Abstract
This study was conducted to investigate the effects of glycerol monolaurate (GML) on lipopolysaccharide (LPS)-induced immunological stress and intestinal mucosal injury in broilers and its underlying mechanisms. A total of 144 one-d-old Arbor Acres broilers were allocated to a 2 × 2 factorial arrangement involving dietary treatment (0 or 1,200 mg/kg dietary GML) and LPS challenge (injected with saline or Escherichia coli LPS on d 16, 18, and 20). Samples were collected on d 21. The results revealed that dietary GML augmented serum immunoglobulin A (P = 0.009) and immunoglobulin G (P < 0.001) levels in challenged birds. Dietary GML normalized LPS-induced variations in serum interleukin-6, interferon-gamma, and LPS levels (P < 0.05), jejunal villus height (P = 0.030), and gene expression of interleukin-6, macrophage inflammatory protein-3 alpha, Toll-like receptor 4, nuclear factor kappa-B, caspase-1, tight junction proteins, adenosine monophosphate-activated protein kinase alpha 1 (AMPKα1), nuclear factor-erythroid 2-related factor 2 (Nrf2), and superoxide dismutase-1 (P < 0.05). GML supplementation ameliorated LPS-induced peroxidation by reducing malondialdehyde content and increasing antioxidant enzyme activity (P < 0.05). Dietary GML enhanced the abundances of Anaerostipes, Pseudoflavonifractor, and Gordonibacter and reduced the proportion of Phascolarctobacterium in challenged birds. Dietary GML was positively correlated with alterations in antioxidant enzyme activities and AMPKα1, Nrf2, and zonula occludens-1 expressions. The genera Anaerostipes, Lachnospira, Gordonibacter, Lachnospira, Marvinbryantia, Peptococcus, and Pseudoflavonifractor were linked to attenuated inflammation and improved antioxidant capacity of challenged birds. In conclusion, dietary GML alleviated LPS-induced immunological stress and intestinal injury of broilers by suppressing inflammation and oxidative stress. Dietary GML regulated cecal microbiota and activated the AMPK/Nrf2 pathway in LPS-challenged broilers.
Keywords: Glycerol monolaurate, Lipopolysaccharide challenge, Inflammation, Antioxidation, Gut microbiota, Adenosine monophosphate-activated protein kinase
1. Introduction
Poultry production is restricted by various environmental, nutritional, and biological pressures, affecting the productive performance and health status of broilers (Surai and Fisinin, 2016). Immunological stress and bacterial infection can induce intestinal inflammation, which contributes to intestinal mucosal injury and gut epithelial dysfunction in birds (Connerton et al., 2018; Jiang et al., 2021). Gram-negative bacterial lipopolysaccharide (LPS) is the main cause of intestinal diseases (Wang et al., 2015). After binding to the extracellular domain of Toll-like reporter 4 (TLR4), LPS initiates a cascade of intracellular signal transduction, promotes the secretion of enormous amounts of proinflammatory cytokines, and induces immunological stress. Intestinal barrier dysfunction mediated by LPS leads to increased permeability, which allows pathogenic bacteria or toxins to enter the blood circulation, thereby causing systemic disease (Chen et al., 2018b).
Antibiotics have long been used for growth promotion and disease prevention in poultry. Nonetheless, antibiotic residues and resistance issues have pressured the poultry industry to develop substitutes for antibiotics to maintain poultry flock health by enhancing immune responses (Kogut, 2017). Among these substitutes, plant metabolites, natural by-products, essential minerals, amino acids, medicinal herbs, organic acids, and essential oils have shown some promising effects, which can at least partially alter immune function in poultry (Kogut, 2017).
Glycerol monolaurate (GML), a natural compound composed of glycerol and lauric acid, is considered a safe and efficacious alternative to growth-promoting antibiotics with distinguished antimicrobial, antiviral, and anti-inflammatory properties (Zhao et al., 2020). Dietary GML has been shown to improve performance, intestinal morphology, and muscle amino acid content in yellow-feathered broilers by regulating the community, function, and metabolites of intestinal microbiota (Liu et al., 2020). Moreover, GML has been found to inhibit immune activation and the production of chemokines and cytokines in human vaginal epithelial cells under the action of staphylococcal toxins (Peterson and Schlievert, 2006), supporting the hypothesis of an immunoregulatory effect during infection (Li et al., 2009). The immunosuppressive properties of GML are conducive to attenuating intestinal inflammation (Zhang et al., 2018). Previous findings demonstrated that GML ameliorated intestinal barrier and immunity in birds by regulating intestinal inflammation, antioxidant balance, and intestinal microbiota (Kong et al., 2021). Although the beneficial effect of GML is gradually being recognized, information on whether GML supplementation can attenuate immunological stress and protect intestinal barrier from LPS challenge in broilers is insufficient.
Dietary GML has been shown to affect immunity in mice and ameliorate high-fat-diet-induced metabolic disorder, obesity, and inflammation by targeting intestinal microbiota (Jiang et al., 2018; Zhao et al., 2019). Based on these findings, the present study was designed to investigate the hypothesis that dietary GML supplementation could alleviate immunological stress and intestinal injury induced by LPS challenge through the regulation of intestinal microbiota in broiler chickens.
2. Materials and methods
2.1. Animal ethics
All the experimental procedures applied in this study were reviewed and approved by Shandong Agricultural University and carried out following the Guidelines for Experimental Animals of the Ministry of Science and Technology (Beijing, People's Republic of China).
2.2. Birds, management, and experimental design
A total of 144 one-d-old Arbor Acres broilers with similar hatching weights were randomly allocated into 2 dietary treatments consisting of a basal diet and the basal diet with 1,200 mg/kg GML. The purity of GML was more than 90%, which was purchased from Henan Zhengtong Food Technology Co., Ltd (Henan, China). The supplemental GML dosage was based on previous findings (Kong et al., 2021). The basal diet met the National Research Council (NRC, 2012) recommendations for growing broilers (Appendix Table 1). All birds were randomly assigned to metal cages (70 cm × 70 cm × 40 cm) equipped with a feeder and nipple drinker and were reared in an environmentally controlled room. Each group contained 6 replicates (cages) with 12 birds per cage. The initial temperature was 34 °C and then gradually decreased to 26 °C by 21 d of age. The average relative humidity was maintained at approximately 70% in the first 3 d and thereafter maintained between 55% and 65%. Birds were kept under 23 h of light and 1 h of darkness in the first week, followed by 20 h of light and 4 h of darkness for the subsequent period.
A 2 × 2 two-factor completely random design was adopted in this study. Dietary GML (diet with 0 or 1,200 mg/kg GML) was considered one factor, and LPS challenge (injection with LPS or saline) was considered another. The LPS challenge was performed in accordance with reported procedures (Kamboh and Zhu, 2014). On d 16, 18, and 20, half of the birds in each replicate were intraperitoneally injected with Escherichia coli LPS (L2880, Sigma–Aldrich Inc., St. Louis, MO, USA) at a dosage of 1 mg/kg of body weight. The remaining birds were injected with 0.9% saline. Feed consumption in each replicate was recorded on d 14 and 21 to calculate the average feed intake (AFI). Spilled feed was carefully collected and weighed to correct the final feed intake data. Birds were weighed to calculate the average body weight gain (ABWG). The feed conversion ratio (FCR) was defined as AFI:ABWG.
2.3. Sample collection
Two birds per replicate were randomly selected for sampling after growth performance was recorded on d 21. Approximately 4 mL of blood samples were retrieved from wing veins with sterile syringes. One milliliter of blood samples was transferred to glass tubes coated with ethylenediaminetetraacetic acid for blood cell analysis. The other 3 mL were transferred to glass tubes without anticoagulants to separate serum by centrifugation at 3,000 × g at 4 °C for 10 min. Birds were euthanized by cervical dislocation. Approximately 2 cm segments were excised from the entry point of the bile duct to Meckel's diverticulum and immediately immersed in 4% paraformaldehyde solution for histological examination. Approximately 1 to 2 g jejunum samples and complete ceca were collected on ice, rapidly frozen in liquid nitrogen, and stored at −80 °C for further analysis.
2.4. Hematology determination
Leukocyte, lymphocyte, intermediate cells, and granulocyte counts in blood samples were determined using an automatic blood counter according to the manufacturer's instructions (KT6200, Genrui Biotech Inc., Shenzhen, China).
2.5. Assay of immune parameters in serum and jejunum
Serum interleukin 1 beta (IL-1β), interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), immunoglobulin A (IgA), and immunoglobulin G (IgG) levels were detected using Enzyme-linked immunosorbent assay (ELISA) kits (MLBIO Co., Shanghai, China). Jejunum samples (0.3 g) were homogenized in 2.7 mL of phosphate-buffered saline and centrifuged at 1,000 × g at 4 °C for 10 min. Then, the supernatant was collected to detect the levels of phospho-AMPK (p-AMPK) and total AMPK by ELISA assays (MLBIO Co., Shanghai, China). The results were normalized to protein concentration in each jejunal homogenate. All determination procedures were performed strictly according to the manufacturer's instructions. The inter- and intra-assay coefficients of variation (CV) were less than 10%.
