Summary
Background
The protective immunity against omicron following a BNT162b2 Pfizer booster dose among elderly individuals (ie, those aged >65 years) is not well characterised.
Methods
In a community-based, prospective, longitudinal cohort study taking place in France in which 75 residents from three nursing homes were enrolled, we selected 38 residents who had received a two-dose regimen of mRNA vaccine and a booster dose of Pfizer BNT162b2 vaccine. We excluded individuals that did not receive three vaccine doses or did not have available sera samples. We measured anti-S IgG antibodies and neutralisation capacity in sera taken 56 (28-68) and 55 (48-64) days (median (range)) after the 2nd and 3rd vaccine doses, respectively. Antibodies targeting the SARS-CoV-2 Spike protein were measured with the S-Flow assay as binding antibody units per milliliter (BAU/mL). Neutralising activities in sera were measured as effective dilution 50% (ED50) with the S-Fuse assay using authentic isolates of delta and omicron BA.1.
Findings
Among the 38 elderly individuals recruited to the cohort study between November 23rd, 2020 and April 29th, 2021, with median age of 88 (range 72-101) years, 30 (78.95%) had been previously infected with SARS-CoV-2. After three vaccine doses, serum neutralising activity was lower against omicron BA.1 (median ED50 of 774.5, range 15.0-34660.0) than the delta variant (median ED50 of 4972.0, range 213.7-66340.0), and higher among previously infected (ie, convalescent; median ED50 against omicron: 1088.0, range 32.6-34660.0) compared with infection-naive residents (median ED50 against omicron: 188.4, range 15.0-8918.0). During the French omicron wave in December 2021-January 2022, 75% (6/8) of naive residents were infected, compared to 25% (7/30) of convalescent residents (P=0.0114). Anti-Spike antibody levels and neutralising activity against omicron BA.1 after a third BNT162b2 booster dose were lower in those with breakthrough BA.1 infection (n=13) compared with those without (n=25), with a median of 1429.9 (range 670.9-3818.3) BAU/mL vs 2528.3 (range 695.4-8832.0) BAU/mL (P=0.029) and a median ED50 of 281.1 (range 15.0-2136.0) vs 1376.0 (range 32.6-34660.0) (P=0.0013), respectively.
Interpretation
This study shows that elderly individuals who received three vaccine doses elicit neutralising antibodies against the omicron BA.1 variant of SARS-CoV-2. Elderly individuals who had also been previously infected showed higher neutralising activity compared with naive individuals. Yet, breakthrough infections with omicron occurred. Individuals with breakthrough infections had significantly lower neutralising titers compared to individuals without breakthrough infection. Thus, a fourth dose of vaccine may be useful in the elderly population to increase the level of neutralising antibodies and compensate for waning immunity.
Funding
Institut Pasteur, Fondation pour la Recherche Médicale (FRM), European Health Emergency Preparedness and Response Authority (HERA), Agence nationale de recherches sur le sida et les hépatites virales – Maladies Infectieuses Emergentes (ANRS-MIE), Agence nationale de la recherche (ANR), Assistance Publique des Hôpitaux de Paris (AP-HP) and Fondation de France.
Keywords: SARS-CoV-2, Antibodies, Omicron, Vaccine, Elderly individuals
Research in context.
Evidence before this study
Compared to the general population, elderly individuals have a decreased response to vaccination, an increased risk of severe forms of COVID-19 and an accelerated waning of their antibody levels. The omicron variant of SARS-CoV-2 has been associated with decreased vaccine efficacy, most likely due to its capacity to escape antibody neutralisation. On December 2021 and January 2022, we searched in Pubmed, BiorXiv and MedrXiv for studies analysing the neutralisation activity of sera from elderly individuals against omicron. We observed that there was little information available.
Added value of this study
Here, we show that a booster dose of the Pfizer BNT162b2 vaccine increases SARS-CoV-2 specific antibody levels and is required to elicit neutralising antibodies against omicron in the elderly population naïve to SARS-CoV-2 infection. Elderly individuals with a history of COVID-19 have higher antibody levels compared to non-convalescents, and their sera are capable of neutralising omicron already after the second dose of the primary vaccine series. Nevertheless, we observed breakthrough infections with omicron after the booster dose, more frequently in the elderly individuals with lower levels of anti-Spike antibodies and with a lower capacity to neutralise omicron.
