Abstract
Introduction:
Postoperative pain remains a significant challenge with the growing number of abdominoplasties every year. Opioids are currently considered the mainstay modality for controlling postoperative pain. However, opioid-related side effects raise the need for a safer and more effective approach. In this study, we aimed to investigate these alternative evidence-based postoperative pain relief modalities following abdominoplasty.
Methods:
This systematic review was designed and conducted using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The literature was systematically searched in December 2021 using the following databases: MEDLINE, Cochrane, and EMBASE. The MeSH terms used to aid the search were the following: abdominoplasty, postoperative pain management, postoperative analgesia, pain control, analgesia, and pain.
Results:
Reviewing the literature resulted in a total of 851 publications. After implementing our criteria, only 13 articles were included in this study, with 990 patients. A continuous infusion pump was the most commonly used method of analgesia (n = 3), followed by a transversus abdominis plane block (n = 2). The postoperative pain assessment scale was mentioned in nine out of the 13. Compared to controls, all interventions resulted in considerably lower pain levels in all the patients. Patient satisfaction was reported in three studies, and all studies reported higher satisfaction rates than the control groups.
Conclusions:
The authors performed a systematic review of the existing database of high-quality research on pain management after cosmetic abdominoplasty to determine the best pain management options currently available. However, future studies are recommended to assess the optimum dosing and administration methods.
Takeaways
Question: What are the alternative nonopioid postoperative pain relief modalities following abdominoplasty?
Findings: Alternative pain relief modalities seem to be promising for abdominoplasty patients with reported shorter hospital stays, decreased opioid consumption, and higher patient satisfaction.
Meaning: Our search yielded promising results with favorable postoperative satisfaction, pain control, and minimal complications.
INTRODUCTION
Abdominoplasty surgery involves removing excess skin and fat from the abdominal region, rectus sheath plication, and umbilicus transposition. The number of cosmetic abdominoplasty procedures has been increasing over the past years. The number of abdominoplasties performed in 2020 in the United States was 163,073, compared with 130,081 abdominoplasties performed in 2018.1,2 Due to excessive tissue manipulation and major incisions performed during the surgery, postoperative pain became a challenge. Patients are reported to experience pain after surgery in up to 80% of cases, but less than half of those patients receive quality pain relief.3 Around 20%–40% of patients described severe pain and reported it as a significant issue which necessitate the use of pain medications‚ especially opioids.4 Effective postoperative pain management methods reduce severe postoperative pain, which significantly affects the patient’s satisfaction. Moreover, it helps shorten hospital length of stay and enhance patient mobilization, subsequently lowering hospital costs.5,6 After significant surgery, the main modality of managing postoperative pain is morphine by patient-controlled analgesia (PCA).7 Morphine has shown numerous significant adverse effects, such as nausea and vomiting, constipation, restriction of regular activities, and delayed postoperative rehabilitation.8 The development of continuous opioid use is another emerging risk. Opioid addiction has been reported in some patients undergoing abdominoplasties.9 The abdominoplasty practice in the United States has shifted to an outpatient setting as the standard of care. Using narcotic pills has led to concerns regarding prescribing practices and the possibility of overprescribing, which are contributing to the opioid epidemic.10 Many authors propose novel solutions to opioid use. There has been a rising trend toward the administration of interfascial plane blocks [eg, transversus abdominis plane (TAP) blocks, pectoralis (PECS I and II) blocks, serratus anterior plane blocks, and erector spine plane blocks].11 A study shows long-term success in relieving pain associated with abdominoplasty by combining intercostal, ilioinguinal, iliohypogastric, and pararectus blocks.12 A reduced postoperative pain score or decreased opioid consumption has been associated with propofol total intravenous anesthesia use in nine of 16 trials. Five clinical trials did not show a significant difference.13 The use of bilateral tranversus abdominis plane block for abdominoplasty was associated with a longer postoperative analgesia duration in the last 24 hours and lower morphine consumption in the first 24 hours than other surgically infiltrated anesthetic techniques.14 Liposomal bupivacaine (Exparel) was introduced as a safe method and an alternative to invasive postoperative pain management options such as PCA, epidurals, peripheral nerve catheters, or intravenous narcotics.15,16 Moreover, the use of 250 mg (0.025%) of bupivacaine with their wetting solution and its persistence in the plasma up to 2 days after surgery has shown benefits. Likewise, the use of a lidocaine dose of up to 37.7 mg/kg in the first 24 hours after surgery is reported to be safe.17 Despite the previously published studies, no consistency exists regarding the most effective nonopioid postoperative pain relief modality. Therefore, we aimed to offer a comprehensive review and investigate the alternative evidence-based postoperative pain relief modalities in abdominoplasty that may be applied in clinical practice.
