Abstract
The outbreak of the B.1.1.529 lineage of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Omicron) has caused an unprecedented number of Coronavirus Disease 2019 (COVID-19) cases, including pediatric hospital admissions. Policymakers urgently need evidence of vaccine effectiveness in children to balance the costs and benefits of vaccination campaigns, but, to date, the evidence is sparse. Leveraging a population-based cohort in Chile of 490,694 children aged 3–5 years, we estimated the effectiveness of administering a two-dose schedule, 28 days apart, of Sinovac’s inactivated SARS-CoV-2 vaccine (CoronaVac). We used inverse probability-weighted survival regression models to estimate hazard ratios of symptomatic COVID-19, hospitalization and admission to an intensive care unit (ICU) for children with complete immunization over non-vaccination, accounting for time-varying vaccination exposure and relevant confounders. The study was conducted between 6 December 2021 and 26 February 2022, during the Omicron outbreak in Chile. The estimated vaccine effectiveness was 38.2% (95% confidence interval (CI), 36.5–39.9) against symptomatic COVID-19, 64.6% (95% CI, 49.6–75.2) against hospitalization and 69.0% (95% CI, 18.6–88.2) against ICU admission. The effectiveness against symptomatic COVID-19 was modest; however, protection against severe disease was high. These results support vaccination of children aged 3–5 years to prevent severe illness and associated complications and highlight the importance of maintaining layered protections against SARS-CoV-2 infection.
Subject terms: Epidemiology, Viral infection
CoronaVac protects young children from severe COVID-19 during a SARS-CoV-2 Omicron surge, supporting the effectiveness and importance of vaccinating this pediatric population.
Main
The emergence and spread of the B.1.1.529 lineage of SARS-CoV-2, the cause of COVID-19, has caused an unprecedented number of infections worldwide in a short period1,2. Emerging evidence suggests that Omicron causes less severe disease than previous variants of concern (VOCs), probably due to lower virulence, infection-acquired immunity and higher vaccination coverage3–6. However, its high transmissibility and ability to partially evade the immune response induced has been associated with a substantial increase in severe COVID-19 cases globally2. The absolute number of pediatric hospital admissions has also surpassed previous waves4,7,8, straining healthcare systems even further. The increase may be related to higher transmissibility of Omicron, less use of face masks in children and, especially concerning, lower vaccination rates among children.
Policymakers urgently need evidence of the effectiveness of vaccines in preventing severe clinical presentations of COVID-19 in children to balance the costs and benefits of mass vaccination campaigns in this population. Although the risk of severe COVID-19 in healthy children is substantially lower than among adults, vaccinating children may reduce community transmission, avoid potentially life-threatening presentations such as multisystemic inflammatory syndrome in children (MIS-C) or pediatric inflammatory multisystem syndrome (PIMS) and prevent long-term consequences of SARS-CoV-2 infection9. Although many countries are vaccinating children, few have authorized COVID-19 vaccines for children under 5 years of age, and some have restricted vaccines for children older than 12 years10. Evidence of the efficacy or effectiveness of COVID-19 vaccines in children is limited, primarily related to mRNA vaccines, and only two studies were conducted during the Omicron outbreak11–15. To our knowledge, there is no published evidence of vaccine effectiveness against COVID-19 in young children under 5 years of age. Furthermore, recent research suggests that several COVID-19 vaccine platforms provide limited protection against infection and symptomatic disease caused by the Omicron variant but were more effective against severe disease16–18. These studies have examined vaccine protection against Omicron in adult populations but are consistent with preliminary, unpublished results from a study in children aged 5–12 years13.
Leveraging a population-based cohort of children aged 3–5 years, we estimated the effectiveness of the complete primary immunization schedule (two doses, 28 days apart) of an inactivated SARS-CoV-2 vaccine, CoronaVac, to prevent laboratory-confirmed, symptomatic COVID-19, hospitalization and admission to an ICU. We estimated vaccine effectiveness using inverse probability-weighted survival regression models to estimate hazard ratios of complete immunization (starting 14 days after the second dose) over the unvaccinated status, accounting for time-varying vaccination exposure and available clinical, demographic and socioeconomic confounders at baseline.
