Summary
There is a strong relationship between stress and metabolism. Because acute traumatic- and chronic stress events are often accompanied with metabolic pathophysiology, it is important to understand the details of the metabolic stress response. In this study we directly compared metabolic effects of acute stress with chronic repeated- and chronic unpredictable stress in mouse models. All types of adversities increased energy expenditure, chronic stress exposure decreased body weight gain, locomotor activity and differentially affected fuel utilization. During chronic exposure to variable stressors, carbohydrates were the predominant fuels, whereas fatty acids were catabolized in acutely and repeatedly restrained animals. Chronic exposure to variable stressors in unpredictable manner provoked anxiety. Our data highlight differences in metabolic responses to acute- repeated- and chronic stressors, which might affect coping behavior and underlie stress-induced metabolic and psychopathologies.
Subject areas: Biological sciences, Physiology, Immunology
Graphical abstract
Highlights
-
•
All forms of stress exposure increase energy expenditure and resting metabolic rate
-
•
Increased energy expenditure is fueled in challenge-specific manner
-
•
Acute restraint increases, chronic stress decreases locomotor activity
-
•
Chronic variable stress, but not repeated restraint provokes anxiety/depression
Biological sciences; Physiology; Endocrinology
Introduction
Coping with stress needs energy. In an acute stress situation metabolic resources are mobilized to serve increased energy demand for “fight or flight” (Berthoud, 2002). Blood glucose level is elevated, lipids are mobilized from the fat depots, body temperature, heart rate, blood pressure and respiration is increased to overcome threatening situations (Goldstein, 1987). All of these changes are adaptive responses for survival, whereas physiological variables return to normal range after the stressor is no longer detected (Roy et al., 2001).
If the stressors occur too frequently or persist continuously, the body remains in the alarm state. As the adaptive capacity of an organism is limited, lack of full recovery pays: stressful challenges lead to development of metabolic, cardiovascular and mental disorders (Seeman et al., 2001). There is a strong association between stressful life events, obesity, metabolic X syndrome (Pyykkonen et al., 2010) and type2 diabetes (Tamashiro et al., 2011; Renzaho et al., 2014). However, the details of chronic stress-induced metabolic changes are not fully revealed. There are two major types of chronic adversity: chronic repeated stress (CRS), when subjects are repeatedly exposed to the same stressor (i.e. restraint) and chronic variable stress (CVS), during which different stressors are applied in a random, unexpected order. While an organism may habituate to CRS, chronic variable stress is non-habituating (Herman et al., 1995; Herman and Tasker, 2016). The physiological effects of chronic adversities have not been compared directly, although it has a significant translational value. The aim of this study was to compare changes in metabolic/behavioral variables in mice, exposed to acute-, repeated (homotypic)- and chronic variable stress (CVS) paradigm to understand the mechanism of stress-related metabolic disorders.
Results
Chronic stress exposure decreases body weight gain and alters body composition
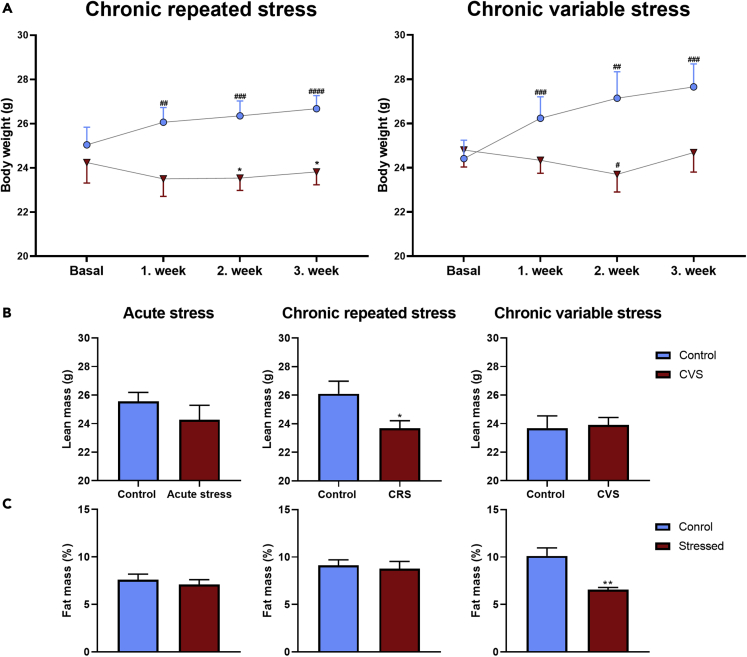
One of the most obvious changes that occurs in stressed animals and humans is the change in body weight. First, we followed weekly the body weight of chronically stressed mice. Although control animals gained 2.48 ± 0.33 g weight during 3 weeks, mice exposed to chronic stress lost 0.42 ± 0.48 g weight in CRS- and 0.11 ± 0.28 g in CVS groups. Figure 1A shows the time course changes in body weights of control and chronically stressed mice.
Figure 1.
Chronic stress exposure decreases body weight gain and alters body composition
Body weight was measured weekly in chronically stressed mice, body composition was determined at the end of stress protocols by EchoMRI.
(A) Body weight data of control animals (blue dots) compared to those of exposed chronic stress paradigms (red triangles). Mean + or – SEM values are shown. Datasets were analyzed by repeated two-way ANOVA followed by Sidak multiple comparison test, ∗p < 0,05 vs. control group on the appropriate week, #p < 0,05; ##p < 0,01; ###p < 0.001;####p < 00,001 vs. basal body weight.
(B) Absolute lean mass weight of control animals (blue columns) and mice exposed to stress (red columns).
(C) Relative fat mass, expressed as % of body weight in control (blue columns) and stressed animals (red columns).
The body composition did not change in the acute- and chronic repeated stress mice (control-blue columns; stressed-red columns), however, the CVS exposed mice represented reduced fat and increased lean mass (B and C). Data are presented as mean ± SEM. Significance was calculated by unpaired t-test. ∗∗p < 0,01 vs. control group.
Body weight loss in chronically stressed mice might occur at the expense of lean or fat body mass. Next, we analyzed body composition of experimental animals using EchoMRI. Acute restraint stress did not elicit significant changes in body composition. Repeated restraint decreased the amount of lean mass, although the lean mass relative to whole body mass was not changed. By contrast, chronic variable stress decreased the proportion of fat mass and increased the proportion of lean mass (Figures 1B and 1C).
Chronic variable stress transiently suppresses food intake
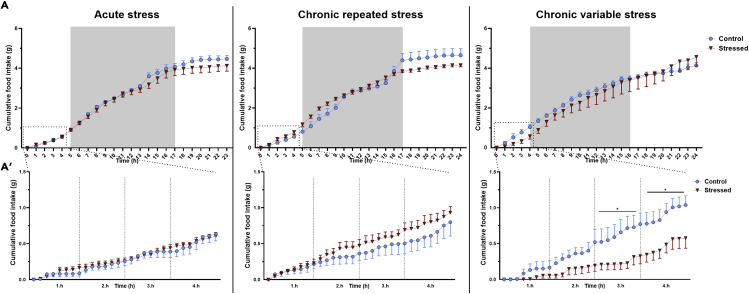
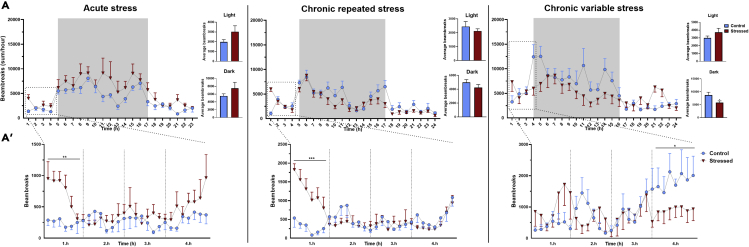
Regulation of food intake in stress is rather complex. Suppression of eating may occur due to stress-malaise. By contrast, stress-induced corticosterone increases appetite and motivates replenishment of bodily resources. To compare the effects of acute and chronic stress on eating patterns, we measured cumulative food intake and found suppression of consumption in mice exposed to CVS, but not in CRS group or acutely stressed mice. Interestingly, suppression of eating in CVS mice was limited to the first 4 h post-stress (Figure 2) then the intake returned to the control’s level by the end of the dark period.
