Introduction
Neuroendocrine prostate cancer (NEPC) is an aggressive variant characterized by poor prognosis, increased frequency of visceral metastasis, and diminished response to hormone therapy.1-3 The term NEPC generally encompasses a spectrum of histology and molecular characteristics ranging from pure histologic small-cell carcinoma to mixed tumors with prostate adenocarcinoma and carcinoma with neuroendocrine features.4-6 Transformation of adenocarcinoma to neuroendocrine or small-cell typically emerges later in the disease course after hormonal treatment as a mechanism of resistance.7,8 De novo neuroendocrine or small-cell prostate cancer is rare, occurring in < 1%-2% of cases at diagnosis3; however, treatment-emergent neuroendocrine differentiation is present in up to 17%-25% of patients with castration-resistant prostate cancer (CRPC).9-11 DNA damage repair gene mutations including BRCA2 are common in metastatic prostate cancer, and identifying them is crucial since they predict sensitivity to therapeutics such as poly-(ADP ribose) polymerase (PARP) inhibitors and platinum chemotherapy.12-16 However, there have been conflicting reports regarding the frequency of BRCA2 mutations in NEPC.10,17 Here, we report the case of a patient with metastatic prostate cancer initially diagnosed with adenocarcinoma which later transdifferentiated into morphologic small-cell carcinoma. He was found to have biallelic loss of BRCA2 and was successfully treated with platinum/etoposide, followed by maintenance olaparib with prolonged response. We additionally report data from our institution which suggest that BRCA2 alterations and NEPC are not mutually exclusive and may co-occur more frequently than previously recognized.
Case
The patient was diagnosed with localized prostate cancer at age 64 years with a prostate-specific antigen (PSA) of 3.9 ng/mL and biopsy which showed Gleason 4 + 3 = 7 adenocarcinoma. His history is also notable for male breast cancer diagnosed at age 47 years, basal cell carcinoma at age 63 years, and a spinal cord tumor (presumed low-grade astrocytoma). He is of Ashkenazi descent, and family history was notable for a sister with uterine cancer, brother with lung cancer (never smoker), paternal uncle with prostate cancer, paternal uncle with colon cancer, and a paternal cousin with breast cancer. Multiple family members also had basal cell carcinomas. He had germline genetic testing performed using multiple platforms including Color Genomics and the University of Washington BROCA test18 which did not reveal an explanatory germline mutation.
The patient underwent definitive treatment with brachytherapy at the time of prostate cancer diagnosis. Four years later he was found to have local recurrence and initiated intermittent androgen deprivation therapy. After 2 years, he developed nonmetastatic, castration-resistant disease which was treated initially with ketoconazole and prednisone. He then developed biochemical and local progression and transitioned to enzalutamide. After 26 months on enzalutamide, his PSA rose to 2.17 ng/mL, and imaging revealed seminal vesicle enlargement and nodal metastasis. Given the low PSA, biopsy was performed of the nodal metastasis, and pathology demonstrated morphologic small-cell carcinoma of prostatic origin consistent with transdifferentiation on the basis of morphology and uniformly positive synaptophysin staining and focal positive NK3.1 staining by immunohistochemistry. Next-generation sequencing using UW-OncoPlex19 was also performed, which showed BRCA2 homozygous copy loss. Subsequent fluorodeoxyglucose positron emission tomography imaging showed liver and lung metastases. He was treated with six cycles of carboplatin and etoposide with complete biochemical and radiographic response. He then initiated olaparib maintenance therapy, which he tolerated well and had ongoing complete response (CR).
Eighteen months after initiation of olaparib, he developed worsening cytopenias. Bone marrow biopsy showed > 20% abnormal myeloid blasts/promyelocytes with fluorescent in situ hybridization positive for translocation 15;17 consistent with secondary acute promyelocytic leukemia (APML). He received induction therapy with all-trans retinoic acid and arsenic and achieved a complete remission. He started consolidation chemotherapy, but arsenic was discontinued during the first cycle because of neuropathy. He then completed three cycles of all-trans retinoic acid/idarubicin and remains in remission from APML 22 months after diagnosis. Olaparib was resumed, and he maintains a CR of his prostate cancer with undetectable PSA and no radiographic evidence of disease 48 months after diagnosis of metastatic small-cell carcinoma of the prostate.