Implications of all the available evidence
Overall, our data show that elderly individuals are at risk of omicron infection even after a booster dose, potentially due to the considerable immune escape of this variant. Elderly individuals with previous COVID-19 infection have a stronger immune response, but their antibody levels will likely decline over time. Thus, a fourth dose of vaccine may be useful in the elderly population to further increase the level of SARS-CoV-2 specific antibodies and compensate for the waning of immunity.
Alt-text: Unlabelled box
Introduction
The neutralisation capacity of sera from vaccinated or previously infected (i.e., convalescent) individuals against the omicron (B.1.1.529) (BA.1) variant of SARS-CoV-2 has been well studied among several population groups, and has been shown to be lower against omicron compared to other variants.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Information on the vaccine efficacy and the neutralisation capacity of sera from elderly individuals against omicron is more limited,13, 14, 15, 16 despite decreased immunogenicity, increased risk of severe forms of disease and accelerated waning of immunity in this population.17, 18, 19 Here, we evaluated the capacity of a booster dose of BNT162b2 to elicit neutralising antibodies against omicron BA.1 and examined levels of humoral immunity before omicron breakthrough infections among residents living in nursing homes.
Methods
Clinical investigation
Thirty-eight residents from three nursing homes were recruited from the Covid-Oise study. Their baseline characteristics are depicted in Supplementary Table 1. This community-based cohort (NCT04644159) started on November 13th 2020 and is ongoing. It comprises four sessions of epidemiological data and biological specimen collection. Inclusion of participants occurred during the three first sessions, in winter 2020, spring 2021 and winter 2021. The criteria of inclusion was to live, work and/or study in the area of the city of Crépy-en-Valois (Oise, France) at the time of study initiation. No exclusion criteria were applied. The goal of this longitudinal cohort study is to monitor the immunological response following SARS-CoV-2 infection and Coronavirus Disease 2019 (COVID-19) vaccination, in participants of a wide age range, starting from 5 years old, up to nursing home residents.
Past infection of the thirty-eight residents included in the present study was determined based on clinical data and detection of SARS-CoV-2 specific antibodies using two serological assays, as previously described.20,21 All sera samples available since inclusion of the residents in the study were evaluated with both assays. Detection of past infection relied on anti-Spike (S) and anti-Nucleocapsid (N) antibodies for pre-vaccination sera, and only on anti-N antibodies for post-vaccination sera.
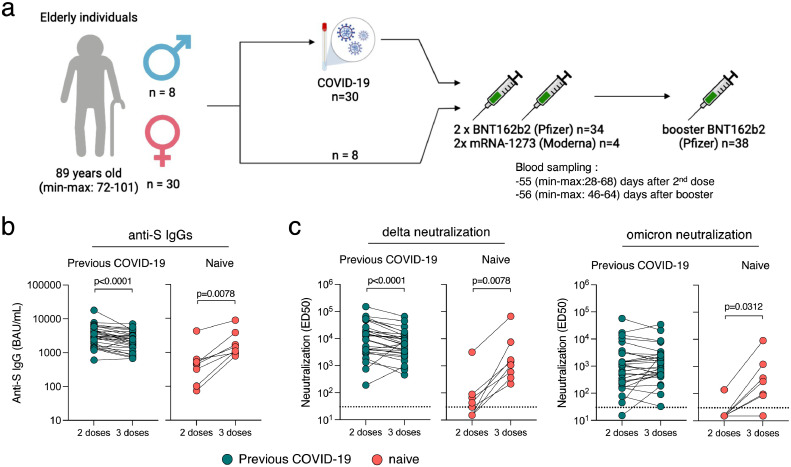
Sera were obtained two months (median 56 days, range 28-68 days) after the second dose, and two months (median 55 days, range 48-64 days) after the third dose of mRNA vaccine (Figure 1a). 37 out of the 38 residents (97.3%) participated to the first sampling session and all participated to the second one (100.0%). All residents were immunised with BNT162b2 for their primary series except four who received mRNA-1273. The two initial doses were received three weeks apart (median 21 days, range 18-31 days). All residents received a booster dose of BNT162b2 eight months after the second dose (median 236 days, range 194-250 days).