Figure 2.
Chronic variable stress transiently suppresses food intake
Food intake was automatically registered for 24h post stress in TSE Phenomaster setup.
(A) Cumulative food intake of acutely restrained mice, animals exposed to repeated restraint or chronic variable stress.
(A’) Details of food intake in the first 4 h post-stress.
Data were analyzed by repeated two-way ANOVA. In case of the first 4 h, data were analyzed separately hour by hour. Data are presented as mean ± SEM. ∗p < 0.05 vs. control group.
All types of stress increase energy expenditure (EE)
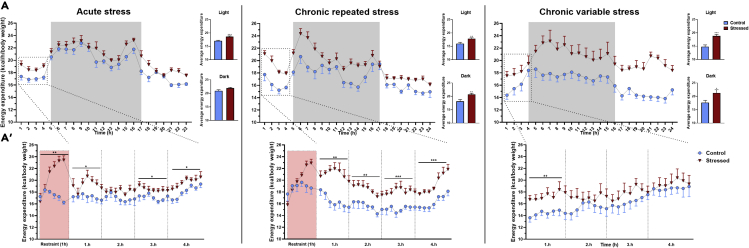
Estimation of energy expenditure (EE) in the Phenomaster indirect calorimetry system is based on the measurement of O2 consumption and CO2 production. There are four components of EE: resting EE, thermic effects of food, physical activity and arousal. Because EE is an important indicator of the metabolic stress response, we followed this parameter in acutely and chronically stressed animals. As expected, control animals displayed a clear circadian rhythmicity in energy expenditure with higher values measured during the dark phase (Figure 3). Although this circadian rhythm was also seen in stressed animals, all types of stress exposure increased energy expenditure. EE started to increase shortly after placing animals into the restrainer. In this case, when the food intake and locomotion is “restrained”, stress-induced arousal might be the driver of EE. Even acute restraint stress resulted in lasting increase of EE in the light phase of the circadian cycle. By contrast, we detected significant increases of EE values throughout the day in chronically stressed animals (Figure 3A). Although EE returned to the control level in CRS animals, it remained higher than that of the control in case of CVS mice 24h post-stress (Figure 3A).
Figure 3.
All types of stress increase energy expenditure
Energy expenditure (EE) was calculated from O2 consumption and CO2 production data, measured in TSE Phenomaster setup, using the Weir equation.
(A) 24h registrations of EE in acutely restrained mice and in animals exposed to repeated restraint or chronic variable stress. Data from control mice are shown as blue dots, data of stressed animals are shown as red triangles. Columns on the right represent the sum of EE during the light and the dark phase of the circadian cycle.
(A’) Details of EE in the first 4 h post-stress. The red areas show EE data during restraint.
Data are presented as mean ± SEM. Significance was analyzed by repeated two-way ANOVA. In case of the first five hours, data were analyzed separately hour by hour. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. control group.
Energy expenditure at rest (in periods, when locomotion was zero) was higher in all stressed groups (acute restraint: 20.92 ± 0.42; CRS: 19.14 ± 0,19; CVS: 20,63 ± 0.35 kcal/day/kg lean body mass) compared to that of the controls (17.52 ± 0.17 kcal/day/kg lean body mass p < 0.0001). All the above data support the conclusion that stressful challenges increase energy expenditure and CVS results in a long-lasting elevation of EE.
Stress exposure differentially affects fuel utilization
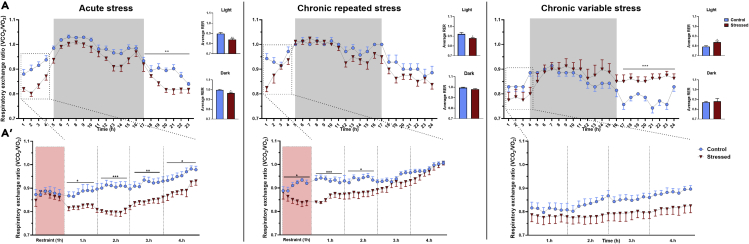
Next, we asked which substrates are utilized for the increased energy production during stress. The respiratory exchange ratio (the ratio between the amount of carbon dioxide produced- and oxygen used in metabolism) is the best estimate for this purpose. RER values close to one indicate preferential use of carbohydrates, while value 0.7 means predominant fat metabolism. RER values in our present study indicated mixed utilization of both carbohydrates and fatty acids, with a clear circadian variation in control animals, showing a trend using more carbohydrates during the dark (active) phase than during light (inactive) phase, when fatty acids are the predominant fuel (Figure 4A). A similar circadian RER variation was seen in acutely stressed mice and in those animals exposed to CRS. By contrast, after CVS, RER remained elevated in the light phase.
Figure 4.
Stress exposure differentially affects fuel utilization
Respiratory exchange ratio (RER) was calculated using the formula: CO2/O2. RER values close to one indicate preferential use of carbohydrates, while value 0.7 means predominant fat metabolism.
(A) Graphs showing 24h RER values following acute restraint, repeated restraint and chronic variable stress. Values from control mice are shown as blue dots, values from stressed animals are shown as red triangles. Columns on the right show average values calculated for the light and dark periods.
(A’) Details of RER during restraint (red-labeled area) and 4 h post-stress period.
Data are presented as mean ± SEM. Data were analyzed by repeated two-way ANOVA. In case of the first 4 h, data were analyzed separately hour by hour.∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. control group.
During acute restraint, RER was not significantly different compared to controls. However, immediately after 1 h acute stress exposure, RER dropped and remained significantly decreased throughout the 4 h post-stress period. Furthermore, the average RER values measured during both phases of the circadian cycle were smaller than that of the controls.
In CRS animals, RER values were significantly lower during the (last) restraint than that of the controls and this difference was maintained up to 2h after stress. The average RER values from the light phase were lower than those obtained in the dark.
In case of CVS exposed animals, the difference compared to the control, was not significant in the first 4 h after stress, although RER values of chronically stressed mice were below the control, non-stressed animals. By contrast, RER of CVS exposed mice was significantly elevated between 15 and 23 h after stress, i.e. in the next light phase.
These results indicate that fat is the major fuel to drive acute and repeated restraint stress-induced energy expenditure, while a fuel switch between fat to carbohydrate is seen in CVS animals.
