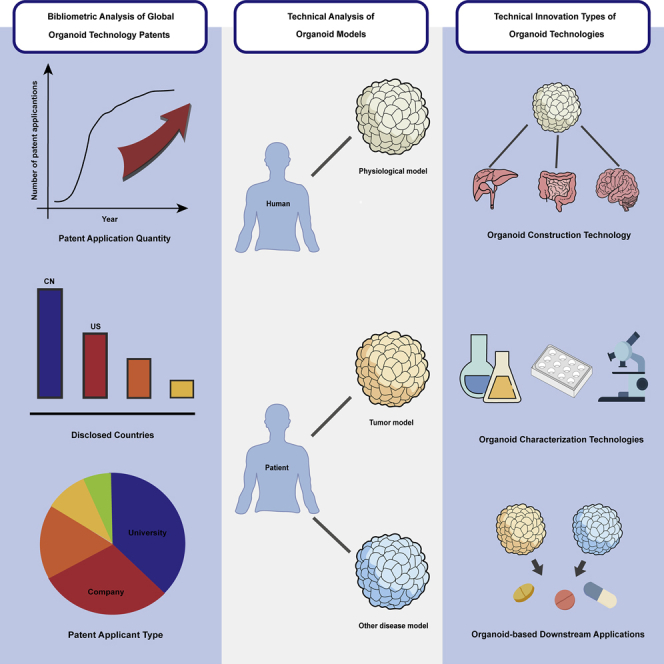
Summary
Organoids are considered a game-changing paradigm of research models for human physiology and disease, which provides unsurpassed opportunities across disciplines in basic medical research, drug development, and personalized medicine. Here, we made a deep investigation for global patents of organoid technologies in the past decade using bibliometric analysis for the first time. We have identified a total of 672 patents related to organoid technology. The number of annual patent applications exhibits an overall upward growth trend over the past decade, especially entering an exponential growth since 2015. Notably, 76.64% of patents are related to the construction of organoid models. Liver, brain, and intestinal models take up the first three places in the physiological models, while tumor models account for 76.30% of the total patents for disease models. Furthermore, drug screening is the most preferred application, revealing the great commercial value of organoid technologies in precision medicine and preclinical drug screening.
Subject areas: Biological sciences, Bioengineering, Tissue engineering, Biotechnology
Graphical abstract
Highlights
-
•
Global innovation of organoid technologies has entered an exponential growth stage
-
•
Organoid construction is currently the main innovation direction in the organoid field
-
•
New technologies emerge for organoid culture and characterization
-
•
Drug screening is the major focus in organoid-based downstream applications
Biological sciences; Bioengineering; Tissue engineering; Biotechnology
Introduction
Organoids are increasingly considered by the global academia and industry to be a revolutionary technology that will profoundly change the paradigm of research model systems used in basic medical research and drug discovery in the past decade and will become the gold standard for disease mechanistic research and preclinical drug screening. Additionally, organoids will provide a unique opportunity to diverse divisions in the biomedical industries, such as precision medicine and regenerative medicine. As the milestone discovery of the Lgr5+ stem cell-derived intestine organoids, the basic research on organoids has been performed for more than one decade and has entered an exponential growth stage in terms of annually published articles (Wang et al., 2020). Notably, many organoid-based start-up companies and businesses have emerged in the recent several years, resulting in the initial formation of an organoid industrial chain that covers the production of reagents and consumables for organoid culture, the R&D of organoid-based physiological and disease models, and downstream applications based on these organoid-based models.
Organoids refer to a category of stem cell-derived three-dimensional (3D) cell aggregates that partially emulate the developmental trajectory of natural organs as well as the tissue-specific structure and function (Schutgens and Clevers, 2020). In 2009, Hans Clevers and colleagues created the first organoid using Lgr5+ adult stem cells isolated from mouse intestine tissue (Sato et al., 2009). Since then, various types of tissue-specific organoids have been reported, such as liver (Takebe et al., 2013), kidney (Taguchi et al., 2014), brain (Lancaster and Knoblich, 2014a), pancreas (Boj et al., 2015), and lung (Dye et al., 2015) organoids. Using these organoids, a variety of human diseases have been modeled in vitro, including infectious diseases (Lamers et al., 2020; Qiao et al., 2020), genetic diseases (Ramli et al., 2020; Schwank et al., 2013), and cancers (Bian et al., 2018; Shi et al., 2020; Yan et al., 2018). These disease models facilitate human disease mechanistic studies and preclinical drug development. Compared with traditional two-dimensional cell culture systems, organoids can better simulate complex interactions of cell-to-cell and cell-to-extracellular matrix in the native 3D microenvironments and maintain the original cell phenotype and function regardless of cell passaging (Schutgens and Clevers, 2020). Compared with animal models, organoids are more humanized models and can reproduce the developmental trajectory of human organs and the occurrence and progression of human diseases (Schutgens and Clevers, 2020). Overall, organoids exhibit unique advantages over traditional cell culture and animal models in basic medical research, drug screening, and other translational research, and thus organoid technologies show a great value in commercialized application in the biomedical industries.
