Abstract
In this study, we developed a novel analysis method based on liquid chromatography/tandem mass spectrometry (LC–MS/MS) to allow the simultaneous identification of 20 coccidiostats in eight matrix categories, including the muscles of chicken, swine, cow, and fish as well as chicken eggs, bovine milk, and porcine viscera. In the pretreatment procedure, acetonitrile/methanol (95:5, v/v) containing 1% formic acid, 5 g of sodium acetate, and 6.0 g of anhydrous magnesium sulfate was used for extraction, followed by a clean-up procedure using n-hexane saturated with ACN to facilitate the elimination of analytes from high lipid samples. Chromatographic separations were achieved using a Poroshell 120SB C18 column and operated with a gradient mobile phase system consisting of methanol (with 0.1% formic acid) and 5 mM ammonium formate, and the MS detection was monitored simultaneously. The method was validated in accordance with the Guidelines for the Validation of Food Chemical Methods by the Taiwan Food and Drug Administration. The limit of quantitation among 8 matrices were 0.5–2 ng g−1. The proposed method proved highly effective in detecting the presence of targeted veterinary drugs, providing a high degree of precision and accuracy over a broad range of matrices.
Keywords: Coccidiostats, LC-MS/MS, Multi-residue analysis, QuEChERS, Veterinary drugs
1. Introduction
Veterinary drugs are used to prevent, treat diseases, and promote growth in livestock, poultry, and fish production. Unfortunately, the overuse and/or improper application of veterinary drugs can result in high residual drug levels in tissue and the surrounding environment. The presence of antibiotic residue is a major contributor in the development of antibiotic resistance, which is amajor concern for human and animal health worldwide [1–3]. This has led to the adoption of maximum residue limits (MRL) for several animals species and target tissues with the aim of guaranteeing food safety. MRLs have been established for several coccidiostat drugs in Taiwan, and in some countries, the use of coccidiostats drugs as feed additives has been restricted or even banned [4]. As a result, many food analysis laboratories are tasked primarily with the detection of residual coccidiostats in food matrices. At present, LC–MS/MS is the preferred method for the detection and analysis of coccidiostats. Several analytical methods based on LC–MS/MS have been used to detect one or more coccidiostats in various matrices; however, the clean-up procedures used in some of these methods are highly complex, particularly when conducted for confirmatory analysis [5]. Most of the recent work conducted in this field has focused on LC–MS/MS methodologies, due to its high sensitivity, rapid detection, and the capacity to monitor multiple compounds in a variety of matrices simultaneously [6,7]. Sample preparation is generally based on generic extraction due to economic considerations [8]. Researchers have developed a range of techniques, such as solid-phase extraction (SPE), derivatization, on-line pretreatment, low-temperature partitioning, supercritical fluid extraction, matrix solid-phase dispersion, isotope dilution, and QuEChERS [9]. Nonetheless, many of these protocols are relatively expensive, laborious, and ill-suited to high-throughput analysis.
Coccidiosis is an infection of the intestinal tract by parasitic protozoa of the phylum Apicomplexa. Parasites belonging to the genus Eimeria commonly affect swine, poultry, cattle, sheep, and rabbits [10] when intensively farmed in warm humid conditions. Overcrowding, poor hygiene practices, and a failure to isolate infected animals allow disease proliferation. Parasites are transmitted via oocysts, which are shed in the feces of infected hosts and ingested by uninfected animals [11]. The disease can lead to intestinal lesions, diarrhea, poor weight gain, poor feed conversion, and in some cases death. Intensive poultry production imposes a particularly high risk of disease occurrence, with the result that coccidiosis ranks among the diseases with the greatest impact in terms of economic losses [12]. At present, it is considered more financially viable to administer coccidiostats to broiler chickens as a feed additive for nearly their entire lives (28–48 days) rather than treating coccidiosis therapeutically [13]. This explains the widespread use of coccidiostats in poultry production. The intensive use of these drugs is a serious concern from the perspectives of sanitation and health, due primarily to concerns that this could lead to the emergence of antimicrobial resistance. In fact, the emergence of Coccidia resistance has been reported in areas around the world. This has led to the use of multiple agents in combination in order to reduce the risk of treatment failure and increase the efficiency of therapeutic regimens [14].
In this paper, we present a multi-residue analysis method based on the QuEChERS method to quantify the concentration of 20 coccidiostat compounds in various matrix categories, including the muscles of chicken, swine, cow, and fish as well as chicken eggs, bovine milk, and porcine liver and kidney. Our objective was to formulate a simple, fast, and inexpensive method for sample preparation in standard laboratories.
2. Materials and methods
2.1. Reagents and chemicals
Ultra-pure water (18.2 MΩ cm−1) was obtained in-house using a Millipore water purification system (Cork, Ireland). Acetonitrile (ACN), methanol (MeOH), and formic acid (FA) were purchased from Merck Ltd. (Darmstadt, Germany). Ammonium formate was supplied by Wako (Osaka, Japan) and sourced by Nacalai Tesque Inc. (Kyoto, Japan).
Analytical standards of clopidol, closantel, decoquinate, diaveridine, diminazene aceturate, ethopabate, metronidazole, praziquantel, pyrantel pamoate, pryimethamine, isotope-labeled internal standard (IS), 4,4′-dinitrocarbanilide-d8 (DNC-d8), and decoquinate-d5 were purchased from Fluka (Kasnas, MO, USA). Buquinolate, dimetridazole, halofuginone hydrobromide, levamisole hydrochloride, nicarbazin, robenidine hydrochloride, and robenidine-d8 hydrochloride were purchased from Sigma–Aldrich (St. Louis, MO, USA). Zoalene (dinitolmide), dimetridazole-d3, and Metronidazole-d4 (MNZ-d4) were purchased from Toronto Research Chemicals Inc. (Toronto, ON, Canada). Isometamidium chloride was purchased from Wako (Osaka, Japan). Imidocarb was purchased from Dr. Ehrenstorfer (Augsburg, Germany). Diclazuril and novobiocin sodium salt were purchased from U.S. Pharmacopeia Convention, Inc. (Rockville, MD, USA).
Individual stock solutions were prepared by dissolving 10 mg of each standard in 10 mL of an appropriate solvent (ACN, MeOH, or DMF). Standard working solutions were prepared for fortification and calibration curves by diluting an appropriate quantity of stock solution with methanol to a final concentration of 1 μg mL−1. The mixtures were stored in amber glass vials at −20 °C, where they remained stable for at least two months.
2.2. Samples
Muscle samples of chicken, domestic swine, cow, and fish as well as porcine liver and kidney, chicken eggs, and bovine milk were purchased from traditional local markets or supermarkets in Taipei, Taiwan. Each sample type was homogenized using an electric food processor and stored at −20 °C prior to analysis.
2.3. Sample preparation
Tissue samples in 2.0 g aliquots were placed in 50 mL polypropylene centrifuge tubes, respectively and spiked with 0.1, 1, and 10 μg mL−1 of standard solutions and 10 μg mL−1 of stable-isotope-labeled (SIL) internal standard to attain concentration levels of 0.5–25 μg kg−1 and 5 μg kg−1, respectively at room temperature for 10 min. 10 mL of cooled water and extraction solvent (ACN/methanol (95:5, v/v) containing 1% formic acid) were then added to the tube to undergo homogenization via vortexing at 1000 rpm for 1 min using a Geno-Grinder 2010 (ATS Scientific, Burlington, Ont., Canada). Following with the addition of QuEChERS powder (6 g of magnesium sulfate and 1.5 g of sodium citrate), the tube was vortexed at 1000 rpm for 1 min, and then centrifuged at 5000 × g for 1 min. The supernatant was subsequently transferred into 50 mL centrifuge tubes with 10 mL of ACN-saturated n-hexane before vortexing at 1000 rpm for 1 min (clean-up). Following with centrifugation at 5000 × g for 1 min, the supernatant was removed and the cleaning process was repeated. The hexane layer was subsequently transferred into a 15 mL polypropylene centrifuge tube with 50 μL of DMSO before being evaporated to dryness under a continuous stream of nitrogen at 50 °C. The residue was reconstituted with 950 μL of 80% methanol (containing 0.1% FA) and filtered through a 0.22 μm PTFE membrane (Millipore, Cork, Ireland) prior to analysis by LC–MS/MS.
2.4. Instrument parameters
LC separations were obtained using ekspert™ ultraLC 100 ultra-performance liquid chromatograph (SCIEX, Framingham, MA, USA) equipped with an Agilent Poroshell 120SB–C18 column (2.7 μm, 3.0 mm × 150 mm) at a temperature of 40 °C. The gradient was applied using 0.1% FA in 5 mmol L−1 of ammonium formate (A) and methanol (B) containing 0.1% FA at a flow rate of 0.3 mL min−1. The initial condition was (A):(B) = 95:5. The concentration of B was maintained for 1min and then increased to 100% over a period of 14 min, where it was maintained for 6 min. Finally, the mobile phases were re-equilibrated to the initial concentration for 1 min. The total run time for this analysis was 22 min. The injection volume was 10 μL.
MS detection was performed using a QTRAP 5500 instrument (SCIEX, Framingham, MA, USA) with positive and negative electrospray ionization (ESI) at voltages of 5.5 and −4.5 kV, respectively. The MS instrument was controlled using Analyst software version 1.6.2. The vaporizer temperature was set at 500 °C, with curtain gas pressure of 20 psi, collision gas pressure of 8 psi, and ion source gas 1 and 2 pressures of 50 psi. The optimal MRM parameters are summarized in Table 1.
Table 1.
