Highlights
-
•
Six (0.93%) rodents carried antibodies against Yersinia pestis fraction 1 antigens.
-
•
There is evidence of Y. pestis circulation in small mammals in Morogoro.
-
•
Plague continues to persist in small mammals in Mbulu.
-
•
There is ongoing quiescence in Lushoto and Iringa plague foci.
Keywords: Plague, Yersinia pestis, ELISA, Small mammals, Rodents, Tanzania
Abstract
Objectives
Plague has been a threat to human health in Tanzania since 1886. This zoonotic disease has established several endemic foci in the country, posing a risk of outbreaks. This study was conducted to investigate the presence of Yersinia pestis in small mammals in five districts. These districts were selected because of recent (Mbulu), past (40-18 years ago: Lushoto) and historic (>100 years ago: Iringa and Kilolo) human cases of plague. In addition, one region that has not had any reported human cases of plague was included (Morogoro-Mvomero).
Methods
Blood from 645 captured small mammals was screened for antibodies against the fraction 1 (F1) antigen of Y. pestis using indirect enzyme-linked immunosorbent assay (ELISA) and competitive-blocking ELISA.
Results
Specific antibodies against Y. pestis F1 antigens were detected in six (0.93%) animals belonging to Mastomys natalensis. Of these, four animals were captured in the active focus in Mbulu, and two animals were captured from an area with no history of human plague (Morogoro-Mvomero).
Conclusion
These results provide evidence of the circulation of Y. pestis in small mammals in Tanzania. Furthermore, evidence of the circulation of Y. pestis in Morogoro-Mvomero highlights the importance of carrying out plague surveillance in areas with no history of human plague, which can help to predict areas where future outbreaks may occur.
Introduction
Plague is an ancient disease caused by the Gram-negative bacterium Yersinia pestis which is transmitted by flea vectors. It is primarily a disease of rodents, but secondarily affects humans resulting in large epidemics. Y. pestis is responsible for causing some of the world's worst pandemics, and its societal impacts have been compared with the ongoing coronavirus disease 2019 (COVID-19) pandemic (Glatter and Finkelman, 2021). In addition to being classified as a re-emerging infectious disease with biowarfare potential (Byard, 2020), plague is also ranked amongst emerging infectious diseases in Africa in the 21st Century (Fenollar and Mediannikov, 2018). Although eradicated in most countries, Tanzania is among the handful of countries in Africa where human plague still persists. For more than a century, wave after wave of plague swept through Tanzania wreaking havoc where natural foci exist in the country (Kilonzo and Mhina, 1982; Makundi et al., 2008; Spinage, 2012; Ziwa et al., 2013a). Of particular note are the recurrent plague epidemics that occurred between 1980 and 2011 in Lushoto (Kilonzo and Mhina, 1982), Mbulu (Makundi et al., 2008), Karatu (Kilonzo et al., 2006) and Singida (Temu, 1991), with nearly 9000 reported cases and over 700 deaths. Available evidence indicates that the disease persists in sylvatic enzootic cycles involving rodents and their fleas and environmental factors that are poorly understood, making it more difficult to eradicate (Kilonzo et al., 2005; Makundi et al., 2008).
The emergence and epidemic spread of plague in Tanzania has been linked to wild mammals, specifically rodents (Kilonzo et al., 2005), but despite more than a century of research, it remains unknown how Y. pestis persists in endemic foci between epidemics (Koch, 1898; Msangi, 1969; Ziwa et al., 2013b). Plague follows a pattern of seasonal outbreaks separated by periods of quiescence, giving the misleading impression that it has disappeared completely. Hence, its presence in small mammal populations often goes unnoticed until outbreaks occur in human populations, specifically in zoonotic foci. Given the public health significance of plague and the risk of its re-emergence, it is crucial to maintain constant epidemiological surveillance of small mammal populations in endemic foci in the country. Plague surveillance and monitoring is not only essential in identifying cases and epizootics as quickly as possible, but also ensures that steps can be taken to control and avoid spillover into human populations. Several natural foci of plague exist in the country, and persistence of these zoonotic foci increases the risk of re-emergence as people living in these areas are increasingly in contact with rodents and fleas.
