Abstract
Increasing evidence has linked the humoral immune response with the development of various cancers. Therefore, there is growing interest in investigating the predictive value of antibodies to assess overall and tissue site-specific cancer risk. Given the large amount of antibody types and the broad scope of the search (i.e. cancer risk), the primary aim of this systematic review was to present an overview of the most researched antibodies (i.e. immunoglobulin (Ig) isotypes (IgG, IgM, IgA, and IgE), tumour and self-antigen-reactive antibodies, infection-related antibodies) in relation to overall and site-specific cancer risk. We identified various antibody types that have been associated with the risk of cancer. While no significant associations were found for IgM serum levels, studies found an inconsistent association among IgE, IgA, and IgG serum levels in relation to cancer risk. When evaluating antibodies against infectious agents, most studies reported a positive link with specific cancers known to be associated with the specific agent recognized by serum antibodies (i.e. helicobacter pylori and gastric cancer, hepatitis B virus and hepatocellular carcinoma, and human papillomavirus and cervical cancer). Several reports identified autoantibodies, as single biomarkers (e.g. anti-p53, anti-MUC1, and anti-CA125) but especially in panels of multiple autoantibodies, to have potential as diagnostic biomarkers for specific cancer types. Overall, there is emerging evidence associating certain antibodies to cancer risk, especially immunoglobulin isotypes, tumour-associated antigen-specific, and self-reactive antibodies. Further experimental studies are necessary to assess the efficacy of specific antibodies as markers for the early diagnosis of cancer.
Keywords: antibodies, biomarkers, cancer, early detection, tumor -associated antigens, immunoglobulin
The humoral immune response has been consistently linked with the development of various cancers. Therefore, increasing evidence has investigated the predictive value of antibodies to assess site-specific cancer risk. In the current study, we review the current evidence for the association between the most researched antibodies and risk of site-specific cancers
Graphical Abstract
Graphical Abstract.
Introduction
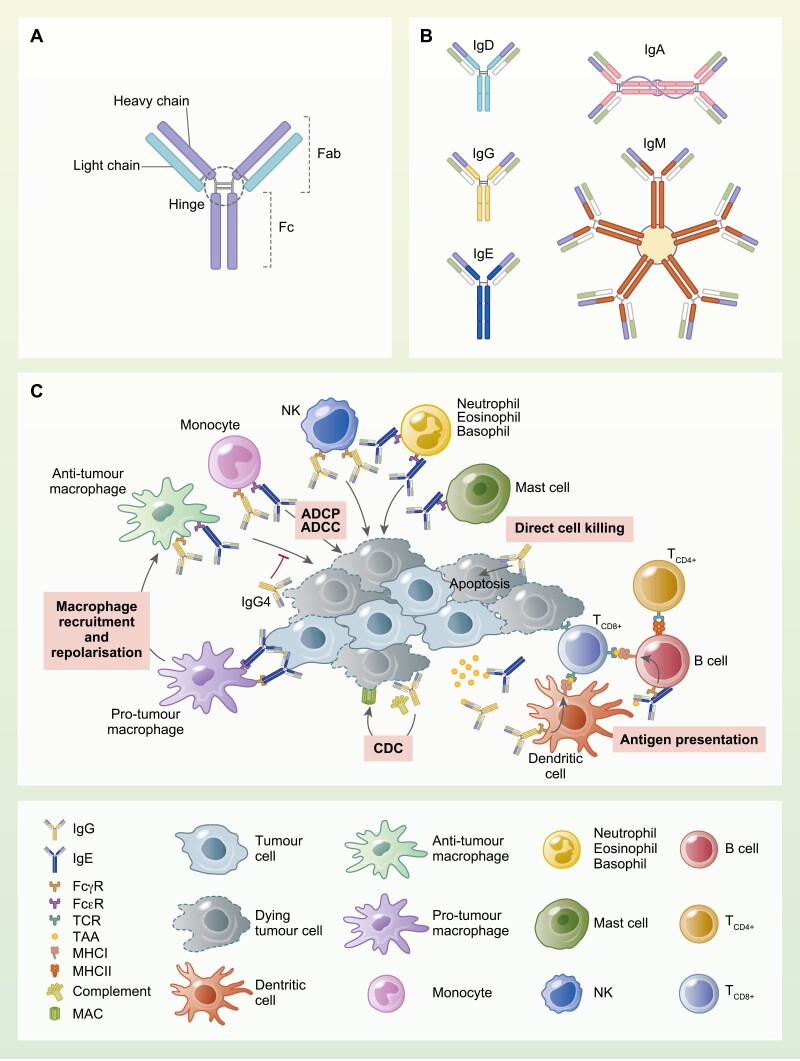
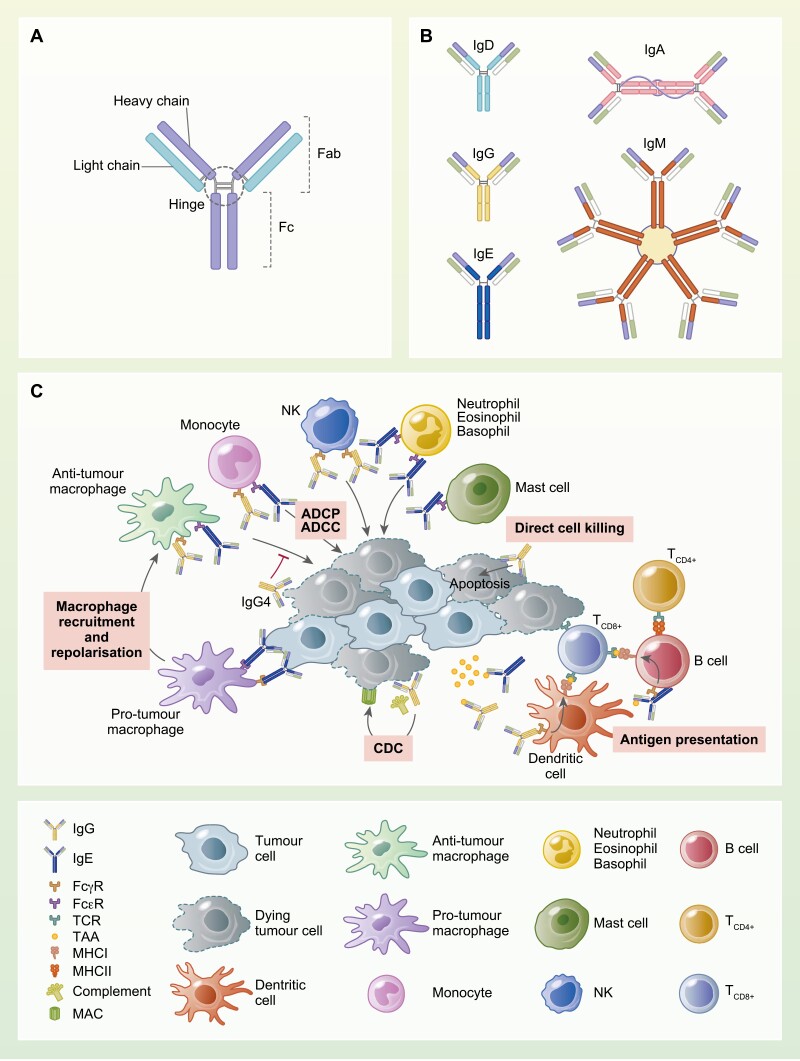
Immunoglobulins (Ig) are tetrameric glycoproteins produced by B cells as part of the humoral immune response. Their structure is composed of a Fab region, consisting of two identical Fab fragments, including the light chain and part of the heavy chain; a fragment crystallizable (Fc) region formed by the constant portion of the two heavy chains; and a hinge region, joining the Fab and Fc regions (Fig. 1). The heavy chain defines the isotype of the antibody, and the Fc portion can bind cognate Fc receptors (FcRs) on immune cells and members of the complement cascade including complement component 1q (C1q) and is responsible for antibody-mediated effector functions such as antibody-dependent cell cytotoxicity (ADCC), antibody-dependent cell phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) [1]. Antibodies, binding to FcRs expressed on immune cells, can also influence immune cell phenotype, and polarization and once complexed with antigens to form immune complexes, they can be internalized to facilitate antigen presentation. Human B cells can express five antibody classes (divided into nine antibody isotypes, IgD, IgM, IgG [1–4], IgA [1, 2], and IgE). Each class recognizes specific cognate FcRs or C1q with different affinity and thus differ in their abilities to trigger effector functions such as ADCC, ADCP, and CDC. Therefore, antibody isotype may significantly influence the immune response that may protect not only against external pathogens but also from the rise of cancer. The IgM isotype is involved in primary immune response and in its secreted form it can assemble in high avidity pentamers. IgG is the predominant class of antibodies in the human serum. IgG subclasses like IgG1 and IgG3 have a high affinity for activating FcγRs and C1q resulting in a high capacity to trigger ADCC and activate the complement cascade. IgG2 and IgG4 subclasses have instead poor capacity to fix complement and lower ability to bind activating FcγRs compared to IgG1, and IgG4 has a relatively high affinity for the inhibitory receptor FcγRIIb resulting in negative immune effector cell activating signals and lower ability to trigger effector functions. IgA is the predominant isotype in mucosal surfaces and in secretions, and its neutralizing capacity is crucial for protecting mucosal surfaces from toxins, viruses, and bacteria. It has a low capacity to activate complement but can engage neutrophils and trigger strong ADCC. IgE antibodies are usually associated with hypersensitivity and allergic reactions as well as responses to parasitic worm infections. IgE can trigger ADCC and ADCP as well as being able to facilitate antigen presentation and, in the context of cancer immune surveillance, being able to repolarize pro-tumour macrophages into pro-inflammatory, anti-tumour phenotypes [2]. In addition to antibody isotypes, another feature that can influence antibody effector function is antibody glycosylation, which might modulate Fc receptors’ affinity and consequently antibody effector function. This has been widely studied for IgG isotypes, with interesting findings on the effects of fucosylation, galactosylation, and sialylation [3]. Of notice, alterations in IgG galactosylation have been reported as a biomarker for multiple cancer types [4]. Antibodies can also have a direct effect by binding to the target antigen. For cell surface antigens involved in downstream signalling, antibody-target engagement can sometimes have an agonistic effect on the target which could result in activation of a signalling cascade, but most often the binding of the antibody could have an antagonistic/inhibitory effect on the target’s downstream signalling functions. This can result in impaired cell growth and apoptosis; for cell surface antigens involved in cell–cell interactions or adhesion, antibody binding could impair or prevent these processes resulting in inhibition of tumour progression (Fig. 1) [1].
Figure 1:
(A) Schematic representing antibody structure with heavy and light chains, and Fab, hinge, and Fc regions. (B) Heavy chain constant regions of different isotype are labelled in: light blue (IgD), yellow (IgG), blue (IgE), pink (IgA), red (IgM); IgM and IgA J chain is in blue. (C) Antibody-mediated anti-tumour or pro-tumour effector functions. Antibodies can exert several anti-tumour effector functions: mediating ADCC, ADCP, and CDC. Antibodies engaged with FcRs on immune effector cells and bound to tumour-derived antigens to form immune complexes, can (a) repolarize immune cells such as NK cells and pro-tumour macrophages into pro-inflammatory, anti-tumour phenotypes and (b) facilitate antigen internalization, processing, and presentation to activate T cells. Antibodies can also exert direct cell killing, via antigen neutralization and blocking of downstream signalling, resulting in block of tumour growth and induction of apoptosis. Some IgG subclasses, such as IgG4, can exert pro-tumour functions. IgG4 has poor capacity to fix complement and lower ability to bind activating FcγRs and therefore lower ability to trigger effector functions compared to IgG1, and relatively high affinity for the inhibitory receptor FcγRIIb, resulting in negative immune effector cell activating signals, potentially blocking IgG1 mediated effector functions. See online supplementary material for a colour version of this figure.