MRM parameter and retention times for 21 coccidiostats.
| Analyte | RTa (min) | DPb (V) | Precursor ion (m/z) | For quantification | For conformation | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Product ion (m/z) | CEc (eV) | CXPd (V) | Product ion (m/z) | CE (eV) | CXP (V) | ||||
| Buqinolate | 15.8 | 50 | 361.8 | 204.0 | 50 | 20 | 260.2 | 40 | 20 |
| Clopidol | 7.7 | 60 | 192.0 | 101.0 | 38 | 15 | 103.0 | 39 | 10 |
| Closantel | 17.6 | −40 | 660.9 | 127.1 | −46 | −10 | 315.0 | −46 | −20 |
| Decoquinate | 17.6 | 50 | 418.2 | 372.4 | 35 | 20 | 204.2 | 58 | 15 |
| Diaveridine | 7.3 | 50 | 261.0 | 245.2 | 37 | 15 | 123.0 | 37 | 20 |
| Dicalzuril | 15.1 | −40 | 407.0 | 335.9 | −26 | −20 | 334.1 | −26 | −20 |
| Dimetridazole | 6.3 | 50 | 142.0 | 96.0 | 24 | 15 | 81.0 | 39 | 10 |
| Diminazene aceturate | 7.4 | 30 | 282.2 | 119.2 | 26 | 15 | 135.2 | 13 | 15 |
| Ethopabate | 11.5 | 30 | 238.0 | 135.9 | 39 | 15 | 206.0 | 16 | 15 |
| Halofuginone | 11.4 | 50 | 416.1 | 100.1 | 30 | 15 | 120.2 | 30 | 15 |
| Imidocarb | 6.9 | 60 | 349.2 | 145.0 | 76 | 20 | 188.2 | 46 | 15 |
| Isometamidium | 10.4 | 60 | 460.3 | 313.2 | 29 | 20 | 298.3 | 31 | 20 |
| Levamisole | 6.8 | 50 | 204.8 | 123.0 | 40 | 15 | 117.2 | 40 | 15 |
| Metronidazole | 6.2 | 45 | 172.1 | 128.3 | 45 | 15 | 82.1 | 32 | 10 |
| Nicarbazin | 14.4 | −60 | 301.0 | 137.2 | −20 | −15 | 107.1 | −50 | −15 |
| Novobiocin | 16.1 | 65 | 613.1 | 189.3 | 65 | 20 | 396.3 | 22 | 20 |
| Praziquantel | 14.3 | 50 | 313.1 | 203.3 | 50 | 10 | 174.2 | 40 | 15 |
| Pyrantel pamoate | 7.9 | 50 | 207.1 | 150.1 | 50 | 15 | 136.1 | 42 | 15 |
| Pyrimethamine | 10.6 | 40 | 249.0 | 177.1 | 40 | 10 | 198.2 | 55 | 25 |
| Robenidine | 14.1 | 30 | 334.2 | 110.9 | 61 | 25 | 138.1 | 35 | 20 |
| Zoalene | 9.0 | −40 | 224.1 | 181.1 | −15 | −20 | 151.3 | −22 | −20 |
RT: Retention time (min).
DP: Declustering potential.
CE: Collision energy.
CXP: Collision cell exit potential.
2.5. Method validation
Neat standard calibration curves were obtained by diluting standard solutions with 0.1% FA of 80% methanol to a final concentration ranging from 0.5 to 25.0 μg L−1. Matrix-matched standard calibration curves were prepared using standard solutions spiking with reconstituted matrices to final concentrations ranging from 0.5 to 25.0 μg L−1 in accordance with the sample preparation procedure described in Section 2.3. The limit of detection (LOD) and limit of quantification (LOQ) were estimated at signal-to-noise (S/N) ratios of 3 and 10, respectively. Precision and accuracy of each standard were assessed by determining the coccidiostat content in spiked sample muscles at levels of 1.0, 5.0, and 10.0 μg kg−1, the results of which were estimated from five replicates.
3. Results and discussion
3.1. Optimization of LC–MS/MS conditions
We first optimized the MS parameters for ESI positive and negative ion modes through the direct infusion of 21 standard solutions. In 14 of the compounds, it was found that singly charged precursor ions [M+H]+ were the most abundant, whereas the deprotonated ion [M–H]− was most abundant in 4 of the compounds (closantel, diclazuril, nicarbazin, and zoalene). Conversely, Levamisole and robenidine were found to form the hydrochloride adduct ion [M+HCl+H]+ rather than [M+H]+. These results were used to optimize the characteristic MS/MS parameters specifically for each analyte.
We also examined the LC conditions. The aqueous mobile phases were used for multi-class veterinary drugs. In a comparison of two organic solvents (acetonitrile and methanol), higher intensities were obtained from higher polarity analytes (e.g., clopidol) when using methanol. When using acetonitrile, lower polarity analytes (e.g., polyethers) resulted in peak areas with unsatisfactory reproducibility; therefore, we opted for methanol as the final solvent. Finally, Poroshell 120SB–C18 (2.7 μm, 3.0 mm × 150 mm; Agilent, Milford, MA, USA) device achieved higher intensities and shorter run times than did the remaining two columns. Most previous studies on the detection of coccidiosis used C18 or C8 [7,15] columns as well as mobile phases consisting of acidified aqueous solutions of acetonitrile [5] or methanol [16]. To ensure complete separation and preservation of all the analytes in the column, we opted for a Poroshell 120SB–C18 column for UHPLC in accordance with the methods outlined.
The Poroshell 120SB–C18 column enables good retention of polar compounds with tolerance for a wide pH range. We did not observe a significant difference in the separation of methanol–water and acetonitrile–water systems; however, the mobile phase of methanol–water produced analyte peaks that were sharper and more symmetrical. We therefore selected methanol–water as the mobile phase. In an effort to improve separation performance, make the peaks more symmetrical, and increase the retention time of the target analyte(s), we examined the influence of adding 0.1%, 0.15%, or 0.2% (v/v) FA on the sensitivity of each analyte in the aqueous phase. The most pronounced responses from the 21 target analytes were obtained using 0.1% FA. This also improved the shape of the peaks and separation effects. As described by Clarke et al. [17], we found that the addition of ammonium formate at 5 mmol L−1 to the aqueous phase significantly reduced peak tailing. Thus, the optimized MRM and LC parameters are listed in Table 1.
We developed analysis methods for 21 coccidiostats commonly found in the 8 categories matrices. When feasible, a stable-isotope-labeled (SIL) internal standard is the best approach to quantitative analysis in mass spectrometry, due to the fact that the behavior is very similar to that of the analytes [12]. In this work, we used five SIL internal standards (decoquinate-d5, dimetridazole-d3, DNC-D8, MNZ-d4, and robenidine-d8) for calibrations of the relative recovery of analytes among various categories matrices.
3.2. Optimization of sample preparation methods
Sample preparation is a crucial aspect of any analytical method. A variety of pretreatment methods have been developed for monitoring the illegal use of coccidiostats. Salts and endogenous compounds cannot be removed entirely, due to the complexity of the biological matrices and the presence of trace levels in real samples, which could lead to matrix effects. Furthermore, most existing techniques are time consuming and require large quantities of organic solvents, including acetonitrile and methanol, which can contribute to environmental pollution. In this study, we sought to optimize the sample extraction and clean-up procedures involved in liquid–liquid purification and clean-up. The extraction and cleanup efficiency were based on relative recovery Optimizations were based on results obtained using chicken muscle during extractions since its low lipid content, was omitted to eliminate any possible contribution between analyte and matrix, and using porcine liver during clean-up evaluation since its complexities of enzyme and high lipid to selected analytes for robust evaluation of routine analysis.
3.2.1. Extraction
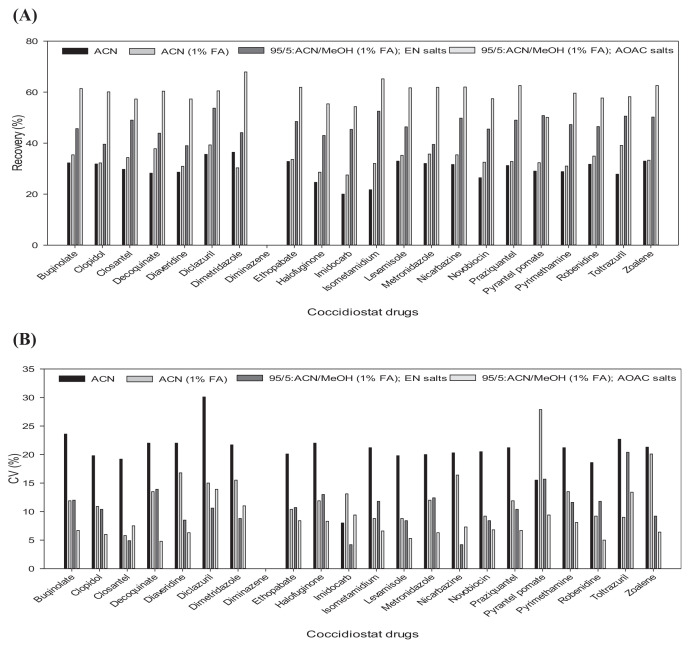
Extraction efficiency was evaluated by analyzing chicken muscle (2 g) using various extraction solvents and powders: (1) ACN with salt, (2) ACN/methanol (95:5, v/v) containing 1% FA with salt, (3) ACN/methanol (95:5, v/v) containing 1% FA, followed by QuEChERS EN powder (MgSO4 4 g; NaCl 1 g; Na Citrate 1 g; disodium citrate sesquihydrate 0.5 g), (4) ACN/methanol (95:5, v/v) containing 1% FA, followed by QuEChERS AOAC powder (MgSO4 6 g; Na Acetate 1.5 g). Fig. 1 presents a comparison of extraction efficiency in terms of recovery and CV (%) for 21 veterinary drugs.
Fig. 1.
Comparison of recoveries (A) and CV (B) for 21 coccidiostats fortified at 0.1 μg g–1 into chicken muscle using different extraction method. The data show the mean of 5 replicates and the error bars indicate RSD (%) values. ACN; ACN containing 1% FA; 95/5: ACN/MeOH containing 1% FA, follow by QuEChERS EN powder; 95/5: ACN/MeOH containing 1% FA, follow by QuEChERS AOAC Q powder.
