft sediments due to tickler‐chain SumWing trawl vs. electro‐fitted PulseWing trawl. ICES Journal of Marine Science, 76(1), 312–329. 10.1093/icesjms/fsy124 [DOI] [Google Scholar]
- Depestele, J. , Ivanović, A. , Degrendele, K. , Esmaeili, M. , Polet, H. , Roche, M. , Summerbell, K. , Teal, L. R. , Vanelslander, B. , & O'Neill, F. G. (2015). Measuring and assessing the physical impact of beam trawling. ICES Journal of Marine Science, 73(suppl_1), i15–i26. 10.1093/icesjms/fsv056 [DOI] [Google Scholar]
- Diesing, M. , Kroger, S. , Parker, R. , Jenkins, C. , Mason, C. , & Weston, K. (2017). Predicting the standing stock of organic carbon in surface sediments of the North‐West European continental shelf. Biogeochemistry, 135(1), 183–200. 10.1007/s10533-017-0310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesing, M. , Thorsnes, T. , & Bjarnadóttir, L. R. (2021). Organic carbon densities and accumulation rates in surface sediments of the North Sea and Skagerrak. Biogeosciences, 18(6), 2139–2160. 10.5194/bg-18-2139-2021 [DOI] [Google Scholar]
- Dolmer, P. , Kristensen, T. , Christiansen, M. L. , Petersen, M. F. , Kristensen, P. S. , & Hoffmann, E. (2001). Short‐term impact of blue mussel dredging (Mytilus edulis L.) on a benthic community. Hydrobiologia, 465, 115–127. 10.1023/A:1014549026157 [DOI] [Google Scholar]
- Dounas, C. , Davies, I. , Triantafyllou, G. , Koulouri, P. , Petihakis, G. , Arvanitidis, C. , Sourlatzis, G. , & Eleftheriou, A. (2007). Large‐scale impacts of bottom trawling on shelf primary productivity. Continental Shelf Research, 27(17), 2198–2210. 10.1016/j.csr.2007.05.006 [DOI] [Google Scholar]
- Duarte, C. M. (2017). Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences, 14(2), 301–310. 10.5194/bg-14-301-2017 [DOI] [Google Scholar]
- Duarte, C. M. , Losada, I. J. , Hendriks, I. E. , Mazarrasa, I. , & Marbà, N. (2013). The role of coastal plant communities for climate change mitigation and adaptation. Nature Climate Change, 3(11), 961–968. 10.1038/nclimate1970 [DOI] [Google Scholar]
- Duplisea, D. E. , Jennings, S. , Malcolm, S. J. , Parker, R. , & Sivyer, D. B. (2001). Modelling potential impacts of bottom trawl fisheries on soft sediment biogeochemistry in the North Sea. Geochemical Transactions, 2(14), 112. 10.1186/1467-4866-2-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrieu de Madron, X. , Ferré, B. , Le Corre, G. , Grenz, C. , Conan, P. , Pujo‐Pay, M. , Buscail, R. , & Bodiot, O. (2005). Trawling‐induced resuspension and dispersal of muddy sediments and dissolved elements in the Gulf of Lion (NW Mediterranean). Continental Shelf Research, 25(19–20), 2387–2409. 10.1016/j.csr.2005.08.002 [DOI] [Google Scholar]
- Eigaard, O. R. , Bastardie, F. , Breen, M. , Dinesen, G. E. , Hintzen, N. T. , Laffargue, P. , Mortensen, L. O. , Nielsen, J. R. , Nilsson, H. C. , O’Neill, F. G. , Polet, H. , Reid, D. G. , Sala, A. , Sköld, M. , Smith, C. , Sørensen, T. K. , Tully, O. , Zengin, M. , & Rijnsdorp, A. D. (2016). Estimating seabed pressure from demersal trawls, seines, and dredges based on gear design and dimensions. ICES Journal of Marine Science, 73(suppl_1), i27–i43. 10.1093/icesjms/fsv099 [DOI] [Google Scholar]
- Eigaard, O. R. , Bastardie, F. , Hintzen, N. T. , Buhl‐Mortensen, L. , Buhl‐Mortensen, P. , Catarino, R. , Dinesen, G. E. , Egekvist, J. , Fock, H. O. , Geitner, K. , Gerritsen, H. D. , González, M. M. , Jonsson, P. , Kavadas, S. , Laffargue, P. , Lundy, M. , Gonzalez‐Mirelis, G. , Nielsen, J. R. , Papadopoulou, N. , … Rijnsdorp, A. D. (2017). The footprint of bottom trawling in European waters: Distribution, intensity, and seabed integrity. ICES Journal of Marine Science, 74(3), 847–865. 10.1093/icesjms/fsw194 [DOI] [Google Scholar]
- Ekdale, A. , Bromley, R. , & Pemberton, S. (1984). Effects of bioturbation on sediment properties. In Ichnology: The use of trace fossils in sedimentology and stratigraphy. Special Publications of SEPM: Society for Sedimentary Geology, USA. [Google Scholar]
- Eleftheriou, A. , & Robertson, M. R. (1992). The effects of experimental scallop dredging on the fauna and physical environment of a shallow sandy community. Netherlands Journal of Sea Research, 30, 289–299. [Google Scholar]
- Estes, E. R. , Pockalny, R. , D’Hondt, S. , Inagaki, F. , Morono, Y. , Murray, R. W. , Nordlund, D. , Spivack, A. J. , Wankel, S. D. , Xiao, N. , & Hansel, C. M. (2019). Persistent organic matter in oxic subseafloor sediment. Nature Geoscience, 12(2), 126–131. 10.1038/s41561-018-0291-5 [DOI] [Google Scholar]
- Falcão, M. , Gaspar, M. B. , Caetano, M. , Santos, M. N. , & Vale, C. (2003). Short‐term environmental impact of clam dredging in coastal waters (south of Portugal): Chemical disturbance and subsequent recovery of seabed. Marine Environmental Research, 56(5), 649–664. 10.1016/s0141-1136(03)00069-2 [DOI] [PubMed] [Google Scholar]
- Fanning, K. A. , Carder, K. L. , & Betzer, P. R. (1982). Sediment resuspension by coastal waters: A potential mechanism for nutrient re‐cycling on the ocean's margins. Deep Sea Research Part A. Oceanographic Research Papers, 29(8), 953–965. 10.1016/0198-0149(82)90020-6 [DOI] [Google Scholar]