It has been suggested that the humoral immune system plays an important role in both the support and suppression of carcinogenesis [5]. For instance, several studies have reported the ability of B cells to inhibit tumour development through the production of tumour-reactive antibodies [6]. However, B cells can also contribute to immune tolerance and allow tumour development by producing immunosuppressive cytokines and antibodies which are ineffective in mediating immune effector functions [6]. Moreover, the humoral immune system is crucial for protection against invading pathogens and plays a critical role in the control and suppression of malignant cells via immunosurveillance. Therefore an imbalance in the immune system homeostasis may have an effect in carcinogenesis. There is ample evidence linking prior and chronic exposure to several infectious agents with a higher risk of cancer (i.e. human papillomavirus (HPV), Epstein-Barr virus (EBV), and Helicobacter pylori (HP)). Moreover, epidemiological evidence has pointed to significant associations between autoimmune disorders and cancer risk. An increased risk of malignancies has been observed previously in different autoimmune disorders.
Furthermore, immunoglobulins against self-antigens and tumour-associated antigens (TAAs) have been found both in the serum of patients with cancer and in the tumour microenvironment [7, 8]. Tumours can produce TAAs either by mutational mechanisms (mutated tumour-specific antigens, mTSAs) or by non-mutational mechanisms (non-mutational TAAs, nmTAAs), which could be overexpressed in cancer compared to normal tissue or may be cancer-specific. TAAs may induce an immune response. Humoral immune surveillance mechanisms may be protective against tumour cells and inhibit cancer growth, however, if the antigens are not tumour specific, the immune system can also recognize antigen-expressing non-malignant cells resulting in autoimmune reactions [7, 9, 10]. However, the propensity of tumour cells to escape immune surveillance may be a key step in tumorigenesis [6].
The presence, specificity, and isotype distribution of Igs in patients with cancer likely have an impact on tumour progression and could potentially inform on early detection of cancer and even predict the survival of the patient [6, 11]. Procedures to test the presence of antibodies, especially serum antibodies, are minimally invasive and easy to measure, and for this they harbour potential as biomarkers for cancer. Therefore, evaluating the link between antibodies and cancer risk and validating antibodies as biomarkers for diagnostic purposes are crucial. This may be especially beneficial in relation to cancers for which screening tests are currently lacking, but for which earlier detection would provide a substantial chance to treat promptly and offers a better chance of prolonged survival. In the present study, we aimed to outline the current evidence for the associations between the most researched immunoglobulin types and the risk of tissue site-specific cancers, and for the utility of Igs as biomarkers for cancer detection.
Methods
Data sources and searches
The current systematic review was performed in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [12]. We performed a literature search of epidemiological studies using PubMed with the search terms presented in Table 1. We included human studies published in English between 1 January 2000 and 9 September 2021. After preliminary screening of titles and abstracts, five independent reviewers (MM, NL, KB, SC, and AS) assessed the full text and reference lists of relevant publications for final inclusion; articles cited as references that were considered to be potentially relevant were also reviewed.
Table 1.
Search strategy followed in the search engine PubMed on 9th September 2021.
| Search terms | Hits | |
|---|---|---|
| Restrictions | Humans | |
| Full-text | ||
| English | ||
| Adult | ||
| From 01/01/2000 | ||
| Malignancy | ||
| #1 | “Cancer” [Title/Abstract] OR “Leukemia” [Title/Abstract] OR “Lymphoma” [Title/Abstract] OR “Myeloma” [Title/Abstract] OR “Leukaemia” [Title/Abstract] OR “Carcinoma” [Title/Abstract] OR “Neoplasm” [Title/Abstract] OR “Malignant tumor” [Title/Abstract] OR “Malignant tumour” [Title/Abstract] | 605,152 |
| #2 | “Neoplasms” [Mesh] | 816,183 |
| #3 | #1 AND #2 | 557,416 |
| #4 | “cancer risk” [Title/Abstract] | 639,508 |
| #5 | “risk*” [Title/Abstract] | |
| #6 | #4 OR #5 | 859,604 |
| #7 | #3 AND #6 | 146,542 |
| Immunoglobulin | ||
| #8 | “Immunoglobulin*” [Title/Abstract] OR “Serum Immunoglobulin*” [Title/Abstract] OR “serum Ig*” [Title/Abstract] OR “Antibod*” [Title/Abstract] OR “Autoantibod*” [Title/Abstract] OR “IgG” [Title/Abstract] OR “IgE” [Title/Abstract] OR “IgM” [Title/Abstract] OR “IgA” [Title/Abstract] OR “IgD” [Title/Abstract] | 136,508 |
| #9 | #7 AND #8 | 3,874 |
| Exclusions | ||
| #10 | ((“Therapeutics” [Mesh]) OR “Pharmacology” [Mesh]) OR “Therapeutic Uses” [Mesh] OR “Treatment” [Title/Abstract] | 1,812,442 |
| #11 | #9 NOT #10 | 2,126 |
Study selection
Only epidemiological studies looking at the association between any serum immunoglobulin antibodies and cancer risk were included. No publications exploring antibodies as potential markers of cancer survival or cancer prognosis were included. We also excluded publications using immunoglobulins as molecular markers in experimental studies such as imaging techniques and/or treatments.
The inclusion criteria considered studies on adults only. Single case studies were excluded. No other restrictions were placed on publication type, with all systematic reviews, narrative reviews, meta-analyses, original research articles (experimental, observational, and clinical trials), commentaries, letters, and editorials identified in the PubMed search, being considered eligible. Non-English publications, duplicate studies, preprints, errata, and animal studies were excluded. Moreover, only publications with full text available were included.
For each selected study, the following study characteristics were extracted into a designated datasheet: name of the first author, year of publication, study location, study design, number of participants, exposure, outcome (i.e. cancer type), main findings, and other observations.
Results
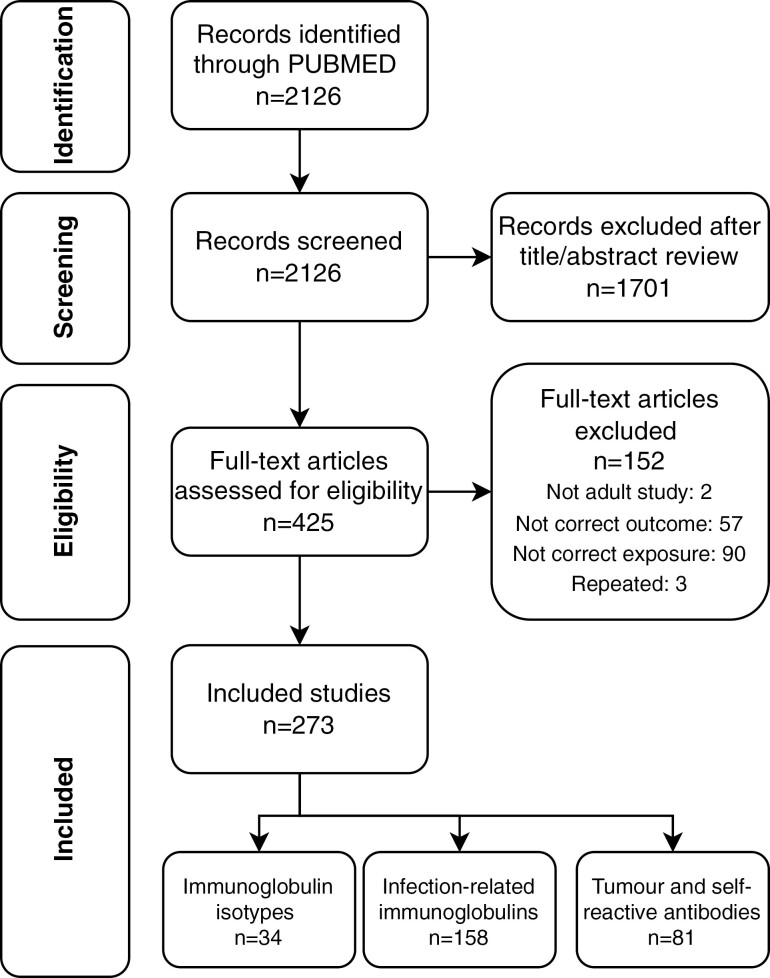
Figure 2 shows the PRISMA flowchart illustrating the study selection procedure. Our PubMed search resulted in a total of 2126 studies. A full-text review was undertaken on 425 potentially eligible articles after title and abstract screening. Following full-text review, 273 publications were included. Of the 152 full-text articles excluded, 2 were looking at paediatric populations, 56 explored a different outcome (i.e. not cancer risk), 90 investigated a different exposure (i.e. antibodies), and 3 were repeated studies. Moreover, information on publications referenced in Tables 2–6 can be found in Supplementary Table S1.
Figure 2.
PRISMA diagram representing the systematic review strategy.
Table 2.
Summary of results of associations between immunoglobulin isotypes and site-specific cancer risks. The strength of association is defined by the number of studies reporting on the association, the range of the hazard ratio/odds ratio/relative risks/standardized incidence ratio reported in each study, and the statistical significance.
| Exposure | Population | Outcome | Number of studies | Main findings | |
|---|---|---|---|---|---|
| Total IgE | IgE deficiency | General population | Overall | 2 | Strong negative association |
| High total IgE | Overall | 4 | Intermediate positive association | ||
| Pancreatic cancer | 1 | No significant association | |||
| Lymphoma, leukaemia, myeloma | 2 | Strong positive association | |||
| Prostate (high PSA) | 1 | Weak positive association | |||
| Head and neck | 1 | Intermediate positive association | |||
| High allergen-specific IgE (serum) | Skin cancer | 1 | No significant association | ||
| Lung | 1 | No significant association | |||
| Breast | 2 | No significant association | |||
| Prostate | 1 | Intermediate positive association | |||
| Lymphoma | 1 | No significant association | |||
| Colon, rectum | 1 | No significant association | |||
| Brain (Glioma) | 1 | Intermediate negative association | |||
| Pancreatic | 1 | No significant association | |||
| Skin cancer | 1 | Intermediate positive association | |||
| Self-reported allergies | Overall | 1 | No significant association | ||
| Lung | 1 | Intermediate negative association | |||
| Breast | 1 | No significant association | |||
| Prostate | 1 | No significant association | |||
| Lymphoma | 1 (pooled analysis of 13 studies) | Strong negative association | |||
| Pancreatic | 1 | No significant association | |||
| High asthma-specific IgE (SR) | Overall | 1 | No significant association | ||
| Lung | 1 | Intermediate positive association | |||
| Breast | 1 | No significant association | |||
| Prostate | 1 | No significant association | |||
| Lymphoma | 1 (pooled analysis of 13 studies) | Strong negative association | |||
| IgA | High total IgA | General population | Overall | 3 | No significant association |
| Pancreatic | 1 | No significant association | |||
| Melanoma | 1 | No significant association | |||
| Bladder | 1 | No significant association | |||
| Solid cancers | 1 (meta-analysis 14 studies) | Strong positive association | |||
| Lymphoma | 2 | Strong negative association | |||
| Gastrointestinal | 2 | Strong negative association | |||
| IgG | High total IgG | General population | Overall | 1 | No significant association |
| Pancreatic | 1 | Weak negative association | |||
| Melanoma | 1 | No significant association | |||
| Bladder cancer | 1 | No significant association | |||
| Solid cancers | 1 (meta-analysis 14 studies) | No significant association | |||
| Patients with IgG4 RD | Overall | 2 | Intermediate positive association | ||
| Lymphoma | 1 | Intermediate positive association | |||
| IgM | High total IgM | General population | Overall | 1 | No significant association |
| Pancreatic | 1 | No significant association | |||
| Melanoma | 1 | No significant association | |||
| Bladder | 1 | No significant association | |||
| Solid cancers | 1 (meta-analysis 14 studies) | No significant association | |||
| Leukaemia | 1 | Intermediate positive association | |||
| High SCCA-IgM | Patients with cirrhosis | Hepatocellular carcinoma | 3 | No significant association | |
Table 6.
