Abstract
In severe COVID-19, the levels of iron (Fe), copper (Cu), zinc (Zn) and selenium (Se), do not only regulate host immune responses, but modify the viral genome, as well. While low serum Fe concentration is an independent risk factor for the increased death rate, Zn controls oxidative stress, synthesis of inflammatory cytokines and viral replication. Therefore, Zn deficiency associates with a worse prognosis. Although Cu exposure inactivates the viral genome and exhibits spike protein dispersal, increase in Cu/Zn due to high serum Cu levels, are correlated with enhanced risk of infections. Se levels are significantly higher in surviving COVID-19 patients. Meanwhile, both Zn and Se suppress the replication of SARS-CoV-2. Since the balance between the deficiency and oversupply of these metals due to a reciprocal relationship, has decisive effect on the prognosis of the SARS-CoV-2 infection, monitoring their concentrations may facilitate improved outcomes for patients suffering from COVID-19.
Keywords: SARS-CoV-2, Iron, Zinc, Copper, Selenium, Oxidative stress
1. Introduction
Bioinformatics has estimated about 50% of all enzymes depend on a metal ion for catalysis. The body concentrations of metal ions are controlled by their affinities for their ligands. Their levels in daily diet are important in determining the success of the immune response against invading pathogens due to their high reactivity and catalytic potential (Maret, 2016). Metalloproteins can be implicated for the attachment of the virus to the host and the severity of viral infections (Chasapis, 2018). In fact, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome consists of 10 open reading frames (ORF), and recently, mutations in the nt28144 of ORF8, nucleocapsid (N) region of SARS-CoV-2 were observed (Wang et al., 2020). Most of the metalloproteins interact with the accessory protein SARS-CoV-2 ORF8, which is a region with high variability leading to the structural alterations, closely associated with the virus's ability to invade and spread. The remaining small number of metalloproteins interact with the SARS-CoV-2 membrane (M), SARS-CoV-2 nonstructural protein 12 (nsp12) and nsp13 (Chasapis et al., 2021). It is thought that the alteration of trace metal metabolism, which have prominent role in innate immunity, may have considerable effects on SARS-CoV-2 immune surveillance mechanisms and the viral spread in patient with coronavirus disease 2019 (COVID-19). Zinc (Zn) (Kumar et al., 2020), iron (Fe) (Liu et al., 2020), and copper (Cu) (Andreou et al., 2020) are the most prevalent metals that bind to proteins linked with viral infections, whereas the maintenance of selenium (Se) levels has vital importance in combating SARS-CoV-2 virulence for the survival of COVID-19 patients (Moghaddam et al., 2020, Seale et al., 2020). The trace metals mentioned above are mostly responsible for modulating the inflammatory reaction and cytokine production in COVID-19 patients. The proinflammatory environment that triggers the cytokine storm is strongly associated with oxidative stress and severe tissue damage. In this context, trace metal deficiencies appear to be responsible for the increased risk of death among COVID-19 patients (Akhtar et al., 2021, Delgado-Roche and Mesta, 2020). Therefore, four redox active trace metals that are thought to be prognostically important in COVID-19 patients are discussed.
2. Inflammatory response and cytokine storm in COVID-19
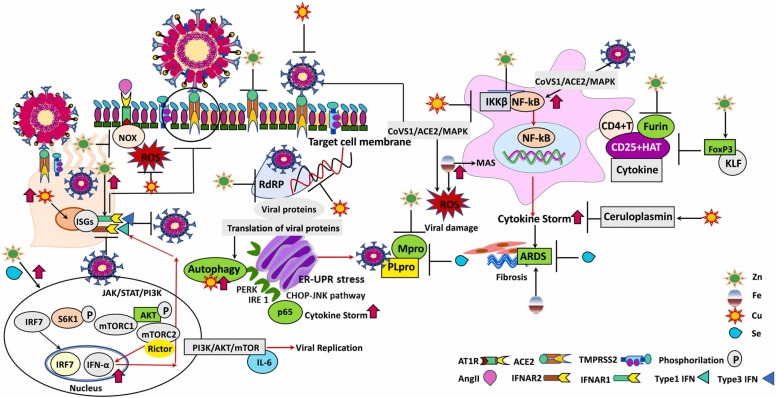
In COVID-19 patients, bronchial epithelial cells respond to viral infection by producing nuclear factor kappa B (NF-κB)-mediated cytokines as the first barrier in defense against the virus. Rising in plasma levels of several cytokines, interleukin-2 (IL-2), IL-6, IL-7, IL-10, tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein 1 (MCP-1), granulocyte-colony stimulating factor (G-CSF), interferon-gamma (IFN-γ)-induced protein 10 (IP-10) and increased macrophage inflammatory proteins are sign of a cytokine storm in severe COVID-19 patients, hospitalized in intensive care units (Y. Fu et al., 2020). Especially, IL-6 is a key player of the exacerbated inflammatory response in COVID-19 (G. Chen et al., 2020). Increased expression of the IL-6 receptor (IL6R) gene that is induced by IL-6, reflects the activation of IL-6 signaling pathway. While IL-6 is not detected in peripheral blood at the transcriptional level, TNF-α is up-regulated to a limited extend only. On the other hand, both circulating IL-6 protein and TNF-α protein are significantly increased (Hadjadj et al., 2020). Hyperinflammation, hyperpyrexia and organ failure that occur in the COVID-19 patients are the indicators of a poor prognosis and become life-threatening clinical manifestations that are associated with the severity of the infection (Y. Fu et al., 2020). It has been observed that the complex of immunoglobulin G (IgG) with SARS-CoV-2 spike (S) protein of COVID-19 patients triggers hyperinflammatory response in macrophages (Hoepel et al., 2020). This severe COVID-19 associated cytokine profile is described as macrophage activation syndrome (MAS). MAS is characterized by cytokine storm, ultimately multiple organ failure, and is evidence of an increased risk of mortality (C. Huang et al., 2020; Karakike and Giamarellos-Bourboulis, 2019; McGonagle et al., 2020; Mehta et al., 2020; P. Xu et al., 2020). At the late stage of the infection, by the time immune response augments and the adaptive response becomes dominant over the innate response, cytokine storm emerges due to the massive cytokine and chemokine release (Pal et al., 2021). Massive release of cytokines contributes to the acute respiratory distress syndrome (ARDS) and leads to vascular hyperpermeability, diffuse coagulopathy, multi-organ failure, and ultimately death (Nile et al., 2020, Ye et al., 2020). In COVID-19 patients, innate immune and infected tissue cells produce type-I IFNs, IFN-α and IFN-β enhancing leukocytes recruitment into the infection site. Consecutively, chemokines and other proinflammatory cytokines are released. Actually, the response to SARS-CoV-2 is not properly balanced in terms of keeping the virus replication under control versus activation of the adaptive immune response. In these patients, low amounts of type I and type III IFNs are simultaneously synthesized and released into the circulation with the high levels of chemokines and IL-6 expressions (Blanco-Melo et al., 2020). In COVID-19 patients, natural killer (NK) cell infiltration is increased by C-X-C motif chemokine receptor 3 (CXCR3)-ligand-producing monocytes in the lungs (Liao et al., 2020), whereas in the peripheral blood the number of cytotoxic NK cell is reduced (Wenjun et al., 2020, Zheng et al., 2020). In fact, the immune response is dependent on the availability of sufficient trace metals in the medium. However, the amount of T-helper 2 (Th2) cytokines is not affected from trace metal deficiency, whereas T-helper 1 (Th1) cytokines are decreased. Thus, shift of Th1 to Th2 function leads to the cell-mediated immune failure and decrease in IL-2 production that leads to diminished activities of NK cells and cytotoxic T cells (Prasad, 2007). Dramatic down-regulation of IFN-stimulated genes (ISGs) is observed in critical SARS-CoV-2 patients ( Fig. 1). Lack of IFN-β mRNA and absence of circulating IFN-β are characteristic features in all COVID-19 patients. Alteration of ISG and plasma levels of IFN-α2 display that the low type I IFN response precedes the clinical deterioration of patients to critical status (Hadjadj et al., 2020). High blood viral load in addition to the excessive NF-κB–driven inflammatory response-related elevated TNF-α and IL-6 with the lack of type I IFN response is a marked clinical presentation of severe and critical COVID-19 patients (Hadjadj et al., 2020). In these patients the severity of the disease is closely associated with a cytokine storm with strikingly elevated expression of IL-6 (Zhao, 2020). Interestingly, IL-6 can activate mammalian target of rapamycin (mTOR) (Pinno et al., 2016). Activation of the Akt/mTOR signaling and suppression of hypoxia-inducible factor 1-alpha (HIF-1α) following SARS-CoV-2 infection increases viral replication (Appelberg et al., 2020). In principle, SARS-CoV-2 evades immune detection by suppressing human immune responses. In this context, the presence of the imbalance between pro-inflammatory cytokine and IFN expression is an important checkpoint in SARS-CoV-2 infection (Engin et al., 2021).
Fig. 1.
SARS-CoV-2 binds to ACE2 receptors on the respiratory epithelium and infiltrating macrophages, leads to activation of the inducible transcription factor, NF-κB. Subsequently, it induces “cytokine storm” and eventually provokes ARDS. Labile iron in the cell that promotes MAS is characterized by cytokine storm and contributes to the formation of ROS. In PI3K-Akt-mTOR pathway nuclear translocation of IRF7 leads to transcriptional activation of type I interferon (IFN) genes. The key component of mTORC2, Rictor regulates IFN-α production. The produced IFN-α binds to IFN-α receptors (IFNAR1-IFNAR2) and provides immune protection against SARS-CoV-2. Zn, Cu and Se target multiple pathways to hamper the functional and structural consequences of inflammatory response caused by SARS-CoV-2 and inactivates the viral genomes of SARS-CoV-2 and exhibits irreversible effects on virus morphology. Thus, envelope disintegration and dispersal of spike protein occurs. Zinc and Se on the one hand suppress the life cycle of SARS-CoV-2 by inhibiting Mpro and PLpro of SARS-CoV-2, on the other hand strengthen the immune defense and counteract to the complications of SARS-CoV-2 infection (Abbreviations. ACE2: the cell receptor angiotensin-converting enzyme II; AKT: protein kinase B; AngII: angiotensin II; ARDS: acute respiratory distress syndrome; AT1R: angiotensin II receptor type 1; CHOP: CCAAT/enhancer-binding protein (C/EBP)-homologous protein; CoVS1: SARS-CoV-2 Spike protein; Cu: copper; ER: endoplasmic reticulum; Fe: iron; FoxP3: the transcription factor forkhead box P3; HAT: CD25 + hyperactivated T-cells; IFNAR: IFN α receptor; IFNα: (type-1 interferon) interferon alpha; IKKβ: IκB kinase β; IL-6: interleukin-6; IRE1: inositol-requiring enzyme 1; IRF7: interferon regulatory factor 7; ISG: interferon-stimulated gene; JAK: janus kinase; JNK: c-Jun N-terminal kinase; KLF: Krüppel-like transcription factor; MAPK: p38-mitogen-activated protein kinases; MAS: macrophage activation syndrome; Mpro: a key enzyme of SARS-CoV-2 and has a pivotal role in mediating viral replication and transcription; mTORC1: mammalian target of rapamycin complex 1; mTORC2: mammalian target of rapamycin complex 2; NFκB: nuclear factor-κB; NOX: nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; p: phosphorylation; PERK: protein kinase r-like endoplasmic reticulum kinase; PI3K: phosphoinositide 3-kinase; PLpro: papain-like protease of SARS-CoV-2; RdRp: RNA dependent RNA polymerase; Rictor: The rictor-mTOR complex directly phosphorylated AKT; ROS: reactive oxygen species; S6K1: s6 kinase 1; Se: selenium; STAT: signal transducers and activators of transcription; TMPRSS2: the serine protease of SARS-CoV receptor ACE2 in S protein priming for entry to target cell; UPR: unfolded protein response; Zn: zinc).
3. COVID-19 and oxidative stress
SARS-CoV-2-induced oxidative stress is a novel insight into the etiology and mechanism which are responsible for serious manifestations of COVID-19. COVID-19 patients with moderate and severe disease have decreased concentrations of glutathione, higher reactive oxygen species (ROS) levels, in addition to the greater redox status (ROS/reduced glutathione (GSH)) than the patients with mild illness. Endogenous GSH deficiency seems to be one of the most potent key determinants for augmenting the SARS-CoV-2-induced oxidative damage. Consequences of this redox imbalance leads to serious events, such as ARDS, multiorgan failure, and death in COVID-19 cases (Polonikov, 2020). In these patients, increased SARS-CoV-2 S protein-induced selective activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) (Fig. 1) decreases the levels of free NADPH that is essential to diminish the oxidized glutathione (GSSG) to GSH. Deficient amounts of GSH further increases the oxidative stress in the cell, which in turn facilitates S protein- angiotensin-converting enzyme 2 (ACE2) interactions and enhances the severity of infection. The affinity of SARS-CoV-2-S for the ACE2 receptor is increased by the oxidation of cysteine residues in both proteins. More viruses bind to receptors, and this interaction reduces the steady state levels of free ACE2 (Suhail et al., 2020, Youn et al., 2021). Increase in the concentration of angiotensin II (Ang II) and decrease in the concentration of angiotensin 1–7 (Ang 1–7) leads to the generation of oxidative stress and higher concomitant fatality rate (Engin et al., 2020). The decline in ACE2 bioavailability following SARS-CoV-2 binding enables Ang II to be available to interact with angiotensin type 1 receptor (AT1R), which mediates signals to activate NOX and induce oxidative stress, as well as inflammatory responses (Beltrán-García et al., 2020). When Ang II binds to AT1R, Ang II markedly enhances NOX activation and consequent ROS generation. Angiotensin II, high glucose, high fat, or hypoxia may lead to the excessive production of mitochondrial ROS (mtROS), and these result in the feed-forward redox stimulation of NOXs (Dikalov and Nazarewicz, 2013, Valente et al., 2012, Wei et al., 2006). Thus, NOX increases the severity of COVID-19 via contributing to NF-κB overexpression. In this case, endothelial cells can activate NOX proteins through immune stimuli associated with SARS-CoV-2 infection, and the resultant local oxidative stress causes endothelial dysfunction (Libby and Lüscher, 2020). In connection with the endothelial dysfunction, autopsy findings confirmed the venous thrombosis and pulmonary embolism which are not suspected before, are responsible for the death of more than half of COVID-19 patients (Wichmann et al., 2020).
