Abstract
Objectives
In response COVID‐19, re‐establishing safe elective services was prioritised in the UK. We assess the impact on face‐to‐face hospital attendance, cost and efficiency of implementing a virtual sleep clinic (intervention 1) to screen for children requiring level 3 ambulatory sleep studies using newly implemented ENT‐UK guidelines for obstructive sleep apnoea (OSA) investigation (intervention 2). Objectives: (1) compare the proportion of children attending sleep clinic undertaking a sleep study before and after implementation of these interventions; (2) compare clinic cancellations and first‐time success rates of sleep studies before and after intervention.
Design
Retrospective analysis.
Setting
District general hospital paediatric sleep clinic.
Participants
Children aged 3 months to 16 years referred to sleep clinic by ENT for investigation of OSA over 3 months immediately following interventions (1 June 2020 – 1 September 2020) to the same period in the previous year (1 June 2019 – 1 September 2019).
Main outcome measures
Number of children attending sleep clinic, date of birth/age of children attending sleep clinic, number of children undergoing sleep study, diagnostic outcomes, number of appointment cancellations, number of first‐time sleep study failures.
Results
Post intervention, there was a significant reduction in the proportion of children undertaking ambulatory sleep studies, and nonsignificant reductions in appointment cancellations and in first‐time sleep study failures.
Conclusions
The introduction of the virtual sleep clinic meant that only those children requiring a sleep study attended a face‐to‐face appointment, which led to reduced face‐to‐face attendance. There were also unintended cost‐effectiveness and efficiency benefits, with potential longer‐term learning implications for the wider sleep community and other diagnostic services.
Keywords: children, COVID‐19, health service, quality improvement, sleep
Key points.
In response to COVID‐19, re‐establishing safe elective services remains priority in the UK.
The introduction of a virtual paediatric sleep clinic to screen children aged 3 months to 16 years referred by ENT for investigation of OSA is an effective way of reducing face‐to‐face consultations in this age group.
The implementation of ENT‐UK guidelines for obstructive sleep apnoea (OSA) investigation facilitates the introduction of such a clinic.
Virtual paediatric sleep clinics can result in reductions in the proportion of children undertaking ambulatory sleep studies, in appointment cancellations and in first‐time sleep study failures.
There are also cost‐effectiveness and efficiency benefits, with potential longer‐term learning implications for the wider sleep community and other diagnostic services.
1. BACKGROUND
The WHO declared the COVID‐19 global pandemic a public emergency on 20 January 2020. 1 The UK went into lockdown on 16 March 2020 with all unnecessary social contact ceasing and a suspension of elective services in hospital trusts across the UK. 2
Our busy ambulatory paediatric sleep‐disordered breathing service set in a district general hospital sees a range of children with obstructive sleep apnoea (OSA) secondary to adenotonsillar hypertrophy, obesity or more complex issues (e.g. craniofacial abnormalities, neuromuscular disease or Down's syndrome). Our service uses ambulatory cardiopulmonary/level 3 sleep studies for the diagnosis of OSA. Level 3 devices record three channels of data (pulse oximetry, airflow and respiratory effort) and are becoming more commonly used by sleep services due to their cost‐effectiveness and accessibility. There is no requirement for inpatient stay, and the skillset required to interpret the data is less than for full inpatient polysomnography. The studies are sufficient to diagnose sleep‐disordered breathing (SDB) in children 3 , 4 and in our experience more representative, particularly for noncomplex cases as children generally sleep better at home. After the initial suspension of the sleep service during the lockdown period and the redeployment of staff to acute services, getting this service up and running again became a priority with waiting times increasing from prelockdown referrals.
With social distancing measures in place and new cases of SARS‐CoV‐2 still emerging, we were forced to completely reshape our paediatric sleep service with the aim of reducing face‐to‐face hospital attendance. In this regard, ambulatory sleep studies have an obvious immediate advantage over inpatient studies. But there were still opportunities to potentially reduce hospital attendance.
Here, we describe two interventions we implemented in May 2020 to our paediatric SDB service, which aimed to reduce face‐to‐face hospital attendance. The primary aim of this study is to look at the impact of the interventions on face‐to‐face hospital attendance. The secondary aim is to evaluate the impact on the cost and efficiency of the service.