- Fariñas‐Franco, J. M. , Allcock, A. L. , & Roberts, D. (2018). Protection alone may not promote natural recovery of biogenic habitats of high biodiversity damaged by mobile fishing gears. Marine Environmental Research, 135, 18–28. 10.1016/j.marenvres.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Ferré, B. , Durrieu de Madron, X. , Estournel, C. , Ulses, C. , & Le Corre, G. (2008). Impact of natural (waves and currents) and anthropogenic (trawl) resuspension on the export of particulate matter to the open ocean: Application to the Gulf of Lion (NW Mediterranean). Continental Shelf Research, 28(15), 2071–2091. 10.1016/j.csr.2008.02.002 [DOI] [Google Scholar]
- Ferguson, A. J. P. , Oakes, J. , & Eyre, B. D. (2020). Bottom trawling reduces benthic denitrification and has the potential to influence the global nitrogen cycle. Limnology and Oceanography Letters, 5(3), 237–245. 10.1002/lol2.10150 [DOI] [Google Scholar]
- Field, C. B. , Behrenfeld, M. J. , Randerson, J. T. , & Falkowski, P. (1998). Primary production of the biosphere: Integrating terrestrial and oceanic components. Science, 281(5374), 237–240. 10.1126/science.281.5374.237 [DOI] [PubMed] [Google Scholar]
- Fiordelmondo, C. , Manini, E. , Gambi, C. , & Pusceddu, A. (2003). Short‐term impact of clam harvesting on sediment chemistry, benthic microbes and meiofauna in the Goro Lagoon (Italy). Chemistry and Ecology, 19(2–3), 173–187. 10.1080/0275754031000119924 [DOI] [Google Scholar]
- Fragkopoulou, E. , Serrão, E. A. , Horta, P. A. , Koerich, G. , & Assis, J. (2021). Bottom trawling threatens future climate refugia of rhodoliths globally. Frontiers in Marine Science, 7, 1246. 10.3389/fmars.2020.594537 [DOI] [Google Scholar]
- Gattuso, J. P. , Gentili, B. , Duarte, C. M. , Kleypas, J. A. , Middelburg, J. J. , & Antoine, D. (2006). Light availability in the coastal ocean: Impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences, 3(4), 489–513. 10.5194/bg-3-489-2006 [DOI] [Google Scholar]
- Geraldi, N. R. , Ortega, A. , Serrano, O. , Macreadie, P. I. , Lovelock, C. E. , Krause‐Jensen, D. , Kennedy, H. , Lavery, P. S. , Pace, M. L. , Kaal, J. , & Duarte, C. M. (2019). Fingerprinting blue carbon: Rationale and tools to determine the source of organic carbon in marine depositional environments. Frontiers in Marine Science, 6, 263. 10.3389/fmars.2019.00263 [DOI] [Google Scholar]
- Gogarty, B. , McGee, J. , Barnes, D. K. A. , Sands, C. J. , Bax, N. , Haward, M. , Downey, R. , Moreau, C. , Moreno, B. , Held, C. , & Paulsen, M. L. (2020). Protecting Antarctic blue carbon: As marine ice retreats can the law fill the gap? Climate Policy, 20(2), 149–162. 10.1080/14693062.2019.1694482 [DOI] [Google Scholar]
- Goldberg, R. , Rose, J. M. , Mercaldo‐Allen, R. , Meseck, S. L. , Clark, P. , Kuropat, C. , & Pereira, J. J. (2014). Effects of hydraulic dredging on the benthic ecology and sediment chemistry on a cultivated bed of the Northern quahog, Mercenaria mercenaria . Aquaculture, 428, 150–157. 10.1016/j.aquaculture.2014.03.012 [DOI] [Google Scholar]
- Gruber, N. , Clement, D. , Carter, B. R. , Feely, R. A. , van Heuven, S. , Hoppema, M. , Ishii, M. , Key, R. M. , Kozyr, A. , Lauvset, S. K. , Monaco, C. L. , Mathis, J. T. , Murata, A. , Olsen, A. , Perez, F. F. , Sabine, C. L. , Tanhua, T. , & Wanninkhof, R. (2019). The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science, 363(6432), 1193–1199. 10.1126/science.aau5153 [DOI] [PubMed] [Google Scholar]
- Hale, R. , Godbold, J. A. , Sciberras, M. , Dwight, J. , Wood, C. , Hiddink, J. G. , & Solan, M. (2017). Mediation of macronutrients and carbon by post‐disturbance shelf sea sediment communities. Biogeochemistry, 135(1), 121–133. 10.1007/s10533-017-0350-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern, B. S. , Frazier, M. , Afflerbach, J. , Lowndes, J. S. , Micheli, F. , O’Hara, C. , Scarborough, C. , & Selkoe, K. A. (2019). Recent pace of change in human impact on the world’s ocean. Scientific Reports, 9(1), 11609. 10.1038/s41598-019-47201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton, I. A. , Heneghan, R. F. , Bar‐On, Y. M. , & Galbraith, E. D. (2021). The global ocean size spectrum from bacteria to whales. Science Advances, 7(46), eabh3732. 10.1126/sciadv.abh3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway, J. D. , Rothman, D. H. , Grant, K. E. , Rosengard, S. Z. , Eglinton, T. I. , Derry, L. A. , & Galy, V. V. (2019). Mineral protection regulates long‐term global preservation of natural organic carbon. Nature, 570(7760), 228–231. 10.1038/s41586-019-1280-6 [DOI] [PubMed] [Google Scholar]
- Hiddink, J. G. , Jennings, S. , Sciberras, M. , Szostek, C. L. , Hughes, K. M. , Ellis, N. , Rijnsdorp, A. D. , McConnaughey, R. A. , Mazor, T. , Hilborn, R. , Collie, J. S. , Pitcher, C. R. , Amoroso, R. O. , Parma, A. M. , Suuronen, P. , & Kaiser, M. J. (2017). Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. Proceedings of the National Academy of Sciences of the United States of America, 114(31), 8301–8306. 10.1073/pnas.1618858114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, H. , Prieto, V. , & Kaiser, M. J. (2009). Trawl disturbance on benthic communities: Chronic effects and experimental predictions. Ecological Applications, 19(3), 761–773. 10.1890/08-0351.1 [DOI] [PubMed] [Google Scholar]
- Howard, J. , Sutton‐Grier, A. , Herr, D. , Kleypas, J. , Landis, E. , Mcleod, E. , Pidgeon, E. , & Simpson, S. (2017). Clarifying the role of coastal and marine systems in climate mitigation. Frontiers in Ecology and the Environment, 15(1), 42–50. 10.1002/fee.1451 [DOI] [Google Scholar]