The results of extraction efficiency were as follows: (1) 20.0–36.4%, (2) 27.5–39.3%, (3) 43.9–53.7%, and (4) 50.1–67.9%. Extraction precision was as follows: (1) 8–30.1%, (2) 5.8–27.9%, (3) 4.2–20.4%, and (4) 5–13.9%. The best recovery performance was obtained using 5% methanol in 95% ACN (as an extraction solvent) followed by QuEChERS AOAC powder. Previous studies [18] reported lower recovery rates using ACN; however, in the present study, repeated extraction using FA-acidified ACN in water achieved high extraction efficiency with only minimal co-extraction of lipids and the highly efficient denaturation of proteins, which is in agreement with the findings in Ref. [19]. Acidifying the extraction solvent proved particularly beneficial to the extraction of coccidiostats. Our use of ACN as an extraction solvent was in concurrence with the methods adopted in previous studies [20,21]. The inclusion of FA was shown to enhance the extraction of coccidiostats, compared with the same mixture without FA.
Diminazene aceturate was not detected in any of the four extracts, due to poor recovery. In previous studies, diminazene aceturate assays of biological fluids by LC have been associated with poor peak shape (tailing peaks), complex mobile phases, and recovery [22]. The presence of two highly basic amidino groups (pKa 11) in diminazene aceturate renders it highly susceptible to residual interactions with silanol groups of silica-based reversed-phase liquid chromatographic stationary phases. The adsorption of basic compounds, such as amidines, to laboratory glassware and equipment can greatly hinder recovery efficiency [23]. Diminazene aceturate was excluded from subsequent investigations, due to poor extraction performance.
3.2.2. Clean-up procedure
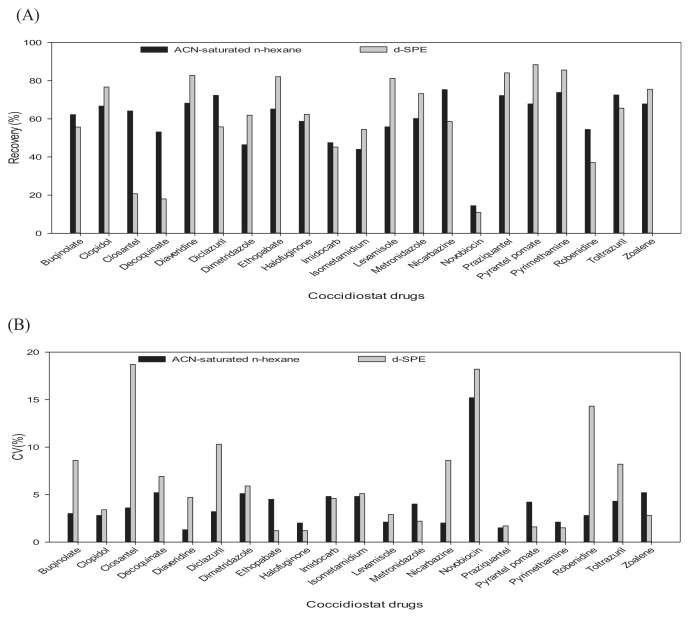
Most clean-up methods are based on dispersive solid phase extraction (d-SPE) or liquid–liquid extraction [24]. A number of studies have demonstrated that attempts to identify multiple multiclass veterinary drugs without a clean-up step can lead to co-extraction and signal suppression or enhancement [25]. When dealing with porcine liver samples, clean-up of the d-SPE and ACN-saturated n-hexane can be enhanced by using solvent to remove the fat phase and reduce the matrix effect in order to improve recovery performance. Fig. 2 presents a comparison of clean-up efficiency using ACN-saturated n-hexane and d-SPE (containing PSA 400 mg, C18 400 mg, and MgSO4 1200 mg) to improve the recovery of veterinary drugs from porcine liver. Recovery of the 19 veterinary drugs under the two clean-up methods was as follows: liquid–liquid extraction (44.0–73.8%) and d-SPE technique (18.0–88.4%). CV (%) in the detection of the same drugs were as follows: liquid–liquid extraction (1.2–5.2%) and d-SPE technique (1.2–18.7%). Liquid–liquid extraction clearly outperformed d-SPE cleanup in terms of recovery and CV%.
Fig. 2.
Comparison of recoveries (A) and CV (B) for 21 coccidiostats fortified at 0.1 μg g–1 into porcine liver using different clean-up method. The data show the mean of 5 replicates and the error bars indicate RSD (%) values. ACN-saturated n-hexane; d-SPE: dispersive solid phase extraction powder.
Thus, we adopted ACN-saturated n-hexane as a clean-up scheme. Diminazene aceturate provided poor efficiency using either of these methods: liquid–liquid extraction (4.2–8.8%) and d-SPE cartridge (1.1–18.2%). Nebot et al. [26] reported findings pertaining to a loss of recovery when a clean-up procedure was implemented.
However, in the present study, a combination of ACN-saturated n-hexane (defatting step) prior to nitrogen drying and reconstitution with solvent proved highly effective in reducing the lipid content from porcine liver samples and thereby reducing interference during analysis.
3.2.3. Matrix effects
Components other than the analyte in a sample can strongly undermine the accuracy of the analytic results. This type of interference is referred to as the matrix effect, as shown in Table 2. We added a given analyte at a given concentration to blank matrices in eight different categories and to pure methanol under the same injection conditions. Matrix effect was evaluated through comparison between the areas obtained by given concentration prepared in blank matrices and in solvent under tested chromatographic conditions. We evaluated the matrix effects at a concentration of 5 μg kg−1 using five replicates. Overall, the matrix effects of the 20 veterinary drugs ranged from −52.0% to 403.8%. This included 27 cases of suppression and 20 cases of enhancement. The major suppression cases found from matrix of bovine muscle, porcine liver and kidney, respectively. Analytes remained consistency of suppression/enhancement among various matrices. Clopidol, closantel, diaveridine, halofuginone, and levamisole exhibited matrix enhancement in all of matrices, whereas decoquinate, dimetridazole, imidocarb, metronidazole, nicarbazin, and zoalene exhibited matrix suppression.
Table 2.
Evaluation of matrix effects (ME, %) for 20 coccidiostats.
| Compound | Chicken muscle | Porcine muscle | Bovine muscle | Fish muscle | Chicken egg | Milk | Porcine liver | Porcine kidney |
|---|---|---|---|---|---|---|---|---|
| Buquinolate | 13.0 | 12.0 | −21.7 | −15.1 | −12.2 | 14.5 | −52.0 | −9.3 |
| Clopidol | −12.5 | −14.5 | −22.9 | −2.3 | −3.2 | −15.2 | −33.2 | −28.7 |
| Closantel | −20.5 | −28.1 | −37.3 | −30.3 | −36.9 | 8.3 | −47.8 | −42.9 |
| Decoquinate | 9.6 | 25.6 | 7.1 | 29.7 | 5.2 | 5.8 | 9.9 | 5.3 |
| Diaveridine | −45.7 | −26.0 | −32.0 | −2.0 | −6.6 | −20.7 | −34.7 | −31.8 |
| Diclazuril | 5.2 | 12.8 | −0.2 | −1.2 | −2.2 | 9.2 | 2.4 | 21.1 |
| Dimetridazole | 10.5 | 4.0 | 14.3 | 6.1 | 17.9 | 6.3 | 3.9 | 1.9 |
| Ethopabate | −16.4 | 0.5 | −8.8 | 12.8 | −6.7 | 4.7 | −23.4 | −3.6 |
| Halofuginone | −20.8 | −23.2 | −29.6 | −0.7 | −9.3 | −7.3 | −19.6 | 0.4 |
| Imidocarb | 119.1 | 279.0 | 403.8 | 256.1 | 113.2 | 132.6 | 88.4 | 106.1 |
| Isometamidium | 24.2 | 14.8 | 27.2 | 29.5 | 11.4 | 3.4 | −12.5 | 17.2 |
| Levamisole | −8.9 | −13.7 | −12.6 | −1.2 | −0.9 | −4.4 | −21.6 | −7.2 |
| Metronidazole | 11.4 | 12.8 | 2.8 | 2.4 | 12.1 | 22.6 | 1.9 | 2.5 |
| Nicarbazin | 62.0 | 10.9 | 6.2 | 8.2 | 13.5 | 8.6 | 7.4 | 10.7 |
| Novobiocin | 19.5 | 5.3 | 1.8 | −0.5 | −32.4 | 9.8 | −1.7 | 10.3 |
| Praziquantel | −6.8 | −2.4 | −13.8 | 6.0 | −1.7 | −4.4 | −12.6 | 3.5 |
| Pyrantel pamoate | −3.7 | −3.0 | −8.8 | 3.9 | −7.9 | −14.4 | −2.7 | 3.7 |
| Pyrimethamine | −13.0 | −17.5 | −26.6 | 1.1 | 2.8 | −3.1 | −33.4 | −26.3 |
| Robenidine | 10.7 | 11.2 | 6.7 | 6.6 | 15.8 | 5.3 | −1.9 | 0.5 |
| Zoalene | 19.8 | 36.4 | 29.8 | 8.9 | 14.9 | 4.5 | 41.2 | 36.3 |
| Numbers of ME <−20% | 3 | 3 | 6 | 1 | 2 | 1 | 7 | 4 |
| Number of ME >20% | 3 | 3 | 3 | 3 | 1 | 2 | 2 | 3 |
| Total numbers of suppression | 27 | |||||||
| Total numbers of enhancement | 20 |
We found an intriguing case in imidocarb with ME (%) from 88.4 to 403.8% among all matrices. We assume the two possible explanations: (1) The adsorption of basic compounds such as amidines to laboratory glassware with chemical properties would greatly hinder recovery efficiency which was similar with diminazene aceturate [23]. However, adsorptions were blocked due to the proportion of the matrix in sample was relative high, as result of high MEs (%) of imidocarb were found among matrices. (2) The retention time of imidocarb was 6.9 min, which was approximately 70% of ammonium formate of water and 30% methanol containing 0.1% FA during LC condition. The mobile phase increasing the solvent concentration during a gradient run. As the solvent increases the evaporation of the mobile phase is enhanced resulting in better ionization. Better ionization as the relative concentration of additives (formate, ammonium buffer) change, again due to gradients of mobile phases [27].
