Summary
Natural killer (NK) cells exert an important role in cancer immune surveillance. Recognition of malignant cells and controlled activation of effector functions are facilitated by the expression of activating and inhibitory receptors, which is a complex interplay that allows NK cells to discriminate malignant cells from healthy tissues. Due to their unique profile of effector functions, the recruitment of NK cells is attractive in cancer treatment and a key function of NK cells in antibody therapy is widely appreciated. In recent years, besides the low-affinity fragment crystallizable receptor for immunoglobulin G (FcγRIIIA), the activating natural killer receptors p30 (NKp30) and p46 (NKp46), as well as natural killer group 2 member D (NKG2D), have gained increasing attention as potential targets for bispecific antibody-derivatives to redirect NK cell cytotoxicity against tumors. Beyond modulation of the receptor activity on NK cells, therapeutic targeting of the respective ligands represents an attractive approach. Here, novel therapeutic approaches to unleash NK cells by engagement of activating NK-cell receptors and alternative strategies targeting their tumor-expressed ligands in cancer therapy are summarized.
Keywords: NKG2D, NKp30, NKp46, FcγRIIIA, natural killer cells, bispecific antibody, CAR NK, CAR T
Graphical Abstract
Graphical Abstract.
Natural Killer (NK) cells exert an important role in cancer immune surveillance and recognition of malignant cells as well as their controlled activation is facilitated by expression of activating and inhibitory receptors, which in a complex interplay allow NK cells to discriminate malignant cells from healthy tissues. In recent years, activating natural killer receptors and their ligands have gained increasing attention as potential targets in cancer immunotherapy. Here, novel therapeutic approaches to unleash NK cells by engagement of activating NK cell receptors and alternative strategies targeting their tumor-expressed ligands in cancer therapy are summarized.
Introduction
Immunotherapy is meanwhile an established treatment option in cancer therapy. A variety of approaches have been preclinically investigated and selected concepts matured into clinical practice. Many approaches that recently achieved clinical approval such as bispecific antibodies, immune checkpoint inhibitors, or chimeric antigen receptor (CAR) T cells are dealing with concepts of redirecting T cells or directly triggering T responses to establish cancer immunity ideally in a self-reinforcing immunity cycle [1–4]. However, despite encouraging success in certain tumor entities, overall response rates are still unsatisfactory. In the tumor microenvironment, other effector cell populations including natural killer (NK) cells contribute to tumor immunity by counteracting immune-evasion or promoting T cell responses [5]. Although clinically less advanced concepts modulating the innate immune system hold great promise to further broaden therapeutic options for cancer patients. Analogous to modulating T cells, activating and inhibitory immune checkpoints on myeloid cells or NK cells have been identified and various agents targeting these receptors are in different stages of preclinical and clinical development [6–9].
NK cells share similarities with CD8-positive T cells in certain aspects, but do not require the presentation of tumor antigens by major histocompatibility complex (MHC) class I molecules for activation. In contrast, NK cells are activated by germline-encoded, stress-inducible marker molecules, which they recognize by sets of activating surface receptors (Fig. 1). Activating NK-cell receptors and their cognate ligands are one class of receptor/ligand systems with great promise in cancer immunotherapy and are evaluated in academia and industry [8, 10, 11]. Here, selected candidate activating receptors and their ligands are introduced and advances in novel approaches in modulating their activity for therapeutic intervention are outlined.
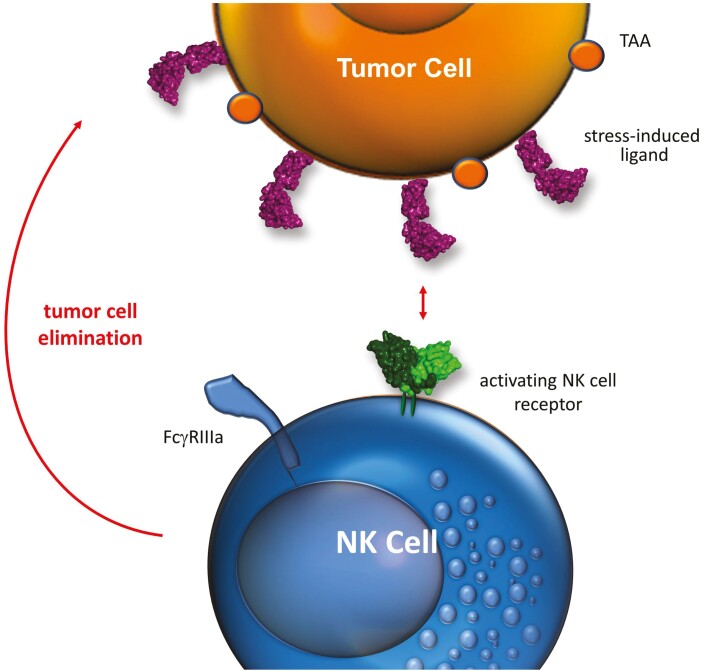
Figure 1:
Tumor cell elimination by triggering activating NK-cell receptors. Upon malignant transformation tumor cells upregulate stress-induced ligands, which can be recognized by activating NK-cell receptors such as NKG2D or NKp30, and are eliminated by cytotoxic attack. Tumor cells are able to evade NK cell attack by shedding or downmodulation of these surface-exposed danger ligands.
NK cells in cancer immunosurveillance and cancer therapy
NK cells are innate immune cells that exert spontaneous cytotoxicity and play a key role in the immune surveillance of tumors [8, 12, 13]. In vitro, NK cells are able to kill tumor cells from different entities, and in animal models an enhanced propensity for tumor development was demonstrated in mutated mice with reduced NK cell functions or in mice depleted of NK cells [14–16]. In humans, an increased risk of cancer was observed in subjects with low natural cytotoxicity in a long-term prospective epidemiological study [17]. Moreover, NK cell defects are found frequently in different tumor types and are associated with progression and metastasis [8, 13, 18–20]. NK cells were suggested to play important roles in different treatment modalities including antibody therapy [21–24]. Here, NK cells are regarded as important effector cells for therapeutic antibodies since the majority of peripheral blood NK cells express the low-affinity Fc receptor for immunoglobulin G (FcγRIIIA, CD16A), which enables them to eliminate target cells by antibody-dependent cell-mediated cytotoxicity (ADCC) [22]. The potential importance of NK cells was suggested by clinical observations. Thus, patients with homozygous expression of the FcγRIIIA 158 V allelic variant with high affinity to the antibody Fc domain had improved responses to antibody therapy than patients expressing the low-affinity FcγRIIIA 158 F variant [25, 26]. Moreover, high NK cell counts were associated with improved survival in non-germinal center (GC) diffuse large B cell lymphoma (DLBCL) patients receiving R-CHOP chemoimmunotherapy, although this was not observed in GC DLBCL patients [27]. In neuroblastoma patients, a contribution of NK cells to anti-GD2 dinutuximab antibody therapy was suggested by improved survival rates observed in patients with unlicensed NK cells lacking inhibitory killer cell immunoglobulin-like receptor (KIR) reactive to self-human leukocyte antigen (HLA) molecules [28].