Patent bibliometric analysis is an essential method to investigate the current status and future trends of emerging technologies, especially for those with commercialized value. Although bibliometric analysis has been performed to investigate academic trends of organoids based on published academic articles, bibliometric analysis has not been conducted to examine organoid technologies based on patents. In this work, we study the global trend of organoid technologies using patent bibliometric analysis based on the incoPat database. We conduct deep data mining, analysis, and interpretation from the aspects of patent technology competition, organoid types, models, construction technology, and downstream application. We expect this work will provide a unique perspective for understanding the evolution of organoid technologies as well as a useful guide for the patent protection of organoid technologies for global researchers and developers in this field.
Bibliometric analysis of global organoid technology patents
Patent application quantity
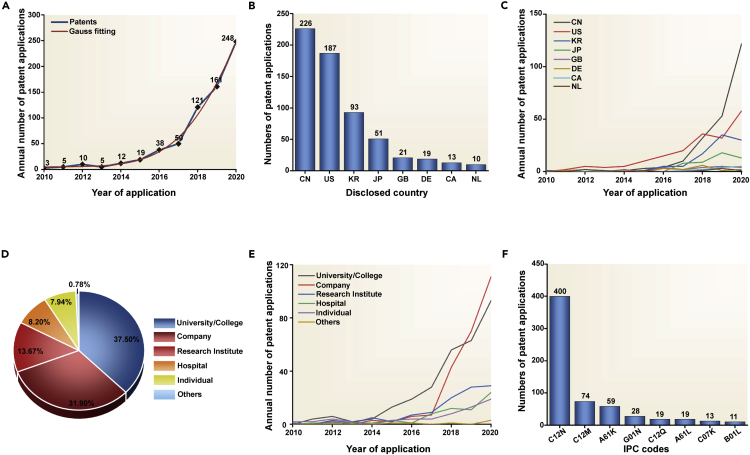
To clarify the technological development in the organoid field in the past eleven years, we analyzed the application quantity of global organoid technology patents per year. Generally, organoid technology patent applications demonstrated an overall upward growth trend over the past decade in Figure 1A. Specifically, the annual application quantity of organoid technology patents experienced two different stages, including a baseline phase with annual patent applications fewer than or around ten cases between 2010 and 2014 and a rapid growth phase started in 2015 with an average annual growth rate (AAGR) of 67.16%.
Figure 1.
Bibliometric analysis of global organoid technology patents
(A) Application trends of global organoid technology patents (Gauss fitting R-squared: 0.99288).
(B) Main disclosed countries or regions of global organoid technology patents.
(C) Application trends of global organoid technology patents for main disclosed countries or regions.
(D) The organizational attributes of the patent applicants.
(E) Application trends of global organoid technology patents for varied organizational attributes.
(F) Statistical analysis of organoid technology patents under different IPC codes.
Disclosed countries
To reveal the technological development of organoids in the different countries, we conducted a statistical analysis of patents across the varied disclosed countries or regions in the past eleven years. The results indicated that the top eight countries or regions were China, the United States (U.S.), South Korea, Japan, Britain, Germany, Canada, and the Netherlands, as shown in Figure 1B.
Particularly, the patents in China and U.S. grew rapidly and accounted for 33.63 and 27.83% of the total number of global organoid technology patents. To investigate the different developmental stages of organoid technologies in individual countries, we defined the rapid growth stage as a period in which a country has annual patent applications of more than ten cases. Using this method, we found organoid technologies in the U.S. have entered a rapid growth stage since 2017, which was two years earlier than that in China. Meanwhile, the organoid technologies in South Korea and Japan have entered the rapid growth stage in 2018 and 2019, respectively, as illustrated in Figure 1C.
Patent applicant type
To analyze the organizational attributes of the patent applicants in the organoid field, we classified the patent applicants into six types, including university/college, company, research institute, hospital, individual, and others.
Statistically, the top three organization types are university/college, company, and research institute, which accounted for 37.50%, 31.90%, and 13.67% of the total patents, respectively, in Figure 1D. The growth curves of the patent application quantity for each type of organization were shown in Figure 1E, indicating that annual organoid patents of universities/colleges have outbroken since 2015. Interestingly, organoid patents applied by companies, research institutes, and hospitals simultaneously outbroke in 2018, manifested by annual patent applications of more than ten.
We listed applicants with ten or more patents shown in Table 1, including four universities, two companies, one research institute, and one hospital. Specifically, the top three applicants were Accurate International Biotechnology CO LTD (China), Children’s Hospital Medical Center (U.S.), Royal Netherlands Academy of Arts and Sciences (KNAW, Netherlands), which reserved 18, 16, and 14 organoid patents, respectively.
Table 1.