A possible explanation for the matrix-induced signal enhancement phenomenon is the co-elution of the analyte with compounds that facilitate the release of analyte ions within the ion-source. Such compounds can be bipolar molecules that, having a surfactant activity, can reduce the surface tension of the ion-spray droplets especially at highly aqueous compositions thus facilitating the release of analyte ions from the ion-spray micro-droplets (either via direct release from the droplet surface or indirectly through the facilitation of coulomb explosions). This results in a higher yield of free ions released into space within the ion-source [28,29]. In this study, we assumed the ME of imidocarb obtained by chemical properties than real ME.
3.3. Method validation
3.3.1. Validation results: muscle samples
The validation results obtained from the analysis of chicken, porcine, bovine, and fish muscle are listed in Table 3. The results indicate satisfactory recovery for almost all of the substances within an acceptable range of 50%–125% according to spike levels. At a spiked level of 1–5 μg kg−1, the recovery performance was as follows: chicken muscle (75.1–118.9%), porcine muscle (83.1–121.6%), bovine muscle (71.0–127.5%), and fish muscle (77.9–124.9%). The recovery of imidocarb in chicken, porcine, and fish muscle was low (16.3–62.5%) in samples with spike levels of 0.5 and 1.0 μg kg−1. Recovery of the other analytes was favorable in comparison with that in previous studies. There have been a number of reports on the analysis of coccidiostats in various category muscles [17,26,30–33]. The comparisons of selected methods with sample preparations, detection, LODs, and LOQs were summarized in Table 5.
Table 3.
Recoveries and coefficient of variance (CV (%)) of 20 coccidiostats in different category muscles.
| Analyte | Spiked level (μg kg−1) | Chicken muscle | Porcine muscle | Bovine muscle | Fish muscle | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||||
| Intraday precision | Interday precision | LOQ (μg kg−1) | Intraday precision | Interday precision | LOQ (μg kg−1) | Intraday precision | Interday precision | LOQ (μg kg−1) | Intraday precision | Interday precision | LOQ (μg kg−1) | ||||||
|
|
|
|
|
|
|
|
|
||||||||||
| Recovery (%) | CV (%) | CV (%) | Recovery (%) | CV (%) | CV (%) | Recovery (%) | CV (%) | CV (%) | Recovery (%) | CV (%) | CV (%) | ||||||
| Buquinolate | 0.5 | 89.7 | 2.9 | 3.7 | 0.5 | 100.2 | 6.4 | 4.1 | 0.5 | 91.4 | 4.7 | 7.1 | 0.5 | 100 | 3.2 | 6.8 | 0.5 |
| 1 | 91.2 | 3.1 | 95.2 | 5.7 | 92.9 | 3.4 | 94.5 | 2.9 | |||||||||
| 2.5 | 96.7 | 5.6 | 106.6 | 4.2 | 100.5 | 2.7 | 104.3 | 3.7 | |||||||||
| 5 | 92.2 | 2.5 | 105.2 | 2.4 | 100.8 | 2.2 | 102.9 | 3.9 | |||||||||
| Clopidol | 0.5 | 86 | 2.2 | 3.4 | 0.5 | 86.9 | 6.9 | 7.4 | 0.5 | 85.5 | 7.9 | 5.3 | 0.5 | 101.3 | 3.8 | 4.2 | 0.5 |
| 1 | 90 | 3 | 103.8 | 4.6 | 95 | 3.4 | 91 | 3.6 | |||||||||
| 2.5 | 83.4 | 3.8 | 97.1 | 4.4 | 100.2 | 4 | 98.1 | 2 | |||||||||
| 5 | 86.3 | 6.4 | 90.7 | 2.7 | 100.9 | 2.7 | 97.4 | 2 | |||||||||
| Closantel | 0.5 | 85.8 | 6.3 | 7 | 0.5 | 93.5 | 4.2 | 9.5 | 0.5 | 82 | 8 | 16.5 | 0.5 | 105.7 | 11.6 | 9.4 | 0.5 |
| 1 | 90 | 3.2 | 94 | 7.7 | 90.7 | 5.2 | 101.4 | 5.3 | |||||||||
| 2.5 | 106.4 | 7.2 | 101.5 | 5.7 | 95.2 | 6.6 | 98.3 | 6.9 | |||||||||
| 5 | 103.3 | 4.1 | 105.2 | 3.4 | 101.4 | 4.4 | 102 | 10 | |||||||||
| Decoquinate | 0.5 | 95.2 | 2.4 | 6 | 0.5 | 111.3 | 4.6 | 6.7 | 0.5 | 110.9 | 1.8 | 4.3 | 0.5 | 124.9 | 5.7 | 8 | 0.5 |
| 1 | 97 | 2.8 | 105.3 | 3 | 101.5 | 4.1 | 113.1 | 3.9 | |||||||||
| 2.5 | 104.5 | 5.9 | 105.7 | 2.9 | 98 | 3.4 | 112.9 | 7.5 | |||||||||
| 5 | 99.5 | 3.3 | 95.4 | 2.3 | 94.2 | 3.7 | 116.1 | 6.6 | |||||||||
| Diaveridine | 0.5 | 93.1 | 4.5 | 4.9 | 0.5 | 102.3 | 4.6 | 10.9 | 0.5 | 85.2 | 4.5 | 6 | 0.5 | 115 | 1 | 4.8 | 0.5 |
| 1 | 80.1 | 1.8 | 96 | 2.3 | 88.8 | 3.7 | 105.3 | 2.8 | |||||||||
| 2.5 | 82.8 | 2.6 | 104.1 | 2.1 | 97.4 | 4.2 | 110.3 | 1.7 | |||||||||
| 5 | 79.3 | 3.4 | 94.1 | 0.5 | 99.7 | 3.4 | 105.5 | 4.1 | |||||||||
| Diclazuril | 0.5 | 96.7 | 6 | 5.6 | 0.5 | 103.8 | 6.9 | 8.1 | 0.5 | 92.8 | 8.8 | 14.5 | 0.5 | 91.7 | 10 | 6.5 | 0.5 |
| 1 | 96.3 | 8.5 | 98.6 | 7.1 | 89.4 | 11.7 | 94.3 | 2.4 | |||||||||
| 2.5 | 96.3 | 5.3 | 100.9 | 5.5 | 84.5 | 6.6 | 117.5 | 4.2 | |||||||||
| 5 | 92.8 | 7.4 | 101.8 | 6.7 | 82.9 | 9.4 | 111.6 | 2.4 | |||||||||
| Dimetridazole | 0.5 | 101.8 | 18.3 | 10.7 | 0.25 | 116.3 | 23 | 9.5 | 0.25 | 97.1 | 19.9 | 8.9 | 0.25 | 100.8 | 6.2 | 7.3 | 0.25 |
| 1 | 81.9 | 10 | 102.9 | 17.2 | 88.1 | 21.1 | 99.2 | 3.7 | |||||||||
| 2.5 | 97.9 | 11.9 | 102.2 | 6.6 | 97.2 | 2.9 | 106.8 | 5.3 | |||||||||
| 5 | 99.1 | 7.5 | 94.1 | 7.8 | 102.1 | 6.6 | 103.6 | 4.9 | |||||||||
| Ethopabate | 0.5 | 85.2 | 4.7 | 4.9 | 0.5 | 90.5 | 3.6 | 7.1 | 0.5 | 81 | 5.5 | 4.6 | 0.5 | 84.6 | 2.7 | 4.1 | 0.5 |
| 1 | 85 | 1.7 | 93.4 | 1.7 | 94.1 | 4.1 | 91.9 | 1.6 | |||||||||
| 2.5 | 91.7 | 2 | 106 | 4.1 | 107.4 | 3 | 108.3 | 3.5 | |||||||||
| 5 | 87.4 | 3.8 | 98.8 | 1.7 | 108.2 | 1.1 | 108.1 | 1.9 | |||||||||
| Halofuginone | 0.5 | 83.4 | 4.3 | 7.9 | 0.5 | 104.4 | 5.2 | 9.3 | 0.5 | 90.9 | 5.2 | 7.4 | 0.5 | 106.6 | 2.3 | 3 | 0.5 |
| 1 | 89 | 2.2 | 97.9 | 3.7 | 96.8 | 5.8 | 95.9 | 2.2 | |||||||||
| 2.5 | 96.4 | 2.3 | 105.8 | 4.2 | 104.5 | 4.1 | 102.6 | 2.3 | |||||||||
| 5 | 95.2 | 5.1 | 102.4 | 2.7 | 105.1 | 2.8 | 100.1 | 1.9 | |||||||||
| Imidocarb | 0.5 | N.Da | N.D. | 17.9 | 0.25 | N.D.a | N.D. | 5.2 | 0.25 | 71.9 | 14.2 | 7.5 | 0.25 | 46.6 | 6.5 | 6.2 | 0.25 |
| 1 | 16.3 | 16.9 | 25 | 4.7 | 60.9 | 4.6 | 62.5 | 5.3 | |||||||||
| 2.5 | 81.9 | 4.1 | 96.5 | 5.8 | 98.5 | 4.2 | 99.3 | 4.9 | |||||||||
| 5 | 84 | 4.1 | 90.6 | 1.8 | 96.9 | 2.4 | 102.4 | 3.8 | |||||||||
| Isometamidium | 0.5 | 118.9 | 4.8 | 6 | 0.5 | 83.1 | 4.2 | 6.2 | 0.5 | 90.2 | 8.3 | 5.8 | 0.5 | 101.3 | 3.4 | 6.9 | 0.5 |
| 1 | 94.9 | 3.7 | 121.6 | 4.2 | 80.4 | 5.8 | 89.9 | 4.9 | |||||||||