2.6. Intestinal permeability and morphology analysis
Intestinal permeability was evaluated based on serum diamine oxidase (DAO) and LPS levels using ELISA assays (MLBIO Co., Shanghai, China). The jejunum segments were dehydrated and embedded in paraffin after fixation in a 4% paraformaldehyde solution for 24 h. Tissue coated with paraffin was sectioned at 5 μm thickness using a microtome (Leica RM2235, Leica Biosystems Inc., Buffalo Grove, USA), fixed on slides, and stained with hematoxylin and eosin. Images of the jejunum were acquired using a Nikon Eclipse 80i microscope (Nikon Inc., Tokyo, Japan) and analyzed with ImageJ analysis software (version 1.47, Bethesda, MD, USA). Villus height (VH) was gauged from the tip of the villus to the villus–crypt junction. Crypt depth (CD) was defined as the depth of the invagination between adjacent villi. The villus height-to-crypt depth ratio (VCR) was calculated. Ten sections of the proper microscopic fields were selected randomly from each sample for morphology measurement. The average of 10 values from individual birds was used in statistical analysis.
2.7. Assessment of oxidative status
The oxidative status of serum and jejunal homogenate was evaluated by determining malondialdehyde (MDA) levels, total antioxidant capacity (T-AOC), catalase (CAT) capacity, total superoxide dismutase (T-SOD) activity, and glutathione peroxidase (GSH-px) capacity. The protein content of jejunal homogenate was measured with a bicinchoninic acid protein assay kit. All diagnostic kits (intra-assay CV < 5%, inter-assay CV < 8%) were purchased from Nanjing Jiancheng Biotechnology Institute (Nanjing, China). All determination procedures were performed in strict accordance with the manufacturer's instructions. The results were normalized to the protein concentration in each jejunal homogenate.
2.8. RNA isolation and real-time quantitative PCR
Jejunal RNA was isolated using RNA-Easy Isolation Reagent (Vazyme Biotech, Nanjing, China) according to the manufacturer's instructions. RNA quality was evaluated using 1% agarose gel electrophoresis. The purity of total RNA was determined by NanoDrop 2000 (NanoDrop, ThermoFisher Scientific, Waltham, MA, USA). Reverse transcription of 1 μg of total RNA was performed using a PrimeScript RT reagent kit (RR047A, Takara Bio Inc., Dalian, China) after genomic DNA was removed by gDNA Eraser. The reverse-transcribed cDNA was diluted 5 times in nuclease-free water. Quantitative PCR was performed with a real-time PCR system (ABI 7500, Applied Biosystems, Foster City, CA, USA) using TB Green Premix Ex Taq (RR820A, Takara Bio Inc., Dalian, China). The reaction program was as follows: predenaturation at 95 °C for 10 s, then a total of 40 cycles of denaturation at 95 °C for 5 s, and annealing at 60 °C for 34 s. Each reaction was repeated 3 times. The primer sequences are shown in Appendix Table 2. A standard curve was used to verify the amplification efficiency per pair of primers. The specificity of the amplified products was verified with a melting curve. The relative expression of the target gene was analyzed through the 2−ΔΔCt method after normalization against the geomean of the expression of β-actin, glyceraldehyde-3-phosphate dehydrogenase, and TATA-binding protein.
2.9. 16S rRNA gene sequencing and analysis
Total genomic DNA from cecal contents was extracted using an E.Z.N.A. Soil DNA Kit (Omega Bio-Tek, Norcross, CA, USA) following the manufacturer's protocol. The extracted DNA was checked for quantity and purity by NanoDrop 2000 (NanoDrop, ThermoFisher Scientific, Waltham, MA, USA). The integrity of DNA was detected by 1% agarose gel electrophoresis. The V3–V4 hypervariable regions of bacterial 16S rRNA genes were amplified with the specific primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA, USA). Amplification was conducted using the following program: 3 min at 95 °C; 27 cycles of 30 s at 95 °C, 55 °C for 30 s, and 72 °C for 45 s; and 72 °C for 10 min. PCR was performed in triplicate using a 20 μl mixture containing 10 ng of template DNA. The PCR products were extracted from a 2% agarose gel, purified by an AxyPrep DNA Gel Recovery Kit (Axygen Biosciences, Union City, CA, USA), and then quantified with a blue fluorescence quantitative system (QuantiFluo-ST, Promega, Madison, WI, USA). Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA) following standard protocols. The original contributions presented in the study are publicly available. This data can be found here: http://www.ncbi.nlm.nih.gov/bioproject/774633.
The raw data FASTQ files were imported into the format which could be operated by the QIIME2 system. Demultiplexed sequences from each sample were quality filtered and trimmed, de-noised, merged, and then the chimeric sequences were identified and removed using the QIIME2 dada2 plugin to obtain the feature table of amplicon sequence variant (ASV) (Callahan et al., 2016). The QIIME2 feature-classifier plugin was then used to align ASV sequences to a pre-trained Silva 16S rRNA database (trimmed to the V3–V4 region bound by the 338F/806R primer pair) to generate the taxonomy table. Any contaminating mitochondrial sequences were filtered using the QIIME2 feature-table plugin. Diversity metrics were calculated using the core-diversity plugin within QIIME2. Feature level alpha diversity indices, such as Chao1 richness estimator, Faith-phylogenetic diversity (Faith-pd), Shannon diversity index, and Simpson indices were calculated to estimate the microbial diversity within an individual sample. Beta diversity analysis based on unweighted Unifrac distance was performed to investigate the structural variation in microbial communities across samples and then visualized by principal coordinate analysis (PCoA). Linear discriminant analysis effect size (LEfSe) was employed to identify the bacteria with different abundances among samples and groups. Redundancy analysis (RDA) was performed to reveal the association of microbial communities with environmental factors. Spearman's rank correlations between predominant taxa were calculated to perform a co-occurrence analysis.
2.10. Statistical analysis
All data were checked for normality with the Shapiro–Wilk test (95% confidence level) and subjected to two-way ANOVA using the general linear model procedure in SPSS software (IBM SPSS Statistics 26.0, Armonk, NY, USA). The main factor effects and interactions of LPS challenge and dietary GML were evaluated. Significant variations between the treatments were compared using Tukey's multiple comparisons or nonparametric factorial Kruskal–Wallis test. Data are presented as the mean values with the total standard errors of the mean (SEM). Differences were considered significantly different at P < 0.05.
3. Results
3.1. Growth performance
The effects of experimental treatments on growth performance are presented in Table 1. The AFI (P = 0.016) and ABWG (P = 0.002) of birds were reduced by LPS challenge. Thus, the highest FCR was observed in the LPS group (P = 0.021). Dietary GML did not affect the AFI, ABWG, or FCR of birds during the entire experimental period (P > 0.05). No interaction between dietary GML and LPS challenge was observed on growth performance in birds (P > 0.05).
Table 1.
Effects of dietary glycerol monolaurate on growth performance of lipopolysaccharide-challenged broilers.1,2
| Item | Treatments |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | GML | LPS | LPS + GML | Diet | Challenge | Interaction | ||
| AFI, g/bird | 477.06 | 469.14 | 448.68 | 450.29 | 3.76 | 0.684 | 0.016 | 0.541 |
| ABWG, g/bird | 345.91 | 340.30 | 313.60 | 312.81 | 4.32 | 0.805 | 0.002 | 0.819 |
| FCR | 1.37 | 1.36 | 1.43 | 1.44 | 0.01 | 0.827 | 0.021 | 0.695 |
Means are based on 6 replicates per treatment with 6 birds per replicate.
SEM = standard error of the mean; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS; AFI = average feed intake; ABWG = average body weight gain; FCR = feed conversion ratio.
3.2. Blood cell counts
Table 2 reports the effects of the experimental treatments on blood cell counts. No interactions between dietary GML and LPS challenge were observed (P > 0.05). LPS challenge increased the counts of leukocytes (P = 0.025), intermediate cells (P = 0.001), and granulocytes (P = 0.001) in the blood compared to the non-challenged birds. Birds offered the GML-supplemented diet had fewer leukocytes than those fed the basal diet (P < 0.001).
Table 2.
Effects of dietary glycerol monolaurate on blood cell counts (109 cells/L) of lipopolysaccharide-challenged broilers.1,2
| Item | Treatments |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | GML | LPS | LPS + GML | Diet | Challenge | Interaction | ||
| Leukocytes | 85.81 | 72.28 | 87.43 | 81.03 | 1.11 | <0.001 | 0.025 | 0.118 |
| Lymphocytes | 65.46 | 61.83 | 65.02 | 60.46 | 1.65 | 0.223 | 0.787 | 0.889 |
| Intermediate cells | 6.84 | 6.30 | 7.47 | 7.74 | 0.14 | 0.649 | 0.001 | 0.162 |
| Granulocytes | 9.77 | 7.22 | 11.51 | 11.17 | 0.38 | 0.070 | 0.001 | 0.115 |
Means are based on 6 replicates per treatment with 2 birds per replicate.
SEM = standard error of the mean; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS.