NK cells can eradicate tumor cells by secretion of perforins and granzymes upon the formation of a lytic synapse between the NK cell and the target cell. In addition, NK cells can kill tumor cells by death receptor activation through the expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or CD95 ligand [12, 29]. NK cells also modulate diverse immune responses by releasing various cytokines and chemokines or by crosstalk with other immune cells including T cells, macrophages, or dendritic cells. To maintain self-tolerance the reactivity state of individual NK cells is fine-tuned (NK cell education) and NK cell activities are tightly regulated by a complex interplay between sets of germline-encoded activating or inhibitory receptors [30, 31]. Activating receptors that allow recognition of tumor cells and mediate natural cytotoxicity include NKG2D and members of the group of natural cytotoxicity receptors (NCR) [8, 31, 32]. The group of NCR comprises natural killer receptors p46 (NKp46, NCR1), p30 (NKp30, NCR3), and p44 (NKp44, NCR2) [33–36]. In particular, NKG2D and NKp30 bind danger signaling self-antigens, which can be either induced or upregulated in response to malignant transformation or other forms of cellular stress (induced self-recognition). Inhibitory receptors include inhibitory members of the polymorphic KIR family and the heterodimeric CD94-NKG2A complex recognizing MHC class I molecules as well as various co-receptors such as CD96, T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT), programmed cell death-1 (PD-1) and others [37–48].
Although antibody therapies have improved patient outcomes, not all patients benefit, and relapses and progression of the disease remain urgent issues. Therefore, the improvement of antibody therapy for cancer is a major issue of current translational research. Regarding their important functions in immunosurveillance of cancer and in antibody therapy, strategies are developed to further improve NK-cell recruitment against tumors. These include agonistic antibodies to co-activating receptors such as CD137, immune checkpoint antibodies for blockade of inhibitory receptor signaling, and tumor-targeting antibodies or bispecific antibody derivatives for NK-cell recruitment and activation by engagement of stimulatory receptors [10, 11, 39].
Engagement of NK lysis receptors
Canonical IgG antibodies can engage FcγRIIIA to elicit ADCC by NK cells, but the interaction between the antibody and FcγRIIIA is of low affinity. Further improvements can be obtained by strengthening the interaction with the Fc receptor using Fc engineered antibodies or bispecific antibody-derivatives [49, 50]. Bispecific antibodies, which combine the antigen-binding sites from two different antibodies in one single protein, can be designed as Fc-less molecules to avoid FcγR ligation and allow to selectively engage a distinct lysis receptor. Currently, one bispecific antibody, the bispecific T cell engager blinatumomab for T cell recruitment via CD3, is marketed [1]. In different attempts to recruit NK cells different formats were employed, including small-sized fusion proteins only consisting of two single-chain fragments variable (scFv) and more complex bispecific IgG-like antibodies harboring a functional Fc domain [10, 50]. Besides, recombinant immunoligands were developed. These molecules typically consist of a tumor cell-directed scFv antibody moiety and a ligand of an activating NK-cell receptor (Fig. 2).
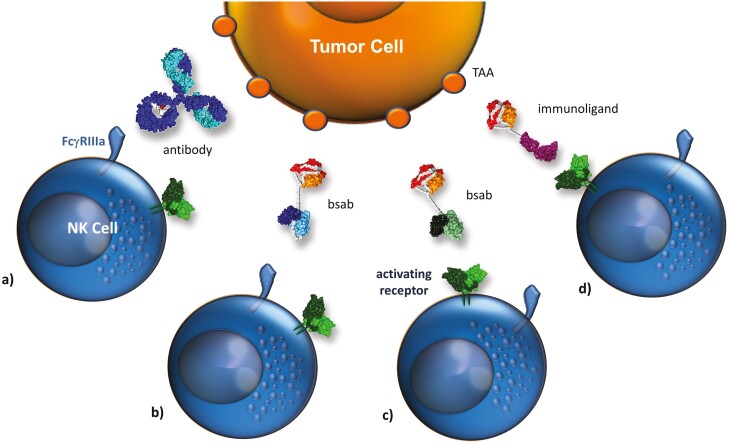
Figure 2:
Strategies to restore/ enhance tumor cell recognition by NK cells. A variety of strategies to restore or enhance tumor cell recognition by NK cells have been evaluated. Selected concepts based on antibodies are summarized: (a) monoclonal antibodies against a tumor-associated antigen (TAA) are capable in triggering ADCC via engagement of the FcγRIIIa, (b) bispecific antibodies (bsab) targeting a TAA and engaging FcγRIIIa, (c) bispecific antibodies targeting a TAA and an alternative activating NK-cell receptor, and (d) recombinant immunoligands based on stress-ligands genetically fused to an antibody fragment targeting a TAA. Note: the molecule structures represent conceptual drawings. The concept could be realized by a variety of molecule designs, including more IgG-like structures [50].
FcγRIIIA
In therapy with tumor-targeting antibodies, the role of NK cells as mediators of ADCC is widely accepted, albeit antibodies mediate a plethora of effector functions [22]. In peripheral blood NK cells are the dominant effector cell population that expresses FcγRIIIA, of which relevance has been suggested by clinical observations [22, 25]. FcγRIIIA is associated with homo- or heterodimers of the adaptor proteins FcƐRI γ and TCR ζ carrying intracellular immunoreceptor tyrosine-based activation motifs (ITAM). In humans, the receptor is also expressed in a subset of peripheral monocytes, macrophages, dendritic cells, and certain T cells. To improve FcγRIIIA engagement and NK cell-mediated ADCC, the antibody`s Fc domain was engineered to achieve a higher affinity [49]. Two Fc glycoengineered antibodies with reduced fucose content, mogamulizumab and obinutuzumab, and an Fc-engineered ADC (belantamab-mafodotin), are approved for clinical use in the treatment of leukemia or lymphomas and more are in different stages of pre-clinical or clinical development. The enhanced affinity to FcγRIIIA by Fc glycoengineered antibodies may reduce the relative impact of inhibitory receptor signaling. Thus, ADCC by rituximab was diminished by inhibitory interactions between KIR and HLA molecules, whereas the inhibitory effect of KIR signaling on ADCC was negligible when the Fc engineered CD20 antibody obinutuzumab was employed [51].
Significant improvements in NK-cell-mediated tumor cell lysis were also obtained with bispecific antibodies harboring FcγRIII-specific Fab or scFv domains with high affinity. Such bispecific CD16 antibodies were highly efficient in inducing NK-cell-mediated lysis of tumor cells. A variety of CD16-engaging bispecific antibodies and antibody derivatives have been described [52–54].