On the other hand, the change in mitochondrial dynamics caused by SARS-CoV-2 after entering the cell results in oxidative stress, proinflammatory response and increased cytokine production that all contribute to the enhanced death rate (de Las Heras et al., 2020). It was demonstrated that the presence of oxidative stress and a high neutrophil to lymphocyte ratio in patients suffering from COVID-19 help to identify the individuals who are at high-risk in the early phases of the disease. Therefore, the use of these parameters is indicated to be beneficial to prevent the patients’ abrupt progression into a worse state. Thus, the high neutrophil to lymphocyte ratio due to NOX activation in critically ill patients with COVID-19 is another parameter enhancing in-hospital mortality, if not treated at an early stage (de Las Heras et al., 2020; Fu et al., 2020, Fu et al., 2020; Laforge et al., 2020). The cascade of events initiated by the oxidative stress in SARS-CoV-2 infection are important factors contributing to the severity of disease (Laforge et al., 2020). Respiratory distress that can cause oxidative stress and ARDS in moderate to severe COVID-19 patients can only be compensated by oxygen therapy (Mach et al., 2011, Park et al., 2009). However, it was shown that hyperoxia contrarily induces ROS generation in mitochondria (Turrens, 2003), thus inhibits oxidative phosphorylation and decreases the adenosine triphosphate (ATP) level (Das, 2013). Inflammatory reactions in macrophages during COVID-19 is triggered by non-structural coronavirus 3a (nsp3a) protein (Chen et al., 2019). Previously, it was presented that SARS-CoV-1 3a protein activates NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammation in macrophages, accompanied by IL-1β activation and increased mtROS (Chen et al., 2019). Although it is not precisely known that the 3a protein of SARS-CoV-2 activates NLRP3 inflammasome in macrophages, it is anticipated that the SARS-CoV-2 3a protein may act similarly, as it displays 72% similarity to its SARS-CoV-1 (J. Xu et al., 2020).
SARS-CoV-2 can infect monocytes and induce the synthesis of the pro-inflammatory cytokines IL-1β, IL-6, and TNF. During macrophage activation, succinate accumulates as a pro-inflammatory metabolite, which affects HIF-1α activity. Since succinate accumulation drives HIF-1α stabilization (Tannahill et al., 2013), succinate oxidation is important for SARS-CoV-2 replication. In the process of metabolic reprogramming, the mitochondria are considerable sources of intracellular ROS . ROS is a strong inducer of HIF-1α (Mills et al., 2016), which is essential for the initiation of glycolysis and the resultant proinflammatory state of SARS-CoV-2-infected monocytes. Stabilization of HIF-1α increases the expression of ACE2, IL-1β, TNF-α, IL-6, and IFN-α, β, and λ in SARS-CoV-2-infected monocytes. High glucose concentrations directly induce viral replication, as well as proinflammatory cytokine expression. Glycolytic flux is necessary for SARS-CoV-2 replication. SARS-CoV-2-induced mtROS release stabilizes HIF-1α, which in turn upregulates glycolytic genes and IL-1β expression. The mtROS/HIF-1α/glycolysis axis is an indicator for severity of COVID-19 disease (Codo et al., 2020). As noted above, IL-6 induced activation of the Akt/mTOR signaling and suppression of HIF-1α following SARS-CoV-2 infection increases viral replication (Appelberg et al., 2020). In brief, SARS-CoV-2-infected monocytes increase mtROS production. These metabolic adaptation results in mtROS-induced HIF-1α stabilization to encourage SARS-CoV-2 replication and monocyte inflammatory response. In high-risk individuals suffering from COVID-19, initial high neutrophil/lymphocyte ratio and excessive ROS generation exacerbates the host immunopathological response, leading to the more severe clinical presentation. In addition to neutrophil infiltration and production of ROS, a decrease in antioxidant defense worsens the clinical course. In these patients, checking oxidative stress markers, and neutrophil migration is of great importance in decreasing the risk of mortality.
4. Are serum iron levels friend or foe in patients with COVID-19?
Iron is an essential micronutrient for humans, as well as pathogens. The innate immune response could restrain Fe availability in the case of infections to limit the Fe consumption of pathogens, however, this may also result in anemia in the host (Ganz, 2019). The available level of hemoglobin is the major factor in defining the oxygen-carrying capacity of the blood. Elevated concentrations of circulating ferritin in blood, in addition to reflecting an acute-phase response, may present a decisive role in inflammation by contributing to the progression to cytokine storm (Kernan and Carcillo, 2017). It has been shown that SARS-CoV-2 RNAs, activate NF-κB, leading to the synthesis of pro-inflammatory mediators. High serum ferritin levels in patients with MAS, may stimulate these signaling pathways and contribute to cytokine storm in severe COVID-19. Thus, a vicious cycle is induced by ferritin and pro-inflammatory cytokines (Franco-Martínez et al., 2021, Pujani et al., 2021, Ruscitti et al., 2020). In hospitalized patients, high serum ferritin is significantly associated with death and hyperferritinemia is an independent risk factor for ARDS in severe COVID-19 (Parimoo et al., 2021). In this respect, serum Fe, ferritin, transferrin, and total Fe-binding capacity are demonstrated to be associated with elevated risk of ARDS, coagulopathy, and multi-organ damage, due to severity of COVID-19 and elevated cytokine concentrations (Lv et al., 2021). While low serum Fe level is an independent risk factor for the increased death rate, elevated level of serum ferritin is an indicator of poor outcome in COVID-19 patients (I. Huang et al., 2020; Zhao et al., 2020). In accordance with the data from 189 studies and 57,563 COVID-19 patients, the mean level of hemoglobin represents an important indicator for anemia diagnosis in men as described by World Health Organization (WHO) (Taneri et al., 2020). Low hemoglobin in COVID-19 patients, particularly in populations with a high risk of complications and mortality, decreases the capability of hemoglobin to meet the increased oxygen starvation of the peripheral tissue (F. Zhou et al., 2020). Indeed, decrease in the bioavailability of iron in COVID-19 patients results in the activation of hepcidin, sequestration of Fe within cells, elevated concentrations of ferritin and diminished hemoglobin, culminating in hypoxia (Taneri et al., 2020). It was demonstrated that the S, N, M proteins, Nsp3, and Nsp7 of the SARS-CoV2 could interact with hemoglobin and its metabolites, heme, hemin and protoporphyrin. Consequently, the S protein receptor binding domain (RBD motifs) and N-terminal domain (NTD) of SARS-CoV-2 bind hemoglobin (Lechuga et al., 2021). Indeed, COVID-19 patients demonstrate impaired hemoglobin biosynthesis, augmented generation of Zn-protoporphyrine IX, heme-CO2, and CO-hemoglobin in addition to the degradation of Fe-heme (Kronstein-Wiedemann et al., 2022). It is proposed that the replication of SARS-CoV-2 depends on the Fe availability. Since the RNA of the virus have porphyrin binding domains, it readily synthesizes heme. In addition to SARS-CoV-2's S protein, the accessory protein ORF3a, an integral membrane protein that functions as an ion channel and may promote virus release, can bind to hemoglobin. ORF3a has heme-Fe binding sites and degrades trapped heme into Fe and porphyrin. In that case, even if hemoglobin preserves its native structure, its oxygen delivery function is decreased (Courrol et al., 2021, Liu and Li, 2022, Redondo et al., 2021). Furthermore, in COVID-19 patients, SARS-CoV-2 may cause decomposition of the hemoglobin. Thereby, the dissociation of porphyrins from Fe results in the release of Fe into the circulation. As a result, these destructions lead to rapid multi-organ failures (Liu and Li, 2022). The free Fe released into the circulation may result in Fe overload, which causes oxidative damage, inflammation and immune dysfunction (Cassat and Skaar, 2013, Moreira et al., 2020). Indeed, increased uptake and storage of Fe in Fe-binding proteins causes elevation of ferritin concentrations in the circulation of COVID-19 patients. Thus, concentrations of d-dimer, high-sensitivity cardiac troponin 1, serum ferritin, lactate dehydrogenase, and IL-6 are obviously increased in non-survivors in comparison to the survivors or increased with illness deterioration (Ostro et al., 2015; F. Zhou et al., 2020). Therefore, Fe chelation may be a beneficial adjuvant therapy in treating COVID-19 for not only with sequestering Fe but also binding to receptors used by SARS-CoV-2, thereby blocking their entry into host cells (Habib et al., 2021). Besides, as SARS-CoV-2 requires Fe for viral replication and for its functions, Fe chelation therapy in COVID-19 may be an alternative rational approach to arrest viral replication (Liu et al., 2020).
Hepcidin as a hepatic peptide hormone, regulates systemic Fe metabolism and moreover, it achieves the intestinal Fe absorption, plasma Fe concentrations, and tissue Fe distribution by interacting with ferroportin. This protein exports Fe into plasma from absorptive enterocytes, from macrophages that recycle the Fe of senescent erythrocytes, and from hepatocytes that store Fe (Ganz and Nemeth, 2012). Hepcidin-like activity of SARS-CoV-2 can induce a significant Fe dysmetabolism (Stockwell et al., 2017). The in-silico analysis showed that a SARS-CoV-2 S protein fragment mimics hepcidin and this functional peptide called Covidin, is resistant to the majority of human proteases. The complex mechanism of Covidin binding and the following internalization of ferroportin results in altered Fe metabolism of host (Gupta et al., 2022). Induction of the innate immune system during the infection, promotes the hepcidin production in host, while the bioavailability of iron is diminished by this activation. Despite the decreased availability of free Fe outside of the cell, elevated intracellular Fe supports the viral replication (Birlutiu et al., 2021, Gupta et al., 2022). Ferroptosis has been described as an Fe-dependent version of regulated cell death, which emerges via the lethal accumulation of lipid-based ROS in the case of lipid peroxide repair systems are compromised due to GSH deficiency (Stockwell et al., 2017). Ferroptotic cell death can be inhibited by lipophilic antioxidants, Fe chelators and inhibitors of lipid peroxidation (Kagan et al., 2017, Stockwell et al., 2017).
Hemoglobinopathies and Fe dysmetabolism may seriously compromise the erythrocytes oxygen transport capacity, with hypoxia, while inducing hyperferritinemia-related tissue alterations. Increased ferritin level is an indicator of a strong inflammatory reaction in COVID-19 and in conjunction with the simultaneous viral entry into the human body and its impact on Fe metabolism (Kernan and Carcillo, 2017, Wessling-Resnick, 2018). In the critical cases of COVID-19, in case of MAS with high levels of ferritin (Fig. 1), H-chain of the ferritin has a prominent role in activating macrophages to enhance the secretion of inflammatory cytokines (Shoenfeld, 2020). Moreover, for viral replication, optimal Fe concentrations within host cells are essential (Drakesmith and Prentice, 2008, Wessling-Resnick, 2018). Therefore, the innate immune system will respond by diminishing the bioavailability of Fe to restrict the replication of the virus throughout the acute phase of infection. Cellular Fe can either be stored in ferritin or released into the circulation by ferroportin. A novel gene, Hephaestin (Heph), encoding a transmembrane-bound ceruloplasmin homolog, is a multicopper ferroxidase essential for Fe transport from enterocytes into the circulation. Thus, Heph expression level constitutes an interesting connection between Fe and Cu metabolism (Vulpe et al., 1999). As a protective mechanism, through IL-6 and Toll-like-receptor-4 (TLR-4) dependent pathways, the concentrations of the hepcidin increases (Mazur et al., 2003). Thereby hepcidin regulates the level of Fe in plasma by its capacity to bind with and promote the internalization and subsequent degradation of ferroportin (Nemeth et al., 2004). Thus, the activity of the ferroportin which carries Fe out of the cells, is blocked. The amount of Fe absorbed from the diet decreases. High cellular Fe sequestration leads to an increase of cytosolic ferritin, which stores Fe to prevent Fe-mediated free radical damage (Drakesmith and Prentice, 2008). Since serum ferritin is significantly increased in patients with COVID-19, ferritin assay can still be applied as a screening biomarker for the severity of the inflammatory state in these patients (Taneri et al., 2020). Furthermore, hepcidin and serum ferritin parallel testing sensitivity to predict the COVID-19 severity is 95.7% (C. Zhou et al., 2020).
The percentage of IFN-γ-producing CD8 + T cells is high in both severe and extremely severe patients in comparison to the mild cases, while the percentage of IFN-γ-producing CD4 + T cells is elevated in the extremely severe patients (F. Wang et al., 2020). Actually, activated CD8 + T cells augment ferroptosis-specific lipid peroxidation (Wang et al., 2019). Fe is a key factor for the IFN-γ receptor 2 (IFN-γR2) internalization thus it attenuates the activation of the IFN-γ/signal transducer and activator of transcription 1 (STAT1) pathway in human T cells. Iron chelators up-regulate the expression of IFN- γR2 on the activated T-cell surface and reinstates IFN-γ/STAT1 pathway in proliferating T lymphocytes (Regis et al., 2005). Thereby, T cell response to SARS-CoV-2 infection via reconstituting the sensitivity of T lymphocytes to IFN-γR2 may be restored by the stated chelators and thus the entry of the SARS-CoV-2 into cell may be inhibited (Perricone et al., 2020).