METHODS AND MATERIALS
Literature Search
The current systematic review was developed using Cochrane review methods, and the systematic review was conducted using Preferred Reporting Items for Systematic Reviews and Meta-Analyses policies.18,19 In December 2021, this study systematically reviewed the published literature to demonstrate alternative evidence-based postoperative pain relief modalities following abdominoplasty. The subsequent databases searched were MEDLINE, Cochrane, and EMBASE. The terms and keywords used to aid the investigation were the following: abdominoplasty, lipoabdominoplasty, panniculectomy, circumferential lipectomy, miniabdominoplasty, truncal dermatolipectomy, postoperative pain management, postoperative analgesia, pain control, analgesia, and pain. The search outcome and studies published were reviewed without a time frame. This review is being utilized in the International Prospective Register of Systematic Reviews (ID: CRD42022309681).18
Study Selection
Our inclusion criteria were studies that included outcomes of pain score, were published without timeframe limitations, published in English language, reported a randomized controlled trial (RCT); prospective or retrospective cohort/comparative, case-control, or case series, including an adult population of 18 years or older, reporting outcomes of interest for clinical questions. Eligible nonopioids for our review were nonsteroidal anti-inflammatory drugs, COX-2 inhibitors, acetaminophen, nefopam, metamizole, corticosteroids, and alpha-2 agonists. Our exclusion criteria were studies that were published in a language other than English, used improper methods, or reported no outcome of interest, as well as meta-analyses or systematic reviews, economic analyses, animal studies, cadaver studies, narrative reviews, editorial papers, and studies in which opioids were used as the primary analgesic. We first screened all abstracts of the included studies for inclusion and exclusion criteria using the Rayyan search engine. The studies were then divided into two groups, each with two independent reviewers. A fifth independent reviewer reviewed all selected articles by both groups to resolve any outstanding disagreements between the articles. Afterward, the two groups reviewed the full texts of the studies to assure compliance with the inclusion and exclusion criteria.
Data Extraction
Data parameters extracted from the final included studies were general information (title, author, publication year, country, and study design), demographics (sample size and mean age/age range), which interventions were used for postoperative pain control, which surgical outcomes were measured to determine the efficacy of pain control, the timing of the intervention used (preoperative/intraoperative/postoperative), type of surgery that was performed for the patient, analgesic dose and administration method, complications of treatment in treatment versus placebo groups, patient satisfaction, and a summary of the main findings.
Bias Assessment
RCT studies were assessed for bias using the Cochrane risk-of-bias tool for randomized trials (RoB 2).19,20 Randomization, allocation concealment, participant and employee blinding, observer blinding, incomplete data, and selective reporting were all evaluated, and each study category was given a “low risk,” “high risk,” or “unclear risk” rating. For the nonrandomized studies, the methodological index for the nonrandomized studies (MINORS) assessment tool was used. It is a validated 12-item tool designed to check the quality of nonrandomized surgical studies.21 One reviewer did the bias risk assessment in each of the included articles using the MINORS tool, and the other reviewer matched the assessments.
Statistical Analysis
Due to the general heterogeneity of the reported data, no meta-analysis was accomplished.
RESULTS
Literature Findings
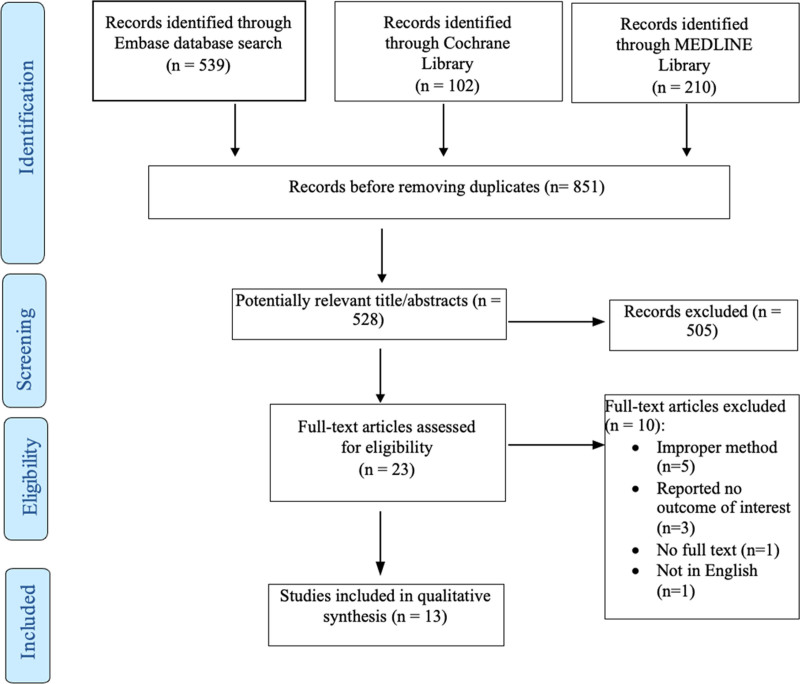
This systematic review investigation found a total of 851 published articles, including 539 articles from EMBASE, 210 from MEDLINE, and 102 articles from the Cochrane library. After removing duplicates, 528 articles remained for review. We initially retrieved 23 full-text publications. However, after implementing the previously set exclusion criteria, only 13 articles published between 2005 and 2018 were included (Fig. 1). For the following reasons, 10 articles were excluded: improper methods (systematic review, review article, letter to editor, and case report), n = 5, reported no outcome of interest (n = 3), the full text was not found (n = 1), and the full text was not in English (n = 1). Table 1 lists the features of each article included. Out of all the included studies, two were prospective cohort studies,22,23 six were RCTs,22–27 and five were retrospective studies.30–34 Table 1 outlines the characteristics of all the included articles. The majority of the studies were done in the United States.22–24,27,28,30,31,33,34 Two studies were conducted in the UK,29,32 one study in Greece,25 and one study in South Africa.26 All the included articles were conducted for cosmetic abdominoplasty surgery, except one paper that included breast augmentation and abdominoplasty.27 Thus, only patients who had undergone cosmetic abdominoplasty were included in the study.