NKG2D
NKG2D (CD314) is a C-type lectin-like receptor. The receptor forms a complex with the signaling molecule DNAX-activating protein of 10 kDa (DAP10), which includes an intracellular tyrosine-based YxxM motif for signal transduction [32, 55]. NKG2D, which in humans is expressed by NK cells, CD8-positive T cells, γδ T cells, and CD4-positive T cell subsets, recognizes multiple cellular ligands. Human NKG2D ligands consist of MHC class I related chain (MIC) A and B and six UL-16 binding proteins (ULBP1 to 6) [32]. In mice, NKG2D ligands include members of the Rae-1 family, which are orthologs of human ULBPs, the H60 family, as well as murine UL16-binding protein-like transcript 1 (MULT1). Normally NKG2D ligands are expressed poorly in healthy tissues. Their expression is regulated at the level of transcription, mRNA or protein stability, and proteolytic cleavage from the cell surface membrane and surface expression is often found upregulated upon cellular stress such as infections with pathogens or malignant transformation. The contribution of NKG2D and its ligands to cancer immunosurveillance has been highlighted in various murine cancer models [56–60]. In cancer patients, the expression of NKG2D ligands has been linked to favorable prognosis and improved NK cell tumor infiltration [61–63]. Acute myeloid leukemia (AML) stem cells have been described as lacking NKG2D ligands and thereby evading immune destruction [64]. However, also contradictious reports exist, according to which high levels of MICA were associated with poor prognosis [65, 66], which may be explained by the occurrence of shed MICA which may act as immunosuppressive [67, 68]. In addition, immunoselection may edit tumor cells to downregulate the expression of ligands for activating receptors as demonstrated for NKG2D and NKp46 in murine cancer models [60, 69]. An important role has been attributed to shedding soluble NKG2D ligands, which may impede NK cell activities by blocking receptor functions or downregulating the cognate receptors’ surface expression levels, as demonstrated for NKG2D in both T and NK cells [70, 71].
For activation of NKG2D, which represents a compelling target for immune intervention, ULBP2 or MICA were fused to IgG antibodies or scFv antibody fragments [72–74]. For example, ULBP2 was fused to a CD138 scFv for targeting of malignant plasma cells [73]. The immunoligand mediated NK cell killing of multiple myeloma cells in vitro and showed therapeutic efficacy in a xenograft model of multiple myeloma. Similarly constructed immunoligands directed against other cell surface antigens such as CD33 or CD20 on blood cancer cells or antigens such as CEA, CD24, and HER2 on solid tumors also have proven efficacy in pre-clinical models [72, 75–78]. Importantly, co-engagement of NKG2D was found to enhance NK cell-mediated ADCC in a synergistic manner. For example, the expression of NKG2D ligands in target cells may be important as evidenced by experiments showing that their expression resulted in higher susceptibility of lymphoma cells to rituximab-mediated ADCC in vitro [79]. Drug-induced expression of NKG2D ligands results in higher in vitro ADCC by an Fc engineered CD33 antibody in AML [80]. In agreement with these findings immunoligands consisting of a CD20 scFv and either ULBP2 or MICA antigen were shown to augment lymphoma cell lysis when combined with rituximab or the CD38 antibody daratumumab [76, 81]. In addition to immunoligands, also bispecific NKG2D engaging antibodies have been described. Recently, the therapeutic efficacy of a bispecific tandem scFv antibody engaging NKG2D and binding to CS1 on malignant plasma cells has been reported [82]. In a recent report, VHH-based bispecific antibodies have been described and showed high cytolytic potential [83].
NKp30
The NCR NKp30 (NCR3, CD337) is expressed on resting as well as activated NK cells, innate lymphoid cells 2 (ILC2), CD8-positive T cells, and γδ T cells [84, 85]. Like FcγRIIIA, NKp30 signals via the ITAM carrying FcƐRI γ and TCR ζ chains [31]. Ligation of NKp30 elicits cytotoxicity and cytokine release functions of NK cells [33]. However, NKp30 exists in different splice variants, which differ in intracellular domains. In contrast to activating NKp30 A and NKp30 B isoforms, the NKp30 C splice variant was reported to exert immunosuppressive functions [86]. In addition to heparan sulfate glycosaminoglycans on cell surfaces or the extracellular matrix, NKp30 recognizes B7-H6, the HLA-B-associated transcript 3 protein (Bat3), and soluble galectin-3 [87–89]. B7-H6 is normally absent from healthy tissues. However, its expression has been found on the cell surface of tumor cells of different origins and its expression has been shown to promote NK cell cytotoxicity [87]. Bat3 normally is localized in the nucleus, but is trafficked to the cell surface upon contact with NK cells and can be secreted in exosomes, and was shown to trigger cytokine release and cytotoxicity [88]. In contrast, soluble galectin-3 inhibited NK cell effector functions [89]. Also, elevated serum levels of soluble B7-H6 were observed in a subgroup of patients with advanced malignant melanoma and were correlated to NKp30 down-modulation, chemotherapy resistance, and metastases in neuroblastoma [90, 91]. Moreover, soluble Bat3 was implicated in immune evasion in chronic lymphocytic leukemia [92].
In cancer patients, a key function for NKp30 has been suggested in neuroblastoma and gastrointestinal stromal tumors (GIST). Thus, in GIST patients receiving activating NK cell KIT tyrosine kinase inhibitor therapy, predominant expression of the immunosuppressive NKp30 C isoform relative to activating NKp30 A and B splice variants was associated with reduced survival [86, 90]. Levels of expression of the NKp30 C isoform were also associated with the risk of relapse in high-risk neuroblastoma patients in remission after induction chemotherapy [90]. Moreover, high NKp30 expression was associated with improved overall survival in AML [93]. To harness NKp30 as a trigger molecule, bispecific immunoligands were generated. Thus, B7-H6 was fused to a CD20 scFv to target lymphoma cells [94]. This recombinant fusion protein triggered NK cell-mediated lysis of lymphoma cells. In particular, a highly synergistic induction of cytotoxicity and cytokine release was observed by a combination of this molecule with a CD20-directed immunoligand containing the NKG2D ligand ULBP2. Similar results were observed with immunoligands consisting of B7-H6 and a HER2-specific scFv [95]. In a recent study, a minimal NKp30 binding domain derived from B7-H6 was affinity maturated and showed in the format of a bispecific, EGFR-directed fusion protein potent NK cell activation capacity. When these novel agents were additionally equipped with a functional Fc domain an even higher cytolytic potential was demonstrated by concomitant FcγRIIIA activation. Besides triggering tumor cell lysis by NK cells, these novel agents triggered significant pro-inflammatory cytokine release demonstrating a unique feature compared to cetuximab [96]. Recently, also NKp30 bispecific antibodies have been preclinically evaluated for their ability to eliminate precursor B-ALL tumor cells or multiple myeloma cells and showed significant cytolytic capacity [10, 97].
NKp46
NKp46 (NCR1, CD335) is another member of the NCR family [34, 98]. NKp46 is displayed by both resting and activated NK cells as well as ILC1, a subset of ILC3, and a minor T cell population [99]. Like NKp30 and FcγRIIIA, NKp46 associates with ITAM-containing FcƐRI γ or TCR ζ chains for intracellular signal transduction. Ligands for NKp46 include hemagglutinin and other viral components, the soluble complement factor P as well as heparan sulfate proteoglycan, which is expressed by different tumors [100, 101]. A specific cellular cell surface ligand for NKp46 however has not been identified yet. Several findings support a cancer-protective role for NKp46. For example, the growth of certain murine lymphoma cells in Ncr1 knock-out mice was increased [102]. Moreover, tumor growth was enhanced upon antibody-mediated NKp46 neutralization in models of malignant melanoma [103]. The engagement of NKp46 induces NK cell cytotoxicity and cytokine release [98], making NKp46 an interesting trigger molecule for immunotherapeutic intervention. Recently, bispecific antibodies engaging NKp46 and a tumor antigen, i.e. either CD19 or CD20 for targeting malignant B cells, have been generated. Bispecific antibodies were produced in a format containing a functional Fc domain to trigger FcγRIIIA in parallel to NKp46. These trifunctional NK cell engagers were highly effective in inducing NK cell cytotoxicity and proved efficacy in a lymphoma xenograft model in mice [104]. These types of molecules were recently also evaluated for their ability to control pediatric B-cell precursor acute lymphoblastic leukemia [97].
