Abstract
PURPOSE:
To describe longitudinal retinal fluid dynamics on spectral domain OCT and to identify imaging biomarkers that predict the worsening of DME with interval extension during anti-vascular endothelial growth factor (VEGF) therapy.
DESIGN:
A post hoc sub-analysis of phase III, VISTA-DME study.
METHODS:
Eyes received either intravitreal aflibercept injection 2 mg every 4 weeks (2q4) or every 8 weeks after 5 initial monthly injections (2q8), and eyes imaged with the Cirrus HD-OCT system were included. The macular cube was analyzed for 10 time-points from baseline through week 100. Retinal OCT images were evaluated using a novel software platform to extract retinal fluid features for calculation of volumetric fluid parameters, including the retinal fluid index (RFI): the percentage of retinal volume that was occupied by intraretinal fluid.
RESULTS:
Fifty-five eyes were included in the 2q4 group, and 58 eyes were included in the 2q8 group. Early RFI volatility with a central macular RFI increase by ≥5 points from week 4 to 8 (P = .004, odds ratio [OR] 31.3, 95% confidence interval [CI] 3.0 to 329) and cumulative RFI volatility with an aggregate increase in macular RFI by ≥10 points from those timepoints with increased RFI between baseline to week 20, P [ .005, OR 10.2, 95% CI 2.1 to 51.3) were both significant predictors for the worsening of DME and visual acuity when the treatment interval was extended to 8 weeks in the 2q8 group.
CONCLUSIONS:
Early fluid dynamics as measured by (1) early RFI volatility and (2) cumulative RFI instability with aggregate increased RFI were associated with intolerance of interval extension.
DIABETIC MACULAR EDEMA (DME) REMAINS THE most common cause of vision loss in individuals with diabetes. DME is characterized by the blood-retina barrier’s breakdown with accumulation of fluid and plasma components in the neurosensory retina.1 Over the last decade, the treatment of DME has evolved dramatically; clinical trials have established the substantial benefit of intravitreal antivascular endothelial growth factor (VEGF) agents in improving visual outcome and reducing the macular thickness and the severity of diabetic retinopathy in eyes with DME.2–4
One of the current challenges in DME management is the significant variability that occurs in the treatment from patient to patient of a given anti-VEGF agent. Some eyes may tolerate significantly longer intervals in which fewer injections are required to achieve or maintain good vision.5 Personalized therapy minimizes overtreatment, reduces the risk of endophthalmitis,6 and limits economic burden.7 Meanwhile, some eyes respond poorly, requiring 6 or more injections before any anatomical improvement.8 This introduces the risk of persistent or recurrent DME, leading to irreversible retinal damage with a lost opportunity for visual recovery.9 Given this high degree of individual variability, there is a clear need for predictive tools and markers for projecting therapeutic outcome and treatment durability following anti-VEGF therapy. Baseline characteristics such as patients at a younger age, eyes with less severe diabetic retinopathy, and absence of surface wrinkling retinopathy have been described as prognostic factors for a better visual improvement.10 However, minimal data are available for predicting the therapeutic interval. Studies have suggested that the various treatment responses are at least partially due to DME’s multifactorial pathology, and there may be early important information related to treatment response in the first few injections.11–15
Spectral-domain optical coherence tomography (SD-OCT) provides noninvasive, rapid visualization of the retinal microstructures and has become an indispensable tool for monitoring eyes with DME. Qualitative descriptors have been used to describe the presence of retinal features such as the integrity of the external limiting membrane or ellipsoid zone (EZ) band and disorganization of retinal inner layers (DRIL). These features may be surrogate biomarkers for retinal health or damage and correlate with visual function.16–27 Basic quantitative features such as central subfield thickness (CST) are widely available as surrogates for fluid severity but do not correlate well with visual acuity. Recent advances in image analysis techniques have enabled volumetric assessment of retinal fluid metrics in eyes with DME.28,29 However, longitudinal dynamics of retinal fluid features and prognostication for recurrence or improvement of macular edema during anti-VEGF therapy are not well established. Using A unique software platform was used for retinal layer segmentation in SD-OCT, which involved performing a post hoc analysis of the VISTA-DME study to evaluate higher-order OCT intraretinal fluid (IRF) and subretinal fluid (SRF) parameters.28 The primary objective of this study was to explore higher-order OCT parameters obtained within the loading phase of anti-VEGF therapy that would predict interval extension intolerance during the management of DME. A secondary objective was to examine the feasibility of a prediction model for long-term intraretinal fluidics in eyes with DME based on the early treatment response and identify any visual acuity and anatomic patterns related to retinal fluid resolution in responders and nonresponders to anti-VEGF therapy.
METHODS
THE VISTA-DME STUDY WAS A DOUBLE-MASKED, RANDOMized phase III clinical trial that investigated the efficacy and safety of intravitreal aflibercept injection (IAI) and compared it to laser photocoagulation in eyes with center involving DME.4,30,31 The study was conducted in 54 sites across the United States according to the principles expressed in the Health Insurance Portability and Accountability Act, the Declaration of Helsinki, and the International Conference on Harmonization. Local institutional review board approval was obtained at each participating institution, and all subjects provided written informed consent prior to study enrollment. The primary inclusion criteria were adults with type 1 or type 2 diabetes presenting center-involving DME with best-corrected visual acuity (BCVA) in ETDRS (Early Treatment Diabetic Retinopathy Study) letter score between 24 and 73 letters (Snellen equivalent of 20 of 320 to 20/40) in the study eye. Only 1 eye per subject was enrolled. Subjects were randomly assigned to 1 of the following 3 treatment groups: IAI, 2 mg every 4 week (2q4); IAI, 2 mg every 8 week after the loading phase of 5 consecutive monthly injections (2q8); or macular laser photocoagulation. From week 24 (IAI: 2q4 and 2q8), groups received rescue treatment with macular laser photocoagulation if pre-specified rescue criteria were met.30
PARTICIPANTS:
In this post-hoc subanalysis of the VISTA-DME study, subjects assigned to the IAI groups that underwent SD-OCT with the Cirrus HD-OCT platform (Zeiss, Oberkochen, Germany) were included. Subjects assigned to the macular laser photocoagulation group were excluded from this pilot study. Likewise, subjects imaged using the Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) were not assessed in the analysis. The specific time points analyzed for this study included week 0, 4, 8, 12, 16, 20, 24, 28, 52, and 100; and subjects were excluded if eyes did not have macular cube scans of sufficient quality for assessment at either week 0, 4, 8, 12, 20, or 24. The full analysis set (ie, eyes that had received study treatment and had at least 1 post-baseline BCVA assessment) in the VISTA-DME study included 154 eyes in the IAI 2q4 group and 151 eyes in the IAI 2q8 group.30 Of these 305 eyes, 138 eyes were evaluated for retinal layer segmentation. Twenty-five eyes were further excluded due to insufficient image quality of their macular cube or unavailable time point at either week 0, 4, 8, 12, 20, or 24.
