Abstract
Background
Refractory disease in primary central nervous system lymphoma (PCNSL) may occur despite adequate initial treatment. There is currently no standard of care for relapsed and recurrent PCSNL. No study to date documents using a combined regimen of radiotherapy, temozolomide, and rituximab. This study aimed to present the clinical course and outcomes of patients with recurrent or refractory disease who were given a combination of radiation, temozolomide, and rituximab.
Methods
Retrospective analysis was employed to evaluate data from recurrent or refractory PCNSL patients who were treated with radiation, temozolomide, and rituximab in two tertiary hospitals in the Philippines. Baseline demographics, treatment regimen, and outcomes were analyzed.
Results
Fifteen patients with a median age of 56 years were included, 11 with refractory disease and 4 with recurrent disease. Patients with bulky disease received either whole brain radiotherapy or partial field radiotherapy with rituximab and temozolomide given during radiation and for 6 months after radiation. Overall response rate to salvage therapy was 93.3% (14/15). Median overall survival from initial diagnosis was not reached (median follow-up: 84 months). Mortality rate was 33.3% (5/15), but only 2 out of 5 mortalities were from disease progression. There were only two reported cases of mild allergic reactions to rituximab, which did not result in treatment interruption.
Conclusion
Rituximab, temozolomide, and radiotherapy can be considered as an effective and safe salvage therapy for relapsed and recurrent central nervous system lymphoma.
Keywords: primary CNS lymphoma, recurrent CNS lymphoma, chemotherapy, radiotherapy, salvage therapy
Key Points.
There is no consensus for standard of care for salvage therapy for PCNSL.
Using radiotherapy with rituximab and temozolomide as salvage therapy is effective.
Salvage therapy with radiotherapy, temozolomide, and rituximab is well-tolerated.
Importance of the Study.
Primary central nervous system lymphoma (PCNSL) is usually responsive to both chemotherapy and radiotherapy. However, there are limited studies evaluating efficacy and safety of multi-modality treatment for recurrent and refractory PCNSL. Current management is based on early-phase clinical trials or the clinical experience of individual institutions. This retrospective case series aims to evaluate the outcomes among patients diagnosed with recurrent or refractory PCNSL following combined modality treatment with radiation, temozolomide, and rituximab. This study demonstrates efficacy and tolerability of radiation, temozolomide, and rituximab as salvage therapy for recurrent and refractory PCNSL, providing an alternative viable treatment option.
Primary CNS Lymphoma (PCNSL) is a rare form of non-Hodgkin lymphoma. The incidence of this disease is documented to be 7 cases per 1,000,000.1 A recent study from Australia noted that the age-standardized incidence of PCNSL to be 0.43 per 100,000,2 while a Korean study found an incidence of 0.17 per 100,000.3 Experts consider high-dose methotrexate and rituximab as the cornerstone in treating PCNSL.4 Despite this, as much as 85% of patients have isolated CNS relapse on follow-up5 and most of these occur in the first 2 years of diagnosis.6 Further, as much as 10–15% proceed to develop refractory disease.4 Refractory disease has been defined as progression occurring during the course of first-line treatment for PCNSL while recurrence refers to relapse following complete response to initial therapy.7
To date, there has been no established treatment guideline or randomized controlled trial on this study population. The National Comprehensive Cancer Network (NCCN) recommends the inclusion of these patients in clinical trials. High-dose methotrexate, other systemic chemotherapy, and stem cell therapy are also considered options.8
Several regimens have been examined for patients with recurrent and refractory disease, especially among those who had treatment failure with high-dose methotrexate (HD-MTX). Salvage therapy with HD-MTX is the most frequently used and is particularly beneficial among patients who responded well to initial treatment with methotrexate. Other agents such as rituximab and temozolomide have also been studied.4 Salvage therapy with a combination of temozolomide and rituximab showed response rates between 14 and 100% and median survival of 8–14 months.5,9 Rituximab monotherapy resulted in 36% response rate and median overall survival of 20.9 months.10 Salvage radiotherapy using whole brain radiotherapy had response in 67–79% of cases and median survival of 10.9–16 months.11–13 At the moment, data on the best treatment option remains limited due to the absence of randomized controlled trials among patients who have recurrent or refractory PCNSL (rrPCNSL).