- Huettel, M. , Berg, P. , & Kostka, J. E. (2014). Benthic exchange and biogeochemical cycling in permeable sediments. Annual Review of Marine Science, 6(1), 23–51. 10.1146/annurev-marine-051413-012706 [DOI] [PubMed] [Google Scholar]
- Hulthe, G. , Hulth, S. , & Hall, P. O. J. (1998). Effect of oxygen on degradation rate of refractory and labile organic matter in continental margin sediments. Geochimica et Cosmochimica Acta, 8, 1319–1328. 10.1016/S0016-7037(98)00044-1 [DOI] [Google Scholar]
- Jennings, S. , Dinmore, T. A. , Duplisea, D. E. , Warr, K. J. , & Lancaster, J. E. (2001). Trawling disturbance can modify benthic production processes. Journal of Animal Ecology, 70(3), 459–475. 10.1046/j.1365-2656.2001.00504.x [DOI] [Google Scholar]
- Jennings, S. , Nicholson, M. D. , Dinmore, T. A. , & Lancaster, J. E. (2002). Effects of chronic trawling disturbance on the production of infaunal communities. Marine Ecology Progress Series, 243, 251–260. 10.3354/meps243251 [DOI] [Google Scholar]
- Jones, J. B. (1992). Environmental impact of trawling on the seabed: A review. New Zealand Journal of Marine and Freshwater Research, 26(1), 59–67. 10.1080/00288330.1992.9516500 [DOI] [Google Scholar]
- Kaiser, M. J. , Clarke, K. R. , Hinz, H. , Austen, M. C. V. , Somerfield, P. J. , & Karakassis, I. (2006). Global analysis of response and recovery of benthic biota to fishing. Marine Ecology Progress Series, 311, 1–14. 10.3354/meps311001 [DOI] [Google Scholar]
- Kaiser, M. J. , Collie, J. S. , Hall, S. J. , Jennings, S. , & Poiner, I. R. (2002). Modification of marine habitats by trawling activities prognosis and solutions. Fish and Fisheries, 3, 114–136. 10.1046/j.1467-2979.2002.00079.x [DOI] [Google Scholar]
- Keil, R. (2017). Anthropogenic forcing of carbonate and organic carbon preservation in marine sediments. Annual Review of Marine Science, 9(1), 151–172. 10.1146/annurev-marine-010816-060724 [DOI] [PubMed] [Google Scholar]
- Khatiwala, S. , Primeau, F. , & Hall, T. (2009). Reconstruction of the history of anthropogenic CO2 concentrations in the ocean. Nature, 462(7271), 346–349. 10.1038/nature08526 [DOI] [PubMed] [Google Scholar]
- Krause‐Jensen, D. , & Duarte, C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nature Geoscience, 9(10), 737–742. 10.1038/ngeo2790 [DOI] [Google Scholar]
- Kristensen, E. , Ahmed, S. I. , & Devol, A. H. (1995). Aerobic and anaerobic decomposition of organic matter in marine sediment: Which is fastest? Limnology and Oceanography, 40(8), 1430–1437. 10.4319/lo.1995.40.8.1430 [DOI] [Google Scholar]
- Kroodsma, D. A. , Mayorga, J. , Hochberg, T. , Miller, N. A. , Boerder, K. , Ferretti, F. , Wilson, A. , Bergman, B. , White, T. D. , Block, B. A. , Woods, P. , Sullivan, B. , Costello, C. , & Worm, B. (2018). Tracking the global footprint of fisheries. Science, 359(6378), 904–908. 10.1126/science.aao5646 [DOI] [PubMed] [Google Scholar]
- Lamarque, B. , Deflandre, B. , Galindo Dalto, A. , Schmidt, S. , Romero‐Ramirez, A. , Garabetian, F. , Dubosq, N. , Diaz, M. , Grasso, F. , Sottolichio, A. , Bernard, G. , Gillet, H. , Cordier, M.‐A. , Poirier, D. , Lebleu, P. , Derriennic, H. , Danilo, M. , Murilo Barboza Tenório, M. , & Grémare, A. (2021). Spatial distributions of surface sedimentary organics and sediment profile image characteristics in a high‐energy temperate marine RiOMar: The west gironde mud patch. Journal of Marine Science and Engineering, 9(3), 29. 10.3390/jmse9030242 [DOI] [Google Scholar]
- LaRowe, D. E. , Arndt, S. , Bradley, J. A. , Estes, E. R. , Hoarfrost, A. , Lang, S. Q. , Lloyd, K. G. , Mahmoudi, N. , Orsi, W. D. , Shah Walter, S. R. , Steen, A. D. , & Zhao, R. (2020). The fate of organic carbon in marine sediments—New insights from recent data and analysis. Earth‐Science Reviews, 204, 103146. 10.1016/j.earscirev.2020.103146 [DOI] [Google Scholar]
- Larsen, T. , Ventura, M. , Andersen, N. , O’Brien, D. M. , Piatkowski, U. , & McCarthy, M. D. (2013). Tracing carbon sources through aquatic and terrestrial food webs using amino acid stable isotope fingerprinting. PLoS One, 8(9), e73441. 10.1371/journal.pone.0073441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, F. , & Sundbäck, K. (2012). Recovery of microphytobenthos and benthic functions after sediment deposition. Marine Ecology Progress Series, 446, 31–44. 10.3354/meps09488 [DOI] [Google Scholar]
- Lee, T. R. , Wood, W. T. , & Phrampus, B. J. (2019). A machine learning (kNN) approach to predicting global seafloor total organic carbon. Global Biogeochemical Cycles, 33(1), 37–46. 10.1029/2018gb005992 [DOI] [Google Scholar]
- Legge, O. , Johnson, M. , Hicks, N. , Jickells, T. , Diesing, M. , Aldridge, J. , Andrews, J. , Artioli, Y. , Bakker, D. C. E. , Burrows, M. T. , Carr, N. , Cripps, G. , Felgate, S. L. , Fernand, L. , Greenwood, N. , Hartman, S. , Kröger, S. , Lessin, G. , Mahaffey, C. , … Williamson, P. (2020). Carbon on the Northwest European Shelf: Contemporary budget and future influences. Frontiers in Marine Science, 7, Article 143. 10.3389/fmars.2020.00143 [DOI] [Google Scholar]
- Lindeboom, H. J. , & de Groot, S. (1998). The effects of different types of fisheries on the north sea and Irish sea benthic ecosystems. IMACT ‐II: NIOZ‐RAPPORT 1998‐1.