Ranking of patent applicants by the number of patent aplications
| Organization | Disclosed country | Organizational attribute | Patents |
|---|---|---|---|
| Accurate International Biotechnology CO LTD | CN | Company | 18 |
| Children’s Hospital Medical Center | US | Hospital | 16 |
| Royal Netherlands Academy of Arts and Sciences | NL | Research institution | 14 |
| Yonsei University | KR | University | 13 |
| Keio University | JP | University | 13 |
| Harvard University | US | University | 12 |
| Beijing K2 Medicine Tech CO LTD | CN | Company | 12 |
| Wake Forest University | US | University | 10 |
Furthermore, we ranked the disclosed countries by the number of patent applicants that own more than three organoid patents (Table 2). The results demonstrate that China, the U.S., and Korea lead the patent applications for organoid technologies. Particularly, China owns 11 companies that have applied for more than three organoid patents, while the U.S. owns 11 universities for more than three organoid patents. Additionally, we listed the top ten patent applicants in each category of organizational attributes (Tables S1, S2, S3, and S4). The top three universities are Yonsei University, Keio University, and Harvard University (Table S1). Specifically, Yonsei University focuses on studying the culture method of salivary gland and taste bud organoids. Keio University tends to study the cultivation methods of brain organoids and the modeling of related diseases. Harvard University develops methods and cultivating devices for brain or bone-marrow organoids. The top two companies are Accurate International Biotechnology CO LTD and Beijing K2 Medicine Tech CO LTD (Table S2), and their research contents are culture methods and media for tumor organoids. Royal Netherlands Academy of Arts and Sciences (KNAW) ranks first among research institutes and constructs various physiological and disease models (Table S3). Children’s Hospital Medical Center ranks first in the category of hospital and focuses on liver and intestinal organoid construction and drug screening (Table S4).
Table 2.
Ranking of disclosed countries by the number of patent applicants that own more than three organoid patent applications
| Disclosed country | Total | Universities | Companies | Research institutes | Hospitals |
|---|---|---|---|---|---|
| CN | 24 | 6 | 11 | 4 | 3 |
| US | 20 | 11 | 3 | 2 | 4 |
| KR | 10 | 4 | 4 | 2 | 0 |
| JP | 7 | 4 | 2 | 1 | 0 |
| SG | 2 | 1 | 0 | 1 | 0 |
| GB | 2 | 0 | 1 | 1 | 0 |
| DE | 1 | 1 | 0 | 0 | 0 |
| AT | 1 | 0 | 1 | 0 | 0 |
| CA | 1 | 0 | 1 | 0 | 0 |
International patent classification
International Patent Classification, also known as the IPC classification method, is currently the only universal patent document classification method in the world (Eisinger et al., 2013). The IPC codes are language-independent symbols for the classification of patents based on the technology areas they pertain to. By statistical analysis of patents based on IPC codes, the hotspots of technology areas can be revealed.
IPC codes with more than ten patent applications are C12N, C12M, A61K, G01N, C12Q, A61L, C07K, and B01L (Figure 1F), the correspondent technology content in Table 3. Notably, the patents pertaining to the C12N category ranked first and accounted for 59.52% of the organoid technology patents. The resting IPC codes individually took up a small fraction of the total patents, indicating that there is still a lot of space for organoid technology to develop in these technology areas.
Table 3.
IPC codes affiliated with more than ten patent applications and their representative technologies
| IPC codes | Counting | Technical contents |
|---|---|---|
| C12N | 400 | The culture or maintenance methods and media of organoids derived from undifferentiated human and animal cells; organoid-related specific gene expression and gene editing methods. |
| C12M | 74 | Equipment applied to the culture, screening, and treatment application of organoids. |
| A61K | 59 | Organoid-related medical preparations containing raw materials or reaction products of unknown structure. |
| G01N | 28 | Organoid-based drug screening and treatment modalities. |
| C12Q | 19 | Evaluate the effects of drugs and cells based on organoids. |
| A61L | 19 | Organoids are used for organ transplantation or tissue repair. |
| C07K | 13 | Peptides and other derivatives for organoid construction. |
| B01L | 11 | Devices and systems for culturing, detecting, and applying organoids. |
Technical analysis of organoid models
We identified 515 patents related to organoid models in the claims of these patents, and we classified these patents into two categories, namely the physiological model and the disease model.
Physiological model
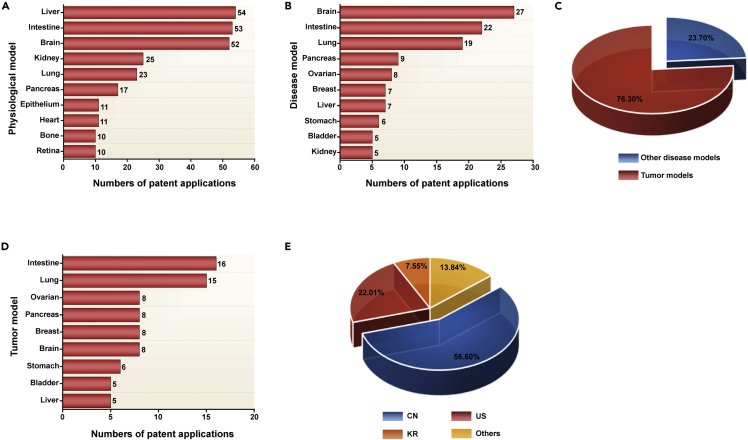
Physiological models refer to microscopic 3D organoids that can reproduce some critical structural and functional characteristics and developmental trajectory of normal human organs. These physiological models may find broad applications in developmental biology, human physiology, and drug toxicity (Rossi et al., 2018). We identified 307 patents involving the use or construction of organoid-based physiological models out of 672 patents. Specifically, liver, brain, and intestinal models ranked in the top three and accounted for 17.59%, 17.26%, and 16. 94% of the total physiological models, respectively, as shown in Figure 2A. Some typical examples of physiological models are provided in Table S5. Additionally, we found that these physiological models covered more than 40 types of human organs, accounting for more than half of the total human organ types.