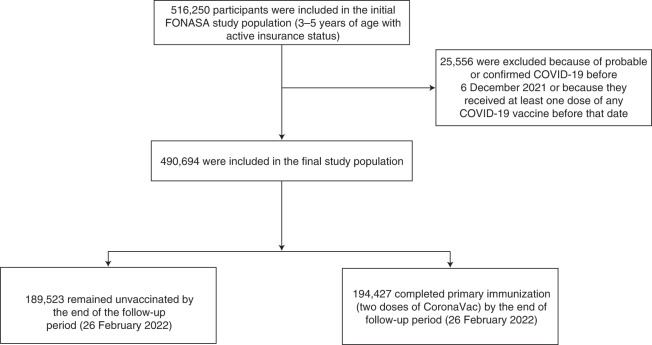
Our study cohort included 516,250 children aged 3–5 years affiliated with the Fondo Nacional de Salud (FONASA), the public national healthcare system of Chile. In total, 490,694 children were included in the final study population; 194,427 had received two doses of CoronaVac, 28 days apart between 6 December 2021 and 26 February 2022; and 189,523 had not received any COVID-19 vaccination by the end of the follow-up period. On 25 November 2021, the Public Health Institute of Chile authorized the emergency use of CoronaVac on young children (3–5 years) and began vaccinating on 6 December 2021. CoronaVac was the only COVID-19 vaccine authorized for young children during the study period. We excluded children who had probable or confirmed COVID-19 according to reverse transcription polymerase chain reaction (RT–PCR) assay for SARS-CoV-2 or antigen test before 6 December 2021, reported to the Ministry of Health (Fig. 1). The cohort characteristics are described in Extended Data Tables 1 and 2. We found statistically significant differences (P < 0.001) in the incidence of COVID-19 and according to vaccination status by children’s sex, age group, comorbidities, nationality, region of residence and insurance category, which justify their inclusion in the models. Vaccination rollout was organized through a public schedule; children needed to show up at their nearest vaccination site with their national ID card (Extended Data Fig. 1). The study period overlapped with that of the Omicron outbreak in Chile (with the Omicron BA.1.1 sublineage predominant), defined by whole-genome sequencing of a sample of the infecting variants circulating over time (Extended Data Tables 3–5 and Extended Data Fig. 2).
Fig. 1. Study participants and cohort eligibility, 6 December 2021 through 26 February 2022.
Participants were 3–5 years of age, affiliated to the FONASA, the public national healthcare system, and received two doses of CoronaVac, 28 days apart between 6 December 2021 and 26 February 2022 or did not receive any COVID-19 vaccination. We excluded children who had probable or confirmed COVID-19 according to RT–PCR assay for SARS-CoV-2 or antigen test before 6 December 2021.
Extended Data Table 1.
Characteristics of the study cohort of children affiliated to FONASA, overall, with laboratory-confirmed COVID-19 and the proportion receiving one or more doses of COVID-19 vaccines, 6 December 2021 through 26 February 2022a
| Vaccinated | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 | Unvaccinated | One dose | Two doses | |||||||
| Characteristic | No. | Col. % | No. | Row % | No. | Row % | No. | Row % | No. | Row % |
| Total | 490,694 | 100 | 14,512 | 3.0 | 189,523 | 38.6 | 106,744 | 21.8 | 194,427 | 39.6 |
| Sex | ||||||||||
| Female | 241,429 | 49 | 6,815 | 2.8 | 90,586 | 38 | 52,593 | 22 | 98,250 | 41 |
| Male | 249,265 | 51 | 7,697 | 3.1 | 98,937 | 40 | 54,151 | 22 | 96,177 | 39 |
| Cohort location | ||||||||||
| Arica | 7,488 | 1.5 | 304 | 4.1 | 3,739 | 50 | 1,774 | 24 | 1,975 | 26 |
| Tarapacá | 11,165 | 2.3 | 259 | 2.3 | 5,407 | 48 | 2,592 | 23 | 3,166 | 28 |
| Antofagasta | 16,652 | 3.4 | 350 | 2.1 | 6,440 | 39 | 3,937 | 24 | 6,275 | 38 |
| Atacama | 8,687 | 1.8 | 409 | 4.7 | 3,514 | 40 | 2,118 | 24 | 3,055 | 35 |
| Coquimbo | 24,079 | 4.9 | 733 | 3.0 | 9,909 | 41 | 5,578 | 23 | 8,592 | 36 |
| Valparaíso | 49,595 | 10.0 | 1,464 | 3.0 | 18,427 | 37 | 10,662 | 21 | 20,506 | 41 |
| Metropolitana | 181,781 | 37.0 | 3,925 | 2.2 | 67,537 | 37 | 38,348 | 21 | 75,896 | 42 |
| LB O’Higgins | 27,870 | 5.7 | 757 | 2.7 | 8,518 | 31 | 5,820 | 21 | 13,532 | 49 |
| Maule | 33,352 | 6.8 | 1,272 | 3.8 | 10,091 | 30 | 7,409 | 21 | 15,852 | 47 |
| Ñuble | 14,040 | 2.9 | 663 | 4.7 | 4,909 | 35 | 3,223 | 22 | 5,908 | 42 |
| Biobío | 43,107 | 8.8 | 1,857 | 4.3 | 16,564 | 38 | 10,090 | 23 | 16,453 | 38 |