- Liu, X. S. , Xu, W. Z. , Cheung, S. G. , & Shin, P. K. S. (2011). Response of meiofaunal community with special reference to nematodes upon deployment of artificial reefs and cessation of bottom trawling in subtropical waters, Hong Kong. Marine Pollution Bulletin, 63(5–12), 376–384. 10.1016/j.marpolbul.2010.11.019 [DOI] [PubMed] [Google Scholar]
- Lovelock, C. E. , & Duarte, C. M. (2019). Dimensions of Blue Carbon and emerging perspectives. Biology Letters, 15(3), 20180781. 10.1098/rsbl.2018.0781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelock, C. E. , Fourqurean, J. W. , & Morris, J. T. (2017). Modeled CO2 emissions from coastal wetland transitions to other land uses: Tidal Marshes, Mangrove Forests, and Seagrass Beds. Frontiers in Marine Science, 4, 143. 10.3389/fmars.2017.00143 [DOI] [Google Scholar]
- Luisetti, T. , Turner, R. K. , Andrews, J. E. , Jickells, T. D. , Kröger, S. , Diesing, M. , Paltriguera, L. , Johnson, M. T. , Parker, E. R. , Bakker, D. C. E. , & Weston, K. (2019). Quantifying and valuing carbon flows and stores in coastal and shelf ecosystems in the UK. Ecosystem Services, 35, 67–76. 10.1016/j.ecoser.2018.10.013 [DOI] [Google Scholar]
- MacIntyre, H. L. , Geider, R. J. , & Miller, D. C. (1996). Microphytobenthos: The ecological role of the “secret garden” of unvegetated, shallow‐water marine habitats. I. Distribution, abundance and primary production. Estuaries, 19(2), 186–201. 10.2307/1352224 [DOI] [Google Scholar]
- Macreadie, P. I. , Costa, M. D. P. , Atwood, T. B. , Friess, D. A. , Kelleway, J. J. , Kennedy, H. , Lovelock, C. E. , Serrano, O. , & Duarte, C. M. (2021). Blue carbon as a natural climate solution. Nature Reviews Earth & Environment, 2(12), 826–839. 10.1038/s43017-021-00224-1 [DOI] [Google Scholar]
- Mandal, A. , Dutta, A. , Das, R. , & Mukherjee, J. (2021). Role of intertidal microbial communities in carbon dioxide sequestration and pollutant removal: A review. Marine Pollution Bulletin, 170, 112626. 10.1016/j.marpolbul.2021.112626 [DOI] [PubMed] [Google Scholar]
- Mariani, G. , Cheung, W. W. L. , Lyet, A. , Sala, E. , Mayorga, J. , Velez, L. , Gaines, S. D. , Dejean, T. , Troussellier, M. , & Mouillot, D. (2020). Let more big fish sink: Fisheries prevent blue carbon sequestration—half in unprofitable areas. Science Advances, 6(44), eabb4848. 10.1126/sciadv.abb4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marine Conservation Institute . (2021). MPA Atlas. Retrieved from www.mpaatlas.org [Google Scholar]
- Martín, J. , Palanques, A. , & Puig, P. (2006). Composition and variability of downward particulate matter fluxes in the Palamós submarine canyon (NW Mediterranean). Journal of Marine Systems, 60(1–2), 75–97. 10.1016/j.jmarsys.2005.09.010 [DOI] [Google Scholar]
- Martín, J. , Puig, P. , Masque, P. , Palanques, A. , & Sanchez‐Gomez, A. (2014). Impact of bottom trawling on deep‐sea sediment properties along the flanks of a submarine canyon. PLoS One, 9(8), e104536. 10.1371/journal.pone.0104536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín, J. , Puig, P. , Palanques, A. , & Giamportone, A. (2014). Commercial bottom trawling as a driver of sediment dynamics and deep seascape evolution in the Anthropocene. Anthropocene, 7, 1–15. 10.1016/j.ancene.2015.01.002 [DOI] [Google Scholar]
- Martín, J. , Puig, P. , Palanques, A. , Masqué, P. , & García‐Orellana, J. (2008). Effect of commercial trawling on the deep sedimentation in a Mediterranean submarine canyon. Marine Geology, 252(3–4), 150–155. 10.1016/j.margeo.2008.03.012 [DOI] [Google Scholar]
- Mayer, L. M. , Schick, D. F. , Findlay, R. H. , & Rice, D. L. (1991). Effects of commercial dragging on sedimentary organic matter. Marine Environmental Research, 31, 249–261. 10.1016/0141-1136(91)90015-Z [DOI] [Google Scholar]
- McArthur, M. A. , Brooke, B. P. , Przeslawski, R. , Ryan, D. A. , Lucieer, V. L. , Nichol, S. , McCallum, A. W. , Mellin, C. , Cresswell, I. D. , & Radke, L. C. (2010). On the use of abiotic surrogates to describe marine benthic biodiversity. Estuarine, Coastal and Shelf Science, 88(1), 21–32. 10.1016/j.ecss.2010.03.003 [DOI] [Google Scholar]
- McLaverty, C. , Eigaard, O. R. , Dinesen, G. E. , Gislason, H. , Kokkalis, A. , Erichsen, A. C. , & Petersen, J. K. (2020). High‐resolution fisheries data reveal effects of bivalve dredging on benthic communities in stressed coastal systems. Marine Ecology Progress Series, 642, 21–38. 10.3354/meps13330 [DOI] [Google Scholar]
- McLeod, E. , Chmura, G. L. , Bouillon, S. , Salm, R. , Björk, M. , Duarte, C. M. , Lovelock, C. E. , Schlesinger, W. H. , & Silliman, B. R. (2011). A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2 . Frontiers in Ecology and the Environment, 9(10), 552–560. 10.1890/110004 [DOI] [Google Scholar]
- Mengual, B. , Cayocca, F. , Le Hir, P. , Draye, R. , Laffargue, P. , Vincent, B. , & Garlan, T. (2016). Influence of bottom trawling on sediment resuspension in the ‘Grande‐Vasière’ area (Bay of Biscay, France). Ocean Dynamics, 66(9), 1181–1207. 10.1007/s10236-016-0974-7 [DOI] [Google Scholar]
- Mercaldo‐Allen, R. , Goldberg, R. , Clark, P. , Kuropat, C. , Meseck, S. L. , & Rose, J. M. (2016). Benthic ecology of northern quahog beds with different hydraulic dredging histories in long island sound. Journal of Coastal Research, 32(2), 408–415. 10.2112/JCOASTRES-D-15-00055.1 [DOI] [Google Scholar]
- Meseck, S. L. , Mercaldo‐Allen, R. , Rose, J. M. , Clark, P. , Kuropat, C. , Pereira, J. J. , & Goldberg, R. (2014). Effects of hydraulic dredging for mercenaria mercenaria, Northern Quahog, on sediment biogeochemistry. Journal of the World Aquaculture Society, 45(3), 301–311. 10.1111/jwas.12114 [DOI] [Google Scholar]