This is the first study to document the demographic profile and clinical course of the rrPCNSL patients in the Philippines. This study can guide future directions on the treatment of this highly recurrent disease in low-income countries like the Philippines. Radiotherapy, rituximab, and temozolomide are readily available treatment modalities in most tertiary hospitals in the country. In the Philippine setting where therapeutic choices may be limited by financial restrictions, finding the most efficient and tolerated regimen for such disease will not only aid clinicians and families in decision-making but may ultimately guide insurance and healthcare policies.
Methods
The authors did a retrospective case series on the profile and outcomes of patients with rrPCNSL seen in St. Luke’s Medical Center and Philippine General Hospital who are all attended to by the Neuro-Oncology service until April 2021. Inclusion and exclusion criteria were used to minimize selection bias. The following were used to include patients in the study who were or who had: A) aged 18 years old and above; B) biopsy confirmed histopathologic evidence of primary CNS lymphoma with positive CD 20 expression on immunohistochemical studies; C) treated initially with a high-dose methotrexate regimen and had clinical and radiologic evidence of stable disease or complete response; and D) were treated with any combination of rituximab, temozolomide, and/or radiation for their recurrence or relapse. Patients who had either: A) incomplete medical records; B) did not complete initial regimen of high-dose methotrexate; or C) did not proceed with salvage treatment for any reason were excluded from the study.
The demographic, clinical profiles, course, and outcomes of the patients were documented through chart review of in-patient, out-patient files, and follow-up via consult or telephone call. Overall mortality rate and response rates were documented.
Ethical approval for this study was obtained from University of the Philippines Manila Ethics Review Board (UPMREB 2020-535-01) and St. Luke’s Medical Center Institutional Ethics Review Committee (SL-21151).
Results
Records were reviewed and a total of 18 patients were identified with diagnosed cases of rrPCNSL who met the inclusion criteria. Three (3) patients were then excluded for the following reasons: A) one patient was treated with only palliative whole brain radiotherapy due to low KPS and no consent for chemotherapy from the patient; B) one patient who expired prior to planned salvage therapy; and C) one patient who underwent treatment but had no prior CD20 immunohistochemistry done.
From the total analyzed 15 patients, there were 8 males and 7 females with a median age of 56 years (range, 21–78). The median KPS score after initial therapy was 90 (range, 40–100). The median MMSE score was 26 (range, 5–30). The most common comorbidity was hypertension (n = 5, 33.3%).
Initial Treatment
Treatment given to all 15 patients are summarized in Table 1. All of the patients received high-dose methotrexate at 8 g/m2 BSA every 14 days. There were 7 (46.6%) patients who received rituximab at 375 mg/m2 BSA as part of their initial treatment, and 2 (13.3%) patients received temozolomide as part of their initial treatment.
Table 1.
Characteristics of patients with recurrent and refractory primary CNS lymphoma
| Patient | Age/Sex | Initial treatment given | Recurrent or refractory | Treatment on relapse | Time to relapse/ recurrence (months) |
Survival time a (months) |
KPS b | Cognitive outcome (reason for decline) |
Outcome | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39/F | MTX | Refractory | WBRT, TMZ, Rituximab | 3 | 120 | 100 | No cognitive decline | Alive | |
| 2 | 52/F | MTX | Refractory | WBRT, TMZ, Rituximab | 2 | 144 | 100 | No cognitive decline | Alive | |
| 3 | 40/M | MTX | Recurrent | WBRT, TMZ, Rituximab | 72 | 108 | 70 | Mild cognitive decline 2 years after WBRT; Forgetfulness but still able to work (Due to treatment) |