Acute restraint is followed by hyperactivity, chronic stress results in hypoactivity
Next, we investigated locomotor activity. Locomotion, as part of the fight-or-flight stress response, is an important factor, which influences EE. In control, non-stressed animals, locomotion showed circadian variation with increased activity in the dark-, compared to the light phase. Even in the dark phase, there were two peaks, one close to the dark onset and another one before the light onset (Figure 5). Acute exposure to restraint increased the post-stress locomotor activity, while chronic exposure resulted in a decrease in locomotion, which was significantly suppressed in the dark (active) phase of the daily cycle. Acute and repeated restraint (CRS) resulted in a brief period of hyperactivity immediately after the release from the restrainer (Figure 5A’).
Figure 5.
Acute- and chronic repeated restraint transiently increase locomotor activity
X-Y-Z home cage locomotor activity of acutely- or repeatedly restrained mice or animals exposed to chronic variable stress was automatically recorded for 24h.
(A) 24 h registrations of locomotor activity. Control: blue dots, Stressed: red triangles. Bars on the right show the average values obtained in the light and dark phases.
(A’) Details of activity recordings during restraint (red area) and in the first 4 h post-stress.
Data are presented as mean ± SEM. Data were analyzed by repeated two-way ANOVA. In case of the first 4 h, data were analyzed separately hour by hour. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. control group.
These data suggest differential locomotor responses to acute and chronic stress exposure. In short term, escape from the restrainer results in hyperactivity, whereas in long term, chronically stressed mice show hypoactivity to save energy. Furthermore, decreased activity seen in chronically stressed mice can be interpreted as a habituated response.
Effects of stress exposure on blood metabolic parameters, hypothalamo-pituitary-adrenocortical axis and organ weights
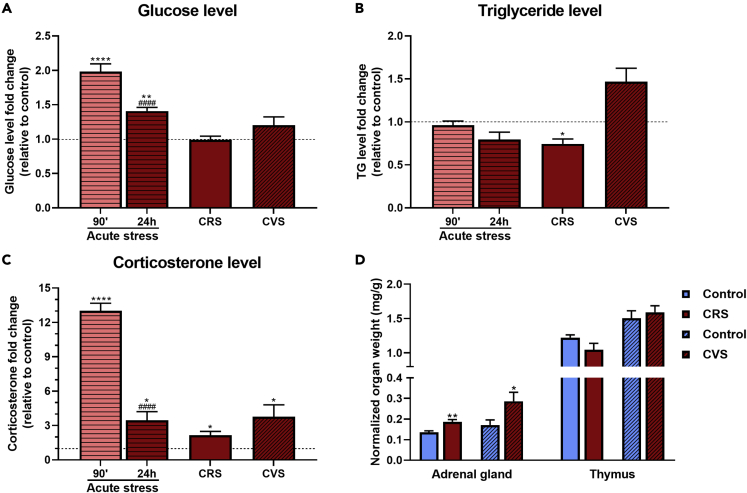
Acute stress significantly elevated blood glucose levels as measured at 30 min following 60 min restraint, which remained slightly elevated 24h post-stress. Glycaemia was not significantly different in chronically stressed animals compared to their controls as measured 24h after the last stress (Figure 6A). We did not detect significant changes in serum triglyceride (TG) level in acutely restrained animals, however, fold change in TG level was significantly lower in CRS mice. In CVS mice, TG levels were increased, however the difference did not reach significance (p = 0.053) (Figure 6B). Corticosterone levels were significantly increased during acute restraint stress with 13-fold increase at 90 min after initiation of stress and significantly higher levels were measured even after 24h (Figure 6C). Both chronic stress paradigms resulted in elevated corticosterone levels measured 24h after the last stress exposure (Figure 6C).
Figure 6.
Validation of stress exposures by metabolic and stress markers
(A–C) Fold changes values, relative to controls. A: Plasma glucose; B: Plasma triglyceride; C: Corticosterone.
(D) Weight of the adrenal glands and thymus, relative to body weight.
Data of acute stress were analyzed by one-way ANOVA followed by Tukey multiple comparison test. In case of CRS and CVS, unpaired t-test was used to calculate significance. Data are presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001 vs. control group. ####p < 0.0001 vs. 90’ after acute stress.
To confirm that chronic exposure to repeated- or variable stressors change body physiology, we have measured the weight of the adrenal glands (as a measure of chronic ACTH load) and thymus weight (as a measure of chronic adrenal steroid load). Normalized adrenal weight was increased in mice exposed either to CRS or CVS (Figure 6D). Comparison of thymus weight did not reveal any significant differences between chronic stress-exposed and non-stressed controls (Figure 6D).
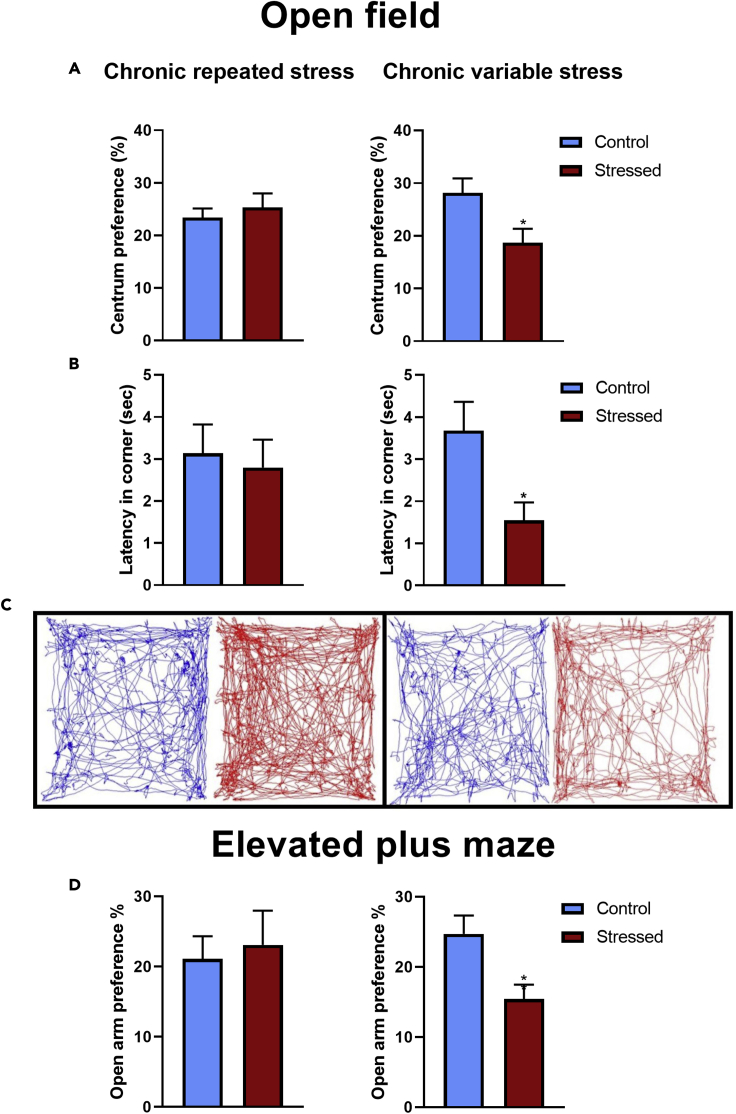
Anxiety develops in response to heterotypic (CVS), but not to homotypic (CRS) chronic stress exposure
Chronic stress is one of the major external factors, which precipitates anxiety or depression. Therefore, we aimed to elucidate differences in anxiety-related behavior of mice exposed to homotypic (CRS) vs heterotypic (CVS) chronic stress. Chronic unpredictable stress (CVS) protocol resulted in anxiety-like behavior with a decrease of centrum preference and in the latency to first time entrance into the corners of open field arena. In elevated plus maze, open arm preference was significantly attenuated in CVS animals. No such changes were observed in mice that have been exposed to repeated restraint (Figure 7).