3.3. Cytokines and immunoglobulin levels
Table 3 illustrates the effects of dietary GML on the inflammatory cytokines and immunoglobulins in the serum of LPS-challenged birds. Significant interactions were observed between dietary GML and LPS challenge in the serum cytokine and immunoglobulin levels of birds (P < 0.05). LPS challenge increased the levels of IL-1β (P = 0.010), IL-6 (P = 0.016), TNF-α (P = 0.002), and IFN-γ (P < 0.001) in serum. Dietary GML reduced the serum IL-1β (P = 0.040) and IFN-γ (P = 0.004) levels and reversed the increase in IL-6 (P = 0.047) and IFN-γ (P = 0.007) levels caused by LPS challenge. Birds in the LPS + GML group exhibited higher serum immunoglobulin A (P = 0.009) and immunoglobulin G (P < 0.001) levels compared to other 3 groups.
Table 3.
Effects of dietary glycerol monolaurate on serum immune parameters (pg/mL) of lipopolysaccharide-challenged broilers.1,2
| Item | Treatments |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | GML | LPS | LPS + GML | Diet | Challenge | Interaction | ||
| IL-1β | 637.90 | 599.65 | 660.78 | 645.94 | 6.05 | 0.040 | 0.010 | 0.345 |
| IL-6 | 25.71b | 29.18b | 40.78a | 30.76b | 1.62 | 0.323 | 0.016 | 0.047 |
| TNF-α | 62.86 | 58.26 | 63.42 | 66.00 | 0.55 | 0.371 | 0.002 | 0.419 |
| IFN-γ | 60.93b | 60.73b | 70.77a | 64.32b | 1.09 | 0.004 | <0.001 | 0.007 |
| IgA | 223.83b | 231.83b | 229.33b | 255.75a | 1.68 | <0.001 | <0.001 | 0.009 |
| IgG | 1,846.11b | 1,740.56b | 1,777.22b | 2,066.67a | 25.18 | 0.076 | 0.015 | <0.001 |
a, b Within a row, means with no common superscripts differ significantly (P < 0.05).
Means are based on 6 replicates per treatment with 2 birds per replicate.
SEM = standard error of the mean; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS; IL-1β = interleukin 1 beta; IL-6 = interleukin 6; TNF-α = tumor necrosis factor alpha; IFN-γ = interferon gamma; IgA = immunoglobulin A; IgG = immunoglobulin G.
3.4. Inflammatory gene expression
To further determine the genetic basis of the ameliorated inflammatory reaction in LPS-challenged birds, the expression of genes related to cytokines and apoptosis was investigated by quantitative PCR (Table 4). The interaction between GML supplementation and LPS challenge notably affected the gene expression of IL-6 (P = 0.010), macrophage inflammatory protein (MIP)-3α (P = 0.024), TLR4 (P = 0.012), nuclear factor kappa-B (NF-κB) (P = 0.001), and caspase-1 (P = 0.023) in the jejunum of birds. LPS challenge upregulated IL-6 (P = 0.016), MIP-3α (P = 0.018), NF-κB (P = 0.033), and caspase-1 (P = 0.001) expression. Dietary GML significantly reduced jejunal MIP-3α (P = 0.016) and NF-κB (P = 0.020) expression and reversed the enhancement of proinflammatory and apoptotic gene expression mediated by LPS challenge (P < 0.05). The results revealed that dietary GML ameliorated jejunal inflammation and apoptosis in LPS-challenged birds.
Table 4.
Effects of dietary glycerol monolaurate on jejunal inflammation associated genes of lipopolysaccharide-challenged broilers.1,2
| Gene | Treatments |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | GML | LPS | LPS + GML | Diet | Challenge | Interaction | ||
| IL-1β | 1.00 | 1.15 | 1.20 | 1.01 | 0.05 | 0.861 | 0.304 | 0.585 |
| IL-6 | 1.00b | 1.34ab | 1.69a | 0.98b | 0.08 | 0.289 | 0.346 | 0.010 |
| TNF-α | 1.00 | 1.13 | 1.12 | 1.07 | 0.06 | 0.873 | 0.716 | 0.683 |
| IFN-γ | 1.00 | 0.64 | 1.07 | 0.91 | 0.09 | 0.158 | 0.342 | 0.603 |
| MIP-3α | 1.00b | 0.94b | 1.67a | 0.96b | 0.06 | 0.016 | 0.018 | 0.024 |
| TLR4 | 1.00b | 1.26ab | 1.95a | 1.06b | 0.10 | 0.144 | 0.083 | 0.012 |
| IκBα | 1.00 | 1.09 | 0.76 | 0.90 | 0.05 | 0.266 | 0.044 | 0.795 |
| NF-κB | 1.00b | 1.20b | 1.61a | 0.98b | 0.04 | 0.020 | 0.033 | 0.001 |
| Caspase-1 | 1.00b | 0.97b | 1.51a | 1.09b | 0.05 | 0.052 | 0.001 | 0.023 |
a, b Within a row, means with no common superscripts differ significantly (P < 0.05).
Means are based on 6 replicates per treatment with 1 bird per replicate.
SEM = standard error of the mean; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS; IL-1β = interleukin 1 beta, IL-6 = interleukin 6; TNF-α = tumor necrosis factor alpha, IFN-γ = interferon gamma; MIP-3α = macrophage inflammatory protein-3 alpha; TLR4 = Toll-like receptor; IκBα = inhibitor kappa-B alpha; NF-κB = nuclear factor kappa-B.
3.5. Intestinal morphology and jejunal AMPK
The effects of the experimental treatments on intestinal morphology and jejunal AMPK levels are presented in Table 5. A significant interaction between dietary GML and LPS challenge was observed in serum LPS levels (P = 0.031), jejunal VH (P = 0.030), p-AMPK (P = 0.021), total AMPK (P = 0.001), and the ratio of p-AMPK and total AMPK (P < 0.001) in the jejunum of birds. LPS challenge led to an increase in serum DAO (P = 0.029) and LPS (P = 0.017) levels and a decrease in jejunal VH (P = 0.007) and VCR (P = 0.029). Dietary GML restored serum LPS levels (P = 0.031) and jejunal VH (P = 0.030) in LPS-challenged birds. GML supplementation increased the jejunal p-AMPK (P = 0.021) and total AMPK (P = 0.001) contents of challenged birds compared to the LPS group. The ratio of p-AMPK and total AMPK was higher in the GML groups than in the other 3 groups (P = 0.027). These results suggested that GML supplementation promoted the activation of AMPK signaling and ensured recovery of intestinal barrier function that had been injured by LPS challenge.
Table 5.
Effects of dietary glycerol monolaurate on jejunal barrier function and histomorphology of lipopolysaccharide-challenged broilers.1,2
| Item | Treatments |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | GML | LPS | LPS + GML | Diet | Challenge | Interaction | ||
| DAO, ng/mL | 26.87 | 29.26 | 36.31 | 32.57 | 1.41 | 0.813 | 0.029 | 0.283 |
| LPS, EU/mL | 88.64b | 92.41ab | 95.83a | 91.77b | 0.65 | 0.607 | 0.017 | 0.031 |
| VH, μm | 799.00a | 805.50a | 669.80b | 790.38a | 12.59 | 0.017 | 0.007 | 0.030 |
| CD, μm | 71.20 | 57.55 | 70.43 | 62.25 | 1.59 | 0.001 | 0.539 | 0.393 |
| VCR, μm | 11.53 | 14.39 | 9.63 | 12.35 | 0.40 | 0.004 | 0.029 | 0.934 |
| p-AMPK, ng/mg prot | 14.20ab | 14.59ab | 12.72b | 15.64a | 0.26 | 0.003 | 0.682 | 0.021 |
| AMPK, ng/mg prot | 16.39ab | 15.47b | 14.22b | 18.14a | 0.32 | 0.024 | 0.691 | 0.001 |
| p-AMPK/AMPK | 0.87b | 0.95a | 0.90b | 0.87b | 0.01 | 0.027 | 0.047 | <0.001 |
a, b Within a row, means with no common superscripts differ significantly (P < 0.05).
Means are based on 6 replicates per treatment with 2 birds per replicate.
SEM = standard error of the mean; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS; DAO = diamine oxidase; VH = villus height; CD = crypt depth; VCR = villus height-to-crypt depth ratio; p-AMPK = phospho-adenosine monophosphate-activated protein kinase; mg prot = milligrams of protein; AMPK = adenosine monophosphate-activated protein kinase.
3.6. Gene expression of tight junction proteins and AMPK
Table 6 presents the effects of the experimental treatments on gene expression of tight junction proteins and AMPK in the jejunum of birds. There was a significant interaction between dietary GML and LPS challenge (P < 0.05). GML supplementation rescued the reduction in the expression of tight junction proteins, such as zonula occludens 1 (ZO-1) (P = 0.039), occludin (OCLN) (P = 0.027), and claudin-2 (CLDN2) (P = 0.039), induced by LPS challenge. Jejunal AMPKα1 (P = 0.038) and AMPKα2 (P = 0.039) expression was downregulated in the challenged birds. A two-way diet × challenge interaction was observed for AMPKα1 expression (P = 0.046), whereby GML supplementation prevented the decrease in jejunal AMPKα1 expression in challenged birds. Collectively, these data suggested that the recovery of intestinal injury by GML supplementation in LPS-treated birds may be related to the enhancement of tight junction protein expression and AMPK activation.
Table 6.