Figure 1.
Immunogenicity of a booster dose of BNT162b2 vaccine in elderly individuals. (a) Thirty-eight elderly individuals from three nursing homes, (30 females and 8 males) were included in the analysis. All received a two-dose regimen of mRNA vaccine (Pfizer BNT162b2; n=34 or Moderna; n=4) and a booster dose (Pfizer BNT162b2; n=38) 8 months apart. Thirty were diagnosed with COVID-19 prior to their booster dose. (b) Anti-Spike IgGs were measured using the S-Flow assay 2 months after the second dose and 2 months after the booster dose. Data are provided as Binding Arbitrary Unit per mL (BAU/mL), the standardised WHO unit. The limit of detection is 3 BAU/mL. Comparisons were performed using the Wilcoxon matched-pairs signed rank test. (c) Neutralisation of delta and omicron were measured using the live-virus S-Fuse assay 2 months after the second dose and 2 months after the booster dose. Data are provided as Effective Dilution 50 (ED50), indicating the dilution of serum capable of inhibiting 50% of viral infection. Green dots indicate individuals with an history of COVID-19 prior to their booster dose of vaccine. Pink dots indicate individuals with no previous COVID-19. The dashed line indicates the limit of detection. Comparisons were performed using the Wilcoxon matched-pairs signed rank test.
We also traced breakthrough infections among residents from the three nursing homes during the French omicron BA.1 epidemic wave in December 2021-January 2022. These breakthrough infections occurred at a median (range) of 53 (34-63) days after the second blood sampling, which was 55 (48-64) days after the booster dose. Following the first cases recorded in the nursing homes in December 2021, for whom PCR testing was motivated by symptoms, all the other residents were submitted to PCR screening, independently of symptoms. When viral load was sufficient, RT-PCR positive test results were reanalysed with a second round of RT-PCR screening to identify SARS-CoV-2 variants of concern based on mutations E484K, E484Q and L452R, or K417N. Presumed omicron infection was defined as detection of a strain not harboring E484Q/N and L452R mutations, or harboring the K417N mutation. The individual characteristics of the nursing home residents who developed breakthrough infections are depicted in Supplementary Table 2. Report of clinical investigation was performed in accordance with the Strobe (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.
Laboratory analyses
Antibodies targeting the Spike protein of SARS-CoV-2 were measured with the S-Flow assay (N=75). This assay uses 293T cells stably expressing the Spike (S) protein (GenBank: QHD43416.1; later to refer as 293T-S cells) and 293T control cells as control to detect anti-Spike antibodies by flow cytometry.22 Cells were cultivated in DMEM containing 10% fetal calf serum and 1% PenStrep (ThermoFisher). On the day of the assay, cells were detached with PBS-EDTA, transferred into U-bottom 96-well plates (50,000 cells per well) and incubated at 4°C for 30 min with sera (1:300 dilution, unless otherwise specified) in PBS containing 0.5% BSA and 2 mM EDTA. Then, cells were washed with PBS, and stained using an anti-IgG Fc Alexa Fluor 647 (Jackson ImmunoResearch). Cells were washed with PBS and fixed for 10 min using 4% paraformaldehyde (PFA). Data were acquired on an Attune NxT instrument (Life Technologies). The sensitivity (95% confidence interval) is 99.2% (97.7–99.8) and the specificity is 100% (98.5–100).23 The assay is standardised with WHO international reference sera (20/136 and 20/130) and cross-validated with two commercially available ELISA (Abbott 147 and Beckmann 56) to allow calculation of binding antibody units per milliliter (BAU/mL).24
Neutralising activities in sera were measured with the S-Fuse assay using authentic isolates of delta and omicron. This assay uses U2OS-ACE2 GFP1-10 and GFP 11 reporter cells, also termed S-Fuse cells, that become GFP+ upon infection with SARS-CoV-2.25,26 On the day of the assay, cells were mixed (ratio 1:1) and plated in μClear 96-well plate (Greiner Bio-One; 20,000 cells per well). The indicated SARS-CoV-2 strains were incubated with sera for 15 min at room temperature and added to S-Fuse cells. All sera were heat-inactivated for 30 min at 56 °C before use. Eighteen hours later, cells were fixed with 4% paraformaldehyde (PFA), washed and stained with Hoechst (dilution 1:1,000, Invitrogen). All sera were tested in limiting dilution to determine Effective Dilution 50% (ED50 or titer) values. In each well, the number of GFP+ syncytia was scored with an Opera Phenix high-content confocal microscope (PerkinElmer). The GFP area and the number of nuclei were quantified using Harmony software version 4.9 (PerkinElmer). The percentage of neutralisation was calculated using the number of syncytia as value with the following formula: 100 × (1 − (value with serum − value in ‘non-infected’)/(value in ‘no serum’ − value in ‘non-infected’)). ED50 were calculated with a reconstructed curve using the percentage of the neutralisation at the different concentrations. Viral stock were produced on Vero E6 cells, titrated on Vero E6 or S-Fuse cells and sequenced to confirm viral lineages (GISAID accession ID: EPI_ISL_2029113 and EPI_ISL_7413964 for delta and omicron BA.1 isolates, respectively).
