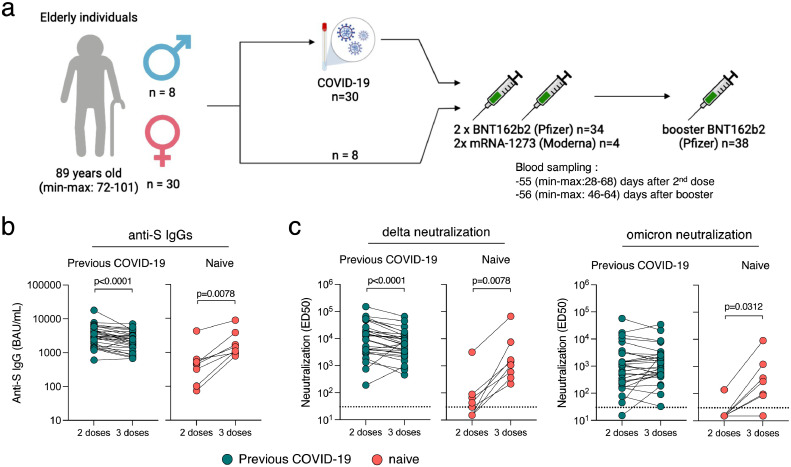
Figure 1.
Immunogenicity of a booster dose of BNT162b2 vaccine in elderly individuals. (a) Thirty-eight elderly individuals from three nursing homes, (30 females and 8 males) were included in the analysis. All received a two-dose regimen of mRNA vaccine (Pfizer BNT162b2; n=34 or Moderna; n=4) and a booster dose (Pfizer BNT162b2; n=38) 8 months apart. Thirty were diagnosed with COVID-19 prior to their booster dose. (b) Anti-Spike IgGs were measured using the S-Flow assay 2 months after the second dose and 2 months after the booster dose. Data are provided as Binding Arbitrary Unit per mL (BAU/mL), the standardised WHO unit. The limit of detection is 3 BAU/mL. Comparisons were performed using the Wilcoxon matched-pairs signed rank test. (c) Neutralisation of delta and omicron were measured using the live-virus S-Fuse assay 2 months after the second dose and 2 months after the booster dose. Data are provided as Effective Dilution 50 (ED50), indicating the dilution of serum capable of inhibiting 50% of viral infection. Green dots indicate individuals with an history of COVID-19 prior to their booster dose of vaccine. Pink dots indicate individuals with no previous COVID-19. The dashed line indicates the limit of detection. Comparisons were performed using the Wilcoxon matched-pairs signed rank test.