SD-OCT IMAGE ANALYSIS:
As part of the VISTA-DME study, a macular cube consisting of 128 B-scans by 512 A-scans covering a nominal 6 × 6-mm scan area centered on the macula were obtained by using a Cirrus HD-OCT unit (Zeiss, Oberkochen, Germany). SD-OCT data were exported in Digital Imaging and Communications in Medicine (DICOM) format and transferred to a previously described customized retinal layer segmentation tool developed at the Cleveland Clinic which provided semiautomated segmentation of the internal limiting membrane, EZ band, retinal pigment epithelium (RPE) band, IRF, and SRF object extraction (Figure 1, A).16,32,33 All image analysts completed training focused on fluid features and retinal layer abnormalities for a standardized approach to OCT segmentation corrections. Each eye’s scans were analyzed by the same image analyst for each time point to minimize variability for a single subject. Following the initial assessment, a senior image analyst (project leader) provided consistency assessment of every image frame following initial segmentation by the image analysts for quality assessment. EZ-RPE thickness was computed by calculating the mean distance between the EZ and RPE lines. En face and 3-dimensional retinal fluid maps were generated to visualize regional fluid accumulation (Figure 1, B). The central subfield and central macula were defined as the inner concentric circles of 0.5 mm and 1.0 mm radii from the fovea (corresponding to inner and outer circles, respectively, on the en face map). “Actual” retinal tissue thickness or volume parameters were defined as the true retinal tissue thickness or volume by means of excluding cystic IRF and SRF spaces (Figure 1, C). The retinal fluid index (RFI) was defined as the following: [RFI = 100 × IRF volume/(total retinal volume SRF volume)] (Supplemental Figure). RFI represents a relative amount of IRF volume relative to the retinal volume in a designated area (eg, central macula) in percentage.
FIGURE 1.
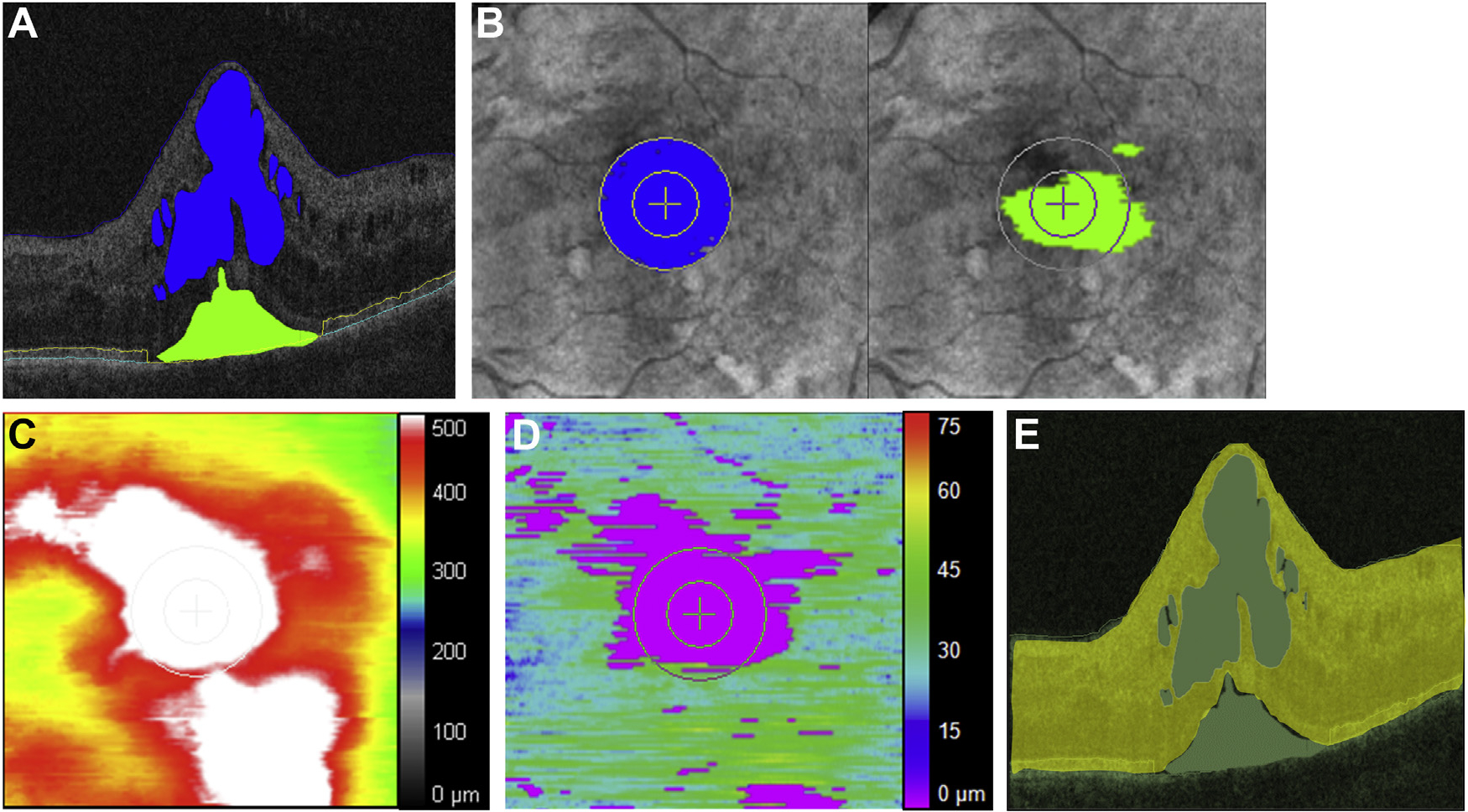
Exemplary OCT images with segmented retinal fluid in eyes with diabetic macular edema, en face retinal fluid mapping, and EZ mapping. (A) Horizontal B-scan crossing the central fovea displaying semiautomatically segmented visually discernable fluid and retinal layer boundaries. Intraretinal fluid within the central macula (an area filled in blue), subretinal fluid (an area filled in green), the internal limiting membrane (blue line), EZ band (yellow line), and RPE (turquoise line) are displayed. Each boundary was reviewed by experienced reviewers, and segmentation errors were carefully corrected manually. (B) En face retinal fluid mapping demonstrating intraretinal fluid (an area filled in blue) and subretinal fluid (an area filled in green) in the central macular zone (i.e., central 2-mm). (C) En face retinal thickness mapping. (D) En face EZ mapping illustrating the topographical thickness between EZ band and RPE. (E) Actual retinal tissue thickness/volume parameters defined as the true retinal tissue thickness/volume by means of excluding cystic intraretinal fluid and subretinal fluid space. This represents residual retinal tissue and any associated diffuse thickening of retinal tissue. EZ = ellipsoid zone; OCT = optical coherence tomography; RPE = retinal pigment epithelium.