Figure 7.
Chronic variable stress (CVS) but not chronic repeated restraint stress (CRS) increases anxiety-like behavior
(A and B) At the end of chronic stress protocols, open field (OF) and elevated plus maze (EPM) tests were performed. In the OF test, reduced centrum preference (A) and latency of the first entrance into the corner (B) were recorded in CVS exposed mice, while these parameters were unchanged in CRS exposed mice.
(C and D) Representative trajectory diagrams for control (blue) and chronically stressed (CRS and CVS) mice are shown on (C). Reduced open arm preference of CVS exposed mice in EPM test is demonstrated on (D).
Unpaired t-test was used to calculate significance. Data are presented as mean ± SEM. ∗p < 0.05 vs. the control group.
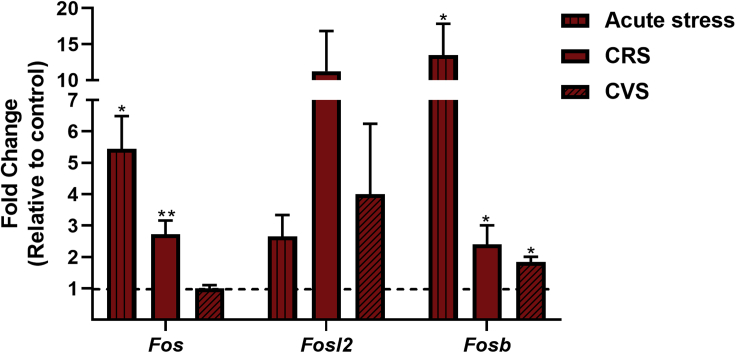
Fos-related transcription factors are differentially expressed in the stressed hypothalamus
To reveal the molecular mechanism underlying the metabolic-, hormonal- and behavioral response to acute and chronic stress, we have measured hypothalamic expression of specific stress-related transcription factors of AP1 family, which are responsible for acute- and lasting adaptations in target gene expression (Kovacs, 2008). We have confirmed that c-fos mRNA was induced in acutely restrained mice. By contrast, repeated restraint evoked significantly attenuated c-fos response, while no response was detected in CVS exposed mice (Figure 8). These results suggest that c-fos response is desensitized by chronic stress exposure.
Figure 8.
Acute- and chronic stress exposure differentially affects expression of neuronal activation markers in the hypothalamus
Hypothalamic mRNA levels of Fos, Fosl2 and Fosb were measured by RT qPCR. Fold change values, relative to controls, are shown in acutely restrained animals and mice exposed to repeated restraint (CRS) or chronic unpredictable variable (CVS) stress. Unpaired t-test was used to calculate significance. Data are presented as mean ± SEM data. ∗p < 0.05; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 vs. control group. ##p < 0.01; #####p < 0.0001 vs. acute stress.
Dramatic elevation of Fosl2 expression was detected exclusively in the CRS group. Fosl2 encodes Fra2, a Fos-related protein, which has been shown to mediate gene expression changes in response to repeated immobilization stress (Nankova et al., 2000).
FosB has been previously identified as a factor critical for long-term neural and behavioral plasticity to chronic stimuli and in stress resilience (Perrotti et al., 2004; Vialou et al., 2010). All stress paradigms increased hypothalamic expression of FosB, however the responses were significantly lower in chronically stressed groups, than that of acutely restrained groups. Altogether, these data indicate that in chronically stressed mice, induction of transient transcription factor (cFos) is desensitized, while mRNA of chronic markers are differentially activated in CRS vs CVS exposed mice.
Discussion
Restraint, by constraining subject’s movement, is generally held as a psychological stressor (Kovács et al., 2005), while it has many physical aspects (heat, mild hypoxia etc.) as well. Restraint activates hypothalamo-pituitary-adrenocortical (HPA) axis as well as the sympathetic nervous system and results in elevation of plasma corticosteroids and adrenaline/noradrenaline, respectively (Pacak and Palkovits, 2001; Nair et al., 2021). These hormones immediately increase lipolysis, glycogenolysis and glyconeogenesis to provide substrates for increased energy demand during stress. Increases of energy expenditure during acute restraint and in early post-stress periods are likely because of elevated stress hormone concentration. As shown in humans, infusion of cortisol or adrenaline increased resting energy expenditure (Brillon et al., 1995; Ratheiser et al., 1998). Noradrenaline injection increases O2 consumption via non-shivering thermogenesis and activation of BAT-UCP1 in mice kept below thermoneutrality (Cannon and Nedergaard, 2011).
We have registered metabolic changes in calo cages during acute restraint stress, revealing that RER values are not different from those of non-stressed controls during the period when animals are in the restrainer. However, the fuel for post-stress increase in EE originates from the increased fat oxidation rather than from carbohydrate, although plasma glucose is still high. Part of the increased post-stress EE might be because of increased locomotor activity seen in the first hour after escape from the restrainer. The other component of stress-induced EE might be the “emotional fever” i.e. stress-induced hyperthermia (Miyamoto et al., 2017). Intriguingly, stress-induced energy expenditure and increased locomotion in acutely stressed mice was not followed by significant increase of food intake to replenish energy stores.
Repeated exposure to the same (homotypic) stressor often results in (behavioral/hormonal) habituation, i.e. decreased response amplitude to the next challenge (Zelena et al., 2004; Herman, 2013). Here we found that stress-induced increase of EE is not habituating, but rather increased in the CRS paradigm. This finding parallels some earlier observations in rats (Harris et al., 2006). In our present results, increased energy expenditure in CRS mice is compensated by increased food intake and decreased home cage locomotor activity. By contrast, repeated restraint (3h daily for 3 days) in rats resulted in uncompensated, sustained weight loss (Harris et al., 2006). We found a major difference in substrate utilization during single vs. repeated restraint. During the 3 weeks restraint, fat is the predominant energy source, whereas in stress naive mice, mixed utilization of carbohydrates and fat is observed.
Chronic variable stress paradigm, although performed in many different ways/schedules, applies different, mostly psychogenic stressors in a random, unexpected order, produces a chronic state of HPA hyperactivity (Flak et al., 2009; Monteiro et al., 2015; Nair et al., 2021). Indeed, CRH expression in the hypothalamic paraventricular nucleus, POMC expression in the pituitary are increased, adrenal gland enlarged and plasma corticosterone elevated (Herman et al., 1995; Flak et al., 2011). Here we confirmed chronic activation of the HPA axis by enlarged adrenals, which was in correlation with increased corticosterone levels in CVS. Chronic HPA axis activity may be involved in maintaining increased energy expenditure in chronically stressed mice (Coccurello et al., 2018). By contrast, using another CVS paradigm, Castaneda et al. did not detect increased energy expenditure following 2 weeks exposure (Castaneda et al., 2011). Lack of change in energy expenditure in this report is interesting, since these authors used mostly physical stressors, directly challenging the energy homeostasis such as cold exposure, warm swim and hypoxia.