Summary of results of associations between autoimmune diseases and site-specific cancer risk. The evidence of association is defined by the number of studies reporting on the association, the range of the hazard ratio/odds ratio/relative risks/standardized incidence ratio reported in each study, and the statistical significance.
| Autoimmune diseases | Cancer risk | Serum antibody | Main findings |
|---|---|---|---|
| Autoimmune diseases (any) | Any cancer, specific cancers | Anti-Ro/SSA | Positive association for risk of any cancer, melanoma, lymphoma, breast cancer |
| Celiac disease (Undiagnosed) | Any cancer | IgA-TTG; IgG TTG levels | Positive association |
| Autoimmune encephalitis | Any cancer | Anti-MNDAW | Cancer prevalence 6% |
| Autoimmune myopathies | Any cancer (concomitant) | Positive Anti-SRP; positive anti-HMGCR | Positive association for anti- HMGCR for necrotizing myopathies |
| Any cancer (concomitant) | Myositis specific antibodies (MSAs): anti-TIF1-γ, anti-NXP2, anti-SAE1, | Positive association for inflammatory myositis with any single MSA +ve; positive association for MSAs -ve | |
| Any cancer (concomitant) | Anti-MJ/NXP-2, anti-MDA5, and Anti-TIF1γ/α | Positive association for anti-TIF1-γ in dermatomyositis | |
| Lung Ca | Anti-TIF1; anti-NXP2; anti-RNAP3 |
No association | |
| Scleroderma | Any cancer (concomitant) | Anti-centromere | Negative association [3], No association [1] |
| Anti-RNAP-3 | Positive association | ||
| Anti-TOPO | Weaker positive association | ||
| Anti-RNAP-1 (large subunit) | Inverse association | ||
| Anti–RNPC-3 | Positive association | ||
| Lung cancer | Anti-centromere Anti-TOPO |
No association | |
| Breast cancer (concomitant) | Anti-RNAP3 | Positive association | |
| Sjogren’s syndrome | Hodgkin disease | Anti-centromere | No association |
| Lymphoma | Ro/SSA and La/SSB | No Association | |
| MALT NHL | Ro/SSA and La/SSB | No Association | |
| Myeloma | Ro/SSA and La/SSB | Positive (suggestive) | |
| Autoimmune Thyroiditis | Thyroid ca | Anti-Tg | Positive association No association |
| Anti-TPO | Positive association No Association |
||
| TPOAb and TgAb combined | Strong positive association | ||
| Autoimmune Vasculitis | Any cancer | ANCA- vasculitis | No association/inconclusive |
| Autoimmune oral phemphoid patients | Any cancer | Anti-alpha6-integrin | Inverse association |
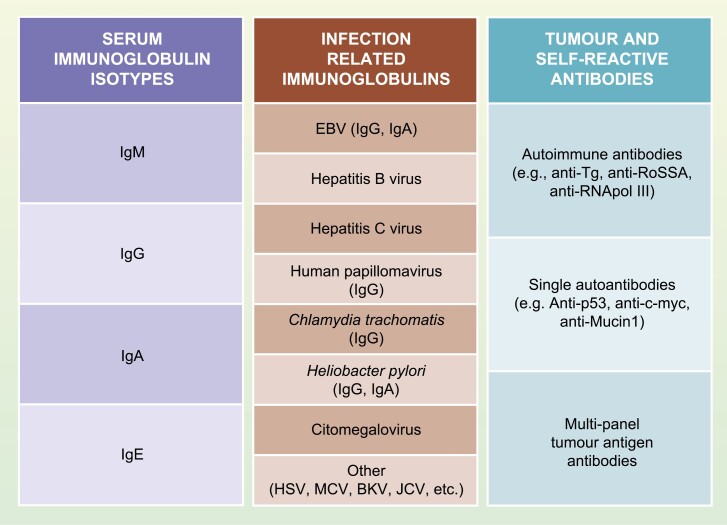
We observed three main categories in the publications, namely, serum immunoglobulins (n = 34), infectious agent-associated immunoglobulins (n = 158), and tumour and self-antigen reacting antibodies (n = 81). Therefore, the systematic review is structured following these main groupings. An overview of the main antibodies identified in the current review is illustrated in Fig. 3.
Figure 3.
Overview of antibodies associated with cancer risk described in the review.
Immunoglobulin M, G, A, and E
We identified 34 papers that assessed the risk of cancer in relation to the different immunoglobulin isotypes: IgE (n = 15), IgA (n = 7), IgG (n = 6), and IgM (n = 6). All studies followed an observational type of study design (case-control or cross-sectional designs). No clinical trials were identified. Most studies investigated the general population, except for three papers exploring IgM in patients with cirrhosis, and one exploring patients with IgG4-related diseases (IgG4-RD). An overview of the main findings is given in Table 2.
Immunoglobulin M (IgM)
Six studies were found looking into the association between IgM levels and the risk of cancer. One study reported an increased risk of chronic lymphocytic leukaemia in patients with increased levels of IgM [13]. No associations were found with other cancer types (i.e. overall, pancreatic, melanoma, bladder, and hepatocellular carcinoma) [14–16].
Immunoglobulin G (IgG)
Six studies have looked into the link between serum IgG levels and risk of site-specific cancer, however, not many studies explored the association with overall cancer. One cohort study found no association between serum IgG and overall cancer risk [17]. When looking at site-specific cancers, a large cohort study reported a negative association between serum IgG and risk of pancreatic cancer [16]. No associations were found for other cancer types (i.e. melanoma, bladder) [5, 14, 15]. Furthermore, a study focused on the association between IgG4-related disease (IgG4-RD), an inflammatory condition, and the risk of numerous cancer types. This observational study reported that the patients with IgG4-RD disease were at higher risk of overall cancer and lymphoma [18].
Immunoglobulin A (IgA)
Epidemiological studies have reported an inconsistent relationship between IgA levels and the risk of cancer [19, 20]. A meta-analysis of 14 studies found a strong positive association with solid cancers [5]. However, a strong negative association was also found between IgA and the risk of gastrointestinal cancer and lymphoma [19]. Moreover, no significant associations were found in various studies looking at overall, pancreatic, melanoma, and bladder cancer risk, in relation to IgA levels [14–16].
Immunoglobulin E (IgE)
Atopy and allergies are defined by exaggerated IgE responses to environmental allergens. We found 12 studies looking at the association between overall IgE (total concentration of IgE in serum) and the risk of various cancer types. Two cohort studies looking at total serum IgE reported a negative association with overall risk of cancer while two large cohorts found no significant associations with overall cancer risk [21, 22]. Moreover, four large cohort studies, including two with data from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, found that IgE-deficiency and ultra-low IgE levels were strongly associated with an increased risk of overall cancer [23–25]. One of these studies reported a strong positive association between low levels of serum IgE and the risk of chronic lymphocytic leukaemia, lymphomas, and multiple myeloma [25]. On the other hand, a large case-control study reported a strong positive association between IgE and head and neck cancers [26]. No other significant associations were found with other cancer types (i.e. pancreatic, prostate) [27].
Several studies have investigated the association between self-reported allergies and allergen-specific IgE, with varying results. A cohort study reported a positive association between serum allergen-specific IgE and risk of prostate cancer [28]. Moreover, a population-based case-control study found an increased risk of squamous cell carcinoma of the skin in patients with high levels of allergen-specific IgE [29]. On the other hand, a nested case–control from the EPIC cohort found a strong negative association between allergen-specific IgE and risk of glioma [30]. No associations were found between this allergen-specific IgE and overall and other specific cancer types (i.e. lung, breast, lymphoma, colon and rectum, pancreatic) [22, 28, 31]. When looking at asthma-specific IgE, a strong positive association was found with lung cancer [32]. On the other hand, a pooled analysis of 13 case-control studies found a negative association between asthma-specific IgE or self-reported food allergies, and risk of non-Hodgkin lymphoma [33]. No associations were found with other cancers (i.e. overall, breast, prostate, and pancreatic) [32].
Cancer-promoting infectious agents
We identified 158 studies assessing the risk of cancer in relation to different antibodies against various infectious agents. All studies followed an observational type of study design. No clinical trials were identified. The most commonly described associations were for antibodies against Epstein–Barr Virus (n = 25), hepatitis B virus (HBV, n = 15), hepatitis C virus (HCV, n = 14), human papillomavirus (n = 29), H. pylori (n = 29), and chlamydia trachomatis (n = 6). No consistent associations were found with other infectious agents. An overview of the main findings is given in Table 3.
Table 3.
Summary of results of associations between infection-related immunoglobulins with site-specific cancer risks. The strength of association is defined by the number of studies reporting on the association, the range of the hazard ratio/odds ratio/relative risks/standardized incidence ratio reported in each study, and the statistical significance.
| Exposure | Antigen/ Immunoglobulin | Outcome | Main findings |
|---|---|---|---|
| EBV (IgG, IgM and IgA) |
VCA | Gastric cancer | Intermediate positive association |
| EBNA | No significant associations | ||
| ZEBRA | No significant associations | ||
| EA | No significant associations | ||
| EBNA | Nasopharyngeal carcinoma | Very strong positive association | |
| VCA | Very strong positive association | ||
| EA | Weak positive association | ||
| Gp350 | Weak positive association | ||
| VCA | Breast cancer | No significant association | |
| EBNA | No significant association | ||
| VCA | Lymphoma (all) | No significant association | |
| EBNA | No significant association | ||
| EA | No significant association | ||
| VCA | Ovarian cancer | Moderately positive association | |
| Hepatitis B virus | Anti-HBs (IgG and IgM) | Hepatocellular carcinoma | Very strong positive association |
| Anti-HBc (IgM) | Strong positive association | ||
| Anti-HBc (IgG) | Strong positive association | ||
| Anti-HBs (IgG and IgM) | Pancreatic cancer | Strong positive association | |
| Anti-HBc (IgM) | Weak positive association | ||
| Anti-HBs (IgG and IgM) | Extrahepatic bile duct cancer | Weak positive association | |
| Anti-HBc (IgM) | Oropharyngeal | Weak positive association | |
| Hepatitis C virus (IgM) |
Anti-HCV | Hepatocellular carcinoma | Very strong positive association |
| Anti-HCV | Pancreatic cancer | No significant association | |
| Anti-HCV | Lymphoma (all) | No significant associations | |
| Anti-HCV | Renal | Weak positive association | |
| Anti-HCV | Cholangiocarcinoma | Weak positive association | |
| HPV (IgG) | 16 | Overall cancer | No significant association |
| 16 | Oesophageal cancer | No significant association | |
| 18 | No significant associations | ||
| 6 | Oropharyngeal cancer | Strong positive association | |
| 11 | No significant associations | ||
| 16 | No significant association | ||
| 18 | Weak positive association | ||
| 16 | Lung cancer | No significant associations | |
| 5 | Non-melanoma skin cancer | No significant associations | |
| 6 | No significant associations | ||
| 8 | No significant associations | ||
| 16 | Intermediate positive association | ||
| 18 | Intermediate positive association | ||
| 16 | Cervical cancer | Very strong positive association | |
| 18 | No significant association | ||
| 16 | Prostate cancer | No significant association | |
| 18 | No significant association | ||
| 33 | No significant association | ||
| 16 | Anogenital cancers (anus, vulvar, vaginal, penile) | Strong positive association | |
| 18 | Anogenital cancers (anus, vulvar, vaginal, penile) | Strong positive association | |
| C. Trachomatis (IgG) | Ovarian cancer | Strong positive association | |
| Cervical cancer | No significant associations | ||
| Prostate cancer | Low negative association | ||
| H. Pylori | IgG | Gastric cancer | Intermediate positive association |
| IgA | Strong positive association | ||
| CagA | Strong positive association | ||
| VacA | Low positive association | ||
| CagA | Pancreatic cancer | Low positive association | |
| CagA | Colorectal carcinoma | Intermediate positive association | |
| Anti-H. Pylori | Lymphoma | No significant association | |
| Herpes simplex virus 2 (IgG) | Prostate cancer | No significant associations | |
| Herpes simplex virus 1 | Oropharyngeal carcinoma | Weak positive association | |
| Cervical cancer | |||
| Human herpes virus -8 (Kaposi sarcoma) | Prostate cancer | No significant associations | |
| Non-Hodgkin Lymphoma | No significant associations | ||
| Varicella zoster virus | Glioma | Weak negative association | |
| T. vaginalis | Prostate cancer | No significant associations | |
| CMV | Anti-CMV | Gastrointestinal cancer | No significant associations |
| Breast cancer | No significant associations | ||
| MCV | Anti-MCV | Merkel cell carcinoma | Weak positive association |
| Bladder cancer | Weak positive association | ||
| BKV | Bladder cancer | Weak positive association | |
| JCV | Bladder cancer | No significant association | |
| Colorectal cancer | No significant association | ||
| Porphyromonas gingivalis | Pancreatic cancer | Weak positive association | |
| Oropharyngeal cancer | Weak positive association | ||
| Chlamydia pneumoniae | IgA | Lung cancer | Weak positive association |
| Polyomavirus | Anti-polyomavirus | Non-Hodgkin lymphoma | No significant association |
| GBV | Anti-GBV | Non-Hodgkin lymphoma | No significant association |
| Propionibacterium Acnes | Prostate cancer | Intermediate positive association |
VCA, viral capsid antigen; EA, early antigen; HBs, hepatitis B specific antigen; HBc, hepatitis B core antigen; \
Epstein–Barr virus
Antibodies against four major EBV antigens (viral capsid antigen (anti-VCA) IgA, IgM and IgG, early antigen (anti-EA) IgG, EBV nuclear antigen (EBNA), and ZEBRA IgM) have been studied in association with the risk of various cancers. Several studies looking into the association between EBV immunoglobulins and nasopharyngeal carcinomas (NPC) have found a positive association with all EBV antigens. For instance, a cohort study found that anti-EBNA1 neutralizing antibodies may be a sensitive biomarker for risk of NPC [34]. This was supported by 2 other population-based studies [35, 36]. Moreover, a large cohort looking at anti-VCA IgA found a strong positive association with the risk of NPC [37]. These results were also supported by two other cohort studies [38, 39]. Of the five studies that examined the risk of gastric cancer (GC), two epidemiological studies reported an increased risk of GC in patients with positive anti-VCA IgG [40, 41]. However, no significant associations were found between anti-EA antibodies and risk of GC [42]. One case-control study with 321 cases of ovarian cancer reported a positive association with anti-EBV IgG, however, no association was found for anti-EBV IgA [43]. No significant associations have been reported between EBV antibodies and risk of lymphoma and breast cancer [44].