Fig. 1.
PRISMA flow chart for the systematic review.
Table 1.
Basic Features of the Included Articles
| Article Number | Study | Country | Methodology | Risk of Bias | Sample Size | Age Mean/Range | Gender |
|---|---|---|---|---|---|---|---|
| 1 | Patel et al, 200822 | USA | Prospective cohort | NA | Intervention = 29, control = 25 | Intervention = 48, control = 45 | Intervention group (f = 27) (m = 2) 2/27, control group = (f = 23) (m = 2) 2/23 |
| 2 | Edwards et al, 201523 | USA | Prospective cohort | NA | 49 | Abdominoplasty ± breast surgery (43 ± 13)/breast surgery (33 ± 10) | F (n = 48)/M (n = 1) |
| 3 | Mentz et al, 200524 | USA | RCT | High | 20 | W Pump = 38.2Y, WO = 34.5Y | F (n = 20) 100% |
| 4 | Kakagia et al, 200725 | Greece | RCT | High | 46 | Group A (NS) 36.80 ± 4.33, Group B (R) 37.20 ± 6.84, Group C (L) 40.27 ± 7.83 | NA |
| 5 | Widgerow et al, 200826 | South Africa | RCT | Some concerns | 31 | NA | NA |
| 6 | Sun et al, 200827 | USA | RCT | Some concerns | 112 | Control = 42, postoperative = 42, perioperative = 43 | Control = 2/34, postoperative = 0/37, perioperative = 4/35 |
| 7 | Singla et al, 201828 | USA | RCT | Some concerns | 219 | 38.9 | F (n = 215)/M (n = 4) |
| 8 | Sforza et al, 201129 | UK and Serbia | RCT | Low | 28 | NA | NM |
| 9 | Michaels et al, 200930 | USA | Retrospective | NA | Group 1 = 39, Group 2 = 29 | Group 1 = 44 years old, Group 2 = 43 | NM |
| 10 | Chavez-Abraham et al, 201131 | USA | Retrospective | NA | 215 | 48.5 | F (n = 414)/M (n = 1) |
| 11 | Gravante et al, 201132 | UK | Retrospective | NA | 51 | 42 | F (n = 51) |
| 12 | Morales et al, 201333 | USA | Retrospective | NA | 64 | 42 | F (n = 64) |
| 13 | Fiala, 201534 | USA | Retrospective | NA | 32 | 41.4 | F (n = 30)/M (n = 2) |
Study Characteristics
A total of 990 patients were included. The mean age of the patients was 43 years old. Out of all the included patients, women were the majority (n = 892, 90.10%); however, in four articles, the gender was not mentioned.25,26,29,30 The authors divided analgesia delivery methods into three categories: preoperative, intraoperative, and postoperative interventions. In the preoperative intervention section, Sun et al27 compared three groups: the first group received placebo capsules before surgery, followed by two placebo capsules after surgery, and the second group received two placebo capsules preoperatively and two celecoxib 200 mg capsules postoperatively. The last group received two celecoxib 200 mg capsules preoperatively and two placebo capsules 1 hour postoperatively. On the other hand, eight studies investigated different methods of intraoperative analgesia. Bupivacaine-containing anesthetic solutions were utilized in seven studies as an intraoperative intervention.23,25,29,30,32–34 Furthermore, Chavez-Abraham et al31 used plain lidocaine intraoperatively in the rectus plication space. Four studies were found for postoperative analgesia. Postoperatively, continuous infusion pumps were used in two articles using bupivacaine.22,24 One study by Widgerow et al26 utilized ropivacaine-containing aesthetic solution lavage drain washout. Furthermore, Singla et al28 compared postoperative intravenous (IV) meloxicam to placebo. Adrenaline was added in two studies.29,30 One study added steroids.34 Table 2 summarizes the articles that report the postoperative pain relief modalities in abdominoplasty.
Table 2.