Results
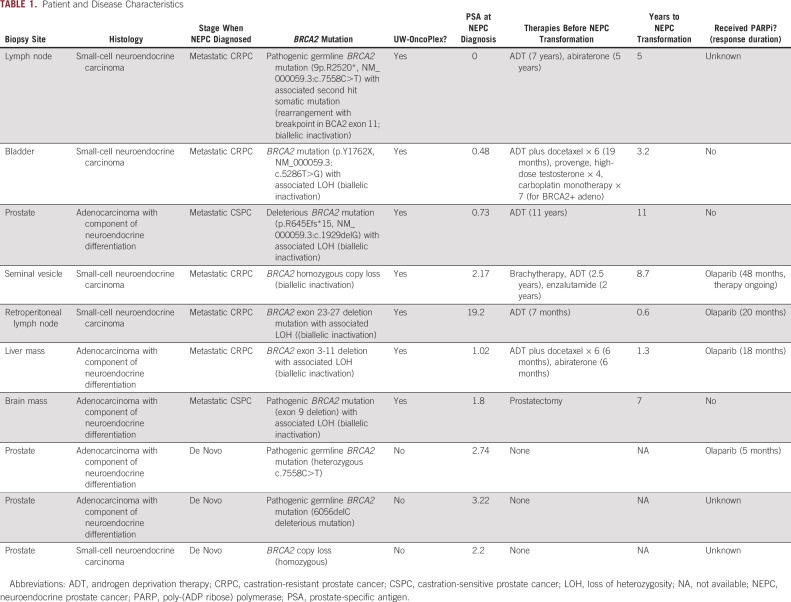
We reviewed institutional data to identify patients with both NEPC (as defined previously4,20) and pathogenic biallelic BRCA2 alterations. Between February 4, 2014, and February 22, 2021, there were 381 patients with prostate cancer who underwent next-generation sequencing by UW-OncoPlex, a multiplexed mutation assay that assesses mutations in over 350 genes including single-nucleotide variants, small insertions and deletions, gene amplifications, and selected gene fusions. In the UW-OncoPlex sequencing set, there were 354 patients with prostate cancer who also had pathology available for review. All patients had metastatic disease. Overall, 37 of 354 (10%) cases had biallelic BRCA2 alterations and 31 of 354 (9%) cases had neuroendocrine or small-cell histology. There were 8 cases (2.3%) that had concurrent NEPC histology and biallelic BRCA2 alterations of whom 4 patients had morphologic small-cell carcinoma and four patients had NEPC.4,20 An additional three patients were identified with coexisting NEPC and BRCA2 alterations sequenced through other platforms. In total, we identified 11 patients with concurrent biallelic BRCA2 inactivation and NEPC/SCNC. Of the subset of UW-OncoPlex patients positive for NEPC histology, 8 of 31 (26%) had biallelic BRCA2 alterations, which was significantly higher than the incidence of BRCA2 mutations identified in those without NEPC histology (29 of 323, 9%; P = .003; Table 1). Additional features are described in Table 1.
TABLE 1.
Patient and Disease Characteristics
Discussion
NEPC is an aggressive variant of prostate cancer that most commonly occurs in the setting of castration-resistant disease. As NEPC often shares clinical and molecular features of other small cell-carcinomas,21 first-line treatment often involves platinum-based chemotherapy regimens.22-24 However, even after platinum-based treatment, the prognosis is poor with a median survival of < 12 months.2 Additional treatment strategies are needed to improve outcomes, and the recognition that biallelic BRCA inactivation is more common in NEPC than previously recognized may provide avenues to improve outcomes.
PARP inhibitors effectively treat BRCA2-deficient tumors by blocking salvage DNA repair pathways, resulting in synthetic lethality.25 PARP inhibitors now play an important role in the treatment of metastatic CRPC, and both olaparib and rucaparib have been US Food and Drug Administration–approved for use in patients with BRCA1/2 alterations.13,26,27 Identifying patients with these mutations has become crucial as it may expand effective treatment options.
Somatic mutations in DNA repair pathway genes, including BRCA2, are common, with 13% of patients with mCRPC harboring somatic and/or and germline BRCA2 alterations, and approximately 90% of these demonstrate biallelic inactivation.15 However, data regarding the frequency of DNA repair pathway gene alterations in NEPC remain conflicting. Aggarwal et al previously reported that the presence of deleterious mutations and/or copy number loss in DNA repair pathway genes (eg, BRCA2) was nearly mutually exclusive in the setting of treatment-emergent small-cell neuroendocrine prostate cancer (t-SCNC).10 They conducted a multi-institutional prospective study to characterize the features of t-SCNC and found that only 1 of 12 (8%) of t-SCNC biopsy specimens had evidence of DNA repair inactivation (all types of any DNA repair gene) versus 29 of 73 (40%) of biopsy specimens without t-SCNC, P = .035. However, in a phase II clinical trial of 60 patients with metastatic prostate cancer who met predefined criteria for NEPC, Beltran et al17 found genomic alterations BRCA2 in 29% of patients, although only four (8%) patients had identifiable biallelic alterations.
