Abstract
Prebiotics are non-digestible carbohydrates which can be used as prime source of energy for gut microflora. These can be naturally occurring in fruit and vegetables or can be made synthetically by enzymatic digestions. New versatile sources of prebiotics had been found nowadays for economic commercialization. This review will decipher on highlighting the importance of prebiotics in immunomodulation and nutrient absorption abilities of gut, as it is important for the anti-effective capacity of the organism especially in the neonatal period. Moreover, new prebiotics transmission strategies with higher penetrating capacity such as microencapsulation and immobilization have been discussed. In addition to this, literature had shown the modulation of gut microflora by the continuous use of prebiotics in many disorders so here, the role of prebiotics in health-related issues such as diabetes and inflammatory bowel disease (IBS) have been explained.
Keywords: Immobilization, Immunomodulation, Gastrointestinal tract, Prebiotics
1. Introduction
The addition of beneficial components to diet plays an important role in manipulating gut microflora. This gut microflora is responsible for maintaining good health of the individual and the health of these microbes directly influenced by the intake of prebiotics [1]. They can nourish the microflora which gets affected during gut dysbiosis in disease conditions. These can be found naturally in various substances such as fruits (watermelon, nectarine, and pear, blueberry etc) and vegetables (scallion, white onion, garlic, spring garlic, and leek) [2]. The study is still going on in finding some new natural sources having prebiotic potentials. Some of the enzymatic synthesized commercially available prebiotics with degree of polymerization DP2 to DP10 are fructooligosaccharides and xylooligosaccharides. Their higher flexibility in the gut environment against different pathogens and easy fermentation ability into short chain fatty acid has made them wonderful compounds utilized by various food and medical industries [3]. They gradually decrease the number of harmful gut microflora and increase the concentration of useful ones. Different studies on boilers have suggested that when these boilers were fed with chicory fructans, there was an increase in the lactobacilli counts and decrease in the Campylobacter and Salmonella in the gastrointestinal tract [4]. Various experimental studies have been proved that prebiotics can help in reducing the severity of particular diseases such as diabetes, IBS, neural disorders and other infectious diseases [5]. Prebiotics are necessary to study as they are related not only with the health aspects but in the food industries; health professionals, scientists, regulators as well as consumers have a great interest in exploring the other beneficial effects of them. Healthy diet which includes all the required nutrients and prebiotics which controls lifestyle diseases by directly increasing the gut microflora populations in large intestine where fermentation of these non-digestible compounds takes place. The proper balance of these in diet may lead to a healthy immune system reducing the risk of inflammations and dyspepsia [7]. There is a huge challenge in identifying some of the new methods for prebiotics and probiotics transmission, also scientists are doing rigorous efforts in expending the advantages of these more than a healthy life and well being. To engineer probiotics and identifying their specific metabolic pathways for prebiotics which can carry pathogen inhibiting activity, immunomodulation and disease specific activities are still a challenging process [6].
This review is focused on various transmission strategies to increase the life of prebiotics and surviving abilities of these in the gut environment. In some cases direct intake of prebiotics is not possible so, to overcome these difficulties various strategies such as encapsulation, entrapment and immobilization has come into play [8]. Moreover, this review has covered various aspects of prebiotics mechanisms of actions such as immunomodulatory effects and enhancement in nutrient absorption. It has been seen in various studies that prebiotics positively affects the immune system by modulating the gut microflora [9]. They directly increase the resistance effects against the infection and microbial ability to fight against allergic reactions. Also, regular use of prebiotics can enhance the nutrients abortion ability of the intestinal linings and the microflora present there. This review can also emphasize on various health aspects of human by prebiotics, they induce health of the individual by targeting to human intestinal microflora [10]. They can rejuvenate the affected microflora in the disease condition and increase their populations. Prebiotics has diverse effects on humans in the diseased state; various studies have proved the positive role of prebiotics in different disorders such as cardiovascular disorders, Crohn’s disease, alcoholic fatty liver disease etc. In this, we have discussed on two disorders i.e. diabetes and inflammatory bowel disease (IBS), as these are worldwide disorders and may lead to other chronic diseases like cardiovascular disease, colorectal cancer etc [11].
2. New potential sources of prebiotics
Some of the naturally occurring prebiotics are Jerusalem artichoke, chicory, rye, milk, honey, onion, barley and salsify etc. in which prebiotic concentration range between 0.3 and 6% of fresh weight. Various natural sources of fructooligosaccharide are honey, banana, barley, tomato, Asparagus, sugar beet, garlic, wheat, mushrooms and rye [12]. Some natural sources of xylooligosaccharides are found in a variety of fruits, vegetables, milk, bamboo shoots, and honey [13]. Galactooligosaccharides are naturally found in bovine and human milk. Primary sources of raffinose oligosaccharides are Seeds of legumes, peas, lentils, beans, mustard and chickpeas. Algal polysaccharide and oligosaccharides are better oligosaccharides than other sources for prebiotics production as these can be included in food, feed or used as pills. Production of XOS and AXOS from agricultural residues offers excellent exposure to nutraceutical industries, having low price products and a significant amount of raw material [14].