| 2.5 | 103.3 | 4.4 | 102.2 | 2.7 | 107.4 | 2.2 | 114.2 | 2.6 | |||||||||
| 5 | 94.2 | 3.2 | 107.3 | 0.8 | 107 | 2.4 | 106.8 | 4.5 | |||||||||
| Levamisole | 0.5 | 75.1 | 2.2 | 5.2 | –b | 95.4 | 5.6 | 7.5 | –b | 89.3 | 9 | 4.7 | –b | 111.2 | 2.8 | 5.4 | –b |
| 1 | 80.3 | 3.9 | 103.7 | 4.6 | 97.7 | 7.6 | 98.1 | 3 | |||||||||
| 2.5 | 88.6 | 2.9 | 106.7 | 3.6 | 108.3 | 4.6 | 106.8 | 4.9 | |||||||||
| 5 | 89.2 | 4.7 | 96.9 | 3 | 99.5 | 5.4 | 98.4 | 5.1 | |||||||||
| Metronidazole | 0.5 | 97.7 | 2.5 | 6.6 | 0.5 | 110.6 | 3.4 | 8.9 | 0.5 | 92.7 | 5.2 | 7.1 | 0.5 | 101.1 | 10.8 | 6.3 | 0.5 |
| 1 | 96.0 | 4.3 | 94 | 3.2 | 100.5 | 4.5 | 103 | 8.6 | |||||||||
| 2.5 | 109.5 | 7.3 | 106.6 | 5.3 | 105.4 | 4.6 | 107.1 | 4.1 | |||||||||
| 5 | 99.9 | 3.7 | 102 | 4.5 | 104.8 | 2.3 | 104.6 | 4.6 | |||||||||
| Nicarbazin | 0.5 | 120.8 | 16.7 | 43.3 | –c | 112.3 | 2.4 | 5.7 | 0.5 | 92.3 | 4 | 5.2 | 0.5 | 100.8 | 6.2 | 4.2 | 0.5 |
| 1 | 76.2 | 40.5 | 97.8 | 3.8 | 97.8 | 5.9 | 99.2 | 3.7 | |||||||||
| 2.5 | 101.9 | 6.1 | 105.3 | 2.2 | 104.5 | 4.6 | 106.8 | 5.3 | |||||||||
| 5 | 102.2 | 6.3 | 102.2 | 4.1 | 103.3 | 3.9 | 103.6 | 4.9 | |||||||||
| Novobiocin | 0.5 | 66.9 | 6.9 | 4.4 | 0.5 | 107.4 | 8.9 | 6.3 | 0.5 | 71.9 | 30 | 37.7 | –d | 59.2 | 13 | 12.4 | 0.5 |
| 1 | 79.6 | 5.8 | 99.3 | 7.5 | 110.5 | 17.6 | 77.9 | 14.2 | |||||||||
| 2.5 | 98.7 | 4.3 | 108.5 | 4.9 | 127.5 | 12.2 | 88.9 | 4.4 | |||||||||
| 5 | 100 | 2.6 | 108.8 | 3.9 | 124.4 | 15 | 96.1 | 9.7 | |||||||||
| Praziquantel | 0.5 | 67.2 | 4.3 | 3.5 | 0.5 | 87.5 | 4.5 | 6 | 1 | 84.8 | 2.7 | 5.7 | 0.5 | 97.2 | 4.7 | 5.9 | 0.5 |
| 1 | 80.7 | 3.7 | 105.3 | 4.3 | 94.7 | 2.6 | 104.1 | 3.6 | |||||||||
| 2.5 | 95.6 | 1.9 | 107.1 | 3.9 | 104.8 | 1.7 | 106 | 2 | |||||||||
| 5 | 97.3 | 3.6 | 93.4 | 3.2 | 104.6 | 1.9 | 94.6 | 1.3 | |||||||||
| Pyrantel pamoate | 0.5 | 78.3 | 9.3 | 5.5 | 1 | 108.5 | 5.9 | 7.4 | 1 | 121.6 | 4.5 | 5.1 | 0.5 | 104.4 | 4.4 | 6.6 | 0.5 |
| 1 | 82.1 | 3.9 | 99.6 | 3.9 | 119.5 | 2.2 | 106.3 | 4.3 | |||||||||
| 2.5 | 91.6 | 2.8 | 96.1 | 3.4 | 112.2 | 4.4 | 94.8 | 3.9 | |||||||||
| 5 | 90.6 | 3.5 | 103.2 | 2.3 | 103.4 | 2.9 | 102.5 | 3.7 | |||||||||
| Pyrimethamine | 0.5 | 87.8 | 4.2 | 5.4 | 0.5 | 106.5 | 4.9 | 10.2 | 0.5 | 90.1 | 4.5 | 6 | 0.5 | 108 | 3.8 | 5.3 | 0.5 |
| 1 | 88.9 | 2.2 | 104.1 | 3.7 | 94.8 | 4 | 104.3 | 3.4 | |||||||||
| 2.5 | 96.5 | 3.2 | 94.2 | 2.7 | 104 | 2.8 | 96.4 | 2.4 | |||||||||
| 5 | 94.4 | 3 | 95.2 | 2.2 | 103 | 2.3 | 96.5 | 1.4 | |||||||||
| Robenidine | 0.5 | 100.6 | 6.2 | 5.5 | 0.5 | 116.4 | 2.8 | 5.3 | 0.5 | 95 | 3.6 | 6.4 | 0.5 | 104.7 | 2.1 | 4.5 | 0.5 |
| 1 | 98.5 | 4.7 | 97.4 | 3.6 | 97.5 | 6.9 | 101.1 | 2.3 | |||||||||
| 2.5 | 106.8 | 4.2 | 104.6 | 2.7 | 102.9 | 3.9 | 100.6 | 8.5 | |||||||||
| 5 | 104 | 1.9 | 107.8 | 2.6 | 100.4 | 3.2 | 105.9 | 4 | |||||||||
| Zoalene | 0.5 | 94.1 | 7.8 | 4.4 | 1 | 107.8 | 5.4 | 4.7 | 1 | 105.9 | 11.3 | 10.6 | 1 | 107.5 | 11.4 | 9.6 | 0.5 |
| 1 | 84.5 | 9.6 | 93.6 | 8.7 | 96.9 | 8.7 | 99.3 | 5.8 | |||||||||
| 2.5 | 91.8 | 9.2 | 101.7 | 5 | 98 | 5.9 | 107.3 | 2.5 | |||||||||
| 5 | 88.6 | 6 | 96.9 | 5.6 | 98.4 | 5.8 | 104.8 | 4.3 | |||||||||
Not detected.
The confirmation transition of levamisole has no specificity.
Residue of nicarbazin in chicken cause the failure of evaluating LOQ.
Novobiocin has not stable recoveries and coefficient of variations in bovine muscle. Modified extraction or clean-up method should be investigated for the consideration of method stability.
Table 5.
Comparison of LODs and LOQs in selected methods.
| Analytes | Matrix | Sample preparation | Detection | LOD (mg/kg) | LOQ (mg/kg) | Reference |
|---|---|---|---|---|---|---|
| Coccidiostats (21) | Muscles (4), milk, egg, viscera | Modified QuEChERS approach | LC–MS/MS | – | 0.002–0.005 | Our work |
| Amprolium decoquinate | Chicken, bovine muscles | Acidic extraction, SPE/C18-clean up | HPLC–UV/VIS | 0.04–0.13 | 0.13–0.42 | [31] |
| Coccidiostats (7) | Porcine muscle | Acidic extraction, SPE/C18-clean up | LC–MS/MS | 0.05 | 0.007 | [26] |
| Coccidiostats (17) | Bovine muscle | QuEChERS | LC–MS/MS | – | 0.02 | [17] |
| Coccidiostats (13) | Chicken muscle | Acidic extraction, SPE/C18-clean up | LC–MS/MS | – | 0.2–1 | [33] |
| Coccidiostats (14) | Muscles (4) | – | LC–QToF–MS | – | 0.012 | [30] |
| Coccidiostats (14) | Egg and milk | – | LC–QToF–MS | – | 0.012 | [30] |
| Coccidiostats (5) | Chicken egg | – | LC–MS/MS | – | 0.05 | [34] |
| Coccidiostats (11) | Milk | – | LC–MS/MS | – | 1.25–21 | [20] |
| Coccidiostats (4) | Milk | Acidic extraction | LC–MS/MS | – | 0.002 | [35] |
| Coccidiostats (4) | Liver | – | LC–MS/MS | 0.002–0.004 | [37] | |
| Coccidiostats (12) | Chicken liver | – | LC–MS/MS | 0.001–0.027 | [38] | |
| Coccidiostats (4) | Kidney | – | LC–MS/MS | 0.001–0.002 | [36] |
Overall, the results obtained using the proposed method are similar or superior to those obtained in previous studies, despite the fact that our method covers a larger number of analytes and matrices. A literature review revealed several methods for the detection and/or quantification of multiple coccidiostats in poultry tissue; however, few of the existing methods are applicable to other animal species [17].
A non-specific shadow peak in the confirmation transition of levamisole in the matrices prevented our determination of the LOQ. The CV of nicarbazin detection in chicken muscle at spike levels of 1 μg kg−1 (40.5%) and 5 μg/kg (43.3%) exceeded the tolerance required to determine the LOQ. The LOQ of novobiocin could not be derived due to unstable recovery and CV from bovine muscle at a spike level of 5 μg/kg (127.5% and 37.7%, respectively). The extraction or clean-upmethod applied to the detection of novobiocin should be modified for the sake of stability. The precision of the proposed method was satisfactory in the detection of the remaining analytes, with CVs ranging from 0.5% to 11.6% in all of the muscle samples.