DEFINITION OF REBOUNDER AND RETINAL FLUID INDEX VOLATILITY:
Week 24 was the visit corresponding to the first 8-week challenge where the interval was extended from 4 to 8 week in the 2q8 group. Anatomical worsening of DME from week 20 to 24 was defined as an increase in central macular RFI by ≥5 points (ie, the difference in percentage), and eyes that fulfilled this criterion were specifically termed “rebounders”; the threshold of “≥5 points” was set based on the comparative assessment in central macular RFI in the 2q4 and 2q8 groups during this period (Figure 2). Early RFI volatility was defined as an increase in central macular RFI by ≥5 points from week 4 to 8. Cumulative RFI volatility was defined as an increase of ≥10 points of RFI during the loading phase (from baseline to week 20). This was determined by the sum of any RFI increases at any of those time points without any consideration of any RFI decreases and pattern of RFI changes. The following are examples of cumulative RFI volatility:
Patient A changes in RFI at each time point through week 20 = −10, −5, +7, +2, −2 (total RFI increase + 9; not considered cumulative RFI volatility);
Patient B: −1, −1, −1, −1, +10 (total RFI increase +10; considered cumulative RFI volatility);
Patient C: −15, +9, −16, +6, −5 (total RFI increase +15; considered cumulative RFI volatility);
Patient D: +1, +2, +2, +2, +1 (total RFI increase +8; not considered cumulative RFI volatility).
FIGURE 2.
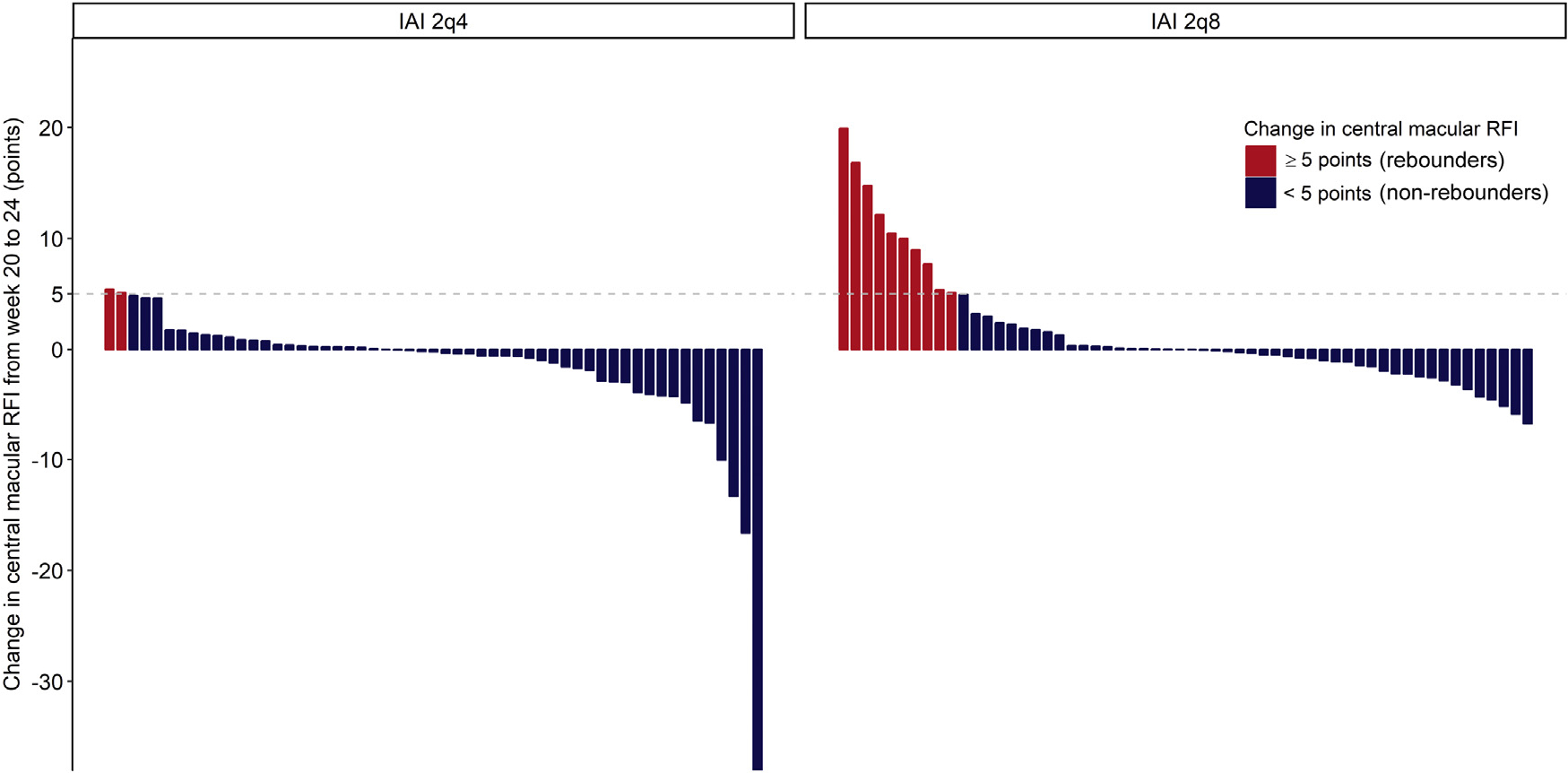
Waterfall plot shows the changes in the central macular RFI from week 20 to 24 in the 2q4 and 2q8 groups. The eyes with an increase in RFI ≥5 points from week 20 to 24 were defined as “rebounders,” shown in red. Of all eyes, the percentage of rebounders was 2.5% (2 of 55) in the 2q4 group and 17% (10 of 58) in the 2q8 group. This difference can be theoretically attributed to the extended treatment interval of 8 week in the 2q8 group. From week 20 to 24, the rebounders in the 2q8 group lost a mean of 4.9 ± 5.4 ETDRS letters with a mean increase in central macular RFI by 11.1 ± 4.9 points. ETDRS = Early Treatment Diabetic Retinopathy Study; RFI = retinal fluid index.
CLASSIFICATION BASED ON EARLY TREATMENT RESPONSE:
Eyes were divided into 3 subgroups based on early treatment response in the central macular RFI at week 4, 8, and 12. Eyes were classified as “early responders” if the central macular RFI was <10% at both week 4 and 8. The remaining eyes were classified as either “delayed responders” if central subfield RFI was <20% at week 12 or “indeterminate responders” if the central macular RFI was ≥20% at week 12. These thresholds were set based on exploratory cluster analysis in which all study eyes were separated into 3 clusters using the K-means clustering method (variables included the central macular RFI at week 4 and 8). By observing the patterns of RFI changes in each cluster, the authors set the thresholds (<10% at both week 4 and 8 and ≥20% at week 12) with the intention of potentially replicating the results of the cluster analysis.
STATISTICAL ANALYSIS:
All statistical analyses were performed using R version 3.4.1 software (R Project for Statistical Computing; www.r-project.org). Data missing due to unavailable time points at week 16, 28, 52, and 100 were excluded from the analysis and were not imputed using the last observation-carried-forward method. The assumption that data were normally distributed was verified by applying the Shapiro-Wilk W-test. Continuous variables were analyzed with the parametric Student t-test. One-way analysis of variance models with post hoc Bonferroni multiple comparison procedures was also used to compare age, HbA1c concentrations, blood pressure, BCVA, and OCT measurements. Categorical variables were analyzed using the χ2 test or Fisher exact test. Correlation analysis was performed using the parametric Pearson correlation test. Data are presented as mean ± standard deviation. All P values were 2-sided, and a P value <.05 was considered statistically significant.