Figure 2.
Technical analysis of organoid models
(A) Organoid technology patents for physiological models.
(B) Organoid technology patents for disease models.
(C) Disease model classification.
(D) Organoid technology patents for tumor models.
(E) Main disclosed countries or regions of organoid technology patents for tumor models.
Disease model
Disease models refer to the organoid models that can simulate some important disease characteristics and phenotypes in human disease onset and progression (Lancaster and Knoblich, 2014b). Disease models are usually constructed by exposing normal organoids to physical, chemical, or pathogenic conditions. In addition, organoid disease models can also be generated from patient-derived cells, which can closely preserve the genetic background of the disease and may closely reproduce some pivotal pathological features of disease onset and progression (Fan et al., 2019).
We identified 208 patents involving the use or construction of organoid-based disease models from all the patents. Specifically, brain, intestine, and lung are the top three targeted organs of these disease models, with each organ type involved in 15 or more patents (Figure 2B). Furthermore, we categorized these disease models into tumor and other disease models.
Tumor organoids are generated from patient-derived tumor tissues or cells and can simulate corresponding tumors' genomic and histological characteristics (Yuki et al., 2020). The patents related to tumor models were 161, accounting for 76.30% of the total patents for disease models, as shown in Figure 2C. The cancer types with more than five patent applications in these tumor models were intestinal cancer, lung cancer, ovarian cancer, pancreatic cancer, breast cancer, brain cancer, stomach cancer, bladder cancer, and liver cancer, as shown in Figure 2D. Typical examples of tumor models are given in Table S6. Interestingly, more than half of tumor organoid patents were disclosed in China, while nearly one-third are disclosed in the U.S., indicating that these two countries were leading the technological advances in the tumor organoid field (Figure 2E). In addition, more than 20 types of cancer have been successfully constructed into tumor organoid models.
In addition to tumor models, brain, intestine, and lung take the top three targeted organs of the resting disease models. Specifically, Alzheimer’s disease, Parkinson’s disease, cerebral ischemia, and infantile autism were the hotspots for patent applications of brain organoids. Enteritis, viral enteropathy, and pulmonary fibrosis were extensively studied in the intestine and lung organoids (Table S7).
Technical innovation types of organoid technologies
We investigated the technical innovation in the claims of the organoid patents under the context of the organoid industry chain and classified these innovations into three categories, including organoid construction technology, characterization technology, and downstream application. The number of organoid construction technologies ranked first among these categories and accounted for 79.46% of the total organoid patents. The downstream application ranked second and involved the patents nearly three times more than that in the category of characterization technology.
The above results indicate that organoid construction technology is the mainstream innovation direction in the organoid field, while the organoid characterization technology is still at an early stage of development.
Organoid construction technology
We further categorized organoid construction technology into method innovation and material innovation according to the claims in the patents. Specifically, the method innovation refers to the novel methods and steps in the operating procedures for the preparation or construction of organoids, while the material innovation refers to the novel devices or other biochemical products involved in manufacturing organoids. Among them, the material innovation can be further subdivided into cultural equipment/system, biological scaffold, culture medium, and reagent/kit.
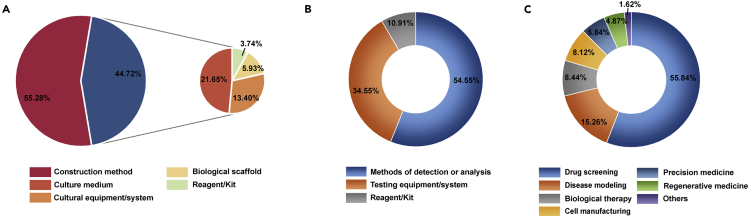
We identified 534 organoid construction patents and accounted for 79.46% of all organoid technology patents. Notably, the method innovations accounted for as high as 55.28% of all organoid construction patents. In the material innovation, the patents for culture medium and culture equipment/system accounted for 35.05% of the total organoid construction patents (Figure 3A). Typical examples of different organoid construction technologies are provided in Table S8. Overall, these results demonstrated that the innovation of organoid construction technology mainly focused on methods, culture equipment/system, and culture medium.
Figure 3.
Technical innovation types and development trends of organoid technologies
(A) Technical innovative types in the organoid construction technology.
