Abstract
The ideal gas equation of state (EOS) model is a well-known low-density limiting model. Recently, an ideal dense matter EOS model for the high-density limit symmetric to the ideal gas model has been developed. Here, by mathematically interpolating between the ideal gas and ideal dense matter limiting models, we establish a global model containing two EOS in the form of P-V-T and P-S-T for arbitrary ranges of densities. Different from empirical or semi-empirical EOS, the coefficients in the global EOS have a clear physical meaning and can be determined from a priori knowledge. The proposed global model is thermodynamically consistent and continuous. It reduces to the ideal gas model when approaching the low-density limit and to the ideal dense matter model when approaching the high-density limit. Verifications for 4He show that the global model reproduces the large-range behavior of matter well, along with providing important insight into the nature of the large-range behavior. Compared to the third-order virial EOS and the Benedict–Webb–Rubin EOS, the global P-V-T EOS has higher descriptive accuracy with fewer coefficients over a wide range of data for N2. The global model is shown to work well in extreme applied sciences. It predicts a linear, inverse relationship between entropy and volume when the temperature-to-pressure ratio is constant, which can explain the entropy-production behavior in shock-Hugoniots.
Subject terms: Engineering, Applied physics, Thermodynamics
Introduction
Various extreme dynamic processes such as explosive and impact loading of materials, shock wave propagation, and planetary or stellar interior evolution have attracted increasing attention. In these processes, matter may experience large ranges of densities, with correspondingly large ranges of behaviors exhibited1. A global equation of state (EOS) model is needed to describe these large-range behaviors of matter. It was often obtained by interpolating between different EOS models2–7. However, since the models used for interpolation usually cover only a restricted range of densities1, the models obtained by interpolating between them still cover only a restricted range of densities and are therefore not truly global. Moreover, since the models used for interpolation were usually based on different assumptions about internal structure of matter or derived by different theoretical tools, interpolating between these distinctly different models suffered from thermodynamic inconsistencies and discontinuities1,4,6. Consequently, the interpolation operation was more empirical1,7, making the interpolation EOS difficult to build or, if developed, the original physical meaning lost.
Interpolation between limits can lead to truly global results8–11. For example, Planck8,9 developed his global radiation formula available for arbitrary wavelengths based on the mathematical interpolation between the long- and short-wave limits. To obtain a truly global EOS model, the models used for interpolation should be related to the low- and high-density limits. Moreover, to satisfy thermodynamic continuity and avoid being trapped in local properties of individual substances, the models used for interpolation should be general in nature and thermodynamically compatible. As we know, the ideal gas EOS,
| 1 |
is a well-known low-density limiting model and all matter shares this simple relation at ultra-low densities12. Recently, it has been found that there is thermodynamic symmetry between the features of the two extremes of density, and thus an ideal dense matter EOS symmetric to the ideal gas EOS was presented13
| 2 |
where is a constant, called the ideal dense matter constant. It is a high-density limiting model and all matter shares this simple relation at ultra-high densities13. Ideal gas and ideal dense matter are a pair of symmetric theoretical concepts. Both the ideal gas and ideal dense matter models are general and they play parallel roles in thermodynamics. Here, by mathematically interpolating between them, we build a global EOS model for arbitrary ranges of densities. Two global EOS in the form of P–V-T and P-S-T are generated. The coefficients in the global EOS are all parameters related to limiting states and have a clear physical meaning. The proposed global model is thermodynamically consistent and continuous. It reduces to the original simple results in either the low- or high-density limit and thus is applicable to both low- and high-density substances. This distinctive feature of the global model enables itself to cover the large-range behavior of matter. The global model is shown to work well in extreme applied sciences such as explosive and shock-Hugoniot.