RESULTS
A TOTAL OF 113 EYES OF 113 SUBJECTS WERE INCLUDED. SPEcifically, 55 eyes of 55 subjects were included in the 2q4 group, and 58 eyes of 58 subjects were included in the 2q8 group. Baseline characteristics of subjects are presented in Table 1. The mean age (years) at baseline was 62.3 ± 12.3 (range: 26–87 years old) in the 2q4 group and 64.3 ± 8.8 (range: 33–81 years old) in the 2q8 group. The central macular RFI at baseline was 16.7 ± 11.0% and 20.4 ± 12.3% in the 2q4 and 2q8 group, respectively. The CST at baseline was 433 ± 159 μm and 465 ± 134 μm in the 2q4 and 2q8 groups, respectively. The mean BCVA (ETDRS letters) at baseline was 58.1 ± 11.9 (Snellen equivalent 20/80) in the 2q4 group and 59.0 ± 11.4 (Snellen equivalent 20/63) in the 2q8 group.
TABLE 1.
Baseline Characteristics of Subjects
| Treatment Group | IAI 2q4 | IAI 2q8 | All IAI |
|---|---|---|---|
|
| |||
| Number of eyes | 55 | 58 | 113 |
| Mean ± SD age, y | 62.3 ± 12.3 | 64.3 ± 8.8 | 63.3 ± 10.6 |
| Females | 27 (49) | 34 (59) | 61 (54) |
| Mean ± SD HbA1c % | 7.8 ± 1.8 | 7.7 ± 1.3 | 7.7 ± 1.6 |
| Mean ± SD diastolic blood pressure, mm Hg | 75.7 ± 8.4 | 75.4 ± 9.4 | 75.5 ± 8.9 |
| Mean ± SD systolic blood pressure, mm Hg | 135.6 ± 15.6 | 135.6 ± 15.4 | 135.6 ± 15.5 |
| Mean ± SD BCVA, ETDRS letters | 58.1 ± 11.9 | 59.0 ± 11.4 | 58.6 ± 11.6 |
| Mean ± SD central macular RFI, % | 16.7 ± 11.0 | 20.4 ± 12.3 | 18.6 ± 11.8 |
| Mean ± SD CST, μm | 433 ± 159 | 465 ± 134 | 450 ±147 |
BCVA = best-corrected visual acuity; CST = central subfield thickness; ETDRS = Early Treatment Diabetic Retinopathy Study; IAI = intra- vitreal aflibercept injection; RFI = retinal fluid index.
Table values are n (%) or mean ± SD. The central macula is equivalent to a distance of 1.0 mm from the fovea (outer circle on en face mapping).
INTRARETINAL FLUID REBOUND WITH TREATMENT INTERVAL EXTENSION:
The first 8-week challenge occurred at week 24 in the 2q8 group. Changes in central macular RFI from week 20 to 24 in all study eyes are presented in a waterfall plot (Figure 2). In the 2q8 group, 10 of 58 eyes (17%) were identified as rebounders and did not tolerate the first 8-week challenge. Those eyes initially gained 10.2 ± 4.9 ETDRS letters of vision from baseline to week 20 and then lost 4.9 ± 5.4 ETDRS letters of vision with a mean increase in central macular RFI of 11.1 ± 4.9 points during the first 8-week challenge. The remaining 48 eyes (defined as “nonrebounders”) gained 11.1 ± 6.6 ETDRS letters of vision from baseline to week 20, demonstrated stability of visual acuity (0.0 ± 4.0 ETDRS letters of vision) and a mean decrease in central macular RFI of 0.73 ± 2.4 points during the initial 8-week challenge (P = .002 and P <.001, respectively).
EARLY RETINAL FLUID INDEX VOLATILITY:
Assessment of longitudinal central macular RFI in eyes with rebounders and nonrebounders (Figure 3) demonstrated a noticeable increase in central macular RFI from week 4 to 8 in the rebounders of the 2q8 group (Figure 4, A). Of eyes that demonstrated early RFI volatility based on an increase in central macular RFI by ≥5 points from week 4 to 8, 80% were ultimately rebounders, which was significantly higher than those eyes without early RFI volatility (80% vs. 13%, respectively; P = .002). Eyes with early RFI volatility had a 31.3-fold chance of intolerance of interval extension (P = .004; odds ratio [OR]: 31.3; 95% confidence interval [CI]: 3.0 to 329) compared to eyes with RFI stability or improvement between week 4 and 8.
FIGURE 3.

Comparison of the central macular RFIs between rebounders and nonrebounders. The rebounders in the 2q8 group exhibited a significant increase in central macular RFI from week 4 to 8 and noticeable volatility in central macular RFI from baseline to week 20 compared with nonrebounders. RFI = retinal fluid index.
FIGURE 4.
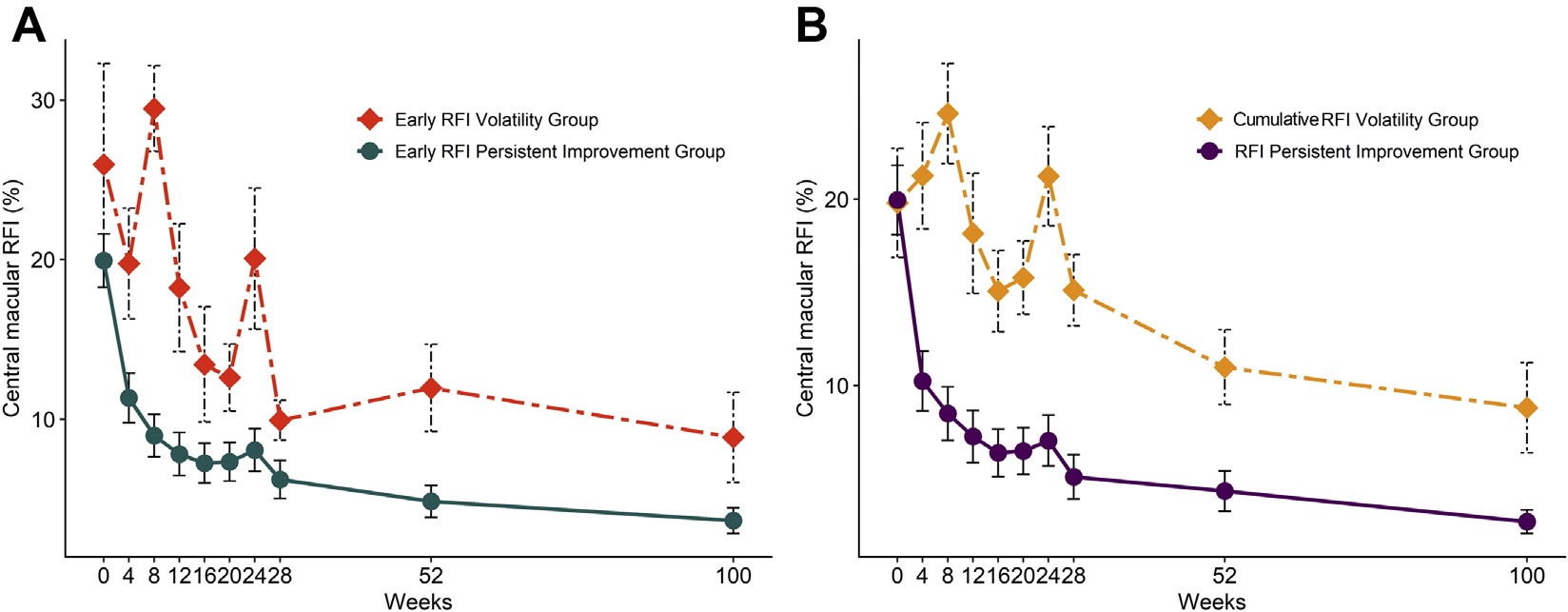
Impact of RFI volatility on tolerance of interval extension. (A) Early RFI volatility. eyes with an increase of more than 5 points in central macular RFI from week 4 to 8 were included in the early RFI volatility group, and eyes with a decrease or an increase less than 5 points in RFI were included in early RFI persistent improvement group. Fluid rebound was noted in 80% of early RFI volatility group compared to 13% of early RFI persistent improvement group (P = .002). (B) Cumulative RFI volatility. Eyes with a cumulative increase in central macular RFI by ≥10 points during visits demonstrating increased RFI from baseline to week 20 were included in cumulative RFI volatility group and demonstrated rebound in 56% of eyes compared to 11% in the remaining eyes (P = .006). RFI = retinal fluid index
CUMULATIVE RETINAL FLUID INDEX VOLATILITY:
In addition, 56% of eyes in the 2q8 group that exhibited cumulative RFI volatility (defined as a ≥10-point of RFI increase between all time points between baseline and week 20) became rebounders, compared to only 11% of the eyes without similar cumulative RFI increases (P = .006) (Figure 4, B). Eyes that demonstrated cumulative RFI volatility from baseline to week 20 exhibited a 10.2-fold chance of becoming rebounders (P = .005; OR: 10.2; 95% CI: 2.1 to 51.3).