(B) Technical innovative types in the organoid characterization technology.
(C) Technical innovative types in the organoid-based downstream application.
Organoid characterization technologies
According to the claims in the patents, the innovation in the organoid characterization technologies can be further divided into method innovation and material innovation. Specifically, the method innovation refers to novel methods and steps in the characterization procedures for detecting or analyzing organoids. Meanwhile, the material innovation refers to novel equipment/system or reagent/kit involved in the process of organoid characterization.
We identified 96 organoid characterization patents, accounting for only 14.30% of all organoid technology patents. Among them, the method innovation accounted for 54.55% of the organoid characterization technology patents, and the equipment/system innovation accounted for 34.55%, as shown in Figure 3B. Typical examples for organoid characterization patents are given in Table S9.
Organoid-based downstream applications
According to the claims in the patents, the downstream applications of organoid technologies can be further divided into seven aspects, including drug screening, disease modeling, biological therapy, cell manufacturing, precision medicine, regenerative medicine, and others.
We identified 251 patents related to the downstream applications and accounted for 37.35% of all organoid technology patents. Specifically, the top three downstream applications were drug screening, disease modeling, and biological therapy, which accounted for 55.84%, 15.26%, and 8.44% of the patents in this category, respectively (Figure 3C). Typical examples for downstream applications are given in Table S10.
Patent value evaluation
We evaluated 672 patents of organoid technologies from the aspects of technology stability, technology superiority, and commercial value using the incoPat’s internal patent evaluation system.
Technology stability refers to the capability of a patent to resist invalidity lawsuit after authorization. Technology superiority is to evaluate the superiority and exclusivity of patent technology. The commercial value is measured by the incoPat patent value index (IPVI), which is calculated based on more than 20 objective parameters (such as patent type, times of citation, cited times, and the number of patents under the same). Generally, patents with more licensing, lawsuits, more patents under the same family, more claims, or cited times are regarded as high value. Conversely, a patent is identified as a low value on the conditions including (i) a patent is abandoned within a short period after publication; (ii) a patent has not been transferred and licensed; (iii) a patent that previously experienced patent litigation; (iv) a patent with a patentee who usually maintains patent rights for a short period.
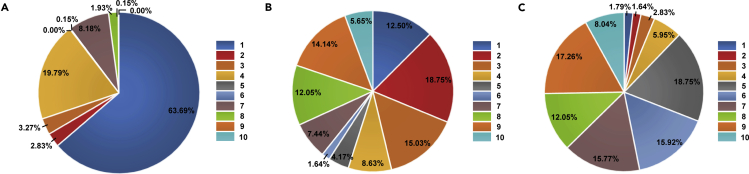
As illustrated in Figure 4A, 89.58% of organoid patents had technical stability scored between one to five, while the left 10.42% of organoid patents had an equal or more than six. These results indicate that these organoid patents generally have a low ability to resist patent invalidity litigation. Technology superiority analysis demonstrated that 59.08% of organoid patents were scored from one to five, as shown in Figure 4B. IPVI analysis showed that 69.05% of organoid patents scored six to ten, implying that organoid patents have substantial commercial value (Figure 4C).
Figure 4.
Patent value evaluation of organoid technologies
(A) Technology stability of the organoid technologies.
(B) Technology superiority of the organoid technologies.
(C) IPVI of organoid technologies.
Discussion
Global innovation of organoid technologies has entered an exponential growth stage. Patent bibliometric analysis indicated that the number of annual organoid applications experienced an outbreak with an AAGR of 67.16% from 2015 to 2020 (Figure 1A). Particularly, China and the U.S. are the top two players in organoid technology innovation, considering their accumulative quantity and annual growth rate of patent applications (Figures 1B and 1C). Patent applicant analysis further indicates that the rapid growth of organoid technologies was majorly contributed by innovation activities from universities and companies (Figures 1D and 1E). Interestingly, we found that universities majorly drove the growth of organoid technology in the U.S., while companies majorly drove the growth of organoid technology in China (Table 2). As evidence, six American universities ranked within the top ten in the category of university-type applicants (Table S1). Technology innovation by these universities majorly focused on the development of culture methods and cultivating devices for physiological organoids and related disease models. Meanwhile, five Chinese companies ranked within the top ten in the category of company-type applicants (Table S2). The main research direction of these companies was to develop culture media and methods for tumor organoids. These results imply that the R&D of organoid technologies in China is strongly related to the productization and commercialization of organoid technologies for precision cancer therapies, while the R&D of organoid technologies in the U.S. is closely correlated with basic stem cell and developmental biology research.
