Abstract
Background
This exploratory study investigates if intra‐articular injected gold microparticles in knee osteoarthritis (KOA) reduce immunomodulatory‐based pain via proteomic changes in the synovial fluid (SF) and serum.
Methods
Thirty patients with moderate KOA were included. Intraarticular injections with 20 mg gold microparticles (72.000 particles, 20–40 µm in diameter) using the patient's synovial fluid (SF) as carrier were performed. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) subscores for pain, stiffness, and function were assessed at inclusion, 8 weeks and 2 years The PainDetect questionnaire, pain pressure threshold (PPT), temporal summation (TS), and conditioned pain modulation (CPM), and pain diary were assessed at inclusion and 8 weeks. Proteome analysis was performed on SF and blood samples before and after 8 weeks of treatment.
Results
A decrease in WOMAC scores (pain (p = 0.0001), stiffness (p = 0.0088), activity (p = 0.0001)), PainDetect (p = 0.0002) and increase in PPT (p = 0.001) and CPM (p = 0.021) and a decrease in TS (p = 0.03) were found after 8 weeks compared to inclusion assessments. At 2 years follow‐up compared to baseline there was a decrease in WOMAC scores (pain (p = 0.0001), stiffness (p = 0.007), activity (p = 0.0001)) and PainDetect (p = 0.0001). In SF, 28 different proteins were downregulated and 11 upregulated (p < 0.05) mainly associated immune response. Similarly, 31 proteins were downregulated and 1 upregulated in serum (p < 0.05) reflecting key immune response and anatomical structure development processes. No adverse effects related to the treatment were recorded.
Conclusions
Gold microparticles injected intra‐articular in KOA joints may provide pain relief and an inflammatory modulatory effect based on proteome changes found in SF and serum. A randomized, controlled, double‐blind study is needed to infer a conclusion.
Significance
This study indicates that intra‐articular gold may provide advantages in clinical practice for managing knee osteoarthritic pain. The use of intraarticular gold can add new knowledge to the treatment of inflammation and pain.
1. INTRODUCTION
Metallic gold and gold‐containing drugs used in medicine are traceable for several thousand years and the use of goldthiocompounds in the treatment of rheumatoid arthritis (RA) became significant in the first third of the last century (Berners‐Price & Filipovska, 2011; Clark et al., 2010; Lehman et al., 2005). The released gold ions reduce pain, joint swelling, and inflammation, and increase joint mobility (Clark et al., 2010; Lehman et al., 2005). However, the use of gold is limited by the toxicity to the kidney, liver, and skin (Clark et al., 2010).
Macrophages attach to the surface of the gold implants and, and by dissolucytosis, release gold ions into the intercellular space (Danscher, 2002; Larsen et al., 2008). These animal studies demonstrate that macrophages, mast cells, and fibroblasts take up the released gold ions and change the behaviour of cells that causes the inflammatory process to diminish, and the gold‐loaded mast cells stop releasing histamine. The gold ions do not spread from the gold implants and therefore the toxic effects of implants are believed to be absent (Danscher, 2002; Danscher & Larsen, 2010; Larsen et al., 2008). Several studies indicate that gold ions selectively modulate the local inflammatory process of pain (Danscher, 2002; Havarinasab et al., 2007; Hazer et al., 2011; Märki et al., 2018), such as the NF‐kB pathway that regulates expression of pro‐inflammatory genes, including those encoding cytokines and chemokines, and also participates in inflammasome regulation (Khan & Khan, 2018). One study used hyaluronic acid a carrier, into the middle carpal joint of nine healthy horses and found a higher pain threshold after 4 days of treatment (Märki et al., 2018). Similar findings have been reported in animals with osteoarthritis (OA) (Jæger et al., 2007, 2012; Lie et al., 2011) and this calls for further studies including clinical studies on humans to clarify this anti‐inflammatory and pain‐reducing mechanism of gold ions.
It seems evident that a subset of patients with knee OA (KOA) display signs of low‐grade inflammation (Giordano et al., 2020; Siebuhr et al., 2014), which might be due to synovitis (Petersen et al., 2016). Pro‐inflammatory cytokines can sensitize the peripheral nerves, which can be assessed as hyperalgesia, and studies on KOA have found both localized and widespread pressure hyperalgesia (Arendt‐Nielsen et al., 2015), which based on preclinical evidence suggest changes in central pain mechanisms (Arendt‐Nielsen & Graven‐Nielsen, 2011). The wind‐up process reflects the excitability of dorsal horn neurons (Arendt‐Nielsen & Graven‐Nielsen, 2011; Arendt‐Nielsen et al., 2015). Temporal summation (TS) is the human surrogate model for the wind‐up process in animal dorsal horn neurons and is facilitated in patients with KOA compared with healthy subjects (Petersen et al., 2015). Conditioned pain modulation (CPM) is the human surrogate assessment of the balance of descending pain inhibitory and facilitatory mechanisms that are often impaired in patients with KOA compared with healthy subjects (Arendt‐Nielsen et al., 2015). KOA patients with facilitated or impaired CPM might not respond well to standard KOA treatment. This is why treatments targeting these mechanisms are needed (Petersen et al., 2015). A previous study found that treatment with a COX‐2 inhibitor in KOA provided improvements in Pressure Pain Threshold (PPT), TS, and pain relief, suggesting that anti‐inflammatory treatments might interact with central pain mechanisms by targeting inflammation mechanisms and thereby providing pain relief (Petersen et al., 2021).
No human studies have investigated the effect of intraarticular gold micro particle implants for the treatment of pain and inflammation in KOA. The present open, exploratory study investigated whether gold ions can act as a KOA treatment option through modulation of inflammatory mediators, pain sensitivity, and central pain mechanisms.
The primary aim of this study was to measure the effect on pain and function after intra‐articular injection of 20 mg gold microparticles >20 µm into the KOA joint using the patient's synovial fluid (SF) as a carrier. The secondary aim was to measure proteomic changes in the SF and serum. The changes in PPTs, TS, and CPM were included as exploratory outcomes.
2. METHODS
2.1. Study flow
We evaluated clinical function, pain profile, and the synovial and serum fluid proteome at baseline and eight weeks. We measured the stability of pain and function at a two‐year follow‐up.
2.2. Participants
From January 2017, through March 2018, we enrolled 30 patients with radiographically confirmed KOA (Kellgren & Lawrence, 1957) (Kellgren‐Lawrence grade ≥2), pain for more than three months and maximal pain intensity VAS (Visual Analogue Scale, 0–10) ≥5 during the last week, and knee joint effusion on MRI that can be aspirated. Eligible patients were enrolled at the specialized, public outpatient clinic at Aalborg University Hospital, Denmark. The exclusion criteria were (1) active adjuvant treatment for any malignancy, (2) active infection and antibiotic treatment, (3) active treatment with steroids, biological or other anti‐rheumatic medication, (4) chronic pain and other than knee joint pain, (5) inability to comply with the protocol and, (6) inadequacy in written and spoken national language (Figure 1).
FIGURE 1.

Enrollment, treatment and follow‐up
2.3. Study treatment
Pure gold particles, 20 mg sterile 99.99%, a total of 72.000 particles, 20–40 µm in diameter (BerlockMicroImplants (BMI), Berlock ApS) (Danscher and Larsen, 2010; Märki et al., 2018) were injected intra‐articulary into the knee joint using the patient's SF as the carrier. Two ml SF aspirated from the knee, was mixed with the sterile gold microparticles and re‐injected into the patient's knee.
2.4. Sample collection and biobanking
SF from the knee was centrifugated for 10 minutes at 2200G to remove cell debris, and the supernatant was frozen in aliquots at −80℃ for future analysis. Besides, a 6 ml venous whole blood sample was taken and centrifugated at 3000 RPM for 15 min. The serum fraction was stored at −80℃ for future analysis. All sample data were collected and kept following Danish legislation on data handling at the Department of Biomedicine at Aalborg University Hospital. Each patient sample was divided into aliquots to avoid unnecessary freezing and thawing cycles.