Effects of dietary glycerol monolaurate on jejunal barrier functions associated genes of lipopolysaccharide-challenged broilers.1,2
| Gene | Treatments |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | GML | LPS | LPS + GML | Diet | Challenge | Interaction | ||
| ZO-1 | 1.00a | 0.93a | 0.60b | 0.99a | 0.07 | 0.555 | 0.087 | 0.039 |
| OCLN | 1.00a | 0.90ab | 0.67b | 0.86ab | 0.03 | 0.467 | 0.010 | 0.027 |
| CLDN1 | 1.00 | 0.82 | 0.56 | 0.92 | 0.06 | 0.507 | 0.205 | 0.057 |
| CLDN2 | 1.00a | 0.81ab | 0.31b | 0.75ab | 0.07 | 0.376 | 0.017 | 0.039 |
| CLDN3 | 1.00 | 1.09 | 1.04 | 1.37 | 0.03 | 0.134 | 0.243 | 0.358 |
| AMPKα1 | 1.00a | 0.67ab | 0.48b | 0.66ab | 0.06 | 0.560 | 0.038 | 0.046 |
| AMPKα2 | 1.00 | 1.20 | 0.63 | 0.98 | 0.07 | 0.053 | 0.039 | 0.561 |
a, b Within a row, means with no common superscripts differ significantly (P < 0.05).
Means are based on 6 replicates per treatment with 1 bird per replicate.
SEM = standard error of the mean; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS; ZO-1 = zonula occludens-1; OCLN = occludin; CLDN1 = claudin-1; CLDN2 = claudin-2; CLDN3 = claudin-3; AMPKα1 = adenosine monophosphate-activated protein kinase alpha 1; AMPKα2 = adenosine monophosphate-activated protein kinase alpha 2.
3.7. Oxidative status
The oxidative status in the serum and jejunum are shown in Table 7. Significant interactions were observed between GML supplementation and LPS challenge in the oxidative status of birds (P < 0.05). The LPS-challenged birds had higher serum MDA levels compared to the non-challenged birds (P = 0.027). Dietary GML reversed the MDA content elevation (P = 0.046) and CAT (P = 0.035) and T-AOC (P = 0.026) reduction induced by LPS challenge. In the jejunum, LPS challenge led to increased MDA content (P = 0.006) and reduced T-AOC (P = 0.008). Increased T-SOD (P = 0.002) and CAT (P = 0.012) activities and T-AOC (P = 0.002) were observed in the GML-treated birds. Dietary GML significantly increased the serum GSH-px (P < 0.001); and jejunal T-SOD (P = 0.006), CAT (P = 0.001), T-AOC (P = 0.038), and GSH-px (P < 0.001) activities. These findings revealed that GML supplementation ameliorated oxidative stress in challenged birds through the improved activity of antioxidant enzymes and maintaining the balance of oxidation and antioxidation.
Table 7.
Effects of dietary glycerol monolaurate on antioxidant capacity of lipopolysaccharide-challenged broilers.1,2
| Item | Treatments |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | GML | LPS | LPS + GML | Diet | Challenge | Interaction | ||
| Serum | ||||||||
| MDA, nmol/mL | 4.86b | 5.03b | 7.76a | 5.19b | 0.33 | 0.077 | 0.027 | 0.046 |
| T-SOD, ng/mL | 7.80 | 7.66 | 7.57 | 7.91 | 0.18 | 0.953 | 0.587 | 0.173 |
| CAT, pg/mL | 2.64a | 2.41ab | 1.77b | 2.57a | 0.11 | 0.232 | 0.138 | 0.035 |
| T-AOC, mmol/mL | 0.52a | 0.45ab | 0.37b | 0.49a | 0.02 | 0.523 | 0.14 | 0.026 |
| GSH-px, ng/mL | 147.47b | 143.63b | 143.42b | 156.3a | 8.03 | 0.029 | 0.037 | <0.001 |
| Jejunum | ||||||||
| MDA, nmol/mg prot | 28.37 | 29.12 | 64.03 | 41.63 | 3.98 | 0.186 | 0.006 | 0.158 |
| T-SOD, ng/mg prot | 1.75b | 1.78ab | 1.61b | 2.01a | 0.03 | 0.002 | 0.478 | 0.006 |
| CAT, pg/mg prot | 12.52b | 12.01ab | 10.93b | 14.33a | 0.26 | 0.012 | 0.492 | 0.001 |
| T-AOC, nmol/mg prot | 9.29a | 9.87a | 6.79b | 9.54a | 0.25 | 0.002 | 0.008 | 0.038 |
| GSH-px, ng/mg prot | 38.49ab | 35.51b | 34.49b | 43.28a | 0.66 | 0.035 | 0.164 | <0.001 |
a, b Within a row, means with no common superscripts differ significantly (P < 0.05).
Means are based on 6 replicates per treatment with 2 birds per replicate.
SEM = standard error of the mean; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS; MDA = malondialdehyde; T-SOD = total superoxide dismutase; CAT = catalase; T-AOC = total antioxidant capacity; GSH-px = glutathione peroxidase; mg prot = milligrams of protein.
3.8. Expressions of antioxidant-related genes
The expressions of antioxidant-related genes are presented in Table 8. LPS challenge led to the downregulation of nuclear factor-erythroid 2-related factor 2 (Nrf2) (P = 0.002), superoxide dismutase 2 (SOD2) (P = 0.020), and CAT (P = 0.011) expression, which contributed to oxidative stress of birds. There was an interaction between dietary GML and LPS challenge in the gene expression of Nrf2 (P = 0.046) and SOD1 (P = 0.041) in the jejunum of birds. Dietary GML rescued the reduction in Nrf2 and SOD1 expression induced by LPS challenge. Moreover, although no interaction existed between dietary GML and LPS challenge (P > 0.05), GML supplementation elevated jejunal heme oxygenase-1 (HO-1) expression in birds (P = 0.023), which is considered a downstream signaling molecule of Nrf2. These data suggested that GML supplementation normalized the expression of antioxidant-related genes and ameliorated LPS-induced oxidative stress by regulating Nrf2 signaling.
Table 8.
Effects of dietary glycerol monolaurate on jejunal antioxidant capacity associated genes of lipopolysaccharide-challenged broilers.1,2
| Gene | Treatments |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | GML | LPS | LPS + GML | Diet | Challenge | Interaction | ||
| Nrf2 | 1.00a | 0.83a | 0.40b | 0.65ab | 0.05 | 0.684 | 0.002 | 0.046 |
| HO-1 | 1.00 | 1.37 | 0.99 | 1.28 | 0.06 | 0.023 | 0.710 | 0.722 |
| SOD1 | 1.00a | 0.91a | 0.53b | 0.95a | 0.05 | 0.163 | 0.075 | 0.041 |
| SOD2 | 1.00 | 0.89 | 0.66 | 0.78 | 0.05 | 0.992 | 0.020 | 0.194 |
| CAT | 1.00 | 1.26 | 0.58 | 0.77 | 0.08 | 0.159 | 0.011 | 0.849 |
| GPX1 | 1.00 | 1.06 | 1.04 | 1.07 | 0.07 | 0.937 | 0.650 | 0.632 |
a, b Within a row, means with no common superscripts differ significantly (P < 0.05).
Means are based on 6 replicates per treatment with 1 bird per replicate.
SEM = standard error of the mean; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS; Nrf2 = nuclear factor-erythroid 2-related factor 2; HO-1 = heme oxygenase-1; SOD1 = superoxide dismutase-1; SOD2 = superoxide dismutase-2; CAT = catalase; GPX1 = glutathione peroxidase 1.
