Abstract
One of the most disabling forms of retinal degeneration occurs in Usher syndrome, since it affects patients who already suffer from deafness. Mutations in the myosin VIIa gene (MYO7A) cause a major subtype of Usher syndrome, type 1B. Owing to the loss of function nature of Usher 1B and the relatively large size of MYO7A, we investigated a lentiviral-based gene replacement therapy in the retinas of MYO7A-null mice. Among the different promoters tested, a CMV-MYO7A chimeric promoter produced wild-type levels of MYO7A in cultured RPE cells and retinas in vivo. Efficacy of the lentiviral therapy was tested by using cell-based assays to analyze the correction of previously defined, MYO7A-null phenotypes in the mouse retina. In vitro, defects in phagosome digestion and melanosome motility were rescued in primary cultures of RPE cells. In vivo, the normal apical location of melanosomes in RPE cells was restored, and the abnormal accumulation of opsin in the photoreceptor connecting cilium was corrected. These results demonstrate that a lentiviral vector can accommodate a large cDNA, such as MYO7A, and mediate correction of important cellular functions in the retina, a major site affected in the Usher syndrome. Therefore, a lentiviral-mediated gene replacement strategy for Usher 1B therapy in the retina appears feasible.
Keywords: Usher syndrome, lentivirus, retina, RPE, MYO7A
Introduction
Usher syndrome is an autosomal recessive disorder of combined deafness and blindness.1 Three clinical subtypes have been defined, with Usher syndrome type 1 (Usher 1) characterized by the most severe deafness at birth.2 The blindness of Usher patients has characteristics of retinitis pigmentosa, although histological analyses of genetically-identified Usher retinas is currently lacking. Usher 1B, which accounts for 30–50% of Usher 1,3–5 is caused by mutations in MYO7A.6 MYO7A encodes an unconventional myosin that is present in a variety of tissues,7,8 but its most critical functions are in the inner ear and retina. In the retina of various vertebrates, including humans, MYO7A protein is present at a high level in the retinal pigment epithelium (RPE),7,9 where a significant proportion is associated with melanosomes.10,11 However, a low level of MYO7A is also detected in the photoreceptor cells.12
Shaker1 mice possess mutant Myo7a,13,14 and are deaf and have vestibular dysfunction. Although they do not appear to undergo photoreceptor degeneration,15,16 several mutant phenotypes have been identified in their retinas at the cellular level. In the shaker1 RPE, the localization and motility of both phagosomes and melanosomes are defective, suggesting roles for MYO7A in the transport of these organelles.11,17–19 The phagosomes result from the phagocytosis of the photoreceptor outer segment tips, as part of the renewal of the photoreceptor disk membranes,20 a critical process for the viability of photoreceptor cells.21 The role of melanosomes in the RPE is not well defined, however, their functions include light absorption, possibly a contribution to the digestion of phagosomes, and protection against lipid peroxidation and the formation of toxic oxiranes from ingested photoreceptor outer segment phospholipids.22 An additional cellular defect in the retina has been observed in the photoreceptor cells of shaker1 mice. Their photoreceptor connecting cilia contain an excess of opsin, indicating an additional role for MYO7A in the renewal of the photoreceptor disk membranes.23
As Usher 1B appears to result from loss of MYO7A function,6 the addition of a functional gene (gene replacement therapy) should provide a therapeutic approach. In recent years, significant progress has been made in gene therapy studies, using animal models of inherited retinal degeneration, especially in viral vector-mediated replacement of genes normally expressed in the RPE. Treatment of Rpe65-mutant dogs and mice with recombinant adeno-associated virus (rAAV), carrying wild-type cDNA for RPE65, has achieved preservation of retinal structure and restoration of physiology and visual behavior in both short and long-term studies.24–29 Likewise, treatments of RCS rats with rAAV, adenoviral, and lentiviral vectors expressing the cDNA of Mertk, a receptor tyrosine kinase involved in RPE phagocytosis of the photoreceptor disk membranes, have resulted in partial preservation of retinal structure, responsiveness to light, correction of the phagocytosis defects, and delayed photoreceptor cell loss.30–32
Gene replacement therapy of Usher 1B presents a special challenge due to the large size of the MYO7A gene, which is over 100 Mb and encodes a protein consisting of 2215 amino-acid residues.33,34 Among the available viral gene delivery vehicles, third generation, recombinant lentiviral vectors can accommodate large transgenes, tissue-specific promoters, transduce postmitotic cells over a broad host range, including humans, and possess a number of important biosafety features.35 In the present study, we have tested the efficacy of lentiviral-mediated MYO7A expression in rescuing mutant phenotypes in the MYO7A-null mice. We demonstrate that current lentiviral vectors can accommodate the large MYO7A cDNA and results in correction of cellular defects in vitro and in vivo, and thus provide a strategy for the retinal therapy of Usher 1B.
Results
Lentiviral-MYO7A expression
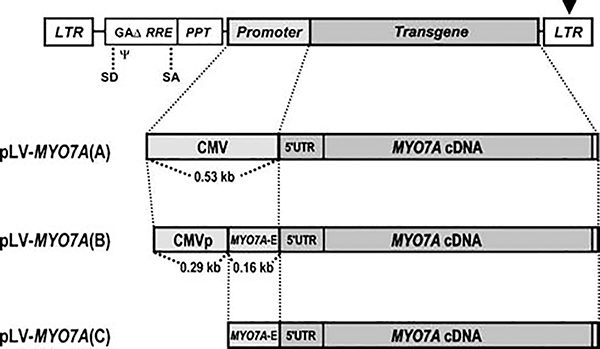
We constructed three HIV-1 derived lentiviral vectors to express human MYO7A protein. They had the same third-generation/self-inactivating backbone,36,37 but differed according to the promoter included to drive expression of full length MYO7A cDNA (Figure 1). The pLV-MYO7A(A) vector contained the 530-bp human cytomegalovirus immediate early promoter (referred to as CMV promoter here after). The pLV-MYO7A(B) encoded a chimeric promoter containing 290 bp of the 5′ end of the CMV promoter fused to 160 bp of the human MYO7A gene sequence that spans the boundary of the first intron and the second exon. The pLV-MYO7A(C) included only the 160-bp MYO7A sequence. These lentiviral vectors were used to produce viruses pseudotyped with the glycoprotein of vesicular stomatitis virus (VSV.G).38,39 Anti-MYO7A labeling of infected HEK 293T cells showed robust expression by LV-MYO7A(A), weak expression by LV-MYO7A(B), and no detectable expression by LV-MYO7A(C) (Supplementary Figure 1a–d).