The recent COVID-19 pandemic has sharpened focus on the importance of zoonotic disease surveillance in wild animals. In Tanzania, much of the current plague surveillance apparatus has developed in a somewhat ad-hoc way, in most cases only done when human cases are reported, thus indicating the gap in effectiveness and the need to improve this. In Mbulu, for instance, available data indicate few reports (Makundi et al., 2008; Haule, 2013; Ziwa et al., 2013b) of wildlife surveillance since the last major outbreak in 2007 that claimed six lives. Despite these reports pointing out the need for ongoing epidemiological surveillance in this area, minimal research has been undertaken to identify factors exacerbating outbreaks in this focus. This defeats the purpose of rodent and vector surveillance, as this should be performed continuously even during periods when no human cases are reported. The Dongobesh plague focus in Mbulu is the most recently established focus (Makundi et al., 2008), and is the only current active plague focus in the country, with sporadic cases of human plague still being reported from time to time. Thus, the insufficient surveillance efforts not only contrast with the recommendations of the World Health Organization (Dennis et al., 1999), but could have dire consequences. The Lushoto plague focus, on the other hand, has a well-documented record of surveillance data on wildlife reservoirs of the disease since the first outbreak in 1980 to 2015 (Kilonzo and Mhina, 1982; Njunwa et al., 1989; Laudisoit, 2009; Laudisoit et al., 2009; Lutege et al., 2015). However, there have been no reports on small mammal plague serosurveys in the last decade, suggesting that surveillance has been discontinued.
It is well known that plague can re-emerge even after decades of silence. Among the most conspicuous examples is the case of Oran, Algeria where plague disappeared for five decades (Bertherat et al., 2007). The most ancient plague focus in Tanzania is Iringa, where plague was authenticated for the first time in 1886 (Koch, 1898). As no human cases of plague have been reported in Iringa for a number of decades, this focus is poorly monitored, or not monitored at all, to establish whether plague has been eradicated completely or whether it is still circulating in reservoir hosts and flea vectors.
Indeed, the establishment of plague foci in Tanzania has been based on outbreaks of the disease among human populations, rather than on substantiation of the disease among natural reservoirs in the particular area (Kilonzo et al., 2005). Most small mammal surveillance for plague has been restricted to endemic foci. In order to address the historic bias of surveillance efforts, which have inadequately covered areas without any reported human cases of plague, the authors undertook a small mammal plague serosurvey in an area (Morogoro-Mvomero) with no history of human plague. This is essential to identify potential plague foci, and to predict areas where the threat of new emergence lurks and understand the distribution of the disease in the country. In addition, the authors conducted a small mammal plague serosurvey in the most ancient foci in the country (Iringa and Kilolo), an 18-year quiescent plague focus (Lushoto), and a currently active plague focus (Mbulu) to determine the presence and persistence of the disease in these areas, and to better understand the distribution of Y. pestis in Tanzania. The data primarily focus on rodents and shrews, as they are the main sentinels of Y. pestis transmission in the country.
Materials and methods
Study areas
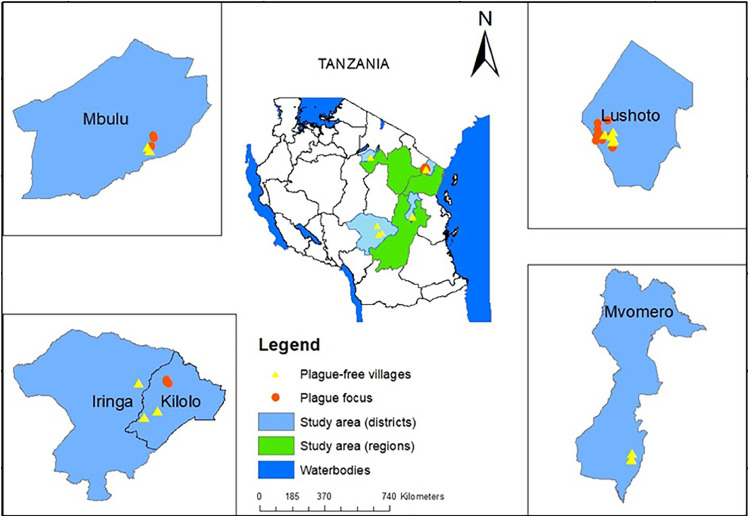
This study was conducted during the hot and rainy season (November–April) and during the dry season (May–October) from 2020 to 2021. The study sites were divided into three categories: active plague foci, quiescent plague foci and plague-free area. The plague-free area category constituted an area (Mgeta division in Mvomero district) in Morogoro region where there has been no record of human plague. Mgeta was chosen because of its similar climatic and ecological conditions to the plague-endemic foci in Tanzania. Quiescent foci comprised areas where human plague has not resurged for years, ranging from 18 years (Lushoto district) to 135 years (Iringa rural and Kilolo districts) since the last recorded outbreaks. In the active focus (Mbulu), sporadic cases of human plague are still being reported. Three different ecotopes – domestic (houses) peridomestic (crop fields) and sylvatic (forest biome) – were explored. In Tanzania, plague outbreaks occur in some villages but not in others in the same vicinity with similar environmental and climatic factors and the same composition of rodent and flea species (Laudisoit, 2009). To this end, villages with a history of human plague and villages with no known history of human plague (plague-free) were sampled in Lushoto, Mbulu and Kilolo districts. Sampling sites are shown in Figure 1.