Cell free DNA were determined in SF and serum by Quant‐iT™ PicoGreen™ dsDNA Assay Kit (ThermoFisher Scientific) according to the manufacture's instruction (Birkelund et al., 2020). A Proximity Extension Assay (PEA) was carried out essentially according to (Giordano et al., 2020) on representative SF samples (n = 6) to quantify low abundance affected by the gold treatment essentially. Sample preparation for proteome analysis was prepared essentially as Birkelund (Birkelund et al., 2020). The protein concentration in biofluids was measured by a microassay. A total of 25 μg SF and serum subject to in‐situ trypsinization (trypsin ratio 1:100) using reduction and alkylation using a Filter Aided Sample Preparation protocol (10 kDa cut‐off) before analysis with tandem mass spectrometry (MS/MS). To expand the protein coverage of the MS analysis, a pH‐fractioning of the enzymatically digested samples was carried out. A total of 500 ng tryptic digest were analysed in technical duplicates in randomized order using a nano UPLC ESI MS/MS setup consisting of a Dionex RSLC nano pump (Dionex RSLC, Thermo Fisher Scientific) connected to a Bruker timsTOF PRO mass spectrometer operated in DIA‐PASEF positive ion mode (Bruker Daltronics, DE).
2.5. Primary outcomes measures
The change in Western Ontario and McMaster Universities Arthritis Index (WOMAC) subscores for pain, stiffness, and function (Bellamy et al., 1988) at 8 weeks follow‐up compared to baseline was the primary outcome. The questionnaire contains 24 questions, 5 pain questions, 2 stiffness questions, and 17 physical function questions. Each question utilizes a 5‐point scale, from 0 (none) to 4 (extreme) (Bellamy et al., 1988). WOMAC is a primary outcome for the current study as The Osteoarthritis Research International (OARSI) Standing Committee for Clinical Trials Response Criteria Initiative recommends that primary outcomes in OA research include assessment of pain and function (Pham et al., 2004).
2.6. Secondary outcomes measures
2.6.1. Pain measures
The additional outcome included the PainDetect questionnaire (Freynhagen et al., 2006), and Quantitative Sensory Testing (QST) (Pressure Pain Threshold (PPT) (assessed over the knee joint) and temporal summation (Petersen et al., 2019) at inclusion and after 8 weeks, a weekly pain diary, and a Global Rating of Change Scale (Kamper et al., 2009) at follow‐up. Outcome measures at 2 years follow‐up were WOMAC (Bellamy et al., 1988), PainDetect questionnaire (Freynhagen et al., 2006), and the Global Rating of Change Scale (Kamper et al., 2009). Adverse events and serious adverse events that occurred within the 2‐year follow‐up were identified by self‐report at follow‐up visits and in‐hospital records. The 2‐year follow‐up was performed by EGSLJ and NKJ.
The PainDetect questionnaire (Freynhagen et al., 2006), comprises 3 major components, gradation of pain, pain course pattern, and radiating pain. Seven questions are evaluating the gradation of pain. Each question is scored by the patient using a 0 to 5 score with 0 = never, 1 = hardly notice, 2 = slightly, 3 = moderately, 4 = strongly and 5 = very strongly. There is one question evaluating pain course patterns. Patients selected from one of four pictures indicating which pattern of pain best describes their course of pain. Each picture is associated with a unique score of 0, −1, or +1 (2 pictures have this score possible). There is one question evaluating radiating pain with a yes (score of +2) or no (score of 0) response option.
PPT was performed using a handheld algometer (Somedic, Hörby, Sweden) with a 1‐cm2 probe (covered by a disposable latex sheath). It was used to record the PPT on 7 sites at the affected knee with two distant sites at the tibialis anterior muscle (5 cm distal to the tibial tuberosity) and at the extensor carpi radialis longus muscle (arm, 5 cm distal to the lateral epicondyle of humeral bone). The sites in the peripatellar regions were 2 cm distal to the inferior medial edge of patella (Site 1); 2 cm distal to the inferior lateral edge of patella (Site 2); 3 cm lateral to the midpoint of the lateral edge of patella (Site 3); 2 cm proximal to the superior lateral edge of patella (Site 4); 2 cm proximal to the superior edge of patella (Site 5); 2 cm proximal to the superior medial edge of patella (Site 6); 3 cm medial to the midpoint of the medial edge of patella (Site 7). An interval of a minimum of 20 seconds was kept between each PPT assessment. The PPT is defined to the subject as “the point at which the pressure sensation just becomes painful.” The pressure is increased gradually at a rate of 30 kPa/s until the pain threshold is reached and the subject presses a button.
The evaluation of temporal summation and deep tissue pain sensitivity (Petersen et al., 2019b), includes cuff pressure stimuli using a computer‐controlled cuff algometer (Cortex Technology, NociTech and Aalborg University, Denmark), a tourniquet cuff (VBM, Sulz, Germany), and an electronic VAS (Aalborg University, Denmark) for the recording of the pain intensity. The place of the cuff is at the level of the head of the gastrocnemius muscle of the leg most affected by knee OA. The electronic continuous VAS (sliding resistor) is 10 cm long, and samples at 10 Hz; 0 cm indicates “no pain” and 10 cm indicates “maximum pain”. The cuff was automatically inflated (inflation rate: 1 kPa/s). The subject was instructed to rate the pain intensity continuously on the VAS from the first sensation of pain and to press the release button at “the pain intensity strong enough to make one feel like interrupting or stopping it”. The pressure detection threshold (PDT) was set at VAS = 1 cm, as in previous studies (Petersen et al., 2019b). The pressure value at the termination of pressure inflation was defined as the pressure‐pain tolerance (PTT).
Ten short‐lasting stimuli (1 s each) at the level of the cuff PTT was given at the lower leg with a 1 s break between stimuli to assess TS. A constant pressure between the individual pressure stimuli of 1 kPa was applied to avoid movement of the cuff. The participants were instructed to continuously rate the pain intensity of the sequential stimuli using the electronic VAS (sliding resistor) (“0” represents “no pain” and “10” “maximal pain”) and not return to zero during the breaks. For each cuff stimulus, a VAS score was extracted. TS was calculated as the absolute difference between the last three stimuli and the first three stimuli (Petersen et al., 2019a). The CPM magnitude was assessed as the absolute changes in cuff PDT with and without a cuff conditioning stimulus. The conditioning stimulus was applied to the contralateral lower leg and the cuff PDT was assessed on the ipsilateral lower leg as described above. The conditioning stimulus was applied as a constant stimulus with an intensity of 70% of the pain tolerance level on the contralateral leg (Petersen et al., 2019b). The CPM effect was calculated as the absolute difference in conditioned and unconditioned PDT (i.e. cuff PDTconditioned minus cuff PDTunconditioned).
Using the Global Rating of Change Scale (Kamper et al., 2009), we asked the question, “Concerning your knee, how will you describe yourself compared to immediately before injection of gold into your knee” and evaluated the answer at an 11‐point scale from very much worse (−5) to complete recovered (5).
2.6.2. Proteomic measures
The analyses of the proteomic primary raw data output were searched against spectral databases generated by fractionated pooled samples using human reference proteome database (05.2021; 20364 entries) in Biognosys Spectronaut (v. 14.10) (Schaab et al., 2012) and using statistical analysis (Koh et al., 2019). Quantitative proteome analysis of SF and serum samples were accomplished by discovery proteomics using DIA‐PASEF (Meier et al., 2020). Functional association of significantly regulated proteins in SF and serum with gene ontology and function were assessed by Metascape (Zhou et al., 2019).
2.7. Statistical analysis
This present exploratory study investigated whether gold ions have a clinical role in treating KOA. Based on preclinical evidence, we estimated medium effect size (Cohen's d = 0.5), and with a power of 80% and a significant level at 0.05, a sample size calculation estimated a total sample of 27 subjects. A total of 30 subjects were included to account for possible drop‐out.
The clinical outcome measures analysis was performed using the Stata software, version 16.0 (StataCorp.) ANOVA was used for the analysis of repeated measures of pain, the pain diary, and QST parameters. Wilcoxon test was used for all other measurements.
2.8. Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable request. Proteomic raw and processed data are available from the Proteome exchange repository with identifier PXD030764 (http://www.proteomexchange.org).