Specific targeting of NK-cell receptor ligands
Regarding their paucity in healthy tissues ligands for activating receptors such as NKG2D or NCR may represent valid target antigens on malignant cells for antibody-based approaches. Of note, the expression of these antigens by different tumor types may result in a broad application spectrum of such strategies and render them particularly attractive. To date, several approaches targeting ligands of NKG2D, NKp30, or NKp46 have been evaluated in pre-clinical studies. These include antibodies, antibody-drug conjugates (ADC), bispecific molecules as well as chimeric antigen receptors (CAR) for cellular therapies with genetically modified effector cells (Fig. 3).
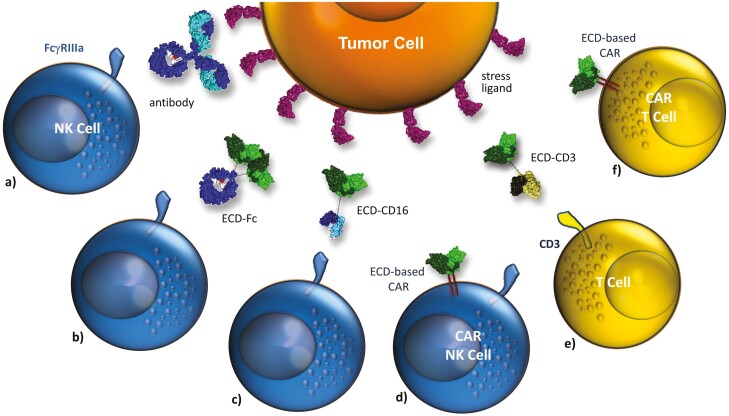
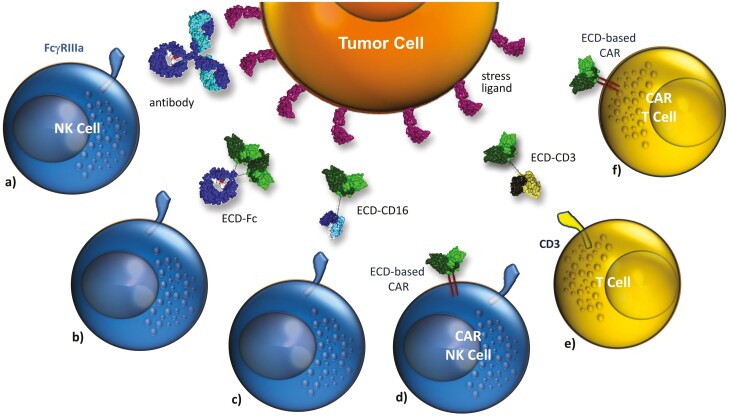
Figure 3:
Targeting the surface-expressed ligands of activating NK-cell receptors. Activating NK-cell receptors can be downregulated in cancer patients. In this situation concepts targeting these quite tumor-restricted ligands may be beneficial: (a) monoclonal antibodies targeting stress-ligands such as MICA/B prevent shedding and trigger ADCC, (b) the extracellular domain (ECD) of an activating receptor (e.g. NKG2D) is used as targeting domain and fused to an IgG-Fc domain thereby redirecting NK cell cytotoxicity by FcγRIIIa engagement. Since macrophages and subsets of monocytes express FcγRIIIa recruitment of other effector cells may be possible, (c) and (e) bifunctional fusion proteins consisting of the ECD of an activating NK-cell receptor and an antibody fragment triggering FcγRIIIa or CD3 for redirecting NK cell or T cell cytotoxicity, respectively, (d) and (f) the ECD of activating NK-cell receptors are used as targeting domains in CAR receptors to generate CAR NK or CAR T cells.
For example, antibodies with a dual specificity for the NKG2D ligands MICA and MICB such as antibody IPH4301 have been designed [105]. These antibodies may mediate dual functions. On the one side, they may target and eradicate cancer cells directly by mediating immune-mediated effector functions such as ADCC or complement-dependent cytotoxicity. On the other side, they may modulate the immune response by neutralizing soluble, shed MICA and MICB molecules, which impair NK and T cell activities in the tumor microenvironment [106]. In another approach, MICA and MICB were employed as targets for ADC to deliver cytotoxic payloads to the tumor cells. Thus, a MICA/B specific antibody was coupled to DNA cross-linking pyrrolobenzodiazepine dimers and the resulting ADC proved therapeutic efficacy in xenograft models of solid tumors in mice [107].
Considering that NKG2D and other NK-cell receptors recognize multiple ligands, the extracellular domain (ECD) of NKG2D was employed as a targeting device as an alternative to antigen-binding domains from antibodies. The rationale for this approach is that the NKG2D receptor domain allows targeting all different NKG2D ligands, whereas antibodies are specific for individual antigens. For example, the NKG2D ECD was fused to an IgG Fc domain. In murine models of Epstein-Barr virus protein LMP1 driven lymphomas, such a murine NKG2D-Fc fusion protein demonstrated therapeutic efficacy in vivo and triggered cytotoxicity against lymphoma cells, but, importantly, not against normal B cells in vitro [108]. To further optimize the efficacy of such constructs, the human NKG2D ECD was fused to a modified Fc domain that was engineered by amino acid sequence alterations to establish a higher affinity to activating FcγR and to achieve a potency to mediate ADCC [109, 110]. This fusion protein efficiently triggered ADCC with NK cells as effector cells against leukemia or breast cancer cells.
Moreover, T cells were redirected to tumor cells expressing danger signaling NK-cell receptor ligands. For example, the NKG2D ECD was fused to a scFv or Fab fragment directed to the activating T cell trigger molecule CD3 [111, 112]. To redirect T-cell cytotoxicity against B7-H6 expressing tumors, a bispecific [anti-B7H6 × CD3] tandem scFv antibody was designed [113]. This BiTE-like molecule triggered human T cells to produce IFNγ in the presence of B7-H6-positive tumor cells. A bispecific variant directed to murine CD3 triggered cytotoxicity of T cells and prolonged survival of mice harboring murine tumors that had been transduced with human B7-H6 expression.
In addition, CAR were designed for genetic T cell and NK cell engineering. Thus CAR based on ECD from NKG2D [114–116], NKp46 [117], NKp30 [118], or DNAM-1 [119] have been generated. In addition, the efficacy of an anti-B7-H6 CAR was described [120, 121]. Most advanced are NKG2D CAR T cells engineered with an expression construct consisting of full-length NKG2D that is fused to the CD3 ζ signaling domain [122]. By pairing with endogenously expressed DAP10 like the natural NKG2D, triggering of this CAR results also in the costimulatory signal. The potential of this concept was suggested in initial studies showing functional in vitro activity and therapeutic activity of murine NKG2D CAR T cells in models of ovarian cancer, lymphoma, and multiple myeloma [116, 123–125], and also human NKG2D CAR T cells demonstrated therapeutic potential in pre-clinical studies [122]. In a phase I clinical study in patients with AML/myelodysplastic syndrome or relapsed/refractory multiple myeloma however no objective responses at a single injection of low cell doses were observed, and persistence of CAR T cells in the patients was limited [126]. Thus, further optimizations are needed to achieve CAR T cell expansion. Whether this may be achieved by other CAR designs in which for example NKG2D ECD was fused to CD3 ζ as well as co-stimulation domains from either 4-1BB or CD28 as suggested in other studies will have to be determined [115, 127].