3.3.2. Validation results: eggs, milk, and viscera
The validation results obtained from samples of chicken egg, bovine milk, porcine liver, and porcine kidney are listed in Table 4. The results indicate satisfactory accuracy for almost all of the substances within an acceptable range of 50%–125% according to spiked levels. At a spiked level of 1–5 μg kg−1, the recovery performance was as follows: chicken egg (61.6–117.5%), bovine milk (54.4–117.1%), porcine liver (83.9–117.6%), and porcine kidney (36.4–71.0%).
Table 4.
Recoveries and CV of 20 coccidiostats in different matrices.
| Analyte | Spike level (μg kg−1) | Chicken egg | Bovine milk | Porcine liver | Porcine kidney | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||||
| Intraday precision | Interday precision | LOQ (μg kg−1) | Intraday precision | Interday precision | LOQ (μg kg−1) | Intraday precision | Interday precision | LOQ (μg kg−1) | Intraday precision | Interday precision | LOQ (μg kg−1) | ||||||
|
|
|
|
|
|
|
|
|
||||||||||
| Recovery (%) | CV (%) | CV (%) | Recovery (%) | CV (%) | CV (%) | Recovery (%) | CV (%) | CV (%) | Recovery (%) | CV (%) | CV (%) | ||||||
| Buquinolate | 0.5 | 83.2 | 7.6 | 9.5 | 0.5 | 109.7 | 3.2 | 3.9 | 0.5 | 101.4 | 1.2 | 7.4 | 0.5 | 64.9 | 7.6 | 3.7 | 0.5 |
| 1 | 102.6 | 3 | 97.4 | 3.2 | 99.1 | 3.3 | 92.1 | 1.6 | |||||||||
| 2.5 | 115.2 | 2.5 | 105.7 | 2.3 | 95.8 | 3.5 | 98.6 | 2.7 | |||||||||
| 5 | 112.6 | 3.1 | 105.5 | 9.8 | 92.3 | 1.6 | 99.2 | 3 | |||||||||
| Clopidol | 0.5 | 93.4 | 8 | 11.2 | 0.5 | 112.6 | 3.8 | 4.3 | 0.5 | 108.9 | 2.7 | 8.3 | 0.5 | 42.8 | 9.8 | 5.4 | 1 |
| 1 | 97.5 | 5.2 | 103.6 | 4.3 | 100 | 3.6 | 84.3 | 3.6 | |||||||||
| 2.5 | 97.8 | 2.3 | 98.4 | 2.3 | 97.6 | 4.7 | 92.5 | 2.9 | |||||||||
| 5 | 94.2 | 4.3 | 102 | 5.5 | 83.5 | 3.6 | 98 | 3.1 | |||||||||
| Closantel | 0.5 | 77.9 | 22.5 | 14.3 | 0.5 | 101.1 | 4.7 | 4.6 | 0.5 | 111.9 | 4.2 | 7.1 | 0.5 | 41.7 | 5.2 | 6.6 | 0.5 |
| 1 | 95.9 | 16.2 | 103.5 | 2.6 | 102.7 | 7 | 88 | 2 | |||||||||
| 2.5 | 102 | 15.8 | 107.5 | 2.1 | 97.2 | 7.1 | 94.7 | 2.3 | |||||||||
| 5 | 99.3 | 15.6 | 116.3 | 6.5 | 91.6 | 4 | 99.1 | 2.8 | |||||||||
| Decoquinate | 0.5 | 97.3 | 10.1 | 5.5 | 0.5 | 112.5 | 8.9 | 7 | 0.5 | 97 | 5.9 | 5.3 | 0.5 | 71 | 8.2 | 6.9 | 0.5 |
| 1 | 94 | 2.2 | 93.1 | 4.9 | 97.5 | 8.1 | 97.2 | 6.1 | |||||||||
| 2.5 | 95.1 | 11.2 | 100.3 | 6.2 | 97 | 6.6 | 104 | 3 | |||||||||
| 5 | 92.3 | 8.6 | 106.9 | 5.4 | 93.3 | 6.2 | 102.9 | 2.6 | |||||||||
| Diaveridine | 0.5 | 91.5 | 5.9 | 16.2 | 0.5 | 116.1 | 6.2 | 4.2 | 0.5 | 109.5 | 3 | 9.9 | 0.5 | 53.7 | 19.6 | 4.8 | 0.5 |
| 1 | 104.3 | 2.7 | 105.8 | 9.1 | 103.4 | 3 | 82.7 | 6.1 | |||||||||
| 2.5 | 104.1 | 3 | 99.6 | 4.4 | 102.7 | 3.1 | 88 | 3.4 | |||||||||
| 5 | 103.8 | 2.9 | 100.4 | 6.8 | 88.7 | 4.1 | 94.1 | 2.8 | |||||||||
| Diclazuril | 0.5 | 91.7 | 10 | 20.8 | 0.5 | 117.1 | 7.6 | 5 | 0.5 | 101.6 | 4.8 | 9.7 | 0.5 | 36.4 | 33.9 | 5.4 | 1 |
| 1 | 94.3 | 2.4 | 107.2 | 5.1 | 91.7 | 5.1 | 102.8 | 4.2 | |||||||||
| 2.5 | 117.5 | 4.2 | 100 | 2 | 83.9 | 5.6 | 103.9 | 4.2 | |||||||||
| 5 | 111.6 | 2.4 | 112.7 | 6.1 | 84.2 | 5.8 | 105.8 | 3.2 | |||||||||
| Dimetridazole | 0.5 | 100.8 | 6.2 | 8 | 0.5 | 112.1 | 19.4 | 6.7 | 2.5 | 117.6 | 18.5 | 11.8 | 2.5 | N.D.a | N.D. | 4.8 | 2.5 |
| 1 | 99.2 | 3.7 | 90.4 | 15.9 | 104.5 | 21.8 | N.D. | N.D. | |||||||||
| 2.5 | 106.8 | 5.3 | 94.1 | 10.2 | 94.7 | 11.1 | 95.9 | 8.8 | |||||||||
| 5 | 103.6 | 4.9 | 96.4 | 11.2 | 93.7 | 3.8 | 95.9 | 5.9 | |||||||||
| Ethopabate | 0.5 | 92.9 | 4.5 | 25.2 | 0.5 | 112 | 1.9 | 5.4 | 0.5 | 111.1 | 1.8 | 7.5 | 0.5 | 54.8 | 8.8 | 3.4 | 0.5 |
| 1 | 104.2 | 2.8 | 104.5 | 4.6 | 105.1 | 1.2 | 96.3 | 2.4 | |||||||||
| 2.5 | 105.6 | 2.7 | 97.4 | 2.9 | 104 | 4.5 | 93.4 | 2 | |||||||||
| 5 | 105.6 | 2.6 | 92.4 | 12.3 | 102.1 | 3.2 | 95 | 5.3 | |||||||||
| Halofuginone | 0.5 | 99.9 | 5.4 | 26 | 0.5 | 107.1 | 2.3 | 4.6 | 1 | 104.1 | 4.1 | 6.2 | 0.5 | 38 | 7.8 | 4.6 | 0.5 |
| 1 | 106.3 | 4.9 | 102.1 | 2.8 | 102 | 3.4 | 95.5 | 3.8 | |||||||||
| 2.5 | 99.5 | 5.2 | 97 | 3.8 | 96.4 | 7 | 100.7 | 4.8 | |||||||||
| 5 | 93.5 | 3.1 | 94.5 | 9.3 | 94.5 | 3.5 | 103.1 | 6 | |||||||||
| Imidocarb | 0.5 | 105.4 | 4.6 | 20.2 | 0.5 | 111.3 | 5.5 | 4 | 0.5 | 97.6 | 7.3 | 13.3 | 2.5 | N.D. | N.D. | 4.9 | 2.5 |
| 1 | 90.5 | 10.8 | 98 | 6.3 | 112.4 | 5.2 | N.D. | N.D. | |||||||||
| 2.5 | 108.3 | 3.2 | 100.2 | 7.8 | 103.3 | 7.5 | 82.7 | 7 | |||||||||
| 5 | 99.9 | 6.1 | 108.2 | 9.7 | 88.6 | 6.8 | 92.5 | 2.9 | |||||||||
| Isometamidium | 0.5 | 107.4 | 5.2 | 20.6 | 0.5 | 106.5 | 1.9 | 3.6 | 0.5 | 95.2 | 1.9 | 8.6 | 0.5 | 59.8 | 9.3 | 5.4 | 0.5 |
| 1 | 98.1 | 3.1 | 96.7 | 5.7 | 104.1 | 5.6 | 91.3 | 2.6 | |||||||||
| 2.5 | 105.8 | 3.6 | 99.6 | 3.1 | 104.6 | 6.8 | 100.5 | 2.8 | |||||||||
| 5 | 101.2 | 3.9 | 109.7 | 7.4 | 93.2 | 4.7 | 102.9 | 4 | |||||||||
| Levamisole | 0.5 | 105.1 | 3.2 | 18.2 | –b | 117.2 | 3.4 | 3.9 | –b | 109.4 | 2.6 | 7.4 | –b | 58.3 | 7.5 | 5.1 | –b |
| 1 | 95.4 | 4.8 | 108.2 | 4.2 | 102.2 | 3.7 | 89.9 | 3.2 | |||||||||
| 2.5 | 94.7 | 7.1 | 98 | 4.6 | 98.3 | 4.2 | 88.9 | 5.8 | |||||||||
| 5 | 90.5 | 6.3 | 101 | 4 | 90.3 | 3 | 86.6 | 5.4 | |||||||||
| Metronidazole | 0.5 | 99 | 7.6 | 8 | 0.5 | 119 | 9 | 4.2 | 0.5 | 104.5 | 4.7 | 3.4 | 0.5 | 53.4 | 3.6 | 7 | 0.5 |
| 1 | 100.8 | 3.3 | 94.3 | 5.4 | 100.4 | 7.4 | 101 | 4.7 | |||||||||
| 2.5 | 96.1 | 8.2 | 89.4 | 4.6 | 105.3 | 9.9 | 99.8 | 4.5 | |||||||||
| 5 | 98.5 | 5.1 | 91.3 | 5.5 | 103.2 | 6.1 | 101.7 | 6.5 | |||||||||
| Nicarbazin | 0.5 | 96 | 6.7 | 4.2 | 0.5 | 105.9 | 6.1 | 5 | 0.5 | 105 | 2.7 | 6.2 | 0.5 | 55.6 | 14.6 | 4.7 | 0.5 |
| 1 | 99.9 | 3.5 | 93.3 | 3.2 | 92.9 | 3.5 | 99.5 | 2.4 | |||||||||