Hepatitis B virus
Antibodies against hepatitis B virus antigens (hepatitis core antigen (anti-HBc) IgG, hepatitis B specific antigen (anti-HBs) IgG and IgM, and anti-hBe IgM) have long been suspected to be predictive factors for hepatocellular carcinoma (HCC). A consistent positive association was found between patients with HBsAg seropositive and risk of HCC [45]. In addition, three large cohort studies reported a stronger positive association in patients who were seropositive both anti-HBs and HBsAg compared with that seropositive for HBsAg [46]. Moreover, two population-based cohort studies found a positive association between positive anti-HBc antibodies and the risk of HCC [47, 48]. In contrast, a large cohort from a hepatitis B-endemic area found no significant association between patients with detected serum anti-HBs IgG and risk of HCC [49]. Furthermore, two case-control studies looking at anti-HCV, HBsAg, andi-HBc, and anti-HBs antibody positivity reported an increased risk of pancreatic cancer [50, 51]. No other associations were found between HBV antibodies and the risk of head and neck cancer and biliary tract cancer [52, 53].
Hepatitis C virus
Several studies have investigated the relationship between hepatitis C virus antibodies and the risk of hepatocellular carcinoma. A case-control study found a strongly increased risk of HCC in patients with positive anti-HCV antibody seropositivity [54]. This positive association was supported by three other epidemiological studies [55–57]. Additionally, a cohort also analyzing HBV antibodies found a higher risk of HCC in individuals who were seropositive for antibodies to both HCV and HBV [55]. Moreover, three epidemiological studies looking into the association between HCV antibodies and lymphomas found no significant associations [58–60]. Lastly, a case–-control from Japan reported a positive association between anti-HCV antibodies and the risk of intrahepatic cholangiocarcinoma [61].
Human papillomavirus
High risk (16 and 18) human papillomavirus antibodies have frequently been linked with cervical and other anogenital cancers (i.e. anus, vulvar, vaginal, and penile). Several epidemiological studies showed that serum antibodies to HPV 16 and 18 are associated with an increased risk of cervical cancer [62, 63]. Moreover, no consistent associations were found between other anogenital cancers and anti-HPV antibodies. Numerous studies investigating the relationship between HPV antibodies and head and neck cancers have found consistently positive associations between positive HPV 16 antibodies and the risk of head and neck cancers [64, 65]. No significant associations were found for other HPV types [64]. Furthermore, two large case-control studies from Sweden and Norway reported an increased risk of non-melanoma skin cancer in patients with detected antibodies for both HPV 16 and 18 [66, 67]. Lastly, no significant associations were found between HPV antibodies and the risk of prostate and lung cancer [68].
Chlamydia trachomatis
Antibodies against chlamydia trachomatis have most commonly been associated with the risk of cancers of the reproductive system (i.e. ovarian, cervical, and prostate). A recent study using data from the EPIC cohort reported an increased risk of ovarian cancer in patients seropositive for antibodies recognizing chlamydia trachomatis [69]. A case-control found a positive association between high titers of antibodies against chlamydia trachomatis and cervical cancer; however, another large population-based case-control study found no significant associations [70]. Lastly, a case-control study with 38 incident cases of prostate cancer reported a protective effect in patients seropositive for chlamydia trachomatis antibodies [71].
Helicobacter pylori
Most studies have focused on the association between H. pylori and the risk of gastric cancer. A recent cohort of 19 106 Japanese men reported an increased risk of GC for patients with undetectable anti-H. pylori IgG titers. However, the increase in risk was dependent on the severity of atrophic gastritis, resulting from persistent H. pylori infection [72]. This was supported by a cross-sectional study that found that serum IgG1 against H. pylori was significantly lower in subjects with GC (n = 62) [73]. On the other hand, a case-control study including 225 incident GC cases and 435 controls reported an increased risk of GC in individuals with elevated titers of IgA and IgG serum antibodies for H. pylori [74]. This positive association between immunoglobulin and risk of GC has been supported by the majority of epidemiological evidence to date [66–77]. Moreover, three epidemiological studies were identified looking at the association between antibodies against H. pylori and risk of colorectal cancer (CRC). However, no significant associations were found [78].
Tumour and self-reactive antibodies
A total of 81 papers were identified assessing the risk of cancer in relation to tumour or self-reactive antibodies. Of the identified papers, 35 studies specifically addressed the risk of any cancer or specific types of cancers in the general population. A further 23 studies were directed towards specific ‘at risk’ populations, such as carriers of mutations in BReast CAncer gene (BRCA) or those with thyroid nodules, while another 23 addressed the risk of cancer in relation to various autoantibodies associated with autoimmune diseases within specific patient cohorts. An overview of the main findings from this section is given in Tables 4–6.
Table 4.
Summary of results of associations between tumour and self-reactive antibodies and site-specific cancer risk, in the general population. The evidence of association is defined by the number of studies reporting on the association, the range of the hazard ratio/odds ratio/relative risks/standardized incidence ratio reported in each study, and the statistical significance.
| Cancer type | Serum Antibodies | Main findings | Diagnostic potential |
|---|---|---|---|
| All/any | Anti-p53 | Positive association | NA |
| Anti-phospholipid | Possible inverse association | NA | |
| Breast | Antibodies to six autoantigens: p53, c-myc, HER2, NY-ESO-1, BRCA2 and MUC1 (assessed individually) |
Positive association for the presence of 1 or more of listed autoantibodies | Autoantibody panel likely to perform better than single marker – but not assessed |
| Anti-thyroid peroxidase | Inverse association | NA | |
| Colorectal | Anti-p53 | Positive association | High specificity but low sensitivity |
| Anti-Neu5Gc (antibodies to meat-derived antigens) | Positive association for total anti-Neu5Gc IgG; Single epitopes no association | NA | |
| IGFBP-2 IgG | Positive association | AUC = 0.92 (when combined with serum IGFBP-2 levels) | |
| Multiple TAA antibodies (8000 potential antigens) |
Positive association: MAPKAPK3, PIM1, STK4, SRC, and FGFR4 Negative association: ACVR2B |
Specificity and sensitivity high for anti-ACVR2B, anti-MAPKAPK3, anti-PIM1 combined | |
| Anti-ASXL2* | Positive association | AUC = 0.67 | |
| Oesophageal | Anti-ASXL2* | Positive association | AUC = 0.76 |
| Anti-p53 | Positive association | NA | |
| Gastric | Panel; p62, c-Myc, NPM1, 14-3-3ξ, MDM2 and p16 | Positive associations | Selected six panel for testing |
| Glioma | Anti-IGFBP-2 | Positive association for astrocytoma | AUC = 0.80 (when combined with serum IGFBP-2 levels) |
| Hepatocellular carcer | Multiple TAA antibodies | Positive associations for autoantibodies to calreticulin, cytokeratin 8, nucleoside diphosphate kinase A, F1-ATP synthase | NA |
| Multiple antibodies | Positive association for antibodies to 21 TAAs; (best performers: IMP-1, KOC, p53 and c-myc, Sui1 and RalA, Calreticulin, and HCC1) | Moderate sensitivity high specificity | |
| 12 antibody panel | Positive association for autoantibodies to HCC1, P16, P53, P90, and Survivin | NA | |
| Lung cancer/NSCLC | Panel: p62, BIRC, Livin-1, p53, PRDX, NY-ESO-1 and Ubiquitin | Positive association | AUC = 0.81 |
| Panel: p53, c-myc, HER2, NY-ESO-1, CAGE, MUC1 ans GBU4-5 | Positive association | High sensitivity for squamous cell lung cancer, moderate sensitivity for all lung cancers | |
| Panel: GAGE7, CAGE, MAGEA1, SOX2, GBU4-5, PGP9.5, and p53 | Positive association | Moderate sensitivity and specificity | |
| Multiple antibodies: (212 selected from immunogenic tumour expressed proteins) |
Positive association for the 5 most immunogenic combined | High sensitivity and specificity | |
| Multiple autoantibodies: p62, p16, Koc, p53, Cyclin B1, Cyclin E, Survivin, HCC1, and RalA | Strongest serological response: Survivin, Cyclin B1, HCC1, and p53 | Low to moderate potential as individual autoantibodies | |
| Anti-BARD1 | Positive association | High sensitivity and specificity | |
| Brain protein autoantibodies | Possible positive association | NA | |
| CD25 and FoxP3 IgGs | Positive association for CD25a; weaker for FoxP3 | NA | |
| Mesothelioma | Panel including PDIA6, MEG3, SDCCAG3, IGHG3, IGHG1 | Positive association | High specificity and sensitivity |
| Anti-p53 | No association |
NA |
|
| Lymphoma/NHL | Anti-cyclic citrullinated peptide (CCP) | No association | NA |
| Autoimmune diseases No individual autoantibodies specified |
Positive association | NA | |
| Ovarian cancer | Panel: anti- MDM2, PLAT, NPM1, 14-3-3 Zeta, p53, and RalA | Positive association | High specificity and sensitivity |
| Anti-MUC1, anti-CA125 | Positive association | NA | |
| Anti-MUC1 | Indirect evidence for inverse association | NA | |
| Anti-p53 and anti-SBP-1** | Positive association for serous ovarian cancer | High specificity and sensitivity for CA125, anti-TP53, and anti-SBP1 combined (AUC 0.96) | |
| Anti-HE4 | Positive association | NA | |
| Pancreatic | Anti-Ezrin | No association | NA |
| Bladder | Anti-UPII | Positive association |
Regarding the target antigens for the autoantibodies evaluated in the general population (Table 4), studies generally showed positive associations for single-antigen targets such as p53, New5Gc, IGFBP-2, BARD1, CD25, FoxP3, MUC1, CA125, SBP1, and HE4, albeit with relatively low sensitivity [79–87]. The remaining 13 studies investigated the general population to assess cancer risk in relation to serum antibodies against multiple antigens, either to identify the most immunogenic of these or to develop and evaluate a panel of biomarkers for diagnostic or screening purposes. These studies indicate strong positive associations for multiple antibodies, with most indicating relatively high specificity and sensitivity for specific antibody panels against tumour specific and self-antigens [88]. For example, Zhang et al. reported a panel of nine candidate autoantibody markers that achieved 94.3% sensitivity and 90.4% specificity for detecting mesothelioma [89]. Similarly, a panel comprising antibodies to the autoantigens, p53, c-myc, human epidermal growth factor receptor 2 (HER2), New York Esophageal Squamous Cell Carcinoma-1 (NY-ESO-1), cancer/testis antigen gene (CAGE), Mucin 1 (MUC1), and GBU4-5, achieved high sensitivity and specificity for the detection of squamous cell lung cancers [90].