In addition to universities and companies, KNAW was the top leading innovator among research institutes (Table S3). Specifically, Hans Clevers, the early pioneer of the organoid field, was affiliated with KNAW and developed a culture method for Lgr5+ stem cell-derived organoids as early as 2009. Notably, KNAW’s patent (publication number: WO2010090513A2) had 157 extended family patents. It is one of the fundamental patents for organoid technologies and is protected by more than 20 countries worldwide. Additionally, Children’s Hospital Medical Center in the U.S. was a leading player among hospitals (Table S4) and applied for its first organoid patent as early as 2011. The Children’s Hospital Medical Center focused on the development of organoid technologies for physiological models and drug screening, which implemented a global patent layout in more than ten countries and regions around the world. The above results demonstrate that the leading applicants in the organoid field have a global vision and protect the commercial interest of their original organoid technologies in the major global markets.
Current innovation in organoid technologies mainly focuses on organoid construction technologies. Briefly, we categorized the innovation of organoid patents into three types, including construction technology, characterization technology, and downstream application. We found that the construction technologies were intensely studied and accounted for 79.46% of the total organoid patents. Meanwhile, the IPC analysis indicated that organoid construction technologies could be further decomposed into construction methods (C12N), culture media (C12N), and culture devices/equipment (C12M), which ranked the top two places in the IPC classification (Table 3).
Organoid construction technologies have been developed to emulate human physiological and disease conditions. Specifically, organoid models have been established to emulate more than 40 types of human organs, among which liver, intestine, and brain have been intensely studied, with more than 50 patents related to generating their corresponding physiological models individually. Additionally, organoid construction methods for the other organs, such as kidney, lung, pancreas, and heart, have also been widely studied, with each organ involved in more than ten organoid patents (Figure 2A). These physiological models will facilitate broad applications in developmental biology, human physiology, and preclinical drug toxicity test.
Compared to the ratio of the physiological model types to human organ types, organoid-based disease model types were much fewer than the number of WHO registered human diseases. The top three targeted organ types for the disease models were brain, intestine, and lung (Figure 2B). The top reconstructed disease type was cancer, which took up more than three-fourths of patents for organoid-based disease models (Figure 2C). The malignant tumor is a devastating disease responsible for more than 19 million cases of occurrence solely in 2020 (Sung et al., 2021). Tumor heterogeneity is the leading failure cause of clinical routine cancer treatment. Therefore, it is urgent to establish a high-fidelity cancer model to conduct cancer mechanistic studies and anti-tumor drug screening. Organoid cancer models can effectively maintain the tumor phenotype and heterogeneity, and emulate the pathophysiologic process of tumor genesis and metastasis, in the original patients with cancer (Fan et al., 2019). According to the patent bibliometric analysis, nearly 20 types of organ-specific tumor organoid models have been successfully constructed. Construction methods for intestinal cancer (9.76%) and lung cancer (9.15%) organoids ranked first and second in the patent applications related to tumor organoid models, respectively (Figure 2D), which can be consistent with the high occurrence rate or mortality rate of the corresponding tumors published by IARC (Sung et al., 2021). Tumor organoids potentially promote cancer mechanistic studies, personalized chemo-/targeted-/immunotherapies, cancer drug discovery, and prognostic marker discovery. In addition to tumor organoid models, the exemplified disease types contain Alzheimer’s disease, enteritis, and pulmonary fibrosis. Overall, there is still a lot of innovation space to develop disease-specific organoid models except for tumors.
In addition to organoid construction technologies, drug screening was the major focus in the category of downstream application. It took up more than half of the total patents in this category (Figure 3C), which implied that organoid technologies have unique advantages in drug screening and potentially facilitate drug evaluation in terms of safety and efficacy in the pharmaceutical industry. Morphological and biological heterogeneity among individual organoids poses critical challenges in precise detection and quantitative analysis of organoids, which hampers widespread downstream applications of organoid technologies. Notably, a recent trend in synergistic engineering of organoid culture with microfabricated devices (publication number: WO2019010587A1; EP3907275A1; CA3000718A1) provides better microenvironmental control over individual organoid growth and reduces inter-organoid heterogeneity. Additionally, AI recognition technologies (publication number: WO2019035766A1; CN110283724A) and biosensors (publication number: IN8576CHENP2010A; CN111961589A; CN211170711U) have been startingly used in in situ detection and analysis of organoids, which facilitates organoid-based quantitative bioassays for downstream applications. Overall, many efforts are still required to develop better organoid characterization technologies and to mature organoid-based downstream applications.
In sum, organoid technologies have entered the rapid growth stage and have already facilitated a wide range of basic research in the biomedical field after more than one decade’s development. We expect that organoid technologies will be acceleratively maturated and commercialized for broad downstream applications.
Limitations of the study
We admit that our study also has its limitation. The incoPat database employs artificial intelligence to translate non-English patent documents into English, thus removing language barriers in investigating global patent applications. However, as the underlying algorithm of the AI in the incoPat is not clear, the natural language processing may not be accurate for the keyword “organoid∗.” Thus, some truly organoid patents from non-English countries may be missed.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| incoPat raw database | This paper; Mendeley Data | https://doi.org/10.17632/fmb43j9pmj.1 |
| incoPat meta database | This paper; Mendeley Data | https://doi.org/10.17632/fmb43j9pmj.1 |
| Software and algorithms | ||
| EXCEL 2019 | Microsoft | https://www.microsoft.com/zh-cn/ |
| Origin 2021 | OriginLab | https://www.originlab.com/ |
| Adobe Illustrator 2020 | Adobe Inc. | https://www.adobe.com/products/illustrator.html |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Pu Chen.