| 2.5 | 102 | 3.3 | 90.9 | 5.1 | 89.5 | 5.8 | 102.7 | 3.8 | |||||||||
| 5 | 104.3 | 1.8 | 97.2 | 2.3 | 90.3 | 2.3 | 100.7 | 2.7 | |||||||||
| Novobiocin | 0.5 | 61.6 | 103 | 46.6 | –c | 108.1 | 9.6 | 5.5 | 0.5 | 115.6 | 3.8 | 12.9 | 0.5 | 40.5 | 10.7 | 6.8 | 0.5 |
| 1 | 97.1 | 58.4 | 100 | 5.9 | 115.5 | 3.6 | 88.6 | 4.4 | |||||||||
| 2.5 | 112.8 | 52 | 96.1 | 2.9 | 115.6 | 5.9 | 99.6 | 3.8 | |||||||||
| 5 | 114.6 | 44.8 | 106.2 | 6.1 | 104.1 | 5.4 | 100.1 | 4.7 | |||||||||
| Praziquantel | 0.5 | 100.8 | 2.3 | 17.9 | 0.5 | 105 | 7.3 | 5.9 | 0.5 | 110.5 | 2.5 | 8 | 0.5 | 48.6 | 28 | 4.7 | 0.5 |
| 1 | 105 | 4.2 | 104.1 | 9.1 | 104.1 | 1.4 | 92.8 | 7 | |||||||||
| 2.5 | 100.6 | 3.6 | 102.1 | 2.1 | 97 | 3 | 96.7 | 1.5 | |||||||||
| 5 | 97.1 | 3.2 | 99.7 | 9.9 | 89.8 | 0.8 | 99.3 | 2.7 | |||||||||
| Pyrantel pamoate | 0.5 | 101.6 | 8.4 | 17.2 | 0.5 | 109.4 | 5.1 | 3.5 | 0.5 | 104.9 | 3.2 | 7.5 | 0.5 | 56.6 | 7.8 | 5 | 0.5 |
| 1 | 103.6 | 4.1 | 104.9 | 9.5 | 102.2 | 2.1 | 92.1 | 5.7 | |||||||||
| 2.5 | 103.8 | 3.3 | 102.1 | 4.3 | 99.2 | 2.9 | 92.5 | 5.8 | |||||||||
| 5 | 100.4 | 5.1 | 111.5 | 6.3 | 95.6 | 3.4 | 98.9 | 7 | |||||||||
| Pyrimethamine | 0.5 | 92.5 | 5.4 | 28.4 | 0.5 | 107.8 | 1.2 | 2.8 | 0.5 | 106.6 | 1.9 | 9.1 | 0.5 | 42.9 | 7.6 | 3.5 | 0.5 |
| 1 | 100.2 | 3.4 | 100.5 | 7.3 | 98.6 | 2.5 | 92.7 | 3.4 | |||||||||
| 2.5 | 100.7 | 2.9 | 99.5 | 3.4 | 93.8 | 5.4 | 96.6 | 2.6 | |||||||||
| 5 | 97.7 | 3.7 | 106.3 | 5.9 | 88.3 | 2.5 | 98.1 | 4.2 | |||||||||
| Robenidine | 0.5 | 99.6 | 5.1 | 4.1 | 0.5 | 118.6 | 9.1 | 6.8 | 0.5 | 111.5 | 3.1 | 7.2 | 1 | 64.2 | 25.3 | 6.4 | 1 |
| 1 | 96 | 4.7 | 97.3 | 2.3 | 102.1 | 3 | 97 | 4.8 | |||||||||
| 2.5 | 92.9 | 5 | 97.2 | 7.6 | 97 | 8.6 | 101.2 | 2.8 | |||||||||
| 5 | 95 | 3.4 | 99.8 | 4.3 | 107.5 | 7.3 | 100.5 | 4.1 | |||||||||
| Zoalene | 0.5 | 107.3 | 16.5 | 21.3 | 0.5 | 54.4 | 46.2 | 7.6 | 0.5 | 103 | 8.8 | 7.9 | 1 | N.D. | N.D. | 10.1 | 1 |
| 1 | 91.5 | 7.7 | 81.7 | 9.8 | 103 | 7 | 83.9 | 12 | |||||||||
| 2.5 | 87.3 | 6.8 | 97.1 | 8.2 | 104.8 | 4.8 | 85 | 5 | |||||||||
| 5 | 106 | 6.8 | 109.8 | 6.4 | 98.3 | 7.6 | 86.7 | 3.2 | |||||||||
Not detected.
The confirmation transition of levamisole has no specificity.
Novobiocin has not stable recoveries and coefficient of variations in chicken egg. Modified extraction or clean-up method should be investigated for the consideration of method stability.
The recovery and CV of nicarbazin detection were favorable: egg (96.0–104.3%), bovine milk (93.3–105.9%), porcine liver (89.5–105.0%), and porcine kidney (99.5–102.7%) at a spike level of 1.0–5.0 μg kg−1. The recovery of novobiocin from chicken egg was poor at a spike level of 0.5 μg/kg, as indicated by a CV of (46.6%). This issue will merit further investigation for the sake of method stability. Kang et al. [30] developed a method for the detection of 14 coccidiostat drugs in chicken egg and bovine milk, which achieved recovery values ranging from 52 to 92% at spike levels of 12.5–100 μg/kg. Buiarelli et al. [34] detected 5 coccidiostat drugs in chicken egg with recovery values ranging from 62 to 95% at spike levels of 1–37.5 μg kg−1.
In the last decade, there have been few reports on the analysis of coccidiostats in milk. Nasz et al. [20] developed and validated an LC–MS/MS method for the detection of 11 coccidiostats in milk with recovery values of 77–118% and an LOQ of 1.25–21 μg kg−1. Thompson et al. [35] developed a method for the measurement of 4 coccidiostats in milk, wherein sample aliquots are injected directly onto the instrument without the need for concentration or cleanup. That method can detect residue down to <1 μg kg−1. Pereira et al. [7] developed an analytical method for the detection of 6 coccidiostat drugs in milk and milk products, which achieved recovery values ranging from 93 to 113% at spiked levels of 0.5–15 μg kg−1. To the best of our knowledge, ours is the first method capable of detecting 20 coccidiostat drugs in milk with high recovery values at low spike levels.
We observed poor recovery of dimetridazole, imidocarb, and zoalene from porcine kidney (36.4–71.0%) at a spike level of 0.5–1.0 μg kg−1. Remaining analytes presented notable recoveries among liver or kidney under comparatively low spiked level (0.5–5.0 μg kg−1) against limited validation reports [36–38].
The LOQ values obtained using the proposed method (Tables 3 and 4) are superior to those obtained using many existing methods. These results have clearly demonstrated the efficacy of the proposed method in the precise quantification of 20 coccidiostats drugs. The comparisons of selected methods with sample preparations, detection, LODs, and LOQs were summarized in Table 5. No existing method covers as many coccidiostats or matrices as that proposed in this paper.
4. Conclusions
This study describes the development and full in-house validation of a highly sensitive and specific LC–MS/MS method for the quantitative determination of coccidiostats in various category matrices. The proposed method features high sample throughput and rapid laboratory turnaround times with low sample preparation costs and very little solvent waste. The proposed method meets the Guidelines for the Validation of Food and Chemical Methods by the Taiwan Food and Drug Administration for routine analysis in food safety control.
Acknowledgments
Financial support from the Food and Drug Administration, Ministry of Health and Welfare of Taiwan, is gratefully acknowledged.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfda.2019.02.004.
Funding Statement
Financial support from the Food and Drug Administration, Ministry of Health and Welfare of Taiwan, is gratefully acknowledged.
