Abstract
An extracellular cytolysin from Vibrio tubiashii was purified by sequential hydrophobic interaction chromatography with phenyl-Sepharose CL-4B and gel filtration with Sephacryl S-200. This protein is sensitive to heat and proteases, is inhibited by cholesterol, and has a molecular weight of 59,000 and an isoelectric point of 5.3. In addition to lysing various erythrocytes, it is cytolytic and/or cytotoxic to Chinese hamster ovary cells, Caco-2 cells, and Atlantic menhaden liver cells in tissue culture. Lysis of erythrocytes occurs by a multihit process that is dependent on temperature and pH. Twelve of the first 17 N-terminal amino acid residues (Asp-Asp-Tyr-Val-Pro-Val-Val-Glu-Lys-Val-Tyr-Tyr-Ile-Thr-Ser-Ser-Lys) are identical to those of the Vibrio vulnificus cytolysin.
Vibrio tubiashii is a marine organism that causes bacillary necrosis in larval and juvenile bivalve mollusks (24, 25). The disease is characterized by a rapid onset of symptoms, such as a generalized reduction in larval motility, an increase in larval quiescence, and extensive soft tissue necrosis. The pathogen has been isolated from hard clam larvae, juvenile hard clams, and Eastern oyster spat and larvae (7, 12, 25). V. tubiashii has been isolated from diseased mollusks in the United States and United Kingdom and has been associated with red tides caused by Mesodinum rubrum along the northwest coast of Spain (12, 23, 24, 25). In spite of the economic importance of V. tubiashii in the cultivation of bivalve mollusks, nothing is known about the virulence mechanisms of this pathogen. Romalde et al. (23) reported that culture supernatants of the pathogen exhibited cytotoxicity towards fathead minnow peduncle cells and mouse lung fibroblasts in tissue culture. We describe the purification and properties of a cytolysin that lyses various types of erythrocytes and Chinese hamster ovary (CHO) cells and is cytotoxic to human intestinal (Caco-2) cells and fish (Atlantic menhaden liver [AML]) cells in tissue culture.
Cytolysin production and purification.
Two V. tubiashii strains (ATCC 19105 and ATCC 19109) were obtained from the American Type Culture Collection (Manassas, Va). Both strains were confirmed to be V. tubiashii using biochemical tests and were stored at −70°C. The ATCC 19105 frozen culture was rapidly thawed and inoculated onto two plates containing Trypticase soy agar (BBL, Cockeysville, Md.) supplemented with 1% NaCl. The plates were incubated at 30°C for 16 to 18 h, and the bacteria were harvested in 5 ml of Casamino Acids-yeast extract broth (3% Casamino Acids, 0.4% yeast extract, 0.05% K2HPO4 [pH 7.4] supplemented with 1% NaCl). A 2-liter flask containing 500 ml of Casamino Acids-yeast extract broth was inoculated with the seed culture suspension (25 optical density units at 650 nm; ca. 1010 CFU), and the culture was incubated for 7 h at 37°C on a rotary shaker at 100 rpm. Culture supernatant fluids (stage 1) were recovered by centrifugation at 16,000 × g (20 min). Disodium hydrogen phosphate and sodium chloride were dissolved in the stage 1 preparation to final molarities of 0.067 and 0.077 M, respectively, and the pH was adjusted to 7.0 with concentrated HCl. The preparation was then subjected to hydrophobic interaction chromatography using a column (1.6 by 30 cm) of phenyl-Sepharose CL-4B (Amersham Pharmacia Biotech, Piscataway, N.J.) equilibrated with phosphate-buffered saline (PBS) (0.067 M Na2HPO4–0.077 M NaCl, pH 7.0). The column was washed first with PBS, then with PBS diluted (1:10) with water, and finally with 25% ethylene glycol in diluted PBS. Washing the column with 50% ethylene glycol in diluted PBS eluted the cytolysin. Peak fractions having hemolytic activity were pooled (stage 2). The stage 2 preparation was subjected to gel filtration using a column (2.6 by 94 cm) of Sephacryl S-200 (Amersham Pharmacia Biotech) equilibrated with PBS. Peak fractions having activity were pooled (stage 3).