2. METHODS
Primary and secondary objectives:
Primary objective:
Compare the proportion of children seen by the paediatric sleep‐disordered breathing service, who were referred by ENT for investigation of OSA, undergoing an ambulatory sleep study pre intervention and post intervention.
Secondary objectives:
Compare cancellation rates to the paediatric sleep‐disordered breathing service pre intervention and post intervention.
Compare the first‐time failure rate of level 3 ambulatory sleep studies pre intervention and post intervention.
-
B.
Interventions:
The first intervention we introduced was the implementation of the new clinical guidelines, which were released by ENT‐UK 5 in July 2019. These guidelines state that in noncomplex children with adenotonsillar hypertrophy and no underlying risk factors, clinical assessment is sufficient to accurately assess the severity of OSA, even in more severe cases of OSA. 79% of new patients seen in our sleep clinic are ear, nose and throat (ENT) referrals of children with simple adenotonsillar hypertrophy. Implementing these guidelines had the potential to reduce the number of ambulatory sleep studies performed, all of which require face‐to‐face contact (as a minimum to collect and return sleep equipment).
The second intervention was the introduction of a virtual video sleep consultation (paediatrician‐led). Before the introduction of the virtual clinic, all ENT‐referred OSA patients were seen in a face‐to‐face paediatrician‐led combined consultation and equipment‐fitting clinic. Using the ENT guidelines, the virtual consultation appointment screened for patients requiring a sleep study, with such patients referred to a separate nurse‐led face‐to‐face ‘equipment‐fitting’ clinic. Because level 3 ambulatory studies involve the use of chest and abdominal respiratory inductance plethysmography (RIP) bands, we recommended that the children being investigated attended the sleep equipment‐fitting clinic appointment for sizing purposes. Guidance from our local infection control team on the quarantining and cleaning of equipment between uses and the trust recommendation that only one parent/carer should accompany their child to the appointment was followed accordingly.
For those children not meeting the criteria for a diagnostic sleep study, the severity of the OSA was assessed in the virtual consultation through clinical history taking which included the modified Epworth Sleepiness Scale. 6 Using ENT‐UK recommendations, 5 local guidelines were developed for paediatricians in the virtual sleep clinic to assist in the process for screening patients eligible for a sleep study (Figure 1A). A combination of clinical experience and the reported literature was used to develop guidance on the clinical assessment of OSA severity (Figure 1B). 6 , 7 , 8
-
C.
Assessing the impact of the interventions:
FIGURE 1.