REFERENCES
- 1.Organization WH. The selection and use of essential medicines: report of the WHO expert committee, 2015 (including the 19th WHO model list of essential medicines and the 5th WHO model list of essential medicines for children) World Health Organization; 2015. [Google Scholar]
- 2. Romero T, Althaus R, Moya VJ, Beltran MDC, Reybroeck W, Molina MP. Albendazole residues in goat’s milk: interferences in microbial inhibitor tests used to detect antibiotics in milk. J Food Drug Anal. 2017;25:302–5. doi: 10.1016/j.jfda.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan K, Zhang H, Hui W, Zhu H, Li X, Zhong F, et al. Rapid screening of toxic salbutamol, ractopamine, and clenbuterol in pork sample by high-performance liquid chromatography-UV method. J Food Drug Anal. 2016;24:277–83. doi: 10.1016/j.jfda.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johansen CH, Bjerrum L, Pedersen K. Impact of salinomycin on the intestinal microflora of broiler chickens. Acta Vet Scand. 2007;49:30. doi: 10.1186/1751-0147-49-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ha J, Song G, Ai LF, Li JC. Determination of six polyether antibiotic residues in foods of animal origin by solid phase extraction combined with liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1017–1018:187–94. doi: 10.1016/j.jchromb.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 6. Clarke L, Fodey TL, Crooks SR, Moloney M, O’Mahony J, Delahaut P, et al. A review of coccidiostats and the analysis of their residues in meat and other food. Meat Sci. 2014;97:358–74. doi: 10.1016/j.meatsci.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 7. Pereira MU, Spisso BF, Jacob Sdo C, Monteiro MA, Ferreira RG, de Carlos BS, et al. Validation of a liquid chromatography-electrospray ionization tandem mass spectrometric method to determine six polyether ionophores in raw, UHT, pasteurized and powdered milk. Food Chem. 2016;196:130–7. doi: 10.1016/j.foodchem.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 8. Ahmad TA, El-Sayed BA, El-Sayed LH. Development of immunization trials against Eimeria spp. Trials Vaccinol. 2016;5:38–47. [Google Scholar]
- 9. de Queiroz Mauricio A, Lins ES. The National Agricultural Laboratories of Brazil and the control of residues and contaminants in food. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:482–9. doi: 10.1080/19440049.2011.620987. [DOI] [PubMed] [Google Scholar]
- 10. Akpo Y, Kpodekon MT, Djago Y, Licois D, Youssao IA. Vaccination of rabbits against coccidiosis using precocious lines of Eimeria magna and Eimeria media in Benin. Vet Parasitol. 2012;184:73–6. doi: 10.1016/j.vetpar.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 11. Sharman PA, Smith NC, Wallach MG, Katrib M. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 2010;32:590–8. doi: 10.1111/j.1365-3024.2010.01209.x. [DOI] [PubMed] [Google Scholar]
- 12. Barreto F, Ribeiro C, Hoff RB, Costa TD. A simple and high-throughput method for determination and confirmation of 14 coccidiostats in poultry muscle and eggs using liquid chromatography – quadrupole linear ion trap – tandem mass spectrometry (HPLC-QqLIT-MS/MS): validation according to European Union 2002/657/EC. Talanta. 2017;168:43–51. doi: 10.1016/j.talanta.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 13. Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci. 2014;93:501–11. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- 14. Olejnik M, Szprengier-Juszkiewicz T, Jedziniak P, Sledzinska E, Szymanek-Bany I, Korycinska B, et al. Residue control of coccidiostats in food of animal origin in Poland during 2007–2010. Food Addit Contam Part B Surveill. 2011;4:259–67. doi: 10.1080/19393210.2011.637238. [DOI] [PubMed] [Google Scholar]
- 15. Piatkowska M, Jedziniak P, Zmudzki J. Multiresidue method for the simultaneous determination of veterinary medicinal products, feed additives and illegal dyes in eggs using liquid chromatography-tandem mass spectrometry. Food Chem. 2016;197:571–80. doi: 10.1016/j.foodchem.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 16. Piatkowska M, Gbylik-Sikorska M, Gajda A, Jedziniak P, Bladek T, Zmudzki J, et al. Multiresidue determination of veterinary medicines in lyophilized egg albumen with subsequent consumer exposure evaluation. Food Chem. 2017;229:646–52. doi: 10.1016/j.foodchem.2017.02.147. [DOI] [PubMed] [Google Scholar]
- 17. Clarke L, Moloney M, O’Mahony J, O’Kennedy R, Danaher M. Determination of 20 coccidiostats in milk, duck muscle and non-avian muscle tissue using UHPLC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30:958–69. doi: 10.1080/19440049.2013.794306. [DOI] [PubMed] [Google Scholar]
- 18. Saxena SK, Rangasamy R, Krishnan AA, Singh DP, Uke SP, Malekadi PK, et al. Simultaneous determination of multiresidue and multi-class antibiotics in aquaculture shrimps by UPLC-MS/MS. Food Chem. 2018;260:336–43. doi: 10.1016/j.foodchem.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 19. Lopez-Garcia E, Mastroianni N, Postigo C, Valcarcel Y, Gonzalez-Alonso S, Barcelo D, et al. Simultaneous LC-MS/MS determination of 40 legal and illegal psychoactive drugs in breast and bovine milk. Food Chem. 2018;245:159–67. doi: 10.1016/j.foodchem.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 20. Nasz S, Debreczeni L, Rikker T, Eke Z. Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of eleven coccidiostats in milk. Food Chem. 2012;133:536–43. doi: 10.1016/j.foodchem.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 21. Tang YY, Lu HF, Lin HY, Shih YC, Hwang DF. Multiclass analysis of 23 veterinary drugs in milk by ultraperformance liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;881–882:12–9. doi: 10.1016/j.jchromb.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 22. Kassaye L, Hymete A, Bekhit AA, Genete G. Validation of an HPLC method for the simultaneous determination of diminazene diaceturate and phenazone in injectable veterinary granules and bulk powders. Pak J Pharm Sci. 2012;25:255–9. [PubMed] [Google Scholar]
- 23. Atsriku C, Watson DG, Tettey JN, Grant MH, Skellern GG. Determination of diminazene aceturate in pharmaceutical formulations by HPLC and identification of related substances by LC/MS. J Pharm Biomed Anal. 2002;30:979–86. doi: 10.1016/s0731-7085(02)00450-8. [DOI] [PubMed] [Google Scholar]
- 24. Gibbs RS, Murray SL, Watson LV, Nielsen BP, Potter RA, Murphy CJ. Development and validation of a hybrid screening and quantitative method for the analysis of eight classes of therapeutants in aquaculture products by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2018;66:4997–5008. doi: 10.1021/acs.jafc.7b05357. [DOI] [PubMed] [Google Scholar]
- 25. Rizzetti TM, de Souza MP, Prestes OD, Adaime MB, Zanella R. Optimization of sample preparation by central composite design for multi-class determination of veterinary drugs in bovine muscle, kidney and liver by ultra-high-performance liquid chromatographic-tandem mass spectrometry. Food Chem. 2018;246:404–13. doi: 10.1016/j.foodchem.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 26. Nebot C, Regal P, Miranda J, Cepeda A, Fente C. Simultaneous determination of sulfonamides, penicillins and coccidiostats in pork by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr Sci. 2012;50:414–25. doi: 10.1093/chromsci/bms021. [DOI] [PubMed] [Google Scholar]
- 27. Mohamed KM, Cromarty D, Steenkamp V. Development and validation of an LC–MS/MS method for determination of p-phenylenediamine and its metabolites in blood samples. J Chromatogr B. 2015;997:1–6. doi: 10.1016/j.jchromb.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Anastassiades M, Kolberg D, Benkenstein A, Eichhorn E, Zechmann S, Mack D, et al. Quick method for the analysis of numerous highly polar pesticides in foods of plant origin via LC-MS/MS involving simultaneous extraction with methanol (QuPPe-method) Stuttgart, Germany: EU Reference Laboratory for Pesticides Requiring Single Residue Methods (EURL-SRM) CVUA; 2015. [Google Scholar]
- 29. Takkis K, Aro R, Kõrgvee L-T, Varendi H, Lass J, Herodes K, et al. Signal enhancement in the HPLC-ESI-MS/MS analysis of spironolactone and its metabolites using HFIP and NH4F as eluent additives. Anal Bioanal Chem. 2017;409:3145–51. doi: 10.1007/s00216-017-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang J, Park SJ, Park HC, Hossain MA, Kim MA, Son SW, et al. Multiresidue screening of veterinary drugs in meat, milk, egg, and fish using liquid chromatography coupled with ion trap time-of-flight mass spectrometry. Appl Biochem Biotechnol. 2017;182:635–52. doi: 10.1007/s12010-016-2350-y. [DOI] [PubMed] [Google Scholar]
- 31. Kim B-J, Ham H-S, Lee J-J, Cheong N-Y, Myung S-W. Determination of coccidiostats (amproliumand decoquinate) in cattle and chicken’s muscle using high performance liquid chromatography. Bull Korean Chem Soc. 2012;33:559–63. [Google Scholar]
- 32. Nakajima T, Nagano C, Sasamoto T, Hayashi H, Kanda M, Kanai S, et al. Development and validation of rapid analysis method for multi-class veterinary drugs in livestock products by LC-MS/MS. Shokuhin Eiseigaku Zasshi. 2012;53:243–53. doi: 10.3358/shokueishi.53.243. [DOI] [PubMed] [Google Scholar]
- 33. Yoshikawa S, Nagano C, Kanda M, Hayashi H, Matsushima Y, Nakajima T, et al. Simultaneous determination of multi-class veterinary drugs in chicken processed foods and muscle using solid-supported liquid extraction clean-up. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1057:15–23. doi: 10.1016/j.jchromb.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 34. Buiarelli F, Di Filippo P, Riccardi C, Pomata D, Giannetti L, Neri B, et al. Liquid chromatography tandem mass spectrometry analysis of synthetic coccidiostats in eggs. Separations. 2017;4:15. [Google Scholar]
- 35. Thompson T, Noot D, Kendall J. Determination of ionophores in raw bovine milk using LC–MS/MS: application to residue surveillance. Food Chem. 2011;127:321–6. [Google Scholar]
- 36. Ai L, Sun H, Wang F, Chen R, Guo C. Determination of diclazuril, toltrazuril and its two metabolites in poultry tissues and eggs by gel permeation chromatography–liquid chromatography–tandem mass spectrometry. J Chromatogr B. 2011;879:1757–63. doi: 10.1016/j.jchromb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 37. Jestoi M, Rokka M, Peltonen K. An integrated sample preparation to determine coccidiostats and emerging Fusarium-mycotoxins in various poultry tissues with LC-MS/MS. Mol Nutr Food Res. 2007;51:625–37. doi: 10.1002/mnfr.200600232. [DOI] [PubMed] [Google Scholar]
- 38. Olejnik M, Szprengier-Juszkiewicz T, Jedziniak P. Multiresidue confirmatory method for the determination of twelve coccidiostats in chicken liver using liquid chromatography tandem mass spectrometry. J Chromatogr A. 2009;1216:8141–8. doi: 10.1016/j.chroma.2009.04.097. [DOI] [PubMed] [Google Scholar]