Hemolytic activity of the cytolysin was measured using sheep erythrocytes (Colorado Serum Company, Denver, Colo.) by a method previously described (3). Briefly, the cytolysin preparations were diluted to 0.5 ml with PBS and added to 0.5 ml of PBS containing 1 mg of bovine serum albumin (BSA) (Sigma, St. Louis, Mo.) per ml. To this mixture, 1 ml of washed erythrocyte suspension in PBS (0.7%, vol/vol) was added, and the tube was incubated at 37°C for 30 min. Unlysed erythrocytes were pelleted by centrifugation, and the absorbance of the supernatant was measured at 545 nm. One hemolytic unit (HU) is defined as the amount which causes the release of 50% of the hemoglobin in the standardized erythrocyte suspension.
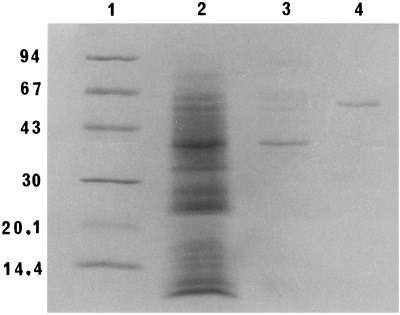
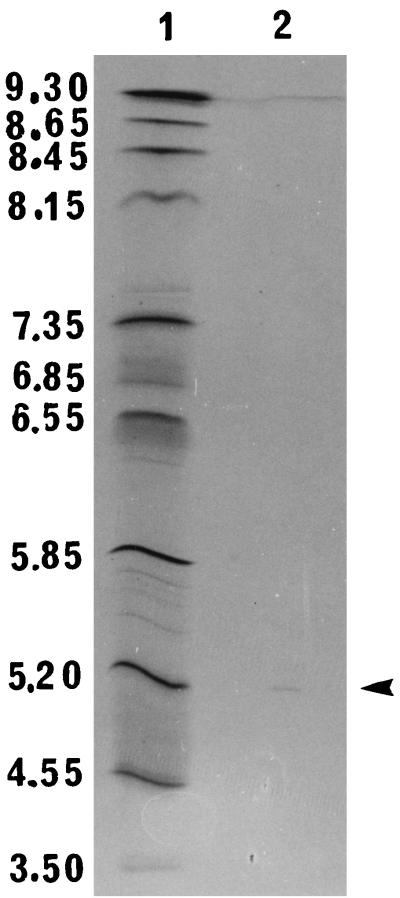
Analysis of the stage 3 preparation using the PhastSystem (Amersham Pharmacia Biotech) showed that the cytolysin was homogeneous by sodium dodecyl sulfate-polyacrylamide gel electrophosporesis (SDS-PAGE) in an 8 to 25% gradient gel (Fig. 1) and by thin-layer isoelectric focusing in a pH 3 to 9 gel (Fig. 2). The molecular weight of the denatured reduced cytolysin, as estimated by the relative mobility method of Weber et al. (26), was 59,000. This molecular weight is much higher than the apparent molecular weight (<10,000) of the native protein as determined by gel filtration (1). Similar observations have been made for the hemolysin of non-O1 Vibrio cholerae and vulnificolysin of Vibrio vulnificus, which also exhibit very low molecular weights by gel filtration but which have molecular weights of 60,000 and 56,000, respectively, as determined by SDS-PAGE (6, 11, 18, 27). The V. tubiashii cytolysin has an isoelectric point of 5.3, which is similar to that of the non-O1 V. cholerae hemolysin (18) but is different from that of vulnificolysin (6).
FIG. 1.
SDS-PAGE of V. tubiashii cytolysin preparations. Lanes: 1, molecular mass markers (values at left are in kilodaltons); 2, stage 1 (4 μg); 3, stage 2 (0.7 μg); 4, stage 3 (0.5 μg). The gel was stained with Coomassie brilliant blue R.
FIG. 2.
Analytical thin-layer isoelectric focusing of V. tubiashii cytolysin. Lanes: 1, pI markers; 2, stage 3 (1 μg). The arrowhead indicates the location of the hemolysin isoform. The gel was stained with Coomassie brilliant blue R.