Figure 1.
Schematic drawings of lentiviral vectors encoding the human MYO7A cDNA. The LV-MYO7A(A) vector encodes the CMV promoter upstream of the human MYO7A cDNA. The LV-MYO7A(B) vector contains a chimeric promoter, consisting of a partial CMV promoter (CMVp) and a 160-bp sequence from the human MYO7A (MYO7A-E). The LV-MYO7A(C) encodes the 160-bp 'MYO7A-E' fragment only. LTR, long terminal repeat; GAΔ, partial HIV1 GAG gene; RRE, Rev responsive element; PPT, polypurine track; ψ, viral packaging sequence; SD, splice donor; SA, splice acceptor. The arrowhead indicates the deletion within the 3′ LTR that causes self-inactivation of the viral LTR enhancer upon integration.
We next examined lentiviral-mediated MYO7A expression in vitro in primary cultures of RPE cells from Myo7a−/− (MYO7A-null mutant) shaker1 mice. Owing to the weak activity of the LV-MYO7A(B) chimeric promoter in the HEK cells, its titer was determined by MYO7A immunostaining in Myo7a−/− RPE cells, infected by serially diluted viral stock. The concentrated LV-MYO7A(B) viral stock was found to be 1 × 109 transducing units per ml (TU/ml). This titer is comparable to that of LV-MYO7A(A) viral stock, determined by infection of the HEK 293T cells (2 × 109 TU/ml). When concentrated LV-MYO7A(A) and LV-MYO7A(B) viruses were used to infect primary Myo7a−/− RPE cells, MYO7A immunolabeling indicated that more than 95% of the cells were transduced by the two viruses. However, treatment with concentrated LV-MYO7A(C) did not yield any detectable expression of MYO7A in RPE cells (data not shown). The LV-MYO7A(B) virus resulted in expression levels and localization of MYO7A that were comparable to that in the Myo7a+/− control cells, as determined by Western blot and immunofluorescence labeling (Figure 2a, b and e). In contrast, infection with the LV-MYO7A(A) resulted in much higher levels of MYO7A expression, such that MYO7A accumulated in large aggregates and cell death was evident after 5 days of infection (Figure 2c and d; Supplementary Figure 1e).
Figure 2.
Lentiviral vector-mediated expression of MYO7A. (a–c) Immunofluorescence shows MYO7A immunolabel in (a) Myo7a+/− RPE, (b) Myo7a−/− RPE, 7 days after infection with LV-MYO7A(B), and (c) Myo7a−/− RPE, 7 days after infection with LV-MYO7A(A). (d) A phase contrast image of (c). (e) Western blot of MYO7A protein (upper panel) in Myo7a+/− RPE (Ctrl), Myo7a−/− RPE (Mut) and Myo7a−/− RPE, 5 days after infection with LV-MYO7A(B) (Mut+B). HSP60 labeling (lower panel) was used as a loading control. (f) Alkaline phosphatase (AP) histochemistry of a retinal section from an albino control mice. The retina was injected at P4 with LV-AP(B) and fixed at P14. Arrows and arrowheads indicate AP staining in the RPE and photoreceptor cells, respectively. Area shown is away from the site of injection. (g, h) MYO7A immunostaining (arrows) of the RPE in the central (g) and peripheral (h) regions of the retina from an albino Myo7a−/− mouse. The retina was injected centrally at P96 with the LV-MYO7A(B) virus and fixed 6 days later. Faint, non-specific staining9 is evident in the photoreceptor synaptic layer. RPE, retinal pigment epithelium; ONL, outer nuclear layer. Scale bars = 20 μm (a–d), 50 μm (f–h).
Similarly, in an established RPE cell line, ARPE-19, immunolabeling indicated higher levels of MYO7A in cells infected with LV-MYO7A(A) than in cells infected with LV-MYO7A(B), 3 days after infection (Supplementary Figure 2a, b). ARPE-19 cells that were exposed to LV-MYO7A(C) exhibited similar levels of MYO7A as non-infected ARPE-19 cells, which express only endogenous MYO7A (Supplementary Figure 2c, d). Combined with the primary RPE cell results, these observations indicate that the chimeric promoter encoded by LV-MYO7A(B) was able to drive relatively normal levels of MYO7A expression in cultured RPE cells, and the 160-bp MYO7A gene sequence alone was insufficient to drive expression in HEK 293T or RPE cells.
To determine the transduction efficiency of VSV.G packaged lentiviruses in vivo, we performed subretinal injection of Myo7a−/− neonatal mice with LV-MYO7A(A) or a lentivirus expressing EGFP from the CMV promoter (LV-CIG).37 Consistent with results obtained from RPE cell cultures in vitro, lentiviruses with the CMV promoter effectively drove the transgene expression in the RPE cell layer in vivo. However, injection of LV-MYO7A(A) into either neonatal or adult mice caused RPE atrophy within a week. Neither neonatal nor adult subretinal injection of lentiviruses containing the CMV promoter resulted in substantial viral transduction of the neural retina.
