Abstract
The ongoing 2022 multicountry outbreak of monkeypox is the largest in history to occur outside of Africa. Monkeypox is an emerging zoonotic disease that for decades has been viewed as an infectious disease with significant epidemic potential because of the increasing occurrence of human outbreaks in recent years. As public health entities work to contain the current outbreak, healthcare professionals globally are aiming to become familiar with the various clinical presentations and management of this infection. We present in this review an updated overview of monkeypox for healthcare professionals in the context of the ongoing outbreaks around the world.
Keywords: emerging infectious diseases, monkeypox, outbreak
Monkeypox is an emerging zoonosis of global health concern. Healthcare professionals worldwide should become familiar with the clinical presentations and management of this viral infection in light of contemporary outbreaks.
The 2022 outbreak of monkeypox involving multiple countries in both endemic and nonendemic regions has generated significant international interest [1, 2]. A once-neglected zoonotic virus endemic to West and Central Africa, monkeypox virus was first identified in 1958 [3] in nonhuman primates kept for research in Denmark [3]. The first case in humans was reported in 1970 in the Democratic Republic of Congo [4]. Over the past 50 years, sporadic outbreaks have been reported mainly in African countries, with several thousand human cases recorded to date. Occasional cases and limited outbreaks linked to travel or importation of animals harboring the virus have also been described in nonendemic countries [5]. It has long been a theoretical concern that monkeypox virus and other zoonotic poxviruses could over time expand to fill the ecological niche once occupied by the closely related variola virus [6, 7]. The combined effects of deforestation, population growth, encroachment on animal reservoir habitats, increasing human movement, and enhanced global interconnectedness have made this possibility more real in the last 20 years [6–8].
With increasing case numbers being reported in the current outbreak, it is important for clinicians everywhere to update their knowledge of this zoonotic infection, including its prevention, clinical management, prophylaxis, and basics of infection control, to understand the broader implications of the current outbreak. In this review, we provide an overview of monkeypox virus infection to serve as a primer for healthcare professionals who may encounter this condition in their practice.
VIROLOGY
The Poxviridae family are double-stranded deoxyribonucleic acid viruses which infect a range of animals including birds, reptiles, insects and mammals. The family consists of 2 subfamilies: Chordopoxvirinae (with 18 genera and 52 species) and Entomopoxvarinae (with 4 genera and 30 species). Monkeypox belongs to the Poxviridae family, the Chordopoxvirinae subfamily, and the genus Orthopoxvirus [9–11]. Several poxvirus species have been shown to cause human infections including Variola (smallpox), Cowpox, Monkeypox, Vaccinia, Camelpox, Alaskapox, Yaba monkey tumor virusTanapox virus, Orf virus, Pseudocowpox virus, Bovine papular stomatitis virus, Buffalopox and Molluscum contagiosum. Humans are the reservoir host of Variola and Molluscum contagiosum viruses [11]. Monkeypox virus (MPXV) has a wide range of potential host organisms, which has allowed it to circulate in wild animals for a prolonged period of time while sporadically causing human disease through spillover events [9]. More importantly, Orthopoxviruses (OPXV) exhibit immunological cross-reactivity and cross-protection, and infection with any member of the genus confers some protection from infection with any other members of the same genus [12, 13].
Orthopoxviruses are large (size range:140–450 nm) viruses with a brick-like structure and a genome consisting of approximately 200-500kbp kb [6, 9, 10] that codes for over 200 genes. Many of the genes encoded by the OPXV genome are not essential for virus replication in cell culture but might play important roles in the host antiviral response [10, 14]. All poxviruses complete their replication cycle in the cytoplasm of infected cells via complex molecular pathways [10, 15]. This intracellular replication cycle has been well characterized for Vaccinia virus, which was used to develop the vaccine that helped to eradicate smallpox globally; key features of this replication cycle are similar for all poxviruses [10, 15]. The infection cycle can be initiated by two distinct forms of the virus: the intracellular mature virion and the extracellular enveloped virion, which differ in their expression of surface glycoproteins. Glycosaminoglycans, which are ubiquitously expressed on the surface of mammalian cells, are thought to be crucial for binding of the virion to the cell membrane, although all cellular receptors have not been fully characterized [10, 15]. A detailed description of the replication cycle is beyond the scope of this review but has been described previously [10, 15].
Smallpox is estimated to have caused millions of fatalities worldwide [13] and was one of the most dreaded infectious diseases in human history. The impact of smallpox serves as a reminder that OPXV can be formidable pathogens. Although the origins of smallpox are not known, there is some evidence which suggests that Variola virus may have evolved from an ancient rodent poxvirus thousands of years ago [16]. The increasing danger of zoonotic OPXV infections such as MPXV has been recognized for a long time [17, 18]. As a consequence of immunization programs against smallpox ending over 40 years ago, a significant proportion of the global population does not have immunity against smallpox and zoonotic OPXV. All of this raises the possibility that given the right conditions, such as increasing incidence of human infections and long-term absence of vaccine immunity, a zoonotic orthopoxvirus like MPXV could acquire the ability to more efficiently transmit between humans and cause larger outbreaks [17].
EPIDEMIOLOGY AND HISTORICAL OUTBREAKS
Monkeypox is endemic in the tropical rainforest regions of Central and West African countries, notably Cameroon, Central African Republic, Cote d’Ivoire, Democratic Republic of the Congo (DRC), Gabon, Liberia, Nigeria, Republic of the Congo and Sierra Leone. Most cases arise sporadically or occur in the context of localized outbreaks [6–8]. Cases outside of endemic countries are typically linked to international travel or importation of animals infected with MPXV [5, 19]. Before 2022, cases outside of Africa had previously been reported in the United States, United Kingdom (UK), Israel, and Singapore [7]. There are 2 distinct genetic clades of the MPXV: the Central African clade and the West African clade [8]. Infection with the West African clade typically results in a more self-limited disease, with case-fatality ratios estimated to be approximately 3–6%, whereas the Central African (Congo Basin) clade has historically been associated with higher transmissibility and case-fatality ratios as high as 10% [8]. Cameroon is the only country where both clades have been confirmed [20, 21]. Cumulatively, more suspected cases of the Central African clade have been reported to date than cases due to the West African clade due to the high number of cases recorded in historical and ongoing outbreaks in the DRC [21]. The West African clade of MPXV has been isolated from cases in newly affected countries in the 2022 multi-country outbreaks
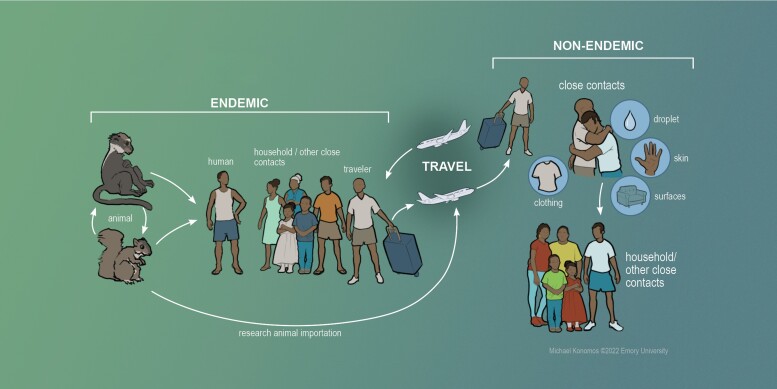
The transmission of monkeypox in endemic and nonendemic settings is summarized in Figure 1. Animal-to-human transmission occurs via bites and scratches from infected animals. Preparation and handling of infected animal products (bushmeat [22]) may also result in transmission. The definitive animal reservoir of MPXV has not been identified. The virus has been isolated from several animal species including small mammals and nonhuman primates. In the reported instances in which MPXV has been isolated from wild animals, the animals demonstrated pox-like lesions consistent with active infection [23–26]. It is not known whether asymptomatic carriage of MPXV occurs in animal reservoir. Serological surveys have been conducted on wild mammals in endemic regions. These studies have found several animal species with detectable antibodies to OPXV in the absence of detectable viremia on polymerase chain reaction (PCR) testing. This suggests exposure to and circulation of zoonotic OPXV in many wild animal species [27]. Human-to-human transmission is thought to occur via direct skin-to-skin contact with lesions on the skin, as well as through indirect contact with contaminated fomites, such as bedding or clothing [8]. Transmission can also occur at close proximity through exchange of respiratory secretions containing live virus.
Figure 1.