Compared to single restraint, chronic variable stress exposure resulted in facilitated EE, which extends up to 24h post-CVS. Furthermore, CVS paradigm reduces home cage locomotor activity, especially in the active phase of the circadian cycle. These results are in agreement with those of Castaneda et al. (Castaneda et al., 2011). Using caloric efficiency, as an indirect measure of energy expenditure, Solomon et al. also found an increase of EE in female rats exposed to CVS (Solomon et al., 2011). CVS animals also display signs of anxiety and depression with decreased activity and exploration in novel environments (i.e., open field arena), decreased number of entries into the center part of the arena and decreased latency to hide into the corners. Mice exposed to CVS show a tendency for utilization of more fat in the first 4 h after the last stressor, however, they change to carbohydrates afterwards. Decrease of respiratory quotient (RQ) was also observed (Castaneda et al., 2011; Coccurello et al., 2018) during the first dark phase in CVS mice, but not thereafter, suggesting RQ being the first metabolic parameter to recover after cessation of chronic stress exposure.
We have observed some differences in metabolic indices of mice exposed to chronic repeated-vs. chronic variable stress. Stressors used in the CVS differ in their length and strength. This raises important questions on comparison of chronic stress paradigms. Although the cumulative exposure time to stressors in CRS (21h) and CVS (82h) paradigms is different, the whole duration (3 weeks) of chronic stress exposure seems to be more important. This is supported by the fact that we did not observe significant differences between corticosterone concentration, adrenal and thymus weight in CVS vs. CRS mice.
Food intake and RER data are those, which show opposite directions in CRS and CVS animals. Mice exposed to CVS, display attenuated food intake at the end of three week stress exposure. By this time, these mice have decreased body fat content, metabolize predominantly carbohydrates in the light (inactive) phase of the circadian cycle and show symptoms of anxiety/depression. It remains to be confirmed if reduced food intake is a result of behavioral changes or due to metabolic reasons, such as stress-induced desensitization of fuel-sensing hypothalamic or brainstem structures or to chronic stress-induced anhedonia. Comparison of energetic and behavioral alterations seen in chronically stressed mice indicate that CRS dispose mice predominantly to metabolic-, whereas CVS to psycho(somatic) abnormalities.
Along this line, metabolic stress response might be closely related to stress coping behavior. If the fuels for muscles are sufficiently mobilized, that might support active coping, whereas paucity of energy rich substrates may result in passive coping. For instance, in the visible burrow system—a model of chronic social stress—weight loss in dominant rats is at the expense of fat depots, whereas subordinates lose more weight which is attributable to lean mass (Tamashiro et al., 2007).
Stress-induced food intake is generally held as the way to replenish depleted energy stores. In case of CRS, (but not in CVS) increases in EE are followed by increases in food intake. Furthermore, stress-induced corticosterone levels can cause cravings for sweet, fatty and salty foods (Laugero et al., 2001; Dallman et al., 2003a, 2003b) and conversely, ingestion of palatable food, reduces the activity of central stress response neuronal network (Foster et al., 2009). Because we fed our animals with common lab chow, they had no choice for qualitative adjustment of food intake.
Central mechanisms that may mediate stress effects on metabolism are rather complex. For instance, corticotropin-releasing hormone (CRH), which initiates the neuroendocrine stress response, is anorectic and increases locomotion. However, the effect of repeated central exposure to CRH is decreased over the time of multiple administration (Krahn et al., 1990), supporting our observation of decreased locomotion in chronically stressed mice. Proopiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus play a central role in regulation of feeding and energy homeostasis via production of anorexigenic αMSH. Chronic stress (CRS and CVS) results in hyperactivity of POMCARC neurons and decrease in body weight (Qu et al., 2020), consistent with our present findings. By contrast, stress-induced noradrenaline release in the arcuate nucleus might promote compensatory food intake via stimulation of orexigenic NPY/AgRPARC neurons (Paeger et al., 2017).
Most of the metabolic-related neuropeptide genes expressed in the hypothalamus have AP-1 binding site and regulated by transcription factors belong to Fos family. Our present data confirm activation of c-Fos mRNA after acute stress, but not in response to chronic challenges. In the latter case, induction of Fosl2 and FosB as markers of chronic neuronal activation is detected.
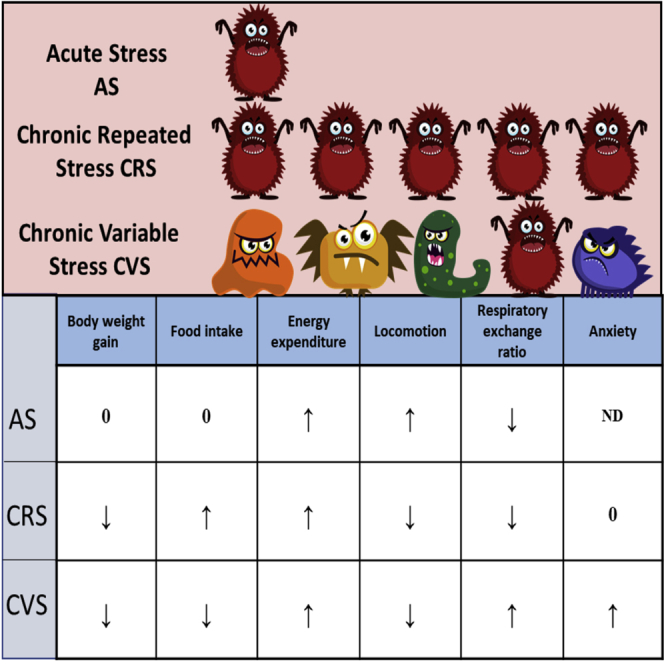
In summary, our study demonstrates that exposure to acute-, repeated- or chronic stress increases energy expenditure, but has different effects on body weight, body composition, food intake, fuel utilization and activity. Repeated stress predisposes rather to metabolic abnormalities, whereas chronic exposure to variable stressors provokes anxiety/depressive symptoms, in addition to changes in metabolism. Summary of stress-induced metabolic changes is shown in Table 1. These data contribute to our understanding of stress-induced metabolic-related disorders.
Table 1.
Summarized effects of acute restraint stress, chronic repeated restraint stress and chronic variable stress on metabolic and stress markers
| STRESS | BW gain | Body fat | Adrenal | Thymus | CORT | Food intake | EE | Locomotion home cage | RER | Anxiety |
|---|---|---|---|---|---|---|---|---|---|---|
| Acute restraint | 0 | 0 | 0 | 0 | ↑ | 0 | ↑ | ↑ | ↓ | ND |
| Chronic repeated restraint (CRS) | ↓ | 0 | ↑ | 0 | ↑ | ↑ | ↑ | ↓ | ↓ | 0 |
| Chronic variable stress (CVS) | ↓ | ↓ | ↑ | 0 | ↑ | ↓ | ↑ | ↓ | ↑ | ↑ |
BW, body weight; CORT, corticosterone; EE, energy expenditure; RER, respiratory exchange ratio; ↑, increased; ↓, decreased; 0, no change; ND, non-determined.
Limitations of the study
One major limitation of our results is that the experiments were carried out at 22°C, the usual temperature of mouse housing. Ambient temperature below the thermoneutrality could represent a mild chronic stress by itself; further studies are required to clarify the dependence of metabolic stress reactions on the housing temperature. Another important issue is that our stress experiments were performed in the late morning, the middle of the inactive phase. Previous studies highlighted the dependence of stress and metabolic responsiveness on the circadian cycle (Koolhaas et al., 2011; Bartlang et al., 2012).