Included Articles Reporting the Alternative Evidence-based Postoperative Pain Relief Modalities
| Article Number | Study | Interventions | Outcomes Measured | Type of Surgery | Treatment (Dose) | Complications | Patient Satisfaction | Pain Scale Used | Summary of Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Patel et al, 200822 | Postoperative continuous bupivicaine infusion pumps | Nausea, LOH stay, pain pills consumption, and pain scores | Abdominoplasty | Bupivicaine 360 mg over 24 h | NA | NA | VAS | The pain scores and number of pills consumed decreased |
| Normal activity by day 3 | |||||||||
| 2 | Edwards et al, 201523 | Intraoperative surgical site liposomal bupivacaine injection | Pain (11-point numerical rating scale), opioid consumption, and overall benefit of analgesic score | Abdominoplasty/breast surgery patients | Liposome bupivacaine 266 mg | NA | High satisfaction, with a low burden of opioid-related side effects | 11-point numerical rating scale | Low pain intensity scores and reduced opioid consumption |
| 3 | Mentz et al, 200524 | Postoperative ambulatory pain pump PCA continuous delivery of 0.5% bupivacaine | Pain, narcotics consumption, discomfort, and mobility | Abdominoplasty | Continuous infusion rates of 0.5–10 ml/h of 0.5% bupivacaine. Bolus programming allows for 1-, 2-, 3-, 4-, or 5-ml delivery | NA | High satisfaction | NA | The experimental group resumed normal activities sooner, voluntarily consumed far fewer postoperative narcotics, and rated their recovery as better |
| 4 | Kakagia et al, 200725 | Intraoperative infiltration of the peri-incisional and dissected area with local ropivacaine | Pain, duration and quality of analgesia, and occurrence of complications | Fleur-de-lis miniabdominoplasty by the technique | 50 ml of 0.75% ropivacaine in 50 ml of 0.9% saline in group b (n = 15), and 60 ml of 0.25% levobupivacaine plus 40 ml of 0.9% saline in group c (n = 16) | NA | NA | VAS | The pain scores in the placebo group were significantly higher than for the patients infiltrated with ropivacaine. |
| 5 | Widgerow et al, 200826 | Postoperative lavage drain washout with ropivacaine | Pain interfering with mobilization, mobilization timing, and difficulty of the lavage process | Abdominoplasty | Ropivacaine 100 ml, 2 mg/ml | Small leakage of fluid around the drain site (n = 6/31) | NA | Pain scale assessing pain that interferes with mobilization | Less pain, earlier mobilization, and shorter hospital stays |
| 6 | Sun et al, 200827 | The control group (n = 40) received two placebo capsules orally 30–90 min before surgery, followed by two placebo capsules 1 h postoperatively in the recovery room; the postoperative group (n = 40) received two placebo capsules orally 30–90 min before surgery and two celecoxib 200 mg capsules 1 h after surgery; and the perioperative group (n = 40) received two celecoxib 200 mg capsules 30–90 min before surgery and two placebo capsules 1 h after surgery | PACU stay (min), resume normal diet, return of normal bowel function (d), resume normal physical activity (d), patient satisfaction, opioid consumption, and pain | Major plastic surgery procedure (breast augmentation and abdominoplasty) | Celecoxib, 400 mg PO | DVT (n = 1, postoperative group), wound complications (n = 13, control group) (n = 9, postoperative group, (n = 4, perioperative group) | Satisfaction was higher in postoperative and perioperative groups | 11-point verbal rating scale | Postoperative administration of celecoxib (200 mg po BID) for 4 days in patients undergoing major plastic surgery procedures decreased postoperative pain and the need for analgesic rescue medication, contributing to improved patient satisfaction with their quality of recovery |
| 7 | Singla et al, 201828 | Postoperative IV meloxicam versus placebo | Pain intensity difference over 24 h postdose/number of doses of opioid rescue analgesia | Abdominoplasty | Meloxicam IV 30 mg | Bleeding (n = 2, one in each group), PE and wound infection in placebo group | NA | Summed pain intensity difference over 24 h postdose (SPID24) | Meloxicam IV provided sustained pain relief and generally was well tolerated in subjects with moderate-to-severe pain following abdominoplasty |
| 8 | Sforza et al, 201129 | Intraoperative TAP block | Presence of pain and pain severity | Abdominoplasty | 20 ml of solution consisting of 10 ml of bupivacaine 0.5% plus 10 ml of lidocaine 1% plus 0.2 ml of adrenaline 1:1000 | NA | NA | Categorical pain scoring system | Reduced postoperative pain scores, both at rest and on movement, and significantly diminished postoperative opioid requirements. Earlier mobilization |
| 9 | Michaels et al, 200930 | Ribs block and IV sedation versus general anesthesia | Time in the operating and recovery rooms, use of narcotics and antiemetics, postoperative nausea and vomiting, and pain | Abdominoplasty | A 2-ml injection of a mixture of 1 ml each of 0.25% bupivacaine and 1% lidocaine with 1:100,000 of epinephrine was made per block | NA | NA | Patient average reported pain scale | Significant decreases in recovery room time, postoperative narcotics, postoperative nausea and vomiting, and pain were achieved using rib blocks |
| 10 | Chavez-Abraham et al, 201131 | Intraoperative lidocaine ECIP into the rectus plication space | Pain and oral narcotic use | Abdominoplasty | 200 ml of 1% plain lidocaine set to deliver 2 ml/h with the option of a 4-ml bolus per hour | NA | NA | Subjective pain scale | Statistically significant decrease in perceived pain and the use of the oral narcotic |
| 11 | Gravante et al, 201132 | Intraoperative TAP block | Use of postoperative analgesia | Abdominoplasty | Bupivacaine (mean = 160 mg) | NA | NA | NA | Decreased pain duration and intensity among all patients |
| 12 | Morales et al, 201333 | Intraoperative liposomal bupivicaine intramuscular injections in an abdominal field block fashion | Pain pill requirement/days until resuming regular day activity | Abdominoplasty | Liposomal bupivicaine injection suspension (Exparel) 20-ml single-use vial, 1.3% (13.3 mg/ml). The total dose of bupivicaine administered in a 20-ml vial is 266 mg | NA | NA | Subjective pain scale of 1–10 | Reduced postoperative pain, less postoperative narcotic used, resumed both earlier and normal activity |
| 13 | Fiala, 201534 | TAP block versus pararectus injections and ilioinguinal/iliohopogastric nerve blocks | Postoperative hydromorphone use, mean time to first request for as-needed pain medication | Abdominoplasty patients | 20 ml of bupivacaine 0.25% mixed with 4 mg of dexamethasone | NA | NA | NA | TAP block provided more effective analgesia than a standard nerve block |
DVT, deep venous thrombosis; ECIP, elastomeric continuous infusion pump; LOH, length of hospital stay; NA, not applicable; PACU, postanesthesia care unit.