Transmission of human monkeypox. In endemic countries, spillover events occur from zoonotic animal reservoirs into humans, potentially leading to limited outbreaks usually facilitated by close human contact. Outbreaks can also occur in nonendemic regions through introduction of the virus via human travel or importation of animals harboring the virus. Subsequent human-to-human transmission can then occur via household contacts and via other close contacts.
In the last 5 decades, the DRC has been most affected by monkeypox outbreaks, whereas the second and third most affected countries have been Nigeria and Republic of the Congo, respectively. Table 1 shows a timeline of outbreaks of human monkeypox cases reported since 1970, when the first human case was detected in a 9-months-old patient in a remote village in the DRC [4]. After the historical success of the global eradication of smallpox during the 1970s, surveillance for pox-like diseases was enhanced in tropical rainforest areas, which has aided in identifying monkeypox outbreaks as they have occurred over time. More importantly, smallpox vaccination, which stopped being universally administered in the 1970s, confers cross-protection against MPXV infection. It is possible that the progressive loss of immunity against smallpox over time combined with the other factors described above has contributed to the increase in sporadic cases and outbreaks globally over the last 15 years [18].
Table 1.
Outbreaks of Monkeypox: 1970–2021
| Decade | 1970–1979 | 1980–1989 | 1990–1999 | 2000–2009 | 2010–2019 | 2020–2021 | Total Cases | Deaths | References |
|---|---|---|---|---|---|---|---|---|---|
| Endemic Countries | Number of Cases Reported* by Decade | ||||||||
| Cameroona | 1 | 1 | —b | — | 16 | — | 18 | N/A | [28–32] |
| Central African Republic | — | 8 | — | — | 61 | — | 69 | 0 | [7, 21, 33–38] |
| Côte d’Ivoire | 1 | — | — | — | — | — | 2 | 0 | [4, 39] |
| DRC* | 38 | 343 | 511 | 10 027 | 18 788 | 7374 | 37 081 | N/A | [4, 18, 22, 39–50] |
| Gabon | — | 4 | 9 | — | — | — | 13 | N/A | [51–53] |
| Liberia | 4 | — | — | — | 6 | — | 10 | N/A | [54, 55] |
| Nigeria | 4 | — | — | — | 228 | 42 | 274 | N/A | [6, 56, 57] |
| Republic of the Congo | — | — | — | 73 | 24 | — | 97 | N/A | [58–60] |
| Sierra Leone | 1 | — | — | — | 2 | — | 3 | 0 | [61–64] |
| South Sudan | — | — | — | — | 19 | — | 19 | 0 | [65] |
| Nonendemic Countries | |||||||||
| Israel | — | — | — | — | 1 | — | 1 | 0 | [66] |
| Singapore | — | — | — | — | 1 | — | 1 | 0 | [67] |
| United Kingdom | — | — | — | — | 4 | — | 4 | 0 | [68, 69] |
| United States | — | — | — | 47 | — | 2 | 49 | 0 | [5, 19, 70, 71] |
Abbreviations: DRC, Democratic Republic of the Congo; N/A, data not available or unclear.
*Reported cases include confirmed, probable, and/or suspected. All DRC cases from 2000 to 2009 and 2010 to 2019 were suspected cases. .
Cameroon is the only country where both viral clades have been reported in humans.
Dash denotes a period without reported outbreaks.
CLINICAL PRESENTATION
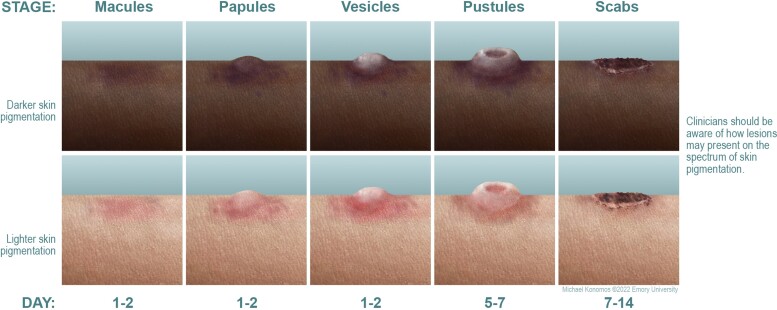
In the context of exposure and in the sylvatic setting persons are at increased risk for developing monkeypox if they live in forested areas and are male gender, are less than 15 years of age, and are not immune to smallpox [18]. Historically, patients have typically presented with prodromal symptoms, including fever, headaches, chills, malaise, and lymphadenopathy, followed by development of a characteristic rash (Figure 2). The rash usually starts in the mouth, and then spreads to the face and extremities, including the palms and soles. Each lesion begins as a macule and then progresses to papules, vesicles, pustules, and scabs (Figure 3). Pain can be prominent, but it is not universally present, and pruritus can occur when the lesions are in the healing stage. Unlike chickenpox, skin lesions due to monkeypox tend to be similar in size and typically present at the same stage. The number of lesions can range from 10 to 150 and can persist for up to 4 weeks [72]. Patients are infectious from the time symptoms start (presumed to include prodromal symptoms before the appearance of the rash) until the lesions scab and fall off, with a new layer of skin being formed [8]. In rare instances, patients can experience complications of monkeypox, such as bacterial superinfection, encephalitis, pneumonitis, and conjunctivitis/keratitis [8, 40]. The timeframe for developing complications and their rates have not been systematically determined [73].
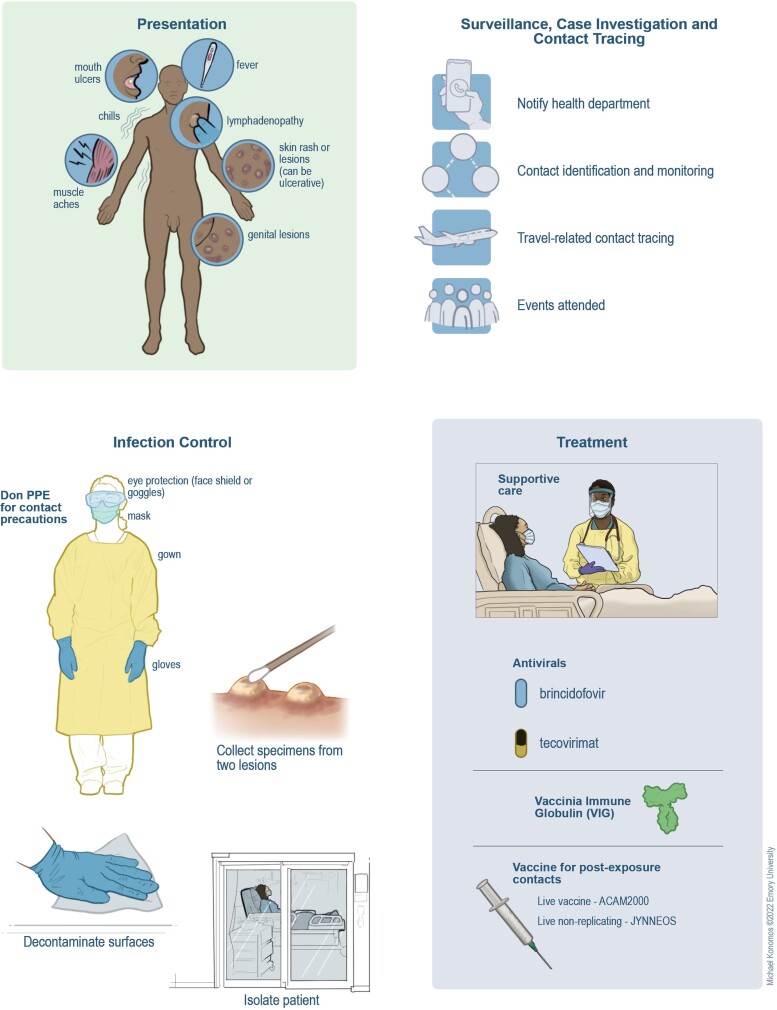
Figure 2.
Clinical protocols and infection control during monkeypox outbreaks. Recognizing the clinical presentation and having a high index of suspicion are crucial for identifying potential cases. Rapid confirmation through testing and isolation of individuals with suspected or confirmed disease are necessary. These actions should be taken alongside notification of public health authorities for contact tracing and other components of the public health response. Although treatment for monkeypox infection is primarily supportive, antiviral agents and immune therapies could be considered for individuals with moderate to severe symptoms or who are at high risk for progression to severe disease. Vaccinating close contacts with high-risk exposures and at-risk individuals is also important for interrupting transmission chains and containing monkeypox outbreaks. Appropriate personal protective equipment (PPE) for medical settings consists of gown, gloves, a fit-tested N-95 or equivalent, and eye protection.