Alive | Progressive disease |
| 4 | 78/F | MTX, Rituximab | Refractory | PFRT, TMZ, Rituximab | 1 | 76 | 80 | Mild cognitive decline, still to converse and answer questions; vegetative post admission for status epilepticus (Due to other medical condition: status epilepticus) |
Alive | |
| 5 | 21/F | MTX, Rituximab | Refractory | WBRT, Spinal RT, TMZ, Rituximab | 3 | 61 | 100 | No cognitive decline | Alive | |
| 6 | 56/M | MTX, Rituximab | Refractory | TMZ, Rituximab | 2 | 84 | 100 | No cognitive decline | Alive | |
| 7 | 53/F | MTX, Rituximab, TMZ | Refractory | WBRT, TMZ, Rituximab | 4 | 74 | 90 | No cognitive decline | Alive | |
| 8 | 60/M | MTX | Recurrent | TMZ, Rituximab, Intraocular MTX | 24 | 84 | 100 | No cognitive decline | Lost to follow-up | |
| 9 | 71/M | MTX | Recurrent | WBRT, TMZ, Rituximab | 24 | 72 | 60 | Gradual cognitive decline 1 month after WBRT, initially with short term memory loss progressing to inability to recognize people and inconsistent answers to questions in the following months (Due to treatment) |
Died | Died of pneumonia |
| 10 | 67/F | MTX, Rituximab | Refractory | PFRT, TMZ, Rituximab | 1 | 31 | 100 | No cognitive decline | Alive | |
| 11 | 64/M | MTX | Refractory | PFRT, TMZ, Rituximab | 2 | 38 | 80 | No cognitive decline | Died | Died from disease progression |
| 12 | 32/M | MTX | Recurrent | WBRT, TMZ, Rituximab | 6 | 11 | 40 | No data | Died | Died from disease progression |
| 13 | 65/M | MTX | Refractory | PFRT, TMZ, Rituximab | 4 | 5 | 80 | No data | Died | Progressive disease; Died of pneumonia |
| 14 | 70/M | MTX, Rituximab | Refractory | WBRT, TMZ, Rituximab | 6 | 23 | 90 | Mild cognitive decline, short term memory loss 6 months after WBRT (Due to treatment) |
Died | Cause of death not specified |
| 15 | 53/F | MTX, Rituximab, TMZ | Refractory | WBRT, TMZ, Rituximab | 29 | 47 | 60 | Short term memory loss after stroke (Due to other medical condition: stroke) |
Alive |
MTX, methotrexate; PFRT, partial field radiotherapy (either FSRT or IMRT); RT, radiotherapy; TMZ, temozolomide; WBRT, whole brain radiotherapy.
a Survival time from diagnosis.
b Karnofsky Performance Score at time of study.
Salvage Therapy
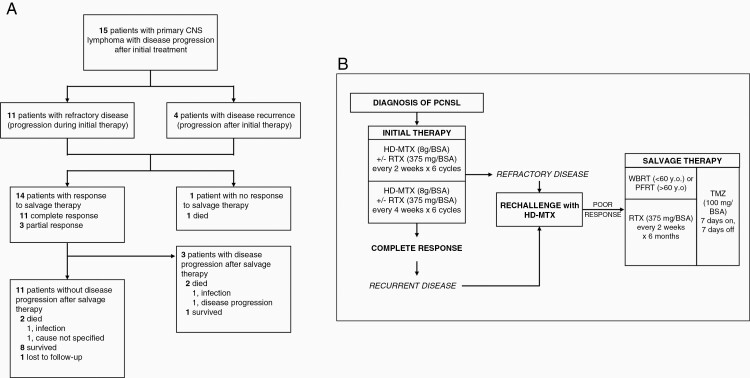
Refractory disease was defined as disease progression during initial treatment with methotrexate, and recurrent disease was defined as clinical disease progression following initial treatment with methotrexate. Of the 15 patients, 11 patients had refractory disease, and 4 patients had recurrence. The median time to disease progression was 14 weeks. For recurrent disease, the median time to recurrence was 24 months. All patients were rechallenged with HD-MTX. Only one patient responded to rechallenge however was noted to have disease recurrence on the 6th month of treatment.
All 15 patients received temozolomide at 100 mg/m2 BSA for 7 days on and 7 days off during and after radiotherapy, and rituximab at 375 mg/m2 BSA every 14 days for 6 months after completing radiotherapy (see Figure 1). In 10 of 11 patients with refractory disease and 3 of 4 patients with recurrence, radiotherapy was given as either whole brain RT or partial field RT with a total dose of 30 Gy, the choice of which was determined by patient age. Patients younger than 60 years old received WBRT, and PFRT was prescribed to those 60 years old and older. PFRT was given to four patients, and WBRT was given to nine patients. There was one patient with refractory disease treated with fractionated stereotactic radiotherapy and one patient with refractory disease who received spinal radiotherapy. Two patients did not receive radiotherapy for the following reasons: A) Patient 6 had small radiologic progression; and B) Patient 8 had local progression of ocular lymphoma only.
Figure 1.
(A) Flow of patients in the study. (B) Flow of treatment regimen given for recurrent and refractory PCNSL.