The quantitative results of the purification are summarized in Table 1. The amount of protein in the preparations was estimated by the method of Bradford (4), using a standard (BSA) and reagent that were purchased from Bio-Rad Laboratories (Richmond, Calif.). About 30% of the cytolysin was recovered in a purified state. The amount of protein in this preparation was only 41 μg, but the specific activity was very high (167,683 HU per mg of protein). Strong binding of the protein to phenyl-Sepharose CL-4B during hydrophobic interaction chromatography suggests that the cytolysin is extremely hydrophobic. The hydrophobic nature of the protein was also apparent during the gel filtration chromatography stage of the purification process: the cytolysin eluted from the column in a very broad peak with an apparent molecular weight of less than 10,000 (results not shown). This elution pattern is suggestive of an interaction between the gel and the protein molecule. The hydrophobic nature of the protein may also have been responsible for the loss of activity that was observed for the various methods (ammonium sulfate precipitation, ultrafiltration, dialysis, and lyophilization) we employed to isolate and concentrate the cytolysin from the culture supernatant fluids.
TABLE 1.
Purification of V. tubiashii cytolysin
| Stage | Vol (ml) | Amt of protein (mg) | Activity (HU) | Sp act (HU/mg) | Recovery (%) |
|---|---|---|---|---|---|
| (1) Culture supernatant fluids | 430 | 9.89 | 21,500 | 2,174 | 100 |
| (2) Hydrophobic interaction chromatography | 19.3 | 0.87 | 9,650 | 11,092 | 45 |
| (3) Gel filtration chromatography | 55 | 0.041 | 6,875 | 167,683 | 32 |
Cytolysin inactivation.
The cytolysin lost its activity when it was incubated at temperatures of 37 to 100°C for 30 min (Table 2). The losses of activity at 37 and 56°C were reduced to 0 and 94%, respectively, when 0.25 mg of BSA per ml was added to the cytolysin solution. Thus, the hemolytic assay was routinely carried out at 37°C for 30 min in the presence of BSA. The cytolysin was stable when incubated at 4°C for 24 h in buffers with pH values of 5 to 10 but lost all its activity at pH 4. The cytolysin was sensitive to digestion with chymotrypsin and subtilisin but not to trypsin and papain. Sensitivity to chelating agents (EGTA and EDTA), dithiothreitol (DTT), cholesterol, and mixed gangliosides was determined by incubating the cytolysin with the reagents for 30 min at 27°C. The organic solvents in which the cholesterol and mixed gangliosides were dissolved were evaporated with a stream of nitrogen, and the reagents were suspended in 0.5 ml of PBS and sonicated for 20 s using a sonicator equipped with a microtip (Tekmar Co., Cincinnati, Ohio). Cytolysin (0.5 ml) was then added to the sonicated suspension, and the mixture was incubated at 27°C for 30 min. Addition of 0.1 μg of cholesterol resulted in a loss of 85% of the cytolysin's activity; amounts greater than 1 μg inhibited 100% of the activity. The addition of 1 mM EGTA and 1 mM EDTA to the reaction mixture and preincubation of the cytolysin with 10 mM DTT and mixed gangliosides did not affect the cytolysin's activity. Results are similar to those reported for vulnificolysin, except that a much smaller amount of cholesterol (1 μg compared to 100 μg) is required to inactivate the V. tubiashii cytolysin (6). The proteases, EGTA, EDTA, DTT, cholesterol, and mixed gangliosides were purchased from Sigma.
TABLE 2.
Inactivation of V. tubiashii cytolysin
| Treatment | Residual activity (%) |
|---|---|
| 27°C | 100 |
| 37°C (PBS) | 50 |
| 37°C (PBS-BSA) | 100 |
| 56°C (PBS) | 0 |
| 56°C (PBS-BSA) | 6 |
| 100°C | 0 |
| Control (PBS, 37°C for 30 min) | 50 |
| Papain, 50 μg | 50 |
| Trypsin, 50 μg | 50 |
| Chymotrypsin, 50 μg | 4 |
| Subtilisin, 50 μg | 4 |
| Control (PBS, 25°C for 30 min) | 100 |
| Cholesterol (μg) | |
| 0.1 | 15 |
| 1 | 8 |
| 10, 25, 50, and 100 | 0 |
Variables influencing erythrocyte lysis.