Figure 3.
Stages of skin presentation and progression of monkeypox rash.
Many features of monkeypox are similar to smallpox [74]; however, in contrast to smallpox, monkeypox is often milder and presents with lymphadenopathy, which was typically absent in smallpox infection. It is also important to note that the cutaneous manifestations of monkeypox can be confused with other infections, including chickenpox, molluscum contagiosum, herpes simplex virus, syphilis, impetigo, measles in the early stages, and rickettsial diseases.
In the current 2022 outbreak, the presentation of monkeypox has had atypical features in many patients. For example, the characteristic rash is still present, but it can be limited to the genital, perigenital, and perianal areas and present at different stages of development [75]. In addition, patients may present with only mild or absent prodromal symptoms which may begin afteerr onset of a localised rash [75]. Therefore, it is crucially important to consider a wide spectrum of disease presentations as clinicians aim to accurately diagnose patients while the world attempts to contain the outbreak.
DIAGNOSIS
It is important to have a high index of suspicion for monkeypox infection and to be aware of the sometimes atypical presentations of the infection that have been described in the ongoing 2022 outbreak. When there is clinical suspicion for monkeypox, clinicians should ask about travel and sexual history and about any close contacts with people with a similar rash or suspected or confirmed monkeypox infection. Behaviors associated with close contact include sleeping in the same room, drinking or eating from the same container, living in the same residence, etc [7]. More importantly, absence of travel history or absence of a specific known close contact with a rash or with suspected or confirmed monkeypox infection should not exclude the possibility of this diagnosis. A thorough skin examination should also be performed.
The optimal diagnostic procedure for a patient with suspected active monkeypox infection is to obtain a specimen from a skin lesion to send for molecular testing by PCR. Ideally, more than 1 specimen should be obtained from 2 separate lesions on different parts of the body, and lesions should be unroofed to adequately sample virus containing secretions. Certain laboratories can perform direct PCR testing for MPXV specifically, whereas others perform generic OPXV testing that requires confirmatory testing for MPXV at a reference laboratory. However, in the context of the current outbreak, a positive OPXV test can reasonably be concluded to represent a diagnosis of monkeypox infection before results from confirmatory testing are available. Testing plans should ideally be coordinated with public health authorities in advance of specimen collection.
Cell culture provides virus strains for further characterization, but it is restricted to accredited biosafety level 3 reference laboratories [76]. Serological testing can potentially be helpful in epidemiologic investigations, retrospective diagnosis of past infections, and diagnosis of late clinical manifestations, such as encephalitis. MPXV serology can cross-react with prior smallpox vaccination, but this is not a concern in unvaccinated individuals [77].
CLINICAL MANAGEMENT
The mainstay of clinical management for typical monkeypox infection is supportive care [73] (Figure 2). Supportive care [73] includes maintenance of adequate fluid balance (because of the possibility of increased insensible fluid losses from the skin, decreased oral intake, and vomiting or diarrhea). Other measures such as hemodynamic support, supplemental oxygen, or other respiratory support and treatment of bacterial superinfections of skin lesions should be considered where indicated. Another aspect of supportive care that has been described with previous OPXV infections is management of ocular infection/complications, specifically resulting in corneal scarring and/or loss of vision [78]. Potential approaches to consider in this situation include early involvement of ophthalmology experts, application of lubricants, topical antibiotics, and possibly topical antivirals such as trifluridine [78].
At the present time, there are no US Food and Drug Administration (FDA)-approved treatments specifically for monkeypox. However, there are antiviral agents that have activity against MPXV [79], including cidofovir, brincidofovir (a lipid-conjugate prodrug of cidofovir), and tecovirimat [80]. In addition to antiviral agents, vaccinia immune globulin intravenous (VIGIV) has been previously approved by the FDA for treatment of complications due to vaccinia vaccination, such as progressive vaccinia and severe generalized vaccinia [81]. A summary of these treatment options is presented in Table 2.
Table 2.
Potential Treatment Options for Monkeypox Infection
| Therapy | Mechanism of Action | Typical Dosing | Formulation | FDA Approval Status | Side Effects and Adverse Events |
|---|---|---|---|---|---|
| Cidofovir | Blocks viral DNA synthesis through competitive inhibition of DNA polymerase | 5 mg/kg per dose once weekly for ≥2 doses (with concomitant probenecid) | IV; off-label: topical, intravesicular | CMV retinitis in patients with AIDS [82] (1996) | Nephrotoxicity; neutropenia; decreased intraocular pressure, nausea, vomiting |
| Brincidofovir | Lipid conjugate prodrug of cidofovir | 4 mg/kg once weekly for 2 doses (max 200 mg/dose) | Oral | Smallpox (2021) [83] | Abdominal pain, nausea, vomiting, diarrhea, elevated liver transaminases and bilirubin |
| Tecovirimat | Inhibits activity of the protein VP37, which prevents creation of virions that can be released from an infected host cell, thereby preventing replication and dissemination within the host | IV: 35 to <120 kg: 200 mg q12 hours ≥120 kg: 300 mg q12 hours Oral: 40 to <120 kg: 600 mg q12 hours ≥120 kg: 600 mg q8 hours All regimens for 14 days |
IV and oral (off-label topical) [84] | Smallpox (2018) [85] | IV: pain and swelling at infusion site; extravasation at infusion site; headache [86] Oral: headache, abdominal pain, nausea, vomiting |
| VIGIV | Passive immunity through OPXV-specific antibodies collected from pooled human plasma of persons immunized with smallpox vaccine | 6000 units/kg as a single dose (up to 9000 units/kg) Dose can be repeated depending upon symptoms | IV | Complications of vaccinia vaccination (progressive vaccinia, severe generalized vaccinia, etc) (2005) [87] | Infusion reaction; local injection-site reaction (contraindicated in persons with IgA deficiency and possible IgA hypersensitivity) |
Abbreviations: AIDS, acquired immunodeficiency syndrome; CMV, cytomegalovirus; DNA, deoxyribonucleic acid; FDA, US Food and Drug Administration; IgA, immunoglobulin A; IV, intravenous; Max, maximum; VIGIV, vaccinia immunoglobulin intravenous.
Currently, tecovirimat, cidofovir, and VIGIV are available from the Strategic National Stockpile under Expanded Access Investigational New Drug (EA-IND) protocols held by the Centers for Disease Control and Prevention (CDC) for treatment of OPXV infections in an outbreak scenario [88]. In the United States, these medications can be accessed through the CDC via requests from state and territorial health departments. As of this writing, the CDC is developing EA-IND for use of brincidofovir for treatment of OPXV infections [88]. The optimal clinical management of human MPXV infection is not clearly established. Large-scale, randomized controlled trials of antivirals for the treatment of OPXV infections are not available. Current drug approvals and treatment approaches are based on in vitro data, animal studies, human pharmacokinetic and pharmacodynamic data, case reports, and case series [80, 81, 89–92]. The historical clinical use of available therapeutic agents in the context of human OPXV infections is reviewed below and summarized in Table 3.
Table 3.
