Abstract
Background:
A rapidly evolving tobacco marketplace highlights the timeliness of the FDA’s authority to regulate tobacco, specifically the role that flavorings in nicotine-containing electronic cigarette (ECIG) liquids have on public health. This study aimed to evaluate the extent to which ECIG liquid flavor and nicotine concentration influenced subjective measures of abuse liability among young adult cigarette (cig) smokers.
Methods:
Young adult (18–21 y.o.) smokers (M=10.1 cig/day, no regular ECIG use history) completed 7 Latin-square ordered conditions each preceded by 12 hrs. nicotine/tobacco abstinence. Conditions were own brand cig (OB) and eGO-style ECIG paired with three liquid flavors (cream, tropical fruit, tobacco/menthol) varying in nicotine concentration (0 or 36 mg/ml). Products were administered in two 10-puff bouts in each condition. Heart rate/blood pressure (HR/BP) and tobacco/nicotine abstinence symptoms, nicotine/general drug effects, and acceptability measures were assessed repeatedly throughout sessions. Mixed linear models were followed-up with Tukey’s HSD t-tests.
Results:
HR/BP indicated nicotine exposure during nicotine-containing conditions. OB and tobacco/menthol 36 mg/ml conditions produced significant decreases in ratings of cig smoking urges. Nicotine/drug effects were elevated significantly for OB and 36 mg/ml ECIG conditions with one exception noted for the tobacco/menthol 0 mg/ml condition. OB had the highest acceptability ratings, and ECIG condition results varied by acceptability item.
Conclusions:
Among young adult smokers, ECIG conditions containing nicotine were positively associated with several subjective measures of abuse liability but not all. Flavors did not consistently mask/enhance effects observed. Results reinforce continued examination of ECIG-delivered nicotine and liquid flavors in relationship to abuse liability.
Keywords: Subjective, Nicotine, Flavor, Abuse Liability, Electronic Cigarette, Cigarette Smoking, Young Adult
1. Introduction
Electronic cigarettes (ECIGs) represent a diverse and growing tobacco product class, defined broadly as devices that heat a liquid (typically nicotine-containing) to produce an inhalable aerosol (Breland et al., 2017). Unlike the unequivocal health harms associated with cigarette smoking, ECIG short- and long-term health effects for individual users are unclear (Breland et al., 2017), and their increased use among youth/young adults (Soneji et al., 2017) as well as potential utility for cigarette smoking cessation (Hartmann-Boyce et al., 2016) creates additional uncertainty for public health advocates and policymakers (Etter, 2015; McMillen et al., 2015a). Specific characteristics of ECIGs may influence their harm potential, including those related to the operating characteristics (liquid reservoir, battery voltage, heater resistance; Shihadeh and Eissenberg, 2014), liquid vehicles (propylene glycol, vegetable glycerin; Spindle et al., 2018; Kosmider et al., 2014; Bitzer et al., 2018a), nicotine concentration (Lopez et al., 2016; Ramôa et al., 2016), and flavorings/additives (St. Helen et al., 2017; Bitzer et al., 2018b). Flavorings and their role in patterns of initiation and subsequent use among vulnerable populations such as young adults, an age group likely to engage in tobacco product experimentation, are of particular interest to the public health community and regulators (Lopez and Eissenberg, 2015; Food and Drug Administration, 2018a; Food and Drug Administration, 2018b).
In contrast to flavored cigarettes, that are banned in the US (Family Smoking Prevention and Tobacco Control Act, 2009) (except menthol), the range of ECIG liquid flavors is vast and includes flavors that are tobacco-like (rich tobacco, cool menthol), fruit-like (apple, banana), food/dessert/spice-like (vanilla, cotton candy, chocolate), drink-like (piña colada, Hawaiian punch) and unlike anything (red rhino, alien sauce; Zhu et al., 2014). Young adults, a group at high risk of tobacco product initiation and use, including ECIGs, may be particularly susceptible to these product characteristics (McMillen et al., 2015b; Wagoner et al., 2016). In one survey of young adult ECIG users in 2014–2015 from Texas, more than 70% reported their first ECIG was flavored to taste like something other than tobacco compared to 44% of older adults (Harrell et al., 2016). Among internet-based surveys of ECIG users, flavoring has been reported as one of the most enjoyed aspects of ECIGs (Etter, 2010). These data and others (Zare et al., 2018) concerning the use of ECIG liquid flavors among young adults suggest a heightened need to further understand the extent to which flavors might influence initiation and subsequent use of ECIGs and other flavored tobacco products.
A related issue to that of flavors is the influence of ECIG nicotine concentration on initiation and subsequent ECIG use. Experienced ECIG users can achieve plasma nicotine concentrations that are comparable to those seen in cigarette smokers (Vansickel and Eissenberg, 2013; Spindle et al., 2015; Ramôa et al., 2016), and available data suggest ECIGs are capable of producing symptoms of nicotine dependence (Etter and Eissenberg, 2015; Hiler et al., 2017). However, the influence of ECIG liquid flavors and nicotine concentration on indicators of dependence potential or abuse liability are not well understood. Tobacco product abuse liability, the degree to which a psychoactive drug or drug formulation would be used for intentional nonmedical purposes and lead to physical and/or psychological dependence (Food and Drug Administration, 2017), can be indexed by well-accepted outcomes including those related to subjective or self-reported effects (Carter et al., 2009).
Several clinical laboratory studies have explored abuse liability indices related to ECIG liquid flavors and nicotine concentrations, but few have included a placebo control for nicotine (but see Van Heel et al., 2017; Goldenson et al., 2016, Litt et al., 2016; Rosbrook and Green, 2016). For example, previous work has held liquid nicotine concentration constant across ECIG liquid flavors tested (Oncken et al., 2015, Kim et al., 2016) or has varied liquid nicotine concentration between flavors/conditions tested exclusive of 0 mg/ml (Audrain-McGovern et al., 2016, St. Helen et al., 2017). Among the studies that included a placebo control, results regarding the effects of ECIG flavor and nicotine have been variable with the most consistent effects noted for menthol flavored ECIGs in producing the greatest reductions in cigarette smoking behavior (Litt et al., 2016) and subjective ratings of airway irritation (Rosbrook and Green, 2016). Missing from this previous work is a focus on young adults as well as a more comprehensive assessment of subjective effects predictive of abuse liability which include measures of drug-related symptoms, product liking, and willingness to take again (Carter et al., 2009).