The effects of temperature, pH, and toxin concentration on erythrocyte lysis were examined by incubating tubes (six for each parameter) containing erythrocyte suspension (final concentration of 0.35%, vol/vol) and the cytolysin and removing one tube every 10 min. The unlysed erythrocytes were centrifuged, and the absorbance of the supernatant was measured. The effects of temperature (27, 32, 37, 42, and 47°C) and pH (7, 8, 9, and 10) were examined by using 2 HU of the cytolysin, while the effect of cytolysin concentration was determined with 0.5, 1.0, 2.0, and 3.0 HU. The effect of erythrocyte concentration on hemolysis was examined by incubating 2 HU with different amounts (0.175 to 3.5% final concentration) of sheep erythrocytes. Optimal hemolysis was observed at temperatures of 37 to 47°C; however, the rate of hemolysis was maximal at higher temperatures. The optimal pH for hemolysis was 7 to 8. The rate and amount of hemolysis were dependent on the amount of cytolysin (Fig. 3A). An increase in the number of erythrocytes (0.175 to 1.05%) resulted in an initial increase of total absorbance (hemolysis) followed by a gradual decrease in the hemolysis (Fig. 3B). However, the percentage of hemolysis (lysed erythrocytes as a proportion of the total erythrocytes) decreased with increased erythrocyte concentration. Results indicate that in the presence of an excess of erythrocytes, the amount of cytolysin that binds to an erythrocyte is not enough to cause its lysis. Further, these results suggest that more than one molecule of the cytolysin is required to lyse an erythrocyte. Such multihit processes have been described for various other cytolysins and hemolysins (6, 10, 13–15, 17, 21).
FIG. 3.
Effects of V. tubiashii cytolysin and erythrocyte concentrations on cytolysin-induced lysis of erythrocytes. Erythrocyte samples (1 ml) were incubated with the cytolysin, and the mixture was centrifuged to pellet unlysed erythrocytes. The absorbance of the supernatant fluids was measured at 545 nm and compared with that of the control. (A) Effect of cytolysin concentration. The control for this experiment was a saponin-lysed 0.35% (vol/vol) erythrocyte suspension. (B) Effect of erythrocyte concentration. The reaction mixture contained 2 HU of cytolysin and different amounts of erythrocytes. The controls for this experiment were saponin-lysed 0.175 to 3.5% (vol/vol) erythrocyte suspensions.
The ability of the cytolysin to bind to the sheep erythrocytes was examined by incubating 1, 2, and 4 HU with the erythrocytes (0.35%, vol/vol) at 4 and 37°C for 1, 2, and 4 min and measuring the amount of activity associated with the erythrocytes. Briefly, after the incubation period, the erythrocytes were removed by centrifugation, resuspended in 1.5 ml of PBS and 0.5 ml of PBS-BSA, and incubated at 37°C for 30 min. Erythrocytes were then centrifuged, and the absorbance of the supernatant was measured at 545 nm. Results indicate that the cytolysin bound to erythrocytes much more rapidly at 37°C than 4°C (Table 3). At 4°C, there was no significant difference between the amounts of toxin bound at 1 and 4 min. In contrast, prolonged incubation at 37°C resulted in more of the toxin binding to the erythrocytes. These results suggest that binding of the V. tubiashii cytolysin to erythrocytes is a temperature-dependent step. In comparison, the binding of vulnificolysin to erythrocytes is independent of temperature (6).
TABLE 3.
Binding of V. tubiashii cytolysin to sheep erythrocytes
| Binding condition | Hemolytic activity (% of control) associated with erythrocytes in the presence of cytolysin at:
|
||
|---|---|---|---|
| 1 HU | 2 HU | 4 HU | |
| 4°C | |||
| 1 min | 10 | 11 | 33 |
| 2 min | 9 | 9 | 32 |
| 4 min | 7 | 9 | 29 |
| 37°C | |||
| 1 min | 11 | 44 | 57 |
| 2 min | 35 | 55 | 61 |
| 4 min | 41 | 56 | 59 |
Biological activity.