| Araucanía | 31,150 | 6.3 | 951 | 3.1 | 14,083 | 45 | 6,460 | 23 | 10,607 | 34 |
| Los Ríos | 10,837 | 2.2 | 513 | 4.7 | 4,894 | 45 | 2,332 | 21 | 3,611 | 33 |
| Los Lagos | 24,781 | 5.1 | 777 | 3.1 | 12,878 | 52 | 5,026 | 22 | 6,877 | 28 |
| Aysén | 2,427 | 0.5 | 119 | 4.9 | 1,073 | 44 | 549 | 23 | 805 | 33 |
| Magallanes | 3,683 | 0.7 | 159 | 4.3 | 1,540 | 42 | 826 | 22 | 1,317 | 36 |
| Age group | ||||||||||
| 3 years | 161,379 | 33 | 4,816 | 3.0 | 76,259 | 47 | 33,096 | 21 | 52,024 | 32 |
| 4 years | 160,829 | 33 | 4,581 | 2.8 | 60,282 | 37 | 35,919 | 22 | 64,628 | 40 |
| 5 years | 168,486 | 34 | 5,115 | 3.0 | 52,982 | 31 | 37,729 | 22 | 77,775 | 46 |
| Comorbidities b | ||||||||||
| None | 445,074 | 90.7 | 12,669 | 2.8 | 174,187 | 39 | 96,221 | 22 | 174,666 | 39 |
| ≥1 | 45,620 | 9.3 | 1,843 | 4.0 | 15,336 | 34 | 10,523 | 23 | 19,761 | 43 |
| Nationality | ||||||||||
| Chilean | 484,715 | 98.8 | 14,404 | 3.0 | 186,988 | 39 | 105,613 | 22 | 192,114 | 39.6 |
| Non-Chilean | 5,979 | 1.2 | 108 | 1.8 | 2,535 | 42 | 1,131 | 19 | 2,313 | 38.7 |
aOn 6 September 2021, the Public Health Institute of Chile authorized the emergency use of CoronaVac for children aged 6 years and older and, on 25 November 2021, extended the age range to children starting at 3 years of age. The first children aged 3–5 years were vaccinated on 6 December 2021, prioritizing immunocompromised children and those with comorbidities, including chronic kidney disease, diabetes mellitus types 1 and 2, cancer, congenital heart disease and HIV. Our study cohort included children 3–5 years of age affiliated to FONASA, the national public health insurance program in Chile. We excluded children with probable or confirmed SARS-CoV-2 infection before 6 December 2021. The model also included health insurance category (a proxy of household income) and location (16 regions). We found statistically significant differences (P < 0.001) between patients with COVID-19 and the vaccinated and unvaccinated groups by sex, age group, comorbidities, nationality, region of residence and health insurance category.
bCoexisting conditions included chronic kidney disease, diabetes mellitus types 1 and 2, cancer, congenital heart disease, HIV, epilepsy, hemophilia, asthma, cystic fibrosis, juvenile idiopathic arthritis and systemic lupus erythematosus (see also Supplementary Table 2).
Extended Data Table 2.
Underlying conditions associated with severe COVID-19 illness in the study cohort of children aged 3–5 years affiliated to FONASAa
| Comorbidity | Number of cases | |
|---|---|---|
| Female | Male | |
| Total participants | 241,429 | 249,265 |
| Chronic kidney disease | 5 | 3 |
| Congenital heart disease | 3,168 | 3,230 |
| Cancer | 318 | 385 |
| Diabetes mellitus types 1 and 2 | 114 | 144 |
| HIV | 534 | 575 |
| Epilepsy | 935 | 1,082 |
| Hemophilia | 68 | 154 |
| Asthma | 15,172 | 21,641 |
| Cystic fibrosis | 24 | 28 |
| Juvenile idiopathic arthritis | 34 | 12 |
| Systemic lupus erythematosus | 0 | 0 |
aThe study cohort included children 3–5 years of age affiliated to FONASA. We excluded children who had probable or confirmed COVID-19 according to RT–PCR assay for SARS-CoV-2 or antigen test before 6 December 2021.
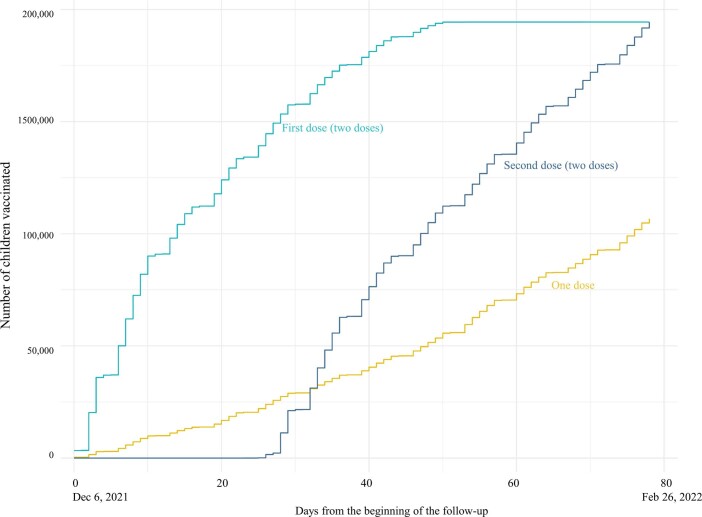
Extended Data Fig. 1. CoronaVac vaccination rollout among children aged 3 to 5 years, by vaccination group.
unvaccinated, vaccinated with one dose, vaccinated with two doses after 28 days. The Public Health Institute of Chile extended the authorization for emergency use of CoronaVac to children starting at three years of age on November 25, 2021. The first children aged 3-5 years were vaccinated on December 6, 2021, prioritizing immunocompromised children and those with comorbidities. The median date of first and second dose for all children in the cohort were 14 and 50 days from the beginning of the follow-up respectively.