2.9. Ethical approvals
The study followed the principles of the declaration of Helsinki and was approved by the local ethics committee of the North Denmark Region (N‐20160045). The regional data protection agency approved the project by July 6, 2016 (2008–58–0028, ID 2016–116) and registered in Clinical Trial Gov (NCT03389906).
We have followed the Consort guideline for reporting non‐randomized pilot and feasibility studies (Lancaster & Thabane, 2019). The first author takes responsibility for the integrity and accuracy of the reported data and the credibility of the study to the protocol.
2.10. Consent
All participants consented to participation in the research via written forms and verbally.
3. RESULTS
3.1. Enrolment and follow‐up
A total of 32 patients were assessed for inclusion and 30 were enrolled and underwent treatment with intra‐articular injection of 20 mg gold microparticles (Figure 1). Table 1 presents the baseline characteristics. No patients presented with symptomatic accumulation or effusion needed aspiration. All 30 patients completed the follow‐up. Between 1 and 2 years after treatment 1 patient with the Kellgren‐Lawrence score IV (Kellgren & Lawrence, 1957) received total knee arthroplasty. All 30 patients were included in the analysis. The follow‐up time was mean 8.2 weeks and a mean of 25.1 months.
TABLE 1.
Baseline characteristics of the 30 patients. Values are median and range; and mean and 95% CI. Scores on the Kellgren–Lawrence scale range from 0 to 4, with a score of 2, 3, or 4 indicating definite osteoarthritis and higher scores indicating more severe disease
| Female/Male sex | 12/20 |
| Age – year | 63 (46–75); 62 (49.5 – 74.2) |
| Body mass index | 28.8 (22.8–41.7); 29.5 (19.7 – 39.3) |
| Clinical sign of effusion | 3/30 |
| Volume (ml) synovial fluid aspirated | 6 (−0.68–12.9); 8 (3 – 18) |
| Kellgren‐Lawrence Score | |
| II | 3 |
| III | 28 |
| IV | 1 |
| Womac Scores | |
| Pain | 9 (6–16); 9.3 (5.1–13.2) |
| Stiffness | 8 (1–4); 3.8 (−0.27–7.3) |
| Activity | 29 (14–51); 29.5 (9.5–49.5) |
| Pain evaluation | |
| Pain Detect | 10 (1–26); 11.3 (−1.2–23.8) |
| Pressure Pain Threshold (kPa) | 598 (276–1043); 572 (380–764) |
| Temporal Summation of Pain (% VAS) | 103 (−69.2–694); 129 (−192–450) |
| Conditioned Modulation of Pain (kPa) | −3.45 (−56.6–125); 8.38 (−77.7–94.4) |
3.2. Outcomes
Patient‐reported outcome measures, WOMAC score (Bellamy et al., 1988), and PainDetect (Freynhagen et al., 2006), at 8 weeks and 2 years are presented in Figure 2. WOMAC scores (pain (p = 0.0001, stiffness (p = 0.0088, activity (p = 0.0001) and PainDetect (p = 0.039) were significantly changed comparing baseline and 8 weeks assessment. At 2 years follow‐up compared to baseline there was a decrease in WOMAC scores (pain (p = 0.0001), stiffness (p = 0.007), activity (p = 0.0001)) and PainDetect (p = 0.0001). The pain diary records on VAS showed significantly decreased pain scores at weeks 5 and 6 compared to baseline (Figure 3) (p<0.0005).
FIGURE 2.
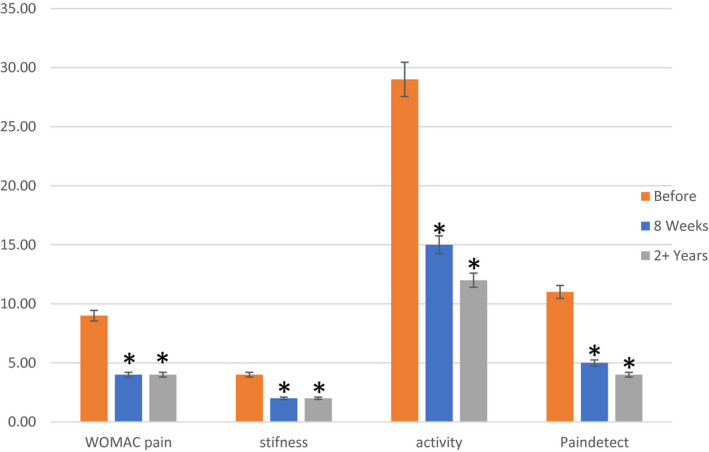
WOMAC pain, stiffness and activity, and PainDetect, before treatment, and 8 weeks and 3 years after intra‐articular injection of 20 mg gold in 30 knee OA patients (Median and quartiles). * represents significance compared to before treatment
FIGURE 3.
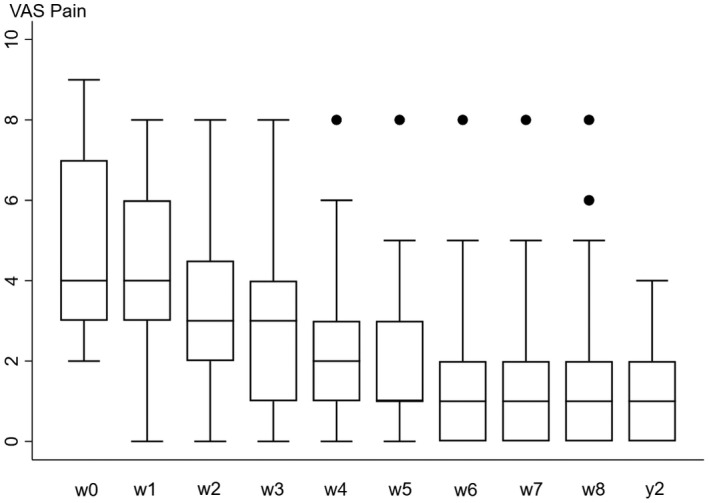
Pain on VAS diary records for 8 weeks and at 2 years after intra‐articular injection of 20 mg gold in 30 knee OA patients. Median and quartiles
At the 8 weeks pain evaluation (Petersen et al., 2019b), PPTs increased by 17.5 (36.3) % (p = 0.001), TS decreased by 51.7 (169) % (p = 0.03), and CPM increased 19.3 (44) % (p = 021) compared to baseline assessments. On the 11‐point Global Rating of Change Scale (Kamper et al., 2009) at 2‐year follow‐up 22 patients reported a positive effect and 6 reported no effect and 2 worse (median 3 (−3; 5)).
Out of the 96‐plex protein panel including chemokines and cytokines tested for in the PEA, 24 proteins were significantly affected (p < 0.05) at eight weeks follow‐up compared to baseline assessments. Figure 4a presents a distinct differentiation of the before and after treatment and indication by unsupervised PCA analysis and heatmap of the level of regulation of the 24 regulated markers proteins directly involved in inflammatory signalling processes and regulated by the NF‐kB pathway.
FIGURE 4.

Proteomic analysis of synovial fluid and serum. (A) Proximity Extension Assay profiling by 92panel array. Unsupervised Principal component analysis (PCA) analysis showing the separation of the patients before and after treatment with gold particles. Clustered image map indicating the 24 significantly regulated proteins in synovial fluid before and 8 weeks after intra‐articular injection of 20 mg gold in 30 knee OA patients. All proteins are associated to the NF‐kB pathway. (B) Functional Enrichment Analysis of regulated proteins in SF and serum with corresponding Gene Ontology classes; (C) Enrichment network analysis based on regulated protein lists from SF and serum. Each pie sector is proportional to the number of hits originated from the gene list (SF, serum) and indicates overlap in biological functions. (D) cell free DNA in SF and serum (p‐value; n.s.)