Figure 1.
Localities of the small mammals surveyed for plague in this study. The black lines in the central Tanzania map delineate regions. The green shaded areas are the four regions where samples were collected, and the light blue areas show the districts. The regions and districts are as follows: Manyara region (Mbulu district), Iringa region (Iringa district and Kilolo district), and Morogoro (Mvomero district). The four inserts show the location (within the districts) of the plague foci and the non-plague villages sampled.
Animal trapping, handling and detection of Y. pestis infection
Sylvatic and commensal rodents were live trapped using Sherman traps (8 × 9 × 23 cm, H.B. ShermanTraps Inc., Tallahassee, FL, USA) and locally made box traps baited with peanut butter and maize meal. The Sherman traps were used in forests and crop fields, while the box traps were used to trap in houses. Traps were inspected in the morning, and captures were removed and taken to a central field processing location. Live captured animals were anaesthetized with diethyl ether, and each animal was bled from the heart using a disposable syringe and needle or from the orbital vein using capillary tubes and capped microtubes; serum separation was done thereafter. The separated serum was collected into sterile vials and preserved at −20°C until use.
Detection of anti-fraction 1 (F1) immunoglobulin G was conducted by indirect enzyme-linked immunosorbent assay (ELISA), as described previously (Dromigny et al., 1998) with modifications. The mean optical density (OD) obtained against the coating buffer alone was subtracted from the OD against F1 antigen (delta OD). In each plate, negative (n=3) and positive sera from wild rodents were included as controls, and sera were scattered at random on the ELISA plates. For result interpretation, the ratio system was used, calculated as the ratio of the delta OD of the sample (OD of plate with serum sample – OD of plate with buffer alone) to the mean delta OD of three negative sera + standard deviations. Samples with OD >0.100 were considered positive, but for Rattus and Mus spp., OD >0.150 was considered positive. The OD thresholds were determined according to the best specificity and sensitivity (Youden's index) from the receiver operating characteristic curve, and the conjugate used (rat or mouse) for each small mammal species, as described by Dromigny et al. (1998).
Samples that reacted positively to the indirect ELISA were subjected to the competitive blocking ELISA to determine the specificity of the antibodies detected, as described by Chu (2000). The protocol involves inhibiting the antibody present in each positive sample with a diluted F1 antigen. The specificity of the reaction is demonstrated by a decrease in OD value according to the amount of F1 antigen added. In contrast, the reaction is considered to be non-specific if the OD value remains the same regardless of the quantity of F1 antigen added prior to the test.
Results
In total, 645 small mammals belonging to 20 species were captured from five districts (Iringa rural, Kilolo, Lushoto, Mbulu and Mvomero) in Tanzania. The small mammals captured included Mastomys natalensis, Praomys delectorum, Lophuromys machangui, Lophuromys makundii, Lophuromys kilonzoi, Grammomy surdaster, Grammomys macmillani, Crocidura hirta, Aethomys kaiseri, Aethomys chrysophilus, Mus minutoides, Mus gratus, Dasymys incomptus, Rattus rattus, Lemniscomys striatus, Lemniscomys zebra, Graphiurus raptor, Arvicanthis neumanni, Arvicanthis nairobae and Steatomys pratensis (Table 1). M. natalensis comprised 50% of the total captures. Of the 645 small mammals, 43 (6.7%) were positive for antibodies to F1 antigen of Y. pestis using indirect ELISA. These 43 samples were further subjected to competitive blocking ELISA to confirm the specificity of the detected antibodies. Only six of the 43 seropositive samples (14.0%) were confirmed to be carrying specific anti-F1 Y. pestis antibodies (Table 2), representing 0.93% of the entire population of small mammals captured in this study. All six samples belonged to M. natalensis, of which four were collected from Mbulu [4/194 (2.1%)] and two were collected from Mgeta-Mvomero [2/71 (2.8%)]. Antibody titres ranged from 1:100 to 1:6400. All confirmed seropositive samples were collected during the rainy season (October–April).