5. Zinc is crucial for the survival of COVID-19 patients
Zinc, following Fe, is the second most prevalent trace metal in the human body. Zn as a versatile element, can stabilize cell membranes in addition to its anti-inflammatory and antioxidant properties (Prasad, 2009). Almost all Zn is available in the intracellular compartments, and it is the constituent of more than 300 metalloenzymes (Vallee and Falchuk, 1993). Although human cells require a constant Zn supply, excessive free Zn ions may be toxic by binding at the active site of enzymes or via allosteric inhibition of enzymes. Thus, the inhibitory Zn ions must be removed in order to activate some enzymes (Maret, 2013). In this context, Zn2+ cations restrict SARS-CoV replication by inhibiting RNA polymerase (RNA dependent RNA polymerase, RdRp) activity (te Velthuis et al., 2010). It is proposed that RdRp (nsp12-nsp7-nsp8 complex) of SARS-CoV-2 contains Zn ions that serves a structural role in maintaining the integrity of the RdRp architecture (J. Chen et al., 2020; Gao et al., 2020; Hillen et al., 2020; Kirchdoerfer and Ward, 2019). The catalytic subunit of the RdRp, nsp12, ligates two Fe-sulfur metal cofactors in sites designed as Zn centers. These metal binding sites are necessary for replication as well as for interaction with the viral helicase (Maio et al., 2021). Since Fe-sulfur clusters are inherently susceptible to destabilization and degradation by oxidants, like oxygen, superoxide, and nitric oxide, Zn is capable of replacing endogenous Fe-sulfur metal cofactors (Imlay, 2006). However, Zn is not likely to be the physiological cofactor in viral replicase activity and supplementation with Zn has been reported to inhibit viral replication (Maio et al., 2021). Thus, Zn ionophores inhibit RdRp and 3 C-like protease (3CLpro), which is necessary for proper viral replication (Hsu et al., 2004, te Velthuis et al., 2010). In this context, it is proposed that Zn-ligating compounds contribute to the coordination of Zn in the catalytic site of 3CLpro, thus inhibiting proteinase activity (Hsu et al., 2004, Lee et al., 2007). Bioinformatics and molecular modeling analysis showed that Zn binds and regulates the enzymatic activities of 3CLpro and RdRp of SARS-CoV-2 and thus inhibits viral replication (Pormohammad et al., 2021, Saakre et al., 2021). In brief, like other RNA viruses, SARS-CoV-2 genome–encoded RdRp is central to SARS-CoV-2 replicative cycle. The RdRp is integrated into a membrane associated viral enzyme complex that regulates the synthesis of negative-strand RNA (Fehr and Perlman, 2015). RdRp plays a crucial role in SARS-CoV-2 replication (Parvez et al., 2020), however Zn2+ directly inhibits the RdRp activity (Rahman and Idid, 2021). Thereby, viral replication can be arrested in cells infected with SARS-CoV-2 if they contain sufficient Zn2+.
Recently, the positive feedback loop between IL-6/STATs and NF-κB signaling called attention to COVID-19 associated mortality (Hojyo et al., 2020). The SARS-CoV-2 spike protein S1 or RBD or SARS-CoV-2-RBD alone induces a more marked production of pro-inflammatory cytokines in addition to the factors associated with epithelial damage. Furthermore, S1-ACE2-mitogen-activated protein kinase (MAPK) signaling pathway induces high levels of NF-κB activation, thereby pro-inflammatory cytokines are produced in human bronchial epithelial cells via activation of endoplasmic reticulum stress (Hsu et al., 2020). When SARS-CoV-2 invades the cytoplasm, in the canonical NF-κB pathway viral-activated inhibitor of κB kinase complex (IKK) is degraded by the proteasome-derived proteolysis. Transcription of NF-κB-dependent proteins is induced following nuclear translocation and by overexpression of NF-κB. Thereby, NF-κB driven virus replication as well as cytokine syntheses, are expedited (Poppe et al., 2017). Increased expression of NF-κB-driven cytokines causes increased risk of multiorgan dysfunction and a worse prognosis of COVID-19 patients (Kircheis et al., 2020). In this respect, Zn takes part in the modulation of the proinflammatory response by targeting NF-κB, since Zn directly binds to inhibitor of NF-κB kinase subunit beta (IKKβ) (Fig. 1).
One mechanism suggested for the free Zn ion is the direct inhibition of the IKK upstream of NF-κB. Zinc transporter ZIP8 (SLC39A8) is up-regulated and transports Zn2+ into monocytes, macrophages, and lung epithelia during an infection to inhibit the NF-κB (Liu et al., 2013). ZIP8 elevates cytosolic Zn levels by promoting extracellular uptake or discharge from subcellular organelles. Imported into the cell by ZIP8, thiol-reactive Zn triggers NF-κB inhibition downstream from MAPKs by blocking IKK complex (Gálvez-Peralta et al., 2014, Liu et al., 2013). Furthermore, Zn also affects the expression of Zn finger protein A20 which is the main negative regulator of NF-κB activation in the tumor necrosis factor receptor- (TNFR) and TLR-initiated pathways (Jarosz et al., 2017). Thus, Zn does not only inhibit the synthesis of inflammatory cytokines, but also provides control of oxidative stress (Gammoh and Rink, 2017, Liu et al., 2013). In this case, Zn inhibits NF-κB activity driven by overexpression of adapter protein myeloid differentiation primary response gene 88 (MyD88), TNF receptor associated factor 6 (TRAF6) and IKKβ (Liu et al., 2013). Inhibition of NF-κB activation via Zn also occurs at DNA nuclear binding levels through increased expression of peroxisome proliferator-activated receptor alpha (PPAR-α). Elevated PPAR-α expression leads to the downregulation of inflammatory cytokines and adhesion molecules expression (von Bülow et al., 2007). In the setting of severe SARS-CoV-2 infection, Zn deficiency increases NF-κB signaling pathway activation, and proinflammatory cytokine expression, causing worsening of COVID-19 patients’ prognosis (Jarosz et al., 2017). As described above, the immunomodulatory function of Zn can be clearly pronounced. In the present pandemic of SARS-CoV-2, Zn supplementation could have a substantial function in treating the COVID-19 patients through enhancing immunomodulatory actions of anti-viral drugs and preventing the replication of SARS-CoV-2 in infected cells (Rahman and Idid, 2021).
Severe COVID-19 patients present with increased serum IL-2 and soluble CD25 (IL-2 receptor α chain) levels (G. Chen et al., 2020). As IL-2 is a potent growth factor for CD25-expressing activated T-cells (Shimizu et al., 1986), the increase in both IL-2 and CD25 shows that a positive feedback loop for T-cell activation in severe patients is present (Kalfaoglu et al., 2020). It is obvious that the number of CD4 + and CD8 + T cells is decisive factor in anti-viral immunity (Jansen et al., 2019; Whitmire and Ahmed, 2000). IFN regulatory factor (IRF)− 1 is a vital negative regulator of CD4 + CD25 + regulatory T (Treg) cells via direct repression of forkhead box P3 (FoxP3) expression (Fragale et al., 2008). SARS-CoV-2 triggers hyperactivation of CD4 + T-cells and immune paralysis. CD4 + T-cells are highly activated and opt unique differentiation pathways in the lung of severe COVID-19 patients. Particularly, those T-cells in severe COVID-19 cases highly express immunoregulatory receptors and CD25, however they are lacking the expression of FoxP3. CD25-expressing hyperactivated T-cells synthesize the protease Furin, that facilitates the viral entry of SARS-CoV-2. In severe COVID-19 patients, CD25 + T cells proliferate vigorously, transforming into multifaceted effector T cells instead of maturing into FoxP3 + Tregs. Therefore, it is suggested that CD25 +FoxP3- T-cells should be defined as hyperactive T-cells. Since CD25 is expressed principally in CD4 + T-cells, T-cells are mainly responsible for the elevated CD25 in severe COVID-19 patients. Furthermore, CD25 + T-cells synthesize and release Furin, which can further augment the entry of SARS-CoV-2 into pulmonary epithelial cells (Kalfaoglu et al., 2020). Zinc binds to RBD in an irreversible fashion and hinders viral entry to host cells (Podsiadlo et al., 2004). Zn induces upregulation of FoxP3 and the Krüppel-like transcription factor (KLF-10) and downregulation of IRF-1 (Maywald and Rink, 2017). IRFs control the kinetics and maintenance of the interferon regulated gene (IRG) response and play essential roles in the defense against viral infections. Overexpression of IRFs induces low-level type I IFNs. The absence of IRF1 unexpectedly enhances p38 MAPK activity (Irving et al., 2020). As mentioned above, SARS-CoV-2-S1-ACE2-MAPK signaling pathway induces high levels of NF-κB activation and cytokine storm.
Dysfunctional humoral and cell-mediated immunity are known as the consequences of Zn deficiency (Tuerk and Fazel, 2009). Consequently, Zn deficiency leads to immunodeficiency accompanied by severe lymphopenia. This status is characterized in part by a considerable reduction in the developing B cell compartments in the bone marrow (Bonaventura et al., 2015, Fukada et al., 2019). In non-hospitalized COVID-19 patients, on the one hand, Zn deficiency increases IL-6 that impairs immunity by producing lymphopenia, on the other hand, inflammation reduces plasma Zn concentration. In fact, the hypozincemia is an independent predictor of hospitalization and is proceeded with a worse clinical outcome in severe COVID-19. In the context of COVID-19 prognosis, detection of Zn deficiency in the early phase of disease for Zn supplementation is important (Dubourg et al., 2021, Fromonot et al., 2021). Moreover, SARS-CoV-2 deploys several mechanisms by using structural and non-structural proteins to antagonize IFN production and signaling. Zn treatment demonstrated to cause an elevation in IFN-α production (Cakman et al., 1997, Skalny et al., 2020) which can be utilized to overcome the IFN antagonism of SARS-CoV-2 proteins (Pal et al., 2021). The mTOR pathway is regulated by IFNs through the course of viral infection as a component of the anti-viral response. Enhanced mTOR expression levels and diminished p53 expression profiles facilitate the virus replication during the SARS-CoV-2 infection. Additionally, viruses inactivate the IFN-α pathway via impairing the IRF-7 mediated activation of IFN-α gene transcription (Appelberg et al., 2020, Ramaiah, 2020).
mTOR is activated during SARS-CoV-2 infection and replication. Whereas the key tumor-suppressor p53 protein is impaired by virus-encoded E3 ubiquitin ligase ring-finger and CHY zinc-finger domain-containing 1 (RCHY1), thus viral survival is increased in the host cells. Therefore, mTOR inhibitors and p53 activators suppress the early stages of viral infection and replication (Ramaiah, 2020). Cellular E3 ubiquitin ligase RCHY1 is an interacting partner of the viral SARS-unique domain (SUD) and papain-like protease (PLpro), both causes more intensive and stronger p53 degradation. So, virus antagonizes the viral inhibitor p53 via stabilizing RCHY1 and promoting RCHY1-mediated p53 degradation (Ma-Lauer et al., 2016). In fact, Zn fingers coordinate one or more Zn ions with a combination of cysteine and histidine residues in order to stabilize the folding. Zinc finger deficiency arrests cells both in the G1 and G2 phases of the cell cycle (Gangwani, 2006). Additionally, the Zn finger of IKKγ, which is a key element of the NF-κB pathway is needed for NF-κB activation in a cell- and stimulus-specific manner (Shifera, 2010). As mentioned above, the NF-κB proteins regulate the expression of large number of cytokines and acute phase proteins in COVID-19 patients. Severe COVID-19 patients show increased amounts of soluble IL–2 receptor alpha (sIL-2Rα)/CD25, which could be released from cell surfaces due to inflammation-induced enhanced proteolytic cleavage, leading to lymphopenia. Moreover, particularly significant decrease in the lymphocyte subsets, a decline in CD4 + and CD8 + T cell counts, in addition to the suppressed IFN-γ production by CD4 + T cells, excluding B cells are often reported in severe COVID-19 pneumonia (G. Chen et al., 2020; Quartuccio et al., 2021). Thus, Zn finger family of transcription factors have critical functions in lymphocyte development, differentiation (Swamynathan, 2010). On the other hand, KLFs are the members of the Zn finger family of transcription factors. The reduced expression of KLF2 in COVID-19 patients plays a prominent role in the development of SARS-CoV-2-induced vascular disease (Maywald and Rink, 2017, Swamynathan, 2010, Xu et al., 2021). Endothelial KLF is significantly decreased in lung autopsy specimens from patients with COVID-19 pneumonia, presenting the evidence that ARDS due to SARS-CoV-2 is a vascular phenotype convincingly attributable to KLF downregulation (D. Wu et al., 2021). Loss of KLF is the predominant molecular incidence in the development of COVID-19-induced vascular disease. KLF activation has therapeutic potential in restricting the endothelial dysfunction in COVID-19 (Xu et al., 2021). Endothelial KLF2 activation is associated with decreased mortality of patients suffering from COVID-19 (X.-J. Zhang et al., 2020). Zn most probably protects the patients against vascular complications via upregulating the FoxP3 and the KLF, while repressing the CD25 expression (Figure).
Since Zn deficiency induces IL-6 and IL-1β overproduction and resultant SARS-CoV-2 replication, Zn supplementation reduces inflammatory cytokines, particularly IL-6 and IL-1β, and also slightly increases the type I IFN response (Beck et al., 1997). Conversely, excess Zn inhibits T cell activity and IL-1β-stimulated IFN-γ expression (Wellinghausen et al., 1997). Moreover, Th1 and Th2 responses are mutually inhibited by their respective signature cytokines. During Zn deficiency, the expression of Th1 cell products, IFN-γ and IL-2, also decreases, while the expression of Th2 cells’ products, IL-4, IL-6, and IL-10, remains unchanged. This kind of imbalance between Th1 and Th2 leads to dysfunctions in cell-mediated immune response (Hönscheid et al., 2009, Prasad, 2009). Zn is an essential element in the production of IL-2 and IFN-γ and stimulates the macrophages to synthesize IL-12. IL-12 activates the NK cells and cytotoxic T cells (Prasad, 2008). Zn deficiency impairs adaptive immunity by inhibiting the function of B lymphocytes, CD4-expressing Th cells and CD8-expressing cytotoxic T lymphocytes (Whitmire et al., 2000). Thus, the cell lysis activity of NK cells decreases, as well (Tapazoglou et al., 1985).