These experiments were performed on male animals. The metabolic stress response in females might be more complex, due to the estrogen dependent changes in food intake and energy consumption (Mauvais-Jarvis et al., 2013).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| EvaGreen® Dye, 20X in Water | Biotium | Cat# 31000 |
| LightCycler 480 Probes Master | Roche | Cat# 04887301001 |
| Critical commercial assays | ||
| Corticosterone RIA kit | Institute of Isotopes Budapest, Hungary | Cat# RK-240CT |
| Glucose Colorimetric Detection Kit | Invitrogen | Cat# EIAGLUC |
| High-Capacity cDNA Reverse Transcription Kit | ThermoFisher Scientific | Cat# 4368814 |
| Multi-Care-in; Triglyceride | Biochemical Systems International S.p.A. | Cat# BC10-MULTICAREIN |
| Total RNA Mini Kit (tissue) | Central European Biosystems GmbH | Cat# RT100 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | The Jackson Laboratories | RRID:IMSR_JAX:000664 |
| Oligonucleotides | ||
| Gapdh qPCR target sequence forward: 5′-TGACGTGCCGCCTGGAGAAA-3′ reverse: 5′-AGTGTAGCCCAAGATGCCCTTCAG-3′ | Microsynth AG (custom order) | N/A |
| Fos qPCR target sequence forward: 5′-GAGAGCCTTTCCTACTACCATTCC-3′ reverse: 5′-GGACAGATCTGCGCAAAAGTC-3′ | Microsynth AG (custom order) | N/A |
| Fosl2 qPCR target sequence forward: 5′-CACCGCGGATCATGTACCAG-3′ reverse: 5′-TATCTACCCGGAACTTCTGCTG-3′ | Microsynth AG (custom order) | N/A |
| FosB qPCR target sequence forward: 5′-CAGATCGACTTCAGGCGGAAAC-3′ reverse: 5′-AATCTCTCACCTCGGCCAGC-3′ | Microsynth AG (custom order) | N/A |
| Software and algorithms | ||
| EthoVision XT | Noldus | https://www.noldus.com/ethovision-xt |
| Prism 6 | GraphPad | https://www.graphpad.com/ |
| StepOne™ Software v2.3 | Applied Biosystems | https://www.thermofisher.com/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Krisztina J. Kovács (kovacs.krisztina@koki.hu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
All experiments were performed on adult male C57BL/6J mice (n = 60; 8–9 weeks old at the beginning of experiment). Breeding pairs were kept at the Specific Pathogen Free (SPF) facility of Medical Gene Technology Unit at the Institute of Experimental Medicine. After weaning, mice were housed at the Minimal Disease (MD) level of the same facility under standard environmental parameters: 12 h light/dark cycle (lights on 06 a.m.), 21–22°C and 65% humidity. Animals received standard pelleted rodent chow (VRF1, Special Diets Services (SDS), Witham, Essex, UK) containing 19,1 g% protein, 55,3 g% carbohydrate and 4.8 g% fat. Chow and water were provided ad libitum. Experiments were complied with the ARRIVE guidelines and performed in accordance with the guidelines of European Communities Council Directive (86/609 EEC), EU Directive (2010/63/EU) and the Hungarian Act of Animal Care and Experimentation (1998; XXVIII, Sect. 243/1998). All procedures and experiments were approved by the Animal Care and Use Committee of the Institute of Experimental Medicine and the Animal Health and Food Control Station, Budapest (permit numbers: PEI/001/29-4/2013 and PE/EA/52-2/2019).
Method details
Experiment protocols
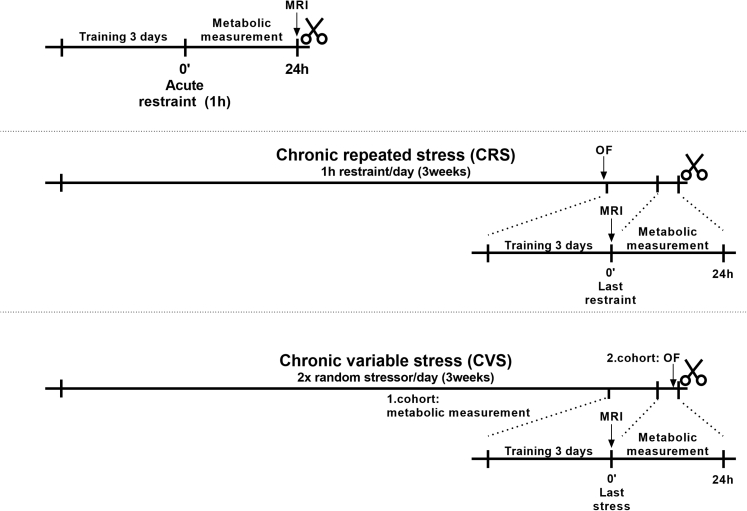
Experimental mice were randomly distributed into the following groups: 1.) acute restraint stress; 2.) chronic repeated restraint stress (CRS); 3.) chronic variable stress (CVS). Control animals (4.) were left undisturbed. Experimental design and timeline diagram for each stress paradigm is shown on below figure.
Flow charts of the experiment
Schematic representation of the implementation of the different stress protocols. CRS: Chronic repeated stress (1h daily restraint), CVS: Chronic variable stress (2x stress daily), MRI: body composition analysis.
Acute restraint stress
Mice were singly housed for one week. 3 days prior to metabolic measurements, mice (n = 8) were transferred to training cages of the TSE Phenomaster setup (TSE, Germany) to accommodate to this novel environment and to learn the usage of feeders and drinking bottles. 50 mL Falcon tubes were used for restraining the animals, in which the conical end of the tube was cut and small holes were made on the sides, to provide free breathing and ventilation. The other end of the tube was tightly filled with paper towel to prevent turning of mice. Restrained animals were placed into the metabolic (calo) cages, measured for one hour, then mice were released from the restrainers and the metabolic measurement continued for 24h. After measurement, mice were sacrificed and blood was collected.
Due to the rapid effect of restraint on various measures of acute stress, another cohort of mice (n = 5 restrained and n = 5 control) was used to measure serum corticosterone, triglyceride (TG), glucose levels and gene expression in the hypothalamus, 90 minutes after the beginning of one hour restraint stress. Mice were decapitated, trunk blood was collected and hypothalamus was dissected, then serum and the tissue were stored until assays. All data were compared to data of control mice, which were left undisturbed.
Chronic repeated restraint stress (CRS)
During CRS, mice (n = 8) were exposed daily to one hour restraint stress for three weeks. Body weight was measured once a week. Control animals (n = 8) were left undisturbed except for changing the bedding and measuring body weight. One week before metabolic measurement, mice were singly housed and transferred into training cages in the last 3 days before testing. Before training, open field test was performed on all experimental mice. Body composition was determined by EchoMRI (700 Whole Body Composition Analyzer; E26-233RM, Echo Medical Systems, Houston, TX, USA). At the end of the chronic stress procedure, animals were transferred to metabolic (calo) cages of the Phenomaster Metabolic Phenotyping system (TSE Systems Gmbh, Berlin, Germany), where the last restraint was performed and metabolic variables were measured for 24 hours. Then mice were decapitated for serum collection, and for measurement of organ weights (adrenals and thymus). Another set of mice (n = 7/groups) were exposed to the same CRS protocol and 24 hours after the last restraint stress, elevated plus maze (EPM) test was performed on these mice and 90 minutes after the behavior test, mice were decapitated and the hypothalamus was dissected and stored until assay.