Clinical Use of Antiviral Agents and Vaccinia Immunoglobulin for Treatment of Orthopoxvirus Infections
| Type of OPXV | Year | Clinical Scenario | Cidofovir | Brincidofovir | Tecovirimat | VIGIV | Reference |
|---|---|---|---|---|---|---|---|
| Vaccinia | 1987 | Progressive vaccinia in a patient with undiagnosed HIV/AIDS | (−) | (−) | (−) | (+) (given IM) | [93] |
| Vaccinia | 2008 | 28-month-old with severe eczema vaccinatum after contact with family member who received smallpox vaccination | (+) | (−) | (+) | (+) | [94] |
| Vaccinia | 2012 | Progressive vaccinia in a patient with undiagnosed AML | (−) | (+) | (+) (also administered topically) | (+) | [84] |
| Vaccinia virus | 2013 | Cluster of cases with secondary and tertiary transmission after sexual contact with a recipient of smallpox vaccination (2 total cases) | (−) | (−) | (−) | (+) | [95] |
| Cowpox | 2015 | Severe ocular infection in a patient with underlying atopic dermatitis | (−) | (−) | (+) | (−) | [96] |
| Cowpox | 2016 | 17-year-old with a kidney transplant with fatal disseminated infection | (+) | (+) | (−) | (+) | [97] |
| Vaccinia | 2019 | Prophylactic treatment of potential severe vaccinia infection in a patient with undiagnosed AML | (−) | (−) | (+) | (+) | [98] |
| Vaccinia | 2019 | Patient with Crohn’s disease with secondary vaccinia lesions | (−) | (−) | (+) | (+) | [99] |
| Vaccinia | 2019 | Patient with acne and secondary vaccinia lesions | (−) | (−) | (+) | (+) | [98, 100] |
| Cowpox | 2019 | Severe keratoconjunctivitis due to cowpox | (−) | (−) | (+) | (−) | [98, 100] |
| Vaccinia | 2019 | Laboratory worker who had a needlestick exposure | (−) | (−) | (+) | (+) | [101] |
| Cowpox | 2021 | Severe orbital infection due to contact with an infected cat | (−) | (−) | (+) | (−) | [102] |
| MPXV | 2022 | Travel-associated case of MPXV infection in the United States | (−) | (−) | (+) | (−) | [19] |
| MPXV | 2022 | MPXV infection in travelers returning to the United Kingdom (7 total patients) |
(−) | (+) (3 of 7 patients) | (+) (1/7 patients) | (−) | [89] |
| MPXV | 2022 | Initial report on cases related to 2022 outbreak of MPXV infections in the United States (17 total patients) | (−) | (−) | (+) (1/ 17 patients) |
(−) | [103] |
Abbreviations: AIDS, acquired immunodeficiency syndrome; AML, acute myeloid leukemia; HIV, human immunodeficiency virus; MPXV, monkeypox virus; IM, intramuscular; VIGIV, vaccinia immunoglobulin intravenous; (+), medication used; (−), medication not used.
Cidofovir
Cidofovir was approved by the FDA in 1996 for the treatment of patients with retinitis caused by cytomegalovirus (CMV) in patients with the acquired immunodeficiency syndrome. Cidofovir has broad antiviral activity against viruses from different families, including herpes viruses, adenovirus, and OPXV. With regards to its use in OPXV infections, cidofovir was used as part of the treatment regimen for a 28-month-old boy with refractory atopic dermatitis who developed severe eczema vaccinatum after being in contact with his father, who had received smallpox vaccination [94]. The child survived without long-term sequelae [94].
Brincidofovir
Brincidofovir was approved by the FDA for the treatment of smallpox infection in June 2021 [83]. It has previously been used in patients with CMV infection [104], adenovirus [105], and OPXV infections. Brincidofovir was used as part of a combination therapy regimen for a patient who received smallpox vaccination and was diagnosed with acute myeloid leukemia (AML) shortly thereafter [84]. After induction chemotherapy, the patient developed progressive Vaccinia and was treated with multiple drugs, including 6 doses of brincidofovir. This agent was also used in the treatment of a 17-year-old kidney-transplant recipient with ultimately fatal disseminated cowpox virus infection [97]. In May 2022, Adler et al [89] described clinical management of 7 patients with MPXV infection in the UK. In this case series, 3 patients received brincidofovir and all of 3 patients developed elevated liver enzymes, a well described side effect associated with brincidofovir use, which led to cessation of treatment [89].
Tecovirimat
Tecovirimat was approved by the FDA in 2018 for the treatment of smallpox infection [106]. It was also approved by the European Medicines Agency in January 2022 for treatment of smallpox and cowpox [107]. It has been used in several case reports for the treatment of disseminated and ocular infections with cowpox [96, 97, 102] and Vaccinia infection as part of a multidrug treatment regimen [84, 94, 100]. Tecovirimat was also used as prophylaxis to prevent development of progressive Vaccinia in a 19-year-old man who had received smallpox vaccination and was diagnosed with AML soon after vaccination [98]. In this case, tecovirimat was used continuously for 61 days specifically as prophylaxis while the patient was receiving chemotherapy for leukemia. The patient developed skin lesions due to inadvertent autoinoculation after vaccination, without progression or dissemination. Tecovirimat has also been used in a patient with keratoconjunctivitis due to cowpox, although a detailed clinical description of this case is not available [100]. Tecovirimat was also used in a laboratory worker who had a needlestick exposure to Vaccinia virus [101]. Other instances in which tecovirimat has previously been used are outlined in Table 3. With regards to MPXV infections specifically, tecovirimat was used to treat a patient with a travel-associated case of monkeypox in the United States in 2021 [19]. In July 2021, an expanded access protocol was announced for the Central African Republic, with a plan for 500 courses of tecovirimat to be used for treatment of monkeypox [108]. In the recent case series by Adler et al [89], 1 of 7 patients received tecovirimat for 2 weeks. The patient experienced no adverse effects and had a shorter duration of viral shedding and illness [89]. Finally, in the CDC’s report on the initial 17 patients with confirmed MPXV infection in the United States during the ongoing 2022 outbreak, 1 patient received tecovirimat [103].
Vaccinia Immune Globulin Intravenous
Vaccinia immune globulin intravenous was approved by the FDA in 2005 for treatment of complications due to vaccination with the Vaccinia virus [109]. Before this, Vaccinia immune globulin was administered as an intramuscular (IM) injection. The historical use of IM Vaccinia immune globulin has been extensively reviewed and summarized [110]. The FDA approval of the intravenous formulation of vaccinia immunoglobulin (VIGIV) has been used in several published reports for human OPXV infections. Many of the patients described in the case reports outlined in Table 3 also received VIGIV in combination with antivirals for treatment of their OPXV infections [84, 94, 95, 98–101]. Vaccinia immune globulin administered intravenously was additionally used in a patient with inflammatory bowel disease who developed infection after eexposureee to a vaccinia-rabies glycoprotein recombinant virus used in animal bait to help control the spread of rabies in the animal population. It was also used to treat two patients who developed symptomatic Vaccinia infection through secondary and tertiary transmission after initial sexual contact between a smallpox vaccine recipient and one of the case-patients [95].
Overall, the natural history of MPXV infection in humans is mild to moderate disease with a self-limited course for many patients. Antiviral therapy should be considered for the following; severe illness requiring hospitalization; ocular, oral, and/or perineal involvement; and in patients considered at higher risk for progression to severe disease (immunocompromised, children <8 years of age, pregnant or breastfeeding persons, and the presence of atopic dermatitis or other active exfoliative skin conditions) [88]. The most practical clinical experience is with tecovirimat, which is the preferred antiviral drug. Treatment for MPXV infection should ideally be given in the context of a clinical trial where feasible, to generate long-term evidence that could inform on how best to treat patients in the future. Clinicians are encouraged to coordinate treatment plans and approaches with infectious disease experts and public health authorities.
IMMUNIZATION
Infection with OPXV can confer immunological cross-protection between viruses of the same genus [9, 12, 13]. There are no vaccines specifically designed to protect against monkeypox infection and disease. The vaccines being considered for use (Vaccinia virus-based vaccines) to prevent MPXV were developed for smallpox. In a study conducted in the DRC in the late 1980s [111], the unvaccinated household contacts of individuals with MPXV disease had a secondary attack rate of 9.28% compared to 1.31% for vaccinated contacts. This yielded a rough estimate of 85% protection conferred by prior smallpox vaccination against monkeypox [15, 111].
Before 2019, ACAM2000 was the only OPXV vaccine available in the United States. ACAM2000 is made from a live, replication-competent Vaccinia virus, a member of the OPXV genus. Due to its replication competent property, there is a risk for serious adverse events associated with use of ACAM2000 (eg, progressive vaccinia [84, 112], eczema vaccinatum [113], and myopericarditis [114, 115]).Vaccinia can also be transmitted from a vaccinated person to unvaccinated individuals through close contact with the vaccination site.
By contrast, Jynneos (also known as Imvamune and Imvanex) is a nonreplicating modified Vaccinia Ankara virus vaccine. It was licensed for both prevention of monkeypox and smallpox in the United States in 2019. Unlike ACAM2000, Jynneos does not lead to the production of live virus in vaccinated individuals and, as such, is considered safer for use in immunocompromised individuals. It is important to note, however, that the immune response to Jynneos vaccine can be diminished in immunocompromised patients; therefore, protection might not be as robust as in immunocompetent individuals [116]. Both vaccines are authorized for use in individuals older than 18 years. There are limited data on the efficacy of Jynneos in preventing MPXV in humans. Its efficacy is inferred from vaccine efficacy studies using animal models (prairie dogs and cynomolgus macaques) [117, 118] and safety and immunogenicity studies in humans [119]. A third vaccine, Aventis Pasteur Small Pox Vaccine, is an experimental smallpox vaccine made from replication-competent Vaccinia virus, similar to ACAM2000. It may be used in the United States under Investigational New Drug protocol or via emergency use authorization in circumstances where the other 2 vaccines are not available.