Various studies are still going on for identifying new sources having the properties of prebiotics. Prebiotics can be developed by using various cereal grains such as corn, wheat, rice, barley, and oats economically. Fructose and fructooligosaccharide can be produced by immobilized inulinase-based reusable biocatalysts and bioreactors [15]. Aloe Vera can be used as a source of prebiotics due to some antibiotic activities. Studies have revealed that aloe vera fructans enhance the microbial populations than that of inulin [16]. Seaweed such as laminarin could, red seaweed (Gelidium sesquipedale) and brown seaweed (Osmundea pinnatifida, Ecklonia radiate) are good sources of prebiotics and able to increase the gut microflora communities [17]. Dark roasted coffee beans brewed coffee and spent coffee grounds have a wide range of oligosaccharide which can be used as prebiotics [18]. Stevia rebaudiana have a high content of steviol glycosides in its leaves and can be used for the higher production of inulin and fructooligosaccharides. Cashew apple (Anacardium occidentale L.) powder has the potential to be used as prebiotics, some evidences have been seen in which there is an increase in the population of Lactobacillus species by the continues use of CAP powder [19]. Microorganisms also have a potential to produce prebiotics for example Penicillium oxalicum can be used for inulinase production which can be further used for the production of inulin having prebiotic properties [20]. Agave salmiana spp have fractions of agave fructans which when supplemented, can optimize the populations of Lactobacillus casei and Lactobacillus paracasei. Increase in five enzymes (cysteine-arylamidase, α-chymotrypsin, β-galactosidase, N-acetyl-β-glucosaminidase and α-fucosidase) grown on agave fructans can be seen when enzyme activity of both bacteria was checked using API ZYM galleries [21].
Milk is considered to be a good source for prebiotic transmission as it contains a large number of bioactive compounds such as bioactive peptides, lipids, minerals, vitamins, immunoglobins, growth factors and cytokines which have good health benefits, it is a traditional food and can be readily available [22]. Foods derived from dairy products such as fermented milk, ice cream and other milk beverages contain a large number of fibrous compounds and oligosaccharide. Prebiotics present in these flavored products are taste enhancing and beneficial for increasing the population of the Bifidobacterium, also they can increase the probiotics in fermented milk products especially those present in yoghurt. In some studies, it has been seen that the raffinose family of oligosaccharide when mixed with milk the fermented milk product formed by it have a significant advantage over each other. The use of these in an adequate manner shows high acidification rate which directly increases to the growth of probiotics in the gut by reducing the doubling time. Milk fortification also helps in increasing the populations of lactic acid bacteria and other probiotics in fermented milk [23].
3. New generation prebiotics and its transmission
Prebiotics can be generated by different means depending upon the availability of sources. New techniques can be used for making prebiotics more efficient and good for human consumption. Various experimental studies are still going on in improving the quality of digestive prebiotics and their utilization. For example, chemical degradation of citrus peel consisting of pectin can give fractions of pectin oligosaccharides. Changing the concentration of the different chemicals such as trifluoroacetic acid (TFA) and hydrogen peroxide (H2O2) can change the prebiotic activity [24]. In potato galactan-rich rhamnogalacturonan I, a bi-enzymatic system having two multi-enzymatic preparations (Depol 670 L and Gamanase 1.5 L) are used to investigate the production of prebiotic galacto, arabino-oligosaccharides and oligomers with a well-defined degree of polymerization (DP) [25]. Immobilization is a new strategy for developing cost-effective prebiotics, catalyst such as levansucrases, β-frutofur-anosidases and β-galactosidases can be used for developing high-quality prebiotics. Immobilization can increase reaction yields, instigating a microenvironment around the enzyme. Covalently immobilized β-galactosidase on macrospheres of chitosan can also be used for the synthesis of lactosucrose. In this approach β-galactosidase from B. circulans was immobilized on chitosan. Through this study, one can investigate the positive effects of immobilization approach on thermal stability, retention activity and operational stability [26].
Prebiotics chocolates are a new way of transmitting the prebiotics and probiotics as a functional food. Many studies have been done to check the bioavailability and bioaccessibility characteristics of prebiotic, probiotic and synbiotics chocolates by in vivo and in vitro methods. Moreover, a metagenomic era has created a clear vision to prebiotics and the host gut microflora symbiotic relationships. Through genomic studies, new potential prebiotics with unique metabolic trait can be biosynthesized by giving a glance at environmental variations [27]. Plant cell wall polysaccharides have a high potential for generating new potent prebiotics. Enzyme membrane reactors can be used for pectic oligosaccharides having prebiotic properties. Encapsulation is a new strategy to study the effect and potential of different prebiotics. Various examples can be seen, such as modified starch can be replaced by emulsification. In this encapsulation plays an important role as oregano extract was encapsulated with five portions of inulin and altered starch as wall materials, while microparticle size and inulin proportion are inversely proportional to each other. Encapsulation can also be studied for probiotics as these have the potential to protect them from the adverse environment of the gut. For example, Bacillus coagulans survivability can be improved by entrapping it into Bio-nanocomposite made up of bacterial nanocellulose [28]. Various computational studies can be done for different prebiotic synthesizing enzymes such as quantum mechanics/molecular mechanics (QM/MM) can be used to study the hydrolysis and transfructosylation reactions catalyzed by A. japonicus FT by means of sucrose as donor and acceptor substrates [29]. Some more informative and related studies are given in Table 1.
Table 1.