Studies performed within specific target populations included populations at high cancer risk (e.g. BRCA mutation carriers, populations identified with high risk of oesophageal cancer for screening), cohorts with specific organ disease populations (e.g. lung and thyroid disease, and populations with autoimmune or paraneoplastic syndromes, including autoimmune myopathies, scleroderma, Sjorgens syndrome, autoimmune thyroiditis, autoimmune vasculitis, and autoimmune phemphoid) (Tables 5 and 6). The most consistent positive associations were seen for the association between overall cancer risk in scleroderma patients with anti-RNA polymerase-3 (RNAP-3) antibodies and for thyroid cancer among people with autoimmune thyroiditis who had high anti-thyroglobulin (Tg) serum antibody titres [91, 92]. Among people undergoing thyroidectomy for any reason, thyroid cancer risk was also associated with the presence of anti-Tg antibodies, though the evidence was mixed regarding anti-thyroid peroxidase (TPO) seropositive status [93]. Antibody panels applied in high-risk populations again appeared to have relatively high sensitivity and specificity in relation to identifying patients with cancer or individuals with premalignant disease [8].
Table 5.
: Summary of results of associations between tumour and self-reactive antibodies and site-specific cancer risk, in specific populations. The evidence of association is defined by the number of studies reporting on the association, the range of the hazard ratio/odds ratio/relative risks/standardized incidence ratio reported in each study, and the statistical significance.
| Target Population | Cancer risk | Serum antibody | Main findings | Diagnostic potential |
|---|---|---|---|---|
| High risk (BRCA) carriers | Breast cancer | MUC1 IgG | No association | NA |
| High risk oesophageal cancer (screening) population | Oesophageal cancer | Panel of eight autoantibodies: p53, IMP1, P16, cyclin B1, P62, c-myc, Survivn and Koc NY-ESO-1 STIP1 |
Positive association | High specificity and moderate sensitivity |
| Lung disease | Premalignant lung lesions Atypical adenomatous hyperplasia/ squamous cell dysplasia |
Panel of nine autoantibodies | Positive association | Moderate specificity and moderate sensitivity for premalignancy |
| Endometrial cancer patients | Endometrial cancer | Anti-p53 | Positive association for serous histology | NA |
| Ovarian cancer patients | Ovarian cancer | NY-ESO-1 | 48% seropositive | NA |
| Thyroid disease: (patients having nodule FNAB or thyroidectomy) |
Thyroid cancer (papillary carcinoma) |
Anti-Tg | Positive association No association |
High specificity and low sensitivity (ref 80) |
| Anti-TPO | Positive association No association |
NA | ||
| Autoimmune thyroiditis (either Tg or TPO Ab +ve) |
No association | NA |
Discussion
There is a growing body of evidence linking antibodies to cancer risk, especially for specific immunoglobulin isotypes and for both TAA-reactive and self-reactive antibodies. B cells can suppress tumour growth by producing antibodies able to facilitate CDC and antigen presentation or engage effector cells to mediate ADCC and ADCP; however, have shown the ability to also support tumour growth by expressing antibodies with poor ability to mediate the above anti-tumour responses, such as isotypes like IgG2 and IgG4 [6]. Antibodies have great potential as biomarkers for cancer since procedures to test for their presence are minimally invasive and antibodies are fairly stable and easy to measure ex vivo. Research identifying antibodies that might be appropriate biomarkers for early detection of cancer, based on our systematic review, still appears to be an emerging field. Most of the studies reviewed were observational, and largely attempted to identify potential markers in association with cancer. Few of the studies test the predictive potential of antibodies to detect an overall cancer risk or risk for site-specific cancers, specifically for one or more autoantibodies (autoantibody panels). Larger studies are required to validate these antibodies as cancer biomarkers and to apply them in clinical practice. The current systematic review presents a broad landscape of potential biomarkers for early diagnosis of cancer and our findings highlight the importance of this newly emerging research topic in cancer biomarker discovery.
A consistent association between serum levels of certain immunoglobulin isotypes and risk for certain cancers was found. However, studies have been conducted in several methods and in different settings and have reported diverse results. It is therefore difficult to point to antibody class-specific associations with cancer risk. While no association was found between general IgG and IgM levels, several studies we found report associations between altered serum levels of IgE and cancer risk [13, 15, 19, 21, 22]. Some studies report that high titres of allergen-specific IgE are associated with an increased risk of prostate cancer and squamous cell carcinoma, and conversely these show a negative association with glioma incidence [29, 30]. Low IgE titres have also been associated with a high risk of overall cancer and an increased risk of haematological malignancies [25]. High IgA titres have been found associated with the risk of a range of solid tumours, but have a strong negative association with risk of gastrointestinal cancer and lymphoma diagnosis, while other studies show no significant associations with overall, pancreatic, melanoma, and bladder cancer risk, in relation to IgA levels [19, 20].
Different types of cancers have different aetiology. This might explain the controversial results on different effects of IgE and IgA antibody titres on different types of cancers [19, 24]. IgE can exert anti-tumour functions, but can be also associated with systemic chronic inflammation, which can instead promote tumourigenesis [21]. This could explain the association between general low serum levels of IgE and increased cancer risk for cancers located far from the site of inflammation. On the other hand, high IgE levels as part of the local chronic inflammatory milieu could predispose to cancers developing at that site of inflammation; for example lung cancer in patients with asthma and non-melanoma skin cancers in patients with atopic dermatitis [28]. IgA may have a protective role in certain tissues such as mucosal areas and the gastrointestinal tract, which would explain the strong negative correlation between high IgA levels and positive associations with the development of gastrointestinal cancer [14, 19]. However, IgA can be associated with specific B cell subsets and their regulatory functions, such as the production of immunosuppressive cytokines like IL-10, which can support a pro-tumour microenvironment [19]. Together, these studies may suggest the importance of considering the inflammatory environment in disparate anatomical sites and its relation to the nature of the humoral response and how this might relate to carcinogenesis.
With the exception of antibodies against EBV which have shown a positive association with NPCs, and possibly with gastric and ovarian cancers, most studies focused on investigating the association between the presence of anti-virus or anti-bacteria antibodies and cancer risk, report a positive association with specific cancers known to be associated to that specific virus/bacterial infection site (for instance, HBV and HCV with hepatocellular carcinoma, HPV with anogenital cancers), but no association with other cancers [34, 39, 42]. Testing the presence of specific anti-viral or anti-bacterial antibodies is often the only method to assess if there is or there has been a specific infection, and it is therefore difficult to distinguish whether it is the presence of the antibodies that is associated with cancer risk and not the virus/bacterial infection itself [72]. In addition, only one study relating to infectious agents described the presence of neutralizing antibodies (i.e. EBV) [34]. The presence of neutralizing antibodies implies that the humoral response generated is already protective against a pathogen, compared to an antibody response that recognizes the epitope but which still allows the pathogen to infect, survive, and replicate. Therefore, further studies looking into the association between neutralizing antibodies against various infectious agents in association to cancer risk are required.
The risk of cancer appeared to be increased among people who present with various autoimmune diseases (i.e. in most cases autoimmune diseases involve the body producing antibodies toward ‘self’ (autoantigens) which can lead to local or systemic inflammation and specific or systemic organ damage) [90, 93]. However, despite the increased risk of cancer, those with autoimmune diseases often have a better prognosis, leading to the hypotheses that such immune responses may be protective against the development of autoantigen-expressing cancer cells at an early stage of carcinogenesis, and therefore preventing cancer from developing and progressing [94]. Evidence of a better prognosis has been found for the development of vitiligo, denoting an immune attack on melanocytes, in patients with melanoma, and of thyroiditis in patients with thyroid cancer [10].
Several studies indicate a high risk of cancer diagnosis within 3 years of diagnosis of specific autoimmune diseases, specifically scleroderma and autoimmune myopathies, suggesting that some autoimmune pathologies may actually represent a ‘paraneoplastic process’ [91]. One hypothesis is that the loss of tolerance to ‘self’ may be due to cross-reaction with tumour autoantigens, leading to the immune system targeting non-cancerous tissue in the process of mounting an immune response to the tumour. Hence the development of autoimmune disease could be an early marker for cancer development prior to becoming clinically detectable [95]. This has prompted calls for cancer screening in patients recently diagnosed with specific autoimmune diseases. However, we did not find examples of any specific autoantibodies being trialled as biomarkers to evaluate cancer risk among patient populations with autoimmune diseases. This may be due to their rather low sensitivity for use as a stand-alone screening tool.
Over the past few decades, it is evident that a substantial body of work has been undertaken to identify the most immunogenic TAAs and their associated tumour-reactive antibodies and autoantibodies, with the end goal being the development of assays to measure the presence of such antibodies (alone or in combination with other markers) for use in cancer screening and diagnostics [94]. Many of these studies have focussed on cancers where screening tests are currently lacking and, or in tumour types such as e.g. in lung cancers, hepatocellular carcinomas, and ovarian cancers in which earlier detection and prompt clinical intervention could provide substantial survival benefits [82, 83, 89, 90].
Some autoantibodies were frequently observed in association with multiple cancer types for example anti-p53 antibodies are detected in breast, colorectal oesophageal, lung, and ovarian cancers [88, 96]. While the detection of anti-p53 antibodies alone is not a very sensitive marker for cancer, a combination with other antibody biomarkers and clinical characteristics could provide additional value for risk stratification [85]. Anti-MUC1 antibodies mark another autoantibody found to be associated with several cancer types and appear to correlate with a more favourable prognosis [82]. MUC1 (also known as CA 15.3) is a transmembrane mucin, a glycoprotein with O-glycosylated tandem repeats, overexpressed in cancer, in particular in breast cancer. MUC1 has been also found aberrantly glycosylated in cancer compared to normal tissue, and some reported anti-MUC1 antibodies are actually against these aberrantly glycosylated variants [97]. Anti-MUC1 antibodies MUC1 have shown a positive association with breast cancer when in combination with 1 or more of the listed autoantibodies p53, c-myc, HER2, NY-ESO-1, BRCA2 [87]. Anti-MUC1 IgG1 antibodies have also shown a positive association with ovarian cancer [81]. Another report on lung cancer shows a positive association with high sensitivity for squamous cell lung cancer and moderate sensitivity for all lung cancers, for an autoantibody panel which includes MUC1 (p53, c-myc, HER2, NY-ESO-1, CAGE, and MUC1, GBU4-5) [90]. Of interest are also antibodies targeting cyclin B1, a protein involved in the transition from G2 to M phase of the cell cycle. Anti-cyclin B1 antibodies have been found to increase in lung cancer [98], with low to moderate prediction potential as individual autoantibody, and oesophageal carcinoma, with high specificity and moderate sensitivity in predicting cancer risk when in a panel with other 7 autoantibodies (p53, IMP1, P16, P62, c-myc, Survivn, and Koc) [99]. However, in patients with breast cancer they have been found to decrease compared to healthy volunteers and they were actually considered to have a protective role in breast cancer development [100]. Given that many identified autoantibodies to one antigen have low sensitivity and/or specificity as biomarkers for individual cancer types, the development of panels of multiple autoantibodies may provide better sensitivity for cancer or specific cancer types.