Statistical analyses
All continuous variables were described using median and range. Percentage and P-values were either exact or rounded to two and five digits, respectively. Proportions were compared using a Fisher exact test. The BAU/mL and neutralisation titers of sera were compared before and after the booster dose using the Wilcoxon matched-pairs signed rank test. When comparing individuals with previous COVID-19 to naive individuals a Mann-Whitney rank test was performed. Comparisons of BAU/mL and neutralisation in individuals with or without subsequent breakthrough infection were performed using the Mann-Whitney rank test. No multiple comparisons correction was applied. Individuals with missing data were excluded from paired analysis (Wilcoxon matched-pairs signed rank test). No sample size calculation was conducted prior to the study, all individuals willing to participate were included. Investigators were not blinded with respect to the origin of the samples and randomisation was not applicable. All analyses were performed using Stata (Stata Corp., College Station, Texas, USA) or GraphPad Prism 8 (GraphPad Software, LLC).
Ethical considerations
The COVID-Oise cohort was registered with ClinicalTrials.gov (NCT04644159) and received ethical approval by the Comité de Protection des Personnes Nord Ouest IV. A written informed consent was obtained from all participants enrolled in the cohort, including those selected for the present study. For the nursing home residents who did not have full capacity to sign legal documents, written informed consent was obtained from their relatives.
Role of the funding source
The funding sources had no role in the study (design, data collection, data analysis and data interpretation) and were not involved in the writing of the manuscript or the decision to submit it for publication. TB, LP, OS and AF had access to dataset and had final responsibility for the decision to submit for publication
Results
Among the 38 elderly individuals (30 females and 8 males) enrolled in this cohort study between November 23rd 2020 and April 29th 2021, with a median (range) age of 88 (72-101) at the final time of sampling, 30 (78.95%) had been previously infected, based on past clinical history and serological findings (Supplementary Table 1). 29 residents were infected before the primary series of vaccination, while for one resident anti-N antibodies, indicating a past infection, were detected in sera sampled post-primary series and thus the time of infection could not be estimated due to a lack of prior samples. Among the 29 infections that occurred before the primary series, 27 could be dated back to the first SARS-CoV-2 epidemic wave, with positive serology obtained in May 2020. The two remaining residents infected before vaccination had either a positive PCR test in November 2020, or a positive serology in December 2020.
Following the second dose of vaccine, anti-Spike IgG levels were higher among convalescents compared with naive residents (Table 1 & Figure 1b). The third dose increased the antibody titers for naive residents to levels similar to those of the convalescents after 2 doses (Table 1 & Figure 1b). The ED50 of neutralisation was lower against omicron BA.1 compared to delta, and higher among convalescents compared to naive residents (Table 1 & Figure 1c). For the eight naive residents, neutralisation was detectable for only five (62.50%) and one (12.50%) individuals against delta and omicron BA.1 after the second dose, respectively. The number of residents displaying neutralising antibodies increased to eight (100%) against delta and six (75.00%) against omicron BA.1 after the third dose. All 30 convalescents except one neutralised delta and omicron after the second and third dose.
Table 1.