To determine the efficiency of transgene expression from lentiviral vectors encoding the CMV-MYO7A chimeric promoter in vivo, we constructed and produced a lentivirus, LV-AP(B), in which the alkaline phosphatase (AP) reporter gene replaced the MYO7A cDNA in LV-MYO7A(B). A viral stock of LV-AP(B) with a titer of 107 TU/ml was injected subretinally at P4. At P14, the majority of injected eyes showed positive AP histochemical staining in the RPE (6 out of 8), and all eyes that showed RPE transduction also contained AP-positive photoreceptor cells. The AP signals in the RPE cells ranged from 70–80% (3/8) to 10–30% (3/8) of the entire RPE layer on cross sections. Moreover, the AP activity as detected by histochemistry indicated that the chimeric promoter resulted in significantly higher expression levels in the RPE than in the photoreceptor cells (Figure 2f).
Immunocytochemical labeling of neonatal Myo7a−/− retinas, injected with LV-MYO7A(B) virus, showed that 30–50% of the RPE was MYO7A positive, with most of the negative cells located furthest from the injection site. The MYO7A expression level varied from cell to cell (Figure 2g and h). Despite LV-AP(B) transduction of photoreceptor cells, significant MYO7A labeling could not be detected in these cells by immuno light (HRP) or electron (immunogold) microscopy, following injection of LV-MYO7A(B).
Together, these results show that lentiviral vectors are capable of expressing the large MYO7A cDNA, and that VSV.G pseudotyped lentiviruses, in conjunction with appropriate promoters, can transduce RPE cells as well as photoreceptor cells.
Correction of mutant phenotypes in primary cultures of RPE cells
As the level of expression of MYO7A by the LV-MYO7A(B) virus in Myo7a−/− RPE cells was similar to that in control cells, we tested whether transduction by this virus could correct previously identified mutant phenotypes.
Myo7a−/− RPE cells do not digest ingested rod outer segments (ROSs) as well as control cells, due to retarded transport to the lysosomes in their basal region.18 In testing for correction of this phenotype, we measured the rate of digestion of ingested ROSs by RPE cells, following a 20-min exposure to ROSs in the medium. ROSs remaining in the RPE cells (and thus defined as undigested) were detected by opsin immunolabeling. At 2 h after the exposure to ROSs, there were significantly fewer remaining ROSs in Myo7a−/− cells that had been pretreated with LV-MYO7A(B) than in untreated mutant cells (5–10 fold). The number of ROSs was similar to that detected in Myo7a+/− control cells (Figure 3a–d).
Figure 3.
Lentiviral correction of Myo7a-mutant phenotypes in RPE primary cultures. (a–c) Immunofluorescence of ROSs remaining in Myo7a+/− RPE (a), Myo7a−/− RPE (b) and Myo7a−/− RPE infected with LV-MYO7A(B) (c). The ROSs are represented by green dots from opsin labeling (e.g. arrows). Nuclei are stained blue. (d) Bar graph showing the total number of ROSs per cell in Myo7a+/− RPE (Ctrl), Myo7a−/− RPE (Mut) and Myo7a−/− RPE infected with LV-MYO7A(B) (Mut+B). (e–g) Kymographs (showing distance traveled in relation to time) illustrate the differences in movements of individual melanosomes from Myo7a+/− RPE (e), Myo7a−/− RPE (f) and Myo7a−/− RPE infected with LV-MYO7A(B) (g). The more constrained movements of melanosomes in control and corrected RPE are evident by less displacement. Each line represents the movement of an individual melanosome. (h) Bar graph showing the average distance per 5 min, traveled by randomly selected individual melanosomes measured from Myo7a+/− RPE (Ctrl), Myo7a−/− RPE (Mut) and Myo7a−/− RPE infected with LV-MYO7A(B) (Mut+B). Scale bars (a–c) = 20 μm. Error bars in (d, h) represent ±s.e.m.
In Myo7a−/− RPE cells, melanosomes undergo rapid movements over much longer ranges than they do in control RPE cells.11 Thus, we tested whether treatment with LV-MYO7A(B) could correct this defect in melanosome motility. To monitor the movements of melanosomes, time-lapse imaging of live cells and particle tracking was used to record the displacement of individual melanosomes. Figure 3e–g illustrates a series of resulting kymographs from different melanosomes. In control cells (Figure 3e) the majority of individual melanosome traces showed little or no displacement over time and thus appear largely as smooth vertical lines, with only a few small sloping regions. In contrast, melanosome traces from mutant cells (Figure 3f) showed much larger and more frequent displacements (sloped regions) over time. Mutant cells treated with LV-MYO7A(B) (Figure 3g) had melanosome traces comparable to control cells. A quantitative analysis of these melanosome tracks confirmed that the long-range movements of melanosomes found in the mutant cells were absent in treated cells; melanosome movements became restricted as in control cells (Figure 3h).
Therefore, lentiviral vector-mediated MYO7A expression effectively corrected two mutant phenotypes, defective phagocytosis and abnormal melanosome motility, found in Myo7a−/− RPE cells.
Correction of melanosome mislocalization in MYO7A-null mice in vivo
A readily apparent mutant phenotype in shaker1 mouse retinas is the complete absence of melanosomes from the apical regions of the RPE cells.17 We thus tested for correction of this phenotype following subretinal injection of LV-MYO7A(B). Semithin sections were examined by light microscopy 4–19 days after injection. No correction or MYO7A was detected 4 days after injection. By 6 days or later, some, but not all RPE cells contained melanosomes in their apical processes (Figure 4a–c). More corrected cells were observed near the site of injection, but, even here, some cells that were not corrected were evident.
Figure 4.