Significant studies on prebiotics transmission strategies.
| Prebiotics/Probiotics | Sources | Transmission strategy or delivery system | References |
|---|---|---|---|
| Bakery products including L. plantarum, L. brevis and Saccharomyces cerevisiae Fructooligosaccharides, inulin | Yacon flour, Green dwarf banana flour, Wheat-taro composite flour | Microencapsulation | [50] |
| Beads of a mixture of alginate and inulin | Lactobacillus salivarius (LS), Lactobacillus reuteri (LR) and Pediococcus acidilactici UL5 (UL5), | Encapsulation studied by specific PMA-qPCR counting | [51] |
| Lactobacillus, Bifidobacterium, lactic acid bacteria, synbiotics | polysaccharide food-addictive such as Corn-derived maltodextrin etc | Oral gavage or water bottle and syringe-feeding as a refinement method on rats | [52] |
| Inulin nanoparticle | inulin nanoparticle (IN)-internalized Pediococcus acidilactici (PA) | Encapsulation into alginate/chitosan/alginate (ACA) microcapsules (MCs) | [8] |
| Colony-stimulating growth factor 1 (CSF-1), Fibroblast growth factor 2 (FGF-2) and interleukin 4 (IL-4) | Streptococcus, Lactococcus, Leuconostoc, Lactobacillus and Pediococcus. | Food-grade (LAB) lactic acid bacteria as vehicles | [53] |
| soy protein isolate (SPI) and acylated soy protein (SPA) | Lactobacillus plantarum CECT 220 and Lactobacillus casei CECT 475 coacervation of soybean protein concentrate (SPC) | spray drying Encapsulation | [54] |
| Combinations of prebiotics and probiotics | α-glucans – amylose and amylopectin free fatty acids and lysophopholipids present in cereals e.g. maize wheat, rice and potato etc | Encapsulation on Starch granules | [55] |
| Combinations of probiotics and prebiotics | L. plantarum L. lactis Escherichia coli, Lactobacillus jensenii, fermented pomegranate juice | Vesicular systems such as nanoparticles, liposomes etc | [56] |
| Bifidobacterium animalis ssp. lactis BB-12 as powdered probiotic passion fruit juice | Microencapsulation | [57] | |
| Lactobacillus casei, Lactobacillus brevis and Lactobacillus plantarum Resistant starch | Rice | Microencapsulation | [58] |
4. Immunomodulation by prebiotics
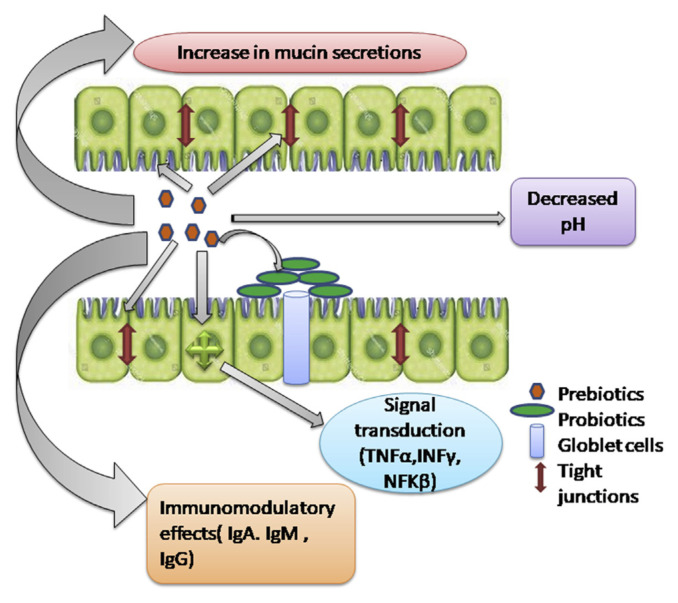
The health of the individual can be improved by increasing their immunity. Some prebiotics can modulate immunity by binding to the G protein receptors within gut-associated lymphoid tissue (GALT). Prebiotics can affect the immune functioning directly, indirectly or both. They increase the number of beneficial microflora and reduce the accumulation of disease-causing pathogens. In various studies, it has been seen that in salmonella infections, pathogen adherence to GIT is diminished by mannose monomers [30]. Prebiotics can positively affect the functioning of the immune system by changing the expression of cytokines. For example, the accumulation of proinflammatory cytokines interferon (IFN) -γ and interleukin (IL) −2 in rat mesenteric lymph nodes was inhibited by microbial residues mainly butyrate. In addition to this, inhibition in the production of proinflammatory IL-2 and IFN-γ by butyrate has been seen in some cell culture studies. Instead of this, propionate and acetate amplify the production of anti-inflammatory cytokine IL-10. The immune response is affected by cytokine production by a range of events. Interactions of pattern recognition receptors, with pathogen-associated molecular patterns can play a significant role in health maintanance maintenance of the individual, as they can directly modify the expressions of cytokines. Prebiotics has a direct effect on the immune system by varying the expression of genes. The compiled immunomodulatory effects are shown in Fig. 1. The effect of OSCs varies by diverse degree of polymerization (DP) which can openly effect on cytokine production in CD4+ T cells [31]. Prebiotics with a range of DP (DP16, DP8 and DP4) can modulate intestinal immune systems which further amplify the number of lactobacilli population in the large intestine results in the higher production of cytokines, such as IFN-γ and IL-10 [32]. Different studies on FOS (DP4) fed mice showed an increase in the production of IL-10 and IFN-γ in CD4+ T cells. Moreover, PP lymphocytes of rats consuming oligofructose (fructans degree of polymerization 2 to 8) having high amounts of inulin have an elevation in the production of IL-10 and IFN-γ under concanavalin stimulation [33]. The direct and indirect effects of prebiotics on innate immunity can be estimated in fishes and other aquatic organisms. Some of the immunosaccharide are fructooligosaccharide, manoligosaccharide, inulin or βglucan boost various responses such as phagocytic activation, neutrophil activation, commencement of the alternative complement system, amplified lysozyme activity etc. Both pattern recognition receptors (PRR) and microbe-associated molecular patterns (MAMPs) are activated in innate immunity cells [34].