Besides CAR T cells also NKG2D-based CAR NK cells have been pre-clinically evaluated. Recently, CAR NK cells targeting multiple myeloma have been described [128]. In addition, NK cells derived from induced pluripotent stem cells (iPSC) were engineered to express an NKG2D CAR containing the 2B4 co-stimulatory as well as the CD3ζ signaling domain [129]. These NKG2D CAR NK cells inhibited tumor growth, prolonged survival and may provide attractive engineered “off-the-shelf” lymphocytes for tumor immunotherapy that deserve further investigation. In a recent study, NKG2D CAR NK cells have been combined with NKG2D-directed bispecific antibodies. In an immunocompetent mouse glioblastoma model mimicking low or absent NKG2D ligand expression, the combination of CAR NK cells and an NKG2D-ErbB2-directed bispecific antibody effectively suppressed the outgrowth of tumors, resulting in treatment-induced endogenous antitumor immunity [130, 131].
Conclusions
Activating NK-cell receptors and their ligands play an important role in the immunosurveillance of cancer cells under physiological conditions. In cancer patients, these receptor systems represent promising target structures for therapeutic intervention. Various approaches are at different stages of pre-clinical and clinical development.
Limitations of approaches aiming at engaging NK cells against tumor cells may occur by tumor-induced NK cell dysfunction resulting from down-regulation of activating NK-cell receptors and induced NK cell anergy. Reduced expression of individual activating NK-cell receptors has been observed frequently in tumor patients. Also, shed ligands may hamper the efficacy of selected approaches because in addition to inducing receptor internalization they may compete for receptor binding. The therapeutic efficacy of the described strategies may be further enhanced by combination with compounds that interfere with the shedding of ligands or promote expression levels. For example, the combination with immune checkpoint blockade, cytokines, or immunomodulatory agents may lead to further improvements and may allow overcoming these limitations. In situations in which NK cells remain dysfunctional and cannot be sufficiently activated by the outlined strategies, approaches making use of the ECD of activating NK-cell receptors for the targeting of their cognate tumor-expressed ligands may represent promising alternative approaches. In principle, the described concepts may perfectly complement actual approaches in modulating T-cell activity and myeloid effector cells. Combining such strategies triggering complex anti-tumor responses may be possible. It will be interesting to see how these novel approaches perform in the clinical situation and how they can be integrated into existing treatment regimens.
Acknowledgments
The Deutsche José Carreras Leukämie-Stifung and the Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie are gratefully acknowledged for the José Carreras-DGHO scholarship for doctoral research to TZ.
Glossary
Abbreviations
- ADC
antibody-drug conjugate
- ADCC
antibody-dependent cell-mediated cytotoxicity
- DLBCL
diffuse large B cell lymphoma
- ITAM
intracellular immunoreceptor tyrosine-based activation motif
- KIR
killer cell immunoglobulin-like receptor
- MHC
major histocompatibility complex
- NCR
natural cytotoxicity receptor
- NK
natural killer
- NKG2D
natural killer group 2 member D
- PD-1
programmed cell death-1
- scFv
single-chain fragment variable
- TIGIT
T-cell immunoreceptor with immunoglobulin and ITIM domains
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- CAR
chimeric antigen receptor
- ECD
extracellular domain
Contributor Information
Matthias Peipp, Division of Antibody-Based Immunotherapy, Department of Internal Medicine II, Christian Albrechts University and University Hospital Schleswig-Holstein, Kiel, Germany.
Katja Klausz, Division of Antibody-Based Immunotherapy, Department of Internal Medicine II, Christian Albrechts University and University Hospital Schleswig-Holstein, Kiel, Germany.
Ammelie Svea Boje, Division of Antibody-Based Immunotherapy, Department of Internal Medicine II, Christian Albrechts University and University Hospital Schleswig-Holstein, Kiel, Germany.
Tobias Zeller, Division of Transfusion Medicine, Cell Therapeutics and Haemostaseology, University Hospital, LMU Munich, Munich, Germany.
Stefan Zielonka, Protein Engineering and Antibody Technologies, Merck Healthcare KGaA, Darmstadt, Germany.
Christian Kellner, Division of Transfusion Medicine, Cell Therapeutics and Haemostaseology, University Hospital, LMU Munich, Munich, Germany.
Funding
The work was supported by research funds to MP within the clinical research unit CATCH-ALL funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 444949889 to MP.
Conflicts of interest
The authors declare no conflicts of interest. SZ is an employee of Merck Darmstadt KGaA developing effector cell engaging molecules. MP received research funding by Merck Darmstadt KGaA.
Author contributions
All authors contributed to writing, editing and proofreading the manuscript.
Data availability
Not applicable.
References
- 1. Goebeler ME, Bargou RC.. T cell-engaging therapies—BiTEs and beyond. Nat Rev Clin Oncol 2020, 17, 418–34. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 2. Wei SC, Duffy CR, Allison JP.. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018, 8, 1069–86. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 3. Larson RC, Maus MV.. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer 2021, 21, 145–61. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen DS, Mellman I.. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013, 39, 1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 5. Huntington ND, Cursons J, Rautela J.. The cancer-natural killer cell immunity cycle. Nat Rev Cancer 2020, 20, 437–54. doi: 10.1038/s41568-020-0272-z. [DOI] [PubMed] [Google Scholar]
- 6. Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL.. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer 2019, 19, 568–86. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lentz RW, Colton MD, Mitra SS, Messersmith WA.. Innate immune checkpoint inhibitors: the next breakthrough in medical oncology?. Mol Cancer Ther 2021, 20, 961–74. doi: 10.1158/1535-7163.MCT-21-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morvan MG, Lanier LL.. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2016, 16, 7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 9. van den Berg TK, Valerius T.. Myeloid immune-checkpoint inhibition enters the clinical stage. Nat Rev Clin Oncol 2019, 16, 275–6. doi: 10.1038/s41571-018-0155-3. [DOI] [PubMed] [Google Scholar]
- 10. Demaria O, Gauthier L, Debroas G, Vivier E.. Natural killer cell engagers in cancer immunotherapy: Next generation of immuno-oncology treatments. Eur J Immunol 2021, 51, 1934–42. doi: 10.1002/eji.202048953. [DOI] [PubMed] [Google Scholar]
- 11. Chester C, Fritsch K, Kohrt HE.. Natural killer cell immunomodulation: targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy. Front Immunol 2015, 6, 601. doi: 10.3389/fimmu.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiossone L, Dumas PY, Vienne M, Vivier E.. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 2018, 18, 671–88. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 14. Gorelik E, Wiltrout RH, Okumura K, Habu S, Herberman RB.. Role of NK cells in the control of metastatic spread and growth of tumor cells in mice. Int J Cancer 1982, 30, 107–12. doi: 10.1002/ijc.2910300118. [DOI] [PubMed] [Google Scholar]
- 15. Riccardi C, Santoni A, Barlozzari T, Puccetti P, Herberman RB.. In vivo natural reactivity of mice against tumor cells. Int J Cancer 1980, 25, 475–86. doi: 10.1002/ijc.2910250409. [DOI] [PubMed] [Google Scholar]
- 16. Talmadge JE, Meyers KM, Prieur DJ, Starkey JR.. Role of NK cells in tumour growth and metastasis in beige mice. Nature 1980, 284, 622–4. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- 17. Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K.. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000, 356, 1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 18. Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011, 121, 3609–22. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sibbitt WL Jr., Bankhurst AD, Jumonville AJ, Saiki JH, Saiers JH, Doberneck RC.. Defects in natural killer cell activity and interferon response in human lung carcinoma and malignant melanoma. Cancer Res 1984, 44, 852–6. [PubMed] [Google Scholar]