Correction of melanosome localization in the RPE in vivo. Semithin (a–c) and ultrathin (d–f) LR White sections of (a) a Myo7a+/+ retina, (b) a Myo7a−/− retina, and (c–f) a Myo7a−/− retina, infected with LV-MYO7A(B) at P1 and analyzed at P16. In a–c, brackets indicate the RPE apical processes. Arrows indicate some of the melanosomes localized in the RPE apical processes. Arrows in (d, e) indicate the RPE cell boundaries. Note that the central RPE cell in the field does not contain any melanosomes in the apical region, whereas the two flanking cells do. The section has been immunogold-labeled for MYO7A. Boxed areas in d and e were enlarged in (e, f) respectively, to show immunogold particles (all have been circled). The cytoskeleton of the zonula adherens is evident in (d) across the entire profile of the central cell (bottom right arrow), indicating that the section is near the periphery of the cell. The lack of melanosomes evident in the apical RPE is not due to the plane of the section. In control RPE, melanosomes are obvious in the apical processes of cells sectioned in this manner. Scale bars: a–c, 10 μm; d–f, 1 μm. Bar graph (g) shows the relationship between the density of MYO7A immunogold particles and the observed correction of melanosome localization (data for each bar were obtained from 15 to 18 cells). Error bars represent ±s.e.m.
To correlate the level of MYO7A expression with correction of melanosome distribution, we quantified immunogold label of MYO7A on sections of LV-MYO7A(B)-treated Myo7a−/− retinas. The mosaic effect of the correction was also evident by electron microscopy, with corrected RPE cells neighboring uncorrected cells, based on the presence or absence of apical melanosomes (Figure 4d). In retinas infected at P1 and analyzed at P16, 94% of the cells, within 1.0 mm of the injection site, contained above background labeling, indicating they had at least been infected. Of these cells, 55% had corrected melanosome distribution. These corrected cells had a mean concentration of gold labeling that was comparable to Myo7a+/+ retinas, whereas the uncorrected cells (those that had been transduced but expressed lower levels of the transgene) had a mean concentration that was 65% lower than the wild-type level (Figure 4e–g). These results demonstrate that correction of the normal melanosome distribution in vivo was correlated with LV-MYO7A(B)-mediated MYO7A expression. Moreover, it is evident that a threshold level of MYO7A is necessary for correction.
Correction of opsin accumulation in the photoreceptor cilia of MYO7A-null mice
MYO7A-null mice were found to have a 2.6-fold higher concentration of opsin immunoreactivity in the connecting cilia of their photoreceptor cells.23 To test if this mutant phenotype had been corrected, we immunogold-labeled EM sections with opsin antibodies and counted the gold particles in the connecting cilia of photoreceptors underlying corrected RPE cells (i.e. cells containing apical melanosomes). For negative and positive controls, we also quantified opsin labeling in the connecting cilia of photoreceptors distant from the site of injection, where no correction of melanosome distribution was evident, as well as photoreceptors in control and untreated mutant retinas. Connecting cilia of photoreceptor cells associated with corrected RPE cells showed, on average, normal opsin labeling, indicating correction (Figure 5a–e).
Figure 5.
Correction of opsin distribution in the connecting cilia (arrows) of photoreceptor cells, following in vivo injection of LV-MYO7A(B). Opsin immunogold labeling of sections of photoreceptors from (a) a Myo7a+/+retina, (b) a Myo7a−/− retina, (c, d) a Myo7a−/− retina, infected with LV-MYO7A(B) at P1 and analyzed at P16. The photoreceptors in (c) are beneath an RPE cell that had correctly distributed melanosomes. Those in (d) are distant from the injection site, where the RPE melanosomes are all distributed as in MYO7A-null RPE cells. Scale bars: 500 nm. (e) Bar graph showing the concentration of opsin immunogold labeling in the cilia of photoreceptors like those in (a–d) (n = 43, 63, 28, and 17 cells, respectively). Error bars represent ±s.e.m.
Discussion
We have tested the efficacy of lentiviral gene therapy in a mouse model for the recessive combined deafness and blindness syndrome, Usher 1B. We demonstrate that recombinant lentivirus-mediated expression of the human MYO7A cDNA leads to effective rescue of several mutant phenotypes in MYO7A-null RPE and photoreceptor cells in vitro and in vivo. The correction of cellular abnormalities in the RPE is critically dependent upon the expression level of MYO7A protein. These findings demonstrate the therapeutic potential of lentiviral vectors for the retinal dystrophy of Usher 1B.
Expression of a large gene by a lentiviral vector
The most successful and widely used viral vector for retinal gene therapy has been recombinant AAV. However, its carrying capacity is limited to 5.2 kb.40 Here, we chose the third generation, recombinant lentiviral vector, in large part because of its high packaging capacity.35,36 Our results show that the current lentiviral vector can accommodate the 6962-bp human MYO7A cDNA plus at least 600 bp of promoter sequence. Furthermore, protein and functional analyses indicate that the lentiviral vector produced MYO7A of the expected molecular weight and cellular activity. To our knowledge, this is the largest transgene expressed by a viral vector in the RPE, or elsewhere in the retina. Our results thus further establish the lentiviral-based gene transfer approach in treating retinal and other inherited diseases caused by loss of function of large genes.
LV-MYO7A expression and correction of cellular events in photoreceptor and RPE cells
MYO7A is normally present in photoreceptor cells as well as the RPE as detected by immunoelectron microscopy, although the amount in the photoreceptors appears to be only a small fraction of that in the RPE. This difference in expression levels is most evident in immunofluorescence images of rodent retinas, where labeling of the photoreceptor cells is nearly undetectable, despite a very strong signal in the RPE cells.7,9,41 Most of the MYO7A in the RPE is associated with melanosomes.10,11,19 Quantitative studies have shown that this proportion (70–80%) is similar among mouse, pig and human RPE.11 A major focus of the present study was on RPE cell correction, especially the role of MYO7A in melanosome motility and localization, for which we have the most tractable assays. However, an ideal treatment of Usher 1B patients would effect MYO7A expression in both RPE and photoreceptor cells – at normal levels.