LONGITUDINAL FLUID OUTCOMES AND PREDICTIVE ASSESSMENT OF OUTCOMES AT WEEKS 52 AND 100:
Sixty of 113 eyes (53%) were classified as early responders, 34 of 113 eyes (30%) were delayed responders, and 19 of 113 eyes (17%) were indeterminate responders (Table 2). The proportion of eyes that achieved a central macular RFI <10% at weeks 52 and 100 was 52 of 53 (98%) and 46 of 49 (94%) in the early responders; 25 of 32 (78%) and 24 of 27 (89%) in the delayed responders; and 5 of 17 (29%) and 4 of 17 (24%) in the indeterminate responders (both P < .001). Of the 58 eyes in the 2q8 group, the proportions of rebounders in the early responders, delayed responders, and indeterminate responders were 4 of 31 (13%), 2 of 18 (11%), and 4 of 9 (44%), respectively (P = .062).
TABLE 2.
Classification Based on the Treatment Response in the Central Macular Retinal Fluid Index From Week 4 to 12
| Classification Based On Treatment Response | Early Responders | Delayed Responders | Indeterminate Responders | P |
|---|---|---|---|---|
|
| ||||
| Baseline (week 0) | ||||
| Number of eyes | 60 | 34 | 19 | |
| Mean ± SD age, y | 62.1 ± 11.3 | 64.4 ± 9.7 | 65.1 ± 10.2 | .45 |
| Females | 31 (52) | 19(56) | 11 (58) | .86 |
| Mean ± SD HbA1c, % | 7.7 ± 1.6 | 7.6 ± 1.5 | 7.9 ± 1.6 | .88 |
| Mean ± SD diastolic blood pressure, mm Hg | 76.1 ± 9.0 | 75.4 ± 10.0 | 74.1 ± 6.5 | .71 |
| Mean ± SD systolic blood pressure, mm Hg | 135.6 ± 16.0 | 136.8 ± 14.9 | 133.4 ± 15.3 | .74 |
| Central macular RFI, | 14.0 ± 11.1b,c | 21.4 ± 7.8a | 28.4 ± 12.9a | < .001 |
| <5 | 13 (22)b,c | 0a | 0a | .002 |
| <10 | 24 (40)b,c | 1 (3)a | 0a | < .001 |
| Mean ± SD CST, mm | 417 ± 159c | 470 ± 95 | 516 ± 162a | .023 |
| <300 | 15 (25)b,c | 0a | 0a | < .001 |
| <400 | 35 (58)b,c | 8 (24)a | 5 (26)a | .001 |
| Mean ± SD BCVA, ETDRS letters | 59.9 ± 11.2c | 59.7 ± 11.3 | 52.3 ± 12.0a | .034 |
| Week 20 to 24 | ||||
| Change in central macular RFI, points | 0.0 ± 3.9 | 0.1 ± 5.0 | -1.8 ± 12.9 | .51 |
| ≥5 | 4 (6.7) | 3 (8.8) | 5 (26) | .056 |
| Week 52 | ||||
| Number of eyes | 53 | 32 | 17 | |
| Central macular RFI, % | 2.1 ± 2.5b,c | 6.1 ± 6.2a,c | 18.1 ± 10.6a,b | < .001 |
| <5 | 45 (85)c | 21 (66)c | 2 (12)a,b | < .001 |
| <10 | 52 (98)b,c | 25 (78)a,c | 5 (29)a,b | < .001 |
| Mean ± SD CST, μm | 238 ± 43b,c | 280 ± 72a | 324 ± 102a | < .001 |
| <300 μm | 48 (91)c | 25 (78)c | 6 (35)a,b | < .001 |
| <400 μm | 53 (100)c | 29(91) | 13 (77)a | < .001 |
| Mean ± SD BCVA, ETDRS letters | 69.3 ± 15.1 | 71.9 ± 9.9 | 63.4 ± 11.8 | .10 |
| ≥79 letters | 15(28) | 10(31) | 2(12) | .31 |
| Gain from baseline, ETDRS letters | 10.3 ± 11.1 | 12.4 ± 8.6 | 10.5 ± 6.6 | .62 |
| ≥15 letters gain from baseline | 16(30) | 12 (38) | 6 (35) | .77 |
| Week 100 | ||||
| Number of eyes | 49 | 27 | 17 | |
| Mean ± SD Central macular RFI, % | 2.6 ± 4.1c | 4.9 ± 6.3c | 17.3 ± 15.6a,b | < .001 |
| <5% | 41 (84)c | 17 (63)c | 3 (18)a,b | < .001 |
| <10% | 46 (94)c | 24 (89)c | 4 (24)a,b | < .001 |
| Mean ± SD CST, μm | 239 ± 45c | 252 ± 57c | 337 ± 148a,b | < .001 |
| <300 μm | 44 (90)c | 22 (82) | 8 (47)a | < .001 |
| <400 μm | 49 (100)c | 27(100) | 13 (77)a | < .001 |
| BCVA, ETDRS letters | 73.4 ± 12.2 | 71.8 ± 10.7 | 66.8 ± 8.9 | .12 |
| ≥79 letters | 21 (43) | 6 (22) | 2(12) | .033 |
| Gain from baseline, ETDRS letters | 12.9 ± 8.8 | 13.5 ± 11.6 | 13.3 ± 9.9 | .96 |
| ≥15 letters gain from baseline | 17 (35) | 10(37) | 8 (47) | .66 |
BCVA = best-corrected visual acuity; CST = central subfield thickness; ETDRS = Early Treatment Diabetic Retinopathy Study; IAI = intra- vitreal aflibercept injection; RFI = retinal fluid index.