Table 1.
Indirect enzyme-linked immunosorbent assay results for detection of antibodies against the fraction 1 antigen of Yersinia pestis in the sera of small mammals
| Species | Number of animals tested for antibodies and % of positive samples in each district |
||||
|---|---|---|---|---|---|
| Mbulu | Lushoto | Iringa and Kilolo | Mvomero | Total | |
| Praomys delectorum | 59 (1.7) | 17 (5.9) | 0 | 3 | 79 |
| Rattus rattus | 19 | 43 | 1 | 11 | 74 |
| Mastomys natalensis | 61 (16.4) | 157 (3.2) | 72 (13.9) | 33 (18.2) | 323 |
| Lophuromys Kilonzoi | 0 | 43 | 0 | 15 | 58 |
| Lophuromys makundii | 24 (4.2) | 0 | 0 | 0 | 24 |
| Lophuromys machangui | 0 | 0 | 1 | 0 | 1 |
| Grammomys surdaster | 0 | 17 (5.9) | 1 | 0 | 18 |
| Grammomys macmillani | 3 | 0 | 0 | 0 | 3 |
| Crocidura hirta | 1 | 5 | 1 | 5 (60) | 12 |
| Steatomys pratensis | 0 | 0 | 1 | 0 | 1 |
| Graphiurus raptor | 2 | 0 | 2 | 0 | 4 |
| Aethomys chrysophilus | 0 | 0 | 7 | 0 | 7 |
| Aethomys kaiseri | 19 (21.1) | 0 | 0 | 0 | 19 |
| Dasymys incomptus | 0 | 0 | 0 | 2 | 2 |
| Lemniscomys striatus | 3 | 0 | 2 | 0 | 5 |
| Lemniscomys zebra | 2 | 0 | 0 | 0 | 2 |
| Mus minutoides | 0 | 0 | 5 (20.0) | 2 | 7 |
| Mus gratus | 1 | 0 | 0 | 0 | 1 |
| Arvicanthis neumanni | 0 | 0 | 4 | 0 | 4 |
| Arvicanthis nairobae | 0 | 1 | 0 | 0 | 1 |
| Total | 194 (7.7) | 283 (2.5) | 97 (11.3) | 71 (12.7) | 645 |
Table 2.
Competitive blocking enzyme-linked immunosorbent assay (cELISA) results of all seropositive indirect ELISA samples
| Animal species | Number tested | Number of cELISA positive | 95% CIa |
|---|---|---|---|
| Praomys delectorum | 2 | 0 | |
| Rattus rattus | 0 | 0 | |
| Mastomys natalensis | 31 | 6 (19.3%) | 0.07–0.37 |
| Lophuromys Kilonzoi | 0 | 0 | |
| Lophuromys makundii | 1 | 0 | |
| Lophuromys machangui | 0 | 0 | |
| Grammomys surdaster | 1 | 0 | |
| Grammomys macmillani | 0 | 0 | |
| Crocidura hirta | 3 | 0 | |
| Steatomys pratensis | 0 | 0 | |
| Graphiurus raptor | 0 | 0 | |
| Aethomys chrysophilus | 0 | 0 | |
| Aethomys kaiseri | 4 | 0 | |
| Dasymys incomptus | 0 | 0 | |
| Lemniscomys striatus | 0 | 0 | |
| Lemniscomys zebra | 0 | 0 | |
| Mus minutoides | 0 | 0 | |
| Mus gratus | 1 | 0 | |
| Arvicanthis neumanni | 0 | 0 | |
| Arvicanthis nairobae | 0 | 0 | |
| Total | 43 | 6 (14%) |
CI, confidence interval.
Clopper–Pearson exact CI method was used.
Discussion
Based on the ELISA results, it is clear that plague is far from eradicated in Tanzania, hence supporting the long-standing hypothesis that plague circulates in wildlife reservoirs during interepizootic periods (Fedorov, 1944; Quan and Kartman, 1962). The presence of specific antibodies in rodent sera against the Y. pestis F1 antigen in 0.93% of the samples suggests current or recent exposure to Y. pestis. Rodents carrying antibodies for Y. pestis appeared healthy and showed no observable clinical manifestation of plague. This observation supports the hypothesis of the presence of an enzootic cycle involving relatively resistant host populations and flea vectors. Similar observations have been made in Mbulu and Lushoto (Kilonzo et al., 2005; Makundi et al., 2008; Ziwa et al., 2013b). According to the enzootic host model, susceptible hosts are decimated during an epizootic, while resistant hosts survive and develop antibodies to plague (Graham et al., 2014). It is indeed resistant reservoir hosts and their fleas that maintain plague circulation in endemic foci between epizootics (Quan and Kartman, 1962). Experimental infections have shown that M. natalensis is resistant to plague infections (Isaäcson et al., 1983; Shepherd et al., 1986). Rattus norvegicus and R. rattus have also been found to be resistant to plague infections (Rahalison et al., 2003; Andrianaivoarimanana et al., 2018), although none of the R. rattus in the current study showed evidence of Y. pestis infection.