The goal of this study was to build upon previous work examining ECIG nicotine delivery among cigarette smokers (Hiler et al., 2017) and ECIG liquid flavor preferences among experienced ECIG users (Soule et al., 2016) to compare three popular ECIG liquid flavors with and without nicotine (0, 36 mg/ml) in comparison to own brand cigarette smoking on a broad set of subjective measures predictive of abuse liability among a sample of young adult (18–21 years) cigarette smokers under conditions of tobacco/nicotine deprivation.
2. Material and Methods
2.1. Participants
The Virginia Commonwealth University Institutional Review Board approved this clinical laboratory study in its entirety. A total of 28 participants provided informed consent and attended at least one session. Among those enrolled, 3 self-withdrew and 5 were withdrawn by the investigator due to subsequent ineligibility (e.g., initiation of prescription medication use unrelated to study). Thus, there were 20 completers (50% male, 50% female; 40% White Non-Hispanic, 35% Black Non-Hispanic, 25% Other race/ethnicity). Participants were eligible if they were healthy, aged 18–21 (Mean [M]=19.9 years, Standard deviation [SD]=1.1), reported smoking at least five cigarettes per day (CPD; M=10.1 CPD, SD=5.5) for the past three months (M=18.3 months, SD=15.7) and provided a semi-quantitative urine cotinine result of at least 3 of 6 at screening (NicAlert test; M=5.7, SD=0.6). Exclusion criteria included self-reported history of chronic disease/psychiatric condition, interest in quitting cigarette smoking in the next 30 days, regular ECIG use (history of using at weekly or greater frequency for one month or longer), regular prescription medication use (other than vitamins/birth control), current pregnancy (confirmed by urinalysis) or breastfeeding, marijuana or alcohol use greater than 20 days in the past 30 days (marijuana M=5.0 days, SD=6.6; alcohol M=5.6 days, SD=4.6), and past month use of other illicit drugs. Individuals with food/chemical allergies suspected to interact with the ECIG liquid flavors were also excluded. Blood pressure (BP) was also taken at screening; individuals with systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg were excluded and referred to a primary care provider. Among included participants, 30% reported a menthol cigarette preference, 85% reported ever ECIG use, and 10% reported past 30-day use of other tobacco products (not including ECIGs).
2.2. Study Design
Participants attended the lab for seven Latin-square ordered conditions differing by the product used: own brand (OB) cigarette or ECIG cartomizer loaded with 1 ml of one of three liquid flavors (Food/Dessert/Spice, Fruit, or Tobacco/Menthol [Tob/Men]) at either 0 or 36 mg/ml nicotine concentration. Please note 14 condition orders were determined for this study by Latin square; due to recruitment challenges, there was unequal completion of each order (1–2 participants per condition order). The selection of the 36 mg/ml nicotine concentration was guided by preliminary results from Hiler et al. (2017) using the same ECIG device/liquid among ECIG-naïve cigarette smokers. Results suggested relatively equivalent nicotine delivery to an OB cigarette under these conditions. The OB cigarette condition was not blinded, as this was not feasible (being the only cigarette condition), and ECIG conditions were administered double-blind. Primary subjective outcomes were measured before and after product use during each condition.
2.3. Materials and ECIG Liquid Flavor Selection
Participant’s self-reported OB cigarette were purchased locally following enrollment. For all ECIG conditions, the ECIG device and cartomizer used was an eGo 3.3–4.1 V, 1100 mAh battery and a 1.5-Ohm, dual-coil, 510-style cartomizer (SmokTech; Shenzhen, China; as in Hiler et al., 2017). ECIG liquid nicotine concentration was verified by the VCU Bioanalytical Core Laboratory (levels were either below the level of quantification for 0 mg/ml or within 2 mg/ml for 36 mg/ml). All ECIG liquids were labeled as 70% propylene glycol/30% vegetable glycerin (identical to Hiler et al., 2017). To determine the specific ECIG liquid flavors within the Food/Dessert/Spice and Fruit flavor categories, a content analysis of preferred ECIG liquid flavors among adult ECIG users (age 18+ and used an ECIG for at least 1 month) from a previous study was performed (N=114 total flavor responses from N=41/46 participants included in Soule et al., 2016; see Supplementary Material*). Four unique liquid flavors at the solvent and nicotine concentration ratios specified above were sourced from a local ECIG vendor (AVAIL Vapor, LLC, Richmond, VA): Food/Dessert/Spice (Cream), Fruit (Tropical Fruit), Tobacco, and Menthol (see Table 1). Participants were matched to their OB menthol preference during Tob/Men conditions.
Table 1.
ECIG Liquid Flavor Information
| Flavor Category | Liquid Flavor Name | Liquid Flavor Brief Description |
|---|---|---|
|
| ||
| Food/Dessert/Spice (Cream) | White Mouseee (First Class*) | “Creamy Vanilla Custard” |
| Fruit (Tropical Fruit) | Tropic Thunder | “Tropical Fruit Medley” |
| Tobacco | Virginia Pure (Tobacco Row*) | “Subtle Tobacco” |
| Menthol | Port Royal | “Full Flavored Menthol Tobacco” |
Please note the White Mouseee and Virginia Pure products names were changed by the vendor during the course of the study but aside from name did not differ in formula/composition.
Only two of the original purchased e-liquid flavors are still available from the vendor (as of 12/9/2018): https://www.availvapor.com/port-royale.html; https://www.availvapor.com/avail-tobacco-row.html
2.4. Procedure
Participants were asked to abstain from tobacco/nicotine for at least 12 hrs. to reduce the influence of nicotine-related tolerance and/or recent tobacco/nicotine exposure (verified by expired air CO ≤10 ppm; measured with BreathCO monitor, Vitalograph, Lenexa, KS). If participants met the CO criterion, they began each session with continuous heart rate (HR) and BP measurement followed by a 30-minute rest period. Following the rest period, participants completed a baseline set of subjective measures. The first directed 10-puff (30-sec interpuff interval [IPI]) product administration directly followed subjective measures completion. Subjective measures were completed at 5, 15, 30, 45, and 55 min after the first 10-puff bout. At the 60-min interval, the second 10-puff bout was administered. Please note, this two-bout procedure has been used previously to establish reliability of results associated with product administration (Hiler et al., 2017; Lopez et al., 2016; Vansickel et al., 2010). Subjective measures were completed four more times at 65, 75, 90, and 105-min at the identical time points following the second 10-puff bout (10 times in total). Following the last subjective assessment, physiological monitoring was stopped, compensation was distributed, and if necessary the next session was scheduled. Participants were compensated using an ascending schedule after completing each session for total of $420.