There were no major adverse events noted. Allergic reaction (rashes) to rituximab was encountered in 2 patients but was easily treated with hydrocortisone and did not interrupt treatment.
Treatment Response and Survival
There were 14 (93.3%) patients responsive to salvage therapy, of which 11 (73.3%) had complete response (CR) and 3 (20%) had partial response (PR) (see Figure 1). There were 3 (20%) patients that developed progressive disease. Of these, one patient remains alive 74 months from relapse with a KPS of 70.
There were 5 (33.3%) patients that died, and 1 was lost to follow-up. Of these 5 patients, 2 died from disease progression, 2 died from infection, and 1 died without specified cause. There were 2 of 11 patients without disease progression that died. Median KPS among the surviving 9 patients following treatment was 95 (range, 80–100). Median overall survival was not reached (median follow-up: 84 months).
Cognitive Status
A descriptive account on the cognitive status of the patients on their most recent follow-up is included in this study. On review of records, 8 of the 15 patients were noted to have no cognitive decline. Out of the five patients who were noted to have cognitive deficits (see Figure 2), only 3 were attributed to the effect of treatment. Patient 3 had mild cognitive decline manifesting as forgetfulness which started 2 years post WBRT. Patient 9 had gradual cognitive decline presenting initially with short term memory loss progressing to inability to recognize people and to answer questions consistently. Patient 14 had short term memory loss 6 months post WBRT. Two other patients were noted to have cognitive decline due to another medical condition. Patient 4 was still able to converse and answer coherently initially but was vegetative after an admission for status epilepticus. On the other hand, patient 15 presented with short term memory loss attributed to stroke. Patients 12 and 13 expired early hence with no further data on cognitive status.
Figure 2.
Illustrated clinical course of patients with rrPCNSL indicating time to disease recurrence or progression and cognitive decline.
Discussion
There are several challenges in finding the best salvage treatment for patients with rrPCNSL. The variation in terms of site, actual number, and age of patients during the time of recurrence as well as the knowledge gaps in the pathophysiology of PCNSL have been identified as barriers to the conduct of randomized controlled trials in this population.4
All of the 15 patients were given methotrexate as initial therapy to the PCNSL. Eleven of these patients developed refractory disease while 4 developed recurrent disease beyond 6 months following methotrexate therapy (Figure 1). Plotkin and colleagues found the repeat administration of HD-MTX in the treatment of recurrent PCNSL had an 87% response rate (CR 73%, PR 14%), with overall survival of 61.9 months.14 In our study however, after failing to respond to a rechallenge with HD-MTX, all the patients were considered poor responders to methotrexate which prompted introduction of a new treatment regimen. In the NCCN guidelines, the options for such patients include systemic chemotherapy, radiotherapy with or without chemotherapy, or high-dose MTX therapy with stem cell rescue.8
Thirteen patients received radiotherapy with chemotherapy while two patients were not given radiotherapy due to relatively limited tumor bulk. While several single-agent or combination therapies have been studied for this population,7 radiation therapy, specifically WBRT remains to be an important option in some cases. It may be given to patients with rapid progression of disease or those with multiple recurrences of disease especially if systemic therapy is not an option. In the Philippines, radiotherapy, rituximab, and temozolomide are accessible therapeutic options in equipped centers. Availability and previously published evidence of effectiveness favor the use of these agents in our multi-modality regimen.
Radiotherapy has been proven effective as salvage therapy for rrPCNSL. A study in 2005 used salvage WBRT in 27 patients with rrPCNSL with a 74% response rate (CR 37%, PR 37%) and overall survival of 10.9 months, with dose-dependent late treatment-associated neurotoxicity in 14%.12 Khimani and colleagues documented an overall response rate of 67% (CR 50%, PR 17%) and median survival of 11.7 months among 36 patients with primary or secondary CNSL who failed prior MTX-based therapy. Longer overall survival was seen in their patients who received five or more cycles of MTX initially and those with relapse confined to the CNS.11 A retrospective study by Hottinger and colleagues including 48 patients showed a 79% response rate (CR 58%, PR 21%) and overall survival of 16 months. Treatment-related neurotoxicity developed in 22% and was associated with older age and radiotherapy initiated less than 6 months from MTX treatment.13 Patient 9 was given WBRT and was noted to have cognitive decline presenting as short term memory loss progressing to inability to recognize people 1 month post treatment. The patient was more than 60 years old at the time of WBRT. Due to the association of radiotherapy with neurocognitive decline, PFRT was preferred over WBRT in the succeeding patients who were aged 60 years old and above at the time of treatment. Two of these patients (Patients 10 and 11) out of 4 had no cognitive decline on their most recent follow-up. However, it must be noted that the lack of formal tests to objectively measure the possible effect of the treatment regimen on the patients’ cognition is a limitation of this study.