Chronic variable stress (CVS)
Experimental mice (n = 8) were exposed twice daily to different psychogenic stressors: social defeat, water avoidance, light/dark alteration, forced swimming, soaked bedding, slanted cages, isolation, crowding, shaking, restraint, foot shock, rat feces odour, in a semi-random order for three weeks. Daily schedule of the stressors is found in the Supplementary material (Table S1). Body weight was measured weekly. Control animals (n = 8) were left undisturbed except for changing the bedding and measuring body weight. One week before metabolic measurement, mice were singly housed and transferred into training cages in the last 3 days before testing. Animals were placed into calo cages and their metabolic parameters were registered for 24 h after the last stressor exposure. At the end of measurement mice were decapitated and blood was collected. Adrenal glands and thymus were dissected and weighed after autopsy. Open field and elevated plus maze test were done on two different day on another cohort of CVS and control mice (n = 5/groups) after 3 weeks’ CVS procedure. These animals were also single housed for a week, placed to training cages for 3 days 90 minutes after EPM test animals were sacrificed and hypothalamus was dissected and stored.
Metabolic measurements
After acclimatization to single housing (1 week) and training boxes (last 3 days), all experimental mice were transferred to metabolic (calo) cages for metabolic measurement. Data of metabolic- and activity variables (food- and O2 consumption, CO2 production and X-Y-Z locomotor activity) were automatically collected in every 9 minutes in TSE Phenomaster system for 24 hours. Energy expenditure (EE (kcal/h)) was calculated using the Weir equation supplied by TSE Labmaster System: EE = [3.941 (VO2) + 1.106 (VCO2)] x 1.44. The respiratory exchange ratio (RER) was calculated with the following formula: VCO2/VO2.
Resting energy expenditure (REE) was estimated as REE = EE if the animal eats less than 0.1g in the last hour and moves <1% of its maximal ambulatory activity for the last 30 min. Since mice were housed below their thermoneutral temperature, the estimated REE also included energy expenditure for thermoregulation.
Gene expression analysis
Frozen hypothalamus samples were homogenized, then, total mRNA was isolated from the homogenate using a Total mRNA Mini Kit (Geneaid) according the manufacturer’s instruction. To eliminate genomic DNA contamination, DNase I (Fermentas) treatment was used. Sample quality control and the quantitative analysis were carried out by NanoDrop (Thermo Scientific). cDNA synthesis was performed with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-Time PCR was carried out in ABI StepOnePlus instrument (Applied Biosystems) with Fast EvaGreen quantitative PCR master mix (Biotium) and gene-specific primers. Primers (Microsynth) were designed in our laboratory using Primer-BLAST software of the National Center for Biotechnology Information (NCBI). Gene expression was analyzed by the 2-ΔΔCT method using the ABI StepOne Software v2.3 (Applied Biosystems). The amplicons were tested by melt curve analysis on ABI StepOnePlus instrument (Applied Biosystems). Relative changes in gene expression were normalized against GAPDH mRNA expression.
Corticosterone, glucose and triglyceride measurement
Glucose level was determined by Glucose Colorimetric Detection Kit from serum according to the manufacturer’s protocol (Glucose Colorimetric Detection Kit, Invitrogen).
Triglyceride (TG) level was measured by multiparameter diagnostic device for triglycerides (MultiCare-in; Biochemical Systems International Srl).
Corticosterone was measured from 10μL serum by direct RIA as described Zelena et al. (Zelena et al., 2003).
Open field test
To reveal if chronic stress exposures result in anxiety, animals from the CRS and CVS groups were tested in open field. Mice were placed in the centre of a 40x35x15cm, white, non-transparent plastic box and their activity was video-recorded from above for 10 min and then analysed by the Noldus EthoVision XT 10 program.
Elevated plus maze
The apparatus (arm length-30 cm, arm width-7 cm, wall height-30 cm platform height-80 cm) was made of dark-grey painted plexiglass. Open arms were surrounded by 0.3 mm high ledges. Mice were placed into the central area of the platform facing to one of the open arms and were allowed to explore the apparatus for 5 min. Mice were considered to enter a compartment when all four legs crossed the lines separating the compartments. Videos were analysed by Noldus EthoVision XT 10.
Quantification and statistical analysis
Because of the circadian rhythm, data of the light and dark phases were analysed separately. In this case, average data from every hour were used to calculate significance by repeated two-way ANOVA. Data from the first four hours post-stress were also analysed separately. In this case, every nine minutes’ data were used for statistics. The data of metabolic measurement and body weight change were analyzed by repeated measures two-way ANOVA and in case of body weight change, it was followed by Sidak’ post hoc test. Time was regarded as repeated measure. One-way ANOVA was used in case of acute stress-corticosterone, TG, glucose concentration and REE, followed by Tukey’s post hoc test. In these markers of CRS and CVS (CORT, TG, glucose) and the open field data, significance was determined between groups by unpaired t-test. All data were compared to the appropriate control groups. In all cases, differences were considered statistically significant at p < 0.05. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. All data are shown as mean ± SEM.
Acknowledgments
The study was supported by grants from the Hungarian National Research, Development and Innovation Office 124424, National Brain Research Program 2017-1.2.1-NKP-2017-00002 for KJK and RRF-2.3.1-21-2022-00011. National Laboratory for Translational Neuroscience. The authors thank to Dr. Ágnes Polyák for design the graphical abstract.
Author contributions
Dániel Kuti: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Visualization, Writing original draft. Zsuzsanna Winkler: Investigation, Data curation, Investigation, Formal analysis. Krisztina Horváth: Methodology, chronic stress. Balázs Juhász: Methodology, chronic stress. Anett Szilvásy- Szabó: Methodology, metabolic measurements. Csaba Fekete: Methodology, Resources. Szilamér Ferenczi: Original draft-review. Krisztina J. Kovács: Conceptualization, Funding acquisition, Writing- original draft, + review and editing.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as living with a disability.
While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: August 19, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104693.
Supplemental information
Data and code availability
-
•
Data: All data reported in this paper will be shared by the lead contact upon request.