2.5. Measures
2.5.1. Physiological Measures.
HR and BP were measured throughout the entirety of each session to assess nicotine-related cardiovascular effects. HR was taken at 10-sec intervals, and BP was taken at 5-min intervals (Criticare Systems VitalCare Monitor 506N3).
2.5.2. Subjective Measures.
Subjective measures representing four general areas relevant to abuse liability assessment (Carter et al., 2009) were assessed 10 times during each session (similar to previous studies; Hiler et al., 2017; Lopez et al., 2016, Vansickel et al., 2010): 1) Tobacco/nicotine abstinence symptoms and nicotine-related effects, 2) general drug effects, 3) mood effects, and 4) product acceptability-related effects. To assess symptoms of tobacco/nicotine abstinence, five visual analog scale (VAS) items (0=Not at all to 100=Extremely) adapted from the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1986) were administered: “Urges to smoke a cigarette,” “Irritability/frustration/anger,” “Anxious,” “Restlessness,” and “Impatient”. These items were added approximately half-way through recruitment and are available for 10 participants. The Direct Effects of Nicotine Scale (DENS) consisting of 10 VAS items (0=Not at all to 100=Extremely) was developed to describe the effects of nicotine (Evans et al., 2006). Seven VAS items (0=Not at all to 100=Extremely) were used to measure general drug effects (Drug Effects Scale; Eissenberg et al.,1996): “Do you feel a rush?”, “How high are you?”, “Do you feel any drug effects?”, “Do you like the drug effects?”, “Do you dislike the drug effects?”, “Do you feel any good drug effects?”, and “Do you feel any bad drug effects?”. General drug effects were assessed using the short form of the Addiction Research Center Inventory (ARCI) which generates five subscales: amphetamine (AMP), benzedrine group (BG), lysergic acid dyethylamide (LSD), morphine-benzedrine group (MBG), and pentobarbital-chlorpromazine-alcohol group (PCAG) (Martin et al., 1971). The short form of the Profile of Mood States (POMS), a 37-item Likert scale questionnaire (5-level from Not at all to Extremely), was used to assess mood effects (Curran et al., 1995). Following scoring, this measure results in six subscales: Tension, Depression, Anger, Vigor, Fatigue, and Confusion. The Direct Effects of Tobacco Scale consisting of 10 VAS items (0=Not at all to 100=Extremely) was used to assess acceptability (e.g., willingness to use again) of OB and ECIG use (Spindle et al., 2015; Blank et al., 2009).
2.6. Data Analysis
All data analyses were performed using SAS (V9.4, Cary, NC, USA). HR/BP data were prepared for analysis by averaging available data points in time-specific bins. For the baseline value, the last 10 minutes of the rest period was averaged. The 5 minutes prior to each subjective assessment (following the first bout) was also averaged for the duration of the session (similar to Blank et al., 2008; Cobb et al., 2011). These methods result in 10 HR/BP time points (baseline, 0–5 min, 10–15 min, 24–30 min, 40–45 min, 50–55 min, 60–65, 70–75, 85–90, and 100–105 min relative to bout 1). Approximately 7.3% of physiological data were missing due to staff or computer error for each outcome analyzed (e.g., 102/1400 time points missing for HR). Less than 0.5% of subjective effect data were missing across items/scales, but for one measure, POMS, results for one participant from all conditions were removed due to an extreme and illogical response pattern observed during the 1st session. Thus, for the POMS, N=19 were analyzed. Following data preparation/scoring, multilevel linear mixed models were completed for each outcome, item, or subscale. Three factors: condition (7 levels), bout (2 levels), and time (5 levels) were included as random effects to account for within-subject dependence. Due to the hierarchical structure of random effects, Kenward-Roger correction was used for degrees of freedom. Dual Quasi-Newton method was used as an optimization technique for ARCI and POMS scales considering the distribution of responses. Other scales were optimized using the Restricted Maximum Likelihood method. Mean comparisons relative to baseline and between all conditions at each time point were performed using Tukey’s HSD. Significance was set at p<0.05.
3. Results
Results of statistical analysis are presented in Table 2 and summarized by measure. There were no significant three-way interactions for any measure; thus, these non-significant interactions are not included in the table.
Table 2.
Statistical analysis results for physiological and subjective measures
| Condition (C) |
Bout (B) |
Time (T) |
C X B |
C X T |
B X T |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
|
| ||||||||||||
| Heart rate a | 17.6 | <.0001 | 8.0 | 0.005 | 13.5 | <.0001 | 0.1 | 0.995 | 0.8 | 0.75 | 1.3 | 0.262 |