The cytolysin was examined for its activity against various erythrocytes and tissue culture cells. Blood samples from goat, rabbit, calf, goose, chicken, horse, and guinea pig were obtained from the Colorado Serum Company. The cytolysin was active against erythrocytes from all seven animal species tested (Table 4). Erythrocytes from sheep and goat were the most sensitive, while those from guinea pig and horse were the least sensitive.
TABLE 4.
Sensitivities of erythrocytes to V. tubiashii cytolysin
| Source of erythrocytes | Hemolytic activity (HU/ml) | Relative sensitivity (%)a |
|---|---|---|
| Sheep | 25 | 100 |
| Goat | 25 | 100 |
| Rabbit | 10 | 40 |
| Calf | 10 | 40 |
| Goose | 10 | 40 |
| Chicken | 7 | 28 |
| Horse | 5 | 20 |
| Guinea pig | 4 | 16 |
Compared to activity against sheep erythrocytes.
The effects of the toxin against CHO cells, Caco-2 cells, and AML cells in tissue culture were examined using a method previously described for a CHO cell assay (16). CHO cells were grown in Eagle's minimum essential medium (Sigma) supplemented with 10% fetal calf serum (Gibco BRL, Life Technologies, Rockville, Md.) and 10% tryptose phosphate broth, while the Caco-2 cells were grown in Dulbecco's modified Eagle's medium (Gibco BRL) supplemented with 20% fetal calf serum, 1 mM sodium pyruvate, 200 mM l-glutamine, and 1 mM nonessential amino acids. AML cells were grown in a medium previously described by Faisal et al. (5). Briefly, for the assay, the cells were grown to confluence in the growth medium, harvested, and resuspended at a concentration of 10,000 cells per ml of the growth medium containing 1% fetal calf serum. A 100-μl aliquot (1,000 cells) of the cell suspension was added to each well in a microtiter plate. The cytolysin was diluted 2- to 256-fold, and 10 μl of the dilution was added to each well. The cells were incubated either at 37°C (CHO and Caco-2) or at 26°C (AML) in an incubator with 5% CO2 and examined microscopically after 2, 4, 6, 8, and 22 h for cytolytic and/or cytotoxic effects. Activity was visible within 4 to 6 h of incubation of the cells with the cytolysin. The affected CHO cells were lysed, but the Caco-2 and AML cells appeared as very small rounded cells that had lost their characteristic morphology. The minimum amounts of toxin that exhibited cytolytic and/or cytotoxic activity towards ca. 50% of the CHO, Caco-2, and AML cells were 0.01, 0.02, and 0.1 HU, respectively.
The suckling mouse assay, as previously described (16), was used for determining whether the cytolysin has the potential to cause diarrhea in this model. Pregnant ICR mice were ordered from Harlan Sprague-Dawley (Indianapolis, Ind.). Studies were carried out in accordance with an Institutional Animal Care and Use Committee-approved protocol. Purified cytolysin tested at 100 HU (0.6 μg) per mouse failed to elicit any fluid. This result suggests either that the cytolysin does not play a role in the ability of the organism to induce fluid accumulation or that a larger amount of the cytolysin is required for a positive response. For example, 0.5 μg of the El Tor-like hemolysin of non-O1 V. cholerae was sufficient for a positive response, but a higher dose (2 μg) of the hemolysin produced by Vibrio metschnikovii was required to induce a significant amount of fluid in suckling mice (9, 19).
N-terminal amino acid sequence.
The cytolysin preparation was concentrated using a ProSpin cartridge (Applied Biosystems, Foster City, Calif.), and the excised ProSpin membrane was sequenced by Edman degradation using a model 477A protein sequencer (Applied Biosystems). The presence of only one sequence further confirmed that the purified preparation was homogeneous. The same sequence was obtained when a Coomassic brilliant blue R (Sigma)-stained cytolysin band was excised from a Western blot and sequenced. The first 17 N-terminal amino acids of the cytolysin are shown in Table 5. A search of the protein database revealed that the sequence had homology only to vulnificolysin (6, 28); 12 of these 17 amino acids are identical to those of vulnificolysin, and 3 other amino acids are logical substitutions.
TABLE 5.