On the one hand, ACE2 facilitates viral entry of SARS-CoV-2 into cells (Hoffmann et al., 2020), on the other hand, SARS-CoV-2 S induces TNF-α converting enzyme (TACE)-dependent dissemination of ACE2 (Haga et al., 2008). In this context, upregulation of both ACE2 and Zn-metalloprotease, a disintegrin and metalloprotease 17 (ADAM17) constitute a positive feedback-loop triggered by SARS-CoV-2. Thus, excessive systemic ACE2 activity results in the worst COVID-19 outcomes. Zinc-chelating agent, ethylenediaminetetraacetic acid (EDTA) alone protects from SARS-CoV-2 infection with inhibitory activity on Zn-metalloproteases, at different levels (Zamai, 2021). Both Zn and substrate are present in the (ACE2) catalytic site (Towler et al., 2004). Therefore, chelation of Zn or its replacement by calcium (Ca) ions affects both ACE2 conformation and ACE2-mediated viral binding/entry (Fernández et al., 2011).
The SARS-CoV-2 S glycoprotein RGD (Arg-Gly-Asp) lies in the RBD. SARS-CoV-2 RGD is a motif that is present in none of the other previously known SARS-CoVs. The SARS-CoV-2 utilize integrins, that are highly expressed in vital organs, for invading host cells via RGD. Availability of a number of phosphorylation sites that regulates Ca2 + signaling, facilitate the virus binding to host cells via integrins in a Ca-dependent manner (Dakal, 2021, Liu, 2009; Van Agthoven et al., 2014). Therefore, RGD–integrin mediated virus attachment with the host cells reduces the level of Ca and other divalent ions (Dakal, 2021). If the amount of divalent ion is initially lowered, binding of virus-host cells may be prevented. This also can be accomplished by EDTA (Berridge, 2012). Thus, pulmonary EDTA chelation therapy has been suggested to counteract SARS-CoV-2 infection by hindering the RGD-integrin mediated virus attachment to host cells (Dakal, 2021). Therefore, human ACE2 is a Zn metalloprotease and can be inhibited by chelating EDTA (Vickers et al., 2002). Albumin, as the main transporter/reservoir of Zn in plasma, is found only at significantly low amounts in SARS-CoV-2 (Coverdale et al., 2019, Ramadori, 2020, Zamai, 2020). Thus, the free Zn concentration increases, which indicates the bioavailability of functional and toxic forms of plasma Zn2+ (Asadzadeh et al., 2018, Briffa et al., 2020). As the vast majority of Zn in plasma is sequestered by albumin, the total amount of plasma Zn primarily depends on albumin level. Therefore, yet the smallest alterations in albumin’s capacity for Zn binding may result in significant consequences. In this context, decreased levels of plasma albumin is considered to rise the plasma concentrations of free Zn, while decreasing the total plasma Zn levels, which is observed in COVID-19 patients (Dubourg et al., 2021, Fromonot et al., 2021). However, it has been reported that an absolute majority of hospitalized COVID-19 patients are Zn deficient, when 1513 non-COVID-19 patients compared with 139 COVID-19 confirmed cases (Verschelden et al., 2021). Amidst COVID-19 patients at the time of hospitalization, individuals with Zn deficiency had higher frequencies of complications, ARDS, prolonged hospital stay and increased mortality, compared to those without Zn deficiency (Jothimani et al., 2020). ARDS develops in 42% of patients suffering from COVID‐19 pneumonia, and 61–81% of those require intensive care. The typical ARDS-related pathological changes are also detected in patients with COVID‐19 ARDS, which causes diffuse alveolar damage in the lung. COVID‐19 ARDS have worse outcomes than ARDS raised because of other causes. The hospital mortality from typical ARDS is 40%. For COVID‐19 ARDS, mortality rate is between 26% and 61.5% in patients admitted into critical care units. Among these who were supported by mechanical ventilation, the mortality rate can reach to 65.7%–94% (Bellani et al., 2016, Tian et al., 2020, Wu et al., 2020; Z. Xu et al., 2020). Elevated plasma free Zn bioavailability as a result of the decreased albumin concentrations induces Zn entry into cells and probably enhances the cellular functions dependent on cellular Zn levels like ACE and ACE2 (Zamai, 2020). In fact, enzymatic activity of both enzymes is significantly upregulated in ARDS patients. This can be defined by the determination of the plasma Ang peptide levels (Zamai, 2020). Low serum albumin on admission in COVID-19 is associated with a higher incidence of serious outcomes and mortality rate. Increase in the serum albumin level reduces the mortality of COVID-19 ARDS (M. A. Wu et al., 2021, Wu et al., 2021).
As mentioned above, SARS-CoV-2 is able to induce the Zn-carboxypeptidase ACE2 by activating the Zn-metalloprotease ADAM17, finally leading to systemic increase in ACE2 activity in COVID-19 patients (Zamai, 2020).
Zn acts as the cofactor of the Cu/Zn-superoxide dismutase (SOD), that catalyzes the dismutation of superoxide radical into the less detrimental molecules O2 and H2O2. These ROS are further detoxified by catalase (CAT) and glutathione peroxidase (GPx). Besides, Zn inhibits NOXs, leading to the diminished production of ROS (Prasad, 2014). Since Zn binds to the gap immediate alongside the sulfhydryl group in the protein, the conformation of the protein changes which leads to the diminished activity of sulfhydryls (Gibbs et al., 1985). Moreover, Zn antagonizes redox-active transition metals such as Cu and Fe that catalyze formation of free radicals, mainly via Fenton reactions. Indeed, Zn is capable of replacing the Cu and Fe, thus prevents the severe tissue damage, which raised due to oxidative DNA and protein damage (Powell, 2000). However, Zn while inhibiting the SARS-CoV-2 main protease (Mpro), also halts the viral replication (Hussein and Elkhair, 2021, Panchariya et al., 2021). Furthermore, Zn ions efficiently inhibit the RNA-synthesizing process of the replication and transcription complex of SARS-CoV (Nemeth et al., 2004) . Similarly, by inhibiting RdRps of SARS-CoV-2, it blocks viral replication without severely affecting host cellular functions (Pal et al. 2021).
6. Copper-zinc interactions and SARS-CoV-2
Copper is an essential trace dietary mineral and it is found practically in all living organisms (Grass et al., 2011). Although SARS-CoV-2 on stainless steel and plastic surfaces is detected for up to 72 h, the United States National Institutes of Health (NIH) demonstrated that virus can only survive up to 4 h on Cu surfaces (van Doremalen et al., 2020). Indeed, the SARS-CoV-2 infectivity from the Cu oxide (CuO) film is diminished by 99.8% in 30 min and 99.9% in 1 h compared to that from glass (Hosseini et al., 2021). As an independent predictive parameter, urinary creatinine-adjusted Cu of ≥ 25.57 μg/g and ≥ 99.32 μg/g are associated with significantly elevated risk of severe illness and mortality in COVID-19, respectively (Zeng et al., 2021). Cu displays antiviral effect through different mechanisms. Although Cu ions inactivate both enveloped or non-enveloped, single- or double-stranded DNA or RNA viruses (Sagripanti et al., 1993), enveloped viruses are more sensitive to Cu2+ related inactivation than the non-enveloped ones (Sagripanti et al., 1993). Exposure to Cu results in irreversible damage in virus envelope and surface spikes and demolition of the viral genomes (Warnes et al., 2015). Copper complexes bind selectively to a specific DNA sequence or conformation and cause a permanent structural transition or conformational change (Erxleben, 2018). Since, free Cu is toxic, its metal complexes are of particular importance in biological applications. Docking studies of Cu complex-SARS-CoV-2 interactions are rare and the complexes could be used as potential corona virus inhibitors. Since the docking orientation of Cu (II) complex is inside the active site of proteins, the docking scores calculated by noncovalent three-dimensional interactions between the ligand and the protein reflect that Cu (II) complex can interact more efficiently with S and N proteins of SARS-CoV-2. Indeed, the SARS-CoV-2, N protein C-terminal domain is capable of binding to single-stranded RNA, single-stranded DNA as well as double-stranded DNA. Therefore, N protein C-terminal domain (N-CTD) may be a target with two active binding sites (monomer and dimer) that take part in the specific RNA binding and stability (Viola et al., 2022; R. Zhou et al., 2020). However, RNA synthesis steps are also affected by Cu via RNA polymerase inhibition (Novello and Stirpe, 1969). This inhibitory effect is 60% more compared to other metals (Novello and Stirpe, 1969). Viral infection results in exaggerated ROS generation. Thereby, oxidant-susceptible pathways, p38 MAPKs and NF-κB, are stimulated via these reactive species for the regulation of virus replication and the proinflammatory response (Morgan and Liu, 2011). Under normal metabolic conditions, Cu is the other cofactor of Zn/Cu SOD that removes ROS, a byproduct of inflammation (Rani et al., 2021). Nuclear factor erythroid 2p45-related factor 2 (Nrf2)/antioxidant response element or a redox-sensitive transcription factor, activates the transcription of Cu-dependent SOD in response to virus-induced oxidative stress. This activation further leads to the disruption of the induction of the MAPK- and NF-κB related signaling pathways, thus protects the cells against oxidative damage (Lee, 2018, Lin et al., 2016; Rani et al., 2021). Preclinical studies demonstrated that SARS-CoV-2 may cause severe inflammation and organ damages by both directly upregulating p38 MAPK activity and downregulating a key p38 MAPK shut off mechanism by reducing ACE2 activity. Furthermore, it is thought that p38 MAPK activation simultaneously supports the SARS-CoV-2 viral life cycle (Grimes and Grimes, 2020). Indeed, SARS-CoV-2-induced p38 MAPK activation has been found to facilitate viral replication (Cheng et al., 2020).
In COVID-19 patients, the disruption of Cu ion homeostasis leads to an uncontrolled production of ROS and reactive nitrogen species (RNS). Generation of the ROS and RNS cause lipid peroxidation of cell membrane, DNA damage and protein modifications (Valko et al., 2016). DNA oxidation by Cu ions is enhanced by hydrogen peroxide, thereby, ROS-mediated base modifications and breakage of the phosphodiester backbone in DNA occur. Since SARS‐CoV‐2 N protein is an indispensable factor in numerous stages of the viral life cycle, it represents a critical drug target. Functions of N protein consist of, folded N‐terminal domain/CTD (NTD/CTD) depend on the capacity in binding various viral/host‐cell RNA/DNA of diverse sequences (Dang and Song, 2022). In general, considering oxidative stress, Cu mediated inactivation of DNA viruses may be a comparable example for SARS-CoV-2. Indeed, studies of molecular dynamics on main protease SARS-CoV-2 (6M03) and S glycoprotein chain A SARS-CoV-2 (6ZGG) demonstrated that Cu nanoparticles embedded in the active site of SARS-CoV-2 S glycoprotein have potent inactivation capacity. As SARS-CoV-2 protease and S glycoprotein inhibitor, Cu nanoparticles could block the key enzyme that helps viruses replicate (Aallaei et al., 2022). However, there is a potential advantage of Cu-mediated viral killing; the host cell can repair broad spectrum of molecular damage much rapidly for itself than for the virus (Sagripanti et al., 1997). Two hypotheses are valid considering the inhibition of virus replication; either Cu binding to histidines can unfold the Mpro or the protease could be disabled by Cu-sulfur bonding. Thus, viral replication is interrupted (Garza-Lopez et al., 2020).
Cu is involved in the function and maintenance of Th cells, B cells, neutrophils, NK cells and macrophages. Thus, host immune function might have a positive impact from the dietary or therapeutic Cu supplementations and the severe viral infection can be overcome (Fig. 1). Therefore, supplementation of Cu and correction of mineral deficits may have useful outcomes for COVID-19 patients (Raha et al., 2020). Cu supplementation restores the secretion and activity of IL-2 in Cu-deficient state. Elevated production of IL-2 is crucial for Th cell proliferation and NK cell cytotoxicity (Hopkins and Failla, 1997).
In addition to Zn/Cu SOD, several Cu-containing enzymes, lysyl oxidase, dopamine-β-hydroxylase, tyrosinase, cytochrome c-oxidase, and particularly ceruloplasmin, are responsible for the regulation of vital cellular functions (Rani et al., 2021). Among these, it acts as a cofactor for ceruloplasmin, which is a key component of Fe metabolism (Bost et al., 2016). Ceruloplasmin is a Cu-containing glycoprotein produced in the liver and binds about 95% of the Cu in serum. This glycoprotein displays ferroxidase activity and catalyzes the conversion of ferrous to ferric Fe which is then transferred to transferrin (Iakovidis et al., 2011). Elevated IL-6 levels result in increased levels of ceruloplasmin, the major Cu-carrying protein in the blood (Di Bella et al., 2017). Considering that increased IL-6-driven ferritin levels are indicator of a strong inflammatory reaction in COVID-19, Cu ion chelation affects the Fe metabolism at mRNA levels and thereby SARS-CoV-2 related inflammation.