Previous studies have shown that VSV.G packaged lentiviral vectors can transduce rodent photoreceptor cells when encoding a photoreceptor-specific promoter.42,43 Our results of LV-AP(B) infection indicate that lentiviral vectors containing the CMV-MYO7A chimeric promoter can drive differential transgene expression in the RPE and photoreceptor cells. The much higher level of expression in the RPE cells resembles the endogenous expression patterns of MYO7A in the mouse retina, and may be a function of the native enhancer element included in the CMV-MYO7A promoter. However, the proportion of photoreceptors that were transduced, as indicated by the AP reporter, is low. There are several factors that might have contributed to this weaker transduction of photoreceptor cells. VSV.G packaged viral particles might be preferentially taken up by the RPE. Secondly, the titer of the LV-AP(B) virus used was relatively low (1 × 107 TU/ml). Lastly, access of the vector to the photoreceptor cells is likely to have been partly responsible. Gruter et al.,44 showed that removal of the physical barrier around adult photoreceptor cells with neuraminidase greatly increases transduction efficiency. With a view to clinical therapy, it is important to note that the extent of this physical barrier most likely differs between normal and partially degenerated retinas.
The present study demonstrates the importance of transgene expression levels in viral-based gene replacement therapies of the RPE. We found that the level of transgene expression mediated by the lentiviral vector is critical for the correction of melanosome mislocation phenotype in vivo. Quantification of immunogold labeling of MYO7A indicated that uncorrected RPE cells that had nevertheless apparently been transduced (since they contained above background levels of MYO7A) possessed an average of 35% of the wild-type level of MYO7A. There was a range of expression levels among these cells (note error bar in Figure 4f), so that the lower threshold level for correction of the melanosome mislocalization phenotype is likely to be substantially higher than 35%. On the other hand, our data also indicate that excessive levels of MYO7A are detrimental to RPE cells in vitro and in vivo; although it remains undetermined whether the cells are more sensitive to high levels of human MYO7A than they would be to comparable levels of murine MYO7A. In any case, it appears that there is a range of MYO7A expression levels, with upper and lower limits, that needs to be achieved in order to effect correction of mutant phenotypes in the RPE.
Despite the unambiguous AP reporter signals in the photoreceptor cells, we did not detect above background levels of MYO7A transgene expression in the photoreceptor cells by immunocytochemistry in virally transduced retinas. Nevertheless, photoreceptor cells, adjacent to corrected RPE cells, had normal, low levels of opsin label in their connecting cilia, indicating that they, too, had been corrected. The photoreceptor cells may have expressed MYO7A at a level that was lower than that found in wild-type cells by immunolabeling, but still sufficient to effect correction of the opsin distribution (in unpublished observations, we have found that retinas from wild-type (Myo7a+/+) mice, rather than the lower-expressing heterozygous (Myo7a+/−) mice, are needed for reliable MYO7A labeling of photoreceptor connecting cilia). Alternatively, the phenotype correction might have resulted indirectly from LV-MYO7A expression in the RPE cells. More efficient disposal of phagosomes by the RPE, the end stage of the disk renewal process, might have removed inhibition of earlier stages, and thus corrected opsin transport along the photoreceptor connecting cilium and distal migration of disks along the outer segment. But, given the normal presence of MYO7A in the connecting cilium,12 a direct effect on the photoreceptor cells seems more likely. The transduction efficiency of the photoreceptor cells by LV-MYO7A should have been much higher than that by LV-AP, since the titer of the LV-MYO7A(B) was 100-fold greater.
The considerable heterogeneity of lentiviral-mediated transgene expression observed among different RPE and photoreceptor cells likely results from variation in transduction efficiency, including different copy number and site of integration.45,46 Strategies for providing better regulation of the level of transgene expression are therefore an important consideration, especially with a view to potential clinical therapy. Such strategies might include (1) using chromatin insulators47,48 to obviate the effects of different integration sites and allow transgene expression to be regulated only by the virally encoded promoter/enhancer elements, or (2) simply avoiding integration, by using integrase-deficient lentiviruses, which have been shown to mediate effective, stable transduction of retinal cells.49
A potential treatment for blindness in Usher 1B
The shaker1 mice have been an important animal model for characterizing cellular defects that potentially exist in humans with mutant MYO7A. Defects in the renewal of photoreceptor disk membranes (as manifest by opsin accumulation in the photoreceptor connecting cilium and retarded phagosome processing) and melanosome trafficking may be central to the development of the disease pathology found in Usher 1B patients, even though the photoreceptor cells of shaker1 mice do not appear to degenerate significantly during the lifespan of the animal (at least on certain backgrounds).15,16 Lack of photoreceptor cell loss is also found in a number of other mouse models of retinal degeneration, such as the Abca4 knockout mouse, a model for Stargardt macular degeneration,50 and all the known mouse models for the other types of Usher 1.51–53
With regard to diseases caused by loss of gene function, as appears to occur in Usher 1B, the critical question is how well the introduced gene mimics wild-type function. The most direct assessment of this question is by analysis of gene expression level and cell-based assays, rather than measurements of cell loss. Cell death is a downstream event that can be influenced by many factors – for example, the mere act of subretinal injection can promote photoreceptor survival.54 Lack of cell death is clearly an important test for the absence of unintended side effects, but such tests can also be performed independent of efficacy studies with any non-mutant species. From a practical viewpoint, rapid responses to treatment are desirable endpoints in any clinical trial. Inhibition of retinal degeneration is unlikely to be a particularly useful measure because of its relatively slow time course. A task ahead is to develop suitable assays for use in Usher 1B patients.
In conclusion, we have demonstrated that cellular abnormalities, representing primary responses to lack of MYO7A in RPE and photoreceptor cells, can be corrected by lentiviral gene therapy. As a first step towards developing gene therapy as a potential treatment for Usher 1B blindness, we have also provided an assessment of the levels of MYO7A expression required for the correction of mouse retinal cellular phenotypes. More generally, our results demonstrate the utility of lentiviral vectors in the delivery of large transgenes in gene therapy.