3.9. Cecal bacterial quantification
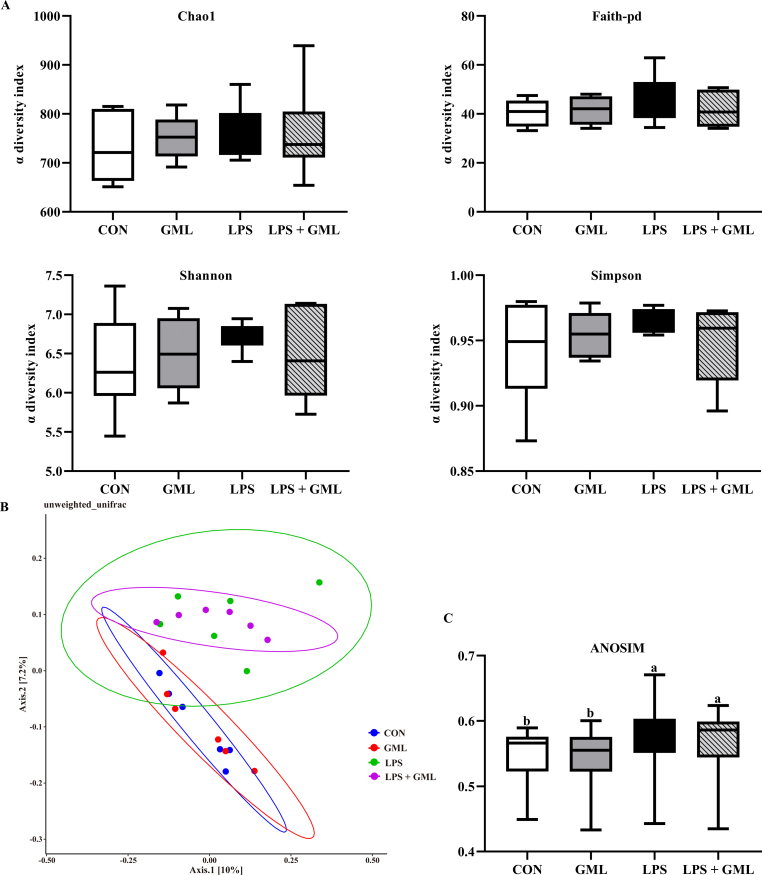
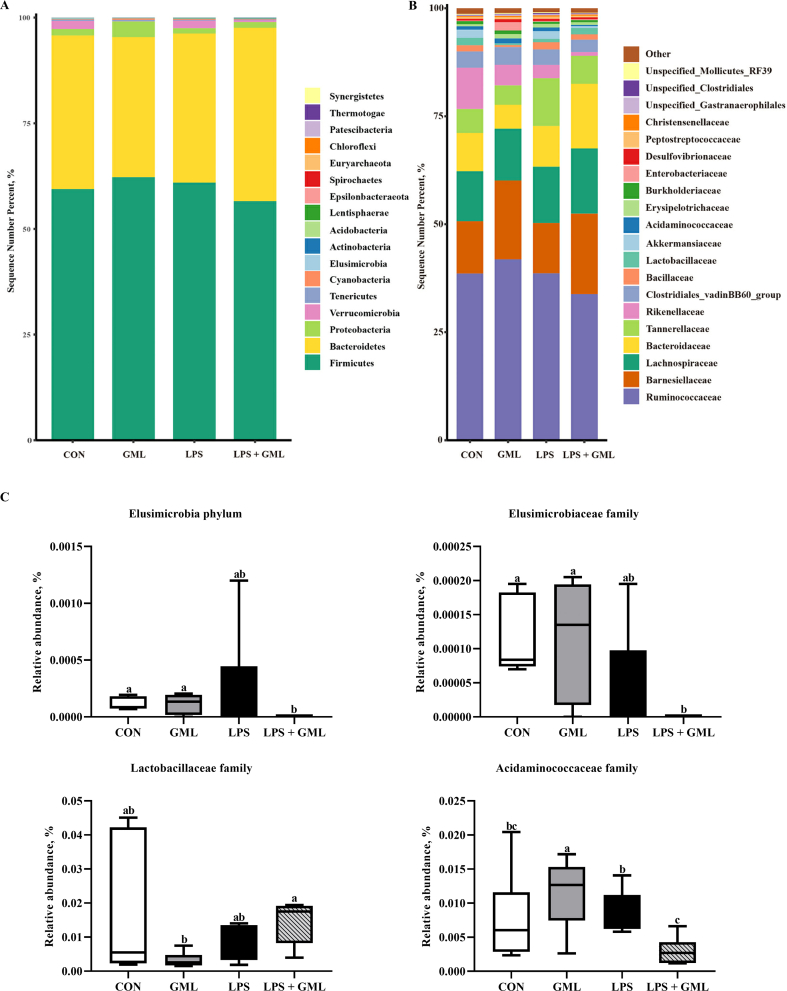
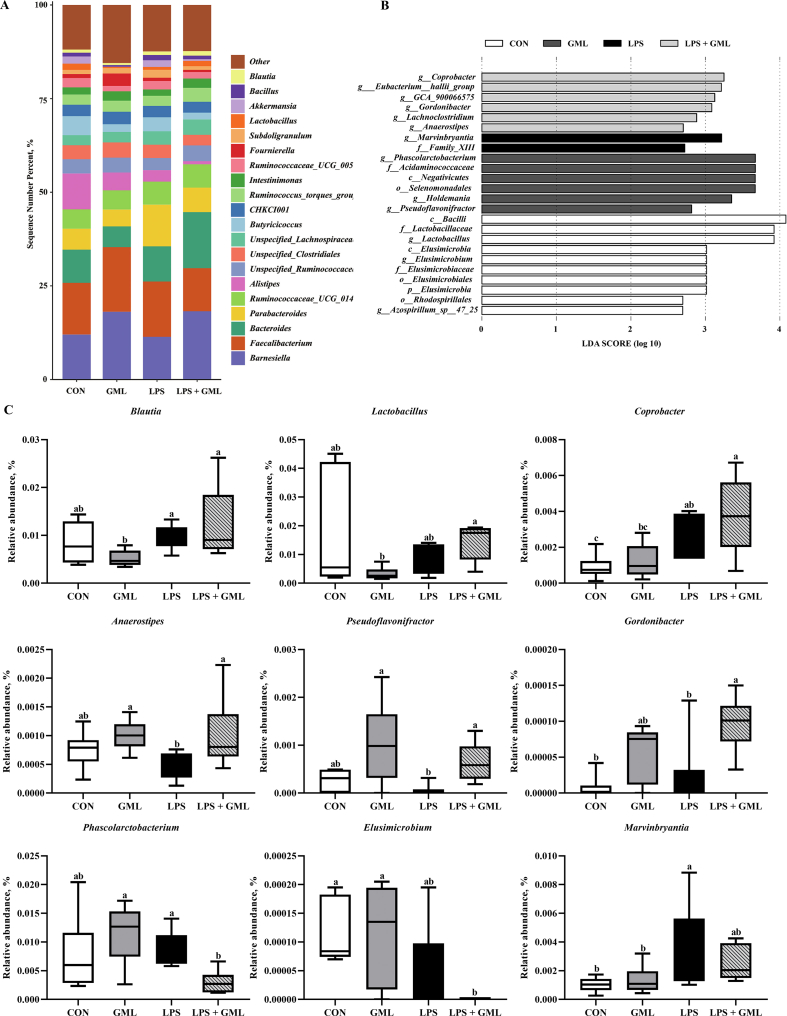
16S rRNA gene high-throughput sequencing was performed to reveal the role of the cecal microbiota in GML attenuation of immunological stress and intestinal injury promoted by LPS challenge. A Venn diagram showed that 1,383, 1,341, 1,379, and 1,311 specific operational taxonomic unit (OUT) existed in the CON, GML, LPS, and LPS + GML groups, respectively, with 654 OTU shared (Fig. 1A). The microbial diversity within an individual sample was assessed by the Chao1, Faith-pd, Shannon, and Simpson indices, but no alteration among all the groups was observed (Fig. 1B). PCoA of the OTU was performed to assess similarities and differences among samples and groups (Fig. 1C). The results revealed that the cecal microbiota in the LPS-challenged groups was separated from that in the CON and GML groups. The differences in species diversity were quantified by ANOSIM based on unweighted Unifrac distance, which indicated that the LPS challenge altered the β diversity index compared to that of the CON and GML groups (Fig. 1D). Collectively, these results indicated that the LPS challenge modulated the cecal microbiota community structure of birds. Taxonomic profiling indicated that Firmicutes, Bacteroidetes, and Proteobacteria accounted for most of the intestinal bacteria of birds (Fig. 2A). Dietary GML increased the proportion of Barnesiellaceae and Bacteroidaceae in the LPS-challenged birds (Fig. 2B). Relative abundance analysis revealed that dietary GML increased the amount of Lactobacillaceae and reduced the proportion of Elusmicrobia, Acidaminococcaceae, and Elusimicrobiaceae in the LPS-challenged birds (Fig. 2C). Moreover, the relative abundance of the 20 predominant genera per group was analyzed to illustrate the specific changes in the microbial taxa. The enhanced abundance of Barnesiella and Bacteroides and reduced Parabacteroides were observed in the LPS + GML group (Fig. 3A). LEfSe analysis indicated that the genera Coprobacter, Gordonibacter, Lachnoclostridium, and Anaerostipes predominated in the LPS + GML group (Fig. 3B). The relative abundances of cecal Blautia, Lactobacillus, and Coprobacter were increased in the LPS + GML group compared to the GML group (Fig. 3C). GML supplementation increased the relative abundances of Anaerostipes, Pseudoflavonifractor, and Gordonibacter and reduced the proportion of Phascolarctobacterium in LPS-challenged birds (Fig. 3C). The abundance of Marvinbryantia was lower in the LPS + GML group than in the LPS group, with an insignificant difference (Fig. 3C).
Fig. 1.
Effects of dietary glycerol monolaurate on cecal microbiota diversity of lipopolysaccharide-challenged broilers. Veen diagram (A); PCoA based on unweighted Unifrac distance (B); ANOSIM based on unweighted Unifrac distance (C). a-b Means with no common superscripts differ significantly (P < 0.05). Means are based on 6 replicates per treatment with 1 bird per replicate. CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS; ANOSIM = analysis of similarities.
Fig. 2.
Effects of dietary glycerol monolaurate on cecal microbiota composition of lipopolysaccharide-challenged broilers. Microbial composition at the phylum and family levels (A, B); relative abundances analysis (C). a-c Means with no common superscripts differ significantly (P < 0.05). Means are based on 6 replicates per treatment with 1 bird per replicate. CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS.
Fig. 3.
Alteration of the cecal microbiota at the genus level. Relative abundances of the top 20 bacterial genera (A); LEfSe of the cecal microbiota (B); relative abundances analysis (C). a-c Means with no common superscripts differ significantly (P < 0.05). Means are based on 6 replicates per treatment with 1 bird per replicate. CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = lipopolysaccharide, birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS.