A total of 529 different proteins were identified in SF samples, 28 were significantly downregulated and 11 were significantly upregulated (Table 2). Across all serum fluid samples, 380 different proteins were identified, 32 were significantly regulated, 31 were downregulated and 1 was upregulated (Table 3). Functional assessment of the up‐ and downregulated proteins in SF and serum as well as local‐systemic relations of the regulated proteomes were made by functional enrichment analysis (Figure 4b,c) (Zhou et al., 2019). Among multiple overlapping enriched biological processes are humoral immune system activation and neutrophil degranulation accounting for sixteen (CTSG, ELANE, FA BP5, GDI2, HSP90AA1, KRT1, LCN2, LTA4H, MPO, PNP, PSMA5, PSMC3, S100A11, SERPINB3, PGLYRP1, CALML5) and twenty‐four proteins (APCS, CTSG, ELANE, KRT1, KRT6A, FCN3, PGLYRP1, IGHV3‐23, IGHV3‐7, IGHV1‐58, IGHV1‐8, H2BC12, LCN2, MPO, DCD, GPX1, HLA‐, HSP90AA1, CDC42, THBS1, IGF2, PNP, SPTA1, ACTB), respectively. Synovial joint associated biological processes include association to wound healing, regulation of immune processes and hydrolase activities. Systemic (serum) enriched processes include biological processes associated to adaptive immune responses and signal transduction. To further determine the local and systemic extend of neutrophil degranulation the level of cell free DNA was measured as indicator of neutrophil extracellular trap (NET) formation linked to modulation of inflammatory processes (Zhou et al., 2019). Absolute concentrations of cfDNA in SF and serum were found to similar before and after treatment (p‐value; n.s).
TABLE 2.
The 49 significantly regulated proteins found in synovial fluid of knee OA patients, 28 downregulated and 11 upregulated, 8 weeks after intra‐articular injection of 20 mg gold in 30 knee OA patients. Proteomic analysis using DIA‐PASEF analysis of 500 ng SF by label free quantification. Functional association of significantly regulated proteins in SF were assessed by STRING analysis
| Genes | Protein descriptions | Function | % Change | Ratio | p‐value | |
|---|---|---|---|---|---|---|
| GOLM1 | Golgi membrane protein 1 | Immune and Infl. | 1907.5 | 20.07 | 6.99E−06 | |
| TYMP | Thymidine phosphorylase | Regenerative | 186.1 | 2.86 | 0.012243 | |
| POC1A | POC1 centriolar protein homolog A | Regenerative | 116.5 | 2.16 | 7.01E−06 | |
| APCS | Serum amyloid P‐component | Immune response | 102.2 | 2.02 | 5.68E−08 | |
| ARHGDIA | Rho GDP‐dissociation inhibitor 1 | Regenerative | 91.5 | 1.91 | 0.011445 | |
| ANXA5 | Annexin A5 | Unknown | 72.1 | 1.72 | 0.000575 | |
| DEFA1;DEFA3 | Neutrophil defensin 1; Neutrophil defensin 3 | Immune response | 69.2 | 1.69 | 0.003064 | |
| CHAD | Chondroadherin | Regenerative | 60.0 | 1.59 | 0.000362 | |
| THBS1 | Thrombospondin−1 | Immune response | 57.0 | 1.57 | 0.002468 | |
| RGSL1 | Regulator of G‐protein signaling protein‐like | Unknown | 53.4 | 1.53 | 0.003183 | |
| IGHV3‐7 | Immunoglobulin heavy variable 3–7 | Immune response | 51.0 | 1.50 | 5.66E−06 | |
| FCN3 | Ficolin−3 | Immune response | −33.3 | 0.66 | 1.44E−05 | |
| LTA4H | Leukotriene A−4 hydrolase | Immune and infl. | −33.7 | 0.66 | 0.015647 | |
| SBSN | Suprabasin | Unknown | −34.7 | 0.65 | 7.16E−07 | |
| IGSF22 | Immunoglobulin superfamily member 22 | Unknown | −35.2 | 0.64 | 0.013466 | |
| CDC42 | Cell division control protein 42 homolog | Immune response | −35.8 | 0.64 | 0.002768 | |
| NUTF2 | Nuclear transport factor 2 | Unknown | −39.2 | 0.60 | 0.010354 | |
| NaN | Immunoglobulin epsilon heavy chain | Sensory percept. | −40.5 | 0.59 | 2.16E−05 | |
| KRT10 | Keratin, type I cytoskeletal 10 | Immune and infl. | −43.8 | 0.56 | 1.35E−05 | |
| CSPG4 | Chondroitin sulphate proteoglycan 4 | Unknown | −46.9 | 0.53 | 0.015744 | |
| MPO | Myeloperoxidase | Immune and infl. | −47.3 | 0.52 | 0.014761 | |
| KRT2 | Keratin, type II cytoskeletal 2 epidermal | Immune and infl. | −48.0 | 0.52 | 2.14E−05 | |
| PGLYRP1 | Peptidoglycan recognition protein 1 | Immune and infl. | −49.5 | 0.50 | 0.004872 | |
| FABP5 | Fatty acid‐binding protein 5 | Immune and infl. | −49.5 | 0.50 | 0.002271 | |
| PNP | Purine nucleoside phosphorylase | Immune and infl. | −49.7 | 0.50 | 0.001185 | |
| MPP1 | 55 kDa erythrocyte membrane protein | Inflammatory | −50.0 | 0.50 | 0.008299 | |
| GPX1 | Glutathione peroxidase 1 | Immune response | −50.5 | 0.49 | 0.014773 | |
| GOT1 | Aspartate aminotransferase, cytoplasmic | Unknown | −52.6 | 0.47 | 0.000849 | |
| LCN1 | Lipocalin−1 | Unknown | −55.6 | 0.44 | 0.00013 | |
| PIP | Prolactin‐inducible protein | Unknown | −56.7 | 0.43 | 2.27E−06 | |
| KRT14 | Keratin, type I cytoskeletal 14 | Immune and infl. | −58.0 | 0.42 | 1.32E−05 | |
| KRT9 | Keratin, type I cytoskeletal 9 | Immune and infl. | −58.4 | 0.41 | 0.000196 | |
| KRT1 | Keratin, type II cytoskeletal 1 | Immune and infl. | −58.5 | 0.41 | 3.71E−10 | |
| RAC2; RAC1 | Ras‐related C3 botulinum toxin substrate 2;1 | Inflammatory | −60.0 | 0.40 | 0.000154 | |
| PSMF1 | Proteasome inhibitor PI31 subunit | Immune and infl. | −62.2 | 0.38 | 0.004097 | |
| SERPINB3 | Serpin B3 | Immune and infl. | −62.5 | 0.37 | 4.56E−06 | |
| DCD | Dermcidin | Immune response | −62.7 | 0.37 | 1.13E−08 | |
| KRT6B | Keratin, type II cytoskeletal 6B | Immune and infl. | −63.7 | 0.36 | 6.08E−09 | |
| SPTA1 | Spectrin alpha chain, erythrocytic 1 | Unknown | −64.0 | 0.36 | 0.001331 | |
| CALML5 | Calmodulin‐like protein 5 | Immune and infl. | −67.9 | 0.32 | 0.000535 | |
| SPTB | Spectrin beta chain, erythrocytic | Unknown | −68.3 | 0.31 | 0.013697 | |
| PSMC3 | 26S proteasome regulatory subunit 6A | Immune and infl. | −75.2 | 0.24 | 0.01387 | |
| CASP14 | Caspase−14 | Unknown | −76.8 | 0.23 | 2.54E−06 | |
| EPB42 | Erythrocyte membrane protein band 4.2 | Unknown | −81.2 | 0.18 | 0.014775 | |
| ELANE | Neutrophil elastase | Inflammatory | −81.7 | 0.18 | 0.003667 | |
| MMRN1 | Multimerin−1 | Unknown | −86.1 | 0.13 | 4.79E−08 | |
| CTSG | Cathepsin G | Immune and infl. | −87.7 | 0.12 | 0.003394 | |
| PSMA5 | Proteasome subunit alpha type 5 | Immune and infl. | −88.1 | 0.11 | 0.003873 | |
| IGM | Immunoglobulin mu heavy chain | Immune response | −88.1 | 0.11 | 1.66E−08 | |
TABLE 3.