Materials and methods
Animals
Shaker1 mice carrying the 4626SB allele, an effective null mutation,15,23 were used on either the C57BL6 or BS (albino) genetic backgrounds, and maintained and genotyped as described.18,23 They were maintained on a 12-h light/12-h dark cycle, with exposure to 10–50 lux of fluorescent lighting during the light phase, and were treated according to NIH, UCLA and UCSD animal care guidelines. Homozygous mutants were distinguished from the heterozygous controls by their hyperactivity, head-tossing and circling behavior,13 and/or by a PCR/restriction digest assay. CD1 albino mice were also used for testing of the chimeric promoter.
Construction of lentiviral vectors
A full-length, human MYO7A cDNA was assembled from three overlapping fragments, pM7-10a,7 and the IMAGE EST clones BE780659 and AI355462, using the pCMV-SPORT6 vector (Invitrogen, Carlsbad, CA, USA). The assembled cDNA was confirmed by complete sequencing. The lentiviral backbone used to construct LV-MYO7A viral vectors was derived from a third generation, self-inactivating vector, LV-CIG.37 The posttranscriptional regulatory element of woodchuck hepatitis virus (WPRE)55 was deleted from the LV-CIG vector. A total of 6962-bp of the MYO7A cDNA, including the entire translated region, 275 bp of 5′UTR, and 39 bp of 3′UTR without the polyadenylation site, was used to replace the cre-IRES-EGFP sequence in the LV-CIG vector.37 For the LV-MYO7A(A) vector, the MYO7A cDNA was under the control of the 530-bp human cytomegalovirus immediate early promoter (referred to as CMV promoter in the text). LV-MYO7A(B) encoded a chimeric promoter containing the 5′ 290 bp of the CMV promoter and a 160-bp MYO7A genomic sequence (Chr. 11q, nt 132114–132273 of AP000752, GenBank), which resides immediately upstream of the start codon and overlaps with a partially characterized MYO7A regulatory sequence.56 LV-MYO7A(C) contained only the 160-bp human MYO7A genomic sequence. The LV-AP(B) contained the same chimeric promoter as LV-MYO7A(B), except that the MYO7A cDNA was replaced by the human placental alkaline phosphatase (AP) cDNA.
Production of lentiviral stocks
Human embryonic kidney (HEK) 293T cells were cotransfected with three packaging plasmids, pLP1, pLP2, pLP/VSVG, and a given lentiviral vector construct, using Lipofectamine 2000, as described36 (Invitrogen). After 24 h, culture medium was replaced by fresh 10% fetal calf serum (FCS)/Dulbecco’s-modified Eagle’s medium (DMEM) or the serum-free CD293 (Invitrogen). Viruscontaining medium was collected at 48 h post transfection, filtered through 0.4 μm Durapore units (Millipore, Billerica, MA, USA), and concentrated by ultracentrifugation, as described.57 Viral titers, defined as TU/ml, were determined by immunostaining of the transgene product in cells infected with serially diluted viral stocks. In the case of LV-MYO7A(B), which gave very weak transgene expression in HEK293T cells, viral titer was determined by anti-MYO7A immunostaining of infected primary mouse Myo7a−/− RPE cells. The titer of LV-AP(B) was determined by AP histochemistry,58 following infection of ARPE19 cells. Concentrated lentiviral stocks used for in vivo and in vitro studies had titers of 2 × 108 TU/ml for LV-CIG,37 2 × 109 TU/ml for LV-MYO7A(A), 1 × 109 TU/ml for LV-MYO7A(B), and 1 × 107 TU/ml for LV-AP(B).
Lentiviral infection of cultured primary RPE cells and ARPE-19 cells
RPE cells from Myo7a+/− and Myo7a−/− mice were isolated as described previously.18,59 Cells were resuspended in 20 μl of concentrated viral stock, seeded into the upper well of 24-well transwell plates (Corning, Corning, NY, USA) and incubated at 37°C for 3 h. The medium was then diluted fivefold with medium, containing high glucose DMEM, 10% FCS, 1 × MEM non-essential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin and 6 μg/ml hexadimethrine bromide (Polybrene; Sigma, St Louis, MO, USA), by bringing the volume up to 0.1 ml. The volume in the lower well was then increased to 0.5 ml and the cells were incubated for 24 h before replacing the medium with fresh virus-free medium without polybrene.
ARPE-19 cells were plated in six-well plates 2 ml per well) for 5 h before the addition of 5 μl per well of concentrated viral stock in medium containing 6 μg/ml hexadimethrine bromide. Cells were fixed at 72 h post infection for immunostaining.
Viral delivery in vivo
Mice were anesthetized with 2.0–3.0% isoflurane inhalation. The injection needle (32 gauge, Hamilton, Reno, NV, USA) was inserted through the temporal limbus and 0.5 μl of viral solution was injected into the ventral subretinal space of neonatal or adult mice. The viral solution consisted of concentrated viral stock with 6 μg/ml Polybrene and 0.025% Fast Green dye (Sigma).
Labeling of cultured RPE cells and retinal cryosections
Cultured cells were fixed and labeled with affinity purified MYO7A antibody, pAb2.2,12 followed by an Alexa Fluor 594 nm secondary antibody (Invitrogen). For Western blots, lysates were obtained from cells cultured on transwell plates for 5 days. After blotting, proteins were labeled using MYO7A pAb2.2 and HSP60 mAb (Nventa, San Diego, CA, USA), and an alkaline phosphatase-conjugated secondary antibody. Thick (14 μm) retinal cryosections were immunolabeled with MYO7A pAb2.2, followed by a biotinylated secondary antibody and horseradish peroxidase (HRP) detection, using the Elite ABC kit (Vector Labs, Burlingame, CA, USA). AP histochemistry was performed as described.58
Analyses of cultured RPE cells
The digestion of mouse ROSs by the RPE cells was assayed as described.18 Briefly, 7 days after viral infection, cells were incubated with ROSs for 20 min, washed repeatedly with cold PBS to remove unbound ROSs, and incubated for a further 2 h. The total number of ROSs remaining in the cells, and the number of DAPI positive nuclei per field were counted in images recorded from five randomly selected fields of view at × 200 magnification. This procedure was repeated on five separate filters per treatment.