Median (range) anti-Spike IgG and ED50 of neutralisation against delta and omicron.
| Naive | Convalescent | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| anti-S IgG | ED50 delta | ED50 omicron | anti-S IgG | ED50 delta | ED50 omicron |
P values |
|||||
| A | B | C | D | E | F | A vs D | B vs E | C vs F | B vs C | E vs F | |
| 2nd dose | 456 (74-4283) | 37 (15-3189) | 15 (0-138) | 3056 (601-17820) | 12393 (189-150000) | 1113 (15-57403) | 0.0001 | <0.0001 | <0.0001 | 0.0625 | <0.0001 |
| 3rd dose | 1256 (784-8832) | 1243 (214-66340) | 188 (15-8918) | 2485 (671-7115) | 6156 (458-64875) | 1088 (33-34660) | 0.3316 | 0.0343 | 0.0163 | 0.0078 | <0.0001 |
P values A vs D, B vs E, C vs F : Mann-Whitney test; B vs C, E vs F : Wilcoxon test.
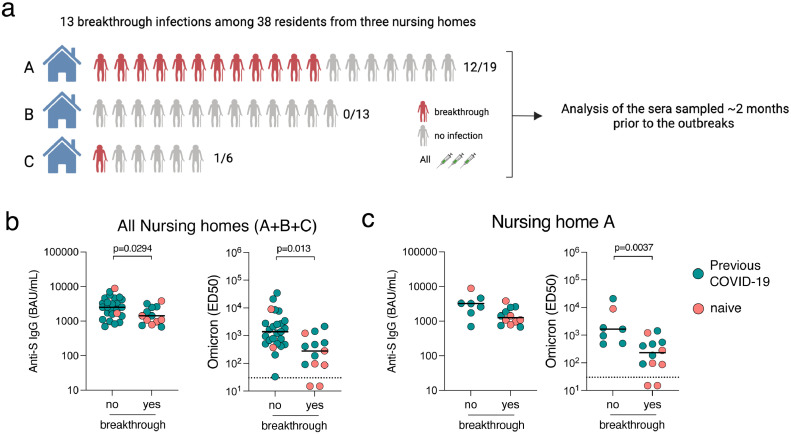
In December 2021-January 2022, a large wave of omicron BA.1 infection spread across France. All of the three nursing homes reported omicron breakthrough infections, but only one displayed a large outbreak across the residents, including some recruited in our study (see Methods and Figure 2a). Among the 38 residents included in our study, 13 breakthrough infections occurred (34.21%), among which 11 were confirmed as presumed omicron infection based on single nucleotide polymorphism PCR analysis (see methods and Supplementary Table 2). The viral load detected for the two other breakthrough infections was not sufficient to identify mutations of interest. The PCR method used to detect positive SARS-CoV-2 cases does not allow to differentiate BA.1 and BA.2, but the frequency of BA.2 was low (<5%) at the time of these outbreaks.27 Seventy-five percent (6/8) of naive residents were infected, compared to 23.33% (7/30) of convalescent (P=0.0114; two-sided Fisher exact test). We compared anti-Spike IgG and ED50 of neutralisation against omicron at the closest sampling point (corresponding to a median (range) of 53 (34-63) days before the breakthrough infections and 55 (48-64) days after the booster dose) between those who subsequently had a breakthrough infection or not (Figure 2b). Median titers were lower in those with compared to those without breakthrough infection: 1429.9 vs 2528.3 BAU/mL (P=0.0294) for anti-Spike IgG; and 281.1 vs 1376.0 (P=0.0013) for ED50 against omicron BA.1, respectively. We performed the same analysis in the nursing home where the virus circulated the most and where most of the breakthrough infections among the residents included in our study were identified (Household A, Figure 2a and 2c). Again, we observed a trend towards lower BAU/mL, and significantly lower neutralisation titers against omicron BA.1, in individuals who had a breakthrough infection compared to those that were uninfected.
Figure 2.