N-terminal amino acid sequences of the V. tubiashii and V. vulnificus cytolysins
| Organism | N-terminal amino acid sequencea |
|---|---|
| 151015 | |
| V. tubiashii | Asp-Asp-Tyr-Val-Pro-Val-Val-Glu-Lys-Val-Tyr-Tyr-Ile-Thr-Ser-Ser-Lys |
| V. vulnificus | Gln-Glu-Tyr-Val-Pro-Ile-Val-Glu-Lys-Pro-Ile-Tyr-Ile-Thr-Ser-Ser-Lys |
Underlined amino acids of the V. tubiashii cytolysin are identical to those present in the V. vulnificus cytolysin.
PCR.
Due to the high degree of homology between the N-terminal amino acid sequences of the V. tubiashii and the V. vulnificus cytolysins, we examined both strains of V. tubiashii for the presence of the cytolysin gene of V. vulnificus by PCR. Two V. vulnificus strains (C7684 and MO6-24) were used as positive controls. A colony from each strain was picked from a plate containing Trypticase soy agar and 1% NaCl, suspended in 25 μl of 0.5 M NaOH, and incubated at room temperature for 30 min. After the addition of 25 μl of Tris-HCl (pH 7.5) and 450 μl of sterile distilled water, the lysates were frozen at −20°C until needed. PCR primers based on sequences within the V. vulnificus cytolysin gene, as described by Hill et al. (8), were used to generate a 519-bp product. PCR was performed in 50-μl volumes containing 45 μl of Platinum PCR supermix (Life Technologies), primers (500 ng), and 5 μl of cell lysate as a DNA template. Following an initial 5-min holding period at 94°C, each of the subsequent 30 PCR cycles consisted of 1.75 min at 94°C, 2 min at 60°C, and 2 min at 72°C. Analysis of the two V. tubiashii strains did not yield the expected 519-bp product. Results suggest that the homology between the hemolysins at the amino acid level (at least at the N-terminal end) does not extend to the DNA sequence using the primers specified by Hill et al. (8). It is quite possible that some homology may be present in the remaining sequence, and the use of different primers may detect both of the pathogens.
Various vibrios cause fatal diseases in both marine and freshwater fish (2). For example, Vibrio anguillarum, V. vulnificus, Vibrio ordalii, Vibrio damsela, V. cholerae, and Vibrio parahaemolyticus cause systemic infections in edible finfish, crustaceans, and shellfish. Extracellular proteins, especially hemolysins, produced by many of the bacterial pathogens are postulated to play an important role in the pathogenesis of disease in fish. Some of these hemolysins have been purified and tested for their virulence in fish (20, 22). However, the role of bacterial virulence factors in diseases of mollusks has not been studied. The cytolytic and cytotoxic properties of the V. tubiashii cytolysin may be important in the mortality of larval and juvenile bivalve mollusks. Interestingly, the cytolysin has various physicochemical properties, including an N-terminal amino acid sequence, that are similar to those of the vulnificolysin of V. vulnificus, a human and fish pathogen (6).
REFERENCES
- 1.Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- 2.Austin B, Austin D A. Bacterial fish pathogens: disease of farmed and wild fish. Chichester, England: Springer Praxis Publishing; 1999. [Google Scholar]
- 3.Bernheimer A W, Schwartz L L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Faisal M, Sami S, Rutan B J. Fish cell lines of leukocytic origin: maintenance and characterization. In: Stolen J S, Fletcher T C, Anderson D P, Knattari S L, Rowley A F, editors. Techniques in fish immunology. Fair Haven, N.J: SOS Publications; 1992. pp. 35–59. [Google Scholar]
- 6.Gray L D, Kreger A S. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect Immun. 1985;48:62–72. doi: 10.1128/iai.48.1.62-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hada H S, West P A, Lee J V, Stemmler J, Colwell R R. Vibrio tubiashii sp. nov., a pathogen of bivalve mollusks. Int J Syst Bacteriol. 1984;34:1–4. [Google Scholar]