Modeling
Equations of state
An ideal gas, as we know, is a high-temperature and low-pressure limiting state and therefore has an infinite specific volume (low-density limit), while the ideal dense matter is a low-temperature and high-pressure limiting state and therefore has an infinitesimal specific volume (high-density limit). The actual matter shows ideal gas behavior near the low-density limit and ideal dense matter behavior near the high-density limit. The thermodynamic state of matter at intermediate densities may have some characteristics of both limiting states simultaneously and is most likely to be some kind of superposition of them. Interpolation is physically feasible.
Note that a classical interpolation technique was used for Planck’s radiation formula8,9. Planck saw that there were two limiting cases for radiation phenomenon, corresponding to two thermodynamic relationships: in the short-wave limit and in the long-wave limit. One of the critical features is that the second order derivatives of entropy with respect to internal energy have exponents of − 1 and − 2, respectively. Thus, Planck constructed the interpolation form of the two limiting relationships, . Combining the thermodynamic relation, , Planck eventually obtained his global radiation formula, .
There is a similar exponential correspondence for the low- and high-density limits of matter. The ideal gas EOS in P-S-T form is
| 3 |
where is the specific heat at constant pressure and the subscript 0 denotes a given thermodynamic state. The ideal dense matter EOS in P–V-T form is13
| 4 |
where is the specific work at constant temperature, defined as14
| 5 |
With pressure, P, and reciprocal temperature, , as independent variables, the differentials of volume based on Eqs. (1) and (4) are
| 6 |
Equation (6) shows that the first order derivatives of volume with respect to pressure (as well as reciprocal temperature) have exponents of − 1 and − 2. With temperature, T, and reciprocal pressure, , as independent variables, the differentials of entropy based on Eqs. (2) and (3) are
| 7 |
Equation (7) shows that the first order derivatives of entropy with respect to temperature (as well as reciprocal pressure) also have exponents of − 1 and − 2. Interpolation between these extremes leads to a global EOS model theoretically available for arbitrary densities. For a two-degree-of-freedom thermodynamic system, two global EOS in the form of P–V-T and P-S-T are derived (See Supplementary Information)
| 8 |
| 9 |
where the superscript, i.s., denotes the ideal dense matter limit and the superscript, i.g., denotes the ideal gas limit. That is, the coefficients in the global EOS remain parameters with respect to the two limiting states. This is because interpolation practically operates on two limiting states and retains their original information11. Since the global model from interpolation removes the constraints (limiting condition) for the two limiting models, the coefficients in Eqs. (8) and (9) are superscripted to indicate that they are still parameters of the corresponding limiting states. Of course, this is not needed for the ideal gas constant, R, and the ideal dense matter constant, , because their physical meaning is not changed in any occasion.
The global model contains the characteristics of both the ideal gas and the ideal dense matter simultaneously. Since both the ideal gas and ideal dense matter models are general, it has generality. The proposed global model is thermodynamically consistent and continuous. It reduces to the ideal gas model when approaching the low-density (high-temperature and low-pressure) limit and to the ideal dense matter model when approaching the high-density (high-pressure and low-temperature) limit, which suggests that the global model can describe the physical properties of either the low- or high-density matter. This distinctive feature of the global model enables itself to cover the large-range behavior of matter experiencing a large range of densities. Yet, since both limiting models can only be applied to homogeneous substances not located in a region with rapid property variations such as near the critical point or the two-phase region, then the global model is not expected to apply in those regions either. The global model reflects the commonality of thermodynamic behavior of matter and could serve as a basis to determine the physical properties of individual substances.
Characteristics
With temperature and pressure as independent variables, the differentials of volume and entropy are derived based on Eqs. (8) and (9), respectively
| 10 |
Equation (10) shows a reciprocal relation between Eqs. (8) and (9), i.e., the Maxwell’s relation corresponding to the Gibbs free energy,
| 11 |
This reflects that Eqs. (8) and (9) are is thermodynamically compatible, and also indicates to some extent the thermodynamic compatibility between the ideal gas and ideal dense matter models. Together Eqs. (8) and (9) provide a complete description for a thermodynamic system. The expressions for various thermodynamic variables can be derived based on them.