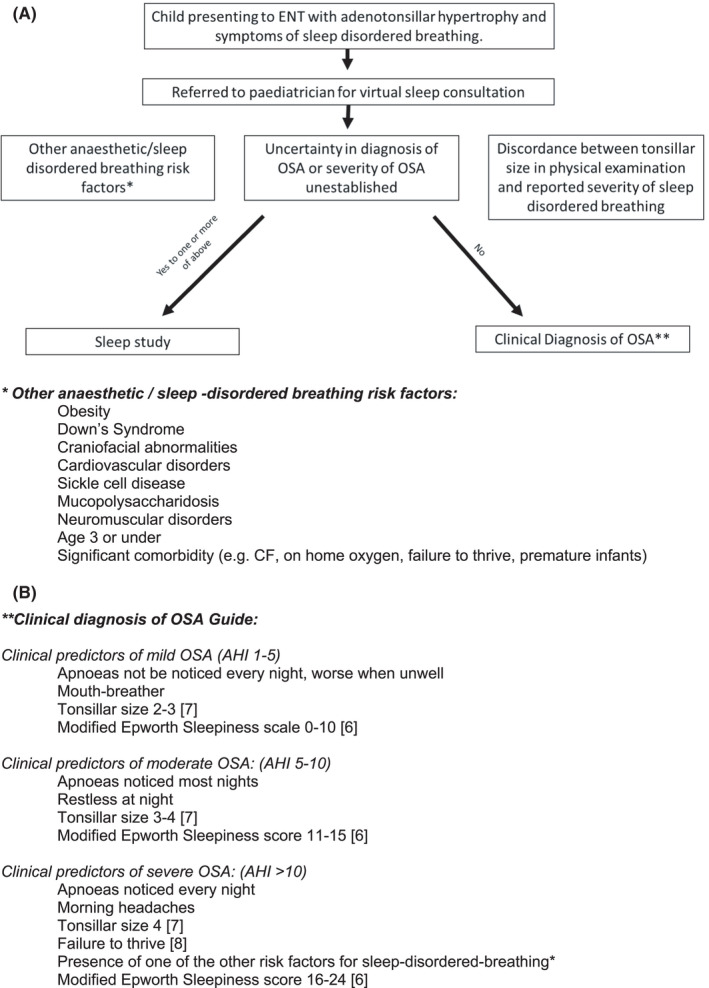
Local guidance on clinical decision‐making for children with adenotonsillar hypertrophy and sleep‐disordered breathing symptoms. Local guidance used by paediatricians to support clinical decision‐making for children with adenotonsillar hypertrophy and sleep‐disordered breathing symptoms. (A) ENT‐UK–derived guidelines 5 on whether a child should be referred for a sleep study. (B) Clinical predictors of mild/moderate/severe OSA based on available evidence 6 , 7 , 8 and local consensus
We compared retrospective data from children aged 3 months to 16 years referred to sleep clinic by ENT for the investigation of OSA over the 3 months immediately following the implementation of the interventions (1 June 2020 – 1 September 2020) to the same period in the previous year pre intervention (1 June 2019 – 1 September 2019). Data collection was as follows: number of children attending sleep clinic (referred by ENT for investigation of OSA), date of birth/age of children attending sleep clinic, number of children undergoing a sleep study, diagnostic outcomes, number of appointment cancellations and number of first‐time sleep study failures. A Priori power analysis was run using G*power. 9 The power analysis determined that a total of 128 participants would be required for an alpha value of 0.05, with a moderate effect size of 0.5 and power of 0.8.
3. RESULTS
Proportion of children undergoing ambulatory sleep studies pre intervention and post intervention:
The proportion of children seen by the paediatric sleep‐disordered breathing service, referred by ENT for investigation of OSA, who underwent an ambulatory sleep study was significantly smaller post intervention (Figure 2). 49% (29/59) of patients had an ambulatory sleep study in the period 1 June 2020 – 1 Sept 2020 compared to 88% (73/83) over the same period in 2019 (p < 0.001 [Welch two‐sample t‐test]). The Welch two‐sample t‐test was used due to significantly different variances (p < 0.001) between the 2 groups using the Levene's Test for Equality of Variances.
FIGURE 2.
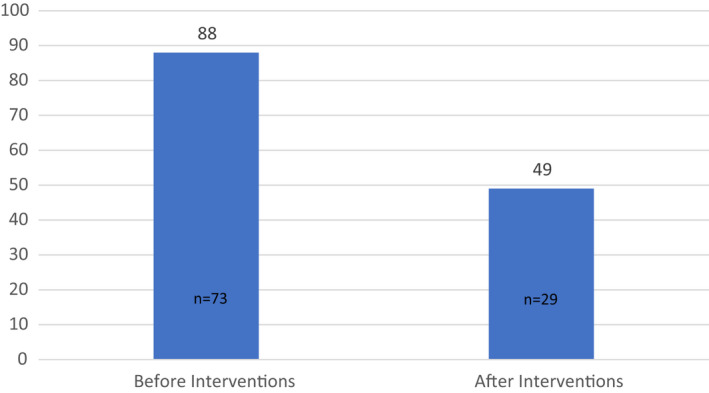
Percentage of children seen in sleep clinic undergoing a sleep study. Graph showing the percentage of children (%) seen by the sleep‐disordered breathing service, referred by ENT for investigation of OSA, undertaking an ambulatory overnight cardiopulmonary sleep study. 88% (73/83 patients) had a sleep study pre intervention compared to 49% (29/59 patients) after the intervention
The mean age of children seen in sleep clinic pre intervention was 4.6 years (range 0.5–15 years). This compared to a mean age of 4.7 years (range 0.4–12 years) in the postintervention group (Figure 3). Both pre intervention and post intervention, the mean age of children who did not undergo a sleep study was higher than the mean age of children who had a sleep study.
FIGURE 3.