Humoral immune response predicts odds of omicron breakthrough infection. (a) Omicron breakthrough infections were reported in the 3 nursing homes included in the study, including 13 breakthrough infections among our study participants. The number of individuals included in our study, as well as the number of breakthrough infections among them are indicated for each household (A, B and C). We collected sera prior to the outbreaks (median (range) of 53 (49-60) days before the breakthrough infection, corresponding to 55 (49-59) days after the booster dose) (b) Levels of anti-Spike IgGs and neutralisation of omicron are indicated for individuals from the three households (A, B and C) having a subsequent confirmed breakthrough infection or not. (c) Levels of anti-Spike IgGs and neutralisation of omicron are indicated for individuals from the household A having a subsequent confirmed breakthrough infection or not. Data are provided as Binding Arbitrary Unit per mL (BAU/mL) (left) and neutralisation titers (ED50) (right). Comparisons were performed using the Mann-Whitney rank test. Green dots indicate individuals with an history of COVID-19 prior to their booster dose of vaccine. Pink dots indicate individuals with no previous COVID-19. The dashed line indicates the limit of detection of the neutralisation assay (30). The limit of detection of the anti-Spike assay is 3 BAU/mL. Black bars indicate the median.
Of note, none of the individuals with an ED50 titer above 2136.0 had a breakthrough infection. The breakthrough infections were either asymptomatic or presented with minor symptoms (no fever or respiratory symptoms), and were considered mild-to-moderate by the physicians in charge, requiring no hospitalisation or oxygenotherapy.
Discussion
This study shows lower levels of anti-Spike IgG and neutralising antibodies against delta and omicron among naive compared to convalescent nursing home residents after two doses of mRNA vaccine. A third dose of BNT162b2 vaccine significantly increased antibody levels for naive residents and elicited serum neutralising activity capable of neutralising omicron. This confirms the importance of receiving at least three doses of vaccine to generate a cross-reactive humoral immune response that covers all VOCs.28 Nevertheless, median antibody levels and neutralisation titers in naive residents remained lower than in convalescents, and were not sufficient to prevent infection with omicron for most of them. This was particularly the case for those with the lowest levels of neutralising antibodies, suggesting that a fourth vaccine dose may be useful in this particularly aged population to prevent infection with omicron, a variant known for its high immune escape properties. Recent data suggest that the fourth dose rescues antibody titers to the levels obtained after the third dose, but does not increase cross-reactivity.29 It remains to determine whether the third dose achieves this kind of “ceiling of immunity” in elderly individuals, in whom vaccine immunogenicity is reduced. A fourth dose will rescue antibody levels and prolong protection, as demonstrated in recent nation-wide clinical trials.30,31 Of note, the absence of severe forms of disease in our omicron-infected group is reassuring, however based on a small sample size.
Anti-Spike IgG and neutralisation titers were lower among residents who had breakthough infections compared to those who had not, regardless of COVID-19 history. However, no antibody level was able to fully discriminate the two groups and to be used as a correlate of protection. This may have to do in part with the very high escape immunity properties of the omicron variant. Furthermore, blood sampling was done 2 months prior to breakthrough and hence antibody levels may have varied by the time of actual infection.
Most previous infections occurred during the first epidemic wave (February-March 2020), so that the primary vaccine series performed in early 2021 was able to boost the production of neutralising antibodies primed one year earlier, even in this elderly population. This is reassuring, and reflects the strength of the so-called hybrid immunity combining the effects of infection and vaccination.32 Interestingly, among these vaccinated-convalescent residents, antibody levels were slightly lower in samples collected two months after the third dose than in samples collected two months after the second dose (Figure 1B and C). This may be explained by the high levels of antibodies elicited by the first injection in convalescent individuals. As a result, antibody levels are already high at the time of the second injection, and may be further increased by this injection. In contrast, due to waning of immune responses, the levels of circulating antibodies are low before the booster dose, so that the levels achieved just after the third dose did not reach those achieved just after the second dose.
Our study has limitations. The small sample size precludes analysis of the characteristics that may further impact vaccine efficacy, such as sex, preexisting conditions, or ongoing medications. The over-representation of females among our participants (30 out of 38 individuals) may have also biased our results, as females are known to differ from males in the induction and waning of antibodies, in both infection and vaccination settings.22,33,34 We did not have access to nasopharyngeal swabs to measure antibody levels at the site of viral entry and replication. We were thus unable to link breakthrough infections to local levels of antibodies, which might represent a better correlate of protection. Further studies are needed to determine the contribution of mucosal immunity on the acquisition of SARS-CoV-2 and the severity of COVID-19. Lastly, we only tested BA.1, the initial omicron clade, which was circulating in France at the time of the investigation and was responsible for the breakthrough infections that occurred in the nursing homes of the study. It will be worth examining the neutralisation activity of the sera against other omicron sub-lineages, such as BA.2, BA.2.12.1, BA.4 and BA.5, at the next blood sampling of the same cohort participants.