Along with salvage radiotherapy, most patients in this study were given a combination of rituximab and temozolomide. Batchelor and colleagues investigated rituximab monotherapy (median of six cycles) as salvage therapy in 12 patients, which achieved a 36% response rate (CR 33%, PR 8%), and a median survival of 20.9 months, with modest toxicity effects (allergic reaction, fatigue, anxiety, back pain).10 A phase II trial including 36 patients evaluated radiographic response of single-agent temozolomide as salvage therapy and yielded a 31% response rate (CR 25%, PR 6%) with negligible toxicity, however median survival was only 3.9 months.15 Another study including 17 patients that failed first-line HD-MTX treatment and were given temozolomide (median of two cycles) showed an objective response of 47% (CR 29%, PR 29%) and a median survival of 6.7 months. Neutropenia and thrombocytopenia developed in 6%.16 A combination of both agents were used as salvage therapy in a 2004 study by Wong and colleagues, which achieved a 100% response rate (CR 71%, PR 29%), however this population-limited study enrolled patients with systemic lymphoma with intracranial recurrence (n = 3) as well as patients with newly-diagnosed (n = 1) and recurrent PCNSL (n = 3). In the same year, Enting and colleagues published a similar study that showed a 53% response rate (CR 40%, PR 13%) and a median survival of 14 months.5 A phase II trial enrolling 16 patients was conducted from 2005 to 2008, which showed an initial response rate of 35.7% (CR 14%, PR 21%), however on subsequent follow-up, CR was found in only 14%. This study was terminated early for reasons of slow accrual and preliminary data showing futility, so median overall survival was not reached.17
Novel agents like ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor which inhibits B-cell receptor-dependent nuclear factor κB (NF-κB) activation, are being studied as well. A retrospective study of 14 patients with lymphoma (13 with rrPCNSL) had a response rate of 50% (CR 21%, PR 29%) with ibrutinib salvage monotherapy. Adverse events included neutropenic fever, diarrhea, and peritumoral hemorrhage after failure of ibrutinib.18 A recent open-label non-randomized clinical trial of ibrutinib monotherapy showed 76.9% response (CR 38%, PR 38%) among 13 patients with rrPCNSL. The median overall survival was 15 months.19 A phase II trial by Soussain and colleagues using the same regimen was recently concluded. Among 44 patients, an overall response rate of 59% was demonstrated with an overall survival of 19.2 months. Adverse events were present in half of patients.20
Our study showed a 93.3% response rate (CR 73.3%, PR 20%), which is comparable to previous studies. This may be owing to the multi-modal treatment approach which employs different yet complementary mechanisms in treating PCNSL (Figure 3). Radiation promotes apoptosis through inducing double-strand breaks in the DNA. It has also been shown to be synergistic with immunotherapy through release of tumor antigens with cell lysis, promoting T-cell cytotoxicity by upregulating MHC (major histocompatibility complex) on tumor cells, and stimulating phagocytosis of damaged tumor cells.21 Rituximab is a CD20-targeted monoclonal antibody that induces apoptosis, causes cytotoxicity via antigen-dependent cell-mediated and complement-mediated pathways, and downregulation of IL-10 transcription, resulting in downstream inactivation of STAT3 and reduced expression of the anti-apoptotic gene Bcl-2.22,23 It is through the latter mechanism that rituximab plays a role in the sensitization of CD20 + B lymphoma cells to cytotoxic chemotherapy, which makes it useful in combination with other regimens on top of its tolerability.9,24 On the other hand, temozolomide is a well-tolerated alkylating agent, whose active form MTIC (5-(3-methyl-1-triazene) imidazole-4-carboxamide) binds to purine sites on DNA, evidently resulting in cell death. It also depletes the DNA repair enzyme MGMT (O-6 methylguanine-DNA methyltransferase) in various cell types and is able to cross the blood-brain barrier. Additionally, it provides additional cytotoxicity when given with concomitant radiotherapy.25
Figure 3.