All rodent sera that tested positive for specific anti-F1 plague antibodies to Y. pestis F1 antigen were collected during the hot rainy season (October–April), which is consistent with previous observations that plague is indeed a seasonal disease in Tanzania (Njunwa et al., 1989; Kilonzo et al., 1992; Makundi and Kilonzo, 1994; Davis et al., 2006). Rodent abundance coupled with species richness and high flea index are important predictors for plague outbreaks in endemic foci (Samia et al., 2011; Sun et al., 2019). However, outbreaks can still happen when there is low abundance of rodents (Davis et al., 2004). The captures in the peridomestic biotopes were dominated by M. natalensis, whereas the forest biotope was dominated by Lophuromys spp. (L. kilonzoi, L. makundii and L. machangui) and P. delectorum.
None of the rodent sera samples collected from the most ancient plague focus (Iringa) in Tanzania were confirmed to be positive for plague. One of the areas sampled was Image ward (Iringa region), where the disease was first authenticated in Tanzania in 1886. These findings raise the important question of whether this is a case of inadequate sampling or did the disease disappear completely from the area? As no follow-up studies or wildlife surveillance have been carried out in this particular focus since the disease was authenticated, it is difficult to ascertain which scenario is correct, although the latter is more probable. However, the probability of the disease resurfacing even after a century of quiescence cannot be ruled out. Moreover, there are no documented records of the reservoirs or species involved in the 1886 outbreak.
Contrary to Iringa, Lushoto is the most extensively studied focus with a well-documented history of plague. With no reported human cases since 2004, Lushoto is no exception to areas where the possibility of re-emergence still lurks. In the current study, no rodent sera from this quiescent focus tested positive for plague infection. These findings are consistent with previous observations reported by Laudisoit (2009) in a study carried out in Lushoto between 2005 and 2008. This particular focus was the hardest hit by plague, accounting for 95% of plague deaths between 1980 and 2011 (Ziwa et al., 2013a). The dynamics of plague in Lushoto have been researched extensively but are still not well understood. Monitoring and surveillance of plague reservoirs and their fleas has been well documented since the first outbreak (Kilonzo and Mhina, 1982; Njunwa et al., 1989). Moreover, factors affecting the persistence of plague in the area have also been identified (Njunwa et al., 1989; Makundi and Kilonzo, 1994; Davis et al., 2006; Kamugisha et al., 2007; Hieronimo et al., 2014). Perhaps the apparent disappearance of plague in this focus can be attributed to housing improvements (i.e. replacement of thatched roofs with iron sheets), coupled with effective control strategies. Control strategies for fleas and rodents include flea killing, chemotherapy, chemoprophylaxis, sanitary improvement and health education (Kilonzo et al., 1992). Furthermore, a permanent plague control team, formed in 1987, ensured that control measures were implemented effectively and cases were reported and treated early, which reduced deaths in 1988 (Kilonzo et al., 1992). In an effort to understand the factors responsible for the persistence of plague in the Lushoto focus, it was observed that plague is associated with high altitudes (Neerinckx, 2006). This was further supported by Kamugisha et al. (2007) and Laudisoit (2009). Villages located at high altitudes (i.e. Magamba, Gologolo, Kiranga, Nywelo and Manolo) were included in the current study, although no samples from these villages were confirmed to be positive; however, this does not mean that surveillance should be discontinued. Complacency regarding small mammal and flea surveillance may breed significant consequences, as was the case in Madagascar (Duplantier et al., 2005). Despite the progress in research in Lushoto, some limitations still exist. For example, no studies have investigated the relationship between the genetic structure of reservoir hosts and plague, which could shed more light on plague circulation and maintenance in the area.