Melanosome motility data sets were recorded, using bright-field time-lapse microscopy, from four or five live RPE cells (from different cultures) per treatment, 7 days after viral infection, as described.11 Kymograph traces and displacement measurements were extracted for 80–90 melanosomes per treatment using the multiple kymograph function in ImageJ (http://rsb.info.nih.gov/ij/).
Light microscopy and immunoelectron microscopy of retinas
Cryosections stained for immunocytochemistry or histochemistry were imaged by DIC optics. Eyecups were processed for embedment in LR White, and semithin and ultrathin sections were prepared, as described previously.11 Ultrathin sections were labeled with affinitypurified MYO7A antibody, followed by a 10-nm gold secondary antibody. Negative control sections processed at the same time included those from Myo7a−/− retinas and those from the same retinas that were incubated with 1 mg/ml of the original antigen fusion protein together with the MYO7A antibody.
MYO7A immunogold density was determined on sections of same-aged Myo7a+/+ retinas and Myo7a−/− retinas that had been injected with LV-MYO7A(B) at P1 and dissected at P16. Cells were determined as corrected or not corrected by the apical localization of melanosomes, at a magnification that was too low to resolve the gold particles (hence there was no bias, based on labeling intensity). For quantification of the immunolabel, images of higher magnification were used, and all the gold particles in a complete section of each RPE cell were counted. The area of each cell’s profile was determined using ImageJ software. For background labeling, the concentration of label in the outer nuclear layer was measured.
The concentration of opsin immunogold labeling in the connecting cilia of photoreceptor cells was determined by counting the gold particles along longitudinal profiles of connecting cilia and measuring the area of each profile. The labeling was quantified in four categories of photoreceptor cell: cells that were subjacent to corrected RPE cells in LV-MYO7A(B)-treated retinas; cells that were distant from the injection site, where RPE melanosome distribution was not corrected in LV-MYO7A(B)-treated retinas; those from MYO7A-null untreated retinas; and those from control (Myo7a+/+) mice.
Supplementary Material
Acknowledgements
We thank Dr Inder Verma for providing the third generation, self-inactivating lentiviral vector, and Dr Tama Hasson for providing the pM7-10a clone, used in the construction of the MYO7A cDNA. This research was supported by grants from the Foundation Fighting Blindness, Research to Prevent Blindness Foundation, and the NIH (EY14440, EY07042, and core Grants EY12598 and EY00331).
Footnotes
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt).
References
- 1.Smith RJ, Berlin CI, Hejtmancik JF, Keats BJ, Kimberling WJ, Lewis RA et al. Clinical diagnosis of the Usher syndromes. Usher Syndrome Consortium. Am J Med Genet 1994; 50: 32–38. [DOI] [PubMed] [Google Scholar]
- 2.Keats BJ, Corey DP. The usher syndromes. Am J Med Genet 1999; 89: 158–166. [PubMed] [Google Scholar]
- 3.Astuto LM, Weston MD, Carney CA, Hoover DM, Cremers CW, Wagenaar M et al. Genetic heterogeneity of Usher syndrome: analysis of 151 families with Usher type I. Am J Hum Genet 2000; 67: 1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharadwaj AK, Kasztejna JP, Huq S, Berson EL, Dryja TP. Evaluation of the myosin VIIA gene and visual function in patients with Usher syndrome type I. Exp Eye Res 2000; 71: 173–181. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang XM, Yan D, Du LL, Hejtmancik JF, Jacobson SG, Nance WE et al. Characterization of Usher syndrome type I gene mutations in an Usher syndrome patient population. Hum Genet 2005; 116: 292–299. [DOI] [PubMed] [Google Scholar]
- 6.Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 1995; 374: 60–61. [DOI] [PubMed] [Google Scholar]
- 7.Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci USA 1995; 92: 9815–9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfrum U, Liu X, Schmitt A, Udovichenko IP, Williams DS. Myosin VIIa as a common component of cilia and microvilli. Cell Motil Cytoskeleton 1998; 40: 261–271. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs D, Williams DS. Usher 1 protein complexes in the retina. Invest Ophthalmol Vis Sci 2004; 45: e-letter (May 26) http://www.iovs.org/cgi/eletters/44/11/5006. [Google Scholar]
- 10.El-Amraoui A, Schonn JS, Kussel-Andermann P, Blanchard S, Desnos C, Henry JP et al. MyRIP, a novel Rab effector, enables myosin VIIa recruitment to retinal melanosomes. EMBO Rep 2002; 3: 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs D, Azarian SM, Lillo C, Kitamoto J, Klomp AE, Steel KP et al. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J Cell Sci 2004; 117: 6473–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Vansant G, Udovichenko IP, Wolfrum U, Williams DS. Myosin VIIa, the product of the Usher 1B syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motil Cytoskel 1997; 37: 240–252. [DOI] [PubMed] [Google Scholar]
- 13.Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M et al. A type VII myosin encoded by mouse deafness gene shaker-1. Nature 1995; 374: 62–64. [DOI] [PubMed] [Google Scholar]
- 14.Mburu P, Liu XZ, Walsh J, Saw D, Jamie M, Cope TV et al. Mutation analysis of the mouse myosin VIIA deafness gene. Genes Funct 1997; 1: 191–203. [DOI] [PubMed] [Google Scholar]
- 15.Hasson T, Walsh J, Cable J, Mooseker MS, Brown SDM, Steel KP. Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton 1997; 37: 127–138. [DOI] [PubMed] [Google Scholar]