Materials availability
The incoPat raw database and incoPat meta database in this study were deposited on Mendeley Data: https://doi.org/10.17632/fmb43j9pmj.1.
Experimental model and subject details
Our study does not use typical experimental models in the life sciences.
Method details
Data sources
The patents used in this study were obtained from the incoPat, which is a global patent database containing complete patent applications from 120 countries and regions. The incoPat database employs natural language processing technology to compile other languages into the English version. Furthermore, the incoPat database can identify the patents applied in the different countries but belonging to the same patent family. Thus it avoids patent duplication across the different countries and is more scientific for bibliometric analysis. Additionally, the incoPat database supports more professional search portals and the raw data export, which facilitate the subsequent manual screen of false-positive results.
Data retrieval and collection
“Organoid” is a very specific and widely-recognized terminology to describe stem cell-derived 3D cell aggregates in the fields of cell biology and development (Lancaster and Knoblich, 2014b). Although a few other words were also used at the very beginning of this field, which was used at a very low frequency. Zhen Wang et al. (Wang et al., 2020) ranked the top ten countries that contributed to organoid research according to total publications, the sum of times cited, and average citations per item of publications. According to the languages of the ranked countries, the search query for organoid technology patents was conducted using “(TIABC=(类器官 OR 오가노이드 OR オルガノイド OR organoïde∗ OR organoid∗)) AND (AD = [20100101 TO 20201231])”, which covered all the conditions that the keywords appears in the document title, abstract, or claim. The period of the search was set AD = [2010-01-01 to 2020-12-31] to get 3,267 patents. These 3,267 items were further refined into 1,140 items, which formed the raw database by merging patents under the same patent family via the internal function of the incoPat database.
We screened the 1,140 patent families based on the inclusion criteria. The inclusion criteria contain: (i) organoids are the main and essential research content of the patent; (ii) organoid technologies are the main and essential research technologies of the patent. Ultimately, a meta database with a total number of 672 patents was generated after the manual screening.
Quantification and statistical analysis
The information of the 672 patents, including the title, abstract, applicant, application date, publication number, publication country, and International Patent Classification (IPC), was exported into EXCEL 2019 software for the bibliometric analysis. To investigate the status and trends of organoid technologies, the claims of these patents were analyzed and classified from the aspects of organoid types, models, construction technology, and downstream application.
Bibliometric analysis of global organoid technology patents
We performed the bibliometric analysis of the organoid patents from the aspects of the number of annual applications, disclosed countries, patent applicant types, and international patent classification. We determined the annual number of patent applications based on the date of application. For disclosed countries, we examined country codes of individual patents using both the incoPat and the SooPAT database. We classified the patent applicants according to organizational attributes into six types: university/college, company, research institute, hospital, individual, and others. The classification criteria are shown in Table S11. We summed the number of patent applications for each applicant type, separately. Each patent was assigned with an IPC by the patent office, and we retrieved the IPC from the incoPat database and conducted statistical analysis.
The AAGR represents the average annual growth rate of patent applications in a given number of years.
| Equation 1 |
where B is the number of patent applications in the last year of a certain period, A is the number of patent applications in the first year, and n is the number of years −1.
Technical analysis of organoid models
We classified the organoid models into two categories: physiological model and disease model (contain tumor model and the other disease model) for statistical analysis. Physiological models are microscopic 3D organoids that can reproduce some physiological characteristics and spatial structure of normal human organs. Disease models can simulate some important disease characteristics and phenotypes in human disease onset and progression. Notably, physiological models were featured with typical physiological characteristics, while disease models were featured with typical pathological disease characteristics. Sometimes, a single patent contains multiple organoid models. We statistically analysed the number of patent applications for each category of organoid models, separately.
Technical innovation types of organoid technologies
We classified technical innovation in the claims of the organoid patents into three categories, including organoid construction technology, characterization technology, and downstream application. For statistical analysis, we further categorized organoid construction technology into method innovation and material innovation according to the core content of the claims in the patents. The innovation in the organoid characterization technology can be further divided into method innovation and material innovation, and the innovation in the organoid-based downstream application can be further divided into six aspects, including drug screening, disease modeling, biological therapy, cell manufacturing, precision medicine, regenerative medicine, and others. Notably, some patents contain multiple technical innovation types. We separately summed the patents for each technical innovation type of organoid technology.
Patent value evaluation
The technology stability, technology superiority, and IPVI were selected and analyzed in the patent information.
The patent data does not have the statistical parameters of significance (i.e., the exact value of n, SEM, SD, etc.) and is not suitable for significance analysis. The statistical data of charts are completed by EXCEL 2019, graphic production by Origin 2021, and group drawing by Adobe Illustrator 2020.
Additional resources
Description: the incoPat database is the resource database in this study (https://www.incopat.com).