- 20. Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 2007, 109, 323–30. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 21. Bracci L, Schiavoni G, Sistigu A, Belardelli F.. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014, 21, 15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Childs RW, Carlsten M.. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov 2015, 14, 487–98. doi: 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 23. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 24. Zingoni A, Fionda C, Borrelli C, Cippitelli M, Santoni A, Soriani A.. Natural killer cell response to chemotherapy-stressed cancer cells: role in tumor immunosurveillance. Front Immunol 2017, 8, 1194. doi: 10.3389/fimmu.2017.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weng WK, Levy R.. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003, 21, 3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 26. Jakubowiak A, Offidani M, Pegourie B, De La Rubia J, Garderet L, Laribi K, et al. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 2016, 127, 2833–40. doi: 10.1182/blood-2016-01-694604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim JK, Chung JS, Shin HJ, Song MK, Yi JW, Shin DH, et al. Influence of NK cell count on the survival of patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood Res 2014, 49, 162–9. doi: 10.5045/br.2014.49.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest 2012, 122, 3260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 2008, 8, 713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brodin P, Karre K, Hoglund P.. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol 2009, 30, 143–9. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 31. Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S.. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013, 31, 227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol Res 2015, 3, 575–82. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med 1999, 190, 1505–16. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, et al. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med 1998, 188, 953–60. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med 1998, 187, 2065–72. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, et al. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med 1999, 189, 787–96. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–43. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119, 3064–72. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poggi A, Zocchi MR.. Natural killer cells and immune-checkpoint inhibitor therapy: Current knowledge and new challenges. Mol Ther Oncolytics 2022, 24, 26–42. doi: 10.1016/j.omto.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA 2009, 106, 17858–63. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu S, Zhang H, Li M, Hu D, Li C, Ge B, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ 2013, 20, 456–64. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol 2014, 15, 431–8. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 43. Martinet L, Smyth MJ.. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol 2015, 15, 243–54. doi: 10.1038/nri3799. [DOI] [PubMed] [Google Scholar]
- 44. Davis ZB, Vallera DA, Miller JS, Felices M.. Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol 2017, 31, 64–75. doi: 10.1016/j.smim.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol 2017, 139, 335–346.e3. doi: 10.1016/j.jaci.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 46. Benson DM Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010, 116, 2286–94. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stojanovic A, Fiegler N, Brunner-Weinzierl M, Cerwenka A.. CTLA-4 is expressed by activated mouse NK cells and inhibits NK Cell IFN-gamma production in response to mature dendritic cells. J Immunol 2014, 192, 4184–91. doi: 10.4049/jimmunol.1302091. [DOI] [PubMed] [Google Scholar]
- 48. Lougaris V, Tabellini G, Baronio M, Patrizi O, Gazzurelli L, Mitsuiki N, et al. CTLA-4 regulates human Natural Killer cell effector functions. Clin Immunol 2018, 194, 43–5. doi: 10.1016/j.clim.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 49. Kellner C, Otte A, Cappuzzello E, Klausz K, Peipp M.. Modulating cytotoxic effector functions by Fc engineering to improve cancer therapy. Transfus Med Hemother 2017, 44, 327–36. doi: 10.1159/000479980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brinkmann U, Kontermann RE.. The making of bispecific antibodies. MAbs 2017, 9, 182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Terszowski G, Klein C, Stern M.. KIR/HLA interactions negatively affect rituximab- but not GA101 (obinutuzumab)-induced antibody-dependent cellular cytotoxicity. J Immunol 2014, 192, 5618–24. doi: 10.4049/jimmunol.1400288. [DOI] [PubMed] [Google Scholar]
- 52. Phung SK, Miller JS, Felices M.. Bi-specific and tri-specific NK cell engagers: the new avenue of targeted NK cell immunotherapy. Mol Diagn Ther 2021, 25, 577–92. doi: 10.1007/s40291-021-00550-6. [DOI] [PubMed] [Google Scholar]
- 53. Ellwanger K, Reusch U, Fucek I, Wingert S, Ross T, Muller T, et al. Redirected optimized cell killing (ROCK(R)): a highly versatile multispecific fit-for-purpose antibody platform for engaging innate immunity. MAbs 2019, 11, 899–918. doi: 10.1080/19420862.2019.1616506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koch J, Tesar M.. Recombinant antibodies to arm cytotoxic lymphocytes in cancer immunotherapy. Transfus Med Hemother 2017, 44, 337–50. doi: 10.1159/000479981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 1999, 285, 730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 56. Cerwenka A, Baron JL, Lanier LL.. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci USA 2001, 98, 11521–6. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH.. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol 2000, 1, 119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 58. Diefenbach A, Jensen ER, Jamieson AM, Raulet DH.. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 2001, 413, 165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y.. NKG2D function protects the host from tumor initiation. J Exp Med 2005, 202, 583–8. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008, 28, 571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Watson NF, Spendlove I, Madjd Z, McGilvray R, Green AR, Ellis IO, et al. Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. Int J Cancer 2006, 118, 1445–52. doi: 10.1002/ijc.21510. [DOI] [PubMed] [Google Scholar]
- 62. de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA, et al. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer 2012, 12, 24. doi: 10.1186/1471-2407-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McGilvray RW, Eagle RA, Watson NF, Al-Attar A, Ball G, Jafferji I, et al. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res 2009, 15, 6993–7002. doi: 10.1158/1078-0432.CCR-09-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paczulla AM, Rothfelder K, Raffel S, Konantz M, Steinbacher J, Wang H, et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 2019, 572, 254–9. doi: 10.1038/s41586-019-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Madjd Z, Spendlove I, Moss R, Bevin S, Pinder SE, Watson NF, et al. Upregulation of MICA on high-grade invasive operable breast carcinoma. Cancer Immun 2007, 7, 17. [PMC free article] [PubMed] [Google Scholar]
- 66. McGilvray RW, Eagle RA, Rolland P, Jafferji I, Trowsdale J, Durrant LG.. ULBP2 and RAET1E NKG2D ligands are independent predictors of poor prognosis in ovarian cancer patients. Int J Cancer 2010, 127, 1412–20. doi: 10.1002/ijc.25156. [DOI] [PubMed] [Google Scholar]
- 67. Liu G, Lu S, Wang X, Page ST, Higano CS, Plymate SR, et al. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J Clin Invest 2013, 123, 4410–22. doi: 10.1172/JCI69369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chitadze G, Lettau M, Bhat J, Wesch D, Steinle A, Furst D, et al. Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int J Cancer 2013, 133, 1557–66. doi: 10.1002/ijc.28174. [DOI] [PubMed] [Google Scholar]
- 69. Elboim M, Gazit R, Gur C, Ghadially H, Betser-Cohen G, Mandelboim O.. Tumor immunoediting by NKp46. J Immunol 2010, 184, 5637–44. doi: 10.4049/jimmunol.0901644. [DOI] [PubMed] [Google Scholar]
- 70. Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D.. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol 2013, 78, 120–9. doi: 10.1111/sji.12072. [DOI] [PubMed] [Google Scholar]