| Systolic BP a | 5.4 | <.0001 | 0.2 | 0.665 | 2.3 | 0.06 | 0.4 | 0.874 | 0.2 | 0.999 | 0.4 | 0.828 |
| Diastolic BP a | 5.5 | <.0001 | 2.9 | 0.09 | 3.6 | 0.006 | 0.2 | 0.963 | 0.7 | 0.877 | 0.7 | 0.611 |
| Adapted-MNWSb | ||||||||||||
| Anxious | 4.5 | 0.0002 | 0.2 | 0.654 | 1.3 | 0.262 | 0.5 | 0.808 | 0.2 | 0.999 | 0.3 | 0.863 |
| Impatient | 5.0 | <.0001 | 0.9 | 0.343 | 0.3 | 0.868 | 0.2 | 0.968 | 0.1 | 0.999 | 0.1 | 0.992 |
| Irritable | 5.8 | <.0001 | 0.0 | 0.969 | 1.3 | 0.288 | 0.4 | 0.882 | 0.1 | 0.999 | 0.2 | 0.965 |
| Restless | 4.2 | 0.0004 | 0.3 | 0.585 | 0.2 | 0.923 | 0.7 | 0.689 | 0.2 | 0.999 | 0.2 | 0.935 |
| Urges to smoke a cigarette | 22.4 | <.0001 | 5.2 | 0.023 | 8.0 | <.0001 | 0.6 | 0.750 | 1.0 | 0.420 | 0.6 | 0.684 |
| Direct Effects of Nicotinec | ||||||||||||
| Nauseous | 1.8 | 0.089 | 0.0 | 0.955 | 1.2 | 0.332 | 0.2 | 0.989 | 0.5 | 0.988 | 1.1 | 0.364 |
| Dizzy | 2.8 | 0.010 | 0.9 | 0.345 | 11.8 | <.0001 | 0.2 | 0.980 | 1.9 | 0.005 | 0.6 | 0.689 |
| Lightheaded | 7.3 | <.0001 | 0.1 | 0.754 | 18.8 | <.0001 | 0.2 | 0.987 | 1.9 | 0.004 | 1.6 | 0.166 |
| Nervous | 0.7 | 0.683 | 0.4 | 0.511 | 0.1 | 0.977 | 0.9 | 0.507 | 0.7 | 0.895 | 0.2 | 0.947 |
| Sweaty | 1.4 | 0.197 | 0.0 | 0.850 | 1.7 | 0.138 | 0.5 | 0.829 | 1.2 | 0.247 | 1.1 | 0.370 |
| Headache | 0.9 | 0.485 | 0.0 | 0.925 | 0.2 | 0.951 | 0.0 | 0.999 | 0.2 | 0.999 | 0.1 | 0.985 |
| Excessive salivation | 0.9 | 0.475 | 0.0 | 0.959 | 2.3 | 0.057 | 0.1 | 0.999 | 0.1 | 0.999 | 1.0 | 0.412 |
| Heart pounding | 2.8 | 0.012 | 0.2 | 0.639 | 8.3 | <.0001 | 0.1 | 0.996 | 1.5 | 0.074 | 0.4 | 0.784 |
| Confused | 1.5 | 0.168 | 0.1 | 0.721 | 1.1 | 0.365 | 0.3 | 0.925 | 0.5 | 0.975 | 0.7 | 0.609 |
| Weak | 1.8 | 0.091 | 0.0 | 0.894 | 1.6 | 0.162 | 0.0 | 0.999 | 0.4 | 0.996 | 0.1 | 0.971 |
| Drug Effects Scalec | ||||||||||||
| Rush | 11.3 | <.0001 | 0.5 | 0.464 | 36.1 | <.0001 | 0.3 | 0.917 | 2.5 | <.0001 | 2.5 | 0.041 |
| High | 8.8 | <.0001 | 0.0 | 0.871 | 24.1 | <.0001 | 0.1 | 0.995 | 2.2 | 0.001 | 1.0 | 0.435 |
| Feel drug effects | 12.1 | <.0001 | 0.0 | 0.949 | 23.6 | <.0001 | 0.1 | 0.997 | 2.0 | 0.004 | 1.5 | 0.192 |
| Like drug effects | 5.8 | <.0001 | 0.0 | 0.885 | 16.3 | <.0001 | 0.2 | 0.978 | 0.8 | 0.729 | 3.5 | 0.007 |
| Dislike drug effects | 1.5 | 0.182 | 0.4 | 0.519 | 3.4 | 0.009 | 0.1 | 0.992 | 0.6 | 0.924 | 0.5 | 0.766 |
| Feel good drug effects | 9.5 | <.0001 | 0.1 | 0.809 | 20.2 | <.0001 | 0.1 | 0.995 | 1.3 | 0.182 | 3.2 | 0.013 |
| Feel bad drug effects | 3.5 | 0.002 | 0.2 | 0.621 | 3.6 | 0.006 | 0.3 | 0.923 | 0.6 | 0.929 | 0.8 | 0.511 |
| ARCId | ||||||||||||
| AMP | 5.3 | <.0001 | 0.0 | 0.918 | 1.1 | 0.356 | 0.3 | 0.925 | 0.2 | 0.999 | 0.2 | 0.928 |
| BG | 8.5 | <.0001 | 0.6 | 0.454 | 1.6 | 0.180 | 0.2 | 0.971 | 0.2 | 0.999 | 0.1 | 0.988 |
| LSD | 1.2 | 0.290 | 0.2 | 0.625 | 0.3 | 0.900 | 0.3 | 0.957 | 0.1 | 0.999 | 0.1 | 0.986 |
| MBG | 3.5 | 0.002 | 0.5 | 0.490 | 1.5 | 0.195 | 0.3 | 0.929 | 0.1 | 0.999 | 0.2 | 0.961 |
| PCAG | 0.7 | 0.733 | 0.3 | 0.672 | 5.8 | 0.0001 | 0.0 | 0.999 | 0.1 | 0.973 | 0.4 | 0.682 |
| POMSe | ||||||||||||
| Tension | 9.0 | <.0001 | 3.5 | 0.061 | 1.6 | 0.177 | 1.0 | 0.446 | 0.4 | 0.665 | 0.2 | 0.939 |
| Depression | 3.3 | 0.003 | 4.1 | 0.043 | 1.7 | 0.138 | 1.5 | 0.181 | 0.5 | 0.989 | 0.7 | 0.588 |
| Anger | 8.7 | <.0001 | 0.0 | 0.929 | 0.7 | 0.591 | 0.5 | 0.794 | 0.5 | 0.987 | 0.2 | 0.964 |
| Vigor | 1.5 | 0.193 | 0.2 | 0.689 | 1.0 | 0.403 | 0.2 | 0.987 | 0.2 | 0.999 | 0.1 | 0.979 |
| Fatigue | 10.3 | <.0001 | 0.4 | 0.515 | 1.0 | 0.428 | 0.6 | 0.757 | 0.2 | 0.999 | 0.4 | 0.789 |
| Confusion | 2.5 | 0.023 | 0.1 | 0.785 | 0.4 | 0.809 | 1.1 | 0.394 | 0.3 | 0.999 | 0.4 | 0.834 |
| Direct Effects of Tobacco Scalec | ||||||||||||
| Satisfy | 42.6 | <.0001 | 17.7 | <.0001 | 26.8 | <.0001 | 0.3 | 0.943 | 0.6 | 0.906 | 21.8 | <.0001 |
| Pleasant | 50.0 | <.0001 | 29.8 | <.0001 | 36.6 | <.0001 | 0.3 | 0.953 | 0.6 | 0.909 | 27.8 | <.0001 |
| Taste good | 27.2 | <.0001 | 24.1 | <.0001 | 36.6 | <.0001 | 0.1 | 0.999 | 0.4 | 0.993 | 31.2 | <.0001 |
| Dizzy | 8.2 | <.0001 | 0.0 | 0.886 | 4.9 | 0.001 | 0.1 | 0.999 | 0.6 | 0.924 | 5.0 | 0.001 |
| Calm | 12.0 | <.0001 | 1.3 | 0.261 | 22.0 | <.0001 | 0.1 | 0.996 | 0.5 | 0.979 | 14.4 | <.0001 |
| Help concentrate | 9.7 | <.0001 | 4.1 | 0.044 | 12.0 | <.0001 | 0.2 | 0.969 | 0.5 | 0.977 | 10.4 | <.0001 |
| Feel awake | 8.4 | <.0001 | 1.9 | 0.165 | 16.7 | <.0001 | 0.2 | 0.986 | 0.4 | 0.996 | 11.5 | <.0001 |
| Reduce hunger | 11.8 | <.0001 | 0.9 | 0.344 | 14.0 | <.0001 | 0.3 | 0.952 | 0.6 | 0.948 | 13.5 | <.0001 |
| Feel sick | 5.3 | <.0001 | 0.0 | 0.948 | 3.2 | 0.013 | 0.2 | 0.988 | 0.5 | 0.974 | 2.5 | 0.039 |
| Like to use another | 5.3 | <.0001 | 0.1 | 0.742 | 5.0 | 0.001 | 0.2 | 0.977 | 1.0 | 0.473 | 26.9 | <.0001 |
Note: Results for C x B X T are not included due to the lack of significant interactions noted. Bolded items had at least one significant main effect or interaction.