- 8.Hill W E, Keasler S P, Trucksess M W, Feng P, Kaysner C A, Lampel K A. Polymerase chain reaction identification of Vibrio vulnificus in artificially contaminated oysters. Appl Environ Microbiol. 1991;57:707–711. doi: 10.1128/aem.57.3.707-711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichinose Y, Yamamoto K, Nakasone N, Tanabe M J, Takeda T, Miwatani T, Iwanaga M. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987;55:1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue K, Akiyama Y, Kinoshita T, Higashi Y, Amano T. Evidence for a one-hit theory in the immune bactericidal reaction and demonstration of a multi-hit response for hemolysis by streptolysin O and Clostridium perfringens theta-toxin. Infect Immun. 1976;13:337–344. doi: 10.1128/iai.13.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwanaga M, Ichinose Y. An aberrant hemolysin of Vibrio cholerae non-O1. Microbiol Immunol. 1991;35:705–715. doi: 10.1111/j.1348-0421.1991.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 12.Jeffries V E. Three vibrio strains pathogenic to larvae of Crassostrea gigas and Ostrea edulis. Aquaculture. 1982;29:201–226. [Google Scholar]
- 13.Johnson M K. Properties of purified pneumococcal hemolysin. Infect Immun. 1972;6:755–760. doi: 10.1128/iai.6.5.755-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson M K, Boese-Marrazzo D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1980;29:1028–1033. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kothary M H, Kreger A S. Purification and characterization of an extracellular cytolysin produced by Vibrio damsela. Infect Immun. 1985;49:25–31. doi: 10.1128/iai.49.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kothary M H, Richardson S H. Fluid accumulation in infant mice caused by Vibrio hollisae and its extracellular enterotoxin. Infect Immun. 1987;55:626–630. doi: 10.1128/iai.55.3.626-630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchlewicz B A, Duncan J L. Lysis of erythrocytes by a hemolysin produced by a group B Streptococcus sp. Infect Immun. 1981;34:787–794. doi: 10.1128/iai.34.3.787-794.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCardell B A, Kothary M H, Madden J M. Two-step purification and partial characterization of a variant of the Vibrio cholerae non-O1 hemolysin. FEMS Microbiol Lett. 1999;180:177–182. doi: 10.1111/j.1574-6968.1999.tb08793.x. [DOI] [PubMed] [Google Scholar]
- 19.Miyake M, Honda T, Miwatani T. Purification and characterization of Vibrio metschnikovii cytolysin. Infect Immun. 1988;56:954–960. doi: 10.1128/iai.56.4.954-960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura S, Fujino M, Yamakawa M, Kawahara E. Purification and characterization of salmolysin, an extracellular hemolytic toxin from Aeromonas salmonicida. J Bacteriol. 1988;170:3694–3702. doi: 10.1128/jb.170.8.3694-3702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberley T D, Duncan J L. Characteristics of streptolysin O action. Infect Immun. 1971;4:683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez L A, Ellis A E, Nieto T P. Purification and characterization of an extracellular metalloprotease, serine protease and hemolysin of Aeromonas hydrophila strain B32: all are lethal for fish. Microb Pathog. 1992;13:17–24. doi: 10.1016/0882-4010(92)90028-m. [DOI] [PubMed] [Google Scholar]
- 23.Romalde J L, Barja J L, Toranzo A E. Vibrios associated with red tides caused by Mesodinium rubrum. Appl Environ Microbiol. 1990;56:3615–3619. doi: 10.1128/aem.56.11.3615-3619.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tubiash H S, Chanley P E, Leifson E. Bacillary necrosis, a disease of larval and juvenile bivalve mollusks. I. Etiology and epizootiology. J Bacteriol. 1965;90:1036–1044. doi: 10.1128/jb.90.4.1036-1044.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tubiash H S, Colwell R R, Sakazaki R. Marine vibrios associated with bacillary necrosis, a disease of larval and juvenile bivalve mollusks. J Bacteriol. 1970;103:2721–2723. doi: 10.1128/jb.103.1.271-272.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber K, Pringle J R, Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto K, Al-Omani M, Honda T, Takeda Y, Miwatani T. Non-Ol Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984;45:192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto K, Wright A C, Kaper J B, Morris J G., Jr The cytolysin gene of Vibrio vulnificus: sequence and relationship to the Vibrio cholerae El Tor hemolysin gene. Infect Immun. 1990;58:2706–2709. doi: 10.1128/iai.58.8.2706-2709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]