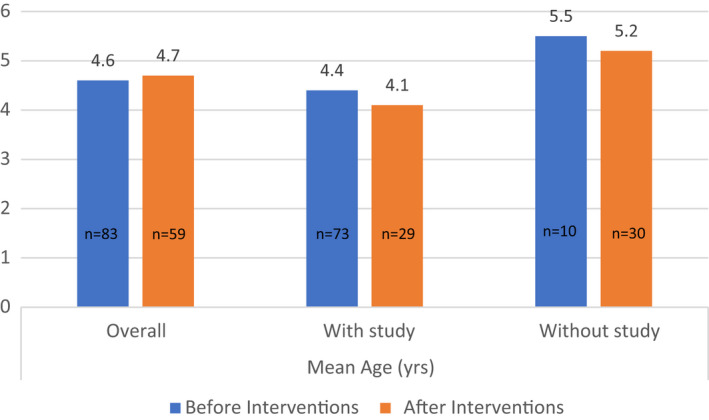
Mean ages of children seen in sleep clinic pre intervention and post intervention. Graph showing the mean ages (years) of ENT‐referred children for investigation of OSA seen in sleep‐disordered breathing clinic before and after intervention
In both the preintervention and postintervention groups, the vast majority of children referred to sleep clinic for investigation of OSA symptoms had simple adenotonsillar hypertrophy. 5/59 children (8%) had risk factors other than adenotonsillar hypertrophy in the preintervention group compared to 6/83 children (7%) in the postintervention group. These risk factors included obesity, Down's Syndrome, neurological conditions leading to hypotonia and premature infants.
The trends in proportions of diagnosed OSA severity (Figure 4) were similar pre intervention and post intervention (p = 0.06 [2‐tailed Student t‐test]). The exception was that there was an overall increase in the proportion of children diagnosed with moderate severity OSA after implementation of the interventions, most of whom were diagnosed clinically, corresponding to reductions in the proportion of patients diagnosed as normal or as having mild OSA.
-
2.
Cancellation rates pre intervention and post intervention:
FIGURE 4.
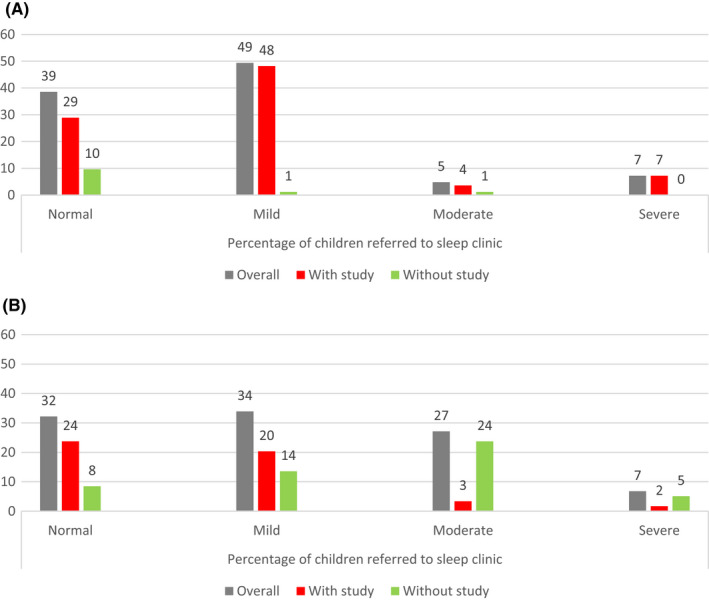
Severity of OSA diagnosed before and after intervention. Graph showing the severity of OSA diagnoses before intervention (A) and after intervention (B) displayed as the percentage of children seen in sleep clinic, referred by ENT for investigation of OSA. With study = patients who were diagnosed with a sleep study, without study = patients who were diagnosed without a sleep study, overall = combined diagnoses with and without sleep studies
The proportion of children referred by ENT to sleep clinic for investigation of OSA whose sleep clinic appointments were cancelled by parents (Figure 5) was greater before intervention (13% [12/95]) than after intervention (5% [3/62]). This result was not significant (p = 0.078 [Welch two‐sample t‐test]). The Welch two‐sample t‐test was used due to significantly different variances (p = 0.001) between the 2 groups using the Levene's Test for Equality of Variances.
-
3.
First‐time failure rate of sleep studies:
FIGURE 5.