Derive the differential expression for internal energy based on Eq. (10)
| 12 |
where is the specific work at constant entropy, defined as14
| 13 |
There is a parametric relationship between and for ideal dense matter13
| 14 |
Equation (12) reveals how these two limiting parameters, and , exist in the physical properties of actual matter
| 15 |
That is, the specific heat at constant volume in the ideal gas limit is exactly the partial derivative of internal energy of actual matter with respect to temperature under isobaric conditions and the specific work at constant entropy in the ideal dense matter limit is exactly the partial derivative of internal energy of actual matter with respect to pressure under isothermal conditions. Further derive the expressions for specific heats as well as specific works
| 16 |
Equation (16) shows the general characteristics of specific heats and specific works that are also the relationships with their limiting counterparts. According to Eq. (16), the specific heats as well as the specific works are all a function of the temperature-to-pressure ratio, , (or the pressure-to-temperature ratio, ) only. The specific heats reduce to the corresponding ones of ideal gas in the high-temperature and low-pressure limit, , and the specific works reduce to the corresponding ones of ideal dense matter in the high-pressure and low-temperature limit, .
The limiting parameters in the global EOS, in turn, can be determined by other accessible parameters. For example, except for the known parameter, R, the other three limiting parameters can be derived inversely based on the expressions for the specific heats and specific works
| 17 |
and are common property parameters for which there are abundant experimental data. The values of and are accessed from the isentropic compressibility coefficient, , and the isothermal compressibility coefficient, , respectively
| 18 |
Therefore, the coefficients in the global EOS are available a priori. In this sense, the global model is an a priori model.
Verifications
Verifying specific heats and specific works
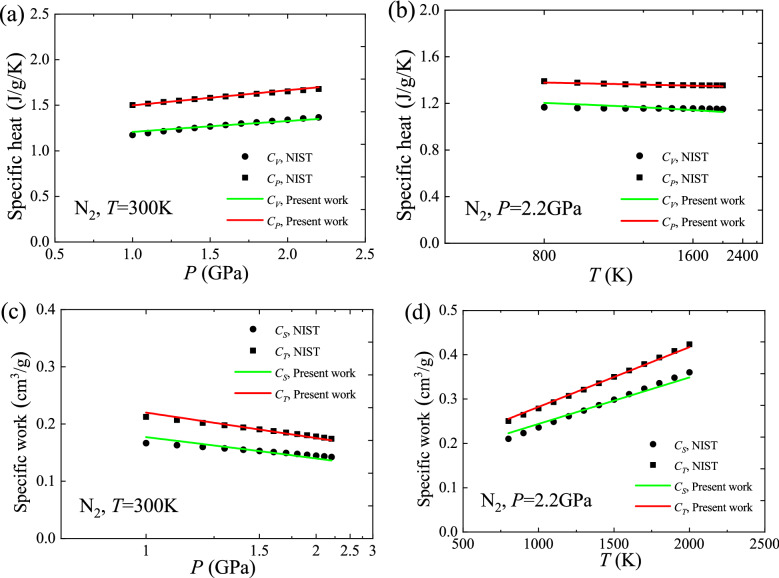
The specific heats and specific works of N2 are calculated using Eq. (16) without free-fitting parameters. Figure 1 shows that these calculated values match well the values from the REFPROP program (DLL version 9.1) developed by the National Institute of Standards and Technology (NIST). The relative deviations are less than 1.1% for , less than 3.2% for , less than 3.5% for , and less than 6.4% for . In addition, the global model predicts that under isothermal conditions, is linear with respect to pressure and is linear with respect to reciprocal pressure; under isobaric conditions, is linear with respect to reciprocal temperature and is linear with respect to temperature, all of which are consistent with the actual features exhibited for N2. This supports the correctness of the global model.
Figure 1.
Verification of the specific heats and specific works using N2 data. (a) Specific heats versus pressure at 300 K; (b) Specific heats versus reciprocal temperature at 2.2 GPa; (c) Specific works versus reciprocal pressure at 300 K; (d) Specific works versus temperature at 2.2 GPa.