The 32 significantly regulated proteins found in serum of knee OA patients, 31 downregulated and 1 upregulated, 8 weeks after intra‐articular injection of 20 mg gold in 30 knee OA patients. Proteomic analysis using DIA‐PASEF analysis of 500 ng serum by label free quantification. Functional association of significantly regulated proteins in serum were assessed by STRING analysis
| Genes | Protein descriptions | Function | % Change | Ratio | p‐value |
|---|---|---|---|---|---|
| IGHV1‐58 | Immunoglobulin heavy variable 1–58 | Unknown | 94.8 | 1.94 | 0.007386875 |
| IGLV3‐1 | Immunoglobulin lambda variable 3–1 | Unknown | −33.8 | 0.66 | 0.000235537 |
| IGF2 | Insulin‐like growth factor II | Unknown | −34.8 | 0.65 | 0.001006413 |
| HLA‐H | Putative HLA class I histocompatibility antigen, alpha chain H | Unknown | −34.9 | 0.65 | 2.27E−07 |
| IL1RAP | Interleukin−1 receptor accessory protein | Immune and infl. | −35.4 | 0.64 | 3.22E−06 |
| P4HB | Protein disulphide‐isomerase | Inflammatory | −36.3 | 0.63 | 0.001831153 |
| IGKJ1 | Immunoglobulin kappa joining 1 | Immune response | −36.6 | 0.63 | 3.28E−06 |
| NaN | Immunoglobulin delta heavy chain | Sensory percept. | −36.9 | 0.63 | 0.00185865 |
| YWHAQ | 14–3–3 protein theta | Unknown | −38.1 | 0.61 | 0.000489174 |
| TUBA1B | Tubulin alpha−1B | Immune and infl. | −38.1 | 0.61 | 0.014099739 |
| IGHV1‐8 | Immunoglobulin heavy variable 1–8 | Unknown | −38.2 | 0.61 | 0.01411 |
| UBB | Polyubiquitin‐B | Immune and infl. | −38.8 | 0.61 | 0.019002538 |
| IGKV2‐40 | Immunoglobulin kappa variable 2–40 | Unknown | −40.1 | 0.59 | 3.06E−05 |
| IGLV1‐36 | Immunoglobulin lambda variable 1–36 | Unknown | −40.4 | 0.59 | 8.19E−07 |
| LCN2 | Neutrophil gelatinase‐associated lipocalin | Immune and infl. | −40.8 | 0.59 | 1.11E−05 |
| GDI2 | Rab GDP dissociation inhibitor beta | Immune response | −41.0 | 0.59 | 1.69E−07 |
| HSP90AA1 | Heat shock protein HSP 90‐alpha | Immune and infl. | −41.5 | 0.58 | 3.07E−07 |
| KRT17 | Keratin, type I cytoskeletal 17 | Immune response | −42.5 | 0.57 | 0.003159425 |
| ENO1 | Alpha‐enolase | Immune and infl. | −44.4 | 0.55 | 0.010242481 |
| KRT6A | Keratin, type II cytoskeletal 6A | Immune response | −44.6 | 0.55 | 1.29E−07 |
| ACTB | Actin, cytoplasmic 1 | Immune response | −47.3 | 0.52 | 7.51E−06 |
| IGKV2‐29 | Immunoglobulin kappa variable 2–29 | Unknown | −48.2 | 0.51 | 7.23E−09 |
| IGHV3‐23 | Immunoglobulin heavy variable 3–23 | Unknown | −49.4 | 0.50 | 1.41E−07 |
| HNRNPA1 | Heterogeneous nuclear ribonucleoprotein A1 | Immune and infl. | −50.4 | 0.49 | 0.005957151 |
| HNRNPC | Heterogeneous nuclear ribonucleoproteins C | Immune and infl. | −55.5 | 0.44 | 0.009134014 |
| HIST1H2AB | Histone H2A | Immune and infl. | −56.1 | 0.43 | 0.001243906 |
| H4C1 | Histone H4 | Unknown | −61.9 | 0.38 | 0.007168713 |
| IGLV2‐8 | Immunoglobulin lambda variable 2–8 | Unknown | −66.2 | 0.33 | 3.21E−10 |
| HIST1H2BK | Histone H2B | Immune and infl. | −67.1 | 0.32 | 3.96E−07 |
| PTMA | Prothymosin alpha | Immune response | −67.3 | 0.32 | 0.010697326 |
| S100A11 | Protein S100‐A11 | Immune and infl. | −67.4 | 0.32 | 0.00122428 |
| IGKV6D−21 | Immunoglobulin kappa variable 6D−21 | Immune response | −73.5 | 0.26 | 2.15E−06 |
4. DISCUSSION
The present open, exploratory, proof‐of‐concept study on patients with painful KOA showed that intra‐articular treatment with gold microparticles provides pain relief, modulation of experimental pain assessment parameters, and changes in the proteomic profile of SF and serum after eight weeks, and continuous clinical benefits throughout the two years follow‐up. The finding of several downregulated proteins directly influenced by the NF‐kB pathway suggests that gold treatment does inhibit this pathway. In addition, these changes might affect both peripheral and central modulatory pain mechanisms.
Similarly, the proteome changes in SF and serum were found during treatment indicates involvement of multiple functional pathways and signalling processes including wound healing, regulation of humoral and adaptive immune system and neutrophil degranulation (Figure 4b,c). In humans, neutrophils increasingly accumulate in the joints of patients with inflammatory pain such as arthritis, and their recruitment was found to be associated with the development of hyperalgesia (Liu et al., 2021).
Previous studies have found inflammation to be associated with pain in KOA (Petersen et al., 2019a, 2016), and this might in part be due to sensitization of peripheral and central pain mechanisms (Arendt. Nielsen et al., 2015; Petersen et al., 2015). Conflicting evidence suggests that anti‐inflammatory treatment with COX‐2 inhibitors does seem to increase PPTs and TS (Arendt‐Nielsen et al., 2016) but traditional treatment with nonsteroidal anti‐inflammatory drugs and paracetamol does not seem to affect PPTs or CPM (Petersen et al., 2019b). The current study indicates that intra‐articular treatment with gold microparticles does modulate PPTs, TS, and CPM and therefore adds to the growing evidence that inflammation and central pain mechanisms might be connecting in KOA.
The significant reduction in pain and sensory testing, and at the same time, the association to the significant proteome changes in SF and serum (Tables 2 and 3, and Figure 4), indicates that some of these biomarkers may be a measurement or a reflection of the changes in pain and inflammatory state. IL6 and IL10 in SF and serum are related to pathological pain sensitivity in a rodent model of osteoarthritis (Bowles et al., 2014; Nees et al., 2019). IL6 is a proinflammatory mediator and increase pain, and IL10 is anti‐inflammatory (Nees et al., 2019). We found a decrease of IL6 in SF (Figure 4a).
Several of the upregulated protein we found in SF (Table 2 and Figure 4b,c) are implicated in the immune cell associated anti‐inflammatory processes. Golgi Membrane Protein 1 (GOLM1) has been implicated in inflammation and immunoregulation (Zhang et al., 2018). Genetic inactivation of GOLM1 in myeloid cells suppresses interleukin (IL)‐12 secretion and polarizes macrophage polarization (Zhang et al., 2018). Serum Amyloid P (APCS) exhibits capabilities in the activation of the innate immune system (Girton et al., 2018).
The elevated Chondroadherin in SF promotes the attachment of chondrocytes and fibroblasts mediated by the integrin α2β1 (Schedel et al., 2004). Previously, gold nanoparticles have been found to induce chondrogenic differentiation of mesenchymal stem cells (Li et al., 2017). This indicates gold particles may induce chondrogenic differentiation of resident mesenchymal stem cells. Increased levels of self‐assembled peptides (SAP) and differential regulation of multiple neutrophil extracellular traps (NETs) associated proteins (myeloperoxidase [MPO], annexin [ANAX], neutrophil elastase [ELANE]) indicates activation of neutrophils and neutrophil granule exocytosis (Zhang et al., 2018). Similarly, proteomic serum profiling (Table 3 and Figure 4b,c) indicates a decreased activity of NETs associated proteins (Enolase (ENO1) and Calgizzarin (S100A11)) and reduced inflammation (Birkelund et al., 2020). Extracellular DNA, called cell‐free DNA (cfDNA), is associated with various pathological conditions that triggers neutrophil degranulation. cfDNA is elevated in peripheral blood and synovial fluid in RA and is associated with disease activity (Birkelund et al., 2020). Our findings indicate that the level of cfDNA in OA patients is less pronounced or less associated to the gold nanoparticle treatment (Figure 4d). Higher levels of cfDNA are reported in patients with intense pain (de Souza Barbosa et al., 2021), however, hitherto no investigations are available for OA patients.