Quality Assessment and Risk of Bias
All articles included had a MINORS score of at least 70%. Seven comparative studies had a mean score of 22.1 (range, 19–23). Table 3 summarizes the MINORS instrument assessment. Of the five included RCTs, two had a high overall risk of bias, and three had some concerns.
Table 3.
MINORS Instrument Assessment for Nonrandomized Comparative Studies (n = 7)
| Item | Patel et al22 | Edwards et al23 | Chavez-Abraham et al31 | Michaels et al30 | Gravante et al32 | Morales et al33 | Fiala34 |
|---|---|---|---|---|---|---|---|
| A clearly stated aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patients | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Prospective collection of data | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Endpoints appropriate to the aim of the study | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Unbiased evaluation of endpoints | 2 | 0 | 1 | 2 | 1 | 2 | 2 |
| Follow-up period appropriate to the aim of the study | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Loss of follow-up less than 5% | 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| Prospective calculation of the sample size | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| An adequate control group | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Contemporary groups | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Baseline equivalence of groups | 1 | 2 | 2 | 1 | 2 | 1 | 2 |
| Adequate statistical analyses | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total score | 22 | 19 | 23 | 23 | 23 | 22 | 23 |
The items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The global ideal score is 16 for noncomparative studies and 24 for comparative studies.
Postoperative Analgesia Need
In all the articles included in this systematic review, postoperative opioids were represented as morphine or equivalent units ingested. In the study conducted by Edwards et al23 which compared between a combination of rib block and IV sedation versus general anesthesia, the authors found that on day 1 after surgery, the median daily dose peaked at approximately three to four tablets/day, decreasing to two to three tablets/day on postoperative days 2 and 3. Sforza et al29 calculated the morphine required in the first 12 hours. In two investigations, TAP blocks were shown to reduce morphine intake.29,32 Intercostal blocks combined with pararectus plus ilioinguinal/iliohypogastric blocks were more effective than the control group.34 The TAP block study times showed longer times for the first analgesia requested compared with rib blocks,29,32,34 lavage drain washout,26 and intradermal and surgical site injections.28
The Postoperative Pain Scale
The postoperative pain assessment scale was mentioned in nine out of the 13 included articles.22,23,25–28,31,33 The visual analog scale (VAS) was used in two included studies.22,25 The 11-point verbal rating scale was utilized in two articles.23,27 One study used the summed pain intensity difference over a 24-hour postdose scale (SPID24).28 Finally, the terms “none,” “mild,” “moderate,” and “severe” were used by Sforza et al.29 Morales et al33 used a subjective pain scale of 1–10. Compared with controls, all interventions resulted in considerably lower pain levels in all the patients.
Complications Related to the Intervention
Three studies reported postoperative complications.26–28 Widgerow et al26 have faced small fluid leakage around the drain site postlavage drain washout among six out of the 31 patients. Sun et al27 found that wound complications (hematoma, seroma, infection, and wound dehiscence) were significant among the control group who received placebo capsules preoperatively, followed by placebo capsules postoperatively. Finally, Singla et al28 found that bleeding-related complications (n = 2/291), one in each group, pulmonary embolism, and wound infection were seen among the placebo group.
Patient Satisfaction Postoperatively
Only three studies reported patient satisfaction. Edwards et al23 reported significantly higher satisfaction with a low burden of opioid-related side effects with intraoperative surgical site liposomal bupivacaine injection. In addition, Mentz et al24 also mentioned that patients had high satisfaction with PCA. In contrast, Sun et al27 reported a higher satisfaction rate in patients who received celecoxib capsules preoperatively or postoperatively compared to the placebo group.
Quantitative Data Analysis
Due to the disparity in the methodologies, types of intervention, and outcomes measured, no quantitative meta-analysis could be performed.
DISCUSSION
The number of abdominoplasties performed each year has increased over the last decade.1 This is mainly for cosmetic reasons and due to the advances in abdominoplasty techniques providing safer outcomes and better recovery and results. Postoperative pain following abdominoplasty is a significant challenge. Morphine has been the mainstay of managing postoperative pain, with a well-documented and significant side-effect profile.3 We comprehensively reviewed and investigated the alternative evidence-based postoperative pain relief modalities in abdominoplasty that may be applied in clinical practice.
Abdominoplasty is one of the most commonly performed elective procedures that requires thorough preoperative planning for satisfactory outcomes. Pain following abdominoplasty remains a significant challenge that comes with several consequences. In the past few years, pain management after abdominoplasty has become a significant interest. Optimizing postoperative analgesia can result in early patient mobilization, reduce the length of hospital stay and its financial sequelae, and make patients feel better, thereby increasing their satisfaction.5,8 For decades, opioids, particularly morphine, have been the mainstay of analgesia in managing postoperative pain following major surgery. Narcotic use during hospital stays serves as a “gateway” to the opioid crisis, with an increased risk of addiction after discharge.35–38 For the past 23 years, approximately 841,000 people have died from drug overdose‚ including prescribed opioids.39 Nearly three-quarters of drug overdose deaths in 2019 were due to opioids.40 In the last decade, there has been a significant increase in the prescription of opioids, while the amount of pain reported by patients has remained constant. Studies show that surgeons tend to prescribe a higher number of opioids than their patients consume, with most patients claiming to finish only about half of the prescription.41 Chu et al42 conducted a retrospective review of 479 plastic surgery patients who underwent various procedures and found that opioids are frequently administered excessively by plastic surgeons, and patients fail to store or dispose of opioids properly, suggesting the need for improved patient education.