- 71. Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, et al. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol 2012, 189, 1360–71. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 72. Wang T, Sun F, Xie W, Tang M, He H, Jia X, et al. A bispecific protein rG7S-MICA recruits natural killer cells and enhances NKG2D-mediated immunosurveillance against hepatocellular carcinoma. Cancer Lett 2016, 372, 166–78. doi: 10.1016/j.canlet.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 73. von Strandmann EP, Hansen HP, Reiners KS, Schnell R, Borchmann P, Merkert S, et al. A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human multiple myeloma in vitro and in vivo. Blood 2006, 107, 1955–62. doi: 10.1182/blood-2005-05-2177. [DOI] [PubMed] [Google Scholar]
- 74. Xie W, Liu F, Wang Y, Ren X, Wang T, Chen Z, et al. VEGFR2 targeted antibody fused with MICA stimulates NKG2D mediated immunosurveillance and exhibits potent anti-tumor activity against breast cancer. Oncotarget 2016, 7, 16445–61. doi: 10.18632/oncotarget.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stamova S, Cartellieri M, Feldmann A, Bippes CC, Bartsch H, Wehner R, et al. Simultaneous engagement of the activatory receptors NKG2D and CD3 for retargeting of effector cells to CD33-positive malignant cells. Leukemia 2011, 25, 1053–6. doi: 10.1038/leu.2011.42. [DOI] [PubMed] [Google Scholar]
- 76. Kellner C, Hallack D, Glorius P, Staudinger M, Mohseni Nodehi S, de Weers M, et al. Fusion proteins between ligands for NKG2D and CD20-directed single-chain variable fragments sensitize lymphoma cells for natural killer cell-mediated lysis and enhance antibody-dependent cellular cytotoxicity. Leukemia 2012, 26, 830–4. doi: 10.1038/leu.2011.288. [DOI] [PubMed] [Google Scholar]
- 77. Rothe A, Jachimowicz RD, Borchmann S, Madlener M, Kessler J, Reiners KS, et al. The bispecific immunoligand ULBP2-aCEA redirects natural killer cells to tumor cells and reveals potent anti-tumor activity against colon carcinoma. Int J Cancer 2014, 134, 2829–40. [DOI] [PubMed] [Google Scholar]
- 78. Kellner C, Lutz S, Oberg HH, Wesch D, Otte A, Diemer KJ, et al. Tumor cell lysis and synergistically enhanced antibody-dependent cell-mediated cytotoxicity by NKG2D engagement with a bispecific immunoligand targeting the HER2 antigen. Biol Chem 2021. doi: 10.1515/hsz-2021-0229. [DOI] [PubMed] [Google Scholar]
- 79. Inagaki A, Ishida T, Yano H, Ishii T, Kusumoto S, Ito A, et al. Expression of the ULBP ligands for NKG2D by B-NHL cells plays an important role in determining their susceptibility to rituximab-induced ADCC. Int J Cancer 2009, 125, 212–21. doi: 10.1002/ijc.24351. [DOI] [PubMed] [Google Scholar]
- 80. Vasu S, He S, Cheney C, Gopalakrishnan B, Mani R, Lozanski G, et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood 2016, 127, 2879–89. doi: 10.1182/blood-2015-11-680546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kellner C, Gunther A, Humpe A, Repp R, Klausz K, Derer S, et al. Enhancing natural killer cell-mediated lysis of lymphoma cells by combining therapeutic antibodies with CD20-specific immunoligands engaging NKG2D or NKp30. Oncoimmunology 2016, 5, e1058459. doi: 10.1080/2162402X.2015.1058459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chan WK, Kang S, Youssef Y, Glankler EN, Barrett ER, Carter AM, et al. A CS1-NKG2D bispecific antibody collectively activates cytolytic immune cells against multiple myeloma. Cancer Immunol Res 2018, 6, 776–87. doi: 10.1158/2326-6066.CIR-17-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Raynaud A, Desrumeaux K, Vidard L, Termine E, Baty D, Chames P, et al. Anti-NKG2D single domain-based antibodies for the modulation of anti-tumor immune response. Oncoimmunology 2020, 10, 1854529. doi: 10.1080/2162402X.2020.1854529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pinheiro PF, Justino GC, Marques MM.. NKp30—A prospective target for new cancer immunotherapy strategies. Br J Pharmacol 2020, 177, 4563–80. doi: 10.1111/bph.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pogge von Strandmann E, Shatnyeva O, Hansen HP.. NKp30 and its ligands: emerging players in tumor immune evasion from natural killer cells. Ann Transl Med 2015, 3, 314. doi: 10.3978/j.issn.2305-5839.2015.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011, 17, 700–7. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 87. Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med 2009, 206, 1495–503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 2007, 27, 965–74. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 89. Wang W, Guo H, Geng J, Zheng X, Wei H, Sun R, et al. Tumor-released galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J Biol Chem 2014, 289, 33311–9. doi: 10.1074/jbc.M114.603464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Semeraro M, Rusakiewicz S, Zitvogel L, Kroemer G.. Natural killer cell mediated immunosurveillance of pediatric neuroblastoma. Oncoimmunology 2015, 4, e1042202. doi: 10.1080/2162402X.2015.1042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schlecker E, Fiegler N, Arnold A, Altevogt P, Rose-John S, Moldenhauer G, et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res 2014, 74, 3429–40. doi: 10.1158/0008-5472.CAN-13-3017. [DOI] [PubMed] [Google Scholar]
- 92. Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M, et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013, 121, 3658–65. doi: 10.1182/blood-2013-01-476606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chretien AS, Fauriat C, Orlanducci F, Rey J, Borg GB, Gautherot E, et al. NKp30 expression is a prognostic immune biomarker for stratification of patients with intermediate-risk acute myeloid leukemia. Oncotarget 2017, 8, 49548–63. doi: 10.18632/oncotarget.17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kellner C, Maurer T, Hallack D, Repp R, van de Winkel JG, Parren PW, et al. Mimicking an induced self phenotype by coating lymphomas with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J Immunol 2012, 189, 5037–46. doi: 10.4049/jimmunol.1201321. [DOI] [PubMed] [Google Scholar]
- 95. Peipp M, Derer S, Lohse S, Staudinger M, Klausz K, Valerius T, et al. HER2-specific immunoligands engaging NKp30 or NKp80 trigger NK-cell-mediated lysis of tumor cells and enhance antibody-dependent cell-mediated cytotoxicity. Oncotarget 2015, 6, 32075–88. doi: 10.18632/oncotarget.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pekar L, Klausz K, Busch M, Valldorf B, Kolmar H, Wesch D, et al. Affinity maturation of B7-H6 translates into enhanced NK cell-mediated tumor cell lysis and improved proinflammatory cytokine release of bispecific immunoligands via NKp30 engagement. J Immunol 2021, 206, 225–36. doi: 10.4049/jimmunol.2001004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Colomar-Carando N, Gauthier L, Merli P, Loiacono F, Canevali P, Falco M, et al. Exploiting natural killer cell engagers to control pediatric B-cell precursor acute lymphoblastic leukemia. Cancer Immunol Res 2022, 10, 291–302. doi: 10.1158/2326-6066.CIR-21-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, et al. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med 1997, 186, 1129–36. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zook EC, Kee BL.. Development of innate lymphoid cells. Nat Immunol 2016, 17, 775–82. doi: 10.1038/ni.3481. [DOI] [PubMed] [Google Scholar]
- 100. Narni-Mancinelli E, Gauthier L, Baratin M, Guia S, Fenis A, Deghmane AE, et al. Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci Immunol 2017, 10, eaam9628. doi: 10.1126/sciimmunol.aam9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hudspeth K, Silva-Santos B, Mavilio D.. Natural cytotoxicity receptors: broader expression patterns and functions in innate and adaptive immune cells. Front Immunol 2013, 4, 69. doi: 10.3389/fimmu.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Halfteck GG, Elboim M, Gur C, Achdout H, Ghadially H, Mandelboim O.. Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J Immunol 2009, 182, 2221–30. doi: 10.4049/jimmunol.0801878. [DOI] [PubMed] [Google Scholar]
- 103. Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest 2009, 119, 1251–63. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 2019, 177, 1701–1713.e16. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 105. Morel A, Viaud N, Bonnafous C, Trichard S, Joulin-Giet A, Mizari S, et al. IPH4301, an antibody targeting MICA and MICB exhibits potent cytotoxic activity and immunomodulatory properties for the treatment of cancer. Cancer Res 2016, 76, 1491. [Google Scholar]
- 106. Ferrari de Andrade L, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 2018, 359, 1537–42. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Blery M, Bregeon D, Gauthier L, Lhospice F, Pouyet L.. Immunomodulatory antibody drug conjugates binding to a human MICA polypeptide. U.S. Patent Application No. US 2020/002307. 2018.