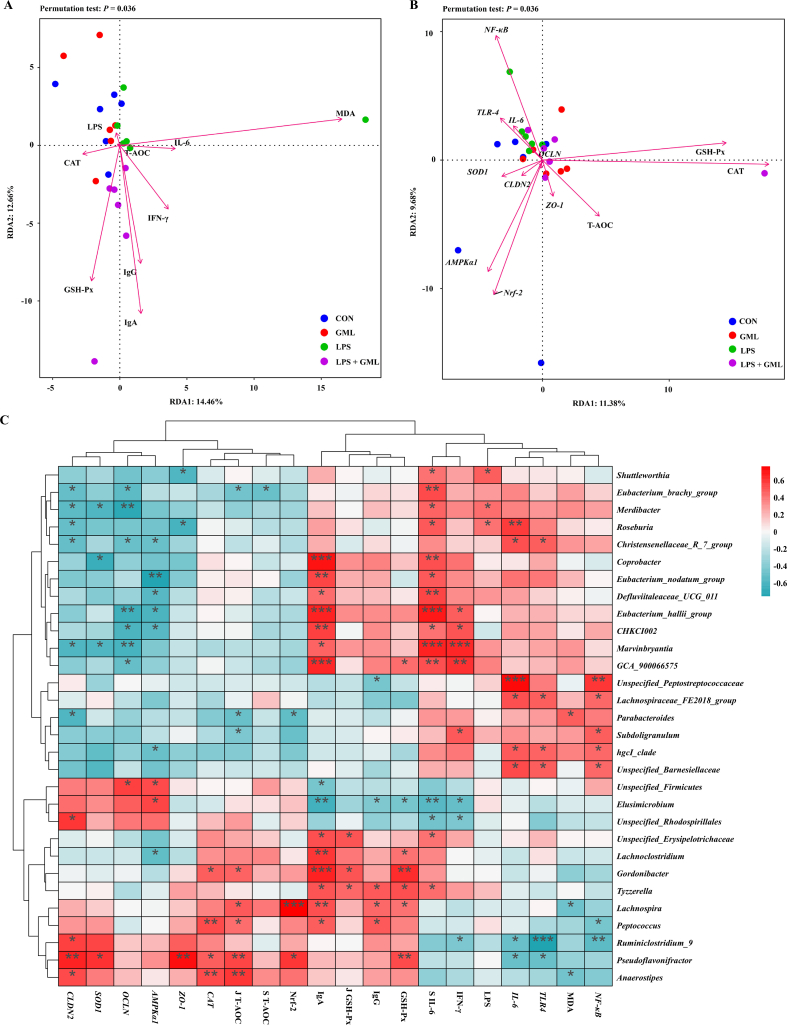
The serum GSH-px, IgA, and IgG levels in LPS-challenged birds were positively correlated with GML supplementation (Fig. 4A). The serum LPS, MDA, and IL-6 levels and jejunal gene expression of TLR4, IL-6, and NF-κB were positively associated with LPS challenge but negatively linked to dietary GML (Fig. 4A). Moreover, the activity of jejunal antioxidant enzymes and the gene expression of AMPKα1, Nrf2, and ZO-1 were positively related to GML supplementation (Fig. 4B). The associations among the intestinal microbiota and immune indices, antioxidant enzymes, tight junction proteins, and AMPK signaling pathway were further analyzed at the genus level and visualized by heatmap (Fig. 4C). The levels of inflammatory factors and NF-κB signaling were positively linked to the genera Roseburia, Marvinbryantia, GCA_900066575, and Subdoligranulum, but they were negatively correlated with Ruminiclostridium_9 and Pseudoflavonifractor. Serum IgA and IgG levels were positively associated with Peptococcus, Lachnospira, Gordonibacter, and Lachnoclostridium abundances. Tight junction proteins and AMPK signaling were possibly correlated with Anaerostipes, Pseudoflavonifractor, and Ruminiclostridium_9 but negatively correlated with the genera Parabacteroides, Marvinbryantia, Merdibacter, and Eubacterium_hallii_group. The abundances of Pseudoflavonifractor, Peptococcus, Lachnospira, Gordonibacter, and Anaerostipes were positively associated with antioxidant capacity and jejunal Nrf2 gene expression.
Fig. 4.
Correlation analysis. The correlation between factors and samples distribution in serum (A) and jejunum (B) by redundancy analysis; heatmap of correlations determined by Spearman analysis among the cecal microbiota, inflammatory factors, tight junctions, antioxidant enzymes, and intestinal barrier parameters (C). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. LPS = lipopolysaccharide; MDA = malondialdehyde; IL-6 = interleukin 6; T-AOC = total antioxidant capacity; CAT = catalase; IFN-γ = interferon gamma; IgG = immunoglobulin G; GSH-px = glutathione peroxidase; IgA = immunoglobulin A; CON = control, birds fed a basal diet; GML = glycerol monolaurate, birds fed a basal diet containing 1,200 mg/kg GML; LPS = birds fed a basal diet and challenged with LPS; LPS + GML = birds fed a basal diet containing 1,200 mg/kg GML and challenged with LPS NF-κB = nuclear factor kappa-B; TLR4 = Toll-like receptor; OCLN = occludin; SOD1 = superoxide dismutase 1; CLDN2 = claudin-2; ZO-1 = zonula occludens-1; AMPKα1 = adenosine monophosphate-activated protein kinase alpha 1; Nrf2 = nuclear factor-erythroid 2-related factor 2; J T-AOC = total antioxidant capacity in jejunum; S T-AOC = total antioxidant capacity in serum.
4. Discussion
Immunological stress threatens the health status of poultry and is thus considered a critical issue in poultry production (Zheng et al., 2020). Birds are inevitably confronted with immunological challenges relevant to bacteria or its products such as LPS (Coble et al., 2011). LPS is widely applied to establish a model of intestinal injury mediated by immunological stress (Jiang et al., 2019). Under stress conditions, growth performance and enteric diseases were affected as dietary nutrients are distributed more to support the immune system (Liu et al., 2015). Numerous studies have demonstrated that the LPS challenge compromises the feed intake of broilers owing to LPS-induced impairment of the intestinal barrier (Chen et al., 2018b; Jiang et al., 2019). This study confirmed the destructive consequences of the LPS challenge on growth performance, as evidenced by decreased AFI and ABWG and increased FCR in challenged birds. No alterations have been reported in the body weight, ABWG, or FCR of birds supplemented with GML in both the first 28 d and all experimental periods (Amer et al., 2021; Liu et al., 2021). In this study, the decreased growth performance induced by LPS challenge was not rescued by GML supplementation. This result confirmed that the growth performance of broilers was insensitive to GML supplementation even under challenge conditions. Peripheral blood lymphocytes are sensitive to immune stress (Dhabhar et al., 1995). LPS challenge can elevate blood leukocyte, lymphocyte, mononuclear cell, and neutrophil counts (Chen et al., 2018b). In the present study, the increased number of leucocytes, lymphocytes, and granulocytes observed in the LPS challenged group, illustrated a successful introduction of immunological stress.
After specific recognition by leukocyte membrane Toll-like receptors and myeloid differential proteins, LPS induces immunological and oxidative stress by stimulating the secretion of numerous cytokines, such as IL-1β and IL-6 (Byun et al., 2013). In this study, LPS challenge elevated the production of serum IL-1β, IL-6, TNF-α, and IFN-γ and upregulated the expression of jejunal IL-6, TLR4, MIP-3α, NF-κB, and caspase-1, which promoted immunological stress and apoptosis. GML is an attractive anti-inflammatory agent with inhibitory activity against the production of MIP-3α and the secretion of proinflammatory cytokines (Peterson and Schlievert, 2006). In vitro, lauric acid and GML could be used to promote resolution following an inflammatory insult (Sivinski et al., 2020). In vivo, GML supplementation reduced the serum IL-6 and TNF-β levels and alleviated the systemic inflammatory response of high-fat-diet-fed mice (Zhao et al., 2019). Dietary GML moderated immunological stress promoted by LPS challenge, which was reflected in the present study by the alleviated levels and gene expression of MIP-3α and proinflammatory cytokines. NF-κB is a central regulator of inflammatory responses involved in most innate immune receptor signaling pathways. The activation of NF-κB is considered one of the central ways that feed compounds affect immunity (Sivinski et al., 2020). Fatty acid treatment has been found to reduce the activation of NF-κB in a dosage-dependent manner under LPS challenge compared with that in the absence of LPS (Nishimura et al., 2018). In this study, dietary GML reduced jejunal NF-κB expression in LPS-challenged birds, which indicated that GML-relieved inflammation may be related to the regulation of NF-κB signaling pathway. Moreover, AMPK has been implicated as an attractive target for inflammation control because activated AMPK has been shown to suppress LPS-induced NF-kB activity and the production of proinflammatory cytokines in multiple cell types (Giri et al., 2004). The induction of AMPK signaling has been reported to inhibit the expression of IL-1β, IL-6, and TNF-α through suppression of the nuclear translocation of NF-κB in mouse neurons (Lu et al., 2010). In the present study, birds in the GML group had a higher ratio of p-AMPK and total AMPK than other 3 groups. Dietary GML enhanced the protein levels of p-AMPK and AMPK in LPS-challenged birds compared to those in the LPS group. Thus, the inhibited NF-κB activity in this study was conjectured to be linked to the activation of AMPK mediated by GML supplementation. The results from the present study indicated that dietary GML attenuated LPS-induced immunological stress by alleviating intestinal inflammation, which was probably linked to specific activation of AMPK and suppression of NF-κB activity.