- Meysman, F. J. R. , Middelburg, J. J. , & Heip, C. H. R. (2006). Bioturbation: A fresh look at Darwin's last idea. Trends in Ecology & Evolution, 21(12), 688–695. 10.1016/j.tree.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Middelburg, J. J. (2018). Reviews and syntheses: To the bottom of carbon processing at the seafloor. Biogeosciences, 15(2), 413–427. 10.5194/bg-15-413-2018 [DOI] [Google Scholar]
- Miller, D. C. , Geider, R. J. , & MacIntyre, H. L. (1996). Microphytobenthos: The ecological role of the “secret garden” of unvegetated, shallow‐water marine habitats. II. Role in sediment stability and shallow‐water food webs. Estuaries, 19(2), 202–212. 10.2307/1352225 [DOI] [Google Scholar]
- Montserrat, F. , Van Colen, C. , Degraer, S. , Ysebaert, T. , & Herman, P. M. J. (2008). Benthic community‐mediated sediment dynamics. Marine Ecology Progress Series, 372, 43–59. 10.3354/meps07769 [DOI] [Google Scholar]
- Morato, T. , Watson, R. , Pitcher, T. J. , & Pauly, D. (2006). Fishing down the deep. Fish and Fisheries, 7(1), 24–34. 10.1111/j.1467-2979.2006.00205.x [DOI] [Google Scholar]
- Morys, C. , Brüchert, V. , & Bradshaw, C. (2021). Impacts of bottom trawling on benthic biogeochemistry: An experimental field study. Marine Environmental Research, 169, 105384. 10.1016/j.marenvres.2021.105384 [DOI] [PubMed] [Google Scholar]
- Nellemann, C. , Corcoran, E. , Duarte, C. M. , Valdés, L. , De Young, C. , Fonseca, L. , & Grimsditch, G. (2009). Blue carbon: A rapid response assessment. United Nations Environment Programme, GRID‐Arendal. [Google Scholar]
- O’Hara, C. C. , Frazier, M. , & Halpern, B. S. (2021). At‐risk marine biodiversity faces extensive, expanding, and intensifying human impacts. Science, 372(6537), 84–87. 10.1126/science.abe6731 [DOI] [PubMed] [Google Scholar]
- Oberle, F. K. J. , Storlazzi, C. D. , & Hanebuth, T. J. J. (2016). What a drag: Quantifying the global impact of chronic bottom trawling on continental shelf sediment. Journal of Marine Systems, 159, 109–119. 10.1016/j.jmarsys.2015.12.007 [DOI] [Google Scholar]
- Oberle, F. K. J. , Swarzenski, P. W. , Reddy, C. M. , Nelson, R. K. , Baasch, B. , & Hanebuth, T. J. J. (2016). Deciphering the lithological consequences of bottom trawling to sedimentary habitats on the shelf. Journal of Marine Systems, 159, 120–131. 10.1016/j.jmarsys.2015.12.008 [DOI] [Google Scholar]
- O'Neill, F. G. , & Ivanović, A. (2015). The physical impact of towed demersal fishing gears on soft sediments. ICES Journal of Marine Science, 73(suppl_1), i5–i14. 10.1093/icesjms/fsv125 [DOI] [Google Scholar]
- O'Neill, F. G. , & Summerbell, K. D. (2016). The hydrodynamic drag and the mobilisation of sediment into the water column of towed fishing gear components. Journal of Marine Systems, 164, 76–84. 10.1016/j.jmarsys.2016.08.008 [DOI] [Google Scholar]
- O'Neill, F. G. , Summerbell, K. , & Ivanović, A. (2018). The contact drag of towed demersal fishing gear components. Journal of Marine Systems, 177, 39–52. 10.1016/j.jmarsys.2017.08.002 [DOI] [Google Scholar]
- Ordines, F. , Ramón, M. , Rivera, J. , Rodríguez‐Prieto, C. , Farriols, M. T. , Guijarro, B. , Pasqual, C. , & Massutí, E. (2017). Why long term trawled red algae beds off Balearic Islands (western Mediterranean) still persist? Regional Studies in Marine Science, 15, 39–49. 10.1016/j.rsma.2017.07.005 [DOI] [Google Scholar]
- Pacoureau, N. , Rigby, C. L. , Kyne, P. M. , Sherley, R. B. , Winker, H. , Carlson, J. K. , Fordham, S. V. , Barreto, R. , Fernando, D. , Francis, M. P. , Jabado, R. W. , Herman, K. B. , Liu, K.‐M. , Marshall, A. D. , Pollom, R. A. , Romanov, E. V. , Simpfendorfer, C. A. , Yin, J. S. , Kindsvater, H. K. , & Dulvy, N. K. (2021). Half a century of global decline in oceanic sharks and rays. Nature, 589(7843), 567–571. 10.1038/s41586-020-03173-9 [DOI] [PubMed] [Google Scholar]
- Palanques, A. , Guillén, J. , & Puig, P. (2001). Impact of bottom trawling on water turbidity and muddy sediment of an unfished continental shelf. Limnology and Oceanography, 46(5), 1100–1110. 10.4319/lo.2001.46.5.1100 [DOI] [Google Scholar]
- Palanques, A. , Martín, J. , Puig, P. , Guillén, J. , Company, J. B. , & Sardà, F. (2006). Evidence of sediment gravity flows induced by trawling in the Palamós (Fonera) submarine canyon (northwestern Mediterranean). Deep Sea Research Part I: Oceanographic Research Papers, 53(2), 201–214. 10.1016/j.dsr.2005.10.003 [DOI] [Google Scholar]
- Palanques, A. , Puig, P. , Guillén, J. , Demestre, M. , & Martín, J. (2014). Effects of bottom trawling on the Ebro continental shelf sedimentary system (NW Mediterranean). Continental Shelf Research, 72, 83–98. 10.1016/j.csr.2013.10.008 [DOI] [Google Scholar]
- Paradis, S. , Goñi, M. , Masqué, P. , Durán, R. , Arjona‐Camas, M. , Palanques, A. , & Puig, P. (2021). Persistence of biogeochemical alterations of deep‐sea sediments by bottom trawling. Geophysical Research Letters, 48(2), e2020GL091279. 10.1029/2020gl091279 [DOI] [Google Scholar]
- Paradis, S. , Puig, P. , Sanchez‐Vidal, A. , Masqué, P. , Garcia‐Orellana, J. , Calafat, A. , & Canals, M. (2018). Spatial distribution of sedimentation‐rate increases in Blanes Canyon caused by technification of bottom trawling fleet. Progress in Oceanography, 169, 241–252. 10.1016/j.pocean.2018.07.001 [DOI] [Google Scholar]
- Paradis, S. , Pusceddu, A. , Masqué, P. , Puig, P. , Moccia, D. , Russo, T. , & Lo Iacono, C. (2019). Organic matter contents and degradation in a highly trawled area during fresh particle inputs (Gulf of Castellammare, southwestern Mediterranean). Biogeosciences, 16(21), 4307–4320. 10.5194/bg-16-4307-2019 [DOI] [Google Scholar]