Fig. 1.
Representation of direct and indirect effects of prebiotics on host innate immunity.
Synbiotics are probiotics combined with prebiotics. These also affect the immune function of various organisms. Different studies on fishes show that they can affect immunity by forcing epithelial cells for secreting cytokines which directly modulate the immune cells. Also, lipopolysaccharides can initiate tumor necrosis factor-α and a toll-like receptor which increases respiratory burst activity, phagocytosis, and nitric oxide production. Various studies on shellfish showed that synbiotics arouse the production and degranulation of hemocytes in shrimp. Melanization and phagocytosis process can be triggered by specific pattern-recognition proteins [35].
Prebiotic properties of mushrooms are also identified as proteoglycans and polysaccharide present in them which can play a vital role in immunomodulation and antitumor actions. Components of both adaptive immunity and innate immunity are modified by producing biologic response modifiers. Many glucans and heteroglycans in mushrooms have properties which can kindle macrophages, splenocytes and thymocytes. Some studies on Wistar rats proved cocoa fibers or inulin as potential prebiotics. Giving rats CF diet can directly increase Bifidobacterium and Lactobacillus counts, the percentage of IgA-coated bacteria, the SCFA concentrations, the TLR2, TLR5, TLR7 and occludin expression, hence improving the immunity [36].
5. Enhancing nutritional absorption by prebiotics
Metabolites in the body can be increased by the perpetual use of prebiotics. They not only increase the concentrations of byproducts while they interact with the gut microflora and increase the levels of molecules in the gastrointestinal tract synergistically. They increase the permeability of the intestinal membrane and modify it according to the size of the molecule. In various studies, it has been seen that they enhance the nutrient absorption in the intestine and may lead to an increase in the levels of RBCs, WBCs and other proteins. Various studies on Channa striata fingerlings proved that an increase in the concentration of prebiotics such as fructooligosaccharide and maltooligosaccharide in the diet increases the hemoglobin concentrations and serum proteins [37]. Combinatorial effect of both prebiotics and probiotics such as Saccharomyces cerevisiae and Lactobacillus acidophilus with FOS and MOS creates resistance against pathogen attack especially Aeromonas hydrophila. In some studies, it has been seen that these combinations affect the SCFA production such as acetate butyrate, propionate etc. [38]. Other product such as flavonoids directly interacts with the environment of the intestine and dietary fibers which further induces the critical change in the absorption and metabolism of the host. They targets on homeostasis of glucose lipid, energy metabolism, and cardiovascular risk factors. They protect from food toxins, can modulate secretions of gut hormones, enzyme activity involved in carbohydrate absorption, immune system and many others. Some investigated mice studies suggested that GOS when supplemented for a long time may affect the lipid and glucose metabolism of the host. About twenty-one metabolites were affected by the incorporation of GOS in the diet which includes oleic acid, arachidic acid and behenic acid [39]. Other prebiotics such as difructose anhydrides (DFA)–IVs directly influence the absorption levels of blood calcium and iron. Some studies on broilers suggested that integration of these into diet increase the digestibility of nutrients also they can increase expression of genes targeted to liver and muscle development. Moreover, it also increases the capability of wound healing in the intestine [40].
6. Prebiotics and diseases
6.1. Role of prebiotics in diabetes
Diabetes is a complex disorder which is influenced by the interaction between genetic, epigenetic and environmental factors. Prebiotics plays an imperative role in modulating the expression of genes and have a high impact on human metabolism. Different carbohydrates and dietary compounds such as fibers creates a wide rapport between polymorphisms which further effects in inactivation of insulin-resistant genes. Advancement in studies of human gut microflora clarifies the actual relationship between the gut microflora and Diabetes mellitus type two (DM2) [41].
β-glucans effect positively on reducing concentrations of plasma cholesterol and the glycemic index (GI). The amount and excellence of carbohydrates influence the value of glycemia and the insulin response commonly known as glycemic load (GL). Elevation in GL and GI of different food has a direct effect on the development of hyperinsulinemia, insulin resistance and risks to increase DM2. Many carbohydrates can affect the population of gut microflora directly by increasing glycemic and insulin response values. In various animal models, it has been seen that inulin-type fructans can modulate lipid and carbohydrate metabolism [42]. Different oligofructose (OFS) shows an antidiabetic effect in both streptozotocin treated rats and high-fat content treated mice.
Prebiotics can directly affect the composition of microbes in the gut when they incorporated in the diet. Some of the newly isolated prebiotics commonly called as a high-fat diet induces the glucose metabolism by modulating microbiome-gut-brain axis. This targeted axis is directly linked to the prevention of induced obesity and diabetes [43]. Some urea, trimethylamine N-oxide (TMAO) (URS) and p-cresyl sulfate, 3-carboxylic acid 4-methyl-5-propyl-2-furan propionic (CMPF) play a major role in abnormalities induced during glucose homeostasis and diabetes. A major role is played in the pathogenesis of CKD and DKD, as a result of the interconnection in gut microflora, kidney, pancreas β cell, and peripheral insulin. The correct characterization of gut microflora and their metabolites play a major role in giving innovative research and find the new clinical treatments for the CKD and DKD [44].