The findings of this study indicate that four of the rodents that were Y. pestis seropositive were captured in the villages of Arri and Mongahay in Mbulu. The potential host M. natalensis has been implicated previously in Mbulu (Makundi et al., 2008; Ziwa et al., 2013b). It was demonstrated by Williams and Cavanaugh (1979) that an antibody titre of 1:128 confers protection in rats against Y. pestis. In the current study, an antibody titre ranging from 1:100 to 1:6400 was observed, which could suggest some level of immunity to the infection. The Dongobesh focus in Mbulu is the most recent plague foci in Tanzania, and although it is still active, it is poorly monitored. The first record of human plague in Mbulu was in 1917, although it is speculated that the disease was prevalent in the area from 1904 (Kilonzo and Mtoi, 1983). Since then, numerous outbreaks have occurred in the area; the most recent was an outbreak in 2007, which occurred after 30 years of quiescence (Makundi et al., 2008).
An interesting phenomenon observed in plague foci in Mbulu and Lushoto is that plague outbreaks occur in some villages and not others in the same vicinity with similar environmental and climatic factors, and the same composition of rodent and flea species (Laudisoit, 2009). In the current study, one of the Y. pestis seropositive samples was collected from Mongahay, a village where no human cases of plague have been reported even though this village is <3 km from Endesh village where sporadic human cases of plague have been being reported to date. This suggests that plague is circulating in the area among the enzootic wildlife reservoirs, even though no human cases have been reported to date in Mongahay. The study undertaken in Lushoto found no significant difference in host density and flea abundance or diversity between plague-endemic and plague-free villages in Lushoto (Laudisoit, 2009). However, it is worth noting that the plague status (plague-free or plague-endemic) in Lushoto and Mbulu was dictated by human plague records rather than data from plague wildlife reservoirs.
Interestingly, two of the sera samples that tested positive for specific anti-F1 Y. pestis antibodies were collected from Morogoro (Mgeta division), a region with no history of plague. This suggests that Mgeta is a potential plague focus, although no human cases of plague have been reported in the area to date. Positive samples from Mgeta were collected from Langali village. It is probable that the rodents contracted the disease locally, so the existence of an endemic focus in Mgeta division cannot be ruled out. A small mammal serosurvey carried out in other areas (Monduli, Chunya and Masasi district) in Tanzania with no records of human plague showed that the distribution of the disease goes beyond the borders of the known foci (Kilonzo et al., 2005). It remains unknown whether these cases were imported from other districts or there are endemic foci in these districts. It is worth noting that the potential reservoirs of plague in Tanzania have been identified based on their seropositivity status. However, they may not be the true reservoirs. Since the true reservoirs of plague are yet to be identified, it is plausible that other wildlife species could contribute to the maintenance of plague in Tanzania. It is therefore worth noting that the danger of plague outbreaks is not limited to the known natural foci.
Conclusion
These results show that plague remains a threat to human health in Tanzania. To better understand the dynamics of sylvatic plague in Tanzania, surveillance should be undertaken continuously, and not only in endemic foci but also in areas with no historical record of human plague. This is essential in identifying new foci, thus improving the predictions of new outbreaks and enabling early warnings. Further, this study found ongoing quiescence in Iringa and Lushoto; however, it should be noted that long-dormant foci can re-emerge. Although this study shed more light on the potential reservoirs of plague, this analysis alone cannot explain the maintenance of plague in any of the foci studied; therefore, experimental infections are recommended to evaluate the sensitivity of these hosts to plague infection.
Acknowledgments
Conflict of interest statement
None declared.
Funding
This work was funded by the Africa Centre of Excellence for Innovative Rodent Pest Management and Biosensor Technology and Development at Sokoine University of Agriculture.
Ethical approval
This study was approved by the Institutional Review Board of Sokoine University of Agriculture, Tanzania (Ref. No. SUA/DPRTC/186/17).
References
- Andrianaivoarimanana V, Rajerison M, Jambou R. Exposure to Yersinia pestis increases resistance to plague in black rats and modulates transmission in Madagascar. BMC Res Notes. 2018;11:e898. doi: 10.1186/s13104-018-3984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertherat E, Bekhoucha S, Chougrani S, Razik F, Duchemin JB, Houti L, et al. Plague reappearance in Algeria after 50 years. Emerg Infect Dis. 2007;13:1459–1462. doi: 10.3201/eid1310.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byard RW. A forensic evaluation of plague – a re-emerging infectious disease with biowarfare potential. Med Sci Law. 2020;60:200–205. doi: 10.1177/0025802420908483. [DOI] [PubMed] [Google Scholar]
- Chu MC. Laboratory manual of plague diagnostic tests. Colorado: World Health Organization; 2000 [Google Scholar]
- Davis S, Begon M, De Bruyn L, Ageyev VS, Klassovskiy NL, Pole SB, et al. Predictive thresholds for plague in Kazakhstan. Science. 2004;304:736–738. doi: 10.1126/science.1095854. [DOI] [PubMed] [Google Scholar]
- Davis S, Makundi RH, Machang'u RS, Leirs H. Demographic and spatio-temporal variation in human plague at a persistent focus in Tanzania. Acta Trop. 2006;100:133–141. doi: 10.1016/j.actatropica.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Dennis DT, Dennis KL, Norman G, Poland, JD, Tikhomirov E. Plague manual: epidemiology, distribution, surveillance and control. Geneva: World Health Organization; 1999.