This systematic review provides a comprehensive qualitative summary of the published epidemiological evidence of the associations between antibodies and the risk of overall and site-specific cancers. A large number of the cohort, case-control studies were included. However, given the broad subject and large amount of antibody types, in this study, we presented an overview of the most researched antibodies in relation to cancer risk, and it is possible that certain studies might have been missed in our literature review. Our systematic review thus presents a broad landscape of different antibodies with the potential of being identified and in the future validated as markers of early diagnosis of cancer. Larger observational studies and clinical trials are necessary to establish the potential prediction capability of such biomarkers or their combinations.
Conclusion
There is consistent evidence associating antibodies to cancer risk, especially for specific immunoglobulin isotypes and for both tumour-associated and self-reactive antibodies. However, research in this field is still in the early stages of development. No clinical trials assessing the utility of antibodies as biomarkers for screening or diagnostic assessment were identified, and most of the studies thus far have reported exploratory findings from observational studies, some involving modeling with validation cohorts with reports of specificity and sensitivity but which still require validation with larger cohorts. Therefore, larger studies and clinical trials are necessary to assess the efficacy of specific antibodies as markers for early cancer diagnosis.
Supplementary Material
Acknowledgements
The authors are solely responsible for study design, data collection, analysis, decision to publish, and preparation for the article.
Glossary
Abbreviations
- ADCC
antibody-dependent cell cytotoxicity
- ADCP
antibody-dependent cell phagocytostis
- ANCA
antineutrophil cytoplasmic antibody
- Anti-EA
early antigen
- Anti-HBc
hepatitis core antigen
- Anti-HBs
hepatitis B specific antigen
- Anti-UPII
uroplakin II antibody
- Anti-VCA
viral capsid antigen
- ASXL2
additional sex combs like 2
- BARD1
BRCA1-associated RING domain protein 1
- BKV
BK virus
- BIRC
baculoviral inhibitors of apoptosis repeat containing
- BRCA
breast cancer gene
- CagA
cytotoxic associated gene A
- CAGE
cancer/testis antigen gene
- CA125
cancer antigen 125
- CDC
complement-dependent cytotoxicity
- CMV
cytomegalovirus
- CRC
colorectal cancer
- C1q
complement component 1q
- EBNA
Epstein-Barr nuclear antigen
- EBV
Epstein-Barr virus
- EPIC
European Prospective Investigation into Cancer and Nutrition
- FcRs
FC receptors
- FNAB
fine needle aspiration biopsy
- FOXP3
forkhead box P3
- GAGE7
G Antigen 7
- GBV
Great Britain virus
- GC
gastric cancer
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HE4
human epididymis protein 4
- HER2
human epidermal growth factor receptor 2
- HP
helicobacter pylori
- HPV
human papillomavirus
- Ig
immunoglobulin
- IgA
immunoglobulin A
- IgE
immunoglobulin E
- IGFBP
insulin-like growth factor-binding protein
- IgG
immunoglobulin G
- IgG4-RD
immunoglobulin G4 related disease
- IgM
immunoglobulin M
- JCV
John Cunningham virus
- La/SSB
Sjögren’s-syndrome-related antigen B
- MAGEA1
melanoma-associated antigen 1
- MCV
molluscum contagiosum virus
- MDM2
mouse double minute 2 homolog
- MEG3
maternally expressed gene 3
- mTSA
mutated tumour specific antigen
- MUC1
mucin 1
- Neu5Gc
N-glycolylneuraminic acid
- nmTAA
non-mutational tumour-associated antigen
- NPC
nasopharyngeal carcinoma
- NPM1
nucleophosmin
- NXP2
nuclear matrix protein
- NY-ESO-1
New York esophageal squamous cell carcinoma-1
- PDIA6
protein disulfide isomerase family A: member 6
- PGP9.5
protein gene product 9.5
- PLAT
tissue plasminogen activator
- PRDX
peroxiredoxins
- PRISMA
preferred reporting items for systematic reviews and meta-analyses
- PSA
prostate-specific antigen
- RalA
Ras-related protein Ral-A
- RD
related disease
- RNAP
ribonucleic acid polymerase
- RNP-3
U11/U12 small nuclear ribonucleoprotein
- Ro/SSA
Sjögren’s-syndrome-related antigen A
- RPA
replication protein A
- SAE
small ubiquitin-like modifier activating enzyme
- SBP1
selenium-binding protein 1
- SCCA
squamous cell carcinoma antigen
- SDCCAG3
serologically defined colon cancer antigen-3
- SR
self-reported
- SRP
anti-signal recognition particle
- TAA
tumour-associated antigen
- Tg
anti-thyroglobulin
- TIF1
transcriptional intermediary factor
- TOPO
topoisomerase
- TPO
anti-thyroid peroxidase
- TTG
tissue transglutaminase
- VacA
vacuolating cytotoxin
Contributor Information
Maria J Monroy-Iglesias, Translational Oncology and Urology Research (TOUR), Centre for Cancer, Society, and Public Health, School of Cancer and Pharmaceutical Sciences, King’s College London, London, UK.
Silvia Crescioli, St. John’s Institute of Dermatology, School of Basic & Medical Biosciences, King’s College London, London SE1 9RT, UK.
Kerri Beckmann, Higher Degree by Research, University of South Australia, Adelaide, Australia; Cancer Epidemiology and Population Health Research Group, University of South Australia, Adelaide, SE, Australia.
Nga Le, Higher Degree by Research, University of South Australia, Adelaide, Australia.
Sophia N Karagiannis, St. John’s Institute of Dermatology, School of Basic & Medical Biosciences, King’s College London, London SE1 9RT, UK.
Mieke Van Hemelrijck, Translational Oncology and Urology Research (TOUR), Centre for Cancer, Society, and Public Health, School of Cancer and Pharmaceutical Sciences, King’s College London, London, UK.
Aida Santaolalla, Translational Oncology and Urology Research (TOUR), Centre for Cancer, Society, and Public Health, School of Cancer and Pharmaceutical Sciences, King’s College London, London, UK.
Funding
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) based at Guy's and St Thomas' NHS Foundation Trust and King's College London (IS-BRC-1215-20006). The authors are solely responsible for decision to publish, and preparation of the manuscript. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
AS, MV, and SK conceived the study idea. MM performed the literature search. AS, MM, and KB developed the core criteria used in this study. AM, MM, KB, NL, and SC extracted the data from revised studies. AS, MM, KB, and SC wrote the first draft of the manuscript. All the authors contributed to the article and approved the submitted version.
Data availability
The data presented in this study are available on request from the corresponding author.
References
- 1. Scott AM, Wolchok JD, Old LJ.. Antibody therapy of cancer. Nat Rev Cancer 2012, 12, 278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 2. Schroeder HW Jr, Cavacini L.. Structure and function of immunoglobulins. J Allergy Clin Immunol 2010, 125, S41–52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shade KT, Conroy ME, Anthony RM.. IgE glycosylation in health and disease. Curr Top Microbiol Immunol 2019, 423, 77–93. doi: 10.1007/82_2019_151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ren S, Zhang Z, Xu C, Guo L, Lu R, Sun Y, et al. Distribution of IgG galactosylation as a promising biomarker for cancer screening in multiple cancer types. Cell Res 2016, 26, 963–66. doi: 10.1038/cr.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peppas I, George G, Sollie S, Josephs DH, Hammar N, Walldius G, et al. Association of serum immunoglobulin levels with solid cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2020, 29, 527–38. doi: 10.1158/1055-9965.EPI-19-0953. [DOI] [PubMed] [Google Scholar]
- 6. Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM.. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol 2020, 20, 294–307. [DOI] [PubMed] [Google Scholar]
- 7. Wang M, Liu F, Pan Y, Xu R, Li F, Liu A, et al. Tumor-associated autoantibodies in ESCC screening: detecting prevalent early-stage malignancy or predicting future cancer risk?. EBioMedicine 2021, 73, 103674. doi: 10.1016/j.ebiom.2021.103674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang S, Qin J, Ye H, Wang K, Shi J, Ma Y, et al. Using a panel of multiple tumor-associated antigens to enhance autoantibody detection for immunodiagnosis of gastric cancer. Oncoimmunology 2018, 7, e1452582. doi: 10.1080/2162402X.2018.1452582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui M, Huang J, Zhang S, Liu Q, Liao Q, Qiu X.. Immunoglobulin expression in cancer cells and its critical roles in tumorigenesis. Front Immunol 2021, 12, 613530. doi: 10.3389/fimmu.2021.613530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zitvogel L, Perreault C, Finn OJ, Kroemer G.. Beneficial autoimmunity improves cancer prognosis. Nat Rev Clin Oncol 2021, 18, 591–602. doi: 10.1038/s41571-021-00508-x. [DOI] [PubMed] [Google Scholar]
- 11. Carneiro B, Petroianu A, Machado JAN, Dos Anjos PMF, da Silva FR, Alberti LR, et al. Clinical and immunological allergy assessment in cancer patients. Sci Rep 2021, 11, 18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009, 339, b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glenn MJ, Madsen MJ, Davis E, Garner CD, Curtin K, Jones B, et al. Elevated IgM and abnormal free light chain ratio are increased in relatives from high-risk chronic lymphocytic leukemia pedigrees. Blood Cancer J 2019, 9, 25. doi: 10.1038/s41408-019-0186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peppas I, Sollie S, Josephs DH, Hammar N, Walldius G, Karagiannis SN, et al. Serum immunoglobulin levels and the risk of bladder cancer in the AMORIS Cohort. Cancer Epidemiol 2019, 62, 101584. doi: 10.1016/j.canep.2019.101584. [DOI] [PubMed] [Google Scholar]
- 15. Kessler A, Sollie S, Karagiannis SN, Walldius G, Hammar N, Van Hemelrijck M.. Serum IgG is associated with risk of melanoma in the Swedish AMORIS study. Front Oncol 2019, 9, 1095. doi: 10.3389/fonc.2019.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sollie S, Santaolalla A, Michaud DS, Sarker D, Karagiannis SN, Josephs DH, et al. Serum immunoglobulin G is associated with decreased risk of pancreatic cancer in the Swedish AMORIS study. Front Oncol 2020, 10, 263. doi: 10.3389/fonc.2020.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li N, Hu H, Wu G, Sun B.. Value of immune factors for monitoring risk of lung cancer in patients with interstitial lung disease. J Int Med Res 2019, 47, 3344–53. doi: 10.1177/0300060519847403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahn SS, Song JJ, Park YB, Lee SW.. Malignancies in Korean patients with immunoglobulin G4-related disease. Int J Rheum Dis 2017, 20, 1028–35. doi: 10.1111/1756-185X.13093. [DOI] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Neovius M, Ye W, Hammarstrom L.. IgA deficiency and risk of cancer: a population-based matched cohort study. J Clin Immunol 2015, 35, 182–8. doi: 10.1007/s10875-014-0124-2. [DOI] [PubMed] [Google Scholar]