We report that in this series, 26% of patients with NEPC had detectable biallelic BRCA2 alterations and that 21% of these occurred in NEPC. This suggests that a higher proportion of patients with NEPC have biallelic BRCA2 alterations than has previously been described and supports sequencing of these patients to detect these alterations. Although we surveyed all patients with prostate cancer whose tumors had been sequenced with the UW-OncoPlex platform, biases regarding which patients underwent sequencing represent significant confounders. We additionally report the case of one of these patients successfully treated with platinum-based chemotherapy followed by maintenance olaparib with an exceptional response and who currently has no evidence of disease 4 years after diagnosis of t-SCNC. His case, to our knowledge, is also the first published case of secondary APML in a patient with prostate cancer treated with olaparib. Remarkably, both t-SCNC and secondary APML remain in CR and complete remission, respectively, the latter consistent with prior reports of excellent outcomes with contemporary treatment of APML.28-30
Of note, there is a prior case report of an exceptional responder with de novo small-cell cancer of the prostate and BRCA2 loss and treated with Olaparib.31 The current report adds a comprehensive institutional sequencing review of the increased frequency of underlying BRCA2 inactivation in NEPC using a single platform to consistently identify BRCA2. In addition, our patient illustrates the potential for an induction/maintenance approach which decreases toxicity to other agents and is the first reported case of secondary APML due to olaparib exposure in a patient with prostate cancer. Both reports emphasize the importance of an interrogation of tissue for BRCA2 in neuroendocrine or small-cell prostate carcinoma where prognosis is poor and treatment options are limited. Additional multi-institutional studies are needed to better understand the prevalence of BRCA2 alterations in neuroendocrine/small-cell carcinoma prostate cancer and to assess the use of induction/maintenance which may prove an effective treatment strategy.
ACKNOWLEDGMENT
We thank our patient for allowing us to participate in his care and share this case. The investigators obtained informed consent to publish information from the participant in this case report. The investigators also acknowledge Elihu Estey, MD, a pioneering researcher in hematologic malignancy who treated our patient and passed away during the preparation of this manuscript but whose legacy will always remain.
Erik Konnick
Consulting or Advisory Role: Roche
Funda Vakar-Lopez
Consulting or Advisory Role: AstraZeneca
Heather H. Cheng
Consulting or Advisory Role: AstraZeneca, AstraZeneca (I)
Research Funding: Sanofi (Inst), Janssen (Inst), Clovis Oncology (Inst), Color Genomics Foundation (Inst), Medivation/Astellas (Inst), Phosplatin Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: Janssen
Michael T. Schweizer
Consulting or Advisory Role: Resverlogix, AstraZeneca, PharmaIn, Sanofi
Research Funding: Janssen (Inst), AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Zenith Epigenetics (Inst), Madison Vaccines, Inc (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Tmunity Therapeutics, Inc (Inst), SignalOne Bio (Inst)
Peter S. Nelson
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Roche/Genentech, Bristol Myers Squibb
Research Funding: Genomic Health (Inst)
Expert Testimony: Venable
Travel, Accommodations, Expenses: Janssen Oncology
Colin C. Pritchard
Consulting or Advisory Role: AstraZeneca, Sana Biotechnology
Research Funding: Color Genomics (I)
Bruce Montgomery
Research Funding: AstraZeneca (Inst), Janssen Oncology (Inst), Clovis Oncology (Inst), Astellas Pharma (Inst), Beigene (Inst)
No other potential conflicts of interest were reported.