We found 4 upregulated proteins in SF related to regenerative processes (Table 2). Thymidine Phosporylase (TYMP) has growth‐promoting activity on endothelial cells and is angiogenic (Dikici et al., 2020). Centriolar protein homolog A (POC1A) is involved in the early steps of centriole duplication (Venoux et al., 2013). Rho GDP‐dissociation inhibitor 1 (ARHGDIA) that inhibits CDC42 associated degeneration (Nomanbhoy & Erickson, 1999). Chondroadherin (Chad) stimulates chondrocyte proliferation (Paracuellos et al., 2017). In another study using a rat model of multiple sclerosis metallic gold shows a regenerative potential by increasing stem cell responses (Pedersen et al., 2012). They found the response of biomarkers playing a role in neural progenitor cells proliferation and viability, proved the stem cell response (Pedersen et al., 2012).
We found a significant downregulation in SF of CDC42 and associated proteins. Increased CDC42 is associated with cartilage degeneration and bone deterioration in a mouse osteoarthritic model (Hu et al., 2018). A recent review discusses CDC42 and related biomarkers that govern chondrocytes dedifferentiation and explains how the induced actin crosslinking and polymerization induces loss of cartilage (Seifert et al., 2012). IL6 production and chondrocyte inflammation is mediated by CDC42 (Lauer et al., 2021).
We are not aware of any other human studies of intra‐articular micro gold treatment and despite several laboratory studies (Danscher, 2002; Danscher & Larsen, 2010; Larsen et al., 2008; Lauer et al., 2021; Seifert et al., 2012) and animal studies (Danscher, 2002; Havarinasab et al., 2007; Hazer et al., 2011; Jæger et al., 2007, 2012; Lie et al., 2011; Märki et al., 2018), only one study involve gold microparticles injected horse carpal joints (Märki et al., 2018). There are several studies on the benefits of intravenous treatment of rheumatoid arthritis with goldthiocompounds like Myocrisine© (Clark et al., 2010; Lehman et al., 2005; Rau, 2005). One human study of extra‐articular placed gold beads for KOA, did not find a clinically relevant difference (Nejrup et al., 2008).
The patients involved in the present study experienced an improvement on most parameters. Twenty‐two of the thirty patients reported a positive effect even after two years, suggesting that the release of gold ions continues unchanged and that the gold microparticles stay in the knee joint. Most of the patients experienced the pain‐relieving effect within the first 6–8 weeks after treatment. Compared to the short‐term effects of single or multiple intra‐articular treatments with steroids or hyaluronic acid (Bellamy et al., 2006; Henrotin et al., 2019) we found this longer‐lasting effect of a single intra‐articular gold injection in KOA.
This explorative study was not blinded and included no control group. Therefore, the findings may overestimate the effects of intra‐articular gold treatment. However, the proteomic analysis of the SF and serum supports the statement that gold might interact with the mechanism involved in osteoarthritis. The pain evaluation and scores obtained before treatment indicated mild to severe pain like previous studies on knee osteoarthritis (Arendt‐Nielsen et al., 2015, 2016; Petersen et al., 2019a; Petersen et al., 2016, 2021) which is the most relevant large clinical group. The study, however, used the patient's SF as the carrier of the gold microparticles, excluding interference from other elements than gold, and not using any agents that may have the possibility to be analgesic. A weakness in the study is that the puncture or the aspiration by itself influences the short‐time clinical outcome measures. However, the volume aspirated was minor and only three patients presented with clinical signs of effusion, not in need of aspiration. We do not consider the puncture, or the aspiration may influence the long‐term clinical outcome measures.
In addition, the intervention was carefully standardized and administered by the same physician (SR), the pain evaluation before and after 8 weeks done by the same research nurse (TJ), and the 2‐year follow‐up performed by two medical students (ES and NKJ).
In conclusion, our results show that gold microparticles injected intra‐articular in KOA joints reduce inflammation and provide pain relief. The pain‐reducing effect may be a result of immunosuppression and regenerative processes. We recorded no adverse effects related to the treatments. However, a large randomized, double‐blind study that comprises patients with KOA, and who have degenerative symptoms for more than three months is needed to infer a conclusion. An extended analysis of the biological material from joints and systemic biofluids in comparison to matched controls will provide more insight into the immunoregulatory processes of the pain relief induced by gold microparticles.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
SR was the principal investigator in the study and participated in data collection, analysis, and interpretation. All authors assisted in analysing and interpreting the data and contributed to writing the manuscript. All authors read and approved the final manuscript. SR take responsibility for the integrity of the work, from inception to finished article. All authors discussed the results and commented on the manuscript.
ACKNOWLEDGMENTS
The authors wish to thank Gregers Gregersen, DVM, Professor Gorm Danscher, DVM, DMSc and Tina Jensen, research nurse, for the assistance during the project.
Rasmussen, S. , Kjær Petersen, K. , Kristiansen, M. K. , Skallerup, J. , Aboo, C. , Thomsen, M. E. , Skjoldemose, E. , Jørgensen, N. K. , Stensballe, A. , & Arendt‐Nielsen, L. (2022). Gold micro‐particles for knee osteoarthritis. European Journal of Pain, 26, 811–824. 10.1002/ejp.1909
Funding information
The Department of Clinical Medicine provided funding for this study. The Center for Neuroplasticity and Pain (CNAP) (Lars Arendt‐Nielsen) provided funding for this study. CNAP is supported by the Danish National Research Foundation (DNRF121) and Danish Rheumatism Association (R204‐A7645). The Danish National Mass Spectrometry Platform for Functional Proteomics (PRO‐MS; grant no. 5072‐00007B). The Obelske family foundation, the Svend Andersen Foundation and the SparNord foundation are acknowledged for grants to the analytical platform, enabling parts of this study.