This study shows that elderly individuals who received three vaccine doses elicited neutralising antibodies against omicron. Protection against omicron was increased in those who had been previously infected in addition to the three vaccine doses, and was associated with higher neutralisation levels. Thus, our results suggest that a fourth vaccine dose may be useful in the elderly population to prevent infection, by augmenting antibody levels and omicron neutralisation.
Contributors
Conceptualisation and Methodology: TB, LP, DP, OS, AF.
Cohort management and sample collection: LP, LT, CD, AK, SFP, LPdF, ER, NJ, MNU.
Serological and seroneutralisation assays: TB, DP, IS, FP, FGB, MA, SP, TW, SC, SvdW, MW.
Data assembly and manuscript writing: TB, LP, DP, LT, OS, AF.
Funding acquisition: OS, AF.
Supervision: OS, AF.
Access and verification of the data: TB, LP, OS, AF.
Decision to submit the manuscript: TB, LP, OS, AF.
Data sharing statement
All data produced in the present study are available with publication with a signed data access agreement upon reasonable request to the authors.
Declaration of interests
SvdW is a member of the Data Safety Monitoring Board of the DISCOVERY trial and the EU-solidact trial, and an associate editor within the Eurosurveillance journal. MW and SP declare a pending patent (US 63/057.471), SvdW declares issued patents (EP1697507, EP1694829, PCT/EP2020055939, US16/809.717 and WO20211176099), and a provisional patent (US63003855). All the other authors declare no competing interests.
Acknowledgements
Work in OS lab is funded by Institut Pasteur, Urgence COVID-19 Fundraising Campaign of Institut Pasteur, Fondation pour la Recherche Médicale (FRM), the European Health Emergency Preparedness and Response Authority (HERA), ANRS-MIE, the Vaccine Research Institute (ANR-10-LABX-77), Labex IBEID (ANR-10-LABX-62-IBEID), ANR/FRM Flash Covid PROTEO-SARS-CoV-2, ANR Coronamito, and IDISCOVR, “TIMTAMDEN” ANR-14-CE14-0029, “CHIKV-Viro- Immuno” ANR-14-CE14-0015-01 and the Gilead HIV cure program. AF lab is funded by the INCEPTION project (PIA/ANR-16-CONV-0005) and the Labex IBEID (ANR-10-LABX-62-IBEID). The COVID-Oise cohort is funded by “Alliance Tous Unis contre le virus” Institut Pasteur, AP-HP and Fondation de France. No pharmaceutical company or other agency was involved in the writing of the manuscript or the decision to submit it for publication. The authors were not precluded from accessing data in the study and all accept responsibility to submit for publication.
We thank the nursing home residents who agreed to participate into the study and the medical teams who were involved in sample and data collection. We thank the teams of Crépy-en-Valois town hall and the Director and technical services of the local hospital for their help in the implementation of the COVID-Oise study. We thank the ICAReB technical team for management and distribution of the samples. We thank William-Henry Bolland for critical reading of the manuscript. We thank members of the Virus and Immunity Unit for discussions and help, Nathalie Aulner and the UtechS Photonic BioImaging (UPBI) core facility (Institut Pasteur), a member of the France BioImaging network, for image acquisition and analysis. The Opera system was co-funded by Institut Pasteur and the Région ile de France (DIM1Health).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101576.
Contributor Information
Timothée Bruel, Email: timothee.bruel@pasteur.fr.
Arnaud Fontanet, Email: arnaud.fontanet@pasteur.fr.