A successful inactivation of virus is achieved with the synergistic action of Cu ion attack, and free Cu ions induce oxidative damage through the generation of ROS/RNS. Viral cellular membranes, nucleic acids, and mitochondria are damaged by Cu-induced oxidative stress (Gaetke and Chow, 2003). In addition to the inhibition of p38 MAPK activation as a cofactor of SOD, Cu ion binds to the strands of genome (Noyce et al., 2007). Pvsn et al. demonstrated that in a total of 150 individuals who were diagnosed with COVID-19, the serum Cu/Zn of patients provides reliable information about the COVID-19 course and survival. Therefore, it is emphasized that quantifying the serum Cu and Zn together with their ratio calculation can be used as routine investigations in COVID-19 patients for profoundly identifying and managing the severe cases of COVID-19 (Pvsn et al., 2022). Systemic inflammation and infections induce decrease of serum Zn level during the acute phase response like COVID-19 (Stafford et al., 2013, Verschelden et al., 2021). Moreover, acute infections result in an elevated level of serum Cu concentration. Both responses consequently give rise to an elevated serum Cu/Zn. Increment of Cu/Zn, particularly high serum Cu levels are correlated with augmented risk of infections (Laine et al., 2020). Thus, Cu exposure inactivates the viral genomes of SARS-CoV-2 and exhibits irreversible effects on virus morphology, including envelope disintegration and S protein dispersal. Despite this, the SARS-CoV-2 virus can remain active on Cu surfaces for up to 4 h (Govind et al., 2021). The behavior of the Cu complexes used for inactivation of the viral genome is controlled by their organic ligands (Iakovidis et al., 2011). Also, when individuals who consume Zn supplements on regular basis are infected with SARS-CoV-2, a severe Cu deficiency is likely to occur. Since, ingesting a high Cu dose is toxic, preventing cytotoxic doses of exogenous Zn intake is important in maintaining the systemic homeostasis of Cu. On the other hand, Cu and Zn can use different routes to evade viral infections. Despite, virus replication and viral genomic RNA packaging dynamics comprising of N proteins and their orthologs require adequate amounts of Zn, excess Cu are unfavorable of virus morphology and infectivity (Monette and Mouland, 2020). Since metal ions control and maintain the structure and functions of viruses (Fernández et al., 2011), modulations in cellular Cu/Zn eventually influence the viral protein conformations and interactions (Monette and Mouland, 2020). Imbalances in plasma Cu/Zn represent a common clinical assessment for Zn2+ deficiencies associated with a wide variety of disorders. In the early stage of disease, high Cu2+ levels may disrupt virus capsid integrity which leads to the premature viral RNA exposure and its subsequent degradation. In late stages of virus replication, high Cu2+ levels may interfere with the biogenesis of Zn2+-mediated viral biomolecular condensates. Cellular metalloproteins and metal-ion carrier proteins during the destruction of the viruses may further cause altered Cu/Zn and the simultaneously promote Cu2+-associated protein aggregates (Monette and Mouland, 2020).
7. Impact of selenium in COVID-19
Selenium is an essential trace element for mammalian redox balance, and the human genome contains at least 25 genes that encode selenoproteins. Selenocysteine is localized in the catalytic centers of many selenoproteins that counteract SARS-CoV-2 infection (Fairweather-Tait et al., 2011; Zhang et al., 2020a). Thus, an association between the rate of recovery of SARS-CoV-2-infected patients and Se status has been reported in humans receiving adequate or insufficient Se (Zhang et al., 2020b). Moghaddam et al. showed that Se concentrations were significantly higher in surviving COVID-19 cases compared to the non-survivors. The Se levels were 23.5% higher in samples from surviving COVID-19 patients as compared with non-survivors. Se level recovers with time in survivors, while in non-survivors it remains low or even significantly declined (Moghaddam et al., 2020). It is well known that significantly increased circulating ferritin and IL-6 are critical predictors for mortality in COVID-19 patients (Ruan et al., 2020). Thus, IL-6 levels are remarkably higher in patients with severe COVID-19 than in those with mild disease (Wan et al., 2020). Therefore, treatment plans should be made considering the positive correlation between the severity of the disease and IL-6 levels (Conti et al., 2020). In SARS‐CoV‐2 infected patients decreased selenoprotein synthesis is associated with the increase of IL‐6 concentrations (Han et al., 2020). Indeed, SARS-CoV-2 suppresses mRNA expression of selenoproteins while increasing gene expression of IL-6 (Wang et al., 2021). Furthermore, presence of excessive ROS cause the release of Fe from the storage in ferritin, especially under the conditions of stressful stimulation (Ismail, 2019, Sturm et al., 2006). Ebselen, a synthetic Se compound that mimics GPx, is a potent inhibitor of the SARS-CoV-2 major protease. It has direct antiviral activity on the SARS-CoV-2 because of its reactive thiol-mediated and peroxiredoxin-like inhibitory effects. Therefore, it is thought that ebselen will have a beneficial effect in inflammatory ARDS occurring in COVID-19 patients (Sies and Parnham, 2020). In these patients, treatment with ebselen not only restores the disturbances in Fe homeostasis but also alleviates the resultant cytotoxicity, thus it could be helpful in the treatment of hyperinflammation due to SARS-CoV-2 infection (Mehta et al., 2020).
The transcription of the genomic RNA in the cell results in the production of a polypeptide which is proteolytically cleaved by the MPro. MPro is recognized as 3CLPro or chymotrypsin-like protease. At 11 different sites, this enzyme is programmed to synthesize proteins that are essential for the replication of the virus (Boopathi et al., 2020; Zhang et al., 2020, Zhang et al., 2020). Thus, the suppression of the SARS-CoV-2 MPro is required for the blockage of the viral replication (Das et al., 2021). It is proposed that SARS-CoV-2 targets GSH biosynthesis as well as selenoprotein thioredoxin reductase 1 and GPx1. In infected cells, deficiency of these crucial antioxidant molecules contributes to the augmentation of the oxidative stress. Indeed, GPx is a substrate of the Mpro, main cysteine protease of SARS-CoV-2, thereby MPro interacts with the essential seleno-enzyme, GPx-1 (Fig. 1). Proteolysis of GPx-1 increases ROS and NF-κB activation. Since NF-κB activates numerous pro-inflammatory cytokines, this mechanism seems to contribute to both enhancing virion production and cytokine storm in COVID-19 (Taylor and Radding, 2020). As stated above, the increased production of ROS in host cells by viral infection leads to oxidative stress, if not balanced by antioxidant defense mechanisms. Excessive oxidative stress leads to the occurrence of mutation on the viral genome which in turn promote the emergence of more virulent strains. Therefore, the interaction between the GPx-1 detoxifying system and the MPro of SARS-CoV-2 is thought to be a novel molecular target, which have antiviral properties against COVID-19 (Seale et al., 2020). In this context, ebselen suppresses the life cycle of SARS-CoV-2 by inhibiting Mpro activity of SARS-CoV-2 (Jin et al., 2020). Similar to SARS-CoV-2 Mpro, the PLpro of SARS-CoV-2 is a cysteine protease, as well and this enzyme has a key role in virus replication. SARS-CoV-2 antagonizes the antiviral innate immune response of patients by using PLpro. Ebselen significantly inhibits the PLpro of SARS-CoV-2 via covalent binding with the sulfhydryl group of the Cys112 in the catalytic triad of the protease (Węglarz-Tomczak et al., 2020). The redox-active Se compounds reduce the replication, transcription, and life cycle of SARS-CoV-2 by reacting with HS-Cys145-Mpro. This should be noted as an important therapeutic target (Zhang et al., 2020a).
Se deficiency significantly increases the susceptibility to lung pathology that is the consequence of the overexpression of pro-inflammatory cytokines (Beck et al., 2001). Supplementing the patients who has suboptimal plasma Se levels (Se<100 µg/L), with 100–200 µg Se/day, with or without cofactors would help them to attain the rapid saturation of selenoproteins. This approach would be a beneficial adjuvant treatment to prevent the aggressive progression of the severe SARS-CoV-2 infection (Alexander et al., 2020). In this respect, Se treatment has been demonstrated to up-regulate the expression of genes for IFNs (IFN-α, IFN-β, and IFN-γ) (Shojadoost et al., 2019). As a Se-dependent antioxidant, the optimal function of the GPxs also depends upon adequate intracellular concentrations of the cofactor GSH. COVID-19 patients with low GSH and selenoprotein levels are more vulnerable to the harmful effects of virus-induced proteolysis (Taylor and Radding, 2020).
Pneumonia with bilateral interstitial infiltrates giving rise to the serious alterations in ventilation-perfusion ratio and severe ARDS result in severe hypoxemia in COVID-19 (Lazzeri et al., 2020). Vascular endothelial growth factor (VEGF) is transcriptionally upregulated by HIF-1α and accumulates during the hypoxic conditions. Accordingly, hypoxia and HIF-1α stabilization may initiate or enhance cytokine storm. Thereby, endothelial cells contribute to the initiation and propagation of severe COVID-19 (Teuwen et al., 2020). Lately, it was exhibited that SARS-CoV-2 induces endotheliitis as a consequence of direct infection of endothelial cells in various organs. The invasion of the endothelial cells by SARS-CoV-2 and the resultant occurrence of the viral components inside the cell, as well as the accumulation of inflammatory cells are indicator of endotheliitis and endothelial dysfunction in COVID-19 (Ackermann et al., 2020, Varga et al., 2020). Given that optimal function of GPxs also depends on sufficient intracellular concentrations of the cofactor GSH, administration of GSH has been demonstrated to relieve hypoxia associated with COVID-19 pneumonia (Horowitz et al., 2020). Selenoenzymes such as GPx and thioredoxin reductases (TrxR) have vital importance in the endothelial cell function (Brigelius-Flohé et al., 2003). The destructive effects of the oxidative stress can be diminished by selenite in human endothelial cells via the TrxR and GPx of stimulation (Lewin et al., 2002, Miller et al., 2001). Besides, ebselen hinders the augmentation in transendothelial electrical resistance and IL-6 release induced in human endothelial cells in the conditions of extended hypoxia (Ali et al., 1999). Optimizing Se status relieves overactive inflammatory response in severe SARS-CoV-2 infection. Another cofactor of selenoenzymes appears to be coenzyme Q10 (CoQ10). CoQ10 supplements can exert an anti-inflammatory effect even when given alone (Alehagen et al., 2016, Zhai et al., 2017). However, SARS-CoV-2 induced excess oxidative stress causes mutation in either one or more of the nine CoQ10 genes, eventuating primary CoQ10 deficiency (Gvozdjakova et al., 2020). Thus, initiation of the use of adequate supplementation of Se and its co-factors is recommended in high-risk regions and/or as early as possible after the time of suspected infection with SARS-CoV-2 (Alexander et al., 2020). Considering the stated available proofs, both selenoproteins and redox-active Se species in the Se metabolic pool employ to alleviate virus-triggered oxidative stress, excessive inflammatory response, and immune system dysfunction, thus may improve the consequences of SARS-CoV-2 infection.
8. Conclusion
Many inflammatory and metabolic changes that occur in the defense mechanism of the host are effective for the continuation of the pandemic by facilitating the replication and entry of the virus into the cells. The antiviral activity of trace elements such as Fe, Zn, Cu and Se, is attributed to their inhibitory effect on viral entry, replication and on the life cycle of SARS-CoV-2. These while regulating the host immune responses, also modify the viral genome due to their dual functions. This process contributes significantly to the COVID-19 pandemic, thereby the prevention of severe SARS-CoV-2 infection seems at least partly depend on critical balances between the deficiency and oversupply of these trace metals.
Funding
This review article did not receive any funding.