df C = (6, 1228), df B = (1, 1228), df T = (1, 1228); df CXB = (6, 1228), df CXT = (24, 1228); df BXT = (4, 1228).
Only available for N=10; df C = (6, 630), df B = (1,630), df T = (4, 630); df CXB = (6, 630), df CXT = (24, 630); df BXT = (4, 630).
df C = (6, 1326), df B = (1, 1326), df T = (4, 1326); df CXB = (6, 1326), df CXT = (24, 1326); df BXT = (4, 1326).
df C = (6, 1330), df B = (1, 1330), df T = (4, 1330); df CXB = (6, 1330), df CXT = (24, 1330); df BXT = (4, 1330).
df C = (6, 1257), df B = (1, 1257), df T = (4, 1257); df CXB = (6, 1257), df CXT = (24, 1257); df BXT = (4, 1257).
3.1. Physiological Measures
Significant main effects of condition, bout, and time were observed for HR. Systolic BP had a significant main effect of condition, and diastolic BP had significant main effects of condition and time. When mean results of HR, systolic BP, and diastolic BP were examined by condition, bout, and time, findings were consistent with nicotine exposure occurring in the nicotine-containing conditions, particularly OB (see Supplementary Material* for more detail).
3.2. Subjective Measures
3.2.1. Tobacco/Nicotine Abstinence Symptoms and Nicotine-Related Effects.
Significant main effects of condition were observed for all adapted-MNWS items, and significant main effects of bout and time were observed for “Urges to smoke a cigarette”. For this latter item, significant decreases relative to baseline were observed during the OB and Tob/Men 36 mg/ml conditions (see Figure 1A); Cream and/or Fruit 0 mg/ml condition ratings were significantly higher than OB at most post administration time points (see Figure 1B; ps<0.05). The Tob/Men 36 mg/ml condition was significantly lower than the Cream and Fruit 0 mg/ml conditions at several post administration time points (ps<0.05). Ratings for other tobacco/nicotine abstinence symptoms followed a relatively similar pattern with the lowest values noted for OB.
Figure 1.
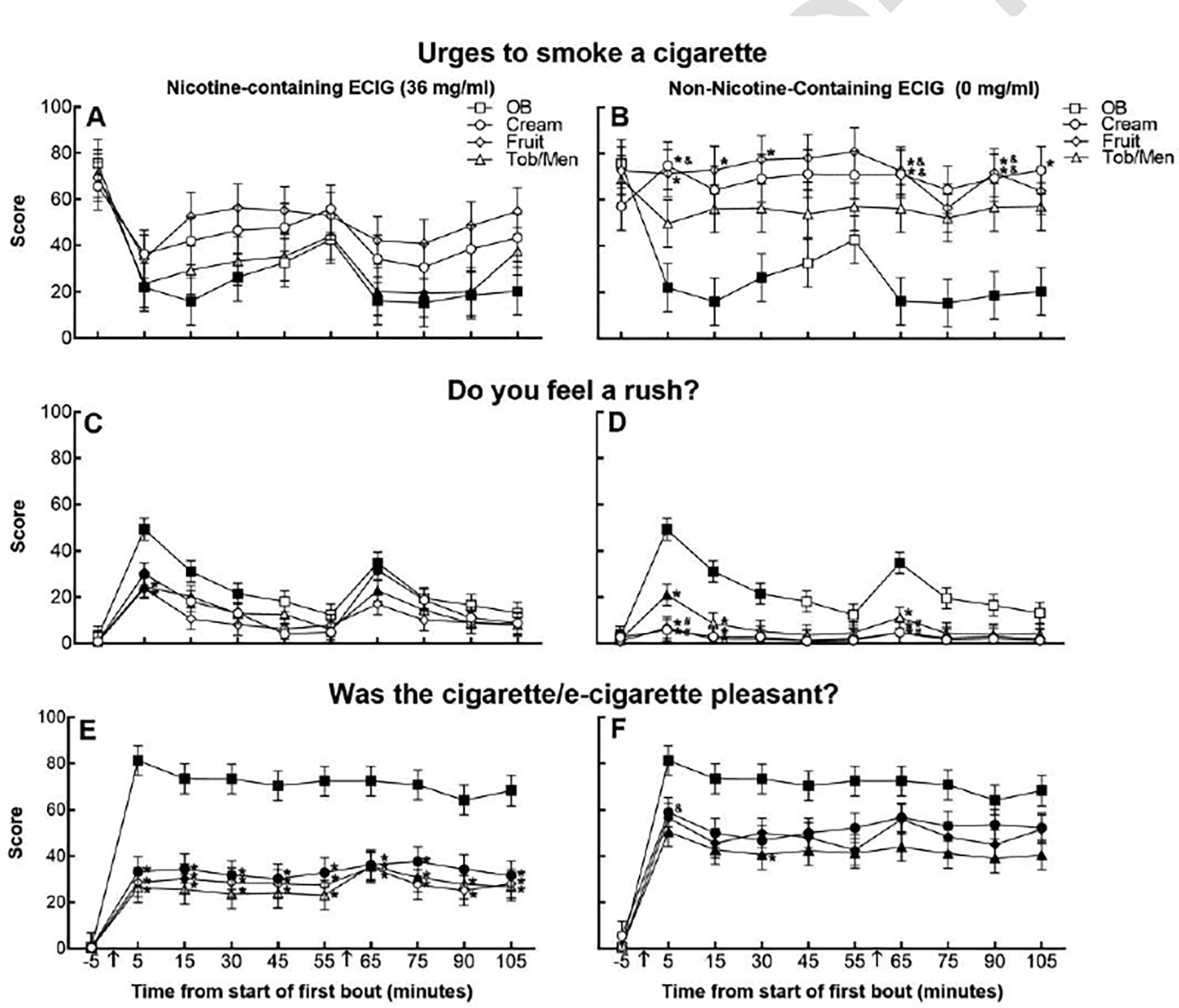
Least squares mean subjective ratings (±standard error) for “Urges to smoke a cigarette” (Panels A and B; N=10), “Do you feel a rush?” (Panels C and D; N=20), “Was the cigarette/e-cigarette pleasant?” (Panels E and F; N=20). Filled symbols indicate a significant difference from baseline (−5); asterisks (*) indicate a significant difference from OB, ampersands (&) indicate a significant difference from Tob/Men 36 mg/ml, number signs (#) indicate a significant difference from Cream 36 mg/ml, at each respective time point (p<0.05, Tukey’s HSD). Arrows indicate product administration (i.e., bout).