Extended Data Table 3.
Main SARS-CoV-2 variants and lineages detected in Chile through genomic surveillance, by detection method, 22 December 2020 through 21 February 2022
| Variant | Genomic sequencing | VAM | Total | Proportion (%) | Subtotal | Proportion (%) |
|---|---|---|---|---|---|---|
| Variants of concern | ||||||
| Alpha (B.1.1.7) | 293 | 196 | 489 | 0.7 | 59,891 | 85.3 |
| Beta (B.1.135) | 4 | 1 | 5 | 0.0 | ||
| Gamma (P.1) | 4,360 | 2,613 | 6,973 | 9.9 | ||
| Delta (B.1.617.2) | 7,666 | 32,787 | 40,453 | 57.6 | ||
| Omicron (BA.1, BA.1.1, BA.2) | 4,211 | 7,760 | 11,971 | 17.1 | ||
| Variants of interest | ||||||
| Lambda (C37) | 1,704 | 25 | 1,729 | 2.5 | 3,618 | 5.2 |
| Mu (B.1.621) | 849 | 1,040 | 1,889 | 2.7 | ||
| Lineages and other variants | ||||||
| Other lineagesa | 1001 | 34 | 1,035 | 1.5 | 1,035 | 1.5 |
| Indeterminate | 0 | 5,642 | 5,642 | 8.0 | 5,642 | 8.0 |
| Total | 20,088 | 50,098 | 70,186 | 100 | 70,186 | 100 |
VAM denotes mutation associated with variant according to RT–PCR assay for SARS-CoV-2.
aCorresponds to other low-frequency lineages and unspecified variants. Preliminary data are in the process of validation.
Source: Department of Epidemiology, Ministry of Health, Chile.
Extended Data Table 5.
Main Omicron sublineages detected through genomic sequencing in Chile during the study period
| Variant of concern | Dec 6–31 2021 | Jan 1–Feb 28, 2022 | Total | ||
|---|---|---|---|---|---|
| n | Proportion (%) | n | Proportion (%) | ||
| Delta (B.1.617.2) | 995 | 39.2 | 159 | 5.7 | 1,154 |
| Omicron | 706 | 27.8 | 1,660 | 59.5 | 2,366 |
| BA.1 | 70 | 49 | |||
| BA.1.1 | 636 | 1,608 | |||
| BA.2 | 0 | 3 | |||
| Unassigned | |||||
| BA.1 sublineages | 810 | 31.9 | 871 | 31.2 | 1,681 |
| BA.2 sublineages | 0 | 40 | 1.4 | 40 | |
| Other lineages | 30 | 1.2 | 60 | 2.2 | 90 |
| Total | 2,541 | 2,790 | 5,331 | ||
Source: Department of Epidemiology, Ministry of Health, Chile
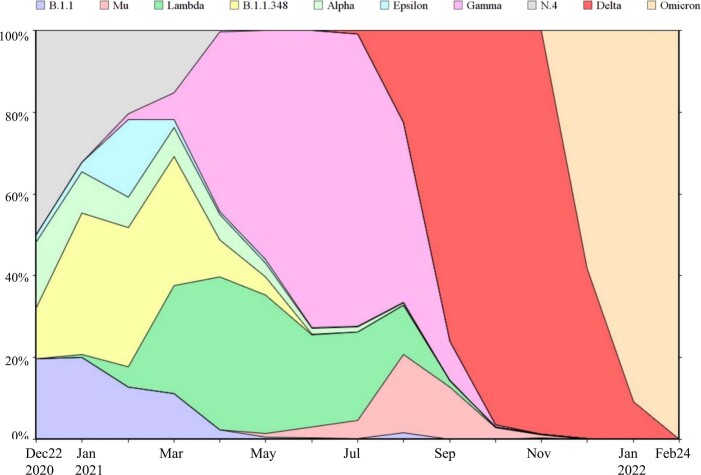
Extended Data Fig. 2. Evolution of the predominant SARS- CoV-2 lineages in Chile, according to data shared on GISAID platform, December 22, 2020, to February 24, 2022.
The Ministry of Health monitors respiratory viruses, including SARS- CoV-2, using genomic surveillance in sentinel centers. Surveillance uses non-probabilistic sampling of SARS-CoV-2 infections focusing on variants of concern (VOC) and variants of interest (VOI) through traveler (imported cases) and community surveillance (hospitalized cases and national core priority studies). The samples are sent for whole-genome sequencing (WGS) and genotyping across the country. Between December 22, 2020, and February 21, 2022, 70,186 SARS-CoV-2 samples were analyzed. Of these, 28.6% (n=20,088) were sequenced and 71.4% (n=50,098) assessed by detection of variant-associated mutations (VAM) using RT- PCR. Of these analyzed samples, 85.3% (n=59,891) correspond to VOC and 5.2% (n=3,618) to variants of interest (VOI).