CRediT authorship contribution statement
Ayse Basak Engin: Writing – original draft. Evren Doruk Engin: Writing – original draft. Atilla Engin: Writing – review & editing, Figure design-preparation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Edited by Dr. M.D. Coleman
References
- Aallaei M., Molaakbari E., Mostafavi P., Salarizadeh N., Maleksah R.E., Afzali D. Investigation of Cu metal nanoparticles with different morphologies to inhibit SARS-CoV-2 main protease and spike glycoprotein using molecular docking and dynamics simulation. J. Mol. Struct. 2022;1253 doi: 10.1016/j.molstruc.2021.132301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar S., Das J.K., Ismail T., Wahid M., Saeed W., Bhutta Z.A. Nutritional perspectives for the prevention and mitigation of COVID-19. Nutr. Rev. 2021;79:289–300. doi: 10.1093/nutrit/nuaa063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alehagen U., Johansson P., Björnstedt M., Rosén A., Post C., Aaseth J. Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur. J. Clin. Nutr. 2016;70:91–96. doi: 10.1038/ejcn.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12 doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.H., Schlidt S.A., Chandel N.S., Hynes K.L., Schumacker P.T., Gewertz B.L. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am. J. Physiol. 1999;277:L1057–L1065. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- Andreou A., Trantza S., Filippou D., Sipsas N., Tsiodras S. COVID-19: the potential role of copper and n-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. Vivo Athens Greece. 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg S., Gupta S., Svensson Akusjärvi S., Ambikan A.T., Mikaeloff F., Saccon E., Végvári Á., Benfeitas R., Sperk M., Ståhlberg M., Krishnan S., Singh K., Penninger J.M., Mirazimi A., Neogi U. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg. Microbes Infect. 2020;9:1748–1760. doi: 10.1080/22221751.2020.1799723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadzadeh F., Maleki-Kaklar M., Soiltanalinejad N., Shabani F. Central composite design optimization of zinc removal from contaminated soil, using citric acid as biodegradable chelant. Sci. Rep. 2018;8:2633. doi: 10.1038/s41598-018-20942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck F.W., Prasad A.S., Kaplan J., Fitzgerald J.T., Brewer G.J. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 1997;272:E1002–E1007. doi: 10.1152/ajpendo.1997.272.6.E1002. [DOI] [PubMed] [Google Scholar]
- Beck M.A., Nelson H.K., Shi Q., Van Dael P., Schiffrin E.J., Blum S., Barclay D., Levander O.A. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001;15:1481–1483. [PubMed] [Google Scholar]
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., Ranieri M., Rubenfeld G., Thompson B.T., Wrigge H., Slutsky A.S., Pesenti A., LUNG SAFE Investigators, ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- Beltrán-García J., Osca-Verdegal R., Pallardó F.V., Ferreres J., Rodríguez M., Mulet S., Sanchis-Gomar F., Carbonell N., García-Giménez J.L. Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants. 2020;9 doi: 10.3390/antiox9100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. Calcium signalling remodelling and disease. Biochem. Soc. Trans. 2012;40:297–309. doi: 10.1042/BST20110766. [DOI] [PubMed] [Google Scholar]
- Birlutiu V., Birlutiu R.M., Chicea L. Off-label tocilizumab and adjuvant iron chelator effectiveness in a group of severe COVID-19 pneumonia patients: a single center experience. Medicine. 2021;100 doi: 10.1097/MD.0000000000025832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura P., Benedetti G., Albarède F., Miossec P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost M., Houdart S., Oberli M., Kalonji E., Huneau J.-F., Margaritis I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS. 2016;35:107–115. doi: 10.1016/j.jtemb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Briffa J., Sinagra E., Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohé R., Banning A., Schnurr K. Selenium-dependent enzymes in endothelial cell function. Antioxid. Redox Signal. 2003;5:205–215. doi: 10.1089/152308603764816569. [DOI] [PubMed] [Google Scholar]
- von Bülow V., Dubben S., Engelhardt G., Hebel S., Plümäkers B., Heine H., Rink L., Haase H. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J. Immunol. 2007;1950(179):4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- Cakman I., Kirchner H., Rink L. Zinc supplementation reconstitutes the production of interferon-alpha by leukocytes from elderly persons. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 1997;17 doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- Cassat J.E., Skaar E.P. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasapis C.T. Interactions between metal binding viral proteins and human targets as revealed by network-based bioinformatics. J. Inorg. Biochem. 2018;186:157–161. doi: 10.1016/j.jinorgbio.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Chasapis C.T., Georgiopoulou A.K., Perlepes S.P., Bjørklund G., Peana M. A SARS-CoV-2 -human metalloproteome interaction map. J. Inorg. Biochem. 2021;219 doi: 10.1016/j.jinorgbio.2021.111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang Xiaoyun, Chen H., Yu H., Zhang Xiaoping, Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.-Y., Moriyama M., Chang M.-F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Malone B., Llewellyn E., Grasso M., Shelton P.M.M., Olinares P.D.B., Maruthi K., Eng E.T., Vatandaslar H., Chait B.T., Kapoor T.M., Darst S.A., Campbell E.A. Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell. 2020;182:1560–1573. doi: 10.1016/j.cell.2020.07.033. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Sun F., Wang L., Gao M., Xie Y., Sun Y., Liu H., Yuan Y., Yi W., Huang Z., Yan H., Peng K., Wu Y., Cao Z. Virus-induced p38 MAPK activation facilitates viral infection. Theranostics. 2020;10:12223–12240. doi: 10.7150/thno.50992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codo A.C., Davanzo G.G., Monteiro L. de B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., Jimenez Restrepo J.L., Vendramini P.H., Reis-de-Oliveira G., Bispo Dos Santos K., Toledo-Teixeira D.A., Parise P.L., Martini M.C., Marques R.E., Carmo H.R., Borin A., Coimbra L.D., Boldrini V.O., Brunetti N.S., Vieira A.S., Mansour E., Ulaf R.G., Bernardes A.F., Nunes T.A., Ribeiro L.C., Palma A.C., Agrela M.V., Moretti M.L., Sposito A.C., Pereira F.B., Velloso L.A., Vinolo M.A.R., Damasio A., Proença-Módena J.L., Carvalho R.F., Mori M.A., Martins-de-Souza D., Nakaya H.I., Farias A.S., Moraes-Vieira P.M. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-dependent axis. Cell Metab. 2020;32:437–446. doi: 10.1016/j.cmet.2020.07.007. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020:34. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Courrol L.C., de Oliveira Silva F.R., Masilamani V. SARS-CoV-2, hemoglobin and protoporphyrin IX: Interactions and perspectives. Photo Photodyn. Ther. 2021;34 doi: 10.1016/j.pdpdt.2021.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverdale J.P.C., Khazaipoul S., Arya S., Stewart A.J., Blindauer C.A. Crosstalk between zinc and free fatty acids in plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:532–542. doi: 10.1016/j.bbalip.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakal T.C. SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19. Immunobiology. 2021;226 doi: 10.1016/j.imbio.2020.152021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang M., Song J. CTD of SARS-CoV-2 N protein is a cryptic domain for binding ATP and nucleic acid that interplay in modulating phase separation. Protein Sci. Publ. Protein Soc. 2022;31:345–356. doi: 10.1002/pro.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K.C. Hyperoxia decreases glycolytic capacity, glycolytic reserve and oxidative phosphorylation in MLE-12 cells and inhibits complex I and II function, but not complex IV in isolated mouse lung mitochondria. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2021;39:3347–3357. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bella L.M., Alampi R., Biundo F., Toscano G., Felice M.R. Copper chelation and interleukin-6 proinflammatory cytokine effects on expression of different proteins involved in iron metabolism in HepG2 cell line. BMC Biochem. 2017;18:1. doi: 10.1186/s12858-017-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S.I., Nazarewicz R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013;19:1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008;6:541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- Dubourg G., Lagier J.-C., Brouqui P., Casalta J.-P., Jacomo V., La Scola B., Rolain J.-M., Raoult D. Low blood zinc concentrations in patients with poor clinical outcome during SARS-CoV-2 infection: is there a need to supplement with zinc COVID-19 patients? J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi. 2021;54:997–1000. doi: 10.1016/j.jmii.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin A.B., Engin E.D., Engin A. Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking. Environ. Toxicol. Pharmacol. 2020;78 doi: 10.1016/j.etap.2020.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin A.B., Engin E.D., Engin A. The effect of environmental pollution on immune evasion checkpoints of SARS-CoV-2. Environ. Toxicol. Pharmacol. 2021;81 doi: 10.1016/j.etap.2020.103520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erxleben A. Interactions of copper complexes with nucleic acids. Coord. Chem. Rev. 2018;360:92–121. doi: 10.1016/j.ccr.2018.01.008. [DOI] [Google Scholar]
- Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Hesketh J.E., Hurst R. Selenium in human health and disease. Antioxid. Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández D., Boix E., Pallarès I., Avilés F.X., Vendrell J. Structural and functional analysis of the complex between citrate and the zinc peptidase carboxypeptidase A. Enzym. Res. 2011;2011 doi: 10.4061/2011/128676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale A., Gabriele L., Stellacci E., Borghi P., Perrotti E., Ilari R., Lanciotti A., Remoli A.L., Venditti M., Belardelli F., Battistini A. IFN regulatory factor-1 negatively regulates CD4+ CD25+ regulatory T cell differentiation by repressing Foxp3. Expr. J. Immunol. 2008;1950(181):1673–1682. doi: 10.4049/jimmunol.181.3.1673. [DOI] [PubMed] [Google Scholar]
- Franco-Martínez L., Cerón J.J., Vicente-Romero M.R., Bernal E., Torres Cantero A., Tecles F., Sánchez Resalt C., Martínez M., Tvarijonaviciute A., Martínez-Subiela S. Salivary ferritin changes in patients with COVID-19. Int. J. Environ. Res. Public. Health. 2021;19:41. doi: 10.3390/ijerph19010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromonot J., Gette M., Ben Lassoued A., Guéant J.-L., Guéant-Rodriguez R.-M., Guieu R. Hypozincemia in the early stage of COVID-19 is associated with an increased risk of severe COVID-19. Clin. Nutr. 2021 doi: 10.1016/j.clnu.2021.04.042. S0261–5614(21)00234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Kong J., Wang W., Wu M., Yao L., Wang Z., Jin J., Wu D., Yu X. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb. Res. 2020;192:3–8. doi: 10.1016/j.thromres.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada T., Hojyo S., Hara T., Takagishi T. Revisiting the old and learning the new of zinc in immunity. Nat. Immunol. 2019;20:248–250. doi: 10.1038/s41590-019-0319-z. [DOI] [PubMed] [Google Scholar]
- Gaetke L.M., Chow C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- Gálvez-Peralta M., Wang Z., Bao S., Knoell D.L., Nebert D.W. Tissue-specific induction of mouse ZIP8 and ZIP14 divalent cation/bicarbonate symporters by, and cytokine response to, inflammatory signals. Int. J. Toxicol. 2014;33:246–258. doi: 10.1177/1091581814529310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammoh N.Z., Rink L. Zinc in infection and inflammation. Nutrients. 2017;9 doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwani L. Deficiency of the zinc finger protein ZPR1 causes defects in transcription and cell cycle progression. J. Biol. Chem. 2006;281:40330–40340. doi: 10.1074/jbc.M608165200. [DOI] [PubMed] [Google Scholar]
- Ganz T. Anemia of inflammation. N. Engl. J. Med. 2019;381:1148–1157. doi: 10.1056/NEJMra1804281. [DOI] [PubMed] [Google Scholar]
- Ganz T., Nemeth E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta. 2012;1823:1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L.W., Wang Q., Lou Z., Rao Z. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Lopez R.A., Kozak J.J., Gray H.B. Copper(II) inhibition of the SARS-CoV-2 main protease. ChemRxiv Prepr. Serv. Chem. 2020 doi: 10.26434/chemrxiv.12673436. [DOI] [Google Scholar]
- Gibbs P.N., Gore M.G., Jordan P.M. Investigation of the effect of metal ions on the reactivity of thiol groups in human 5-aminolaevulinate dehydratase. Biochem. J. 1985;225:573–580. doi: 10.1042/bj2250573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind V., Bharadwaj S., Sai Ganesh M.R., Vishnu J., Shankar K.V., Shankar B., Rajesh R. Antiviral properties of copper and its alloys to inactivate covid-19 virus: a review. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2021;34:1217–1235. doi: 10.1007/s10534-021-00339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes J.M., Grimes K.V. p38 MAPK inhibition: a promising therapeutic approach for COVID-19. J. Mol. Cell. Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y., Maciorowski D., Medernach B., Becker D.P., Durvasula R., Libertin C.R., Kempaiah P. Iron dysregulation in COVID-19 and reciprocal evolution of SARS-CoV-2: natura nihil frustra facit. J. Cell. Biochem. 2022 doi: 10.1002/jcb.30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gvozdjakova A., Klauco F., Kucharska J., Sumbalova Z. Is mitochondrial bioenergetics and coenzyme Q10 the target of a virus causing COVID-19. Bratisl. Lek. Listy. 2020;121:775–778. doi: 10.4149/BLL_2020_126. [DOI] [PubMed] [Google Scholar]
- Habib H.M., Ibrahim S., Zaim A., Ibrahim W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021;136 doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.-A., Merkling S.H., Treluyer J.-M., Veyer D., Mouthon L., Blanc C., Tharaux P.-L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S., Yamamoto Norio, Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto Naoki, Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. USA. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., Jiang Y., Cheng X., Zhu C., Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen H.S., Kokic G., Farnung L., Dienemann C., Tegunov D., Cramer P. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584:154–156. doi: 10.1038/s41586-020-2368-8. [DOI] [PubMed] [Google Scholar]
- Hoepel W., Chen H.-J., Allahverdiyeva S., Manz X., Aman J., Amsterdam UMC COVID-19 Biobank, Bonta P., Brouwer P., de Taeye S., Caniels T., van der Straten K., Golebski K., Griffith G., Jonkers R., Larsen M., Linty F., Neele A., Nouta J., van Baarle F., van Drunen C., Vlaar A., de Bree G., Sanders R., Willemsen L., Wuhrer M., Bogaard H.J., van Gils M., Vidarsson G., de Winther M., den Dunnen J. Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses. Immunology. 2020 doi: 10.1101/2020.07.13.190140. [DOI] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönscheid A., Rink L., Haase H. T-lymphocytes: a target for stimulatory and inhibitory effects of zinc ions. Endocr. Metab. Immune Disord. Drug Targets. 2009;9:132–144. doi: 10.2174/187153009788452390. [DOI] [PubMed] [Google Scholar]