Significant main effects and/or interactions of condition and time for Direct Effects of Nicotine Scale items were observed for “Dizzy”, “Lightheaded”, and “Heart pounding”. For “Lightheaded”, the item with the highest F value for the interaction of condition and time, OB, Cream 36 mg/ml, and Tob/Men 36 condition ratings significantly increased relative to baseline following each bout (ps<0.05); all 0 mg/ml ECIG conditions were significantly lower than OB following each bout (ps<0.05). The Tob/Men 36 mg/ml condition was significantly greater than Fruit 0 mg/ml immediately following each bout (ps<0.05). Results for “Dizzy” and “Heart pounding” followed a similar pattern.
3.2.2. General Drug Effects.
Significant main effects of condition and/or time were observed for all Drug Effects Scale items except “Dislike drug effects”. Significant condition by time interactions were observed for “Rush”, “High”, and “Feel drug effects”, and significant bout by time interactions were observed for “Rush”, “Like drug effects”, and “Feel good drug effects”. For “Rush”, the item with the highest F value for the interaction of condition and time, all nicotine-containing conditions resulted in significantly elevated ratings at the first time point following the first bout (see Figure 1C; ps<0.05). Following the first bout, the Tob/Men 0 mg/ml condition was also significantly elevated relative to baseline (see Figure 1D; p<0.05). Most ECIG conditions were significantly lower than OB at the first post bout time point (ps<0.05). Between ECIG conditions, the Cream 36 mg/ml condition had significantly greater ratings relative to Cream 0 and Fruit 0 mg/ml conditions following the first bout (ps<0.05). For other Drug Effects Scale items, a similar pattern was observed.
Significant main effects of condition or time were observed for AMP, BG, MBG, and PCAG ARCI subscales. The highest F value for the main effect of condition was for the stimulant sensitive scale (BG). When means were examined by condition, bout, and time, no significant mean differences were observed, but nicotine-containing conditions tended to produce increased ratings relative to non-nicotine-containing conditions. Results for other scales followed a similar pattern related to nicotine content.
3.2.3. Mood Effects.
Five subscales from the POMS had a significant main effect of condition and/or bout. When means were examined by condition, bout, and time, no significant mean differences were observed with variable patterns of responding across time and between conditions.
3.2.4. Acceptability-Related Effects.
All Direct Effects of Tobacco Scale items had a significant main effect of condition and time and interaction of bout and time with the highest F values for items assessing positive attributes. The pattern of ratings observed by condition, bout, and time varied by item. For “Satisfy”, OB had the highest ratings, and most ECIG condition time points were significantly lower than OB at post bout time points (ps<0.05) with no significant differences between ECIG conditions. For “Pleasant”, OB was the highest rated condition across time, and nicotine-containing ECIG conditions were significantly lower than OB ratings at almost all post bout time points (see Figure 1E; ps<0.05). Non-nicotine-containing ECIG conditions achieved higher ratings for this item, and the Cream 0 mg/ml condition was significantly higher than Tob/Men 36 mg/ml condition following the first bout (see Figure 1F; p<0.05). Results for “Dizzy”, “Calm down”, “Help concentrate”, “Feel awake”, and “Reduce hunger” items indicated 0 mg/ml ECIG condition ratings were significantly lower than OB across time (ps<0.05). Ratings of “Feel sick” were only significantly increased relative to baseline for the Cream 36 mg/ml condition following the first bout; this condition-specific time point also was significantly higher than the OB, Cream 0 mg/ml and Fruit 0 mg/ml conditions (ps<0.05). For “Like to use another”, the OB and Fruit 0 mg/ml condition ratings were significantly elevated to baseline for most of the post bout time points (ps<0.05), but only the Fruit and Tob/Men 36 mg/ml conditions had no significant increases relative to baseline for this item. Between ECIG conditions, immediately following the first bout, the Fruit 0 mg/ml condition was significantly greater than the Tob/Men 36 mg/ml condition (p<0.05).
4. Discussion
This study systematically examined the subjective effects of three popular ECIG liquid flavors, Cream, Tropical Fruit, and Tob/Men, at two nicotine concentrations (0 and 36 mg/ml) as compared to OB smoking in sample of young adult cigarette smokers under conditions of tobacco/nicotine deprivation. Results indicated that 10 puffs from an OB cigarette and an ECIG containing Tob/Men flavored liquid at 36 mg/ml produced significant decreases in ratings of “Urges to smoke a cigarette” and increases in ratings of nicotine-related side effects and general drug effects. All nicotine-containing ECIG conditions and the Tob/Men 0 mg/ml ECIG produced significant increases relative to baseline in at least one measure of general drug effect responding. Acceptability responses varied for the ECIG conditions.
Consistent with previous work examining the ECIG-related tobacco/nicotine abstinence symptom suppression and nicotine-related responding (Vansickel et al., 2010; Dawkins et al., 2012; Nides et al., 2014), nicotine-containing ECIG conditions in the current study produced the strongest effects for these measures. Subjective ratings of drug effects for the nicotine-containing ECIG conditions did not appear to be significantly enhanced or masked by the ECIG liquid flavors assessed here. Alternatively, among the non-nicotine-containing ECIG conditions, the Tob/Men flavor was the only condition where some drug effect ratings approached those of nicotine-containing conditions. This finding could imply a greater conditioned response to ECIG flavors that more closely simulate conventional cigarette smoking (Rose et al., 2003). Interestingly in a recent study that explored the impact of conditioned stimuli such as “aroma” (i.e., tobacco-flavored vs. apple-flavored ECIG liquid) on measures of craving reduction among cigarette smokers, there were no significant effects of this factor (Van Heel et al., 2017). Another potential hypothesis may relate to flavorant and/or pharmacological effects of menthol when used as an ECIG liquid flavoring. Previous work has indicated menthol ECIGs contributed to sensory effects of ECIGs when nicotine concentration was low (Rosebrook and Green, 2016). Cooling and anesthetic effects of menthol within the ECIG liquids also may have influenced subjective responses in this study. Due to the low proportion of participants who preferred menthol cigarettes in the current study (and thus received menthol flavored ECIG liquid during the Tob/Men ECIG conditions; i.e., N=6), we were unable to explore fully this potential phenomenon. Future work with larger sample sizes and specific comparison of tobacco and menthol ECIG liquid flavors with varying menthol and nicotine concentrations is warranted.