The estimated adjusted vaccine effectiveness for CoronaVac in children aged 3–5 years during the Omicron outbreak was 38.2% (95% CI, 36.5–39.9) for the prevention of COVID-19, 64.6% (95% CI, 49.6–75.2) for the prevention of hospitalization and 69.0% (95% CI, 18.6–88.2) for the prevention of COVID-19-related ICU admission (Table 1). We did not estimate vaccine effectiveness against fatal outcomes because only two deaths were observed in the unvaccinated group as of 26 February 2022, the study end.
Table 1.
Effectiveness of the CoronaVac COVID-19 vaccine in preventing symptomatic COVID-19 outcomes in children 3–5 years of age in the study cohort according to immunization status, 6 December 2021 through 26 February 2022a
| Immunization status | Cases | Vaccine effectiveness (95% CI) | |||
|---|---|---|---|---|---|
| Person-days | No. | Incidence rate per 1,000 person-days | Weighted, standard adjustmentb | Weighted, stratified analysisb | |
| Symptomatic COVID-19 | |||||
| Unvaccinated | 29,404,535 | 7,555 | 0.2569 | – | – |
| CoronaVac | 18,499,492 | 4,562 | 0.2466 | 37.9 | 38.2 |
| (≥14 days after second dose) | (36.1; 39.6) | (36.5; 39.9) | |||
| Hospitalization | |||||
| Unvaccinated | 29,579,595 | 62 | 0.0021 | – | – |
| CoronaVac | 18,990,209 | 23 | 0.0012 | 65.2 | 64.6 |
| (≥14 days after second dose) | (50.4; 75.6) | (49.6; 75.2) | |||
| Admission to ICU | |||||
| Unvaccinated | 29,580,825 | 9 | 0.0003 | – | – |
| CoronaVac | 18,993,888 | 3 | 0.0002 | 68.8 | 69.0 |
| (≥14 days after second dose) | (18.0; 88.1) | (18.6; 88.2) | |||
aWe classified participants’ status into two categories during the study period: unvaccinated and fully immunized (≥14 days after receiving the second dose of CoronaVac). The days between the first dose vaccine administration and the full immunization were excluded from the at-risk person-time. We provide the results for the standard and stratified versions of the Cox hazard models using inverse probability of treatment weighting.
bThe analyses were adjusted for age, sex, region of residence, health insurance category (a proxy of household income), nationality and whether the patient had underlying conditions that have been associated with severe COVID-19 in children, coded as described in Supplementary Table 1. The standard and stratified versions of the extended Cox proportional hazard models were fit to test the robustness of the estimates to model assumptions.
Our estimates provide evidence of vaccination effectiveness in children aged 3–5 years during the Omicron outbreak in Chile (Table 1 and Extended Data Fig. 3). These results are substantially lower than recent preliminary estimates of the effectiveness of two-dose vaccination of CoronaVac in children 6–16 years, in a period when B.1.617.2 (Delta) was the predominant circulating SARS-CoV-2 variant14. In that study, the estimated effectiveness in children 6–16 years was 74.5% (95% CI, 73.8–75.2) for the prevention of COVID-19, 91.0% (95% CI, 87.8–93.4) for the prevention of hospitalization and 93.8% (95% CI, 87.8–93.4) for the prevention of COVID-19-related ICU admission. The estimates for the subgroup of children aged 6–11 years were 75.8% (95% CI, 74.7–76.8) for the prevention of COVID-19 and 77.9% (95% CI, 61.5–87.3) for the prevention of hospitalization14. Although the estimates are not directly comparable, the lower estimated vaccine effectiveness in this study could be due to Omicron or because the cohort included younger children. Vaccine effectiveness was estimated shortly after vaccination. In light of recent evidence suggesting that the effectiveness of a two-dose COVID-19 vaccination against infection and symptomatic disease wanes over time19, our estimates of protection for children aged 3–5 years may be at their highest level.
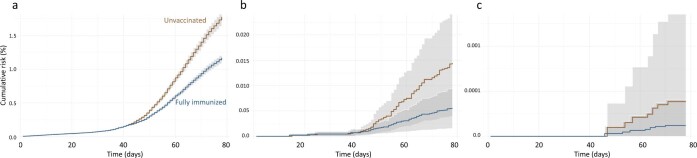
Extended Data Fig. 3. Extended Fig.3 Estimated cumulative incidence of (a) symptomatic COVID- 19, (b) hospitalization, and (c) admission to incentive care unit (ICU) for unvaccinated and fully immunized individuals.
Comparison of the cumulative incidence curves between unvaccinated and fully immunized children (≥ 14 days after receiving the second dose of the CoronaVac COVID-19 vaccine) on January 1, 2022. The estimates are presented as the mean values, with 95% point-wise confidence intervals, for boys, aged 4, affiliated to FONASA insurance type A, and not having comorbidities.