Appendix. Supplementary materials
References
- 1.Carreño JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–688. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- 2.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2021;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Beltran WF, KJSt Denis, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. New Engl J Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. New Engl J Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484.e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 12.Wu M, Wall EC, Carr EJ, et al. Three-dose vaccination elicits neutralising antibodies against omicron. Lancet Lond Engl. 2022;399:715–717. doi: 10.1016/S0140-6736(22)00092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanshylla K, Tober-Lau P, Gruell H, et al. Durability of omicron-neutralising serum activity after mRNA booster immunisation in older adults. Lancet Infect Dis. 2022;22:445–446. doi: 10.1016/S1473-3099(22)00135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haveri A, Solastie A, Ekström N, et al. Neutralizing antibodies to SARS-CoV-2 Omicron variant after 3rd mRNA vaccination in health care workers and elderly subjects and response to a single dose in previously infected adults. Medrxiv. 2021. 10.1101/2021.12.22.21268273 [DOI] [PMC free article] [PubMed]
- 15.Haveri A, Solastie A, Ekström N, et al. Neutralizing antibodies to SARS-CoV-2 Omicron variant after third mRNA vaccination in health care workers and elderly subjects. Eur J Immunol. 2022;52(5):816–824. doi: 10.1002/eji.202149785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alidjinou EK, Demaret J, Corroyer-Simovic B, et al. Immunogenicity of BNT162b2 vaccine booster against SARS-CoV-2 Delta and Omicron variants in nursing home residents: a prospective observational study in older adults aged from 68 to 98 years. Lancet Reg Heal - Europe. 2022;17 doi: 10.1016/j.lanepe.2022.100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefèvre B, Tondeur L, Madec Y, et al. Beta SARS-CoV-2 variant and BNT162b2 vaccine effectiveness in long-term care facilities in France. Lancet Heal Longev. 2021;2:e685–e687. doi: 10.1016/S2666-7568(21)00230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tober-Lau P, Schwarz T, Vanshylla K, et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir Med. 2021;9:e104–e105. doi: 10.1016/S2213-2600(21)00456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anna F, Goyard S, Lalanne AI, et al. High seroprevalence but short-lived immune response to SARS-CoV-2 infection in Paris. Eur J Immunol. 2021;51:180–190. doi: 10.1002/eji.202049058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosado J, Pelleau S, Cockram C, et al. Multiplex assays for the identification of serological signatures of SARS-CoV-2 infection: an antibody-based diagnostic and machine learning study. Lancet Microbe. 2021;2:e60–e69. doi: 10.1016/S2666-5247(20)30197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grzelak L, Velay A, Madec Y, et al. Sex differences in the evolution of neutralizing antibodies to SARS-CoV-2. J Infect Dis. 2021;224:jiab127. doi: 10.1093/infdis/jiab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grzelak L, Temmam S, Planchais C, et al. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci Transl Med. 2020;12(559):eabc3103. doi: 10.1126/scitranslmed.abc3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadjadj J, Planas D, Ouedrani A, et al. Immunogenicity of BNT162b2 vaccine against the Alpha and Delta variants in immunocompromised patients with systemic inflammatory diseases. Ann Rheum Dis. 2022;81:720–728. doi: 10.1136/annrheumdis-2021-221508. annrheumdis-2021-221508. [DOI] [PubMed] [Google Scholar]
- 25.Buchrieser J, Dufloo J, Hubert M, et al. Syncytia formation by SARS-CoV-2 infected cells. Embo J. 2020;38:e106267. doi: 10.15252/embj.2020106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27:917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- 27.France SP. Analyse risque variants. 2022; published online April 20. https://www.santepubliquefrance.fr/content/download/430087/file/analyse_risque_variants_20220420.pdf.
- 28.Wratil PR, Stern M, Priller A, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. 2022;28:496–503. doi: 10.1038/s41591-022-01715-4. [DOI] [PubMed] [Google Scholar]
- 29.Regev-Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against Omicron. New Engl J Med. 2022;386:1377–1380. doi: 10.1056/NEJMc2202542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. New Engl J Med. 2022;386:1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. New Engl J Med. 2022;386 doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crotty S. Hybrid immunity. Science. 2021;372:1392–1393. [Google Scholar]
- 33.Anastassopoulou C, Antoni D, Manoussopoulos Y, et al. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: a mixed effects model across two vaccination periods. PLoS One. 2022;17 doi: 10.1371/journal.pone.0266958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunders MJ, Altfeld M. Implications of sex differences in immunity for SARS-CoV-2 pathogenesis and design of therapeutic interventions. Immunity. 2020;53:487–495. doi: 10.1016/j.immuni.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.