In addition, patients will be at risk of opioids’ undesirable effects, such as sedation, vomiting, and constipation, which can prolong hospital stay and increase complications and costs.39 Therefore, any modality that reduces postoperative pain and opioid use will certainly improve recovery after abdominoplasty. A study conducted by Swanson43 has shown that efficient pain management peripherally would reduce the use of central analgesics and eventually reduce the patient’s recovery time.
The assessment of postoperative analgesia needed revealed lower morphine consumption. The TAP blocks reduced morphine intake.29,32 Intercostal blocks combined with pararectus plus ilioinguinal/iliohypogastric blocks were also more effective than the control group.34 The TAP block study times showed longer times for the first analgesia requested compared with rib blocks,29,32,34 lavage drain washout,26 and intradermal and surgical site injections.28 Therefore, nonopioid analgesics significantly decreased the need for morphine analgesics, and all interventions significantly decreased pain levels in all patients compared with controls. Additionally, all three studies reported that patients’ satisfaction with the nonopioid analgesics group was higher than controls. Thus, nonopioid analgesics could be a promising alternative to excessive morphine analgesics with significant pain relief and high patient satisfaction rates with minimal complications.
Although TAP block significantly reduced postoperative pain and opioid consumption, its evidence in reducing postoperative nausea and vomiting is weak.44 There is not enough data in the literature to reliably recommend TAP blocks as a safe and effective intervention.44 TAP blocks can be expensive.34 Moreover, a few complications of TAP blocks have been previously reported in the literature. In the work by Young et al,45 two patients had liver injury subsequent to accessing TAP space through a lateral approach, even with the use of ultrasound as guidance. There is no optimal dose for bupivacaine in the TAP block.34 Despite the current promising results, much research is needed to inform the most optimal dose for bupivacaine in TAP blocks,34 which poses a limitation. Rectus plication analgesia with TAP block is challenging as current evidence shows that TAP block is ineffective in achieving reliable pain relief in rectus plication, and a different blocking approach may be required.34 As abdominoplasty patients are usually obese, obesity can significantly impact sedation outcomes. Increased body mass index (BMI) could increase the risk of sedation, possibly because sleep apnea, pulmonary hypertension, and restrictive lung disease are more prevalent in the obese population.46 According to Alvarez et al,47 obstructive sleep apnea is commonly found in morbid obesity, which puts morbidly obese patients at greater risk for opioid-induced airway obstruction. Hence, there might be concerns regarding the safety of opioids in obesity. In fact, obese patients may require fewer opioids than nonobese patients for the same degree of pain control.48 In this review, five articles25,28,30,32,33 mentioned the BMIs of abdominoplasty patients. In the work by Kakagia et al,25 the average BMI in the placebo group was 24.98 kg/m2, and the average BMIs in the ropivacaine and levobupivacaine groups were 25.57 and 26.47, respectively. The intervention groups showed favorable outcomes in postoperative pain control, defined as a decreased need for opioids. However, the study did not examine the significance of BMI as a cofactor for determining the required dose of analgesia. Another study30 showed similar averages of BMI ranging from 24 to 25. In the work by Morales et al,33 the average BMI was 27 kg/m2, but no correlations were tested. Similarly, in the work by Singla et al,28 the average BMI was 26.9 in the placebo group and 26.5 in the intervention group, but the correlation with required analgesia was not examined. Interestingly, the Gravante et al32 study found that patients with increased BMI before surgery who underwent greater resection of excess abdominal wall tissue required more morphine. A BMI of 29 was the cutoff for patients needing analgesia within the first day postoperatively. Nevertheless, it was unclear whether this increase in the need for analgesia was mainly due to the high BMI or higher resection, leading to increased pain.
This research provides valuable information about alternatives in managing postoperative pain with nonopioid analgesics. However, there are several limitations. The study’s heterogeneity in the methodologies, type of intervention, and outcomes measured may potentially impact the final results. Also, there is a potential risk of publication bias. Further variable correlations could not be performed due to the variability in reporting postoperative outcomes. The pain assessment scale was mentioned in nine out of the 13 included articles, and only three studies reported patient satisfaction. Furthermore, the study participants’ gender was not mentioned in four articles. In addition, the lack of description of different intervention complications in the majority of the studies affected the reporting of possible complications. There were two articles included in this review that reported conflicts of interest. In the work by Edwards et al,23 the study was financially supported by Pacira Pharmaceuticals, Inc., a manufacturer of liposomal bupivacaine used as the intervention in the study. Similarly, in the work by Singla et al,28 the study was financially supported by Recro Pharma, Inc., which is a manufacturer of IV meloxicam, the drug under investigation in the study. Our protocol for managing postabdominoplasty pain consists of the following. For intraoperative pain management, the senior author (O.F.N.) uses 0.5 ml of bupivacaine and epinephrine in 20 ml of normal saline to infiltrate the surgical site and plicated rectus sheath. Additionally, depending on the patient’s position intraoperatively, TAP block could be administered if the patient was supine. On the other hand, if prone followed by supine, the erector spinae would be administered. Postoperatively, patients will receive opioid analgesia, either by PCA or intermittent, and will be discharged home with tramadol orally.