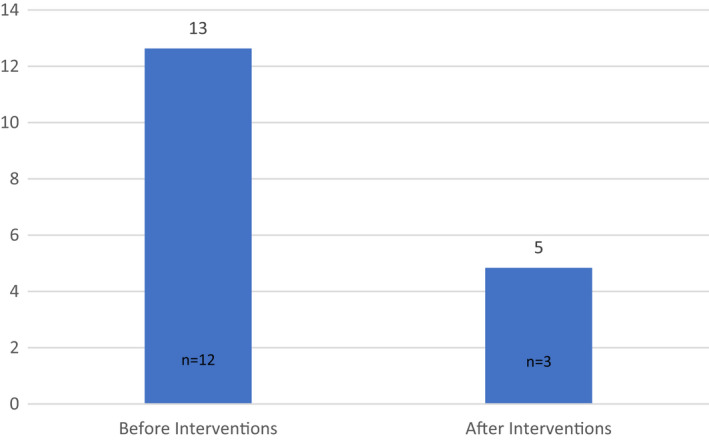
Percentage of sleep clinic appointments cancelled. Graph showing the percentage (%) of ENT‐referred sleep‐disordered breathing clinic appointments (for investigation of OSA) cancelled by parents/carers before and after intervention (13% [12/95] before intervention versus 5% [3/62], after restructure)
The first‐time failure rate (Figure 6) of ambulatory sleep studies for ENT patients referred to sleep clinic (for investigation of OSA) was lower after intervention (7% [2/29]) compared with before intervention (10% [7/73]). This result was not significant (p = 0.226 [2‐tailed Student t‐test]).
FIGURE 6.
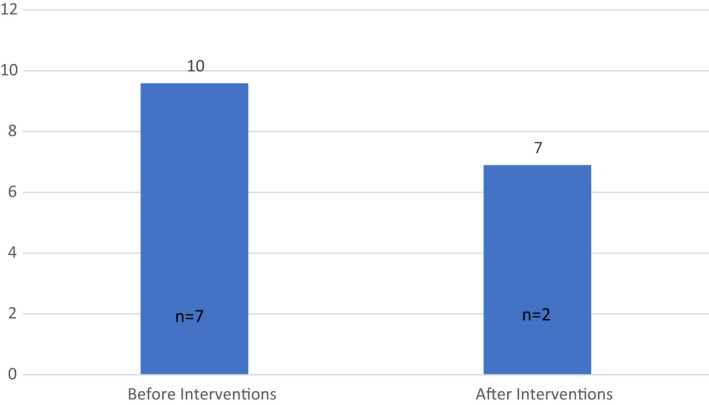
Percentage of sleep studies conducted failing at first attempt. Graph showing the percentage of children, referred by ENT to sleep clinic for investigation of OSA, undergoing a sleep study who had a first–time study failure, before and after intervention (10% [7/73] before intervention compared to 7% [2/29] after intervention)
4. DISCUSSION
Before the pandemic, our paediatric sleep consultations for patients referred by ENT for investigation of OSA symptoms would occur in the same face‐to‐face outpatient appointment as the demonstration to parents/carers of ambulatory sleep study equipment set‐up. The whole face‐to‐face appointment was clinician‐led, and the majority of children attending the sleep clinic would go home with level 3 sleep study equipment.
The implementation of the virtual screening clinic and new ENT‐UK guidelines 5 means that only those children who meet the criteria for a sleep study to investigate their OSA symptoms physically attend the children's outpatient department. This has led to a statistically significant 39% reduction in the number of patients seen face to face. The reduction in the proportion of children undergoing a sleep study is likely to be mainly related to the change in ENT‐UK guidance, but we also believe that having the consultation/screening part of the clinic separate and virtual makes it less likely that equipment will be handed out to patients who do not meet the criteria.
Furthermore, the equipment‐fitting clinic appointments are shorter than previous face‐to‐face combined consultation/equipment‐fitting clinics (20‐min compared to 30‐min appointments). Overall, fewer children are attending face‐to‐face outpatient appointments, for less time, meaning that the interventions implemented have been successful in meeting the main aim of reducing face‐to‐face hospital attendance in the era of the COVID‐19 pandemic.