Table values are n, n (%), or mean ± SD. The central macula is equivalent to a distance of 1.0 mm from the fovea (outer circle on en face mapping).
Significantly different (P < .05) from early responders.
Significantly different (P < .05) from delayed responders.
Significantly different (P < .05) from indeterminate responders
Longitudinal changes in CST by components are shown in Figure 5 (actual retinal tissue thicknesses are shown at the bottom in gray; IRF is shown in the middle in light purple, and SRF is shown at the top in dark purple). In the early responders, all the components were dramatically reduced from baseline to week 4, and those changes were well sustained until weeks 52 and 100. In the delayed responders, there was a slower reduction in IRF and actual retinal tissue thickness compared with the early responders. The indeterminate responders represented eyes with late or nonresponses to IAI that exhibited the highest central macular RFI throughout the follow-up; gradual improvement in actual retinal tissue thickness occurred more rapidly than that of the delayed responders. However, there was no decrease in IRF from baseline to week 8; and after a steady decrease from week 8 to 20, IRF persisted until weeks 52 and 100, particularly in the 2q4 group.
FIGURE 5.

Longitudinal changes in central subfield thickness by components. Actual retinal tissue thickness is shown at the bottom (gray), intraretinal fluid is shown in the middle (light purple) and subretinal fluid at the top (dark purple).
Overall, IAI was successful in reducing actual central subfield retinal tissue thickness (in micrometers). In the long-term follow-up, there were no differences among the early responders, the delayed responders, and the indeterminate responders at baseline (297 ± 62, 302 ± 70, and 283 ± 62, respectively; P = .59) or week 100 (227 ± 38, 224 ± 29, and 218 ± 44, respectively; P = .67), although there was a difference during the follow-up, for instance, at week 12 (236 ± 40, 263 ± 53, and 231 ± 47, respectively; P = .012). At baseline, SRF that existed within central subfield was most frequently present in the early responders with 22 of 60 (37%) compared with 5 of 34 (15%) in the delayed responders and 4 of 19 (21%) in the indeterminate responders (P = .057). Central subfield SRF volume was rapidly reduced with IAI treatment, 23 of 31 eyes (74%) achieved complete resolution of SRF (within the central subfield) by week 12.
FLUID-STRATIFIED VISION OUTCOME AT WEEK 100:
The BCVA (ETDRS letters) at week 100 in the early responders, delayed responders, and indeterminate responders was 73.4 ± 12.2 (Snellen equivalent 20/40), 71.8 ± 10.7 (Snellen equivalent 20/40), and 66.8 ± 8.9 (Snellen equivalent 20/50), respectively (P = .118). The proportion of eyes that achieved very good BCVA (≥79 ETDRS letters, Snellen equivalent ≥20/25) at week 100 was higher in the early responders with 43% (21 of 49) than the delayed responders with 6 of 27 (22%), and the indeterminate responders with 2 of 17 (12%), respectively (P = .033). The mean visual gains (in ETDRS letters) from baseline to week 100 in the early responders, delayed responders, and indeterminate responders were 12.9 ± 8.8, 13.5 ± 11.6, and 13.3 ± 9.9, respectively (P = .96), which were comparable among 3 subgroups.
CORRELATION BETWEEN CENTRAL MACULAR RFI AT BASELINE AND RETINAL PARAMETERS AT WEEK 100:
As shown in Figure 6, central macular RFI at baseline was negatively correlated with actual central subfield retinal tissue thickness at week 100 (r = −0.307; P = .028) and mean central subfield EZ-RPE thickness at week 100 (r = −0.377; P < .001). In addition, baseline central macular RFI was negatively correlated with BCVA at week 100 (r = −0.319; P = .002), and eyes with central macular RFI <20% at baseline had a significantly higher chance of achieving very good BCVA of ≥79 ETDRS letters at week 100 than the remaining eyes (P = .001; OR: 6.1; 95% CI: 1.8 to 27).
FIGURE 6.

Correlation between central macular RFI at baseline and selected OCT metrics at week 100. (A, B) Central macular RFI at baseline was negatively correlated with actual central subfield retinal tissue thickness at week 100 (r = −0.307; P = .028) (A), and central subfield ellipsoid zone to retinal pigment epithelium thickness at week 100 (r = −0.377; P < .001) (B). (C) Central macular RFI at baseline was negatively correlated with best-corrected visual acuity at week 100 (r = −0.319; P = .002). OCT = optical coherence tomography; RFI = retinal fluid index.
DISCUSSION
THIS STUDY IDENTIFIED 2 RISK FACTORS ASSOCIATED WITH the rebounder group that demonstrated the following intolerance for interval extension to 8 weeks with worsening of DME: 1) early RFI volatility (ie, an increase in central macular RFI by ≥5 points from week 4 to 8 (OR: 31.3; P = .004)); and 2) cumulative RFI volatility (ie, ≥10 points of RFI increase between all time points from baseline to week 20 determined by the sum of any RFI increases (OR: 10.2; P = .005)). The underlying pathogenesis for these findings and their association with systemic risk factors remains uncertain. It is possible that these risk factors and anatomic factors represent an upregulation of proinflammatory cytokines that modulate vascular permeability following anti-VEGF therapy due to a compensatory response to VEGF inhibition or possibly a very high VEGF burden.34 Available evidence supports the notion that DME develops primarily from a permeability-driven disease responsive to anti-VEGF therapy and may transition to a multifactorial and inflammation-driven disease less responsive to anti-VEGF therapy.11 In select eyes, DME was observed to recur, whereas intraocular VEGF levels remained low following intravitreal bevacizumab injection, indicating the involvement of other cytokines.35 Several cytokines involved in vascular permeability such as interleukin-6 (IL-6), IL-8, monocyte chemoattractant protein-1, and angiopoietin-2 are elevated in intraocular fluids in eyes with DME.35–38 In particular, the intraocular level of IL-8, a proinflammatory cytokine that induces the accumulation of neutrophils along the vascular wall and increases vascular permeability,39 has been correlated with the responsiveness to anti-VEGF therapy in eyes with DME.37 Whether IL-8 is involved in the rebounders or delayed responders in the present study is unknown. Further investigation is needed to elucidate the mechanism behind the correlations that were observed and to validate whether the 2 risk factors are applicable beyond week 24 in the 2q8 group.
In this report, a classification based on the anatomic response by means of RFI rather than visual acuity response was selected, given that real-world clinical decision making is most frequently dependent on OCT changes. In eyes with DME, the initial visual acuity is the most reliable predictor for the final visual acuity following anti-VEGF therapy,23,40 reflecting the fact that nonspecific central subfield thickness itself has a limited role in predicting visual acuity and initial presence of retinal atrophy such as DRIL or photoreceptor damage pose greater impact on final visual acuity.23–27 However, this does not preclude the importance of RFI because retinal neural injury due to the marked retinal swelling set the stage for later retinal manifestations that threaten visual acuity. Hence, this report focused on exploring the predictors of IRF outcomes and worsening of macular edema that occurs with extended treatment interval in the 2q8 group.