6.2. Role of prebiotics in irritable bowel syndrome (IBS)
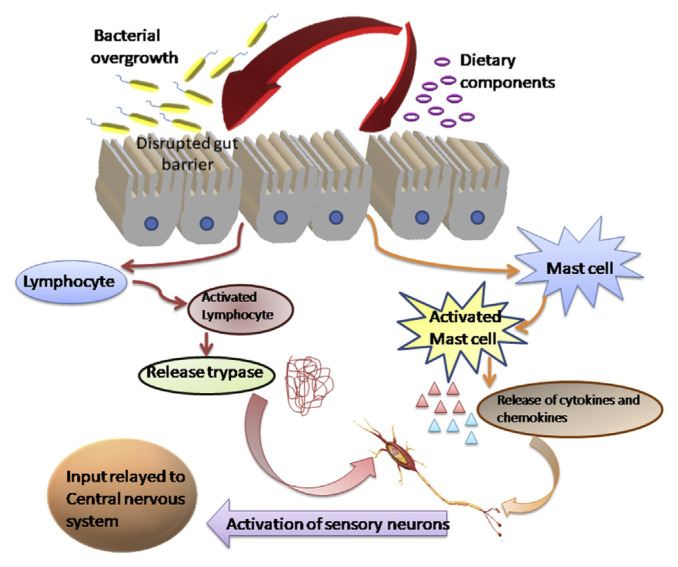
Irritable bowel syndrome is a body illness in which the human gut microbiome affects adversely. This adverse effect is directly linked with the nervous system, mucosal barrier system, neurotransmitters, hormones and immune system. The pictorial representation of the work done by the microbiota-gut-brain axis in the IBS has been described in Fig. 2. Various symptoms such as abdominal pain and blotting get directly affected by the eating habits [45]. All these effects can be reduced by adding the prebiotics into the routine diet. Various studies have suggested that the incorporation of these into the diet may lead to the modulation of the gut microflora, which can help in reducing the symptoms and adverse effects of the disease. Most commonly used prebiotics which can be used for curing symptoms are fructo, oligo-, di-, and monosaccharides and polyols (FODMAPs), they target to intestinal flora and help in getting relief from IBS. Other diets such as gluten-free, guided elimination diets are also helpful for IBS patients. In both animal and human case studies, it has been evaluated that the manipulation of gastrointestinal microbiota by the influence of diet and probiotics improves the symptoms of diseases. Bacteria play both positive and negative mechanistic roles in IBS by offering proper probiotic and dietary choices. Amixture comprising 2′-fucosyllactose (2′-FL) and lacto-N-neotetraose (LNnT) administered for treating a patient with irritable bowel syndrome (IBS) [46]. In addition to this, the main approach in the pathophysiology IBS is visceral hypersensitivity which is normally induced by the presence of Helicobacter pylori [47]. Moreover in IBS patients, Helicobacter pylori is the main cause of increased functional dyspepsia and increase stress, anxiety and depression. There is a pathogenic connection between H. pylori and BMAL1, transcriptional activation of LIN28, breaks the circadian rhythm which leads to the induction in the expression of BMAL1 in vivo and in vitro by H. pylori promoting inflammation [48].
Fig. 2.
The function of the microbiota-gut-brain axis in Irritable bowel syndrome (IBS).
The more futuristic research will focus on improving the identification strategies for the gut microflora so that the exact interactions between the gut microflora and diet can be identified. Moreover, diet therapies can be supplemented as a functional food to induce and increase the populations of beneficial bacteria. In addition to this, biomarker-based diet plans can be induced to give the appropriate treatments to IBS patients [49].
7. Conclusions
Prebiotics are the potential candidates which can influence the gut microflora positively by enhancing their populations. For lowering down the market price of available prebiotics, various enzymatic synthesized biotics are made and discovery of some sources such as seaweeds can directly persuade the cost and quality of them. Moreover, different transmitting strategies for them can help in accelerating the action of prebiotics in the gut environment. In addition to this, the immunomodulatory effects and nutrient absorption in the gut by the means of prebiotics can help in maintaining individual health and gut microflora populations. This review has focused on diabetes and IBS. Furthermore, many other diseases such as cardiovascular diseases, liver disease and colon cancer can be studied. Moreover, the systems biology can be applied in the future studies for a higher understanding of gut microflora and finding the proper food i.e. prebiotics for them. Metabolic engineering of these gut microbes can also help in the generations of new microbes which can reside in the gut environment and metabolize the prebiotics in adequate manner.
Acknowledgments
The authors acknowledge Maharshi Dayanand University, Rohtak, India for providing infrastructure facility. PS acknowledges the grant from DBT, Govt. of India (Grant No. BT/ PR27437/BCE/8/1433/2018) and the infrastructural support from Department of Science and Technology, New Delhi, Govt. of India, FIST grant (Grant No. 1196 SR/FST/LS-I/ 2017/4). PS acknowledges Department of Microbiology, Barkatullah University, Bhopal, India for their infrastructural support for D.Sc. Work.