- Parker, R. W. R. , Blanchard, J. L. , Gardner, C. , Green, B. S. , Hartmann, K. , Tyedmers, P. H. , & Watson, R. A. (2018). Fuel use and greenhouse gas emissions of world fisheries. Nature Climate Change, 8(4), 333–337. 10.1038/s41558-018-0117-x [DOI] [Google Scholar]
- Pendleton, L. , Donato, D. C. , Murray, B. C. , Crooks, S. , Jenkins, W. A. , Sifleet, S. , Craft, C. , Fourqurean, J. W. , Kauffman, J. B. , Marbà, N. , Megonigal, P. , Pidgeon, E. , Herr, D. , Gordon, D. , & Baldera, A. (2012). Estimating global “Blue Carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS One, 7(9), e43542. 10.1371/journal.pone.0043542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershing, A. J. , Christensen, L. B. , Record, N. R. , Sherwood, G. D. , & Stetson, P. B. (2010). The impact of whaling on the ocean carbon cycle: Why bigger was better. PLoS One, 5(8), e12444. 10.1371/journal.pone.0012444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnegar, J. K. , & Engelhard, G. H. (2008). The ‘shifting baseline’ phenomenon: A global perspective. Reviews in Fish Biology and Fisheries, 18(1), 1–16. 10.1007/s11160-007-9058-6 [DOI] [Google Scholar]
- Polymenakou, P. N. , Pusceddu, A. , Tselepides, A. , Polychronaki, T. , Giannakourou, A. , Fiordelmondo, C. , Hatziyanni, E. , & Danovaro, R. (2005). Benthic microbial abundance and activities in an intensively trawled ecosystem (Thermaikos Gulf, Aegean Sea). Continental Shelf Research, 25(19–20), 2570–2584. 10.1016/j.csr.2005.08.018 [DOI] [Google Scholar]
- Puig, P. , Canals, M. , Company, J. B. , Martin, J. , Amblas, D. , Lastras, G. , & Palanques, A. (2012). Ploughing the deep sea floor. Nature, 489(7415), 286–289. 10.1038/nature11410 [DOI] [PubMed] [Google Scholar]
- Pusceddu, A. , Bianchelli, S. , & Danovaro, R. (2015). Quantity and biochemical composition of particulate organic matter in a highly trawled area (Thermaikos Gulf, Eastern Mediterranean Sea). Advances in Oceanography and Limnology, 6(1/2), 21–34. 10.4081/aiol.2015.5448 [DOI] [Google Scholar]
- Pusceddu, A. , Bianchelli, S. , Martin, J. , Puig, P. , Palanques, A. , Masque, P. , & Danovaro, R. (2014). Chronic and intensive bottom trawling impairs deep‐sea biodiversity and ecosystem functioning. Proceedings of the National Academy of Sciences of the United States of America, 111(24), 8861–8866. 10.1073/pnas.1405454111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu, A. , Fiordelmondo, C. , Polymenakou, P. , Polychronaki, T. , Tselepides, A. , & Danovaro, R. (2005). Effects of bottom trawling on the quantity and biochemical composition of organic matter in coastal marine sediments (Thermaikos Gulf, northwestern Aegean Sea). Continental Shelf Research, 25(19–20), 2491–2505. 10.1016/j.csr.2005.08.013 [DOI] [Google Scholar]
- Pusceddu, A. , Grémare, A. , Escoubeyrou, K. , Amouroux, J. M. , Fiordelmondo, C. , & Danovaro, R. (2005). Impact of natural (storm) and anthropogenic (trawling) sediment resuspension on particulate organic matter in coastal environments. Continental Shelf Research, 25(19–20), 2506–2520. 10.1016/j.csr.2005.08.012 [DOI] [Google Scholar]
- Queirós, A. M. , Hiddink, J. G. , Kaiser, M. J. , & Hinz, H. (2006). Effects of chronic bottom trawling disturbance on benthic biomass, production and size spectra in different habitats. Journal of Experimental Marine Biology and Ecology, 335(1), 91–103. 10.1016/j.jembe.2006.03.001 [DOI] [Google Scholar]
- Rajesh, N. , Muthuvelu, S. , Mahadevan, G. , & Murugesan, P. (2019). Impact of bottom trawling on water and sediment characteristics of Cuddalore and Parangipettai coastal waters. Indian Journal of Geo‐Marine Sciences, 48(5), 639–646. [Google Scholar]
- Ramalho, S. P. , Almeida, M. , Esquete, P. , Génio, L. , Ravara, A. , Rodrigues, C. F. , Lampadariou, N. , Vanreusel, A. , & Cunha, M. R. (2018). Bottom‐trawling fisheries influence on standing stocks, composition, diversity and trophic redundancy of macrofaunal assemblages from the West Iberian Margin. Deep‐Sea Research Part I: Oceanographic Research Papers, 138, 131–145. 10.1016/j.dsr.2018.06.004 [DOI] [Google Scholar]
- Riekenberg, P. M. , Oakes, J. M. , & Eyre, B. D. (2020). Shining light on priming in euphotic sediments: Nutrient enrichment stimulates export of stored organic matter. Environmental Science & Technology, 54(18), 11165–11172. 10.1021/acs.est.0c01914 [DOI] [PubMed] [Google Scholar]
- Rijnsdorp, A. D. , Hiddink, J. G. , van Denderen, P. D. , Hintzen, N. T. , Eigaard, O. R. , Valanko, S. , Bastardie, F. , Bolam, S. G. , Boulcott, P. , Egekvist, J. , Garcia, C. , van Hoey, G. , Jonsson, P. , Laffargue, P. , Nielsen, J. R. , Piet, G. J. , Sköld, M. , & van Kooten, T. (2020). Different bottom trawl fisheries have a differential impact on the status of the North Sea seafloor habitats. ICES Journal of Marine Science, 77(5), 1772–1786. 10.1093/icesjms/fsaa050 [DOI] [Google Scholar]
- Roberts, C. M. (2007). The unnatural history of the sea. Island Press. [Google Scholar]