REFERENCES
- Arendt‐Nielsen, L. , Egsgaard, L. L. , & Petersen, K. K. (2016). Evidence for a central mode of action for etoricoxib (COX‐2 inhibitor) in patients with painful knee osteoarthritis. Pain, 157, 1634–1644. 10.1097/j.pain.0000000000000562 [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , & Graven‐Nielsen, T. (2011). Translational musculoskeletal pain research. Best Practice & Research Clinical Rheumatology, 25, 209–226. 10.1016/j.berh.2010.01.013 [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Skou, S. T. , Nielsen, T. A. , & Petersen, K. K. (2015). Altered central sensitization and pain modulation in the CNS in chronic joint pain. Current Osteoporosis Reports, 13, 225–234. 10.1007/s11914-015-0276-x [DOI] [PubMed] [Google Scholar]
- Bellamy, N. , Buchanan, W. W. , Goldsmith, C. H. , Campbell, J. , & Stitt, L. W. (1988). Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology, 15, 1833–1840. [PubMed] [Google Scholar]
- Bellamy, N. , Campbell, J. , Robinson, V. , Gee, T. , Bourne, R. , & Wells, G. (2006). Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Systematic Review, 10, CD005328. [DOI] [PubMed] [Google Scholar]
- Berners‐Price, S. J. , & Filipovska, A. (2011). Gold compounds as therapeutic agents for human diseases. Metallomics, 3, 863–873. 10.1039/c1mt00062d [DOI] [PubMed] [Google Scholar]
- Birkelund, S. , Bennike, T. B. , Kastaniegaard, K. , Lausen, M. , Poulsen, T. B. G. , Kragstrup, T. W. , Deleuran, B. W. , Christiansen, G. , & Stensballe, A. (2020). Proteomic analysis of synovial fluid from rheumatic arthritis and spondyloarthritis patients. Clinical Proteomics, 17. 10.1186/s12014-020-09292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles, R. D. , Mata, B. A. , Bell, R. D. , Mwangi, T. K. , Huebner, J. L. , Kraus, V. B. , & Setton, L. A. (2014). In vivo luminescence imaging of nf‐kb activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis & Rheumatology, 66, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, P. , Tugwell, P. , Bennet, K. , Bombardier, C. , Shea, B. , & Wells, G. S. A. M. (2010). Injectable gold for rheumatoid arthritis (Review). Cochrane Database Systematic Reviews, 1997:CD000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danscher, G. (2002). In vivo liberation of gold ions from gold implants. Autometallographic tracing of gold in cells adjacent to metallic gold. Histochemistry and Cell Biology, 117, 447–452. 10.1007/s00418-002-0400-8 [DOI] [PubMed] [Google Scholar]
- Danscher, G. , & Larsen, A. (2010). Effects of dissolucytotic gold ions on recovering brain lesions. Histochemistry and Cell Biology, 133, 367–373. 10.1007/s00418-010-0681-2 [DOI] [PubMed] [Google Scholar]
- Dikici, S. , Aldemir Dikici, B. , Bhaloo, S. I. , Balcells, M. , Edelman, E. R. , MacNeil, S. , Reilly, G. C. , Sherborne, C. , & Claeyssens, F. (2020). Assessment of the angiogenic potential of 2‐deoxy‐d‐ribose using a novel in vitro 3D dynamic model in comparison with established in vitro assays. Frontiers in Bioengineering and Biotechnology, 7. 10.3389/fbioe.2019.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freynhagen, R. , Baron, R. , Gockel, U. , & Tölle, T. R. (2006). painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Current Medical Research and Opinion, 22, 1911–1920. [DOI] [PubMed] [Google Scholar]
- Giordano, R. , Petersen, K. K. , Andersen, H. H. , Simonsen, O. , & Arendt‐Nielsen, L. (2020). Serum inflammatory markers in patients with knee osteoarthritis: A proteomic approach. Clinical Journal of Pain, 36, 229–237. 10.1097/AJP.0000000000000804 [DOI] [PubMed] [Google Scholar]
- Girton, A. W. , Popescu, N. I. , Keshari, R. S. , Burgett, T. , Lupu, F. , & Coggeshall, K. M. (2018). Serum amyloid P and IgG exhibit differential capabilities in the activation of the innate immune system in response to Bacillus anthracis peptidoglycan. Infection and Immunity, 86. 10.1128/IAI.00076-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havarinasab, S. , Johansson, U. , Pollard, K. M. , & Hultman, P. (2007). Gold causes genetically determined autoimmune and immunostimulatory responses in mice. Clinical and Experimental Immunology, 150, 179–188. 10.1111/j.1365-2249.2007.03469.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazer, D. B. , Hazer, B. , & Dinçer, N. (2011). Soft tissue response to the presence of polypropylene‐g‐poly(ethylene glycol) comb‐type graft copolymers containing gold nanoparticles. Journal of Biomedicine and Biotechnology, 2011, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotin, Y. , Bannuru, R. , Malaise, M. , Ea, H. K. , Confavreux, C. , Bentin, J. , Urbin‐Choffray, D. , Conrozier, T. , Brasseur, J. P. , Thomas, P. , Hick, A. C. , Marinello, A. , Giordan, N. , & Richette, P. (2019). Hyaluronan derivative HYMOVIS® increases cartilage volume and type ii collagen turnover in osteoarhritic knee: Data from MOKHA study. BMC Musculoskeletal Disorders, 20:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. , Ji, X. , Yang, M. , Fan, S. , Wang, J. , Lu, M. , Shi, W. , Mei, L. , Xu, C. , Fan, X. , Hussain, M. , Du, J. , Wu, J. , & Wu, X. (2018). Cdc42 is essential for both articular cartilage degeneration and subchondral bone deterioration in experimental osteoarthritis. Journal of Bone and Mineral Research, 33, 945–958. 10.1002/jbmr.3380 [DOI] [PubMed] [Google Scholar]
- Jæger, G. T. , Larsen, S. , Søli, N. , & Moe, L. (2007). Two years follow‐up study of the pain‐relieving effect of gold bead implantation in dogs with hip‐joint arthritis. Acta Veterinaria Scandinavica, 49. 10.1186/1751-0147-49-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jæger, G. T. , Stigen, Ø. , Devor, M. , & Moe, L. (2012). Gold bead implantation in acupoints for coxofemoral arthrosis in dogs: Method description and adverse effects. Animals, 2, 426–436. 10.3390/ani2030426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper, S. J. , Maher, C. G. , & Mackay, G. (2009). Global rating of change scales: A review of strengths and weaknesses and considerations for design. J Man Manip Ther, 17, 163–170. 10.1179/jmt.2009.17.3.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellgren, J. H. , & Lawrence, J. S. (1957). Radiological assessment of osteo‐arthrosis. Annals of the Rheumatic Diseases, 16(4), 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. A. , & Khan, M. J. (2018). Nano‐gold displayed anti‐inflammatory property via NF‐kB pathways by suppressing COX‐2 activity. Artificial Cells, Nanomedicine, and Biotechnology, 46, 1149–1158. 10.1080/21691401.2018.1446968 [DOI] [PubMed] [Google Scholar]
- Koh, H. W. L. , Zhang, Y. , Vogel, C. , & Choi, H. (2019). EBprotV2: A perseus plugin for differential protein abundance analysis of labeling‐based quantitative proteomics data. Journal of Proteome Research, 18, 748–752. 10.1021/acs.jproteome.8b00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, G. A. , & Thabane, L. (2019). Guidelines for reporting non‐randomised pilot and feasibility studies. Pilot and Feasibility Studies, 5. 10.1186/s40814-019-0499-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, A. , Kolind, K. , Pedersen, D. S. , Doering, P. , Pedersen, M. Ø. , Danscher, G. , Penkowa, M. , & Stoltenberg, M. (2008). Gold ions bio‐released from metallic gold particles reduce inflammation and apoptosis and increase the regenerative responses in focal brain injury. Histochemistry and Cell Biology, 130, 681–692. 10.1007/s00418-008-0448-1 [DOI] [PubMed] [Google Scholar]
- Lauer, J. C. , Selig, M. , Hart, M. L. , Kurz, B. , & Rolauffs, B. (2021). Articular chondrocyte phenotype regulation through the cytoskeleton and the signaling processes that originate from or converge on the cytoskeleton: Towards a novel understanding of the intersection between actin dynamics and chondrogenic function. International Journal of Molecular Sciences, 22. 10.3390/ijms22063279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman, A. J. , Esdaile, J. M. , Klinkhoff, A. V. , Grant, E. , Fitzgerald, A. , & Canvin, J. (2005). A 48‐week, randomized, double‐blind, double‐observer, placebo‐controlled multicenter trial of combination methotrexate and intramuscular gold therapy in rheumatoid arthritis: Results of the METGO study. Arthritis and Rheumatism, 52, 1360–1370. 10.1002/art.21018 [DOI] [PubMed] [Google Scholar]