Much research is needed to further evaluate the effectiveness of nonopioid analgesia following abdominoplasty and its capability to replace morphine use postoperatively, therefore leading to many desirable outcomes that are friendly to both the patient and the healthcare system without the cost of opioid adverse events. The ideal study design would be an RCT, double-blind or triple-blind, and, if feasible, to purely examine the effects of nonopioid analgesia without exposing the intervention group’s patients to opioids. The study should have a large sample size. Multiple studies should be conducted on different populations to account for patient characteristics and surgeon technique variability. Measures of pain following surgery should be consistent, standardized, and closely monitored to minimize bias. This will allow for better, more accurate conclusions when comparing the intervention to the gold standard of care and other interventions. Standardized trials with clear, consistent, and objective outcome measures can allow for meta-analysis and the production of a guide for postoperative pain management in abdominoplasty. We recommend that future research projects focus mainly on prospective studies and RCTs to decrease the disparity in the methodologies and increase the article’s validity by adding meta-analysis. The literature requires further studies to accurately compare the advantages and disadvantages of each form of nonopioid analgesia.
CONCLUSIONS
The main aim of our review was to assess the alternatives to analgesics other than opioids in the postoperative period after cosmetic abdominal contouring surgery. Our search yielded that each of the reported methods was effective, with favorable postoperative satisfaction, pain control, and minimal complications. It is crucial to handle any alternative pain management methods with caution, and each patient should undergo an individual evaluation before beginning therapy. These results can help plastic surgeons select an appropriate analgesic while steering away from unnecessary complications of opioid analgesics. We recommend that future studies be conducted in a fashion that allows precise comparison between the advantages and disadvantages of each form of analgesia.
Footnotes
Published online 22 July 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia.
REFERENCES
- 1.American Society for Aesthetic Plastic Surgery. Cosmetic surgery national data bank statistics. 2018. Available at https://www.plasticsurgery.org/documents/News/Statistics/2018/top. Accessed August 10, 2021. [DOI] [PubMed]
- 2.The Aesthetic Society. American Society for Aesthetic Plastic Surgery. Cosmetic surgery national data bank statistics. 2020. Available at https://cdn.theaestheticsociety.org/media/statistics/aestheticplasticsurgerynationaldatabank-2020stats.pdf. Retrieved August 10, 2021.
- 3.Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol. 2016;33:160–171. [DOI] [PubMed] [Google Scholar]
- 4.Gerbershagen HJ, Aduckathil S, van Wijck AJ, et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934–944. [DOI] [PubMed] [Google Scholar]
- 5.Dzau VJ, Pizzo PA. Relieving pain in America: insights from an Institute of Medicine Committee. JAMA. 2014;312:1507–1508. [DOI] [PubMed] [Google Scholar]
- 6.Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66:2321–2337. [DOI] [PubMed] [Google Scholar]
- 8.Meissner W, Coluzzi F, Fletcher D, et al. Improving the management of post-operative acute pain: priorities for change [published correction appears in Curr Med Res Opin. 2016 May;32(5):979]. Curr Med Res Opin. 2015;31:2131–2143.. [DOI] [PubMed] [Google Scholar]
- 9.Bennett KG, Kelley BP, Vick AD, et al. Persistent opioid use and high-risk prescribing in body contouring patients. Plast Reconstr Surg. 2019;143:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegelman JI, Levine RH. Abdominoplasty: a comparison of outpatient and inpatient procedures shows that it is a safe and effective procedure for outpatients in an office-based surgery clinic. Plast Reconstr Surg. 2006;118:517–522. [DOI] [PubMed] [Google Scholar]
- 11.Machi A, Joshi GP. Interfascial plane blocks. Best Pract Res Clin Anaesthesiol. 2019;33:303–315. [DOI] [PubMed] [Google Scholar]
- 12.Feng LJ. Painless abdominoplasty: the efficacy of combined intercostal and pararectus blocks in reducing postoperative pain and recovery time. Plast Reconstr Surg. 2010;126:1723–1732. [DOI] [PubMed] [Google Scholar]
- 13.Wong SSC, Chan WS, Irwin MG, et al. Total intravenous anesthesia (TIVA) with propofol for acute postoperative pain: a scoping review of randomized controlled trials. Asian J Anesthesiol. 2020;58:79–93. [DOI] [PubMed] [Google Scholar]
- 14.Abo-Zeid MA, Al-Refaey AK, Zeina AM. Surgically-assisted abdominal wall blocks for analgesia after abdominoplasty: a prospective randomized trial. Saudi J Anaesth. 2018;12:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyas KS, Rajendran S, Morrison SD, et al. Systematic review of liposomal bupivacaine (Exparel) for postoperative analgesia. Plast Reconstr Surg. 2016;138:748e–756e. [DOI] [PubMed] [Google Scholar]
- 16.Martinez V, Beloeil H, Marret E, et al. Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Br J Anaesth. 2017;118:22–31. [DOI] [PubMed] [Google Scholar]