Verifying two global EOS
These two global EOS, Eqs. (8) and (9), are verified using 4He data and compared with the ideal gas and ideal dense matter EOS (Fig. 2). The zero-value point of entropy for selected data is the normal boiling point (NBP). The coefficients in the two global EOS are calculated by Eq. (17). The ideal gas EOS are extrapolated to the high-pressure or low-temperature region from correlations of data in the low-pressure or high-temperature region. Results show that both the entropy and volume predicted by the ideal gas EOS are always larger than the data from NIST. The ideal dense matter EOS are extrapolated to the low-pressure or high-temperature region from correlations of data in the high-pressure or low-temperature region. Results show that both the entropy and volume predicted by the ideal dense matter EOS are always smaller than the data from NIST. The ideal gas and ideal dense matter EOS give upper and lower limits for thermodynamic behavior of matter, respectively. Compared with them, the global EOS reproduce the entire selected property data excellently, which indicates that the large-range behavior of matter at intermediate densities can be explained by a superposition of the properties of the low- and high-density limits. The interpolation pattern correctly reveals the respective proportions of the contribution from these two limiting states.
Figure 2.
Verification of the global EOS using 4He data and comparison with the ideal gas and ideal dense matter EOS. (a) Entropy versus pressure at 300 K; (b) Entropy versus temperature at 1.6 GPa; (c) Volume versus pressure at 80 K; (d) Volume versus temperature at 2 GPa.
Applications
Interpolation versus extrapolation
Extrapolation based on the ideal gas limit has been a common approach to developing an EOS for actual matter15,16. However, the EOS yielded by this approach were mostly empirical or semi-empirical for high-density matter due to the complexity and diversity of their molecular interactions17–22. These EOS usually have complex forms or plenty of empirical coefficients. These coefficients are “isolated” and their values can only be determined from a large amount of experimental data. When these EOS are further extrapolated beyond the range where they were fitted to experimental data, the results are generally not reliable23–25.
Extrapolation only yields a local result, while interpolation can lead to a global result. In the following, the global P-V-T EOS, Eq. (8), from interpolation is compared with the third-order virial EOS and the Benedict-Webb-Rubin (BWR) EOS from extrapolation using N2 data at 300 K (Fig. 3). The values of coefficients in the third-order virial EOS and the BWR EOS are taken from the work of Nowak et al.26 and Crain Jr et al.27, respectively. Results show that the third-order virial EOS with three coefficients is valid only for relatively low-pressure region. The BWR EOS was extrapolated to relatively high-pressure region by introducing eight empirical coefficients. Even so, the accuracy of the BWR EOS decreases as the pressure increases further. Since both the third-order virial EOS and the BWR EOS were constructed with the ideal gas EOS as the basic framework, their extrapolation behaviors are certainly deteriorating with increasing pressure or density. Compared to the third-order virial EOS and the BWR EOS, the global P-V-T EOS shows a significant advantage by using both the ideal gas and ideal dense matter EOS as the basic framework. It achieves higher descriptive accuracy with fewer coefficients (two coefficients for isothermal conditions) over a wide range of data for N2, especially in the ultrahigh-pressure region. Additionally, the empirical coefficients in the virial EOS and the BWR EOS need to be determined by fitting a large amount of experimental data26,27, while the coefficients in the global P-V-T EOS have a clear thermodynamic meaning and can be determined from a priori knowledge.
Figure 3.