Acknowledgments
We thank Wen Zhao, Haowen Qiao, and Han Fan for their help in writing the article. This research was funded by the National Key Research and Development Program of China, grant number 2018YFA0109000, and Hubei Key Laboratory of Embryonic Stem Cell Research (Hubei University of Medicine), grant number 2021ESOF003. We also thank for Innovations in stem cell and organoids project (ISCO).
Author contributions
Conceptualization, P.C., L.Z.; methodology, P.C., L.Z.; validation, L.Z.; formal analysis, L.Z., Y.F., X.H., T.C., X.X., F.X.; investigation, L.Z.; resources, L.Z., Y.G.; data curation, P.C.; writing—original draft preparation, L.Z., P.C.; writing—review and editing, L.Z., Y.G. and P.C.; visualization, L.Z.; supervision, P.C.; project administration, P.C.; funding acquisition, P.C. All authors have read and agreed to the published version of the article.
Declaration of interests
The authors declare no conflict of interest.
Inclusion and diversity
While citing references scientifically relevant for this work, we actively worked to promote gender balance in our reference list. The author list of this paper includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Published: August 19, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104728.
Supplemental information
Data and code availability
-
•
Compiled data (incoPat raw data and incoPat meta data) reported in this paper have been deposited at Mendeley Data: https://doi.org/10.17632/fmb43j9pmj.1 and are publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Bian S., Repic M., Guo Z., Kavirayani A., Burkard T., Bagley J.A., Krauditsch C., Knoblich J.A. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods. 2018;15:631–639. doi: 10.1038/s41592-018-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boj S.F., Hwang C.I., Baker L.A., Chio I.I.C., Engle D.D., Corbo V., Jager M., Ponz-Sarvise M., Tiriac H., Spector M.S., et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye B.R., Hill D.R., Ferguson M.A., Tsai Y.H., Nagy M.S., Dyal R., Wells J.M., Mayhew C.N., Nattiv R., Klein O.D., et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4:e05098. doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger D., Tsatsaronis G., Bundschus M., Wieneke U., Schroeder M. Automated patent categorization and guided patent search using IPC as inspired by MeSH and PubMed. J. Biomed. Semantics. 2013;4:S3. doi: 10.1186/2041-1480-4-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Demirci U., Chen P. Emerging organoid models: leaping forward in cancer research. J. Hematol. Oncol. 2019;12:142. doi: 10.1186/s13045-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., Ravelli R.B.G., Paul van Schayck J., Mykytyn A.Z., Duimel H.Q., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Qiao H., Guo M., Shang J., Zhao W., Wang Z., Liu N., Li B., Zhou Y., Wu Y., Chen P. Herpes simplex virus type 1 infection leads to neurodevelopmental disorder-associated neuropathological changes. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramli M.N.B., Lim Y.S., Koe C.T., Demircioglu D., Tng W., Gonzales K.A.U., Tan C.P., Szczerbinska I., Liang H., Soe E.L., et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology. 2020;159:1471–1486.e12. doi: 10.1053/j.gastro.2020.06.010. [DOI] [PubMed] [Google Scholar]
- Rossi G., Manfrin A., Lutolf M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018;19:671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schutgens F., Clevers H. Human organoids: tools for understanding biology and treating diseases. Annu. Rev. Pathol. 2020;15:211–234. doi: 10.1146/annurev-pathmechdis-012419-032611. [DOI] [PubMed] [Google Scholar]
- Schwank G., Koo B.K., Sasselli V., Dekkers J.F., Heo I., Demircan T., Sasaki N., Boymans S., Cuppen E., van der Ent C.K., et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Shi R., Radulovich N., Ng C., Liu N., Notsuda H., Cabanero M., Martins-Filho S.N., Raghavan V., Li Q., Mer A.S., et al. Organoid cultures as preclinical models of non-small cell lung cancer. Clin. Cancer Res. 2020;26:1162–1174. doi: 10.1158/1078-0432.CCR-19-1376. [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Taguchi A., Kaku Y., Ohmori T., Sharmin S., Ogawa M., Sasaki H., Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.R., Ueno Y., Zheng Y.W., Koike N., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- Wang Z., He X., Qiao H., Chen P. Global trends of organoid and organ-on-a-chip in the past decade: a bibliometric and comparative study. Tissue Eng. Part A. 2020;26:656–671. doi: 10.1089/ten.TEA.2019.0251. [DOI] [PubMed] [Google Scholar]
- Yan H.H.N., Siu H.C., Law S., Ho S.L., Yue S.S.K., Tsui W.Y., Chan D., Chan A.S., Ma S., Lam K.O., et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882–897.e11. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Yuki K., Cheng N., Nakano M., Kuo C.J. Organoid models of tumor immunology. Trends Immunol. 2020;41:652–664. doi: 10.1016/j.it.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Compiled data (incoPat raw data and incoPat meta data) reported in this paper have been deposited at Mendeley Data: https://doi.org/10.17632/fmb43j9pmj.1 and are publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.