The disadvantage of a virtual clinic is that clinical examination is not possible. However, we have been able to assess for obesity using height/weight parameters. At the time of writing, COVID‐19 guidance published by the Royal College of Paediatrics and Child Health 10 recommends that throat examination in children is not performed routinely, and in our experience, tonsillar grading can be undertaken via video consultation in a cooperative school‐age child. The average age of children referred to our sleep clinic from ENT services in the postintervention group was 4.7 years and the number of school‐age children (born on or before 31/08/2015) assessed virtually was 34/59 (58%).
The mean age of children undergoing a sleep study was lower than those who received a clinical diagnosis. This is unsurprising post intervention as one of the ENT‐UK criteria for conducting a study is the child being aged 3 years or under, but this was also the case before implementation of the virtual sleep clinic/ENT‐UK guidelines and likely reflects the clinical diagnostic uncertainty in younger age groups, supporting the ENT‐UK guidance.
Interestingly, there was no statistically significant difference in the proportion of children diagnosed as not having OSA (normal) and the spread of diagnosed OSA severities before and after implementation of the interventions. With the higher proportion of the diagnosis of all OSA severities post intervention compared to pre intervention being clinically rather than via sleep study suggests that clinical assessment is an accurate diagnostic tool in the diagnosis of OSA in eligible children. The exception was the higher proportion of children in the moderate OSA severity category after intervention. Most of the children in this category were diagnosed clinically post intervention, and there was a slight reduction in children diagnosed as normal or mild severity OSA accordingly. This could be because our clinical guidelines which have been adapted from the ENT‐UK guidance overestimate the severity of OSA. It could also be due to differences in the patient population in the postintervention group compared to the preintervention group.
In addition to reducing face‐to‐face attendance, we have seen unintended efficiency and cost‐effectiveness benefits. Because the virtual paediatrician‐led consultation is shorter (20 min) than the previously used combined face‐to‐face consultation/equipment‐fitting clinic (30 min), we have capacity to see more patients per clinic. Although not significant, we saw a trend towards a lower first‐time failure rate of ambulatory sleep studies post intervention. Pre intervention, the paediatrician would demonstrate to the caregiver how to attach the sleep study equipment at the end of the clinic consultation. With the time constraint of 30 min for this entire process, we believe that the equipment demonstration was often rushed, increasing the risk of errors when setting up the equipment at home. Post intervention, the separation of the equipment‐fitting service into a designated specialist nurse‐led clinic, with 30‐min slots per patient, we believe, has led to the trend of fewer errors.
Note that in our hospital, the paediatric sleep service is paediatrician‐led rather than ENT‐led. The main reason for this is that, although OSA forms our largest group of referrals, we also see patients with other pathologies referred from paediatrics (e.g. infants with chronic lung disease on home oxygen and children with sleep‐disordered breathing conditions of central origin). In addition, our service is still in its infancy, and we were unclear from the outset exactly what proportion of referred patients would be from ENT. Going forward, ENT will be using the guidance to directly refer eligible OSA patients to the technician, avoiding the need for these patients to see the paediatrician.
Furthermore, although statistically nonsignificant, there was a trend towards a reduction in sleep appointment cancellations by parents/carers, which is likely to be due to the reduced logistical burden for families attending virtual appointments compared to attending face‐to‐face appointments. We have introduced a telephone backup service for contacting parents/carers who forget or are unable to join the virtual clinic which has led recently to a trend towards reduced nonattendance. It is worth noting that the lower cancellation rates post intervention may have been also influenced by the flexibility of working from home policies and home schooling during the period observed.
Limitations of the study were the relatively small numbers of patients in the preintervention and postintervention groups and the use of level 3 ambulatory studies rather than full inpatient polysomnography, the latter which is recommended by the ENT‐UK guidance. In addition, due to limitations of virtual consultation, there is a chance that, cases which did meet the criteria for a sleep study were missed, impacting on the numbers of studies performed post intervention.
5. CONCLUSIONS
These results mean that not only are we able to safely run our diagnostic paediatric sleep‐disordered breathing service during the COVID‐19 pandemic, but also we have developed a more efficient, convenient and cost‐effective service. There is scope to involve the paediatric community nursing team and/or courier services for the distribution and collection of ambulatory sleep equipment, which would avoid the need for patients and their families having to attend the hospital at all. Our ENT services have also now implemented the screening guidelines in their clinics with the potential for minimisation of referral of clinically diagnosable OSA cases to the sleep service, with further cost‐efficiency benefits.