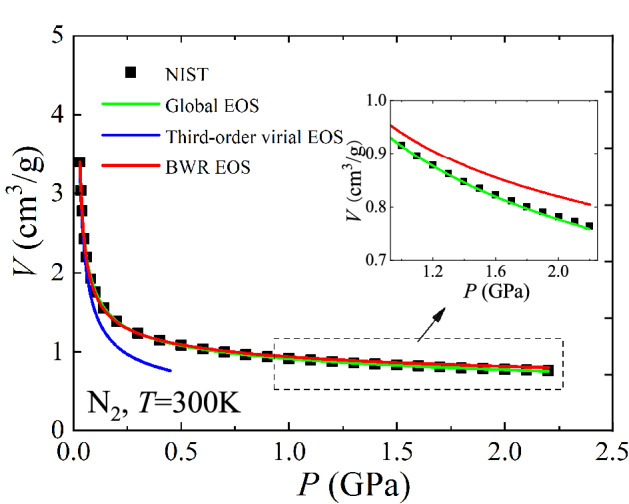
Comparison of the global P-V-T EOS with the third-order virial EOS and the BWR EOS using N2 data.
Explosive physics
The Jones–Wilkins–Lee (JWL) EOS is a well-known empirical EOS in explosive physics and is quite effective in describing the expansion behavior of the detonation products of condensed explosives28,29. It is pressure-explicit and has a simplified form,
| 19 |
where A and R1 are constants. The first term on the right side of Eq. (19) represents the high-pressure contribution and the second term represents the low-pressure contribution. The former is actually the isothermal form of the ideal dense matter P-V-T EOS, while the latter expresses the ideal gas EOS. Therefore, the simplified JWL EOS can be understood as a superposition of the ideal dense matter state and the ideal gas state under the pressure representation. As a comparison, the global P-V-T EOS is volume-explicit and behaves as a superposition of the ideal dense matter state and the ideal gas state under the volume representation. Figure 4 shows further comparison between the global P-V-T EOS and the simplified JWL EOS using the solid CO2 data from the high-pressure experiment of Liu30. Results show that both are in good agreement with the isothermal data of solid CO2. However, with respect to the effect of temperature, the simplified JWL EOS retains only the ideal gas part, while the global P-V-T EOS keeps both the ideal dense matter part and the ideal gas part. Therefore, the global P-V-T EOS may reflect the effect of temperature more accurately. In addition, as mentioned earlier, the coefficients in the global P-V-T EOS can be obtained a priori, while the simplified JWL EOS has empirical coefficients that need to be experimentally fitted.
Figure 4.
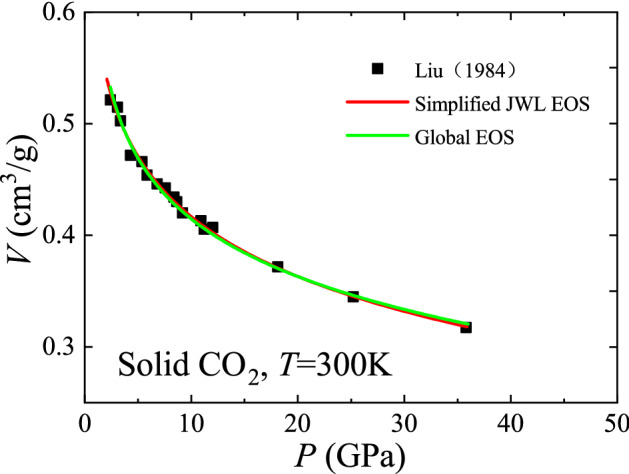
Comparison of the global P-V-T EOS with the simplified JWL EOS using solid CO2 data.