Subjective ratings for acceptability consistently indicated the highest preference for OB smoking with varying results for the ECIG conditions. For example, only the 36 mg/ml ECIG conditions were rated consistently significantly lower than OB for “Pleasant”. For ratings of “Satisfying”, all ECIG conditions resulted in significantly lower ratings relative to OB with no significant differences between ECIG conditions. For other subjective items assessing positive subjective attributes (e.g., “Calm down”) only non-nicotine-containing ECIG conditions were associated with significantly lower subjective ratings relative to OB. All non-nicotine-containing ECIG conditions and Cream 36 mg/ml resulted in significant increases in “Like to use another”. Of note, when this same ECIG device at similar nicotine concentrations (0, 8, 18, 36 mg/ml; Hiler et al., 2017) controlling for flavor (all either Tob or Men) was assessed among ECIG naïve cigarette smokers, results indicated significantly greater levels of acceptability for the nicotine-containing ECIG conditions (e.g., “Satisfying”). Taken together, these findings suggest a need to explore more ECIG-specific acceptability-related items to better understand the influence of ECIG nicotine content and liquid flavor on these outcomes.
Considering these findings, there are several limitations to the current study. The sample size and number of within-subjects factors reduced power to detect effects across condition and time. The lack of a negative control condition (e.g., unflavored 0 mg/ml ECIG liquid) also limits conclusions that can be drawn. Importantly, a supplemental mixed models analysis restricted to only the ECIG conditions and first five time points (i.e., bout 1) that explored the specific contribution of the nicotine and flavor factors confirms the overall conclusions regarding the strength of effects related to nicotine content and, to a lesser extent, for flavor (see Supplementary Material*). Other than the use of CO (an imperfect assessment of recent tobacco/nicotine use), participants were not biochemically screened for other behaviorally-active compounds which could have influenced their responding. The choice of specific ECIG liquid flavors and use of a single ECIG device/components limits generalizability. While similar liquid flavor preferences are found consistently among other examinations of ECIG users (Zare et al., 2018; Russell et al., 2018), the specific flavors chosen for this study originated from an older experienced ECIG user sample (Soule et al., 2016). Young adult ECIG flavor preferences may follow a differential pattern (Harrell et al., 2016; Morean et al., 2018). A factor unexplored in the current study was the role of ECIG liquid flavor “sweetness”, which has been linked to appeal/liking indices (Goldenson et al., 2016; Kroemer et al., 2018). Future work should include measures of perceived sweetness as well as objective measures of glucose, fructose, and sucrose concentration in ECIG liquids to address the influence of this ECIG liquid attribute. Replication of these results using other measures of abuse liability (e.g., choice/purchase tasks) are also needed. Lastly, subjective effects observed might have differed with the use of ad lib smoking instructions versus the 10-puff protocol used here. While this approach provides important control over tobacco use behavior and potentially nicotine delivery, real-world ECIG use patterns may significantly influence subjective abuse liability indices.
In conclusion, these findings support findings in previous work that ECIGs containing nicotine can suppress cigarette smoking urges and produce nicotine and drug-related responding among current cigarette smokers, although they may be less effective in this regard relative to OB cigarettes. ECIG liquid flavors did not appear to significantly enhance or mask these effects, although Tob/Men 0 mg/ml flavored liquid was associated with some drug-related responding. Acceptability ratings were not related consistently to ECIG flavor or nicotine concentration. Unique to this study was the focus on young adult cigarette smokers and the placebo-controlled design to explore the effects of three popular ECIG liquid flavors. These findings reinforce efforts to evaluate the interaction of ECIG-delivered nicotine and liquid flavors on subjective response measures as well as other outcomes relevant to understanding ECIG-associated abuse liability.
Supplementary Material
Highlights.
Nicotine/drug-related subjective effects driven by ECIG nicotine content.
Acceptability results not related consistently to ECIG nicotine/flavor content.
Little evidence that ECIG flavors masked/enhanced subjective effects.
Acknowledgements
We would like to thank Aashir Nasim for his contributions to this study.