ACAM2000 and Jynneos have been studied as postexposure prophylaxis (PEP) using intranasal challenge animal models [118]. Both vaccines conferred some degree of protection against monkeypox at lower inoculum doses. Administration of vaccine at 1 day postexposure was more effective than administration at 3 days postexposure for Jynneos, but ACAM2000 was similarly effective at either postexposure vaccination timepoint [118]. Vaccination of healthcare workers against monkeypox in the DRC was safely conducted as part of real-world feasiibility and immunogenicity studies with ongoing follow-up [120].
Classification of Exposure Risk and Use of Immunization as Pre-Exposure or Postexposure Prophylaxis
In the current outbreak happening in nonendemic countries, vaccination administered as post-exposure prophylaxis (PEP) for close contacts with high-risk exposures and exposed healthcare workers is underway in several European Union countries the UK, the United States, and Canada and being considered in others [72, 121, 122]. Exposure risk can be classified into three categories: high, intermediate, and low/uncertain [19]. High-risk exposures include direct contact between the exposed person’s broken skin or mucous membranes and “materials, skin, lesions, or body fluids” of a patient [19]. Being in close contact to a patient during an aerosol-generating procedure while not wearing respiratory protection is also considered a high-risk exposure [19]. Intermediate-risk exposures include direct contact between the exposed person’s intact skin and “materials, skin, lesions, or body fluids” of the index patient [19]. Intermediate-risk exposures also include being within six feet of a case-patient for more than three hours or delivering medical care to patients with the infection without appropriate personal protective equipment (PPE). A low-risk exposure includes providing medical care to patients while wearing appropriate PPE [19]. More importantly, there are many unique exposure situations that do not clearly fit into one of these categories. In these instances, individual risk assessment should be determined in collaboration with public health authorities.
Vaccination as PEP is recommended for persons who have had high-risk exposures to case-patients during their infectious period. It is additionally recommended on a case-by-case basis for individuals who have had intermediate-risk exposures. Postexposure prophylaxis is not generally recommended for low/uncertain risk exposures. In countries where vaccination has been deployed as PEP, public health officials recommend administering vaccines as soon as possible within four days after an exposure to prevent or attenuate infection [123]. In the United States and UK, vaccination as PEP has been used up to 14 days postexposure given the theoretical possibility that it could still attenuate infection even if it occurs towards the end of the incubation period [122, 123].
Vaccination as pre-exposure prophylaxis (PrEP) is currently recommended by the CDC for laboratory personnel who perform testing for OPXV including MPXV, personnel who handle OPXV cultures or animals infected with OPXV, and certain public health response team members who may require vaccination for preparedness purposes [122, 123]. In the context of the ongoing outbreaks, emerging epidemiologic information identifying groups that may be at higher risk of exposure has led to the expansion of vaccination as PrEP to include gay, bisexual, and other men who have sex with men (MSM) in Montreal, Canada [124].
CONTACT TRACING, ISOLATION, AND WASTE MANAGEMENT
Contact tracing is crucial to controlling the spread of monkeypox (Figure 2). Patients with monkeypox should be interviewed to identify contacts for tracing. Types of contact include face-to-face contact, direct physical contact (including sexual contact), and contact with contaminated fomites such as bedding or other objects with shared use. In the healthcare setting, anyone who has had contact with the patient (staff, roommates, visitors) should be identified. If someone is exposed to a person with monkeypox, they should be monitored for symptoms such as fevers, chills, rash, and lymphadenopathy for 21 days after the last exposure and offered vaccination as PEP where appropriate. Individuals with suspected or confirmed monkeypox should be isolated in a private room in the emergency department or in a hospital room (if admitted) or in a separate area from other family members and pets (if at home). They should also avoid close contacts with others while they are infectious. Isolation should continue until all lesions have resolved and a new layer of skin has formed underneath. Waste from monkeypox is considered a Category A substance (pathogen that is life-threatening or causes permanent disability [125]). As such, handling and management of clinical waste should be done in accordance with US Department of Transportation Hazardous Materials Regulations. With regards to pets, the highest concern for human-to-animal transmission is with pet rodents. Although it is possible that transmission can occur to other pets such as dogs and cats, this risk is not clearly defined and should be further evaluated. Current recommendations are to quarantine pet rodents for 21 days and to exclude infection through testing.
CURRENT TRENDS AND FUTURE DIRECTIONS
On May 14, 2022, a familial cluster of 2 cases of monkeypox was reported in the UK; the case-patients had no history of travel to an endemic region. Since then, thousands of cases have been reported in multiple countries in Europe, South America, the Middle East, Canada, and the United States [1, 2]. A majority of cases have been in young men between the ages of 25–35 years, many of whom self-identify as gay, bisexual, or other MSM [1, 2]. The clinical picture has sometimes been characterized by atypical presentations including genital, perigenital, and perianal lesions, suggesting that close intimate contacts during sex might play an important role in transmission [1, 126, 127]. Whether or not monkeypox can be sexually transmitted in the traditional sense is being actively investigated. For example, small amounts of virus have been isolated from patient semen in Italy [126] and Germany [127]. Until more clarity is gained on the role of sexual transmission, abstinence during active infection and for up 8 weeks after recovery has been recommended by public health authorities in the UK as an additional precaution [128]. Prioritized vaccination of close contacts of case-patients (known as ring-vaccination) as PEP and pre-exposure vaccination of gay/bisexual and other MSM are strategies [124] being advanced currently in some countries to contain the outbreak.
The ongoing global outbreak of monkeypox is one of the largest in history, with chains of transmission chains occurring in multiple countries occurring outside of regions where monkeypox is known to be endemic. This suggests that local transmission leading to sizeable clusters may have gone unnoticed for some time, likely facilitated by the relatively long incubation of MPXV and an initially low index of suspicion by clinicians not familiar with the infection. With the world still in a global pandemic caused by another emerging zoonotic virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and a general public primed by its devastation, parallels have been drawn between the early days of the coronavirus disease 2019 (COVID-19) pandemic and the multicountry outbreaks of monkeypox. The current situation with monkeypox, although serious, is different. It is unlikely that the ongoing monkeypox outbreaks will lead to a global pandemic on the scale of COVID-19. MPXV is not a novel virus, and there is experience from previous outbreaks regarding how to prevent propagation of the infection. More importantly, the transmission of monkeypox is also substantially different from SARS-CoV-2. However, monkeypox is new to many clinicians, who understandably do not have extensive experience in identifying or treating cases of the disease.
Defining the characteristics of the current outbreak will be key in determining how best to utilize the available tools to contain it. Implementing screening tools in healthcare settings and maintaining a high level of suspicion using emerging clinical case definitions will help identify cases and delineate the scope of the outbreak. Early isolation of suspected and confirmed cases and closely monitoring and vaccinating their close contacts and healthcare workers with high-risk exposures as appropriate will be important for limiting new infections and disrupting transmission chains. Monkeypox has a wide host range, and if the ongoing outbreak is prolonged, there is reasonable concern that it could establish new ecological niches in wild animals in geographies outside of Africa, thus broadening its enzootic and endemic range.
Similar to the early days of the human immunodeficiency virus (HIV)/acquired immune deficiency syndrome pandemic, clustering of cases in gay/bisexual and other MSM has sadly led to unacceptable stigma directed towards this group of people. The infectious disease community continues to lead in fighting stigma and discrimination in response to HIV and other infectious diseases. We are once again called upon to do the same in the current outbreak. Although resources should be focused on identifying cases in social networks emerging as having a higher risk for exposure, the core of our public health messaging should be supportive and free of judgment. We should continually remind colleagues and the public that infectious pathogens do not care about race, gender, or sexual orientation.
CONCLUSIONS
In the coming months, we will gain more clarity on the magnitude of the current outbreak as case finding intensifies. Acting quickly and proactively will be crucial for containing it. Ensuring that we learn from recent epidemics and share available resources early and quickly will be the key to success. The warning signals on monkeypox becoming a global public health concern have been present for many years. Now is the time to adopt a truly global approach that addresses this problem definitively not only in wealthy countries but also, critically, in the endemic countries that have been responding to monkeypox for decades.