- Dromigny J, Ralafiarisoa L, Raharimanana C, Randriananja N, Chanteau S. La sérologie anti-F1 chez la souris OF1, test complémentaire pour le diagnostic de la peste humaine. Archives l'Institut Pasteur de Madagascar. 1998;64:18–20. [Google Scholar]
- Duplantier J-M, Duchemin J-B, Chanteau S, Carniel E. From the recent lessons of the Malagasy foci towards a global understanding of the factors involved in plague reemergence. Vet Res. 2005;36:437–453. doi: 10.1051/vetres:2005007. [DOI] [PubMed] [Google Scholar]
- Fedorov VN. On mechanism of plague microbe preservation in non-epizootic years. Vestnik mikrob epidem i parazitol 1944;27–39.
- Fenollar F, Mediannikov O. Emerging infectious diseases in Africa in the 21st century. New Microbes New Infect. 2018;26:e10–e18. doi: 10.1016/j.nmni.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatter KA, Finkelman P. History of the plague: an ancient pandemic for the age of COVID-19. Am J Med. 2021;134:176–181. doi: 10.1016/j.amjmed.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CB, Woods ME, Vetter SM, Petersen JM, Montenieri JA, Holmes JL, et al. Evaluation of the effect of host immune status on short-term Yersinia pestis infection in fleas with implications for the enzootic host model for maintenance of Y. pestis during interepizootic periods. J Med Entomol. 2014;51:1079–1086. doi: 10.1603/me14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haule M. Investigation of fleas as vectors in the transmission of plague during a quiescent period in North-Eastern Tanzania. J Entomol Nematol. 2013;5:88–93. [Google Scholar]
- Hieronimo P, Kimaro DN, Kihupi NI, Gulinck H, Mulungu LS, Msanya BM, et al. Land use determinants of small mammals’ abundance and distribution in a plague endemic area of Lushoto district. Tanzania. Tanzan J Health Res. 2014;16:1–12. doi: 10.4314/thrb.v16i3.8. [DOI] [PubMed] [Google Scholar]
- Isaäcson M, Taylor P, Arntzen L. Ecology of plague in Africa: response of indigenous wild rodents to experimental plague infection. Bull World Health Organ. 1983;61:339–344. [PMC free article] [PubMed] [Google Scholar]
- Kamugisha ML, Gesase S, Minja D, Mgema S, Mlwilo TD, Mayala BK. Pattern and spatial distribution of plague in Lushoto, north-eastern Tanzania. Tanzan J Health Res. 2007;9:12–18. doi: 10.4314/thrb.v9i1.14286. [DOI] [PubMed] [Google Scholar]
- Kilonzo B, Mbise T, Mwalimu D, Kindamba L. Observations on the endemicity of plague in Karatu and Ngorongoro, northern Tanzania. Tanzan J Health Res. 2006;8:1–6. doi: 10.4314/thrb.v8i1.14262. [DOI] [PubMed] [Google Scholar]
- Kilonzo B, Mhina J, Sabuni C, Mgode G. The role of rodents and small carnivores in plague endemicity in Tanzania. Belg J Zool. 2005;135:119–125. [Google Scholar]
- Kilonzo BS, Mbise TJ, Makundi RH. Plague in Lushoto district, Tanzania, 1980–1988. Trans R Soc Trop Med Hyg. 1992;86:444–445. doi: 10.1016/0035-9203(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Kilonzo BS, Mhina JI. The first outbreak of human plague in Lushoto district, north-east Tanzania. Trans R Soc Trop Med Hyg. 1982;76:172–177. doi: 10.1016/0035-9203(82)90269-3. [DOI] [PubMed] [Google Scholar]
- Kilonzo BS, Mtoi RS. Entomological, bacteriological and serological observations after the 1977 plague outbreak in Mbulu District. Tanzania. East Afr Med J. 1983;60:91–97. [PubMed] [Google Scholar]
- Koch R. Julius Springer; Schwarzwasserfieber, German. Berlin: 1898. Reise-Berichte über Rinderpest, Bubonenpest in Indien und Afrika, Tsetse- oder Surrakrankheit, Texasfieber, tropische Malaria. [Google Scholar]