There are multiple potential benefits to using the RFI metric in the management of DME. First, the proposed classification, using RFI, provides an estimation of long-term anatomical outcome during anti-VEGF therapy. Second, the use of RFI may allow physicians to set a specific treatment target or retreatment criteria that can be shared with the patient. Early responders in this analysis study have a slightly different definition from responders in previous studies that defined eyes that achieved 10% to 20% reduction in CST from baseline.12–14 Because the baseline CST is a significant determinant of potential anatomical improvement in DME (referred to as the “floor effect”), responders by conventional definition comprise a subgroup of eyes with greater baseline CST, and eyes may be more likely to achieve greater gain of visual acuity. One limitation of this definition is that eyes with marked retinal swelling at baseline will fulfill the definition of responders, even if post-injection CST is far from ideal (eg, change in CST from 700 to 500 μm) and is not a sufficient clinical response. Interestingly, in this analysis, early responders were often made up of eyes with thinner baseline CST that were likely to achieve greater final visual acuity. RFI and EZ integrity metrics have been correlated with visual acuity and are potential biomarkers of functional outcomes.28 2q4 and 2q8 IAI resulted in longitudinal improvement of both EZ integrity and RFI. Future research should also examine the impact of fluid volatility on EZ recovery and integrity in DME. In this study, baseline central macular RFI was negatively correlated with actual central subfield retinal tissue thickness at week 100 and central subfield EZ-RPE thickness at week 100 (Figure 6, A and B), suggesting that eyes with high RFI at baseline were inclined to eventually develop retinal atrophy or irreversible photoreceptor loss or both. It is possible that greater central macular RFI (eg, ≥20%) is more likely to cause permanent retinal damage if left untreated. Supporting this hypothesis, central macular RFI at baseline was negatively correlated with visual acuity at week 100 (Figure 6, C), and eyes with central macular RFI <20% at baseline had a 6.1-fold increased chance of achieving very good BCVA of ≥79 ETDRS letters at week 100 compared with the remaining eyes. It is possible that maintaining relatively low RFI (eg, central macular RFI <10%) throughout the follow-up is a necessary condition to minimize chronic retinal damage or atrophy and have the best chance of achieving very good visual acuity in the long-term follow-up.
The quantitative assessment of IRF/SRF volume allowed an evaluation of actual retinal tissue (ie, without fluid) thickness or volume parameters that may account for diffuse thickening of the retinal tissue, as shown in Figure 5. Intraretinal fluid represents fluid accumulated in extracellular space, mostly in the inner nuclear layer and the outer plexiform layer, whereas diffuse thickening of retinal tissue represents the intracellular fluid due to swelling of the glial cells.41,42 The RFI response within week 12 following 3 consecutive IAI was positively associated with 2-year anatomical outcomes. Overall, eyes in the early responders and delayed responders achieved favorable anatomical and visual outcomes with IAI treatment. However, consistent with previous studies, a suboptimal response at weeks 4, 8, and 12 did not preclude further meaningful IRF improvement from occurring in many eyes in the delayed responders and indeterminate responders.12 Of note, actual retinal tissue thickness at week 100 was comparable among early responders, delayed responders, and unpredictable, which indicates that VEGF therapy was effective for improving the diffuse retinal thickening in almost all eyes.
This study has several important limitations that should be noted. The study represents a post hoc exploratory subanalysis with a smaller sample size than the original study. The results should be confirmed using larger prospective datasets. Expansion and validation of these findings on the entire VISTA dataset is ongoing, in addition to independent prospective datasets. With regard to the technical specifications of the OCT retinal layer segmentation tool, retinal thickness or volume may be slightly overestimated because retinal thickness was measured along A-scans (ie, parallel to the vertical axis of B-scans) and not perpendicular to the retinal surface or RPE.33,43 In select cases, a shadowing effect due to substantial retinal swelling and lipid deposition also creates a challenge for precision assessment of exact fluid metrics. Moreover, as in select images, speckle noise can degrade the OCT image quality, confounding small foci IRF.44 Opportunities for improved assessments include multiple-frame averaging, which may be enabled with multiple-frame averaging. Future opportunities for additional analysis will include the incorporation of other biomarkers and variables such as DRIL, hyperreflective foci, signal intensity within the retinal fluid, intraretinal lipid exudates, and the duration of DME with an upscaled number of eyes imaged using the Spectralis OCT in the VISTA-DME study, which may allow identifying superior prognostic imaging biomarkers and generate a better prediction model for visual and anatomical outcome. Although RFI and CST are clinically related, previous work has demonstrated that RFI is more closely linked with visual function.28 Future research will compare the potential for fluid rebound prediction in RFI measures compared to CST measurements. There are also important strengths of this study including the prospective randomized design of the VISTA-DME study with a uniform treatment regimen, long-term follow-up with 10 time points, and masked examiners.
The present study identified 2 novel metrics that may serve as potential imaging biomarkers for treatment interval extension: 1) early RFI volatility between weeks 4 and 8; and 2) cumulative RFI volatility. These factors were independent of baseline CST. Eyes with these risk factors may be regarded as poor candidates for extending treatment interval with a higher risk of losing vision between the injections and may provide a unique opportunity for patient education and prognostication. In addition, identifying these at-risk eyes may facilitate clinical trial enrichment for emerging molecules and therapeutics. Further validation and enhanced automation of calculating this imaging biomarker may provide an important opportunity for use in a clinical trial and potentially deployment to clinical care. This study also successfully classified study eyes based on the treatment response in central macular RFI. According to this definition, roughly 1 in 2 eyes was classified as an early responder, and of those, 94% of eyes achieved central macular RFI <10%, and 43% achieved very good BCVA at week 100. Eyes in this category may be candidates for early treatment interval extension.
Supplementary Material
Acknowledgments
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST and none were reported. Publication of this article was supported by Regeneron (to J.P.E.); US National Institutes of Health/National Eye Institute grant K23-EY022947-01A1 (to J.P.E.); Research to Prevent Blindness (Cole Eye Institute grant); an unrestricted travel grant from Alcon Novartis Hida Memorial Award 2015 funded by Alcon Japan Ltd. (to A.U.); and Betty J. Powers Retina Research Fellowship (to A.U.). J.P.E. receives research support from Aerpio, Alcon, Thrombogenics/Oxurion, Regeneron, Genentech, Novartis, Allergan; and is a consultant for Aerpio, Alcon, Allegro, Allergan, Genentech/Roche, Novartis, Thrombogenics/Oxurion, Leica, Zeiss, Regeneron, Santen; and holds a patent with Leica. AU. K.R., A.B., and K.C. are employees of Regeneron. S.K.S. receives research support from Allergan and Gilead; and is a consultant for Regeneron, Novartis, and Bausch & Lomb.
Footnotes
(Study of Intravitreal Aflibercept Injection [IAI; EYLEA; BAY86-5321] in Patients With Diabetic Macular Edema; VISTA-DME; NCT01363440).
Contributor Information
JUSTIS P. EHLERS, Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research, Cleveland, Ohio, USA Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio, USA; Cole Eye Institute, Cleveland, Ohio, USA.
ATSURO UCHIDA, Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research, Cleveland, Ohio, USA; Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio, USA; Cole Eye Institute, Cleveland, Ohio, USA.