- 16.Lillo C, Kitamoto J, Liu X, Quint E, Steel KP, Williams DS. Mouse models for Usher syndrome 1B. Adv Exp Med Biol 2003; 533: 143–150. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Ondek B, Williams DS. Mutant myosin VIIa causes defective melanosome distribution in the RPE of shaker-1 mice. Nat Genet 1998; 19: 117–118. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci USA 2003; 100: 6481–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol Biol Cell 2004; 15: 2264–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 1969; 42: 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bok D, Hall MO. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol 1971; 49: 664–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schraermeyer U, Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res 1999; 12: 219–236. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Udovichenko IP, Brown SDM, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci 1999; 19: 6267–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 2001; 28: 92–95. [DOI] [PubMed] [Google Scholar]
- 25.Narfstrom K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM et al. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci 2003; 44: 1663–1672. [DOI] [PubMed] [Google Scholar]
- 26.Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther 2004; 9: 182–188. [DOI] [PubMed] [Google Scholar]
- 27.Lai CM, Yu MJ, Brankov M, Barnett NL, Zhou X, Redmond TM et al. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65−/− knockout mouse eye results in limited rescue. Genet Vaccines Ther 2004; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang JJ, Chang B, Kumar A, Nusinowitz S, Noorwez SM, Li J et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther 2006; 13: 565–572. [DOI] [PubMed] [Google Scholar]
- 29.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther 2005; 12: 1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A et al. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci USA 2001; 98: 12584–12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith AJ, Schlichtenbrede FC, Tschernutter M, Bainbridge JW, Thrasher AJ, Ali RR. AAV-Mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. Mol Ther 2003; 8: 188–195. [DOI] [PubMed] [Google Scholar]
- 32.Tschernutter M, Schlichtenbrede FC, Howe S, Balaggan KS, Munro PM, Bainbridge JW et al. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther 2005; 12: 694–701. [DOI] [PubMed] [Google Scholar]
- 33.Kelley PM, Weston MD, Chen ZY, Orten DJ, Hasson T, Overbeck LD et al. The genomic structure of the gene defective in Usher syndrome type Ib (MYO7A). Genomics 1997; 40: 73–79. [DOI] [PubMed] [Google Scholar]
- 34.Levy G, Levi-Acobas F, Blanchard S, Gerber S, Larget-Piet D, Chenal V et al. Myosin VIIA gene: heterogeneity of the mutations responsible for Usher syndrome type IB. Hum Mol Genet 1997; 6: 111–116. [DOI] [PubMed] [Google Scholar]
- 35.Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem 2005; 74: 711–738. [DOI] [PubMed] [Google Scholar]
- 36.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc Natl Acad Sci USA 2001; 98: 11450–11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aiken C Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol 1997; 71: 5871–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA 1993; 90: 8033–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol 2005; 79: 9933–9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.el-Amraoui A, Sahly I, Picaud S, Sahel J, Abitbol M, Petit C. Human Usher 1B/mouse shaker-1: the retinal phenotype discrepancy explained by the presence/absence of myosin VIIA in the photoreceptor cells. Hum Mol Genet 1996; 5: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 42.Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA 1997; 94: 10319–10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi M, Miyoshi H, Verma IM, Gage FH. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol 1999; 73: 7812–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gruter O, Kostic C, Crippa SV, Perez MT, Zografos L, Schorderet DF et al. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther 2005; 12: 942–947. [DOI] [PubMed] [Google Scholar]
- 45.Bushman FD. Targeting survival: integration site selection by retroviruses and LTR-retrotransposons. Cell 2003; 115: 135–138. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol 2004; 2: E234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuhn EJ, Geyer PK. Genomic insulators: connecting properties to mechanism. Curr Opin Cell Biol 2003; 15: 259–265. [DOI] [PubMed] [Google Scholar]
- 48.West AG, Fraser P. Remote control of gene transcription. Hum Mol Genet 2005; 14 (Suppl): R101–R111. [DOI] [PubMed] [Google Scholar]
- 49.Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med 2006; 12: 348–353. [DOI] [PubMed] [Google Scholar]
- 50.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardťs disease from the phenotype in abcr knockout mice. Cell 1999; 98: 13–23. [DOI] [PubMed] [Google Scholar]
- 51.Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Chang B et al. Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum Mol Genet 2003; 12: 3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Libby RT, Kitamoto J, Holme RH, Williams DS, Steel KP. Cdh23 mutations in the mouse are associated with retinal dysfunction but not retinal degeneration. Exp Eye Res 2003; 77: 731–739. [DOI] [PubMed] [Google Scholar]
- 53.Ball SL, Bardenstein D, Alagramam KN. Assessment of retinal structure and function in Ames waltzer mice. Invest Ophthalmol Vis Sci 2003; 44: 3986–3992. [DOI] [PubMed] [Google Scholar]
- 54.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 1990; 347: 83–86. [DOI] [PubMed] [Google Scholar]
- 55.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol 1999; 73: 2886–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orten DJ, Weston MD, Kelley PM, Cremers CW, Wagenaar M, Jacobson SG et al. Analysis of DNA elements that modulate myosin VIIA expression in humans. Hum Mutat 1999; 14: 354. [DOI] [PubMed] [Google Scholar]
- 57.Yang X-J. Preparation of Recombinant Retroviruses. In: Rakoczy E (ed). Vision Research Protocols. Human Press: Torowa, NJ, 2001, pp 171–190. [DOI] [PubMed] [Google Scholar]
- 58.Fields-Berry SC, Halliday AL, Cepko CL. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc Natl Acad Sci USA 1992; 89: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibbs D, Williams DS. Isolation and culture of primary mouse retinal pigmented epithelial cells. Adv Exp Med Biol 2003; 533: 347–352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.