Funding Statement
PS acknowledges the grant from DBT, Govt. of India (Grant No. BT/ PR27437/BCE/8/1433/2018) and the infrastructural support from Department of Science and Technology, New Delhi, Govt. of India, FIST grant (Grant No. 1196 SR/FST/LS-I/ 2017/4).
References
- 1.Joshi D, Roy S, Banerjee S. Prebiotics: a functional food in health and disease. In: Mandal Subhash C, Vivekananda Mandal, Tetsuya Konish, editors. Natural products and drug discovery. Elsevier; 2018. pp. 507–23. [Google Scholar]
- 2. Jovanovic-Malinovska R, Kuzmanova S, Winkelhausen E. Oligosaccharide profile in fruits and vegetables as sources of prebiotics and functional foods. Int J Food Prop. 2014;17:949–65. [Google Scholar]
- 3.Di Gioia D, Biavati B. Probiotics Prebiotics Anim Heal Food Saf. Springer; 2018. Probiotics and prebiotics in animal health and food safety: conclusive remarks and future perspectives; pp. 269–73. [Google Scholar]
- 4. Sharma R, Sharma S, Shukla PC, Sharma V, Baghel RPS, Raikwar A, et al. Microbial and functional feed supplement to improve livestock and poultry productivity with special reference to synbiotics: a review. 2018;7(7):62–8. [Google Scholar]
- 5. Maguire M, Maguire G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev Neurosci. 2019;30:179–201. doi: 10.1515/revneuro-2018-0024. [DOI] [PubMed] [Google Scholar]
- 6. Figueroa-González I, Quijano G, Ramírez G, Cruz-Guerrero A. Probiotics and prebiotics—perspectives and challenges. J Sci Food Agric. 2011;91:1341–8. doi: 10.1002/jsfa.4367. [DOI] [PubMed] [Google Scholar]
- 7. Duncan SH, Flint HJ. Probiotics and prebiotics and health in ageing populations. Maturitas. 2013;75:44–50. doi: 10.1016/j.maturitas.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 8. Cui L, Yan C, Li H, Kim W, Hong L, Kang S, et al. A new method of producing a natural antibacterial peptide by encapsulated probiotics internalized with inulin nanoparticles as prebiotics. J Microbiol Biotechnol. 2018;28:510–9. doi: 10.4014/jmb.1712.12008. [DOI] [PubMed] [Google Scholar]
- 9. Yahfoufi N, Mallet JF, Graham E, Matar C. Role of probiotics and prebiotics in immunomodulation. Curr Opin Food Sci. 2018;20:82–91. [Google Scholar]
- 10. Morgan NK, Keerqin C, Wallace A, Wu S-B, Choct M. Effect of arabinoxylo-oligosaccharides and arabinoxylans on net energy and nutrient utilization in broilers. Anim Nutr. 2018:1–7. doi: 10.1016/j.aninu.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rufino MN, Aleixo GFP, Trombine-Batista IE, Giuffrida R, Keller R, Bremer-Neto H. Systematic review and meta-analysis of preclinical trials demonstrate robust beneficial effects of prebiotics in induced inflammatory bowel disease. J Nutr Biochem. 2018;62:1–8. doi: 10.1016/j.jnutbio.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 12. Yadav R, Shukla P. An overview of advanced technologies for selection of probiotics and their expediency: a review. Crit Rev Food Sci Nutr. 2017;57:3233–42. doi: 10.1080/10408398.2015.1108957. [DOI] [PubMed] [Google Scholar]
- 13. Mano MCR, Neri-Numa IA, da Silva JB, Paulino BN, Pessoa MG, Pastore GM. Oligosaccharide biotechnology: an approach of prebiotic revolution on the industry. Appl Microbiol Biotechnol. 2018;102:17–37. doi: 10.1007/s00253-017-8564-2. [DOI] [PubMed] [Google Scholar]
- 14. Ahmad M, Mudgil P, Gani A, Hamed F, Masoodi FA, Maqsood S. Nano-encapsulation of catechin in starch nanoparticles: characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019;270:95–104. doi: 10.1016/j.foodchem.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 15. Neeraj G, Ravi S, Somdutt R, Ravi SK, Kumar VV. Immobilized inulinase: a new horizon of paramount importance driving the production of sweetener and prebiotics. Crit Rev Biotechnol. 2018;38:409–22. doi: 10.1080/07388551.2017.1359146. [DOI] [PubMed] [Google Scholar]
- 16. Quezada MP, Salinas C, Gotteland M, Cardemil L. Acemannan and fructans from aloe vera (aloe barbadensis miller) plants as novel prebiotics. J Agric Food Chem. 2017;65:10029–39. doi: 10.1021/acs.jafc.7b04100. [DOI] [PubMed] [Google Scholar]
- 17. Okolie CL, Rajendran CK, SR, Udenigwe CC, Aryee ANA, Mason B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J Food Biochem. 2017;41:e12392. [Google Scholar]
- 18. Tian T, Freeman S, Corey M, German JB, Barile D. Chemical characterization of potentially prebiotic oligosaccharides in brewed coffee and spent coffee grounds. J Agric Food Chem. 2017;65:2784–92. doi: 10.1021/acs.jafc.6b04716. [DOI] [PubMed] [Google Scholar]
- 19. Duarte FND, Rodrigues JB, da Costa Lima M, Lima M, dos S, Pacheco MTB, et al. Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro-industrial byproduct on Lactobacillus species. J Sci Food Agric. 2017;97:3712–9. doi: 10.1002/jsfa.8232. [DOI] [PubMed] [Google Scholar]