These data highlight the advantages of the introduction of virtual screening clinics using ENT‐UK guidelines in the diagnosis of OSA in children. Future studies should directly compare the accuracy of diagnosis of OSA in children clinically versus via sleep study, as well as comparing management and clinical outcomes of patients diagnosed clinically versus via sleep study.
It took a global pandemic to reshape the way we run our paediatric sleep service, but the benefits we have seen mean that we are likely to continue this new structure longer‐term. The COVID‐19 era is an appropriate time for adult and paediatric sleep services, as well as other diagnostic services, to reassess their guidelines and the way their services are delivered.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
JJ designed the study, collected/analysed the data and wrote the manuscript. KB assisted in data collection and performed statistical analysis on the data. AT edited the manuscript. All authors have read and approved the manuscript.
ETHICS APPROVAL
As per the UK Policy Framework for Health and Social Care Research, 11 this study is classified as clinical audit and therefore does not require ethical approval.
ACKNOWLEDGEMENT
Simone Millership (Ward Clerk Team Manager, East Suffolk and North Essex NHS Foundation Trust) assisted in data collection.
Johnson J‐A, Burrows K, Trinidade A. Running a paediatric ambulatory sleep service in a pandemic and beyond. Clin Otolaryngol. 2022;47:433–439. doi: 10.1111/coa.13918
[Correction added on April 13, 2022, after first online publication: Peer review history is not available for this article, so the peer review history statement has been removed.]
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.
REFERENCES
- 1. Who.int . Timeline: WHO's COVID‐19 response. [online]. 2021. Available at: <https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/interactive‐timeline/> [Accessed 1 November 2021]
- 2. Lacobucci G. Covid‐19: all non‐urgent elective surgery is suspended for at least three months in England. BMJ. 2020;18(368):m1106. [DOI] [PubMed] [Google Scholar]
- 3. Kingshott RN, Gahleitner F, Elphick HE, et al. Cardiorespiratory sleep studies at home: experience in research and clinical cohorts. Arch Dis Child. 2019;104:476‐481. [DOI] [PubMed] [Google Scholar]
- 4. Singh G, Hardin K, Bang H, Nandalike K. The feasibility and utility of level iii portable sleep studies in the pediatric inpatient setting. J Clin Sleep Med. 2019;15:985‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ENTUK.org . Safe Delivery of Paediatric ENT Surgery in the UK: A National Strategy. [online]. 2021. Available at: <https://www.entuk.org/safe‐delivery‐paediatric‐ent‐surgery‐uk‐national‐strategy> [Accessed 1 November 2021]
- 6. Janssen KC, Phillipson S, O'Connor J, Johns MW. Validation of the epworth sleepiness scale for children and adolescents using rasch analysis. Sleep Med. 2017;33:30‐35. [DOI] [PubMed] [Google Scholar]
- 7. Kljajić Z, Roje Ž, Bečić K, et al. Formula for the prediction of apnea / hypopnea index in children with obstructive sleep apnea without polysomnography according to the clinical parameters: Is it reliable? Int J Pediatr Otorhinolaryngol. 2017;100:168‐173. [DOI] [PubMed] [Google Scholar]
- 8. Isaiah A, Shikara M, Pereira KD, Das G. Refining screening questionnaires for prediction of sleep apnea severity in children. Sleep Breath. 2020;24(4):1349‐1356. [DOI] [PubMed] [Google Scholar]
- 9. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175‐191. doi: 10.3758/bf03193146. PMID: 17695343. [DOI] [PubMed] [Google Scholar]
- 10. RCPCH.ac.uk . 2021. [online] Available at: <https://www.rcpch.ac.uk/sites/default/files/generated‐pdf/document/COVID‐19‐‐‐guidance‐for‐paediatric‐services.pdf> [Accessed 1 November 2021]
- 11. Hra‐decisiontools.org.uk . 2021. [online] Available at: <http://www.hra‐decisiontools.org.uk/research/docs/DefiningResearchTable_Oct2017‐1.pdf> [Accessed 1 November 2021]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