Intestinal integrity is the key factor preventing infection of broilers by pathogenic bacteria (Jiang et al., 2019). DAO is considered a sensitive indicator reflecting intestinal injury (Wang et al., 2016). Reduced gene expression of tight junction proteins can affect intestinal permeability and disrupt the intestinal barrier, thereby causing the translocation of LPS into the blood circulation from the intestinal cavity (Zhang and Guo, 2009). In the present study, LPS challenge increased the serum DAO and LPS levels and downregulated the jejunal gene expression of ZO-1, OCLN, and CLDN2 to varying degrees. However, dietary GML reversed the LPS-induced alterations of serum LPS and jejunal tight junction proteins, which revealed a recovery of the intestinal barrier and gut injury. Intestinal morphology reflects the health status of the gut and the response to certain feed substances (Burlikowska, 2012). GML supplementation has been found to improve intestinal morphology (Kong et al., 2021; Liu et al., 2020). In the present study, dietary GML increased jejunal VH and VCR, reduced intestinal CD, and reversed the LPS-induced decrease in VH of the jejunum in birds, which indicated an augmentation of intestinal morphology. The LPS-induced impairment of intestinal morphology is rescued by natural extracts with anti-inflammatory effects (Jiang et al., 2019). Thus, the ameliorative effect of GML on intestinal inflammation may be a potential reason for the improved intestinal integrity in challenged birds. Moreover, AMPK is involved in the tight junction assembly process in challenged epithelial cells, and its activation enhances recovery of epithelial barrier function following injury (Olivier et al., 2019). Intestinal AMPK activity has been notably linked to colitis development in inflammatory mouse models (Chen et al., 2018a). Butyrate promotes tight junction assembly and regulates intestinal barrier function by activating AMPK in human colon carcinoma cells (Elamin et al., 2013). In this study, dietary GML augmented the gene expression of tight junction proteins and activation of AMPK in LPS-challenged birds. These results indicate that dietary GML supplementation ameliorated LPS-induced intestinal injury and barrier dysfunction by attenuating inflammation and activating AMPK signaling.
Oxidative damage in broiler tissues is promoted by LPS challenge owing to the disrupted balance between pro-oxidant and antioxidant systems (Wu et al., 2013). The sophisticated antioxidant defense system, including enzymes T-SOD, CAT, and GSH-px, is crucial to counteract oxidative damage. These antioxidant enzymes are considered sensitive indicators to reflect oxidative stress in animals (Zheng et al., 2020). LPS has been reported to induce oxidative stress in birds, which is characterized by decreased T-SOD, CAT, and GSH-px activities in the serum and liver (Zheng et al., 2020). In the present study, LPS challenge elevated the serum and jejunal MDA contents and reduced the activity and expression of antioxidant enzymes in the jejunum of birds. In contrast, GML supplementation reduced MDA content and augmented the activities of antioxidant enzymes in challenged birds, which revealed that LPS-induced lipid peroxidation was reversed by dietary GML. The peroxidation level in meat is alleviated by GML-supplied diets and to be proportional to the increase in dietary additive concentration (Fortuoso et al., 2019). Dietary GML has been shown to enhance the antioxidant capacity of laying hens by reducing MDA content and increasing SOD activity (Liu et al., 2021). In this study, GML supplementation modulated the delicate balance between oxidants and antioxidants and alleviated oxidative stress in LPS-challenged birds. Oxidative stress is significantly connected with inflammation. These 2 processes induce each other reciprocally, prompting a vicious cycle (Lugrin et al., 2014). Thus, the relieving effect of dietary GML on oxidative stress induced by LPS challenge may be related to the reversal of the inflammatory response. Nrf2 is a master regulator of cellular defenses against oxidative stress and is activated to promote the expression of antioxidant genes such as HO-1 (Zimmermann et al., 2015). AMPK serves as a stress sensor and exerts a beneficial effect on the alleviation of oxidative stress (Wang et al., 2012). AMPK has been found to directly phosphorylate Nrf2 in mouse embryonic fibroblasts (Matzinger et al., 2020). In the present study, dietary GML rescued the reduced expression of Nrf2 and SOD1 induced by LPS challenge, which paralleled the changes in AMPK signaling. Overall, dietary GML exerted antioxidant properties and attenuated LPS-induced oxidative stress, probably by regulating the AMPK/Nrf2 signaling pathway and enhancing antioxidant enzyme activity.
A large number of microorganisms colonize the gastrointestinal tract of poultry, closely and densely interacting with the feed and host. The gut microbiome benefits the host through the modulation of host gut morphology, physiology, and immunity (Pan and Yu, 2014). Although the intestinal microbiota itself can regulate the immune system by releasing LPS, excessive LPS levels disrupt the dynamic balance of the intestinal microbiota (Lucke et al., 2018). In the present study, the community structure of the cecal microbiota in birds was modulated by LPS challenge, similar to the outcomes from previous findings (Jiang et al., 2021; Wang et al., 2016). The rich community of species enhances the stability of the intestinal microecology, leading to decreased sensitivity to bacterial invasion and intestinal inflammation (Ott et al., 2004). Thus, modulation of the microbial community structure by GML supplementation was associated with the alleviation of intestinal inflammation in challenged birds. In the poultry diet, various feed additives are used to reduce the levels of enteric pathogens by modulating the intestinal microbiome (Pan and Yu, 2014), such as xylanase, which enriches gut lactic acid bacteria and reduces the proportion of adverse and pathogenic bacteria (Rodríguez et al., 2012). A previous study revealed that GML supplementation attenuated gut microbiota dysbiosis in mice, with increases in Akkermansia, Bifidobacterium, and Lactobacillus abundances (Zhao et al., 2019). In this study, dietary GML increased the abundance of the genus Lactobacillus in the cecum of challenged birds. Lactobacillus can promote gut health as a probiotic bacterium for animals and plays a vital role in maintaining intestinal metabolic capacities and microbial homeostasis (Angelakis, 2017). A negative correlation has been widely reported between Lactobacillus and inflammatory cytokines (Jiang et al., 2021). The increase in the abundance of Lactobacillus due to GML supplementation may explain the depressed intestinal inflammation in challenged birds. Administration of GML promoted the colonization of some beneficial bacteria (Liu et al., 2020), such as the genera Blautia and Coprobacter, in challenged broilers in the present study. Moreover, GML supplementation reversed the reduction in the relative abundances of Anaerostipes, Pseudoflavonifractor, and Gordonibacter induced by LPS challenge. The promoted colonization of probiotics can result in colonization resistance to intestinal pathogens (Imhann et al., 2016), which partly explained the reduced proportions of the genera Phascolarctobacterium, Elusimicrobium, and Marvinbryantia in LPS-challenged birds fed GML-supplied diet. Enhanced adhesion and colonization of probiotics have been shown to result in a defensive response to LPS challenge, which promoted gut health in broiler chicks (Jiang et al., 2021). In the current study, attenuated inflammation and ameliorated intestinal integrity, and antioxidant capacity reduction in LPS-challenged birds was demonstrated to be positively associated with GML supplementation. Moreover, the augmented immunity, inflammation, intestinal barrier, and antioxidation indices were related to the intestinal microbiota. In mice, dietary GML supplementation has been shown to significantly affect metabolism and immunity through the intestinal microbiota, which considerably impacted host health and physiology (Jiang et al., 2018). The amelioration of metabolic disorders, obesity, and inflammation by increased GML supplementation may occur through targeting the intestinal microbiota (Zhao et al., 2019). Mutual regulatory activities exist in a poultry host and its gut microbiota, particularly during the exchange of nutrients and modulation of intestinal morphology, physiology, and immunity (Pan and Yu, 2014). These results indicated that GML supplementation attenuated immunological stress and intestinal mucosal injury mediated by LPS challenge, probably through the alteration of the cecal microbiota.
5. Conclusion
In conclusion, dietary GML supplementation ameliorated LPS-induced immunological stress and intestinal injury in broilers by suppressing inflammation and oxidative stress. The alterations in the cecal microbiota modulated by GML supplementation were probably one of the reasons for the recovery of intestinal health in LPS-challenged broilers. Moreover, the present results confirmed the immunomodulatory, antioxidant, and anti-inflammatory properties of GML in LPS-challenged broilers, which may be associated with the activation of the AMPK/Nrf2 signaling pathway.