- Hopkins R.G., Failla M.L. Copper deficiency reduces interleukin-2 (IL-2) production and IL-2 mRNA in human T-lymphocytes. J. Nutr. 1997;127:257–262. doi: 10.1093/jn/127.2.257. [DOI] [PubMed] [Google Scholar]
- Horowitz R.I., Freeman P.R., Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Respir. Med. Case Rep. 2020;30 doi: 10.1016/j.rmcr.2020.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini M., Chin A.W.H., Behzadinasab S., Poon L.L.M., Ducker W.A. Cupric oxide coating that rapidly reduces infection by SARS-CoV-2 via solids. ACS Appl. Mater. Interfaces. 2021;13:5919–5928. doi: 10.1021/acsami.0c19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A.C.-Y., Wang G., Reid A.T., Veerati P.C., Pathinayake P.S., Daly K., Mayall J.R., Hansbro P.M., Horvat J.C., Wang F., Wark P.A. SARS-CoV-2 Spike protein promotes hyper-inflammatory response that can be ameliorated by Spike-antagonistic peptide and FDA-approved ER stress and MAP kinase inhibitors in vitro. bioRxiv. 2020 doi: 10.1101/2020.09.30.317818. [DOI] [Google Scholar]
- Hsu J.T.-A., Kuo C.-J., Hsieh H.-P., Wang Y.-C., Huang K.-K., Lin C.P.-C., Huang P.-F., Chen X., Liang P.-H. Evaluation of metal-conjugated compounds as inhibitors of 3CL protease of SARS-CoV. FEBS Lett. 2004;574:116–120. doi: 10.1016/j.febslet.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620937175. 1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein R.K., Elkhair H.M. Molecular docking identification for the efficacy of some zinc complexes with chloroquine and hydroxychloroquine against main protease of COVID-19. J. Mol. Struct. 2021;1231 doi: 10.1016/j.molstruc.2021.129979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I. Iakovidis I. Delimaris S.M. Piperakis. Copper and its complexes in medicine: a biochemical approach Mol. Biol. Int. 2011, (2011), (594529) https://doi.org/10.4061/2011/594529. [DOI] [PMC free article] [PubMed]
- Imlay J.A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006;59:1073–1082. doi: 10.1111/j.1365-2958.2006.05028.x. [DOI] [PubMed] [Google Scholar]
- Irving A.T., Zhang Q., Kong P.-S., Luko K., Rozario P., Wen M., Zhu F., Zhou P., Ng J.H.J., Sobota R.M., Wang L.-F. Interferon regulatory factors IRF1 and IRF7 directly regulate gene expression in bats in response to viral infection. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail H.T.H. Hematobiochemical disturbances and oxidative stress after subacute manganese chloride exposure and potential protective effects of ebselen in rats. Biol. Trace Elem. Res. 2019;187:452–463. doi: 10.1007/s12011-018-1395-x. [DOI] [PubMed] [Google Scholar]
- Jansen J.M., Gerlach T., Elbahesh H., Rimmelzwaan G.F., Saletti G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019;119:44–52. doi: 10.1016/j.jcv.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Jarosz M., Olbert M., Wyszogrodzka G., Młyniec K., Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang Xinglou, You T., Liu Xiaoce, Yang Xiuna, Bai F., Liu H., Liu Xiang, Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P., Manoharan S., Ramani V., Narasimhan G., Kaliamoorthy I., Rela M. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V.E., Mao G., Qu F., Angeli J.P.F., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., Kapralov A.A., Amoscato A.A., Jiang J., Anthonymuthu T., Mohammadyani D., Yang Q., Proneth B., Klein-Seetharaman J., Watkins S., Bahar I., Greenberger J., Mallampalli R.K., Stockwell B.R., Tyurina Y.Y., Conrad M., Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfaoglu B., Almeida-Santos J., Tye C.A., Satou Y., Ono M. T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.589380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front. Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Ward A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019;10:2342. doi: 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircheis R., Haasbach E., Lueftenegger D., Heyken W.T., Ocker M., Planz O. NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.598444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstein-Wiedemann R., Stadtmüller M., Traikov S., Georgi M., Teichert M., Yosef H., Wallenborn J., Karl A., Schütze K., Wagner M., El-Armouche A., Tonn T. SARS-CoV-2 infects red blood cell progenitors and dysregulates hemoglobin and iron metabolism. Stem Cell Rev. Rep. 2022 doi: 10.1007/s12015-021-10322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.-J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine J.T., Tuomainen T.-P., Salonen J.T., Virtanen J.K. Serum copper-to-zinc-ratio and risk of incident infection in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur. J. Epidemiol. 2020;35:1149–1156. doi: 10.1007/s10654-020-00644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Las Heras N., Martín Giménez V.M., Ferder L., Manucha W., Lahera V. Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID-19: therapeutic effects of vitamin D. Antioxidants. 2020;9 doi: 10.3390/antiox9090897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri M., Lanza A., Bellini R., Bellofiore A., Cecchetto S., Colombo A., D’Abrosca F., Del Monaco C., Gaudiello G., Paneroni M., Privitera E., Retucci M., Rossi V., Santambrogio M., Sommariva M., Frigerio P. Respiratory physiotherapy in patients with COVID-19 infection in acute setting: a Position Paper of the Italian Association of Respiratory Physiotherapists (ARIR) Monaldi Arch. Chest Dis. Arch. Monaldi Mal. Torace. 2020:90. doi: 10.4081/monaldi.2020.1285. [DOI] [PubMed] [Google Scholar]
- Lechuga G.C., Souza-Silva F., Sacramento C.Q., Trugilho M.R.O., Valente R.H., Napoleão-Pêgo P., Dias S.S.G., Fintelman-Rodrigues N., Temerozo J.R., Carels N., Alves C.R., Pereira M.C.S., Provance D.W., Souza T.M.L., De-Simone S.G. SARS-CoV-2 proteins bind to hemoglobin and its metabolites. Int. J. Mol. Sci. 2021;22:9035. doi: 10.3390/ijms22169035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxid. Med. Cell. Longev. 2018;2018:6208067. doi: 10.1155/2018/6208067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-C., Kuo C.-J., Hsu M.-F., Liang P.-H., Fang J.-M., Shie J.-J., Wang A.H.-J. Structural basis of mercury- and zinc-conjugated complexes as SARS-CoV 3C-like protease inhibitors. FEBS Lett. 2007;581:5454–5458. doi: 10.1016/j.febslet.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.H., Arthur J.R., Riemersma R.A., Nicol F., Walker S.W., Millar E.M., Howie A.F., Beckett G.J. Selenium supplementation acting through the induction of thioredoxin reductase and glutathione peroxidase protects the human endothelial cell line EAhy926 from damage by lipid hydroperoxides. Biochim. Biophys. Acta. 2002;1593:85–92. doi: 10.1016/s0167-4889(02)00333-6. [DOI] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Wang R., Zou W., Sun X., Liu X., Zhao L., Wang S., Jin M. The influenza virus H5N1 infection can induce ROS production for viral replication and host cell death in A549 cells modulated by human Cu/Zn superoxide dismutase (SOD1) overexpression. Viruses. 2016;8 doi: 10.3390/v8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.-J., Bao S., Gálvez-Peralta M., Pyle C.J., Rudawsky A.C., Pavlovicz R.E., Killilea D.W., Li C., Nebert D.W., Wewers M.D., Knoell D.L. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Radiolabeled cyclic RGD peptides as integrin alpha(v)beta(3)-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug. Chem. 2009;20:2199–2213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li H. COVID-19: attacks the 1-beta chain of hemoglobin to disrupt respiratory function and escape immunity. Biol. Med. Chem. 2022 doi: 10.26434/chemrxiv-2021-dtpv3-v11. [DOI] [Google Scholar]
- Liu W., Zhang S., Nekhai S., Liu S. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr. Clin. Microbiol. Rep. 2020;7:13–19. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y., Chen L., Liang X., Liu X., Gao M., Wang Q., Wei Q., Liu L. Association between iron status and the risk of adverse outcomes in COVID-19. Clin. Nutr. 2021;40:3462–3469. doi: 10.1016/j.clnu.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach W.J., Thimmesch A.R., Pierce J.T., Pierce J.D. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs. Res. Pract. 2011;2011 doi: 10.1155/2011/260482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio N., Lafont B.A.P., Sil D., Li Y., Bollinger J.M., Krebs C., Pierson T.C., Linehan W.M., Rouault T.A. Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets. Science. 2021;373:236–241. doi: 10.1126/science.abi5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Lauer Y., Carbajo-Lozoya J., Hein M.Y., Müller M.A., Deng W., Lei J., Meyer B., Kusov Y., von Brunn B., Bairad D.R., Hünten S., Drosten C., Hermeking H., Leonhardt H., Mann M., Hilgenfeld R., von Brunn A. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc. Natl. Acad. Sci. USA. 2016;113:E5192–E5201. doi: 10.1073/pnas.1603435113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. Inhibitory zinc sites in enzymes. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2013;26:197–204. doi: 10.1007/s10534-013-9613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. The metals in the biological periodic system of the elements: concepts and conjectures. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywald M., Rink L. Zinc supplementation induces CD4+CD25+Foxp3+ antigen-specific regulatory T cells and suppresses IFN-γ production by upregulation of Foxp3 and KLF-10 and downregulation of IRF-1. Eur. J. Nutr. 2017;56:1859–1869. doi: 10.1007/s00394-016-1228-7. [DOI] [PubMed] [Google Scholar]
- Mazur A., Feillet-Coudray C., Romier B., Bayle D., Gueux E., Ruivard M., Coudray C., Rayssiguier Y. Dietary iron regulates hepatic hepcidin 1 and 2 mRNAs in mice. Metabolism. 2003;52:1229–1231. doi: 10.1016/s0026-0495(03)00277-4. [DOI] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S., Walker S.W., Arthur J.R., Nicol F., Pickard K., Lewin M.H., Howie A.F., Beckett G.J. Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin. Sci. 2001;1979(100):543–550. [PubMed] [Google Scholar]
- Mills E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E., Tourlomousis P., Däbritz J.H.M., Gottlieb E., Latorre I., Corr S.C., McManus G., Ryan D., Jacobs H.T., Szibor M., Xavier R.J., Braun T., Frezza C., Murphy M.P., O’Neill L.A. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470. doi: 10.1016/j.cell.2016.08.064. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., Hackler J., Seemann P., Diegmann J., Pilz M., Bachmann M., Minich W.B., Schomburg L. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12 doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monette A., Mouland A.J. Zinc and copper ions differentially regulate prion-like phase separation dynamics of pan-virus nucleocapsid biomolecular condensates. Viruses. 2020;12 doi: 10.3390/v12101179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira A.C., Mesquita G., Gomes M.S. Ferritin: an inflammatory player keeping iron at the core of pathogen-host interactions. Microorganisms. 2020;8 doi: 10.3390/microorganisms8040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M.J., Liu Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E., Tuttle M.S., Powelson J., Vaughn M.B., Donovan A., Ward D.M., Ganz T., Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novello F., Stirpe F. The effects of copper and other ions on the ribonucleic acid polymerase activity of isolated rat liver nuclei. Biochem. J. 1969;111:115–119. doi: 10.1042/bj1110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce J.O., Michels H., Keevil C.W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007;73:2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B., Hu J., Goldberg D., Reynolds P., Hertz A., Bernstein L., Kleeman M.J. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study Cohort. Environ. Health Perspect. 2015;123:549–556. doi: 10.1289/ehp.1408565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A., Squitti R., Picozza M., Pawar A., Rongioletti M., Dutta A.K., Sahoo S., Goswami K., Sharma P., Prasad R. Zinc and COVID-19: basis of current clinical trials. Biol. Trace Elem. Res. 2021;199:2882–2892. doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchariya L., Khan W.A., Kuila S., Sonkar K., Sahoo S., Ghoshal A., Kumar A., Verma D.K., Hasan A., Khan M.A., Jain N., Mohapatra A.K., Das S., Thakur J.K., Maiti S., Nanda R.K., Halder R., Sunil S., Arockiasamy A. Zinc2+ ion inhibits SARS-CoV-2 main protease and viral replication in vitro. Chem. Commun. 2021;57:10083–10086. doi: 10.1039/d1cc03563k. [DOI] [PubMed] [Google Scholar]
- Parimoo A., Biswas A., Baitha U., Gupta G., Pandey S., Ranjan P., Gupta V., Barman Roy D., Prakash B., Wig N. Dynamics of inflammatory markers in predicting mortality in COVID-19. Cureus. 2021;13 doi: 10.7759/cureus.19080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.S., Kim S.R., Lee Y.C. Impact of oxidative stress on lung diseases. Respirol. Carlton Vic. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- Parvez M.S.A., Karim M.A., Hasan M., Jaman J., Karim Z., Tahsin T., Hasan M.N., Hosen M.J. Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach. Int. J. Biol. Macromol. 2020;163:1787–1797. doi: 10.1016/j.ijbiomac.2020.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone C., Bartoloni E., Bursi R., Cafaro G., Guidelli G.M., Shoenfeld Y., Gerli R. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol. Res. 2020;68:213–224. doi: 10.1007/s12026-020-09145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinno J., Bongartz H., Klepsch O., Wundrack N., Poli V., Schaper F., Dittrich A. Interleukin-6 influences stress-signalling by reducing the expression of the mTOR-inhibitor REDD1 in a STAT3-dependent manner. Cell. Signal. 2016;28:907–916. doi: 10.1016/j.cellsig.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Podsiadlo P., Komiyama T., Fuller R.S., Blum O. Furin inhibition by compounds of copper and zinc. J. Biol. Chem. 2004;279:36219–36227. doi: 10.1074/jbc.M400338200. [DOI] [PubMed] [Google Scholar]
- Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020;6:1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- Poppe M., Wittig S., Jurida L., Bartkuhn M., Wilhelm J., Müller H., Beuerlein K., Karl N., Bhuju S., Ziebuhr J., Schmitz M.L., Kracht M. The NF-κB-dependent and -independent transcriptome and chromatin landscapes of human coronavirus 229E-infected cells. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pormohammad A., Monych N.K., Turner R.J. Zinc and SARS‑CoV‑2: a molecular modeling study of Zn interactions with RNA‑dependent RNA‑polymerase and 3C‑like proteinase enzymes. Int. J. Mol. Med. 2021;47:326–334. doi: 10.3892/ijmm.2020.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S.R. The antioxidant properties of zinc. J. Nutr. 2000;130:1447S–1454SS. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Zinc: mechanisms of host defense. J. Nutr. 2007;137:1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008;43:370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS. 2014;28:364–371. doi: 10.1016/j.jtemb.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Pujani M., Raychaudhuri S., Singh M., Kaur H., Agarwal S., Jain M., Chandoke R.K., Singh K., Sidam D., Chauhan V. An analysis of hematological, coagulation and biochemical markers in COVID-19 disease and their association with clinical severity and mortality: an Indian outlook. Am. J. Blood Res. 2021;11:580–591. [PMC free article] [PubMed] [Google Scholar]