Recent research suggests that currently available vaccines may be less effective against Omicron. Consistent with our results, an unpublished study in New York13 found that the effectiveness of two BNT162b2 vaccine doses for the prevention of COVID-19 and hospitalization decreased from 66% to 51% and from 85% to 73% for children aged 12–17 years, respectively. The drop was more considerable among children aged 5–11 years; protection against COVID-19 fell from 68% to 12%; and protection against hospitalization fell from 100% to 48%13. Preliminary, unpublished results from a large cohort of children aged 3–11 years in Argentina show that two doses of Sinopharm’s inactivated SARS-CoV-2 vaccine BBIBP-CorV were 59% effective against hospitalization when Omicron was the predominant variant and 83% effective when Delta and Omicron circulated15. Results among adults tell the same story. Early data from South Africa reported that BNT162b2 protection against COVID-19-related hospitalization decreased from 93% to 70% among adults16. Among adults in the United Kingdom, two doses of ChAdOx1 nCoV-19 provided no detectable protection against the Omicron variant after 20 weeks, and two doses of BNT162b2 were only 8.8% effective against Omicron after 25 weeks17. The study suggests that a BNT162b2 or mRNA-1273 booster substantially increased protection against Omicron17. Similarly, a study that evaluated serum neutralization against Omicron or the D614G variant among adult participants with the mRNA-1273 vaccine primary series observed neutralization titers 35 times lower for Omicron18.
Children’s age could also potentially affect vaccine effectiveness estimates for severe disease, as suggested by older children in recent unpublished studies in New York13 and Chile14. Clinical trials for Moderna’s mRNA-1273 and Pfizer-BioNTech’s BNT162b2 in children 6 months of age to under 5 years of age are being conducted. Preliminary results for two 3-µg doses, 21 days apart, of the BNT162b2 in children 2 years of age to under 5 years of age did not produce an adequate immune response, although the immune response of children between 6 months of age and 2 years of age was similar to that of young adults20. Data from the mRNA-1273 vaccine in children have not yet been released.
Observational studies have limitations. Selection bias could affect vaccine effectiveness estimates if the vaccinated and unvaccinated groups are systematically different. We partially addressed this issue by adjusting our estimates with observable confounders that may affect vaccination and the risk of COVID-19. However, we do not have data to assess whether vaccinated and unvaccinated children or their caregivers differ in some unobservable characteristics, such as compliance with COVID-19 behavioral guidelines. Another limitation in our study relates to genomic surveillance capabilities. The Ministry of Health’s strategy has focused on detecting VOCs through traveler and community surveillance but uses non-probabilistic sampling (Extended Data Fig. 2 and Extended Data Tables 3–5). There were few child admissions to the ICU associated with SARS-CoV-2 infection during our study period, which led to wide CIs in our estimates. Finally, because laboratory-confirmed COVID-19 cases depend on the patients’ healthcare-seeking behavior, it is possible that asymptomatic or mildly symptomatic cases were missed in our study. Although this may occur in both groups, immunized children may be more likely to develop mild symptoms due to vaccine-induced protection than unvaccinated children. If so, we might have overestimated protection against symptomatic infection. However, this potential bias would not have affected our effectiveness estimates for protection against COVID-19-related hospitalization and ICU admission. Our study examined the effectiveness of a two-dose CoronaVac schedule; the results may be different with a homologous or heterologous booster dose, as shown for adults.
Strengths of the study include that data were collected during the Omicron outbreak, with the highest transmission rates since the beginning of the pandemic. Vaccination rollout in Chile was quick and had high uptake (Extended Data Fig. 1). Our estimated vaccine effectiveness reflects a ‘real-life’ situation by including the challenges public health officials face in the field, such as a more diverse set of participants (for example, with underlying conditions), schedule compliance, logistics and cold chains. These estimates may be essential for decision-making as a complement to controlled clinical trials.
Overall, our results show that the effectiveness of a complete primary immunization schedule with CoronaVac in children 3–5 years of age against symptomatic COVID-19 during the Omicron outbreak was limited. However, vaccines were effective against severe disease in young children. These results support the vaccination of children 3–5 years of age to prevent severe illness and associated complications; however, they underscore the importance of maintaining layered protections against SARS-CoV-2 infection in this population. Important next steps include examining how long vaccine protection lasts and whether booster shots will be necessary. We hope that the results from this study inform policymakers in countries considering child vaccination against COVID-19.
Methods
Outcomes
The Ministry of Health in Chile requires that all suspected COVID-19 cases are immediately notified to health authorities through Epivigila, an online platform that centralizes all case notification and test results and represents the case count source used for this study. Suspected COVID-19 cases require laboratory testing with RT–PCR assay or antigen tests. We estimated the vaccine effectiveness of CoronaVac for children aged 3–5 years using three primary outcomes: laboratory-confirmed symptomatic SARS-CoV-2 infection (COVID-19), hospitalization and admission to the ICU associated with SARS-CoV-2 infection. We considered the time to the onset of symptoms from the beginning of the follow-up, 6 December 2021, as the endpoint of each outcome. We used the onset of symptoms as a proxy for the time of infection. We classified participants’ status into two categories along the study period: unvaccinated and fully immunized (≥14 days after receipt of the second dose with CoronaVac). A child was excluded from the unvaccinated group when she or he received the first vaccine dose. The period between the first dose administration and 13 days after the second dose was excluded from the at-risk person-time in our analyses.