Author contributions
Zhigang Song conceived the idea and provided resources. Linglian Kong designed the study, performed the experiment, and wrote the manuscript. Zhenhua Wang provided guidelines. Chuanpi Xiao and Qidong Zhu participated in the experiment. All authors contributed to the article and approved the submitted version.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MC170) and the Shandong Province Agricultural Industry Technology (SDAIT-11-08).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2022.06.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Amer S.A., A-Nasser A., Al-Khalaifah H.S., AlSadek D.M.M., Abdel fattah D.M., Roushdy E.M., et al. Effect of dietary medium-chain α-monoglycerides on the growth performance, intestinal histomorphology, amino acid digestibility, and broiler chickens' blood biochemical parameters. Animals. 2021;11:57. doi: 10.3390/ani11010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb Pathog. 2017;106:162–170. doi: 10.1016/j.micpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Burlikowska K. Dietary fructans and their potential beneficial influence on health and performance parametres in broiler chickens. J Cent Eur Agric. 2012;13:272. [Google Scholar]
- Byun E.B., Sung N.Y., Byun E.H., Song D.S., Kim J.K., Park J.H., et al. The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Int Immunopharm. 2013;15:450–456. doi: 10.1016/j.intimp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang J., You Q., He S., Meng Q., Gao J., et al. Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front Pharmacol. 2018;9:761. doi: 10.3389/fphar.2018.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br J Nutr. 2018;119:1254–1262. doi: 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- Coble D.J., Redmond S.B., Hale B., Lamont S.J. Distinct lines of chickens express different splenic cytokine profiles in response to Salmonella Enteritidis challenge. Poultry Sci. 2011;90:1659–1663. doi: 10.3382/ps.2010-01279. [DOI] [PubMed] [Google Scholar]
- Connerton P.L., Richards P.J., Lafontaine G.M., O'Kane P.M., Ghaffar N., Cummings N.J., et al. The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome. 2018;6:88. doi: 10.1186/s40168-018-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F.S., Miller A.H., McEwen B.S., Spencer R.L. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- Elamin E.E., Masclee A.A., Dekker J., Pieters H.-J., Jonkers D.M. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr. 2013;143:1872–1881. doi: 10.3945/jn.113.179549. [DOI] [PubMed] [Google Scholar]
- Fortuoso B.F., Dos Reis J.H., Gebert R.R., Barreta M., Griss L.G., Casagrande R.A., et al. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: impact on health, performance and meat quality. Microb Pathog. 2019;129:161–167. doi: 10.1016/j.micpath.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Giri S., Nath N., Smith B., Viollet B., Singh A.K., Singh I. 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24:479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhann F., Bonder M.J., Vich Vila A., Fu J., Mujagic Z., Vork L., et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Qi L., Lv Z., Jin S., Wei X., Shi F. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants. 2019;8:575. doi: 10.3390/antiox8120575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Qi L., Wei Q., Shi F. Maternal stevioside supplementation ameliorates intestinal mucosal damage and modulates gut microbiota in chicken offspring challenged with lipopolysaccharide. Food Funct. 2021;12:6014–6028. doi: 10.1039/d0fo02871a. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Zhao M., Zhang H., Li Y., Liu M., Feng F. Antimicrobial emulsifier–glycerol monolaurate induces metabolic syndrome, gut microbiota dysbiosis, and systemic low-grade inflammation in low-fat diet fed mice. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201700547. [DOI] [PubMed] [Google Scholar]
- Kamboh A.A., Zhu W.Y. Individual and combined effects of genistein and hesperidin on immunity and intestinal morphometry in lipopolysacharide-challenged broiler chickens. Poultry Sci. 2014;93:2175–2183. doi: 10.3382/ps.2014-03971. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. Issues and consequences of using nutrition to modulate the avian immune response. J Appl Poultry Res. 2017;26:605–612. [Google Scholar]
- Kong L., Wang Z., Xiao C., Zhu Q., Song Z. Glycerol monolaurate ameliorated intestinal barrier and immunity in broilers by regulating intestinal inflammation, antioxidant balance, and intestinal microbiota. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.713485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Estes J.D., Schlievert P.M., Duan L., Brosnahan A.J., Southern P.J., et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Shen J., Zhao C., Wang X., Yao J., Gong Y., et al. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int J Biol Macromol. 2015;72:624–632. doi: 10.1016/j.ijbiomac.2014.08.057. [DOI] [PubMed] [Google Scholar]
- Liu T., Li C., Zhong H., Feng F. Dietary medium-chain α-monoglycerides increase BW, feed intake, and carcass yield in broilers with muscle composition alteration. Poultry Sci. 2021;100:186–195. doi: 10.1016/j.psj.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Tang J., Feng F. Glycerol monolaurate improves performance, intestinal development, and muscle amino acids in yellow-feathered broilers via manipulating gut microbiota. Appl Microbiol Biotechnol. 2020;104:10279–10291. doi: 10.1007/s00253-020-10919-y. [DOI] [PubMed] [Google Scholar]
- Lu J., Wu D-m, Zheng Y-l, Hu B., Zhang Z-f, Shan Q., et al. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J Pathol. 2010;222:199–212. doi: 10.1002/path.2754. [DOI] [PubMed] [Google Scholar]
- Lucke A., Böhm J., Zebeli Q., Metzler-Zebeli B.U. Dietary deoxynivalenol contamination and oral lipopolysaccharide challenge alters the cecal microbiota of broiler chickens. Front Microbiol. 2018;9:804. doi: 10.3389/fmicb.2018.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugrin J., Rosenblatt-Velin N., Parapanov R., Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395:203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- Matzinger M., Fischhuber K., Pölöske D., Mechtler K., Heiss E.H. AMPK leads to phosphorylation of the transcription factor Nrf2, tuning transactivation of selected target genes. Redox Biol. 2020;29 doi: 10.1016/j.redox.2019.101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Moriyama M., Kawabe K., Satoh H., Takano K., Azuma Y.-T., et al. Lauric acid alleviates neuroinflammatory responses by activated microglia: involvement of the GPR40-dependent pathway. Neurochem Res. 2018;43:1723–1735. doi: 10.1007/s11064-018-2587-7. [DOI] [PubMed] [Google Scholar]
- NRC . 11th revised edition. Natl Acad Press; Washington, DC: 2012. Nutrient requirements of poultry. [Google Scholar]
- Olivier S., Leclerc J., Grenier A., Foretz M., Tamburini J., Viollet B. AMPK activation promotes tight junction assembly in intestinal epithelial Caco-2 cells. Int J Mol Sci. 2019;20:5171. doi: 10.3390/ijms20205171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott S.J., Musfeldt M., Wenderoth D.F., Hampe J., Brant O., Fölsch U.R., et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microb. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M.L., Schlievert P.M. Glycerol monolaurate inhibits the effects of gram-positive select agents on eukaryotic cells. Biochemistry. 2006;45:2387–2397. doi: 10.1021/bi051992u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez M.L., Rebolé A., Velasco S., Ortiz L.T., Treviño J., Alzueta C. Wheat- and barley-based diets with or without additives influence broiler chicken performance, nutrient digestibility and intestinal microflora. J Sci Food Agric. 2012;92:184–190. doi: 10.1002/jsfa.4561. [DOI] [PubMed] [Google Scholar]
- Sivinski S.E., Mamedova L.K., Rusk R.A., Elrod C.C., Swartz T.H., McGill J.M., et al. Development of an macrophage screening system on the immunomodulating effects of feed components. J Anim Sci Biotechnol. 2020;11:89. doi: 10.1186/s40104-020-00497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F., Fisinin V.I. Vitagenes in poultry production: Part 2. Nutritional and internal stresses. World’s Poult Sci J. 2016;72:761–772. [Google Scholar]
- Wang S., Song P., Zou M.-H. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci. 2012;122:555–573. doi: 10.1042/CS20110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li Z., Han Q., Guo Y., Zhang B., D’inca R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br J Nutr. 2016;116:1878–1888. doi: 10.1017/S0007114516004116. [DOI] [PubMed] [Google Scholar]
- Wang X., Li Y., Shen J., Wang S., Yao J., Yang X. Effect of Astragalus polysaccharide and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers. Int J Biol Macromol. 2015;76:188–194. doi: 10.1016/j.ijbiomac.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Zhou Y.M., Wu Y.N., Zhang L.L., Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet Immunol Immunopathol. 2013;153:70–76. doi: 10.1016/j.vetimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang B., Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. 2009;102:687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- Zhang M.S., Tran P.M., Wolff A.J., Tremblay M.M., Fosdick M.G., Houtman J.C.D. Glycerol monolaurate induces filopodia formation by disrupting the association between LAT and SLP-76 microclusters. Sci Signal. 2018;11 doi: 10.1126/scisignal.aam9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Cai H., Jiang Z., Li Y., Zhong H., Zhang H., et al. Glycerol-monolaurate-mediated attenuation of metabolic syndrome is associated with the modulation of gut microbiota in high-fat-diet-fed mice. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201801417. [DOI] [PubMed] [Google Scholar]
- Zhao M., Jiang Z., Cai H., Li Y., Mo Q., Deng L., et al. Modulation of the gut microbiota during high-dose glycerol monolaurate-mediated amelioration of obesity in mice fed a high-fat diet. mBio. 2020;11 doi: 10.1128/mBio.00190-20. e00190-00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.W., Zhang J.Y., Zhou H.B., Guo Y.P., Ma Q.G., Ji C., et al. Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide. Poultry Sci. 2020;99:5389–5398. doi: 10.1016/j.psj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K., Baldinger J., Mayerhofer B., Atanasov A.G., Dirsch V.M., Heiss E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis—a role for the unfolded protein response. Free Radic Biol Med. 2015;88:417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.