Synergism of rituximab, temozolomide, and radiation in the treatment of primary CNS lymphoma. Rituximab acts on the CD20 receptor and induces cytotoxicity by increasing anti-tumor antigenicity, cell apoptosis by inhibiting NF-KB pathway among others, and chemosensitization by downregulating Bcl-2. Temozolomide and radiation inhibit DNA replication, evidently causing cell death. Radiation also increases tumor antigenicity through the release of tumor antigens during apoptosis. Both rituximab and radiotherapy may increase tumor cell detection through their indirect antigenic effect. Chemosensitization from rituximab and tumor irradiation may also potentiate the cytotoxicity of temozolomide. BTK, Bruton tyrosine kinase; IL-10, interleukin-10; JAK, Janus kinase; MTIC, 5-(3-methyl-1-triazene)-imidazole-4-carboxamide; NF-KB, nuclear factor KB; STAT, signal transducer and activator of transcription.
In comparison to previous case series on treatment of rrPCNSL, our study shows relatively higher response and survival rates. It is difficult to attribute this all primarily from treatment efficacy due to intrinsic patient characteristics that may favor a better prognosis prior to salvage therapy. It can be noted that included patients had high KPS scores prior to treatment, which may give selective advantage over rrPCNSL patients who are clinically more advanced. Though there is no consensus on having a low KPS as an absolute contraindication for salvage chemotherapy, there is a theoretical possible induction of more harm to the patient if subjected to toxic effects of chemotherapy without clear benefit for improvement. Secondly, MGMT status is not routinely obtained for lymphoma patients, which when methylated may confer higher sensitivity of the tumor to temozolomide therapy, which is the case with other brain tumors treated with temozolomide.26 Therefore, there might be a possibility that rrPCNSL patients who respond well to temozolomide salvage therapy may harbor this methylation status, and thus may skew the data to have more favorable results. It is thus prudent that in future studies, MGMT methylation status may be obtained prior to temozolomide initiation.
In connection, while our study presented positive findings, there are important limitations that restrict generalizability of the results. The type of study is a primary limitation. Due to its retrospective nature, no comparison to gold standard or placebo treatment is possible, which makes verification of positive results difficult. Secondly, due to the rarity of PCNSL with even lower incidence of recurrence/relapse, a high population size for any study may be challenging to achieve. In addition, upfront treatment of newly diagnosed cases of PCNSL may not always be done in the Philippines since chemotherapy financed mostly out-of-pocket—either the patients decide on palliative care or most do not finish the complete course of HD-MTX especially when rituximab is added to the initial regimen. Therefore, most patients are inadequately treated and by definition cannot be properly identified to be recurrent or relapsed once their disease progresses. Lastly, in this particular study, no formal measurement was done for quality of life (QoL), which limits how the good survival rates can be translated to better quality of life.
While this study is primarily limited by its size, this is the first study to date that documents the treatment response among patients with rrPCNSL given a combination of radiotherapy, rituximab, and temozolomide as salvage therapy. The median overall survival was not reached due to more than half of patients being alive at the time of study completion. Median follow-up time of 84 months among patients given this treatment regimen is significantly longer compared to the studies currently published in literature. More importantly, the regimen is tolerated well by the patients across various age groups.
We conclude that the combination of radiotherapy, rituximab, and temozolomide is a safe and effective regimen among patients with rrPCNSL. Given the documented outcomes from this review, we believe that this treatment regimen should be strongly offered and considered among patients who relapse or progress with the disease. Ultimately, the management should be multidisciplinary, individualized, and based on current available evidence.
Contributor Information
Julette Marie F Batara, Institute for Neurosciences, St. Luke’s Medical Center, Quezon City and Global City, Philippines; Department of Neurosciences, College of Medicine and Philippine General Hospital, University of the Philippines Manila, Manila, Philippines.
Almira Doreen Abigail O Apor, Department of Neurosciences, College of Medicine and Philippine General Hospital, University of the Philippines Manila, Manila, Philippines.
Christianne V Mojica, Institute for Neurosciences, St. Luke’s Medical Center, Quezon City and Global City, Philippines.
Mark Willy L Mondia, Department of Neurosciences, College of Medicine and Philippine General Hospital, University of the Philippines Manila, Manila, Philippines.