Statistical analyses
We used Bonferroni-adjusted Pearson’s χ2 to compare descriptive data and control for multiple comparisons. To estimate hazard ratios, we used extensions of the Cox hazard model that allowed us to account for the time-varying vaccination status of participants21–23. We adjusted for differences in observed individual characteristics by inverse probability of treatment weighting as in marginal structural models24, estimating the weights non-parametrically25. Vaccine effectiveness was estimated based on the hazard ratio between the treated and non-treated status. We reported hazard ratio estimates adjusted for age, sex, region of residence, nationality, health insurance category (a proxy of household income) and underlying conditions (Extended Data Tables 1 and 2) under the standard and stratified versions of the Cox hazard model.
Let Ti be the time-to-event of interest, from 6 December 2021, for the i-th individual in the cohort, . Let be a p-dimensional vector of individual-specific characteristics, such as age and sex, and let zi(t) be the time-dependent treatment indicator. The model assumes that the time-to-events are independent and with probability distribution given by
where
with being a vector of regression coefficients, being the regression coefficient measuring the effectiveness of the kth vaccine and λ0 being the baseline hazard function
where P0 is the baseline probability distribution. A Cox model with time-dependent covariates compares the risk of the event of interest between immunized and non-immunized participants at each event time but re-evaluates which risk group each person belonged to, based on whether they had been immunized by that time.
We also fitted a stratified version of the model26, where the time-to-event distribution is given by
with being the regression coefficient measuring the effectiveness of the kth vaccine, and λx,0 is the predictor-specific baseline hazard function. We fitted a stratified version of the extended Cox proportional hazard model to test the robustness of our estimates to model assumptions. Under the stratified Cox model, each combination of predictors has a specific hazard function that can evolve independently.
We estimated the vaccine effectiveness as . We show the adjusted vaccine effectiveness results, including covariates as controls (age, gender, region, nationality, health insurance category and comorbidities). We show the results for the standard and stratified versions of the Cox hazard model using inverse probability of treatment weighting. We computed standard 95% Wald CIs for the estimates. Inference was based on a partial likelihood approach27. Recall that the effectiveness estimate for the COVID-19 vaccines in the Cox model with time-dependent vaccination status compares the risk of an event for children who received the vaccine and those who were unvaccinated at each event time. The risk group is determined by whether the child had received the vaccine shot or not in a specific calendar time, and the comparison of the risk of an event is made at the same calendar time. Each term in the partial likelihood of the effectiveness regression coefficient corresponds to the conditional probability of an individual to express the outcome of interest from the risk set at a given calendar time.
Under the standard Cox model, all individuals at risk are included in the risk set, and their contribution is weighted based on their covariates (as shown in Extended Data Table 1). Under the stratified version of the Cox model, each stratum has a different risk set determined by the covariates.
We conducted the analysis with the survival package28 of R, version 4.0.5 (ref. 29).
Ethics statement
The research protocol was approved by the Comité Ético Científico Clínica Alemana Universidad del Desarrollo (Santiago, Chile). No human health risks as a result of our study were identified because we analyzed administrative datasets. The study was considered exempt from informed consent.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-022-01874-4.
Supplementary information
Acknowledgements
This research was supported by the Agencia Nacional de Investigación y Desarrollo (ANID) through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), grant 1220907, to A.J.; by the Millennium Science Initiative Program–Millennium Nucleus Center for the Discovery of Structures in Complex Data (MiDaS), grant NCN17_059, to A.J.; by the Advanced Center for Chronic Diseases (ACCDiS) ANID FONDAP, grant 15130011, to R.A.; and by the Research Center for Integrated Disaster Risk Management (CIGIDEN) ANID FONDAP, grant 15110017, to E.U.A. The funders of this study had no role in the study design; in the collection, analysis and interpretation of data; in the writing of this manuscript; or in the decision to submit the article for consideration for publication.
Extended data
Extended Data Table 4.
Proportion of Omicron from all SARS-CoV-2 variants detected in Chile through genomic surveillance from 12 December 2021 to 19 February 2022
| Epidemiological week | Omicron | Proportion | Total |
|---|---|---|---|
| No. | % | No. | |
| Year 2021 | |||
| Dec 12–18 | 211 | 13.2 | 1,594 |
| Dec 19–25 | 711 | 39.5 | 1,798 |
| Dec 26–Jan 1 | 1,746 | 58.6 | 2,980 |
| Year 2022 | |||
| Jan 2–8 | 1,908 | 69.5 | 2,746 |
| Jan 9–15 | 1,993 | 80.8 | 2,467 |
| Jan 16–22 | 1,655 | 79.1 | 2,092 |
| Jan 23–29 | 1,475 | 87.7 | 1,682 |
| Jan 30–Feb 5 | 949 | 97.5 | 973 |
| Feb 6–12 | 946 | 96.2 | 983 |
| Feb 13–19 | 323 | 89.7 | 360 |
| Total | 11,917 | 67.4 | 17,675 |
No. denotes number of cases.