Acknowledgments
Financial support. B. K. T. is funded by the Emory Center for AIDS Research Grant Number P30 AI050409.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Boghuma K Titanji, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, USA.
Bryan Tegomoh, Nebraska Department of Health and Human Services, Lincoln, Nebraska, USA.
Saman Nematollahi, Department of Medicine, University of Arizona College of Medicine, Tucson, Arizona, USA.
Michael Konomos, Visual Medical Education, Emory University School of Medicine, Atlanta, Georgia, USA.
Prathit A Kulkarni, Infectious Diseases Section, Department of Medicine, Baylor College of Medicine, Houston, Texas, USA; Medical Care Line, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA.
References
- 1. European Center for Disease Control . Epidemiological update: monkeypox outbreak. Available at: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-monkeypox-outbreak. Accessed 31 May 2022.
- 2. World Health Organization . Multi-country monkeypox outbreak: situation update. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON392. Accessed 15 June 2022.
- 3. Magnus PV, Andersen EK, Petersen KB, et al. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand 1959; 46:156–76. doi: 10.1111/j.1699-0463.1959.tb00328.x [DOI] [Google Scholar]
- 4. Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970–79. Bull World Health Organ 1980; 58: 165–82. [PMC free article] [PubMed] [Google Scholar]
- 5. Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med 2004; 350:342–50. doi: 10.1056/NEJMoa032299 [DOI] [PubMed] [Google Scholar]
- 6. Alakunle E, Moens U, Nchinda G, et al. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses 2020; 12:1257. doi: 10.3390/v12111257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl Trop Dis 2022; 16:e0010141. doi: 10.1371/journal.pntd.0010141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis 2004; 4:15–25. doi: 10.1016/S1473-3099(03)00856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knipe DM, Howley PM. Fields Virology, 6th Edition: Lippincott Williams & Wilkins, Philadelphia, PA; 2013. [Google Scholar]
- 10. McFadden G. Poxvirus tropism. Nat Rev Microbiol 2005; 3:201–13. doi: 10.1038/nrmicro1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oliveira GP, Rodrigues RAL, Lima MT, et al. Poxvirus host range genes and virus-host spectrum: a critical review. Viruses 2017; 9:331. doi: 10.3390/v9110331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shchelkunov SN, Marennikova SS, Moyer RW. Orthopoxiruses Pathogenic to Humans. New York, NY: Springer, 2005. [Google Scholar]
- 13. Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organization, 1988. [Google Scholar]
- 14. Haller SL, Peng C, McFadden G, et al. Poxviruses and the evolution of host range and virulence. Infect Genet Evol 2014; 21:15–40. doi: 10.1016/j.meegid.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fine PE, Jezek Z, Grab B, et al. The transmission potential of monkeypox virus in human populations. Int J Epidemiol 1988; 17:643–50. doi: 10.1093/ije/17.3.643 [DOI] [PubMed] [Google Scholar]
- 16. Esposito JJ, Sammons SA, Frace AM, et al. Genome sequence diversity and clues to the evolution of variola (smallpox) virus. Science 2006; 313:807–12. doi:10.1126/science.1125134 [DOI] [PubMed] [Google Scholar]
- 17. Shchelkunov SN. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog 2013; 9:e1003756. doi: 10.1371/journal.ppat.1003756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A 2010; 107:16262–7. doi: 10.1073/pnas.1005769107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao AK, Schulte J, Chen TH, et al. Monkeypox in a traveler returning from Nigeria–Dallas, Texas, July 2021. MMWR Morb Mortal Wkly Rep 2022; 71:509–16. doi: 10.15585/mmwr.mm7114a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakazawa Y, Mauldin MR, Emerson GL, et al. A phylogeographic investigation of African monkeypox. Viruses 2015; 7:2168–84. doi: 10.3390/v7042168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berthet N, Descorps-Declère S, Besombes C, et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci Rep 2021; 11:13085. doi: 10.1038/s41598-021-92315-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer H, Perrichot M, Stemmler M, et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol 2002; 40:2919–21. doi: 10.1128/JCM.40.8.2919-2921.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet 1986; 1:98–9. doi: 10.1016/S0140-6736(86)90748-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radonić A, Metzger S, Dabrowski PW, et al. Fatal monkeypox in wild-living sooty mangabey, Côte d’Ivoire, 2012. Emerg Infect Dis 2014; 20:1009–11. doi: 10.3201/eid2006.131329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patrono LV, Pléh K, Samuni L, et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat Microbiol 2020; 5:955–65. doi: 10.1038/s41564-020-0706-0 [DOI] [PubMed] [Google Scholar]
- 26. Falendysz EA, Lopera JG, Doty JB, et al. Characterization of monkeypox virus infection in African rope squirrels (funisciurus sp.). PLoS Negl Trop Dis 2017;11:e0005809. doi: 10.1371/journal.pntd.0005809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doty JB, Malekani JM, Kalemba LN, et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses 2017; 9:283. doi: 10.3390/v9100283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guagliardo SAJ, Monroe B, Moundjoa C, et al. Asymptomatic orthopoxvirus circulation in humans in the wake of a monkeypox outbreak among chimpanzees in Cameroon. Am J Trop Med Hyg 2020; 102:206–12. doi: 10.4269/ajtmh.19-0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tchokoteu PF, Kago I, Tetanye E, Ndoumbe P, Pignon D, Mbede J. [Variola or a severe case of varicella? A case of human variola due to monkeypox virus in a child from the Cameroon]. Ann Soc Belg Med Trop 1991; 71:123–8. [PubMed] [Google Scholar]
- 30. World Health Organization . Regional office for A. Weekly bulletin on outbreak and other emergencies: week 31: 28 July - 3 August 2018. Week Bullet Outbreak Emergen 2018:1–24. [Google Scholar]
- 31. World Health Organization . Regional Office for A. Weekly bulletin on outbreak and other emergencies: week 41: 05-11 October 2020. Week Bullet Outbreak Emergen 1-18
- 32. World Health Organization . Regional office for A. Weekly bulletin on outbreak and other emergencies: week 01: 30 December 2019–05 January 2020. Week Bullet Outbreak Emergen 2020: 1-14.
- 33. Berthet N, Nakouné E, Whist E, et al. Maculopapular lesions in the Central African Republic. Lancet 2011; 378:1354. doi: 10.1016/S0140-6736(11)61142-2 [DOI] [PubMed] [Google Scholar]
- 34. Nakoune E, Lampaert E, Ndjapou SG, et al. A nosocomial outbreak of human monkeypox in the Central African Republic. Open Forum Infect Dis 2017; 4:ofx168. doi: 10.1093/ofid/ofx168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalthan E, Tenguere J, Ndjapou SG, et al. Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Med Mal Infect 2018; 48:263–8. doi: 10.1016/j.medmal.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization . Weekly bulletin on outbreak and other emergencies: week 31: 29. July-04 August 2019. Available at: https://apps.who.int/iris/handle/10665/326159. Accessed May 30, 2022 .
- 37. reliefweb . Monkeypox in Central African Republic. Available at: https://reliefweb.int/report/central-african-republic/monkeypox-central-african-republic. Accessed 31 May 2022.
- 38. reliefweb . Central African Republic: Monkey Pox Outbreak–December 2015. Available at: https://reliefweb.int/disaster/ep-2015-000178-caf. Accessed 31 May 2022.