Role of Funding Source
This work was supported by the Virginia Foundation for Healthy Youth (8521068; 8521236), National Cancer Institute (P30CA016059; R21CA184634), National Institute on Drug Abuse (P50DA036105, R03DA043005, U54DA036105) and the Center for Tobacco Products of the US Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Author Disclosures
Conflict of Interest
TE is a paid consultant in litigation against the tobacco industry and is named on a patent application for a device that measures the puffing behavior of electronic cigarette users. All other authors have no conflicts of interest to report.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audrain-McGovern J, Strasser AA, Wileyto EP, 2016. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend. 166, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MD, Sams C, Weaver MF, Eissenberg T, 2008. Nicotine delivery, cardiovascular profile, and subjective effects of an oral tobacco product for smokers. Nicotine Tob. Res. 10, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T, 2009. Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation. Nicotine Tob. Res. 11, 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer ZT, Goel R, Reilly SM, Foulds J, Muscat J, Elias RJ, Richie JP Jr., 2018a. Effects of solvent and temperature on free radical formation in electronic cigarette aerosols. Chem. Res. Toxicol. 31, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, Muscat J, Richie JP Jr., 2018b. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic. Biol. Med. 120, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T, 2017. Electronic cigarettes: What are they and what do they do? Ann. N. Y. Acad. Sci. 1394, 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK, 2009. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol. Biomarkers Prev. 18, 3241–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Shihadeh A, Weaver MF, Eissenberg T, 2011. Waterpipe tobacco smoking and cigarette smoking: A direct comparison of toxicant exposure and subjective effects. Nicotine Tob. Res. 13, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SL, Andrykowski MA, Studts JL, 1995. Short form of the Profile of Mood States (POMS-SF): Psychometric information. Psychol. Assess. 7, 80–83. [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K, 2012. The electronic-cigarette: Effects on desire to smoke, withdrawal symptoms and cognition. Addict. Behav. 37, 970–973. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML, 1996. Burprenorphine’s physical dependence potential: Antagonist-precipitated withdrawal in humans. J. Pharmcol. Exp. Ther. 276, 449–459. [PubMed] [Google Scholar]
- Etter JF, 2010. Electronic cigarettes: A survey of users. BMC Public Health. 10, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, 2015. E-cigarettes: Methodological and ideological issues and research priorities. BMC Med. 16, 13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Eissenberg T, 2015. Dependence levels in users of electronic cigarettes, nicotine gums, and tobacco cigarettes. Drug Alcohol Depend. 147, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T, 2006. Transdermal nicotine-induced tobacco abstinence symptom suppression: Nicotine dose and smokers’ gender. Exp. Clin. Psychopharmacol. 14, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Family Smoking Prevention and Tobacco Control Act, S. 1776, 111th Cong. (2009). [Google Scholar]
- Food and Drug Administration, 2017. Guidance for industry: Assessment of abuse potential of drugs. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- Food and Drug Administration, 2018a. Advance notice of proposed rulemaking on the regulation of flavors in tobacco products. Federal Register, 83 FR 12294. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- Food and Drug Administration, 2018b. Statement from FDA Commissioner Scott Gottlieb, M.D., on proposed new steps to protect youth by preventing access to flavored tobacco products and banning menthol in cigarettes. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, Pang RD, McBeth JF, Pentz MA, Samet JM, Leventhal AM, 2016. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug Alcohol Depend. 168, 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell MB, Weaver SR, Loukas A, Creamer M, Marti CN, Jackson CD, Heath JW, Nayak P, Perry CL, Pechacek TF, Eriksen MP, 2016. Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Prev. Med. Rep. 11, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P, 2016. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev 9, CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler M, Breland A, Spindle T, Maloney S, Lipato T, Karaoghlanian N, Shihadeh A, Lopez A, Ramôa C, Eissenberg T, 2017. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: Influence of liquid nicotine concentration and user experience. Exp. Clin. Psychopharmacol. 25, 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D, 1986. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry 43, 289–294. [DOI] [PubMed] [Google Scholar]
- Kim H, Lim J, Buehler SS, Brinkman MC, Johnson NM, Wilson L, Cross KS, Clark PI, 2016. Role of sweet and other flavours in liking and disliking of electronic cigarettes. Tob. Control 25, ii55–ii61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML, 2014. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, Veldhuizen MG, Delvy R, Patel BP, O’Malley SS, Small DM, 2018. Sweet taste potentiates the reinforcing effects of e-cigarettes. Eur. Neuropsychopharmacol. 28, 1089–1102. [DOI] [PubMed] [Google Scholar]
- Litt MD, Duffy V, Oncken C, 2016. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tob. Control 25 Suppl. 2, ii67–ii72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AA, Eissenberg T, 2015. Science and the evolving electronic cigarette. Prev. Med. 80, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramôa CP, Karaoghlanian NV, Lipato T, Breland AB, Shihadeh AL, Eissenberg T, 2016. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: A preliminary report. Nicotine Tob. Res. 18, 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinkski DR, 1971. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin. Pharmacol. Ther. 12, 245–258. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Winickoff JP, 2015a. e-Cigarettes— The roles of regulation and clinicians. JAMA Intern. Med. 175, 1603–1604. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD, 2015. Trends in electronic cigarette use among U.S. adults: Use is increasing in both smokers and nonsmokers. Nicotine Tob. Res. 17, 1195–1202. [DOI] [PubMed] [Google Scholar]
- Morean ME, Butler ER, Bold KW, Kong G, Camenga DR, Cavallo DA, Simon P, O’Malley SS, Krishnan-Sarin S, 2018. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PLoS One 13, e0189015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nides M, Leischow S, Bhatter M, Simmons M, 2014. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am. J. Health Behav. 38, 265–274. [DOI] [PubMed] [Google Scholar]
- Oncken CA, Litt MD, McLaughlin LD, Burki NA, 2015. Nicotine concentrations with electronic cigarette use: Effects of sex and flavor. Nicotine Tob. Res. 17, 473–478. [DOI] [PubMed] [Google Scholar]
- Ramôa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, Breland AB, Shihadeh A, Eissenberg T, 2016. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: A preliminary report. Tob. Control 25, e6–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbrook K, Green BG, 2016. Sensory effects of menthol and nicotine in an e-cigarette. Nicotine Tob. Res. 18, 1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, Salley A, 2003. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol. Biochem. Behav. 76, 243–250. [DOI] [PubMed] [Google Scholar]
- Russell C, McKeganey N, Dickson T, Nides M, 2018. Changing patterns of first e-cigarette flavor used and current flavors used by 20,836 adult frequent e-cigarette users in the USA. Harm Reduct. J. 15, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, Yang J, Primack BA, Andrews JA, Miech RA, Spindle TR, Dick DM, Eissenberg T, Hornik RC, Dang R, Sargent JD, 2017. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: A systematic review and meta-analysis. JAMA Pediatr. 171, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule EK, Lopez AA, Guy MC, Cobb CO, 2016. Reasons for using flavored liquids among electronic cigarette users: A concept mapping study. Drug Alcohol Depend. 166, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T, 2015. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob. Res. 17, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Talih S, Hiler MM, Karaoghlanian N, Halquist MS, Breland AB, Shihadeh A, Eissenberg T, 2018. Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug Alcohol Depend. 188, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihadeh A, Eissenberg T, 2015. Electronic cigarette effectiveness and abuse liability: Predicting and regulating nicotine flux. Nicotine Tob. Res. 17, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Helen G, Dempsey DA, Havel CM, Jacob III P, Benowitz NL, 2017. Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug Alcohol Depend. 178, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner KG, Cornacchione J, Wiseman KD, Teal R, Moracco KE, Sutfin EL, 2016. E-cigarettes, hookah pens and vapes: Adolescent and young adult perceptions of electronic nicotine delivery systems. Nicotine Tob. Res. 18, 2006–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heel M, Van Gucht D, Vanbrabant K, Baeyens F, 2017. The importance of conditioned stimuli in cigarette and e-cigarette craving reduction by e-cigarettes. Int. J. Environ. Res. Public Health 14, pii: e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE, 2010. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol. Biomarkers Prev. 19, 1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Eissenberg T, 2013. Electronic cigarettes: Effective nicotine delivery after acute administration. Nicotine Tob. Res. 15, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare S, Nemati M, Zheng Y, 2018. A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS One 13, e0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M, 2014. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob. Control 23, iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