- Roberts, C. M. , O’Leary, B. C. , McCauley, D. J. , Cury, P. M. , Duarte, C. M. , Lubchenco, J. , Pauly, D. , Sáenz‐Arroyo, A. , Sumaila, U. R. , Wilson, R. W. , Worm, B. , & Castilla, J. C. (2017). Marine reserves can mitigate and promote adaptation to climate change. Proceedings of the National Academy of Sciences of the United States of America, 114(24), 6167–6175. 10.1073/pnas.1701262114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, C. M. , O'Leary, B. C. , & Hawkins, J. P. (2020). Climate change mitigation and nature conservation both require higher protected area targets. Philosophical Transactions of the Royal Society B: Biological Sciences, 375(1794), 20190121. 10.1098/rstb.2019.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosli, N. , Leduc, D. , Rowden, A. A. , Clark, M. R. , Probert, P. K. , Berkenbusch, K. , & Neira, C. (2016). Differences in meiofauna communities with sediment depth are greater than habitat effects on the New Zealand continental margin: implications for vulnerability to anthropogenic disturbance. Peerj, 4, 39. 10.7717/peerj.2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffin, K. K. (1998). The persistence of anthropogenic turbidity plumes in a shallow water estuary. Estuarine, Coastal and Shelf Science, 47(5), 579–592. 10.1006/ecss.1998.0366 [DOI] [Google Scholar]
- Rühl, S. , Thompson, C. , Queirós, A. M. , & Widdicombe, S. (2020). Missing links in the study of solute and particle exchange between the sea floor and water column. ICES Journal of Marine Science, 77(5), 1602–1616. 10.1093/icesjms/fsaa060 [DOI] [Google Scholar]
- Saba, G. K. , Burd, A. B. , Dunne, J. P. , Hernández‐León, S. , Martin, A. H. , Rose, K. A. , Salisbury, J. , Steinberg, D. K. , Trueman, C. N. , Wilson, R. W. , & Wilson, S. E. (2021). Toward a better understanding of fish‐based contribution to ocean carbon flux. Limnology and Oceanography, 66(5), 1639–1664. 10.1002/lno.11709 [DOI] [Google Scholar]
- Sabine, C. L. , & Tanhua, T. (2010). Estimation of anthropogenic CO2 inventories in the ocean. Annual Review of Marine Science, 2, 175–198. 10.1146/annurev-marine-120308-080947 [DOI] [PubMed] [Google Scholar]
- Sala, E. , Mayorga, J. , Bradley, D. , Cabral, R. B. , Atwood, T. B. , Auber, A. , Cheung, W. , Costello, C. , Ferretti, F. , Friedlander, A. M. , Gaines, S. D. , Garilao, C. , Goodell, W. , Halpern, B. S. , Hinson, A. , Kaschner, K. , Kesner‐Reyes, K. , Leprieur, F. , McGowan, J. , … Lubchenco, J. (2021). Protecting the global ocean for biodiversity, food and climate. Nature, 592, 397–402. 10.1038/s41586-021-03371-z [DOI] [PubMed] [Google Scholar]
- Sarmiento, J. L. , & Gruber, N. (2006). Ocean biogeochemical dynamics. Princeton University Press. [Google Scholar]
- Schratzberger, M. , Lampadariou, N. , Somerfield, P. J. , Vandepitte, L. , & Vanden Berghe, E. (2009). The impact of seabed disturbance on nematode communities: Linking field and laboratory observations. Marine Biology, 156(4), 709–724. 10.1007/s00227-008-1122-9 [DOI] [Google Scholar]
- Sciberras, M. , Hiddink, J. G. , Jennings, S. , Szostek, C. L. , Hughes, K. M. , Kneafsey, B. , Clarke, L. J. , Ellis, N. , Rijnsdorp, A. D. , McConnaughey, R. A. , Hilborn, R. , Collie, J. S. , Pitcher, C. R. , Amoroso, R. O. , Parma, A. M. , Suuronen, P. , & Kaiser, M. J. (2018). Response of benthic fauna to experimental bottom fishing: A global meta‐analysis. Fish and Fisheries, 19(4), 698–715. 10.1111/faf.12283 [DOI] [Google Scholar]
- Sciberras, M. , Parker, R. , Powell, C. , Robertson, C. , Kröger, S. , Bolam, S. , & Geert Hiddink, J. (2016). Impacts of bottom fishing on the sediment infaunal community and biogeochemistry of cohesive and non‐cohesive sediments. Limnology and Oceanography, 61(6), 2076–2089. 10.1002/lno.10354 [DOI] [Google Scholar]
- Seddon, N. , Turner, B. , Berry, P. , Chausson, A. , & Girardin, C. A. J. (2019). Grounding nature‐based climate solutions in sound biodiversity science. Nature Climate Change, 9(2), 84–87. 10.1038/s41558-019-0405-0 [DOI] [Google Scholar]
- Seiter, K. , Hensen, C. , Schröter, J. , & Zabel, M. (2004). Organic carbon content in surface sediments—Defining regional provinces. Deep Sea Research Part I: Oceanographic Research Papers, 51(12), 2001–2026. 10.1016/j.dsr.2004.06.014 [DOI] [Google Scholar]
- Serpetti, N. , Gontikaki, E. , Narayanaswamy, B. E. , & Witte, U. (2013). Macrofaunal community inside and outside of the Darwin Mounds Special Area of Conservation, NE Atlantic. Biogeosciences, 10(6), 3705–3714. 10.5194/bg-10-3705-2013 [DOI] [Google Scholar]
- Sheridan, P. , & Doerr, J. (2005). Short‐term effects of the cessation of shrimp trawling on Texas benthic habitats. American Fisheries Society Symposium, 41, 571–578. [Google Scholar]
- Siegel, D. A. , DeVries, T. , Doney, S. C. , & Bell, T. (2021). Assessing the sequestration time scales of some ocean‐based carbon dioxide reduction strategies. Environmental Research Letters, 16(10), 104003. 10.1088/1748-9326/ac0be0 [DOI] [Google Scholar]
- Smeaton, C. , Hunt, C. A. , Turrell, W. R. , & Austin, W. E. N. (2021). Marine sedimentary carbon stocks of the United Kingdom’s exclusive economic zone. Frontiers in Earth Science, 9, 50. 10.3389/feart.2021.593324 [DOI] [Google Scholar]
- Smith, C. (2000). Impact of otter trawling on an eastern Mediterranean commercial trawl fishing ground. ICES Journal of Marine Science, 57(5), 1340–1351. 10.1006/jmsc.2000.0927 [DOI] [Google Scholar]
- Smith, J. D. , & McLean, S. R. (1977). Boundary layer adjustments to bottom topography and suspended sediment. In Nihoul J. C. J. (Ed.), Elsevier oceanography series (Vol. 19, pp. 123–151). Elsevier. [Google Scholar]
- Snelgrove, P. V. R. , Soetaert, K. , Solan, M. , Thrush, S. , Wei, C.‐L. , Danovaro, R. , Fulweiler, R. W. , Kitazato, H. , Ingole, B. , Norkko, A. , Parkes, R. J. , & Volkenborn, N. (2018). Global carbon cycling on a heterogeneous seafloor. Trends in Ecology & Evolution, 33(2), 96–105. 10.1016/j.tree.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Soetaert, K. , Herman, P. M. J. , & Middelburg, J. J. (1996). A model of early diagenetic processes from the shelf to abyssal depths. Geochimica et Cosmochimica Acta, 60(6), 1019–1040. 10.1016/0016-7037(96)00013-0 [DOI] [Google Scholar]