DURIYE DAMLA SEVGI, Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research, Cleveland, Ohio, USA; Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio, USA; Cole Eye Institute, Cleveland, Ohio, USA.
MING HU, Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research, Cleveland, Ohio, USA; Cleveland Clinic, Cleveland, Ohio, USA; Department of Quantitative Health Sciences, Cleveland, Ohio, USA.
KIM REED, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA; and the Regeneron, Tarrytown, New York, USA.
ALYSON BERLINER, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA; and the Regeneron, Tarrytown, New York, USA.
ROBERT VITTI, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA; and the Regeneron, Tarrytown, New York, USA.
KAREN CHU, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA; and the Regeneron, Tarrytown, New York, USA.
SUNIL K. SRIVASTAVA, Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research, Cleveland, Ohio, USA Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio, USA; Cole Eye Institute, Cleveland, Ohio, USA.
REFERENCES
- 1.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med 2012;366(13):1227–1239. [DOI] [PubMed] [Google Scholar]
- 2.Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117(6):1064–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120(10):2013–2022. [DOI] [PubMed] [Google Scholar]
- 4.Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology 2016;123(11):2376–2385. [DOI] [PubMed] [Google Scholar]
- 5.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016;123(6):1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu K, Chin EK, Bennett SR, et al. Endophthalmitis after intravitreal injection of vascular endothelial growth factor inhibitors: management and visual outcomes. Ophthalmology 2018;125(8):1279–1286. [DOI] [PubMed] [Google Scholar]
- 7.Lally DR, Shah CP, Heier JS. Vascular endothelial growth factor and diabetic macular edema. Surv Ophthalmol 2016;61(6):759–768. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119(4):789–801. [DOI] [PubMed] [Google Scholar]
- 9.Boyer DS, Nguyen QD, Brown DM, et al. Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE Trials. Ophthalmology 2015;122(12):2504–2513. [DOI] [PubMed] [Google Scholar]
- 10.Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 2012;130(9):1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol 2011;152(4):686–694. [DOI] [PubMed] [Google Scholar]
- 12.Bressler NM, Beaulieu WT, Maguire MG, et al. Early response to antivascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in protocol T. Am J Ophthalmol 2018;195:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugel PU, Campbell JH, Kiss S, et al. Association between early anatomic response to antivascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of protocol in study data. Retina 2019;39(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez VH, Campbell J, Holekamp NM, et al. Early and long-term responses to antivascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol 2016;172:72–79. [DOI] [PubMed] [Google Scholar]
- 15.Shah AR, Yonekawa Y, Todorich B, et al. Prediction of anti-VEGF response in diabetic macular edema after 1 injection. J Vitreoretin Dis 2017;1(3):169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh Y, Vasanji A, Ehlers JP. Volumetric ellipsoid zone mapping for enhanced visualisation of outer retinal integrity with optical coherence tomography. Br J Ophthalmol 2016;100(3):295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Runkle AP, Kaiser PK, Srivastava SK, et al. OCT angiography and ellipsoid zone mapping of macular telangiectasia type 2 from the AVATAR study. Invest Ophthalmol Vis Sci 2017;58(9):3683–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uji A, Murakami T, Nishijima K, et al. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol 2012;153(4):710–717. [DOI] [PubMed] [Google Scholar]
- 19.Forooghian F, Stetson PF, Meyer SA, et al. Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina 2010;30(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alasil T, Keane PA, Updike JF, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology 2010;117(12):2379–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maheshwary AS, Oster SF, Yuson RM, et al. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol 2010;150(1):63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browning DJ, Glassman AR, Aiello LP, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007;114(3):525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Channa R, Sophie R, Khwaja AA, et al. Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye (Lond) 2014;28(3):269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otani T, Yamaguchi Y, Kishi S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina 2010;30(5):774–780. [DOI] [PubMed] [Google Scholar]
- 25.Radwan SH, Soliman AZ, Tokarev J, et al. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol 2015;133(7):820–825. [DOI] [PubMed] [Google Scholar]
- 26.Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol 2014;132(11):1309–1316. [DOI] [PubMed] [Google Scholar]
- 27.Das R, Spence G, Hogg RE, et al. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol 2018;136(2):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehlers JP, Uchida A, Hu M, et al. Higher-order assessment of OCT in diabetic macular edema from the VISTA study: ellipsoid zone dynamics and the retinal fluid index. Ophthalmology Retina 2019;3:1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos AR, Alves D, Santos T, et al. Measurements of retinal fluid by OCT leakage in diabetic macular edema: a biomarker of visual acuity response to treatment. Retina 2019;39(1):52–60. [DOI] [PubMed] [Google Scholar]
- 30.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014;121(11):2247–2254. [DOI] [PubMed] [Google Scholar]
- 31.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122(10):2044–2052. [DOI] [PubMed] [Google Scholar]
- 32.Arepalli S, Traboulsi EI, Ehlers JP. Ellipsoid zone mapping and outer retinal assessment in stargardt disease. Retina 2018;38:1427–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida A, Pillai JA, Bermel R, et al. Outer retinal assessment using spectral domain optical coherence tomography in patients with Alzheimer’s and parkinson’s disease. Invest Ophthalmol Vis Sci 2018;59(6):2768–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forooghian F, Kertes PJ, Eng KT, et al. Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Invest Ophthalmol Vis Sci 2010;51(5):2388–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roh MI, Kim HS, Song JH, et al. Effect of intravitreal bevacizumab injection on aqueous humor cytokine levels in clinically significant macular edema. Ophthalmology 2009;116(1):80–86. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe D, Suzuma K, Suzuma I, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 2005;139(3):476–481. [DOI] [PubMed] [Google Scholar]
- 37.Kwon JW, Jee D. Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PLoS One 2018;13(9):e0203408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh IK, Kim SW, Oh J, et al. Inflammatory and angiogenic factors in the aqueous humor and the relationship to diabetic retinopathy. Curr Eye Res 2010;35(12):1116–1127. [DOI] [PubMed] [Google Scholar]
- 39.Ghasemi H, Ghazanfari T, Yaraee R, et al. Roles of IL-8 in ocular inflammations: a review. Ocul Immunol Inflamm 2011;19(6):401–412. [DOI] [PubMed] [Google Scholar]
- 40.Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology 2015;122(7):1395–1401. [DOI] [PubMed] [Google Scholar]
- 41.Bolz M, Ritter M, Schneider M, et al. A systematic correlation of angiography and high-resolution optical coherence tomography in diabetic macular edema. Ophthalmology 2009;116(1):66–72. [DOI] [PubMed] [Google Scholar]
- 42.Yanoff M, Fine BS, Brucker AJ, Eagle RC Jr. Pathology of human cystoid macular edema. Surv Ophthalmol 1984;28(Suppl):505–511. [DOI] [PubMed] [Google Scholar]
- 43.Hariri A, Lee SY, Ruiz-Garcia H, et al. Effect of angle of incidence on macular thickness and volume measurements obtained by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53(9):5287–5291. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt JM, Xiang SH, Yung KM. Speckle in optical coherence tomography. J Biomed Opt 1999;4(1):95–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