- Pvsn K.K., Tomo S., Purohit P., Sankanagoudar S., Charan J., Purohit A., Nag V., Bhatia P., Singh K., Dutt N., Garg M.K., Sharma P., Misra S., Yadav D. Comparative analysis of serum zinc, copper and magnesium level and their relations in association with severity and mortality in SARS-CoV-2 patients. Biol. Trace Elem. Res. 2022 doi: 10.1007/s12011-022-03124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartuccio L., Fabris M., Sonaglia A., Peghin M., Domenis R., Cifù A., Curcio F., Tascini C. Interleukin 6, soluble interleukin 2 receptor alpha (CD25), monocyte colony-stimulating factor, and hepatocyte growth factor linked with systemic hyperinflammation, innate immunity hyperactivation, and organ damage in COVID-19 pneumonia. Cytokine. 2021;140 doi: 10.1016/j.cyto.2021.155438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha S., Mallick R., Basak S., Duttaroy A.K. Is copper beneficial for COVID-19 patients? Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.T., Idid S.Z. Can Zn be a critical element in COVID-19 treatment. Biol. Trace Elem. Res. 2021;199:550–558. doi: 10.1007/s12011-020-02194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G. Hypoalbuminemia: an underestimated, vital characteristic of hospitalized COVID-19 positive patients? Hepatoma Res. 2020;6:28. doi: 10.20517/2394-5079.2020.43. [DOI] [Google Scholar]
- Ramaiah M.J. mTOR inhibition and p53 activation, microRNAs: the possible therapy against pandemic COVID-19. Gene Rep. 2020;20 doi: 10.1016/j.genrep.2020.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani I., Goyal A., Bhatnagar M., Manhas S., Goel P., Pal A., Prasad R. Potential molecular mechanisms of zinc- and copper-mediated antiviral activity on COVID-19. Nutr. Res. 2021;92:109–128. doi: 10.1016/j.nutres.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo N., Zaldívar-López S., Garrido J.J., Montoya M. SARS-CoV-2 accessory proteins in viral pathogenesis: knowns and unknowns. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.708264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regis G., Bosticardo M., Conti L., De Angelis S., Boselli D., Tomaino B., Bernabei P., Giovarelli M., Novelli F. Iron regulates T-lymphocyte sensitivity to the IFN-gamma/STAT1 signaling pathway in vitro and in vivo. Blood. 2005;105:3214–3221. doi: 10.1182/blood-2004-07-2686. [DOI] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscitti P., Berardicurti O., Barile A., Cipriani P., Shoenfeld Y., Iagnocco A., Giacomelli R. Severe COVID-19 and related hyperferritinaemia: more than an innocent bystander. Ann. Rheum. Dis. 2020;79:1515–1516. doi: 10.1136/annrheumdis-2020-217618. [DOI] [PubMed] [Google Scholar]
- Saakre M., Mathew D., Ravisankar V. Perspectives on plant flavonoid quercetin-based drugs for novel SARS-CoV-2. Beni-Suef Univ. J. Basic Appl. Sci. 2021;10:21. doi: 10.1186/s43088-021-00107-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagripanti J.L., Routson L.B., Lytle C.D. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl. Environ. Microbiol. 1993;59:4374–4376. doi: 10.1128/aem.59.12.4374-4376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagripanti J.L., Routson L.B., Bonifacino A.C., Lytle C.D. Mechanism of copper-mediated inactivation of herpes simplex virus. Antimicrob. Agents Chemother. 1997;41:812–817. doi: 10.1128/AAC.41.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale L.A., Torres D.J., Berry M.J., Pitts M.W. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020;112:447–448. doi: 10.1093/ajcn/nqaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifera A.S. The zinc finger domain of IKKγ (NEMO) protein in health and disease. J. Cell. Mol. Med. 2010;14:2404–2414. doi: 10.1111/j.1582-4934.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Kondo S., Sabe H., Ishida N., Honjo T. Structure and function of the interleukin 2 receptor: affinity conversion model. Immunol. Rev. 1986;92:103–120. doi: 10.1111/j.1600-065x.1986.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojadoost B., Kulkarni R.R., Yitbarek A., Laursen A., Taha-Abdelaziz K., Negash Alkie T., Barjesteh N., Quinteiro-Filho W.M., Smith T.K., Sharif S. Dietary selenium supplementation enhances antiviral immunity in chickens challenged with low pathogenic avian influenza virus subtype H9N2. Vet. Immunol. Immunopathol. 2019;207:62–68. doi: 10.1016/j.vetimm.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Sies H., Parnham M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020;156:107–112. doi: 10.1016/j.freeradbiomed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J., Tsatsakis A., Tinkov A.A. Zinc and respiratory tract infections: perspectives for COVID‑19 (Review) Int. J. Mol. Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford S.L., Bokil N.J., Achard M.E.S., Kapetanovic R., Schembri M.A., McEwan A.G., Sweet M.J. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci. Rep. 2013;33 doi: 10.1042/BSR20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., Noel K., Jiang X., Linkermann A., Murphy M.E., Overholtzer M., Oyagi A., Pagnussat G.C., Park J., Ran Q., Rosenfeld C.S., Salnikow K., Tang D., Torti F.M., Torti S.V., Toyokuni S., Woerpel K.A., Zhang D.D. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm B., Twaroch T., Knapitsch B., Czingraber S., Ternes N., Goldenberg H., Scheiber-Mojdehkar B. Differential response of iron metabolism to oxidative stress generated by antimycin A and nitrofurantoin. Biochimie. 2006;88:575–581. doi: 10.1016/j.biochi.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N., Laatsch B., Narkiewicz-Jodko A., Johnson B., Liebau J., Bhattacharyya S., Hati S. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;39:644–656. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamynathan S.K. Krüppel-like factors: three fingers in control. Hum. Genom. 2010;4:263–270. doi: 10.1186/1479-7364-4-4-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneri P.E., Gómez-Ochoa S.A., Llanaj E., Raguindin P.F., Rojas L.Z., Roa-Díaz Z.M., Salvador D., Groothof D., Minder B., Kopp-Heim D., Hautz W.E., Eisenga M.F., Franco O.H., Glisic M., Muka T. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur. J. Epidemiol. 2020;35:763–773. doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., Zheng L., Gardet A., Tong Z., Jany S.S., Corr S.C., Haneklaus M., Caffrey B.E., Pierce K., Walmsley S., Beasley F.C., Cummins E., Nizet V., Whyte M., Taylor C.T., Lin H., Masters S.L., Gottlieb E., Kelly V.P., Clish C., Auron P.E., Xavier R.J., O.’Neill L.a.J. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapazoglou E., Prasad A.S., Hill G., Brewer G.J., Kaplan J. Decreased natural killer cell activity in patients with zinc deficiency with sickle cell disease. J. Lab. Clin. Med. 1985;105:19–22. [PubMed] [Google Scholar]
- Taylor E.W., Radding W. Understanding selenium and glutathione as antiviral factors in COVID-19: does the viral Mpro protease target host selenoproteins and glutathione synthesis. Front. Nutr. 2020;7:143. doi: 10.3389/fnut.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M., Xiao S.-Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A., Patane M.A., Pantoliano M.W. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279:17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk M.J., Fazel N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009;25:136–143. doi: 10.1097/MOG.0b013e328321b395. [DOI] [PubMed] [Google Scholar]
- Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente A.J., Yoshida T., Murthy S.N., Sakamuri S.S.V.P., Katsuyama M., Clark R.A., Delafontaine P., Chandrasekar B. Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H282–H296. doi: 10.1152/ajpheart.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Jomova K., Rhodes C.J., Kuča K., Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016;90:1–37. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- Van Agthoven J.F., Xiong J.-P., Alonso J.L., Rui X., Adair B.D., Goodman S.L., Arnaout M.A. Structural basis for pure antagonism of integrin αVβ3 by a high-affinity form of fibronectin. Nat. Struct. Mol. Biol. 2014;21:383–388. doi: 10.1038/nsmb.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschelden G., Noeparast M., Noparast M., Goossens M.C., Lauwers M., Cotton F., Michel C., Goyvaerts C., Hites M. Plasma zinc status and hyperinflammatory syndrome in hospitalized COVID-19 patients: an observational study. Int. Immunopharmacol. 2021;100 doi: 10.1016/j.intimp.2021.108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F., Acton S., Patane M., Nichols A., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Viola Null, Muhammad N., Khan I.N., Ali Z., Ibrahim M., Shujah S., Ali S., Ikram M., Rehman S., Khan G.S., Wadood A., Noor A., Schulzke C. Synthesis, characterization, antioxidant, antileishmanial, anticancer, DNA and theoretical SARS-CoV-2 interaction studies of copper(II) carboxylate complexes. J. Mol. Struct. 2022;1253 doi: 10.1016/j.molstruc.2021.132308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulpe C.D., Kuo Y.M., Murphy T.L., Cowley L., Askwith C., Libina N., Gitschier J., Anderson G.J. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat. Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) Med. Biol. 2020 doi: 10.1101/2020.02.10.20021832. [DOI] [Google Scholar]
- Wang C., Liu Z., Chen Z., Huang X., Xu M., He T., Zhang Z. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J. Med. Virol. 2020;92:667–674. doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., Liu W., Zhu Y., Lin Q., Mao L., Fang M., Zhang H., Sun Z. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Green M., Choi J.E., Gijón M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A., Xia H., Zhou J., Li G., Li J., Li W., Wei S., Vatan L., Zhang H., Szeliga W., Gu W., Liu R., Lawrence T.S., Lamb C., Tanno Y., Cieslik M., Stone E., Georgiou G., Chan T.A., Chinnaiyan A., Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Huang J., Sun Y., Stubbs D., He J., Li W., Wang F., Liu Z., Ruzicka J.A., Taylor E.W., Rayman M.P., Wan X., Zhang J. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021;153 doi: 10.1016/j.fct.2021.112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6 doi: 10.1128/mBio.01697-15. e01697-01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Węglarz-Tomczak E., Tomczak J.M., Talma M., Brul S. Ebselen as a highly active inhibitor of PLProCoV2. Biol. Chem. 2020 doi: 10.1101/2020.05.17.100768. [DOI] [Google Scholar]
- Wei Y., Sowers J.R., Nistala R., Gong H., Uptergrove G.M.-E., Clark S.E., Morris E.M., Szary N., Manrique C., Stump C.S. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J. Biol. Chem. 2006;281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- Wellinghausen N., Martin M., Rink L. Zinc inhibits interleukin-1-dependent T cell stimulation. Eur. J. Immunol. 1997;27:2529–2535. doi: 10.1002/eji.1830271010. [DOI] [PubMed] [Google Scholar]
- Wenjun W., Xiaoqing L., Sipei W., Puyi L., Liyan H., Yimin L., Linling C., Sibei C., Lingbo N., Yongping L., Jianxing H. The definition and risks of cytokine release syndrome-like in 11 COVID-19-infected pneumonia critically ill patients. Dis. Charact. Retrosp. Anal. 2020 doi: 10.1101/2020.02.26.20026989. [DOI] [Google Scholar]
- Wessling-Resnick M. Crossing the iron gate: why and how transferrin receptors mediate viral entry. Annu. Rev. Nutr. 2018;38:431–458. doi: 10.1146/annurev-nutr-082117-051749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire J.K., Ahmed R. Costimulation in antiviral immunity: differential requirements for CD4(+) and CD8(+) T cell responses. Curr. Opin. Immunol. 2000;12:448–455. doi: 10.1016/s0952-7915(00)00119-9. [DOI] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.-R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou Xing, Xu S., Huang H., Zhang L., Zhou Xia, Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou Xin, Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Lee T.-H., Huang R.-T., Guzy D., Schoettler R., Adegunsoye N., Mueller A., Husain J., A., I Sperling A., Mutlu G.M., Fang Y. SARS-CoV-2 infection is associated with reduced Krüppel-like factor 2 in human lung autopsy. Am. J. Respir. Cell Mol. Biol. 2021;65:222–226. doi: 10.1165/rcmb.2020-0564LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.A., Fossali T., Pandolfi L., Carsana L., Ottolina D., Frangipane V., Rech R., Tosoni A., Lopez G., Agarossi A., Cogliati C., Meloni F., Marchini B., Nebuloni M., Catena E., Colombo R. Hypoalbuminemia in COVID-19: assessing the hypothesis for underlying pulmonary capillary leakage. J. Intern. Med. 2021;289:861–872. doi: 10.1111/joim.13208. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Wang Y., Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12 doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Liu Y., Ding Y., Luo S., Zheng X., Wu X., Liu Z., Ilyas I., Chen S., Han S., Little P.J., Jain M.K., Weng J. The zinc finger transcription factor, KLF2, protects against COVID-19 associated endothelial dysfunction. Signal Transduct. Target. Ther. 2021;6:266. doi: 10.1038/s41392-021-00690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J.Y., Zhang Y., Wu Y., Cannesson M., Cai H. Therapeutic application of estrogen for COVID-19: Attenuation of SARS-CoV-2 spike protein and IL-6 stimulated, ACE2-dependent NOX2 activation, ROS production and MCP-1 upregulation in endothelial cells. Redox Biol. 2021;46 doi: 10.1016/j.redox.2021.102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L. The Yin and Yang of ACE/ACE2 pathways: the rationale for the use of renin-angiotensin system inhibitors in COVID-19 patients. Cells. 2020;9 doi: 10.3390/cells9071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L. Upregulation of the renin-angiotensin system pathways and SARS-CoV-2 infection: the rationale for the administration of zinc-chelating agents in COVID-19 patients. Cells. 2021;10:506. doi: 10.3390/cells10030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H.-L., Zhang B., Wang X., Yang Q., Cheng L. Urinary trace elements in association with disease severity and outcome in patients with COVID-19. Environ. Res. 2021;194 doi: 10.1016/j.envres.2020.110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J., Bo Y., Lu Y., Liu C., Zhang L. Effects of coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-J., Qin J.-J., Cheng X., Shen L., Zhao Y.-C., Yuan Y., Lei F., Chen M.-M., Yang H., Bai L., Song X., Lin L., Xia M., Zhou F., Zhou J., She Z.-G., Zhu L., Ma X., Xu Q., Ye P., Chen G., Liu L., Mao W., Yan Y., Xiao B., Lu Z., Peng G., Liu M., Yang Jun, Yang L., Zhang C., Lu H., Xia X., Wang D., Liao X., Wei X., Zhang B.-H., Zhang X., Yang Juan, Zhao G.-N., Zhang P., Liu P.P., Loomba R., Ji Y.-X., Xia J., Wang Y., Cai J., Guo J., Li H. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187. doi: 10.1016/j.cmet.2020.06.015. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K., Huang J., Dai D., Feng Y., Liu L., Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa250. ofaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Chen Y., Ji Y., He X., Xue D. Increased serum levels of hepcidin and ferritin are associated with severity of COVID-19. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.926178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Zeng R., von Brunn A., Lei J. Structural characterization of the C-terminal domain of SARS-CoV-2 nucleocapsid protein. Mol. Biomed. 2020;1:2. doi: 10.1186/s43556-020-00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]