- 20. Mellemkjaer L, Hammarstrom L, Andersen V, Yuen J, Heilmann C, Barington T, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol 2002, 130, 495–500. doi: 10.1046/j.1365-2249.2002.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Hemelrijck M, Garmo H, Binda E, Hayday A, Karagiannis SN, Hammar N, et al. Immunoglobulin E and cancer: a meta-analysis and a large Swedish cohort study. Cancer Causes Control 2010, 21, 1657–67. doi: 10.1007/s10552-010-9594-6. [DOI] [PubMed] [Google Scholar]
- 22. Lindelof B, Granath F, Tengvall-Linder M, Ekbom A.. Allergy and cancer. Allergy 2005, 60, 1116–20. doi: 10.1111/j.1398-9995.2005.00808.x. [DOI] [PubMed] [Google Scholar]
- 23. Ferastraoaru D, Rosenstreich D.. IgE deficiency is associated with high rates of new malignancies: results of a longitudinal cohort study. J Allergy Clin Immunol Pract 2020, 8, 413–5. doi: 10.1016/j.jaip.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 24. Helby J, Bojesen SE, Nielsen SF, Nordestgaard BG.. IgE and risk of cancer in 37 747 individuals from the general population. Ann Oncol 2015, 26, 1784–90. doi: 10.1093/annonc/mdv231. [DOI] [PubMed] [Google Scholar]
- 25. Nieters A, Luczynska A, Becker S, Becker N, Vermeulen R, Overvad K, et al. Prediagnostic immunoglobulin E levels and risk of chronic lymphocytic leukemia, other lymphomas and multiple myeloma-results of the European Prospective Investigation into Cancer and Nutrition. Carcinogenesis 2014, 35, 2716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liao HC, Wu SY, Ou CY, Hsiao JR, Huang JS, Tsai ST, et al. Allergy symptoms, serum total immunoglobulin E, and risk of head and neck cancer. Cancer Causes Control 2016, 27, 1105–15. doi: 10.1007/s10552-016-0788-4. [DOI] [PubMed] [Google Scholar]
- 27. Olson SH, Hsu M, Wiemels JL, Bracci PM, Zhou M, Patoka J, et al. Serum immunoglobulin e and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 2014, 23, 1414–20. doi: 10.1158/1055-9965.EPI-13-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang H, Rothenbacher D, Low M, Stegmaier C, Brenner H, Diepgen TL.. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int J Cancer 2006, 119, 695–701. doi: 10.1002/ijc.21883. [DOI] [PubMed] [Google Scholar]
- 29. Wiemels JL, Wiencke JK, Li Z, Ramos C, Nelson HH, Karagas MR.. Risk of squamous cell carcinoma of the skin in relation to IgE: a nested case-control study. Cancer Epidemiol Biomarkers Prev 2011, 20, 2377–83. doi: 10.1158/1055-9965.EPI-11-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schlehofer B, Siegmund B, Linseisen J, Schuz J, Rohrmann S, Becker S, et al. Primary brain tumours and specific serum immunoglobulin E: a case-control study nested in the European Prospective Investigation into Cancer and Nutrition cohort. Allergy 2011, 66, 1434–41. doi: 10.1111/j.1398-9995.2011.02670.x. [DOI] [PubMed] [Google Scholar]
- 31. Hollander P, Rostgaard K, Smedby KE, Chang ET, Amini RM, de Nully Brown P, et al. Autoimmune and atopic disorders and risk of classical Hodgkin lymphoma. Am J Epidemiol 2015, 182, 624–32. doi: 10.1093/aje/kwv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kantor ED, Hsu M, Du M, Signorello LB.. Allergies and asthma in relation to cancer risk. Cancer Epidemiol Biomarkers Prev 2019, 28, 1395–403. doi: 10.1158/1055-9965.EPI-18-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vajdic CM, Falster MO, de Sanjose S, Martinez-Maza O, Becker N, Bracci PM, et al. Atopic disease and risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Cancer Res 2009, 69, 6482–9. doi: 10.1158/0008-5472.CAN-08-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coghill AE, Bu W, Nguyen H, Hsu WL, Yu KJ, Lou PJ, et al. High levels of antibody that neutralize B-cell infection of Epstein-Barr virus and that bind EBV gp350 are associated with a lower risk of nasopharyngeal carcinoma. Clin Cancer Res 2016, 22, 3451–7. doi: 10.1158/1078-0432.CCR-15-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen DY, Chen YM, Lan JL, Chen HH, Hsieh CW, Wey SJ, et al. Polymyositis/dermatomyositis and nasopharyngeal carcinoma: the Epstein-Barr virus connection?. J Clin Virol 2010, 49, 290–5. doi: 10.1016/j.jcv.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 36. Cheng WM, Chan KH, Chen HL, Luo RX, Ng SP, Luk W, et al. Assessing the risk of nasopharyngeal carcinoma on the basis of EBV antibody spectrum. Int J Cancer 2002, 97, 489–92. doi: 10.1002/ijc.1641. [DOI] [PubMed] [Google Scholar]
- 37. Hsu WL, Chen JY, Chien YC, Liu MY, You SL, Hsu MM, et al. Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20-year follow-up study on 9,622 males without family history in Taiwan. Cancer Epidemiol Biomarkers Prev 2009, 18, 1218–26. doi: 10.1158/1055-9965.EPI-08-1175. [DOI] [PubMed] [Google Scholar]
- 38. Cao SM, Liu Z, Jia WH, Huang QH, Liu Q, Guo X, et al. Fluctuations of Epstein Barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One 2011, 6, e19100. doi: 10.1371/journal.pone.0019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klatka J, Hymos A, Szkatula-Lupina A, Grywalska E, Klatka B, Terpilowski M, et al. T-lymphocyte activation is correlated with the presence of anti-EBV in patients with laryngeal squamous cell carcinoma. In Vivo 2019, 33, 2007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Z, Lv Z, Ding H, Xu Q, Sun L, Jing J, et al. Role of serum EBV-VCA IgG detection in assessing gastric cancer risk and prognosis in Northern Chinese population. Cancer Med 2018, 7, 5760–74. doi: 10.1002/cam4.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kayamba V, Monze M, Asombang AW, Zyambo K, Kelly P.. Serological response to Epstein-Barr virus early antigen is associated with gastric cancer and human immunodeficiency virus infection in Zambian adults: a case-control study. Pan Afr Med J 2016, 23, 45. doi: 10.11604/pamj.2016.23.45.8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Varga MG, Cai H, Waterboer T, Murphy G, Shimazu T, Taylor PR, et al. Epstein-Barr virus antibody titers are not associated with gastric cancer risk in East Asia. Dig Dis Sci 2018, 63, 2765–72. doi: 10.1007/s10620-018-5154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Littman AJ, Rossing MA, Madeleine MM, Tang MT, Yasui Y.. Association between late age at infectious mononucleosis, Epstein-Barr virus antibodies, and ovarian cancer risk. Scand J Infect Dis 2003, 35, 728–35. doi: 10.1080/00365540310016556. [DOI] [PubMed] [Google Scholar]
- 44. de Sanjose S, Bosch R, Schouten T, Verkuijlen S, Nieters A, Foretova L, et al. Epstein-Barr virus infection and risk of lymphoma: immunoblot analysis of antibody responses against EBV-related proteins in a large series of lymphoma subjects and matched controls. Int J Cancer 2007, 121, 1806–12. doi: 10.1002/ijc.22857. [DOI] [PubMed] [Google Scholar]
- 45. Liu X, Baecker A, Wu M, Zhou JY, Yang J, Han RQ, et al. Interaction between tobacco smoking and hepatitis B virus infection on the risk of liver cancer in a Chinese population. Int J Cancer 2018, 142, 1560–7. doi: 10.1002/ijc.31181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seo SI, Choi HS, Choi BY, Kim HS, Kim HY, Jang MK.. Coexistence of hepatitis B surface antigen and antibody to hepatitis B surface may increase the risk of hepatocellular carcinoma in chronic hepatitis B virus infection: a retrospective cohort study. J Med Virol 2014, 86, 124–30. doi: 10.1002/jmv.23779. [DOI] [PubMed] [Google Scholar]
- 47. Tanaka K, Nagao Y, Ide T, Kumashiro R, Sata M.. Antibody to hepatitis B core antigen is associated with the development of hepatocellular carcinoma in hepatitis C virus-infected persons: a 12-year prospective study. Int J Mol Med 2006, 17, 827–32. [PubMed] [Google Scholar]
- 48. Imazeki F, Yokosuka O, Fukai K, Hiraide A, Saisho H.. Significance of prior hepatitis B virus infection in the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig Dis Sci 2003, 48, 1786–92. doi: 10.1023/a:1025459431613. [DOI] [PubMed] [Google Scholar]
- 49. Cho EJ, Kwack MS, Jang ES, You SJ, Lee JH, Kim YJ, et al. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non-B non-C hepatocellular carcinoma in a hepatitis B-endemic area. Digestion 2011, 84, 17–22. doi: 10.1159/000333210. [DOI] [PubMed] [Google Scholar]
- 50. Ben Q, Li Z, Liu C, Cai Q, Yuan Y, Wang K, et al. Hepatitis B virus status and risk of pancreatic ductal adenocarcinoma: a case-control study from China. Pancreas 2012, 41, 435–40. doi: 10.1097/MPA.0b013e31822ca176. [DOI] [PubMed] [Google Scholar]
- 51. Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, et al. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer 2012, 131, 461–8. doi: 10.1002/ijc.26376. [DOI] [PubMed] [Google Scholar]
- 52. Hsing AW, Zhang M, Rashid A, McGlynn KA, Wang BS, Niwa S, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer 2008, 122, 1849–53. doi: 10.1002/ijc.23251. [DOI] [PubMed] [Google Scholar]
- 53. Komori MF, Kimura T, Kariya S, Onoda T, Takeda S, Mizukawa N, et al. Epidemiological correlations between head and neck cancer and hepatitis B core antibody positivity. Anticancer Res 2020, 40, 2393–403. doi: 10.21873/anticanres.14209. [DOI] [PubMed] [Google Scholar]
- 54. Amougou MA, Noah DN, Moundipa PF, Pineau P, Njouom R.. A prominent role of hepatitis D virus in liver cancers documented in Central Africa. BMC Infect Dis 2016, 16, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun CA, Wu DM, Lin CC, Lu SN, You SL, Wang LY, et al. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol 2003, 157, 674–82. doi: 10.1093/aje/kwg041. [DOI] [PubMed] [Google Scholar]
- 56. Chang KC, Tsai PS, Hsu MC, Hung SF, Tsai CC, Lu SN.. Chronic hepatitis C increased the mortality rates of patients with hepatocellular carcinoma and diabetes mellitus in a triple hepatitis virus endemic community. J Gastroenterol 2010, 45, 636–45. doi: 10.1007/s00535-009-0189-5. [DOI] [PubMed] [Google Scholar]
- 57. Al-Kubaisy WA, Obaid KJ, Noor NA, Ibrahim NS, Al-Azawi AA.. Hepatitis C virus prevalence and genotyping among hepatocellular carcinoma patients in Baghdad. Asian Pac J Cancer Prev 2014, 15, 7725–30. doi: 10.7314/apjcp.2014.15.18.7725. [DOI] [PubMed] [Google Scholar]
- 58. de Sanjose S, Nieters A, Goedert JJ, Domingo-Domenech E, Fernandez de Sevilla A, Bosch R, et al. Role of hepatitis C virus infection in malignant lymphoma in Spain. Int J Cancer 2004, 111, 81–5. doi: 10.1002/ijc.11727. [DOI] [PubMed] [Google Scholar]
- 59. Franceschi S, Polesel J, Rickenbach M, Dal Maso L, Probst-Hensch NM, Fux C, et al. Hepatitis C virus and non-Hodgkin’s lymphoma: findings from the Swiss HIV Cohort Study. Br J Cancer 2006, 95, 1598–602. doi: 10.1038/sj.bjc.6603472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cocco P, Piras G, Monne M, Uras A, Gabbas A, Ennas MG, et al. Risk of malignant lymphoma following viral hepatitis infection. Int J Hematol 2008, 87, 474–83. doi: 10.1007/s12185-008-0086-3. [DOI] [PubMed] [Google Scholar]