Shock-Hugoniots
Combining Eqs. (8) and (9) yields a new global EOS,
| 20 |
where c is an integration constant. Equation (20) has a particular feature that temperature and pressure are in the form of a ratio, (or ). When the temperature-to-pressure ratio is kept constant, a very simple linear relationship between entropy and volume appears
| 21 |
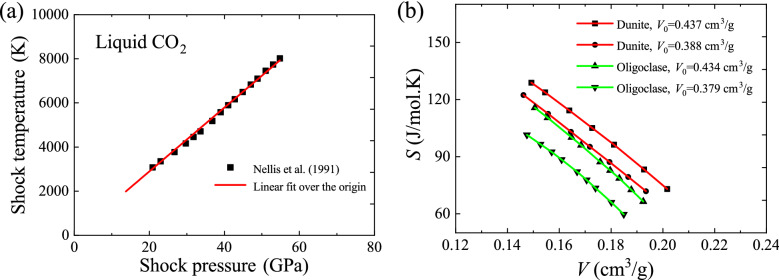
where C is a constant. This new physical property predicted by the global model can be used to describe the shock adiabatic compression (Hugoniot). The temperature-to-pressure ratio is approximately constant for a large number of shock adiabatic processes31–34. For example, the shock experiments of Nellis et al.35 on liquid CO2 shows that the shock temperature is almost linearly proportional to the shock pressure (Fig. 5a). Therefore, according to Eq. (21), the entropy of shock adiabatic process should have linear, inverse relationship with volume, which is consistent with the observations of Ahrens et al.36 for minerals such as dunite and oligoclase (Fig. 5b). This suggests that the shock-induced entropy production depends directly on the degree of volume compression.
Figure 5.
Shock-Hugoniots. (a) Shock temperature versus shock pressure for liquid CO2; (b) entropy versus volume for dunite and oligoclase, where V0 is initial volume.
Conclusions
Interpolation between the low- and high-density limits is needed to gain a truly global EOS model. The ideal gas model is a well-known model for the low-density limit. Recently, an ideal dense matter model for the high-density limit symmetric to the ideal gas model has been developed. In this work, by mathematically interpolating between the ideal gas and ideal dense matter limiting models, we establish a global model containing two EOS in the form of P-V-T and P-S-T for arbitrary ranges of densities. The two global EOS constitute a complete description for the thermodynamic properties of matter.
The proposed global model is thermodynamically consistent and continuous. It returns to the ideal gas model when approaching the low-density limit and to the ideal dense matter model when approaching the high-density limit. The global model can be applied to either low- or high-density matter, and thus can cover the large-range behavior of matter. Verification for 4He shows that the ideal gas model always gives larger predicted values of entropy and volume, and the ideal dense matter model always gives smaller predicted values, while the global model reproduces well the property data of 4He experiencing a large range of densities. The thermodynamic behaviors of matter at intermediate densities show the physical nature of superposition of the low- and high-density limits.
Since interpolation uses information only on the low- and high-density limits, the coefficients in the global EOS are all parameters of limiting states with a clear physical meaning. They can be determined from a priori knowledge. In this sense, the global model is an a priori model. The general expressions for specific heats and specific works are derived and their values for N2 are calculated without free-fitting parameters. The calculated values are verified to be in good agreement with the values from the NIST database.
The global model is shown to work well in applied sciences dealing with high-density matter. Compared to the third-order virial EOS and the BWR EOS, the global P-V-T EOS achieves higher descriptive accuracy with fewer coefficients over a wide range of data for N2, especially in the ultrahigh-pressure region. Interpolation between the ideal gas and ideal dense matter models shows significant advantages over extrapolation on the ideal gas model only. The global P-V-T EOS is also shown to have almost the same effect as the classical JWL EOS in describing the expansion behavior of detonation products. Furthermore, the global model predicts a linear, inverse relationship between entropy and volume when the temperature-to-pressure ratio is constant, which can explain the entropy-production behavior in shock- Hugoniots.
Supplementary Information
Acknowledgements
The authors thank Prof. Yuan-Yuan Duan, Prof. Hai-Dong Wang, Dr. Tian Zhao, Dr. Sheng-Zhi Xu, and Dr. Yang Li for helpful discussions. This research was financially supported by National Natural Science Foundation of China (No. 51356001).
Author contributions
T.-W.X. conceived the research, performed the research and wrote the manuscript. Z.-Y.G. performed the research, revised the manuscript and provided the fund support.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-16016-6.