- Li, J. , Li, X. , Zhang, J. , Kawazoe, N. , & Chen, G. (2017). Induction of chondrogenic differentiation of human mesenchymal stem cells by biomimetic gold nanoparticles with tunable RGD density. Advanced Healthcare Materials, 6, 1–12. 10.1002/adhm.201700317 [DOI] [PubMed] [Google Scholar]
- Lie, K. I. , Jæger, G. , Nordstoga, K. , & Moe, L. (2011). Inflammatory response to therapeutic gold bead implantation in canine hip joint osteoarthritis. Veterinary Pathology, 48, 1118–1124. 10.1177/0300985810381910 [DOI] [PubMed] [Google Scholar]
- Liu, J. A. , Yu, J. , & Cheung, C. W. (2021). Immune actions on the peripheral nervous system in pain. International Journal of Molecular Sciences, 22(3), 1448. 10.3390/ijms22031448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Märki, N. , Witte, S. , Kuchen, S. , Reichenbach, S. , Ramseyer, A. , Gerber, V. , & Spadavecchia, C. (2018). Safety of intra‐articular gold microimplants in horses–a randomized, blinded, controlled experimental study. Journal of Equine Veterinary Science, 60, 59–66.e2. 10.1016/j.jevs.2017.03.005 [DOI] [Google Scholar]
- Meier, F. , Brunner, A. D. , Frank, M. , Ha, A. , Bludau, I. , Voytik, E. , Kaspar‐Schoenefeld, S. , Lubeck, M. , Raether, O. , Bache, N. , Aebersold, R. , Collins, B. C. , Röst, H. L. , & Mann, M. (2020). diaPASEF: parallel accumulation–serial fragmentation combined with data‐independent acquisition. Nature Methods, 17, 1229–1236. 10.1038/s41592-020-00998-0 [DOI] [PubMed] [Google Scholar]
- Nees, T. A. , Rosshirt, N. , Zhang, J. A. , Reiner, T. , Sorbi, R. , Tripel, E. , Walker, T. , Schiltenwolf, M. , Hagmann, S. , & Moradi, B. (2019). Synovial cytokines significantly correlate with osteoarthritis‐related knee pain and disability: Inflammatory mediators of potential clinical relevance. J Clin Med, 8, 1343. 10.3390/jcm8091343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejrup, K. , Fine Olivarius, N. , Jacobsen, J. L. , & Siersma, V. (2008). Randomised controlled trial of extraarticular gold bead implantation for treatment of knee osteoarthritis: A pilot study. Clinical Rheumatology, 27, 1363–1369. 10.1007/s10067-008-0918-9 [DOI] [PubMed] [Google Scholar]
- Nomanbhoy, T. K. , Erickson, J. W. , & Cerione, R. A. (1999). Kinetics of Cdc42 membrane extraction by Rho‐GDI monitored by real‐time fluorescence resonance energy transfer. Biochemistry, 38, 1744–1750. 10.1021/bi982198u [DOI] [PubMed] [Google Scholar]
- Paracuellos, P. , Kalamajski, S. , Bonna, A. , Bihan, D. , Farndale, R. W. , & Hohenester, E. (2017). Structural and functional analysis of two small leucine‐rich repeat proteoglycans, fibromodulin and chondroadherin. Matrix Biology, 63, 106–116. 10.1016/j.matbio.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, D. S. , Fredericia, P. M. , Pedersen, M. O. , Stoltenberg, M. , Penkowa, M. , Danscher, G. , Rungby, J. , & Larsen, A. (2012). Metallic gold slows disease progression, reduces cell death and induces astrogliosis while simultaneously increasing stem cell responses in an EAE rat model of multiple sclerosis. Histochemistry and Cell Biology, 138, 787–802. 10.1007/s00418-012-0996-2 [DOI] [PubMed] [Google Scholar]
- Petersen, K. K. , Arendt‐Nielsen, L. , Simonsen, O. , Wilder‐Smith, O. , & Laursen, M. B. (2015). Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain, 156, 55–61. 10.1016/j.pain.0000000000000022 [DOI] [PubMed] [Google Scholar]
- Petersen, K. K. , Olesen, A. E. , Simonsen, O. , & Arendt‐Nielsen, L. (2019a). Mechanistic pain profiling as a tool to predict the efficacy of 3‐week nonsteroidal anti‐inflammatory drugs plus paracetamol in patients with painful knee osteoarthritis. Pain, 160, 486–492. 10.1097/j.pain.0000000000001427 [DOI] [PubMed] [Google Scholar]
- Petersen, K. K. , Siebuhr, A. S. , Graven‐Nielsen, T. , Simonsen, O. , Boesen, M. , Gudbergsen, H. , Karsdal, M. , Bay‐Jensen, A. C. , & Arendt‐Nielsen, L. (2016). Sensitization and serological biomarkers in knee osteoarthritis patients with different degrees of synovitis. Clinical Journal of Pain, 32, 841–848. 10.1097/AJP.0000000000000334 [DOI] [PubMed] [Google Scholar]
- Petersen, K. K. , Simonsen, O. , Olesen, A. E. , Mørch, C. D. , & Arendt‐Nielsen, L. (2019b). Pain inhibitory mechanisms and response to weak analgesics in patients with knee osteoarthritis. Eur J Pain (United Kingdom), 23, 1904–1912. 10.1002/ejp.1465 [DOI] [PubMed] [Google Scholar]
- Petersen, K. K. , Vaegter, H. B. , Stubhaug, A. , Wolff, A. , Scammell, B. E. , Arendt‐Nielsen, L. , & Larsen, D. B. (2021). The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain, 162, 31–44. 10.1097/j.pain.0000000000002019 [DOI] [PubMed] [Google Scholar]
- Pham, T. , van der Heijde, D. , Altman, R. D. , Anderson, J. J. , Bellamy, N. , Hochberg, M. , Simon, L. , Strand, V. , Woodworth, T. , & Dougados, M. (2004). OMERACT‐OARSI initiative: Osteoarthritis research society international set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr Cartil, 12, 389–399. 10.1016/j.joca.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Rau, R. (2005). Have traditional DMARDs had their day? Effectiveness of parenteral gold compared to biologic agents. Clinical Rheumatology, 24, 189–202. 10.1007/s10067-004-0869-8 [DOI] [PubMed] [Google Scholar]
- Schaab, C. , Geiger, T. , Stoehr, G. , Cox, J. , & Mann, M. (2012). Analysis of high accuracy, quantitative proteomics data in the MaxQB database. Molecular & Cellular Proteomics: MCP, 11. 10.1074/mcp.M111.014068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedel, J. , Wenglén, C. , Distler, O. , Müller‐Ladner, U. , Schölmerich, J. , Heinegård, D. , & Krenn, V. (2004). Differential adherence of osteoarthritis and rheumatoid arthritis synovial fibroblasts to cartilage and bone matrix proteins and its implication for osteoarthritis pathogenesis. Scandinavian Journal of Immunology, 60, 514–523. 10.1111/j.0300-9475.2004.01507.x [DOI] [PubMed] [Google Scholar]
- Seifert, O. , Matussek, A. , Sjögren, F. , Geffers, R. , & Anderson, C. D. (2012). Gene expression profiling of macrophages: Implications for an immunosuppressive effect of dissolucytotic gold ions. J Inflamm (United Kingdom), 9. 10.1186/1476-9255-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebuhr, A. S. , Petersen, K. K. , Arendt‐Nielsen, L. , Egsgaard, L. L. , Eskehave, T. , Christiansen, C. , Simonsen, O. , Hoeck, H. C. , Karsdal, M. A. , & Bay‐Jensen, A. C. (2014). Identification and characterisation of osteoarthritis patients with inflammation derived tissue turnover. Osteoarthr Cartil, 22, 44–50. 10.1016/j.joca.2013.10.020 [DOI] [PubMed] [Google Scholar]
- de Souza, B. Ê. , Santos Ibiapina, H. N. , Rocha da Silva, S. , Costa, A. G. , Val, F. F. , Mendonça‐da‐Silva, I. , de Lima, C. , Ferreira, L. , Sartim, M. A. , Monteiro, W. M. , Cardoso de Melo, G. , & de Almeida Gonçalves Sachett, J. (2021). Association of cfDNA levels and bothrops envenomation. Toxicon, 192, 66–73. [DOI] [PubMed] [Google Scholar]
- Venoux, M. , Tait, X. , Hames, R. S. , Straatman, K. R. , Woodland, H. R. , & Fry, A. M. (2013). Poc1A and Poc1B act together in human cells to ensure centriole integrity. Journal of Cell Science, 126, 163–175. 10.1242/jcs.111203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Kim, H. , Lv, J. , Zhao, N. , & Ma, X. (2018). Golgi phosphoprotein 2 is a novel regulator of IL‐12 production and macrophage polarization. The Journal of Immunology, 200, 1480–1488. 10.4049/jimmunol.1700897 [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Zhou, B. , Pache, L. , Chang, M. , Khodabakhshi, A. H. , Tanaseichuk, O. , Benner, C. , & Chanda, S. K. (2019). Metascape provides a biologist‐oriented resource for the analysis of systems‐level datasets. Nature Communications, 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request. Proteomic raw and processed data are available from the Proteome exchange repository with identifier PXD030764 (http://www.proteomexchange.org).