-
•
Code: This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Bartlang M.S., Neumann I.D., Slattery D.A., Uschold-Schmidt N., Kraus D., Helfrich-Förster C., Reber S.O. Time matters: pathological effects of repeated psychosocial stress during the active, but not inactive, phase of male mice. J. Endocrinol. 2012;215:425–437. doi: 10.1530/joe-12-0267. [DOI] [PubMed] [Google Scholar]
- Berthoud H.R. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Brillon D.J., Zheng B., Campbell R.G., Matthews D.E. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am. J. Physiol. 1995;268:E501–E513. doi: 10.1152/ajpendo.1995.268.3.e501. [DOI] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- Castaneda T.R., Nogueiras R., Muller T.D., Krishna R., Grant E., Jones A., Ottaway N., Ananthakrishnan G., Pfluger P.T., Chaudhary N., et al. Decreased glucose tolerance and plasma adiponectin:resistin ratio in a mouse model of post-traumatic stress disorder. Diabetologia. 2011;54:900–909. doi: 10.1007/s00125-010-2019-y. [DOI] [PubMed] [Google Scholar]
- Coccurello R., Romano A., Giacovazzo G., Tempesta B., Fiore M., Giudetti A.M., Marrocco I., Altieri F., Moles A., Gaetani S. Increased intake of energy-dense diet and negative energy balance in a mouse model of chronic psychosocial defeat. Eur. J. Nutr. 2018;57:1485–1498. doi: 10.1007/s00394-017-1434-y. [DOI] [PubMed] [Google Scholar]
- Dallman M.F., Akana S.F., Laugero K.D., Gomez F., Manalo S., Bell M.E., Bhatnagar S. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol. Behav. 2003;79:3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- Dallman M.F., Pecoraro N., Akana S.F., La Fleur S.E., Gomez F., Houshyar H., Bell M.E., Bhatnagar S., Laugero K.D., Manalo S. Chronic stress and obesity: a new view of "comfort food". Proc. Natl. Acad. Sci. USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak J.N., Jankord R., Solomon M.B., Krause E.G., Herman J.P. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol. Behav. 2011;104:228–234. doi: 10.1016/j.physbeh.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak J.N., Ostrander M.M., Tasker J.G., Herman J.P. Chronic stress-induced neurotransmitter plasticity in the PVN. J. Comp. Neurol. 2009;517:156–165. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M.T., Warne J.P., Ginsberg A.B., Horneman H.F., Pecoraro N.C., Akana S.F., Dallman M.F. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D.S. Stress-induced activation of the sympathetic nervous system. Baillieres Clin. Endocrinol. Metab. 1987;1:253–278. doi: 10.1016/s0950-351x(87)80063-0. [DOI] [PubMed] [Google Scholar]
- Harris R.B., Palmondon J., Leshin S., Flatt W.P., Richard D. Chronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm. Behav. 2006;49:615–625. doi: 10.1016/j.yhbeh.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Herman J.P. Neural control of chronic stress adaptation. Front. Behav. Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Adams D., Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Tasker J.G. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front. Endocrinol. 2016;7:137. doi: 10.3389/fendo.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas J.M., Bartolomucci A., Buwalda B., De Boer S.F., Flügge G., Korte S.M., Meerlo P., Murison R., Olivier B., Palanza P., et al. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kovács K.J. Measurement of immediate-early gene activation- c-fos and beyond. J. Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Kovács K.J., Miklós I.H., Bali B. Psychological and physiological stressors. Handbook of Stress and the Brain. 2005;15:775–792. [Google Scholar]
- Krahn D.D., Gosnell B.A., Majchrzak M.J. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol. Psychiatr. 1990;27:1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- Laugero K.D., Bell M.E., Bhatnagar S., Soriano L., Dallman M.F. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology. 2001;142:2796–2804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Funakami Y., Kawashita E., Nomura A., Sugimoto N., Saeki H., Tsubota M., Ichida S., Kawabata A. Repeated cold stress enhances the acute restraint stress-induced hyperthermia in mice. Biol. Pharm. Bull. 2017;40:11–16. doi: 10.1248/bpb.b16-00343. [DOI] [PubMed] [Google Scholar]
- Monteiro S., Roque S., de Sá-Calçada D., Sousa N., Correia-Neves M., Cerqueira J.J. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatr. 2015;6:6. doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B.B., Khant Aung Z., Porteous R., Prescott M., Glendining K.A., Jenkins D.E., Augustine R.A., Silva M.S.B., Yip S.H., Bouwer G.T., et al. Impact of chronic variable stress on neuroendocrine hypothalamus and pituitary in male and female C57BL/6J mice. J. Neuroendocrinol. 2021;33 doi: 10.1111/jne.12972. [DOI] [PubMed] [Google Scholar]
- Nankova B.B., Rivkin M., Kelz M., Nestler E.J., Sabban E.L. Fos-related antigen 2: potential mediator of the transcriptional activation in rat adrenal medulla evoked by repeated immobilization stress. J. Neurosci. 2000;20:5647–5653. doi: 10.1523/jneurosci.20-15-05647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K., Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr. Rev. 2001;22:502–548. doi: 10.1210/er.22.4.502. [DOI] [PubMed] [Google Scholar]
- Paeger L., Karakasilioti I., Altmüller J., Frommolt P., Brüning J., Kloppenburg P. Antagonistic modulation of NPY/AgRP and POMC neurons in the arcuate nucleus by noradrenalin. Elife. 2017;6:e25770. doi: 10.7554/elife.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti L.I., Hadeishi Y., Ulery P.G., Barrot M., Monteggia L., Duman R.S., Nestler E.J. Induction of FosB in reward-related brain structures after chronic stress. J. Neurosci. 2004;24:10594–10602. doi: 10.1523/jneurosci.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyykkonen A.J., Raikkonen K., Tuomi T., Eriksson J.G., Groop L., Isomaa B. Stressful life events and the metabolic syndrome: the prevalence, prediction and prevention of diabetes (PPP)-Botnia Study. Diabetes Care. 2010;33:378–384. doi: 10.2337/dc09-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu N., He Y., Wang C., Xu P., Yang Y., Cai X., Liu H., Yu K., Pei Z., Hyseni I., et al. A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol. Psychiatr. 2020;25:1006–1021. doi: 10.1038/s41380-019-0506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheiser K.M., Brillon D.J., Campbell R.G., Matthews D.E. Epinephrine produces a prolonged elevation in metabolic rate in humans. Am. J. Clin. Nutr. 1998;68:1046–1052. doi: 10.1093/ajcn/68.5.1046. [DOI] [PubMed] [Google Scholar]
- Renzaho A.M.N., Houng B., Oldroyd J., Nicholson J.M., D'esposito F., Oldenburg B. Stressful life events and the onset of chronic diseases among Australian adults: findings from a longitudinal survey. Eur. J. Publ. Health. 2014;24:57–62. doi: 10.1093/eurpub/ckt007. [DOI] [PubMed] [Google Scholar]
- Roy M., Kirschbaum C., Steptoe A. Psychological, cardiovascular, and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendocrinology. 2001;26:375–391. doi: 10.1016/s0306-4530(00)00061-5. [DOI] [PubMed] [Google Scholar]
- Seeman T.E., Mcewen B.S., Rowe J.W., Singer B.H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl. Acad. Sci. USA. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M.B., Jankord R., Flak J.N., Herman J.P. Chronic stress, energy balance and adiposity in female rats. Physiol. Behav. 2011;102:84–90. doi: 10.1016/j.physbeh.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro K.L.K., Nguyen M.M.N., Ostrander M.M., Gardner S.R., Ma L.Y., Woods S.C., Sakai R.R. Social stress and recovery: implications for body weight and body composition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1864–R1874. doi: 10.1152/ajpregu.00371.2007. [DOI] [PubMed] [Google Scholar]
- Tamashiro K.L., Sakai R.R., Shively C.A., Karatsoreos I.N., Reagan L.P. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14:468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- Vialou V., Robison A.J., Laplant Q.C., Covington H.E., 3rd, Dietz D.M., Ohnishi Y.N., Mouzon E., Rush A.J., 3rd, Watts E.L., Wallace D.L., et al. ΔFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena D., Földes A., Mergl Z., Barna I., Kovács K.J., Makara G.B. Effects of repeated restraint stress on hypothalamo-pituitary-adrenocortical function in vasopressin deficient Brattleboro rats. Brain Res. Bull. 2004;63:521–530. doi: 10.1016/j.brainresbull.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Zelena D., Mergl Z., Földes A., Kovács K.J., Tóth Z., Makara G.B. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1110–E1117. doi: 10.1152/ajpendo.00219.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data: All data reported in this paper will be shared by the lead contact upon request.
-
•
Code: This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.