References
- 1.Young DA, Corey EM. A new global equation of state model for hot, dense matter. J. Appl. Phys. 1995;78:3748. doi: 10.1063/1.359955. [DOI] [Google Scholar]
- 2.Volokitin VS, Golosnoi IO, Kalitkin NN. Wide-range equation of state of matter. I. Analysis of nonideality models. Russ. Phys. J. 1994;37:1029. doi: 10.1007/BF00559211. [DOI] [Google Scholar]
- 3.Volokitin VS, Golosnoi IO, Kalitkin NN. Wide-range equation of state of matter. II. Microfield model. Russ. Phys. J. 1995;38:336. doi: 10.1007/BF00560096. [DOI] [Google Scholar]
- 4.Trainor KS. Construction of a wide-range tabular equation of state for copper. J. Appl. Phys. 1983;54:2372. doi: 10.1063/1.332350. [DOI] [Google Scholar]
- 5.Peterson JH, Honnell KG, Greeff C, Johnson JD, Boettger J, Crockett S. Global Equation of State for Copper. American Institute of Physics; 2012. [Google Scholar]
- 6.Young DA. A new global hydrogen equation of state model. High Press. Res. 2000;16:389. doi: 10.1080/08957950008201438. [DOI] [Google Scholar]
- 7.Luding S. Global equation of state of two-dimensional hard sphere systems. Phys. Rev. E. 2001;63:042201. doi: 10.1103/PhysRevE.63.042201. [DOI] [PubMed] [Google Scholar]
- 8.Planck M. On an improvement of Wien’s equation for the spectrum. Ann. Physik. 1900;1:719. doi: 10.1002/andp.19003060410. [DOI] [Google Scholar]
- 9.Planck M. On the theory of the energy distribution law of the normal spectrum. Verh. Deut. Phys. Ges. 1900;2:237. [Google Scholar]
- 10.Srinivasan G, Jonson M, Lundqvist S. Surface correlation energy—An interpolation formula. II Nuovo Cimento B. 1975;29:285. doi: 10.1007/BF02728312. [DOI] [Google Scholar]
- 11.Sun J-W, Perdew JP, Seidl M. Correlation energy of the uniform electron gas from an interpolation between high-and low-density limits. Phys. Rev. B. 2010;81:085123. doi: 10.1103/PhysRevB.81.085123. [DOI] [Google Scholar]
- 12.Cengel YA, Boles MA. Termodynamics. McGraw-Hill; 2006. [Google Scholar]
- 13.Xue T-W, Guo Z-Y. A general equation of state for high density matter from thermodynamic symmetry. J. Appl. Phys. 2022;131:044902. doi: 10.1063/5.0077707. [DOI] [Google Scholar]
- 14.Bordoni S. Routes towards an abstract thermodynamics in the late nineteenth century. Eur. Phys. J H. 2013;38:617. doi: 10.1140/epjh/e2013-40028-7. [DOI] [Google Scholar]
- 15.van der Waals, J. D. Over de Continuiteit van den Gasen Vloeistoftoestand (Doctoral Dissertation, Leiden: Leiden University, 1873).