- 20. Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gamboa RG, Basurto RIO, Santoyo MC, Madrigal JB, Álvarez BER, Avila MG. In vitro evaluation of prebiotic activity, pathogen inhibition and enzymatic metabolism of intestinal bacteria in the presence of fructans extracted from agave: a comparison based on polymerization degree. LWT. 2018;92:380–7. [Google Scholar]
- 22. Guerin J, Petit J, Burgain J, Borges F, Bhandari B, Perroud C, et al. Lactobacillus rhamnosus GG encapsulation by spray-drying: milk proteins clotting control to produce innovative matrices. J Food Eng. 2017;193:10–9. [Google Scholar]
- 23. Abushelaibi A, Al-Mahadin S, El-Tarabily K, Shah NP, Ayyash M. Characterization of potential probiotic lactic acid bacteria isolated from camel milk. LWT Food Sci Technol. 2017;79:316–25. [Google Scholar]
- 24. Zhang S, Hu H, Wang L, Liu F, Pan S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018;244:232–7. doi: 10.1016/j.foodchem.2017.10.071. [DOI] [PubMed] [Google Scholar]
- 25. Khodaei N, Karboune S. Optimization of enzymatic production of prebiotic galacto/galacto (arabino)-oligosaccharides and oligomers from potato rhamnogalacturonan I. Carbohydr Polym. 2018;181:1153–9. doi: 10.1016/j.carbpol.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 26. Duarte LS, da Natividade Schöffer J, Lorenzoni ASG, Rodrigues RC, Rodrigues E, Hertz PF. A new bioprocess for the production of prebiotic lactosucrose by an immobilized β-galactosidase. Process Biochem. 2017;55:96–103. [Google Scholar]
- 27. Montoro BP, Benomar N, Gómez NC, Ennahar S, Horvatovich P, Knapp CW, et al. Proteomic analysis of Lactobacillus pentosus for the identification of potential markers involved in acid resistance and their influence on other probiotic features. Food Microbiol. 2018;72:31–8. doi: 10.1016/j.fm.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 28. Khorasani AC, Shojaosadati SA. Starch-and carboxymethylcellulose-coated bacterial nanocellulose-pectin bionanocomposite as novel protective prebiotic matrices. Food Hydrocoll. 2017;63:273–85. [Google Scholar]
- 29. Jitonnom J, Ketudat-Cairns JR, Hannongbua S. QM/MM modeling of the hydrolysis and transfructosylation reactions of fructosyltransferase from Aspergillus japonicas, an enzyme that produces prebiotic fructooligosaccharide. J Mol Graph Model. 2018;79:175–84. doi: 10.1016/j.jmgm.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 30. Kanjan P, Sahasrabudhe NM, de Haan BJ, de Vos P. Immune effects of β-glucan are determined by combined effects on Dectin-1, TLR2, 4 and 5. J Funct Foods. 2017;37:433–40. [Google Scholar]
- 31. Gao K, Wang C, Liu L, Dou X, Liu J, Yuan L, et al. Immunomodulation and signaling mechanism of Lactobacillus rhamnosus GG and its components on porcine intestinal epithelial cells stimulated by lipopolysaccharide. J Microbiol Immunol Infect. 2017;50:700–13. doi: 10.1016/j.jmii.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 32. Yadav R, Singh PK, Puniya AK, Shukla P. Catalytic interactions and molecular docking of bile salt hydrolase (BSH) from l. plantarum RYPR1 and its prebiotic utilization. Front Microbiol. 2017;7:1–7. doi: 10.3389/fmicb.2016.02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dargahi N, Johnson J, Donkor O, Vasiljevic T, Apostolopoulos V. Immunomodulatory effects of Streptococcus thermophilus on U937 monocyte cell cultures. J Funct Foods. 2018;49:241–9. [Google Scholar]
- 34. Tuo Y, Song X, Song Y, Liu W, Tang Y, Gao Y, et al. Screening probiotics from Lactobacillus strains according to their abilities to inhibit pathogen adhesion and induction of pro-inflammatory cytokine IL-8. J Dairy Sci. 2018;101:4822–9. doi: 10.3168/jds.2017-13654. [DOI] [PubMed] [Google Scholar]
- 35. Huynh T-G, Cheng A-C, Chi C-C, Chiu K-H, Liu C-H. A synbiotic improves the immunity of white shrimp, Litopenaeus vannamei: metabolomic analysis reveal compelling evidence. Fish Shellfish Immunol. 2018;79:284–93. doi: 10.1016/j.fsi.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez Lagunas MJ, Azagra Boronat I, Saldaña-Ruíz S, Massot Cladera M, Rigo-Adrover M, Sabaté-Jofre A, et al. Immunomodulatory role of probiotics in early life. In: Diego Muñoz-Torrero, Montserrat Riu, Carles Feliu., editors. recent Adv Pharm Sci VII. Kerala: India: Research Signpost Trivandrum; 2017. pp. 19–34. [Google Scholar]
- 37. Munir MB, Hashim R, Nor SAM, Marsh TL. Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: haematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol. 2018;75:99–108. doi: 10.1016/j.fsi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 38. Fernando WM, Flint SH, Ranaweera K, Bamunuarachchi A, Johnson SK, Brennan CS. The potential synergistic behaviour of inter-and intra-genus probiotic combinations in the pattern and rate of short chain fatty acids formation during fibre fermentation. Int J Food Sci Nutr. 2018;69:144–54. doi: 10.1080/09637486.2017.1340932. [DOI] [PubMed] [Google Scholar]