- 108. Zhang B, Kracker S, Yasuda T, Casola S, Vanneman M, Homig-Holzel C, et al. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell 2012, 148, 739–51. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Raab S, Steinbacher J, Schmiedel BJ, Kousis PC, Steinle A, Jung G, et al. Fc-optimized NKG2D-Fc constructs induce NK cell antibody-dependent cellular cytotoxicity against breast cancer cells independently of HER2/neu expression status. J Immunol 2014, 193, 4261–72. doi: 10.4049/jimmunol.1400872. [DOI] [PubMed] [Google Scholar]
- 110. Steinbacher J, Baltz-Ghahremanpour K, Schmiedel BJ, Steinle A, Jung G, Kubler A, et al. An Fc-optimized NKG2D-immunoglobulin G fusion protein for induction of natural killer cell reactivity against leukemia. Int J Cancer 2015, 136, 1073–84. doi: 10.1002/ijc.29083. [DOI] [PubMed] [Google Scholar]
- 111. Godbersen C, Coupet TA, Huehls AM, Zhang T, Battles MB, Fisher JL, et al. NKG2D ligand-targeted bispecific T-cell engagers lead to robust antitumor activity against diverse human tumors. Mol Cancer Ther 2017, 16, 1335–46. doi: 10.1158/1535-7163.MCT-16-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hagelstein I, Lutz MS, Schmidt M, Heitmann JS, Malenke E, Zhou Y, et al. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins as novel treatment option in advanced soft tissue sarcomas. Front Immunol 2021, 12, 653081. doi: 10.3389/fimmu.2021.653081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wu MR, Zhang T, Gacerez AT, Coupet TA, DeMars LR, Sentman CL.. B7H6-specific bispecific T cell engagers lead to tumor elimination and host antitumor immunity. J Immunol 2015, 194, 5305–11. doi: 10.4049/jimmunol.1402517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhang T, Barber A, Sentman CL.. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res 2006, 66, 5927–33. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- 115. Lehner M, Gotz G, Proff J, Schaft N, Dorrie J, Full F, et al. Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One 2012, 7, e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang T, Lemoi BA, Sentman CL.. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood 2005, 106, 1544–51. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tal Y, Yaakobi S, Horovitz-Fried M, Safyon E, Rosental B, Porgador A, et al. An NCR1-based chimeric receptor endows T-cells with multiple anti-tumor specificities. Oncotarget 2014, 5, 10949–58. doi: 10.18632/oncotarget.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Eisenberg V, Shamalov K, Meir S, Hoogi S, Sarkar R, Pinker S, et al. Targeting multiple tumors using T-cells engineered to express a natural cytotoxicity receptor 2-based chimeric receptor. Front Immunol 2017, 8, 1212. doi: 10.3389/fimmu.2017.01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wu MR, Zhang T, Alcon A, Sentman CL.. DNAM-1-based chimeric antigen receptors enhance T cell effector function and exhibit in vivo efficacy against melanoma. Cancer Immunol Immunother 2015, 64, 409–18. doi: 10.1007/s00262-014-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hua CK, Gacerez AT, Sentman CL, Ackerman ME.. Development of unique cytotoxic chimeric antigen receptors based on human scFv targeting B7H6. Protein Eng Des Sel 2017, 30, 713–21. doi: 10.1093/protein/gzx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gacerez AT, Hua CK, Ackerman ME, Sentman CL.. Chimeric antigen receptors with human scFvs preferentially induce T cell anti-tumor activity against tumors with high B7H6 expression. Cancer Immunol Immunother 2018, 67, 749–59. doi: 10.1007/s00262-018-2124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Demoulin B, Cook WJ, Murad J, Graber DJ, Sentman ML, Lonez C, et al. Exploiting natural killer group 2D receptors for CAR T-cell therapy. Future Oncol 2017, 13, 1593–605. doi: 10.2217/fon-2017-0102. [DOI] [PubMed] [Google Scholar]
- 123. Zhang T, Barber A, Sentman CL.. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res 2007, 67, 11029–36. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 124. Barber A, Meehan KR, Sentman CL.. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther 2011, 18, 509–16. doi: 10.1038/gt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Barber A, Zhang T, Sentman CL.. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol 2008, 180, 72–8. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 126. Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase 1 trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res 2018, 7, 100–12. doi: 10.1158/2326-6066.CIR-18-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Han Y, Xie W, Song DG, Powell DJ Jr. Control of triple-negative breast cancer using ex vivo self-enriched, costimulated NKG2D CAR T cells. J Hematol Oncol 2018, 11, 92. doi: 10.1186/s13045-018-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Leivas A, Valeri A, Cordoba L, Garcia-Ortiz A, Ortiz A, Sanchez-Vega L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J 2021, 11, 146. doi: 10.1038/s41408-021-00537-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Li Y, Hermanson DL, Moriarity BS, Kaufman DS.. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 2018, 23, 181–192.e5. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhang C, Roder J, Scherer A, Bodden M, Pfeifer Serrahima J, Bhatti A, et al. Bispecific antibody-mediated redirection of NKG2D-CAR natural killer cells facilitates dual targeting and enhances antitumor activity. J ImmunoTher Cancer 2021, 9, e002980. doi: 10.1136/jitc-2021-002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lazarova M, Wels WS, Steinle A.. Arming cytotoxic lymphocytes for cancer immunotherapy by means of the NKG2D/NKG2D-ligand system. Expert Opin Biol Ther 2020, 20, 1491–501. doi: 10.1080/14712598.2020.1803273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.