- 16.Klein MJ. The historical origins of the van der Waals equation. Physica. 1974;73:28. doi: 10.1016/0031-8914(74)90224-9. [DOI] [Google Scholar]
- 17.Menikoff R. Empirical Equations of State for Solids. Springer; 2007. [Google Scholar]
- 18.Towle LC. Empirical equations of state for solids. Appl. Phys. 1975;8:117. doi: 10.1007/BF00896029. [DOI] [Google Scholar]
- 19.Beattie JA, Bridgeman OC. A New Equation of State for Fluids. American Academy of Arts & Sciences; 1928. [Google Scholar]
- 20.Benedict M, Webb GB, Rubin LC. An empirical equation for thermodynamic properties of light hydrocarbons and their mixtures I. Methane, ethane, propane and n-butane. J. Chem. Phys. 1940;8:334. doi: 10.1063/1.1750658. [DOI] [Google Scholar]
- 21.Benedict M, Webb GB, Rubin LC. An empirical equation for thermodynamic properties of light hydrocarbons and their mixtures II. Mixtures of Methane, Ethane, Propane, and n-Butane. J. Chem. Phys. 1942;10:747. doi: 10.1063/1.1723658. [DOI] [Google Scholar]
- 22.Oh KH, Persson PA. Equation of state for extrapolation of high-pressure shock Hugoniot data. J. Appl. Phys. 1989;65:3852. doi: 10.1063/1.343434. [DOI] [Google Scholar]
- 23.Span R, Wagner W. On the extrapolation behavior of empirical equations of state. Int. J. Thermophys. 1997;18:1415. doi: 10.1007/BF02575343. [DOI] [Google Scholar]
- 24.Brosh E, Shneck RZ, Makov G. Explicit Gibbs free energy equation of state for solids. J. Phys. Chem. Solids. 2008;69:1912. doi: 10.1016/j.jpcs.2008.01.019. [DOI] [Google Scholar]
- 25.Latimer K, Dwaraknath S, Mathew K, Winston D, Persson KA. Evaluation of thermodynamic equations of state across chemistry and structure in the materials project. Mnpj Comput. Mater. 2018;4:1. doi: 10.1038/s41524-017-0060-9. [DOI] [Google Scholar]
- 26.Nowak P, Kleinrahm R, Wagner W. Measurement and correlation of the (p, ρ, T) relation of nitrogen I. The homogeneous gas and liquid regions in the temperature range from 66 K to 340 K at pressures up to 12 MPa. J. Chem. Thermodyn. 1997;29:1137. doi: 10.1006/jcht.1997.0230. [DOI] [Google Scholar]
- 27.Crain RW, Jr, Sonntag RE. Nitrogen constants for the Benedict-Webb-Rubin equation of state. J. Chem. Eng. Data. 1967;12:73. doi: 10.1021/je60032a021. [DOI] [Google Scholar]
- 28.Lee E, Hornig H, Kury J. Adiabatic Expansion of High Explosive Detonation Products. University of California Radiation Laboratory Livermore; 1968. [Google Scholar]
- 29.Baudin, G. & Serradeill, R. Review of Jones-Wilkins-Lee Equation of State Vol. 10 (EPJ Web of Conferences, EDP Sciences, 2010).
- 30.Liu L-G. Compression and phase behavior of solid CO2 to half a megabar. Earth Planet Sci. Lett. 1984;71:104. doi: 10.1016/0012-821X(84)90056-6. [DOI] [Google Scholar]
- 31.Nagayama K, Mori Y, Shimada K, Nakahara M. Shock Hugoniot compression curve for water up to 1 GPa by using a compressed gas gun. J. Appl. Phys. 2002;91:476. doi: 10.1063/1.1421630. [DOI] [Google Scholar]
- 32.Kormer SB. Optical study of the characteristics of shock-compressed condensed dielectrics. Sov. Phys. Usp. 1968;11:229. doi: 10.1070/PU1968v011n02ABEH003814. [DOI] [Google Scholar]
- 33.Lyzenga GA, Ahrens TJ, Nellis WJ, Mitchell AC. The temperature of shock-compressed water. J. Chem. Phys. 1982;76:6282. doi: 10.1063/1.443031. [DOI] [Google Scholar]
- 34.Min SH, Berkowitz ML. A comparative computational study of coarse-grained and all-atom water models in shock Hugoniot states. J. Chem. Phys. 2018;148:144504. doi: 10.1063/1.5011968. [DOI] [PubMed] [Google Scholar]
- 35.Nellis WJ, Mitchell AC, Ree FH, Ross M, Holmes NC, Trainor RJ, Erskine DJ. Equation of state of shock-compressed liquids: Carbon dioxide and air. J. Chem. Phys. 1991;95:5268. doi: 10.1063/1.461665. [DOI] [Google Scholar]
- 36.Ahrens TJ, O'Keefe JD. Shock melting and vaporization of lunar rocks and minerals. The Moon. 1972;4:214. doi: 10.1007/BF00562927. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.