- 39. Cheng W, Lu J, Lin W, Wei X, Li H, Zhao X, et al. Effects of a galacto-oligosaccharide-rich diet on fecal microbiota and metabolite profiles in mice. Food Funct. 2018;9:1612–20. doi: 10.1039/c7fo01720k. [DOI] [PubMed] [Google Scholar]
- 40. Lee SI, Kim IH. Difructose dianhydride improves intestinal calcium absorption, wound healing, and barrier function. Sci Rep. 2018;8:7813. doi: 10.1038/s41598-018-26295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dávila LA, Pirela VB, Villasmil NR, Cisternas S, Díaz W, Escobar MC, et al. New insights into alleviating diabetes mellitus: role of gut microbiota and a nutrigenomic approach. Diabetes Food Plan IntechOpen. 2018:184–202. [Google Scholar]
- 42. Aw W, Fukuda S. Understanding the role of the gut ecosystem in diabetes mellitus. J Diabetes Investig. 2018;9:5–12. doi: 10.1111/jdi.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dahiya DK, Renuka, Puniya M, Shandilya UK, Dhewa T, Kumar N, et al. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review. Front Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koppe L, Fouque D, Soulage CO. Metabolic abnormalities in diabetes and kidney disease: role of uremic toxins. Curr Diab Rep. 2018;18:97. doi: 10.1007/s11892-018-1064-7. [DOI] [PubMed] [Google Scholar]
- 45. Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000 Research. 2018:7. doi: 10.12688/f1000research.14592.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hennet T, McConnell B, Salomonsson E, Vigsnæs LK. Synthetic composition and method for treating irritable bowel syndrome. 2018 [Google Scholar]
- 47. Gerards C, Leodolter A, Glasbrenner B, Malfertheiner PH. Pylori infection and visceral hypersensitivity in patients with irritable bowel syndrome. Dig Dis. 2001;19:170–3. doi: 10.1159/000050673. [DOI] [PubMed] [Google Scholar]
- 48. Li T, Shao W, Li S, Ma L, Zheng L, Shang W, et al. H. Pylori infection induced BMAL1 expression and rhythm disorder aggravate gastric inflammation. EBioMedicine. 2019;39:301–14. doi: 10.1016/j.ebiom.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dolan R, Chey WD, Eswaran S. The role of diet in the management of irritable bowel syndrome: a focus on FODMAPs. Expert Rev Gastroenterol Hepatol. 2018;12(6):607–15. doi: 10.1080/17474124.2018.1476138. [DOI] [PubMed] [Google Scholar]
- 50. Longoria-García S, Cruz-Hernández MA, Flores-Verástegui MIM, Contreras-Esquivel JC, Montañez-Sáenz JC, Belmares-Cerda RE. Potential functional bakery products as delivery systems for prebiotics and probiotics health enhancers. J Food Sci Technol. 2018:1–13. doi: 10.1007/s13197-017-2987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Atia A, Gomaa A, Fernandez B, Subirade M, Fliss I. Study and understanding behavior of alginate-inulin synbiotics beads for protection and delivery of antimicrobial-producing probiotics in colonic simulated conditions. Probiotics Antimicrob Proteins. 2018;10:157–67. doi: 10.1007/s12602-017-9355-x. [DOI] [PubMed] [Google Scholar]
- 52. Tillmann S, Wegener G. Syringe-feeding as a novel delivery method for accurate individual dosing of probiotics in rats. Benef Microbes. 2018;9:311–5. doi: 10.3920/BM2017.0127. [DOI] [PubMed] [Google Scholar]
- 53. Bron PA, Kleerebezem M. Lactic acid bacteria for delivery of endogenous or engineered therapeutic molecules. Front Microbiol. 2018;9:1821. doi: 10.3389/fmicb.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Castro MAA, Alric I, Brouillet F, Peydecastaing J, Fullana SG, Durrieu V. Soy protein microparticles for enhanced oral ibuprofen delivery: preparation, characterization, and in vitro release evaluation. AAPS PharmSciTech. 2018;19:1124–32. doi: 10.1208/s12249-017-0928-5. [DOI] [PubMed] [Google Scholar]
- 55. Qi X, Tester RF. Starch granules as active guest molecules or microorganism delivery systems. Food Chem. 2018;271:182–6. doi: 10.1016/j.foodchem.2018.07.177. [DOI] [PubMed] [Google Scholar]
- 56. Singhvi G, Girdhar V, Patil S, Gupta G, Hansbro PM, Dua K. Microbiome as therapeutics in vesicular delivery. Biomed Pharmacother. 2018;104:738–41. doi: 10.1016/j.biopha.2018.05.099. [DOI] [PubMed] [Google Scholar]
- 57. Dias CO, de Almeida JDSO, Pinto SS, de Oliveira Santana FC, Verruck S, Müller CMO, et al. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: a functional non-dairy product for probiotic delivery. Food Biosci. 2018;24:26–36. [Google Scholar]
- 58. Ashwar BA, Gani A, Gani A, Shah A, Masoodi FA. Production of RS4 fromrice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018;239:287–94. doi: 10.1016/j.foodchem.2017.06.110. [DOI] [PubMed] [Google Scholar]