Source: Department of Epidemiology, Ministry of Health, Chile
Author contributions
A.J. and R.A. conceived and designed the study. J.R.Z. provided support for the analysis. A.J., F.P., T.B. and R.A. managed and analyzed the data. A.J., E.A.U. and R.A. wrote the first draft of the manuscript. A.J., E.A.U., J.Z., C.G., J.A., A.P., V.V., M.S.M., R.G., J.C.F., P.S., P.L., P.E., J.C.R., H.E. and R.A. critically reviewed and edited the manuscript. V.V., H.E. and R.A. had access to vaccine safety data. All authors are responsible for the study design, data collection and data analysis. All authors read and approved the final version of the manuscript. The authors vouch for the accuracy and completeness of the data and accept responsibility for publication. A.J., E.A.U. and R.A. contributed equally to the manuscript.
Peer review
Peer review information
Nature Medicine thanks Laith Abu-Raddad and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Alison Farrell, in collaboration with the Nature Medicine team.
Data availability
Owing to data privacy regulations, the individual-level data used in this study cannot be shared (Law N19.628). Aggregate data on vaccination and COVID-19 incidence are publicly available at https://github.com/MinCiencia/Datos-COVID19/.
Competing interests
R.A. participated in an online, international advisory board organized by AstraZeneca on 21 March 2022.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alejandro Jara, Eduardo A. Undurraga, Rafael Araos.
Extended data
is available for this paper at 10.1038/s41591-022-01874-4.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-022-01874-4.
References
- 1.World Health Organization. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhi, S. A. et al. Population immunity and Covid-19 severity with Omicron variant in South Africa. N. Engl. J. Med.386, 1314–1326 (2022). [DOI] [PMC free article] [PubMed]
- 4.Cloete, J. et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc. Health6, 294–302 (2022). [DOI] [PMC free article] [PubMed]
- 5.Altarawneh, H. N. et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N. Engl. J. Med. 386, 1288–1290 (2022). [DOI] [PMC free article] [PubMed]
- 6.Hoffmann M, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, L. et al. COVID infection severity in children under 5 years old before and after Omicron emergence in the US. Preprint at https://www.medrxiv.org/content/10.1101/2022.01.12.22269179v1 (2022).
- 8.Iuliano AD, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods—United States, December 2020–January 2022. MMWR Morb. Mortal. Wkly Rep. 2022;71:146–152. doi: 10.15585/mmwr.mm7104e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann P, Pittet LF, Finn A, Pollard AJ, Curtis N. Should children be vaccinated against COVID-19? Arch. Dis. Child. 2021;107:e1. doi: 10.1136/archdischild-2021-323040. [DOI] [PubMed] [Google Scholar]
- 10.Coronavirus Vaccine Tracker. The New York Times. https://nyti.ms/3nvAEc4
- 11.Walter EB, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N. Engl. J. Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson SM, et al. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N. Engl. J. Med. 2022;386:713–23.. doi: 10.1056/NEJMoa2117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorabawila, V. et al. Risk of Infection and Hospitalization Among Vaccinated and Unvaccinated Children and Adolescents in New York After the Emergence of the Omicron Variant. JAMA10.1001/jama.2022.7319 (2022). [DOI] [PMC free article] [PubMed]
- 14.Jara, A. et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in children and adolescents: a large-scale observational study. Preprint at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4035405 (2021). [DOI] [PMC free article] [PubMed]
- 15.Gonzalez, S. et al. Effectiveness of BBIBP-CorV, BNT162b2 and mRNA-1273 vaccines against hospitalisations among children and adolescents during the Omicron outbreak in Argentina. Preprint at https://www.medrxiv.org/content/10.1101/2022.04.18.22273978v1 (2022). [DOI] [PMC free article] [PubMed]
- 16.Collie, S., Champion, J., Moultrie, H., Bekker, L.-G. & Gray, G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N. Engl. J. Med.386, 494–496 (2022). [DOI] [PMC free article] [PubMed]
- 17.Andrews, N. et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med.386, 1532–1546 (2022). [DOI] [PMC free article] [PubMed]
- 18.Pajon, R. et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N. Engl. J. Med.386, 1088–1091 (2022). [DOI] [PMC free article] [PubMed]
- 19.Feikin DR, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfizer and BioNTech Provide Update on Ongoing Studies of COVID-19 Vaccine. https://bit.ly/3J3GZHc (2021).
- 21.Jara A, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob. Health. 2022;10:e798–e806. doi: 10.1016/S2214-109X(22)00112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jara A, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox DR. Regression models and life‐tables. J. R. Stat. Soc. Ser. B Stat. Methodol. 1972;34:187–202. [Google Scholar]
- 24.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therneau, T. M. & Grambsch, P. M. Modeling Survival Data: Extending the Cox Model (Springer, 2000).
- 27.Thompson MG, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N. Engl. J. Med. 2021;385:320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therneau, T. M. A Package for Survival Analysis in R. Version 3.3-1. https://cran.r-project.org/web/packages/survival/index.html
- 29.The R Project for Statistical Computing. https://www.r-project.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Owing to data privacy regulations, the individual-level data used in this study cannot be shared (Law N19.628). Aggregate data on vaccination and COVID-19 incidence are publicly available at https://github.com/MinCiencia/Datos-COVID19/.