- Laudisoit A. University of Liège and Antwerpen; Antwerpen: 2009. Diversity, ecology and status of potential hosts and vectors of the plague bacillus Yersinia pestis. Contribution to plague epidemiology in an endemic plague focus: the Lushoto district (Tanzania) [Google Scholar]
- Laudisoit A, Neerinckx S, Makundi RH, Leirs H, Krasnov BR. Are local plague endemicity and ecological characteristics of vectors and reservoirs related? A case study in north-east Tanzania. Curr Zool. 2009;55:200–211. [Google Scholar]
- Lutege BM, Hang'ombe BM, Kilonzo BS. Identification of plague hosts and vectors in Lushoto district of Tanzania. Int J Sci. 2015;24:145–152. [Google Scholar]
- Makundi RH, Kilonzo BS. Seasonal dynamics of rodent fleas and its implication on control strategies in Lushoto district, north-eastern Tanzania. J Appl Entomol. 1994;118:165–171. [Google Scholar]
- Makundi RH, Massawe AW, Mulungu LS, Katakweba A, Mbise TJ, Mgode G. Potential mammalian reservoirs in a bubonic plague outbreak focus in Mbulu district, northern Tanzania, in 2007. Mammalia. 2008;72:253–257. [Google Scholar]
- Msangi AS. Entomological observations after the 1968 plague outbreak in Mbulu district. Tanzania. East Afr Med J. 1969;46:465–470. [PubMed] [Google Scholar]
- Neerinckx S. Catholic University of Leuven; Lushoto, Tanzania. Leuven: 2006. Ecological factors influencing the spread of plague disease. [Google Scholar]
- Njunwa KJ, Mwaiko GL, Kilonzo BS, Mhina JIK. Seasonal patterns of rodents, fleas and plague status in the Western Usambara Mountains. Tanzania. Med Vet Entomol. 1989;3:17–22. doi: 10.1111/j.1365-2915.1989.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Quan SF, Kartman L. Ecologic studies of wild rodent plague in the San Francisco Bay area of California: VIII. Zoonoses Res. 1962;1:121–144. [PubMed] [Google Scholar]
- Rahalison L, Ranjalahy M, Duplantier J-M, Duchemin J.B, Ravelosaona J, Ratsifasoamanana L, et al. Susceptibility to plague of the rodents in Antananarivo, Madagascar. Adv Exp Med Biol. 2003;529:439–442. doi: 10.1007/0-306-48416-1_87. [DOI] [PubMed] [Google Scholar]
- Samia NI, Kausrud KL, Heesterbeek H, Ageyev V, Begon M, Chan K-S, et al. Dynamics of the plague-wildlife-human system in Central Asia are controlled by two epidemiological thresholds. Proc Natl Acad Sci. 2011;108:14527–14532. doi: 10.1073/pnas.1015946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Leman PA, Hummitzsch DE. Experimental plague infection in South African wild rodents. J Hyg. 1986;96:171–183. doi: 10.1017/s0022172400065943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinage CA. Springer Berlin Heidelberg; Berlin: 2012. African ecology. [Google Scholar]
- Sun Z, Xu L, Schmid BV, Dean KR, Zhang Z, Xie Y, Fang X, et al. Human plague system associated with rodent diversity and other environmental factors. R Soc Open Sci. 2019;6 doi: 10.1098/rsos.190216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temu GP. Plague situation in Singida. Unpublished document submitted to the Ministry of Health, Dar es Salaam. No. Ref. HED/110/18, 27 March 1991.
- Williams JE, Cavanaugh DC. Measuring the efficacy of vaccination in affording protection against plague. Bull World Health Organ. 1979;57:309–313. [PMC free article] [PubMed] [Google Scholar]
- Ziwa MH, Matee MI, Hang'ombe BM, Lyamuya EF, Kilonzo BS. Plague in Tanzania: an overview. Tanzan J Health Res. 2013;15:252–258. [PubMed] [Google Scholar]
- Ziwa MH, Matee MI, Kilonzo BS, Hang'ombe BM. Evidence of Yersinia pestis DNA in rodents in plague outbreak foci in Mbulu and Karatu Districts, northern Tanzania. Tanzan J Health Res. 2013;15:152–157. doi: 10.4314/thrb.v15i3.1. [DOI] [PubMed] [Google Scholar]