- 17.Swanson E. Prospective study of lidocaine, bupivacaine, and epinephrine levels and blood loss in patients undergoing liposuction and abdominoplasty. Plast Reconstr Surg. 2012;130:702–722. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Available at https://training.cochrane.org/handbook. 2011. Accessed June 1, 2019. [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- 22.Patel PI, Patel MJ, O’Toole M, et al. Safe, cost-effective pain control using a continuous local anesthetic infusion pump after an abdominoplasty. Plast Reconstr Surg. 2008;121:355–356. [DOI] [PubMed] [Google Scholar]
- 23.Edwards MC, Sorokin E, Brzezienski M, et al. Impact of liposome bupivacaine on the adequacy of pain management and patient experiences following aesthetic surgery: results from an observational study. Plast Surg (Oakv). 2015;23:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mentz HA, Ruiz-Razura A, Newall G, et al. Use of a regional infusion pump to control postoperative pain after an abdominoplasty. Aesth Plast Surg. 2005;29:415–422. [DOI] [PubMed] [Google Scholar]
- 25.Kakagia DD, Fotiadis S, Tripsiannis G, et al. Postoperative analgesic effect of locally infiltrated levobupivacaine in fleur-de-Lys abdominoplasty. Aesthetic Plast Surg. 2007;31:128–132. [DOI] [PubMed] [Google Scholar]
- 26.Widgerow AD, Wittstock C, Candy GP. Lavage drain extension for local anaesthetic instillation into abdominal wounds. S Afr J Surg. 2008;46:14–16. [PubMed] [Google Scholar]
- 27.Sun T, Sacan O, White PF, et al. Perioperative versus postoperative celecoxib on patient outcomes after major plastic surgery procedures. Anesth Analg. 2008;106:950–958. [DOI] [PubMed] [Google Scholar]
- 28.Singla N, Bindewald M, Singla S, et al. Efficacy and safety of intravenous meloxicam in subjects with moderate-to-severe pain following abdominoplasty. Plast Reconstr Surg Glob Open. 2018;6:e1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sforza M, Andjelkov K, Zaccheddu R, et al. Transversus abdominis plane block anesthesia in abdominoplasties. Plast Reconstr Surg. 2011;128:529–535. [DOI] [PubMed] [Google Scholar]
- 30.Michaels BM, Eko FN. Outpatient abdominoplasty facilitated by rib blocks. Plast Reconstr Surg. 2009;124:635–642. [DOI] [PubMed] [Google Scholar]
- 31.Chavez-Abraham V, Barr JS, Zwiebel PC. The efficacy of a lidocaine-infused pain pump for postoperative analgesia following elective augmentation mammaplasty or abdominoplasty. Aesthetic Plast Surg. 2011;35:463–469. [DOI] [PubMed] [Google Scholar]
- 32.Gravante G, Castrì F, Araco F, et al. A comparative study of the transversus abdominis plane (TAP) block efficacy on post-bariatric vs aesthetic abdominoplasty with flank liposuction. Obes Surg. 2011;21:278–282. [DOI] [PubMed] [Google Scholar]
- 33.Morales R, Jr, Mentz H, III, Newall G, et al. Use of abdominal field block injections with liposomal bupivicaine to control postoperative pain after abdominoplasty. Aesthet Surg J. 2013;33:1148–1153. [DOI] [PubMed] [Google Scholar]
- 34.Fiala T. Tranversus abdominis plane block during abdominoplasty to improve postoperative patient comfort. Aesthet Surg J. 2015;35:72–80. [DOI] [PubMed] [Google Scholar]
- 35.Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176:1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172:425–430. [DOI] [PubMed] [Google Scholar]
- 37.Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shafi S, Collinsworth AW, Copeland LA, et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg. 2018;153:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CDC. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, Ga.: CDC, National Center for Health Statistics. 2020. Available at http://wonder.cdc.gov. Retrieved August 10, 2021. [Google Scholar]
- 40.Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torabi R, Bourn L, Mundinger GS, et al. American Society of plastic surgeons member post-operative opioid prescribing patterns. Plast Reconstr Surg Glob Open. 2019;7:e2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu JJ, Janis JE, Skoracki R, et al. Opioid overprescribing and procedure-specific opioid consumption patterns for plastic and reconstructive surgery patients. Plast Reconstr Surg. 2021;147:669e–679e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanson E. A physiologic pain pump for abdominoplasty: an alternative to regional blocks and liposomal bupivacaine. Plast Reconstr Surg. 2015;136:714e–716e.. [DOI] [PubMed] [Google Scholar]
- 44.Petersen PL, Mathiesen O, Torup H, et al. The transversus abdominis plane block: a valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol Scand. 2010;54:529–535. [DOI] [PubMed] [Google Scholar]
- 45.Young MJ, Gorlin AW, Modest VE, et al. Clinical implications of the transversus abdominis plane block in adults. Anesthesiol Res Pract. 2012;2012:731645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jirapinyo P, Thompson CC. Sedation challenges: obesity and sleep apnea. Gastrointest Endosc Clin N Am. 2016;26:527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvarez A, Singh PM, Sinha AC. Postoperative analgesia in morbid obesity. Obes Surg. 2014;24:652–659. [DOI] [PubMed] [Google Scholar]
- 48.Rand CS, Kuldau JM, Yost RL. Obesity and post-operative pain. J Psychosom Res. 1985;29:43–48. [DOI] [PubMed] [Google Scholar]