- 39. Mwanbal PT, Tshioko KF, Moudi A, et al. Human monkeypox in Kasai Oriental, Zaire (1996–1997). Euro Surveill 1997; 2:33–5. doi: 10.2807/esm.02.05.00161-en [DOI] [PubMed] [Google Scholar]
- 40. Rimoin AW, Kisalu N, Kebela-Ilunga B, et al. Endemic human monkeypox, Democratic Republic of Congo, 2001–2004. Emerg Infect Dis 2007; 13:934–7. doi: 10.3201/eid1306.061540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jezek Z, Arita I, Mutombo M, Dunn C, Nakano JH, Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol 1986; 123:1004–12. doi: 10.1093/oxfordjournals.aje.a114328 [DOI] [PubMed] [Google Scholar]
- 42. Jezek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ 1988; 66:459–64. [PMC free article] [PubMed] [Google Scholar]
- 43. McCollum AM, Nakazawa Y, Ndongala GM, et al. Human monkeypox in the Kivus, a conflict region of the Democratic Republic of the Congo. Am J Trop Med Hyg 2015; 93:718–21. doi: 10.4269/ajtmh.15-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whitehouse ER, Bonwitt J, Hughes CM, et al. Clinical and epidemiological findings from enhanced monkeypox surveillance in Tshuapa province, Democratic Republic of the Congo during 2011–2015. J Infect Dis 2021; 223:1870–8. doi: 10.1093/infdis/jiab133 [DOI] [PubMed] [Google Scholar]
- 45. Nolen LD, Osadebe L, Katomba J, et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis 2016; 22:1014–21. doi: 10.3201/eid2206.150579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eltvedt AK, Christiansen M, Poulsen A. A case report of monkeypox in a 4-year-old boy from the DR Congo: challenges of diagnosis and management. Case Rep in Pediatr 2020; 2020:8572596. doi: 10.1155/2020/8572596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fuller T, Thomassen HA, Mulembakani PM, et al. Using remote sensing to map the risk of human monkeypox virus in the Congo basin. Ecohealth 2011; 8:14–25. doi: 10.1007/s10393-010-0355-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoff NA, Doshi RH, Colwell B, et al. Evolution of a disease surveillance system: an increase in reporting of human monkeypox disease in the Democratic Republic of the Congo, 2001-2013. Int J Trop Dis Health 2017; 25:IJTDH.35885 doi: 10.9734/IJTDH/2017/35885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention . Human Monkeypox – Kasai Oriental, Democratic Republic of Congo, February 1996-October 1997. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00050245.htm. Accessed 31 May 2022. [PubMed]
- 50. World Health Organization . 1997-Monkeypox in the Democratic Republic of Congo (former Zaire). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/1997_07_31b-en. Accessed 31 May 2022.
- 51. Meyer A, Esposito JJ, Gras F, Kolakowski T, Fatras M, Muller G. [First appearance of monkey pox in human beings in Gabon]. Med Trop (Mars) 1991; 51:53–7. [PubMed] [Google Scholar]
- 52. Müller G, Meyer A, Gras F, Emmerich P, Kolakowski T, Esposito JJ. Monkeypox virus in liver and spleen of child in Gabon. Lancet 1988; 1:769–70. doi: 10.1016/S0140-6736(88)91580-2 [DOI] [PubMed] [Google Scholar]
- 53. Monkeypox 1991. Gabon. Wkly Epidemiol Rec 1992; 67:101–2. [PubMed] [Google Scholar]
- 54. Outbreak News Ttoday . Monkeypox confirmed in Liberia. Available at: http://outbreaknewstoday.com/monkeypox-confirmed-liberia-57035/. Accessed 31 May 2022.
- 55. World Health Organization . Regional office for Africa HEP. Weekly bulletin on outbreaks and other emergencies: week 1: 30 December 2017-5 January 2018. Brazzaville: World Health Organization. 2018. [Google Scholar]
- 56. Eteng WE, Mandra A, Doty J, et al. Notes from the field: responding to an outbreak of monkeypox using the one health approach-Nigeria, 2017–2018. MMWR Morb Mortal Wkly Rep 2018; 67:1040–1. doi: 10.15585/mmwr.mm6737a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. reliefweb . Monkeypox Outbreak in Nigeria: Situation Report #12 (December 2019). Available at: https://reliefweb.int/report/nigeria/monkeypox-outbreak-nigeria-situation-report-12-december-2019. Accessed 31 May 2022.
- 58. Doshi RH, Guagliardo SAJ, Doty JB, et al. Epidemiologic and ecologic investigations of Monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg Infect Dis 2019; 25:281–9. doi: 10.3201/eid2502.181222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guagliardo SAJ, Doshi RH, Reynolds MG, et al. Do monkeypox exposures vary by ethnicity? Comparison of Aka and Bantu suspected monkeypox cases. Am J Trop Med Hyg 2020; 102:202–5. doi: 10.4269/ajtmh.19-0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Reynolds MG, Emerson GL, Pukuta E, et al. Detection of human monkeypox in the Republic of the Congo following intensive community education. Am J Trop Med Hyg 2013; 88:982–5. doi: 10.4269/ajtmh.12-0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reynolds MG, Wauquier N, Li Y, et al. Human monkeypox in Sierra Leone after 44-year absence of reported cases. Emerg Infect Dis 2019; 25:1023–5. doi: 10.3201/eid2505.180832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ye F, Song J, Zhao L, et al. Molecular evidence of human monkeypox virus infection, Sierra Leone. Emerg Infect Dis 2019; 25:1220–2. doi: 10.3201/eid2506.180296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Foster SO, Brink EW, Hutchins DL, et al. Human monkeypox. Bull World Health Organ 1972; 46:569–76. [PMC free article] [PubMed] [Google Scholar]
- 64. reliefweb . WHO AFRO Outbreaks and Other Emergencies, Week 16: 15–21 April 2017 (Data as reported by 17:00, 21 April 2017). Available at: https://reliefweb.int/report/sierra-leone/who-afro-outbreaks-and-other-emergencies-week-16-15-21-april-2017-data-reported. Accessed 31 May 2022. [Google Scholar]
- 65. Formenty P, Muntasir MO, Damon I, et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg Infect Dis 2010; 16:1539–45. doi: 10.3201/eid1610.100713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Erez N, Achdout H, Milrot E, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis 2019; 25:980–3. doi: 10.3201/eid2505.190076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yong SEF, Ng OT, Ho ZJM, et al. Imported monkeypox, Singapore. Emerg Infect Dis 2020; 26:1826–30. doi: 10.3201/eid2608.191387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill 2018; 23:1800509 doi:10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis 2020; 26:782–5. doi: 10.3201/eid2604.191164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis 2006; 194:773–80. doi: 10.1086/505880 [DOI] [PubMed] [Google Scholar]
- 71. Health Department, Maryland . Travel-associated monkeypox virus infection confirmed in Maryland resident. Available at: https://health.maryland.gov/newsroom/Pages/Travel-Associated-Monkeypox-virus-infection-confirmed-in-Maryland-resident.aspx. Accessed 31 May 2022.
- 72. Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:734–42. doi: 10.15585/mmwr.mm7122e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses 2017; 9:380. doi: 10.3390/v9120380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am 2019; 33:1027–43. doi: 10.1016/j.idc.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Centers for Disease Control and Prevention (CDC) . Monkeypox Virus Infection in the United States and Other Non-endemic Countries—2022. Available at: https://emergency.cdc.gov/han/2022/han00466.asp. Accessed June 14th 2022.
- 76. Centers of Disease Control and Prevention . Biosafety in Microbiological and Biomedical Laboratories. Editors PJ Meechan and J Potts). 6th ed. Available at: https://www.cdc.gov/labs/pdf/CDC-BiosafetyMicrobiologicalBiomedicalLaboratories-2020-P.pdf. Accessed 17 June 2022.
- 77. Hammarlund E, Lewis MW, Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med 2005; 11:1005–11. doi: 10.1038/nm1273 [DOI] [PubMed] [Google Scholar]
- 78. Hughes C, McCollum A, Pukuta E, et al. Ocular complications associated with acute monkeypox virus infection, DRC. Int J Infect Dis 2012; 21:276–7.doi: 10.1016/j.ijid.2014.03.994 [DOI] [Google Scholar]
- 79. Adalja A, Inglesby T. A novel international monkeypox outbreak. Ann Intern Med 2022. doi: 10.7326/M22-1581 [DOI] [PubMed] [Google Scholar]
- 80. Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med 2018; 379:44–53. doi: 10.1056/NEJMoa1705688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wittek R. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int J Infect Dis 2006; 10:193–201. doi: 10.1016/j.ijid.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 82. James JS. Cidofovir recommended for approval for CMV retinitis. AIDS Treat News 1996; (no 244):6–7. [PubMed] [Google Scholar]
- 83. US Food and Drug Administration . FDA approves drug to treat smallpox. Rockville, MD: US Food and Drug Administration; June 4, 2021 (News Release). Available at: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-smallpox. Accessed 15 June 2022.
- 84. Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis 2012; 206:1372–85. doi: 10.1093/infdis/jis510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. US Food and Drug Administration . FDA approves the first drug with an indication for treatment of smallpox. Rockville, MD: US Food and Drug Administration; July 13, 2018 (News Release). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-indication-treatment-smallpox. Accessed 15 June 2022.
- 86. Centers for Disease Control and Prevention . Guidance for Tecovirimat Use Under Expanded Access Investigational New Drug Protocol during 2022 U.S. Monkeypox Cases. Available at: https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html. Accessed 15 June 2022.