- Soulsby, R. (1997). Dynamics of marine sands: A manual for practical applications. Thomas Telford. [Google Scholar]
- Thrush, S. F. , & Dayton, P. K. (2002). Disturbance to marine benthic habitats by trawling and dredging: Implications for marine biodiversity. Annual Review of Ecology and Systematics, 33(1), 449–473. 10.1146/annurev.ecolsys.33.010802.150515 [DOI] [Google Scholar]
- Tiano, J. C. , De Borger, E. , O'Flynn, S. , Cheng, C. H. , van Oevelen, D. , & Soetaert, K. (2021). Physical and electrical disturbance experiments uncover potential bottom fishing impacts on benthic ecosystem functioning. Journal of Experimental Marine Biology and Ecology, 545, 151628. 10.1016/j.jembe.2021.151628 [DOI] [Google Scholar]
- Tiano, J. C. , van der Reijden, K. J. , O'Flynn, S. , Beauchard, O. , van der Ree, S. , van der Wees, J. , Ysebaert, T. , & Soetaert, K. (2020). Experimental bottom trawling finds resilience in large‐bodied infauna but vulnerability for epifauna and juveniles in the Frisian Front. Marine Environmental Research, 159, 104964. 10.1016/j.marenvres.2020.104964 [DOI] [PubMed] [Google Scholar]
- Tiano, J. C. , Witbaard, R. , Bergman, M. J. N. , van Rijswijk, P. , Tramper, A. , van Oevelen, D. , & Degraer, S. (2019). Acute impacts of bottom trawl gears on benthic metabolism and nutrient cycling. ICES Journal of Marine Science, 76(6), 1917–1930. 10.1093/icesjms/fsz060 [DOI] [Google Scholar]
- Tillin, H. M. , Hiddink, J. G. , Jennings, S. , & Kaiser, M. J. (2006). Chronic bottom trawling alters the functional composition of benthic invertebrate communities on a sea‐basin scale. Marine Ecology Progress Series, 318, 31–45. 10.3354/meps318031 [DOI] [Google Scholar]
- Trimmer, M. , Petersen, J. , Sivyer, D. B. , Mills, C. , Young, E. , & Parker, E. R. (2005). Impact of long‐term benthic trawl disturbance on sediment sorting and biogeochemistry in the southern North Sea. Marine Ecology Progress Series, 298, 79–94. 10.3354/meps298079 [DOI] [Google Scholar]
- Trueman, C. N. , Johnston, G. , O'Hea, B. , & MacKenzie, K. M. (2014). Trophic interactions of fish communities at midwater depths enhance long‐term carbon storage and benthic production on continental slopes. Proceedings of the Royal Society B: Biological Sciences, 281(1787), 20140669. 10.1098/rspb.2014.0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, J. T. (2015). Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump. Progress in Oceanography, 130, 205–248. 10.1016/j.pocean.2014.08.005 [DOI] [Google Scholar]
- van de Velde, S. , Van Lancker, V. , Hidalgo‐Martinez, S. , Berelson, W. M. , & Meysman, F. J. R. (2018). Anthropogenic disturbance keeps the coastal seafloor biogeochemistry in a transient state. Scientific Reports, 8(1), 5582. 10.1038/s41598-018-23925-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Molen, J. , Aldridge, J. N. , Coughlan, C. , Parker, E. R. , Stephens, D. , & Ruardij, P. (2012). Modelling marine ecosystem response to climate change and trawling in the North Sea. Biogeochemistry, 113(1–3), 213–236. 10.1007/s10533-012-9763-7 [DOI] [Google Scholar]
- van Nugteren, P. , Moodley, L. , Brummer, G.‐J. , Heip, C. H. R. , Herman, P. M. J. , & Middelburg, J. J. (2009). Seafloor ecosystem functioning: The importance of organic matter priming. Marine Biology, 156(11), 2277–2287. 10.1007/s00227-009-1255-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainright, S. C. , & Hopkinson, C. S. Jr (1997). Effects of sediment resuspension on organic matter processing in coastal environments: A simulation model. Journal of Marine Systems, 11, 353–368. 10.1016/S0924-7963(96)00130-3 [DOI] [Google Scholar]
- Wang, Z. , Leung, K. M. Y. , Sung, Y. H. , Dudgeon, D. , & Qiu, J. W. (2021). Recovery of tropical marine benthos after a trawl ban demonstrates linkage between abiotic and biotic changes. Communications Biology, 4(1), 212. 10.1038/s42003-021-01732-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling, L. , Findlay, R. H. , Mayer, L. M. , & Schick, D. F. (2001). Impact of a scallop drag on the sediment chemistry, microbiota, and faunal assemblages of a shallow subtidal marine benthic community. Journal of Sea Research, 46, 309–324. 10.1016/S1385-1101(01)00083-1 [DOI] [Google Scholar]
- Watson, A. J. , Schuster, U. , Shutler, J. D. , Holding, T. , Ashton, I. G. C. , Landschützer, P. , Woolf, D. K. , & Goddijn‐Murphy, L. (2020). Revised estimates of ocean‐atmosphere CO2 flux are consistent with ocean carbon inventory. Nature Communications, 11(1), 4422. 10.1038/s41467-020-18203-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, R. A. , & Morato, T. (2013). Fishing down the deep: Accounting for within‐species changes in depth of fishing. Fisheries Research, 140, 63–65. 10.1016/j.fishres.2012.12.004 [DOI] [Google Scholar]
- Wilkinson, G. M. , Besterman, A. , Buelo, C. , Gephart, J. , & Pace, M. L. (2018). A synthesis of modern organic carbon accumulation rates in coastal and aquatic inland ecosystems. Scientific Reports, 8(1), 15736. 10.1038/s41598-018-34126-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterwerp, J. C. , & Kranenburg, C. (2002). Fine sediment dynamics in the marine environment (Vol. 5). Elsevier. [Google Scholar]
- Yallop, M. L. , de Winder, B. , Paterson, D. M. , & Stal, L. J. (1994). Comparative structure, primary production and biogenic stabilization of cohesive and non‐cohesive marine sediments inhabited by microphytobenthos. Estuarine, Coastal and Shelf Science, 39(6), 565–582. 10.1016/S0272-7714(06)80010-7 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article or are openly available in figshare at https://doi.org/10.6084/m9.figshare.16776250.