SUPPORT
AUTHOR CONTRIBUTIONS
Conception and design: Peter S. Nelson, Bruce Montgomery
Financial support: Peter S. Nelson
Administrative support: Bruce Montgomery
Provision of study materials or patients: Funda Vakar-Lopez, Heather H. Cheng, Peter S. Nelson, Colin C. Pritchard
Collection and assembly of data: Erik Konnick, Funda Vakar-Lopez, Heather H. Cheng, Colin C. Pritchard, Bruce Montgomery
Data analysis and interpretation: Lynn Symonds, Erik Konnick, Heather H. Cheng, Michael T. Schweizer, Peter S. Nelson, Colin C. Pritchard, Bruce Montgomery
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Erik Konnick
Consulting or Advisory Role: Roche
Funda Vakar-Lopez
Consulting or Advisory Role: AstraZeneca
Heather H. Cheng
Consulting or Advisory Role: AstraZeneca, AstraZeneca (I)
Research Funding: Sanofi (Inst), Janssen (Inst), Clovis Oncology (Inst), Color Genomics Foundation (Inst), Medivation/Astellas (Inst), Phosplatin Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: Janssen
Michael T. Schweizer
Consulting or Advisory Role: Resverlogix, AstraZeneca, PharmaIn, Sanofi
Research Funding: Janssen (Inst), AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Zenith Epigenetics (Inst), Madison Vaccines, Inc (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Tmunity Therapeutics, Inc (Inst), SignalOne Bio (Inst)
Peter S. Nelson
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Roche/Genentech, Bristol Myers Squibb
Research Funding: Genomic Health (Inst)
Expert Testimony: Venable
Travel, Accommodations, Expenses: Janssen Oncology
Colin C. Pritchard
Consulting or Advisory Role: AstraZeneca, Sana Biotechnology
Research Funding: Color Genomics (I)
Bruce Montgomery
Research Funding: AstraZeneca (Inst), Janssen Oncology (Inst), Clovis Oncology (Inst), Astellas Pharma (Inst), Beigene (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Conteduca V, Oromendia C, Eng KW, et al. Clinical features of neuroendocrine prostate cancer Eur J Cancer 1217–182019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal R, Zhang T, Small EJ, et al. Neuroendocrine prostate cancer: Subtypes, biology, and clinical outcomes J Natl Compr Canc Netw 12719–7262014 [DOI] [PubMed] [Google Scholar]
- 3.Beltran H, Tagawa ST, Park K, et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer J Clin Oncol 30e386–e3892012 [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation Am J Surg Pathol 38756–7672014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spetsieris N, Boukovala M, Patsakis G, et al. Neuroendocrine and aggressive-variant prostate cancer. Cancers (Basel) 2020;12:3792. doi: 10.3390/cancers12123792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labrecque MP, Coleman IM, Brown LG, et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer J Clin Invest 1294492–45052019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirano D, Okada Y, Minei S, et al. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy Eur Urol 45586–5922004discussion 592 [DOI] [PubMed] [Google Scholar]
- 8.Berruti A, Mosca A, Porpiglia F, et al. Chromogranin A expression in patients with hormone naïve prostate cancer predicts the development of hormone refractory disease J Urol 178838–8432007quiz 1129 [DOI] [PubMed] [Google Scholar]
- 9.Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets Cancer Discov 1487–4952011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study J Clin Oncol 362492–25032018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aparicio A, Logothetis CJ, Maity SN.Understanding the lethal variant of prostate cancer: Power of examining extremes Cancer Discov 1466–4682011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoma C.Targeting DNA repair defects in prostate cancer Nat Rev Urol 17432–4322020 [DOI] [PubMed] [Google Scholar]
- 13.Mateo J, Boysen G, Barbieri CE, et al. DNA repair in prostate cancer: Biology and clinical implications Eur Urol 71417–4252017 [DOI] [PubMed] [Google Scholar]
- 14.Lozano R, Castro E, Aragón IM, et al. Genetic aberrations in DNA repair pathways: A cornerstone of precision oncology in prostate cancer Br J Cancer 124552–5632021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer Cell 1611215–12282015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng HH, Pritchard CC, Boyd T, et al. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer Eur Urol 69992–9952016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltran H, Oromendia C, Danila DC, et al. A phase II trial of the aurora kinase A inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: Efficacy and biomarkers Clin Cancer Res 2543–512019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirts BH, Casadei S, Jacobson AL, et al. Improving performance of multigene panels for genomic analysis of cancer predisposition Genet Med 18974–9812016 [DOI] [PubMed] [Google Scholar]
- 19.Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens J Mol Diagn 1656–672014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer Nat Med 22298–3052016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rickman DS, Beltran H, Demichelis F, et al. Biology and evolution of poorly differentiated neuroendocrine tumors Nat Med 23664–6732017 [DOI] [PubMed] [Google Scholar]
- 22.Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer Clin Cancer Res 193621–36302013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltran H, Tomlins S, Aparicio A, et al. Aggressive variants of castration-resistant prostate cancer Clin Cancer Res 202846–28502014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papandreou CN, Daliani DD, Thall PF, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate J Clin Oncol 203072–30802002 [DOI] [PubMed] [Google Scholar]
- 25.Nizialek E, Antonarakis ES.PARP inhibitors in metastatic prostate cancer: Evidence to date Cancer Manag Res 128105–81142020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer N Engl J Med 3822091–21022020 [DOI] [PubMed] [Google Scholar]
- 27.Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial Lancet Oncol 21162–1742020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallman MS, Altman JK.How I treat acute promyelocytic leukemia Blood 1145126–51352009 [DOI] [PubMed] [Google Scholar]
- 29.Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet Blood 1331630–16432019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estey EH.Acute myeloid leukemia: 2019 update on risk-stratification and management Am J Hematol 931267–12912018 [DOI] [PubMed] [Google Scholar]
- 31.Chanez B, Chaffanet M, Adélaide J, et al. Poly (ADP-Ribose) polymerase inhibitors for de novo BRCA2-null small-cell prostate cancer JCO Precis Oncol 21–82018 [DOI] [PubMed] [Google Scholar]