Previous Presentations
The abstract of this study was presented as oral presentation at the 6th Quadrennial Meeting of the World Federation of Neuro-Oncology Societies (WFNOS 2022).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest. The authors have nothing to disclose.
Authorship Statement. Conceptualization: JMFB; Data curation: JMFB, CVM, ADAOA, MWM; Interpretation of data: JMFB, CVM, ADAOA, MWM; Formal analysis: JMFB, CVM, ADAOA; Writing-original draft: JMFB, CVM, ADAOA, MWM; Writing-review: JMFB, CVM, ADAOA, MWM; Editing: JMFB, CVM, ADAOA, MWM; Figures: ADAOA.
References
- 1. O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am J Hematol. 2013;88(12):997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farrall AL, Smith JR. Changing incidence and survival of primary central nervous system lymphoma in Australia: a 33-year national population-based study. Cancers. 2021;13(3):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shin S-H, Jung K-W, Ha J, et al. Population-based incidence and survival for primary central nervous system lymphoma in Korea, 1999–2009. Cancer Res Treat. 2015;47(4):569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enting RH, Demopoulos A, DeAngelis LM, Abrey LE. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63(5):901–903. [DOI] [PubMed] [Google Scholar]
- 6. Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80(2):159–165. [DOI] [PubMed] [Google Scholar]
- 7. Holdhoff M, Wagner-Johnston N, Roschewski M. Systemic approach to recurrent primary CNS lymphoma: perspective on current and emerging treatment strategies. Onco Targets Ther. 2020;13:8323–8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan Y, Ding T, Wang S, et al. Current and emerging therapies for primary central nervous system lymphoma. Biomark Res. 2021;9(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong ET, Tishler R, Barron L, Wu JK. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer. 2004;101(1):139–145. [DOI] [PubMed] [Google Scholar]
- 10. Batchelor TT, Grossman SA, Mikkelsen T, et al. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76(10):929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khimani NB, Ng AK, Chen YH, et al. Salvage radiotherapy in patients with recurrent or refractory primary or secondary central nervous system lymphoma after methotrexate-based chemotherapy. Ann Oncol. 2011;22(4):979–984. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen PL, Chakravarti A, Finkelstein DM, et al. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23(7):1507–1513. [DOI] [PubMed] [Google Scholar]
- 13. Hottinger AF, DeAngelis LM, Yahalom J, Abrey LE. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69(11):1178–1182. [DOI] [PubMed] [Google Scholar]
- 14. Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643–5646. [DOI] [PubMed] [Google Scholar]
- 15. Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96(6):864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makino K, Nakamura H, Hide T, Kuratsu J. Salvage treatment with temozolomide in refractory or relapsed primary central nervous system lymphoma and assessment of the MGMT status. J Neurooncol. 2012;106(1):155–160. [DOI] [PubMed] [Google Scholar]
- 17. Nayak L, Abrey LE, Drappatz J, et al. Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk Lymphoma. 2013;54(1):58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chamoun K, Boyle E, Houillier C, et al. Ibrutinib monotherapy in relapsed/refractory CNS lymphoma: a retrospective case-series. Neurology. 2017;88(1):101–102. [DOI] [PubMed] [Google Scholar]
- 19. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase II “proof-of-concept” iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) net. Eur J Cancer. 2019;117:121–130. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Liu Z-G, Yuan H, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res. 2019;25(6):1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tao K, Wang X, Tian X. Relapsed primary central nervous system lymphoma: current advances. Front Oncol. 2021;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pescovitz MD. Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6(5p1):859–866. [DOI] [PubMed] [Google Scholar]
- 24. Jazirehi AR, Bonavida B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti-CD20 mAb) in non-Hodgkin’s lymphoma: implications in chemosensitization and therapeutic intervention. Oncogene. 2005;24(13):2121–2143. [DOI] [PubMed] [Google Scholar]
- 25. Reni M, Mason W, Zaja F, et al. Salvage chemotherapy with temozolomide in primary CNS lymphomas: preliminary results of a phase II trial. Eur J Cancer. 2004;40(11):1682–1688. [DOI] [PubMed] [Google Scholar]
- 26. Alnahhas I, Alsawas M, Rayi A, et al. Characterizing benefit from temozolomide in MGMT promoter unmethylated and methylated glioblastoma: a systematic review and meta-analysis. Neuro-Oncol Adv. 2020;2(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]