- 87. CIDRAP. FDA approves VIG for smallpox shot complications. Available at: https://www.cidrap.umn.edu/news-perspective/2005/02/fda-approves-vig-smallpox-shot-complications. Accessed 16 June 2022.
- 88. Centers for Disease Control and Prevention . Interim Clinical Guidance for the Treatment of Monkeypox. Available at: https://www.cdc.gov/poxvirus/monkeypox/treatment.html. Accessed June 16, 2022 .
- 89. Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis 2022; S1473-3099:00228-6. doi:10.1016/S1473-3099(22)00228-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chan-Tack K, Harrington P, Bensman T, et al. Benefit-risk assessment for brincidofovir for the treatment of smallpox: U.S. Food and Drug Administration’s evaluation. Antiviral Res 2021; 195:105182. doi: 10.1016/j.antiviral.2021.105182 [DOI] [PubMed] [Google Scholar]
- 91. Delaune D, Iseni F. Drug development against smallpox: present and future. Antimicrob Agents Chemother 2020; 64:e01683–19 doi: 10.1128/AAC.01683-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hutson CL, Kondas AV, Mauldin MR, et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. mSphere 2021; 6:e00927–20. doi: 10.1128/mSphere.00927-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Redfield RR, Wright DC, James WD, Jones TS, Brown C, Burke DS. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med 1987; 316:673–6. doi: 10.1056/NEJM198703123161106 [DOI] [PubMed] [Google Scholar]
- 94. Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis 2008; 46:1555–61. doi: 10.1086/587668 [DOI] [PubMed] [Google Scholar]
- 95. Centers of Disease Control and Prevention (CDC) . Secondary and tertiary transmission of vaccinia virus after sexual contact with a smallpox vaccinee–San Diego, California, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:145–7. [PMC free article] [PubMed] [Google Scholar]
- 96. Kinnunen PM, Holopainen JM, Hemmilä H, et al. Severe ocular cowpox in a human, Finland. Emerg Infect Dis 2015; 21:2261–3. doi: 10.3201/eid2112.150621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gazzani P, Gach JE, Colmenero I, et al. Fatal disseminated cowpox virus infection in an adolescent renal transplant recipient. Pediatr Nephrol 2017; 32:533–6. doi: 10.1007/s00467-016-3534-y [DOI] [PubMed] [Google Scholar]
- 98. Lindholm DA, Fisher RD, Montgomery JR, et al. Preemptive tecovirimat use in an active duty service member who presented with acute myeloid leukemia after smallpox vaccination. Clin Infect Dis 2019; 69:2205–7. doi: 10.1093/cid/ciz286 [DOI] [PubMed] [Google Scholar]
- 99. Centers for Disease Control and Prevention (CDC) . Human vaccinia infection after contact with a raccoon rabies vaccine bait - Pennsylvania, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:1204–7. [PubMed] [Google Scholar]
- 100. US Food and Drug Administration (FDA) . Clinical review for new drug application 208627, TPOXX (tecovirimat). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/208627Orig1s000MedR.pdf. Accessed 14 June 2022.
- 101. Whitehouse ER, Rao AK, Yu YC, et al. Novel treatment of a vaccinia virus infection from an occupational needlestick - San Diego, California, 2019. MMWR Morb Mortal Wkly Rep 2019; 68:943–6. doi: 10.15585/mmwr.mm6842a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kiernan M, Koutroumanos N. Orbital Cowpox. N Engl J Med 2021; 384:2241. doi: 10.1056/NEJMicm2033620 [DOI] [PubMed] [Google Scholar]
- 103. Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak - nine states, may 2022. MMWR Morb Mortal Wkly Rep 2022; 71:764–9. doi: 10.15585/mmwr.mm7123e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Marty FM, Winston DJ, Chemaly RF, et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019; 25:369–81. doi: 10.1016/j.bbmt.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Grimley MS, Chemaly RF, Englund JA, et al. Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant 2017; 23:512–21. doi: 10.1016/j.bbmt.2016.12.621 [DOI] [PubMed] [Google Scholar]
- 106. Hoy SM. Tecovirimat: first global approval. Drugs 2018; 78:1377–82. doi: 10.1007/s40265-018-0967-6 [DOI] [PubMed] [Google Scholar]
- 107. European Medicines Agency. Tecovirimat SIGA . 2022. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/tecovirimat-siga. Accessed 17 June 2022.
- 108. GlobeNewswire . SIGA Announces Collaboration with Oxford University to Support Expanded Access Protocol for Use of TPOXX® (Tecovirimat) to Treat Monkeypox in Central African Republic. Available at: https://www.globenewswire.com/en/news-release/2021/07/29/2270930/9738/en/SIGA-Announces-Collaboration-with-Oxford-University-to-Support-Expanded-Access-Protocol-for-Use-of-TPOXX-Tecovirimat-To-Treat-Monkeypox-in-Central-African-Republic.html. Accessed 15 June 2022.
- 109. US Food and Drug Administration . Vaccinia Immune Globulin Intravenous (Human). Available at: https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/vaccinia-immune-globulin-intravenous-human. Accessed 31 May 2022.
- 110. Hopkins RJ, Lane JM. Clinical efficacy of intramuscular vaccinia immune globulin: a literature review. Clin Infect Dis 2004; 39:819–26. doi: 10.1086/422999 [DOI] [PubMed] [Google Scholar]
- 111. Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ 1988; 66:465–70. [PMC free article] [PubMed] [Google Scholar]
- 112. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis 2003; 36:766–74. doi: 10.1086/374244 [DOI] [PubMed] [Google Scholar]
- 113. Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis 2012; 54:832–40. doi: 10.1093/cid/cir952 [DOI] [PubMed] [Google Scholar]
- 114. Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 2003; 289:3283–9. doi: 10.1001/jama.289.24.3283 [DOI] [PubMed] [Google Scholar]
- 115. Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol 2004; 43:1503–10. doi: 10.1016/j.jacc.2003.11.053 [DOI] [PubMed] [Google Scholar]
- 116. Petersen BW, Damon IK, Pertowski CA, et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm Rep 2015; 64(RR-02):1–26. [PubMed] [Google Scholar]
- 117. Hatch GJ, Graham VA, Bewley KR, et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol 2013; 87:7805–15. doi: 10.1128/JVI.03481-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Keckler MS, Salzer JS, Patel N, et al. IMVAMUNE(®) and ACAM2000(®) provide different protection against disease when administered postexposure in an intranasal monkeypox challenge prairie dog model. Vaccines (Basel) 2020; 8:396. doi: 10.3390/vaccines8030396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med 2019; 381:1897–908. doi: 10.1056/NEJMoa1817307 [DOI] [PubMed] [Google Scholar]
- 120. Petersen BW, Kabamba J, McCollum AM, et al. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res 2019; 162:171–7. doi: 10.1016/j.antiviral.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. UK Health Security Agency . Monkeypox cases confirmed in England – latest updates. Available at: https://www.gov.uk/government/news/monkeypox-cases-confirmed-in-england-latest-updates. Accessed May 31, 2022.
- 122. Centers for Disease Control and Prevention . Monkeypox and Smallpox Vaccine Guidance. Available at: https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html. Accessed 15 June 2022.
- 123. UK Health Security Agency . Recommendations for the use of pre and post exposure vaccination during a monkeypox incident. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1080838/Recommendations-for-pre-and-post-exposure-vaccination-during-a-monkeypox-incident-6-june-2022.pdf. Accessed 15 June 2022.
- 124. CTVNews . Montreal public health shifting vaccination plan as monkeypox cases grow to 126. Available at: https://montreal.ctvnews.ca/montreal-public-health-shifting-vaccination-plan-as-monkeypox-cases-grow-to-126-1.5946380. Accessed 15 June 2022.
- 125. NETEC . Waste Management from Patients Being Treated for Monkeypox Virus. Available at: https://netec.org/2021/07/21/monkeypox-waste-management/. Accessed 31 May 2022.
- 126. Antinori A, Mazzotta V, Vita S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill 2022; 27:2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Noe SZ, Seilmaier M, Antwerpen MH, et al. Clinical and virological features of first Monkeypox cases in Germany. Research square 2022. doi: 10.21203/rs.3.rs-1725831/v1 [DOI] [PMC free article] [PubMed]
- 128. BBC . Monkeypox infections rise as guidance advises cases to abstain from sex. Available at: https://www.bbc.com/news/health-61640196. Accessed 15 June 2022.