- 61. Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci 2004, 95, 592–5. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kreimer AR, Brennan P, Lang Kuhs KA, Waterboer T, Clifford G, Franceschi S, et al. Human papillomavirus antibodies and future risk of anogenital cancer: a nested case-control study in the European prospective investigation into cancer and nutrition study. J Clin Oncol 2015, 33, 877–84. doi: 10.1200/JCO.2014.57.8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bettampadi D, Sirak BA, Abrahamsen ME, Reich RR, Villa LL, Ponce EL, et al. Factors associated with persistence and clearance of high-risk oral human papillomavirus (HPV) among participants in the HPV infection in men (HIM) study. Clin Infect Dis 2021, 73, e3227–34. doi: 10.1093/cid/ciaa1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Furniss CS, McClean MD, Smith JF, Bryan J, Applebaum KM, Nelson HH, et al. Human papillomavirus 6 seropositivity is associated with risk of head and neck squamous cell carcinoma, independent of tobacco and alcohol use. Ann Oncol 2009, 20, 534–41. doi: 10.1093/annonc/mdn643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kerishnan JP, Gopinath SC, Kai SB, Tang TH, Ng HL, Rahman ZA, et al. Detection of human papillomavirus 16-specific IgG and IgM antibodies in patient sera: a potential indicator of oral squamous cell carcinoma risk factor. Int J Med Sci 2016, 13, 424–31. doi: 10.7150/ijms.14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Andersson K, Michael KM, Luostarinen T, Waterboer T, Gislefoss R, Hakulinen T, et al. Prospective study of human papillomavirus seropositivity and risk of nonmelanoma skin cancer. Am J Epidemiol 2012, 175, 685–95. doi: 10.1093/aje/kwr373. [DOI] [PubMed] [Google Scholar]
- 67. Andersson K, Luostarinen T, Strand AS, Langseth H, Gislefoss RE, Forslund O, et al. Prospective study of genital human papillomaviruses and nonmelanoma skin cancer. Int J Cancer 2013, 133, 1840–5. doi: 10.1002/ijc.28188. [DOI] [PubMed] [Google Scholar]
- 68. Anantharaman D, Gheit T, Waterboer T, Halec G, Carreira C, Abedi-Ardekani B, et al. No causal association identified for human papillomavirus infections in lung cancer. Cancer Res 2014, 74, 3525–34. doi: 10.1158/0008-5472.CAN-13-3548. [DOI] [PubMed] [Google Scholar]
- 69. Idahl A, Le Cornet C, Gonzalez Maldonado S, Waterboer T, Bender N, Tjonneland A, et al. Serologic markers of Chlamydia trachomatis and other sexually transmitted infections and subsequent ovarian cancer risk: Results from the EPIC cohort. Int J Cancer 2020, 147, 2042–52. [DOI] [PubMed] [Google Scholar]
- 70. Madeleine MM, Anttila T, Schwartz SM, Saikku P, Leinonen M, Carter JJ, et al. Risk of cervical cancer associated with Chlamydia trachomatis antibodies by histology, HPV type and HPV cofactors. Int J Cancer 2007, 120, 650–5. doi: 10.1002/ijc.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anttila T, Tenkanen L, Lumme S, Leinonen M, Gislefoss RE, Hallmans G, et al. Chlamydial antibodies and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2005, 14, 385–9. doi: 10.1158/1055-9965.EPI-03-0325. [DOI] [PubMed] [Google Scholar]
- 72. Inoue M, Sawada N, Goto A, Shimazu T, Yamaji T, Iwasaki M, et al. High-negative anti-helicobacter pylori IgG antibody titers and long-term risk of gastric cancer: results from a large-scale population-based cohort study in Japan. Cancer Epidemiol Biomarkers Prev 2020, 29, 420–6. doi: 10.1158/1055-9965.EPI-19-0993. [DOI] [PubMed] [Google Scholar]
- 73. Vorobjova T, Ren Z, Dunkley M, Clancy R, Maaroos HI, Labotkin R, et al. Response of IgG1 and IgG2 subclasses to helicobacter pylori in subjects with chronic inflammation of the gastric mucosa, atrophy and gastric cancer in a country with high helicobacter pylori infection prevalence. APMIS 2006, 114, 372–80. doi: 10.1111/j.1600-0463.2006.apm_392.x. [DOI] [PubMed] [Google Scholar]
- 74. Knekt P, Teppo L, Aromaa A, Rissanen H, Kosunen TU.. Helicobacter pylori IgA and IgG antibodies, serum pepsinogen I and the risk of gastric cancer: changes in the risk with extended follow-up period. Int J Cancer 2006, 119, 702–5. doi: 10.1002/ijc.21884. [DOI] [PubMed] [Google Scholar]
- 75. Karami N, Talebkhan Y, Saberi S, Esmaeili M, Oghalaie A, Abdirad A, et al. Seroreactivity to helicobacter pylori antigens as a risk indicator of gastric cancer. Asian Pac J Cancer Prev 2013, 14, 1813–7. doi: 10.7314/apjcp.2013.14.3.1813. [DOI] [PubMed] [Google Scholar]
- 76. Kosunen TU, Seppala K, Sarna S, Aromaa A, Knekt P, Virtamo J, et al. Association of helicobacter pylori IgA antibodies with the risk of peptic ulcer disease and gastric cancer. World J Gastroenterol 2005, 11, 6871–4. doi: 10.3748/wjg.v11.i43.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Korodi Z, Dillner J, Jellum E, Lumme S, Hallmans G, Thoresen S, et al. Human papillomavirus 16, 18, and 33 infections and risk of prostate cancer: a Nordic nested case-control study. Cancer Epidemiol Biomarkers Prev 2005, 14, 2952–5. doi: 10.1158/1055-9965.EPI-05-0602. [DOI] [PubMed] [Google Scholar]
- 78. Blase JL, Campbell PT, Gapstur SM, Pawlita M, Michel A, Waterboer T, et al. Prediagnostic helicobacter pylori antibodies and colorectal cancer risk in an elderly, caucasian population. Helicobacter 2016, 21, 488–92. doi: 10.1111/hel.12305. [DOI] [PubMed] [Google Scholar]
- 79. Hellstrom I, Swisher E, Hellstrom KE, Yip YY, Agnew K, Luborsky JL.. Anti-HE4 antibodies in infertile women and women with ovarian cancer. Gynecol Oncol 2013, 130, 629–33. doi: 10.1016/j.ygyno.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu-Rice Y, Edassery SL, Urban N, Hellstrom I, Hellstrom KE, Deng Y, et al. Selenium-Binding Protein 1 (SBP1) autoantibodies in ovarian disorders and ovarian cancer. Reproduction 2017, 153, 277–84. doi: 10.1530/REP-16-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cramer DW, Fichorova RN, Terry KL, Yamamoto H, Vitonis AF, Ardanaz E, et al. Anti-CA15.3 and anti-CA125 antibodies and ovarian cancer risk: results from the EPIC cohort. Cancer Epidemiol Biomarkers Prev 2018, 27, 790–804. doi: 10.1158/1055-9965.EPI-17-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hermsen BB, Verheijen RH, Menko FH, Gille JJ, van Uffelen K, Blankenstein MA, et al. Humoral immune responses to MUC1 in women with a BRCA1 or BRCA2 mutation. Eur J Cancer 2007, 43, 1556–63. doi: 10.1016/j.ejca.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 83. Zhao H, Zhang X, Han Z, Xie W, Yang W, Wei J.. Alteration of circulating natural autoantibodies to CD25-derived peptide antigens and FOXP3 in non-small cell lung cancer. Sci Rep 2018, 8, 9847. doi: 10.1038/s41598-018-28277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pilyugin M, Descloux P, Andre PA, Laszlo V, Dome B, Hegedus B, et al. BARD1 serum autoantibodies for the detection of lung cancer. PLoS One 2017, 12, e0182356. doi: 10.1371/journal.pone.0182356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li Y, Jiang T, Zhang J, Zhang B, Yang W, You G, et al. Elevated serum antibodies against insulin-like growth factor-binding protein-2 allow detecting early-stage cancers: evidences from glioma and colorectal carcinoma studies. Ann Oncol 2012, 23, 2415–22. doi: 10.1093/annonc/mds007. [DOI] [PubMed] [Google Scholar]
- 86. Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA.. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol 2006, 1, 513–9. [PubMed] [Google Scholar]
- 87. Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, et al. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol 2007, 18, 868–73. doi: 10.1093/annonc/mdm007. [DOI] [PubMed] [Google Scholar]
- 88. Pedersen JW, Gentry-Maharaj A, Fourkala EO, Dawnay A, Burnell M, Zaikin A, et al. Early detection of cancer in the general population: a blinded case-control study of p53 autoantibodies in colorectal cancer. Br J Cancer 2013, 108, 107–14. doi: 10.1038/bjc.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang X, Shen W, Dong X, Fan J, Liu L, Gao X, et al. Identification of novel autoantibodies for detection of malignant mesothelioma. PLoS One 2013, 8, e72458. doi: 10.1371/journal.pone.0072458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Tureci O, et al. Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax 2008, 63, 228–33. doi: 10.1136/thx.2007.083592. [DOI] [PubMed] [Google Scholar]
- 91. Lazzaroni MG, Cavazzana I, Colombo E, Dobrota R, Hernandez J, Hesselstrand R, et al. Malignancies in patients with anti-RNA polymerase III antibodies and systemic sclerosis: analysis of the EULAR scleroderma trials and research cohort and possible recommendations for screening. J Rheumatol 2017, 44, 639–47. doi: 10.3899/jrheum.160817. [DOI] [PubMed] [Google Scholar]
- 92. Wu X, Lun Y, Jiang H, Gang Q, Xin S, Duan Z, et al. Coexistence of thyroglobulin antibodies and thyroid peroxidase antibodies correlates with elevated thyroid-stimulating hormone level and advanced tumor stage of papillary thyroid cancer. Endocrine 2014, 46, 554–60. doi: 10.1007/s12020-013-0121-x. [DOI] [PubMed] [Google Scholar]
- 93. Castagna MG, Belardini V, Memmo S, Maino F, Di Santo A, Toti P, et al. Nodules in autoimmune thyroiditis are associated with increased risk of thyroid cancer in surgical series but not in cytological series: evidence for selection bias. J Clin Endocrinol Metab 2014, 99, 3193–8. doi: 10.1210/jc.2014-1302. [DOI] [PubMed] [Google Scholar]
- 94. Shah AA, Laiho M, Rosen A, Casciola-Rosen L.. Protective effect against cancer of antibodies to the large subunits of both RNA polymerases I and III in scleroderma. Arthritis Rheumatol 2019, 71, 1571–9. doi: 10.1002/art.40893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bockle BC, Stanarevic G, Ratzinger G, Sepp NT.. Analysis of 303 Ro/SS-A antibody-positive patients: is this antibody a possible marker for malignancy?. Br J Dermatol 2012, 167, 1067–75. doi: 10.1111/j.1365-2133.2012.11161.x. [DOI] [PubMed] [Google Scholar]
- 96. Ralhan R, Arora S, Chattopadhyay TK, Shukla NK, Mathur M.. Circulating p53 antibodies, p53 gene mutational profile and product accumulation in esophageal squamous-cell carcinoma in India. Int J Cancer 2000, 85, 791–5. doi:. [DOI] [PubMed] [Google Scholar]
- 97. Blixt O, Bueti D, Burford B, Allen D, Julien S, Hollingsworth M, et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res 2011, 13, R25. doi: 10.1186/bcr2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li P, Shi JX, Xing MT, Dai LP, Li JT, Zhang JY.. Evaluation of serum autoantibodies against tumor-associated antigens as biomarkers in lung cancer. Tumour Biol 2017, 39, 1010428317711662. doi: 10.1177/1010428317711662. [DOI] [PubMed] [Google Scholar]
- 99. Zhou SL, Yue WB, Fan ZM, Du F, Liu BC, Li B, et al. Autoantibody detection to tumor-associated antigens of P53, IMP1, P16, cyclin B1, P62, C-myc, Survivn, and Koc for the screening of high-risk subjects and early detection of esophageal squamous cell carcinoma. Dis Esophagus 2014, 27, 790–7. doi: 10.1111/dote.12145. [DOI] [PubMed] [Google Scholar]
- 100. Pandey JP, Kistner-Griffin E, Namboodiri AM, Iwasaki M, Kasuga Y, Hamada GS, et al. Higher levels of antibodies to the tumour-associated antigen cyclin B1 in cancer-free individuals than in patients with breast cancer. Clin Exp Immunol 2014, 178, 75–8. doi: 10.1111/cei.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.