Abstract
Whole-cell suspensions of Cylindrocarpon didymum were observed to transform 2,2′-bimorphine to the compounds 10-α-S-monohydroxy-2,2′-bimorphine and 10,10′-α,α′-S,S′-dihydroxy-2,2′-bimorphine. Mass spectrometry and 1H nuclear magnetic resonance spectroscopy confirmed the identities of these new morphine alkaloids.
The morphine alkaloids are the major alkaloid components of the opium poppy, Papaver somniferum, and these compounds still provide some of the most potent analgesic compounds in clinical use. Many synthetic derivatives of morphine have been produced, and subtle changes in functionality can significantly alter the pharmacological activity of these compounds. Microbial transformations of morphine alkaloids have been investigated as a means of producing opiate drugs and pivotal intermediates that are difficult to produce chemically. These transformations have mainly comprised either N-dealkylation, reduction of the C-7–C-8 vinylic group, oxidation or reduction of the C-6 oxygroup, or hydroxylation of the C-14 position of the morphinan skeleton (10, 11, 14, 15, 21, 22). Several of the morphine alkaloid-transforming enzymes have been purified and characterized (2, 3, 7, 16). Furthermore, the genes encoding a number of these enzymes have been cloned and expressed in Escherichia coli, which has facilitated the engineering of recombinant cells to produce biologically semisynthetic opiate drugs (1, 8, 9, 20). Oxyfunctionalization of morphine and related compounds is of interest as a means of providing both new synthons for the production of existing semisynthetic drugs and new intermediates that have potential as platforms for combinatorial synthesis of new opiate drugs. We recently demonstrated that the fungus Cylindrocarpon didymum is capable of converting morphine to pseudomorphine (2,2′-bimorphine) (19). In this paper we describe the biological conversion of bimorphine to two novel compounds, 10-α-S-monohydroxy-2,2′-bimorphine and 10,10′-α,α′-S,S′-dihydroxy-2,2′-bimorphine.
Biotransformation of morphine and 2,2′-bimorphine.
Mycelia of C. didymum, obtained from the Institute of Biotechnology's culture collection, were grown in mineral medium (pH 7.0) containing (per liter) 10.0 g of KH2PO4, 5.0 g of (NH4)2SO4, and 0.5 g of MgSO4 and supplemented with 10.0 g of yeast extract per liter and trace elements as described by Rosenberger and Elsdon (18) (YL medium). Cultures were incubated in Erienmeyer flasks (2 liters for 400 ml of medium or 250 ml for 40 ml of medium) at 30°C for 48 h with rotary shaking at 180 rpm. Mycelia were harvested by filtration through sterile Whatman no. 1 filter paper in vacuo, washed with 100 ml of sterile mineral medium, and used immediately for whole-cell incubation.
Initially, morphine was transformed with washed mycelia resuspended in 40 ml of mineral medium (typically 12.5 g [wet weight]/liter) supplemented with 10 mM morphine. To monitor the transformation, samples (0.2 ml) were abstracted at regular intervals and diluted five-fold in 50 mM phosphoric acid (pH 3.5) to dissolve any precipitated metabolites. Mycelia were removed by centrifugation at 14,000 × g, and the samples were analyzed by high-performance liquid chromatography (HPLC) as described previously (19). The 2,2′-bimorphine that was produced was not transformed further under these conditions (that is, in a neutral pH medium that contained untransformed morphine).
Subsequently, the effect of pH on the transformation of 2,2′-bimorphine was tested. Whole-cell preparations (400 ml) of mycelia with morphine were used to generate 2,2′-bimorphine. These preparations were harvested after 48 h, and the 2,2′-bimorphine precipitates were collected along with the mycelia. Samples of the harvested mycelia and 2,2′-bimorphine (2.5 mM) were then incubated in 40 ml of mineral medium adjusted to pH 4.0, 6.0, or 8.0. Samples were removed and assayed for 2,2′-bimorphine by HPLC. After 150 h, the 2,2′-bimorphine had disappeared from the pH 4.0 preparation, and the amount of 2,2′-bimorphine was reduced by one-half in the pH 6.0 and 8.0 preparations.
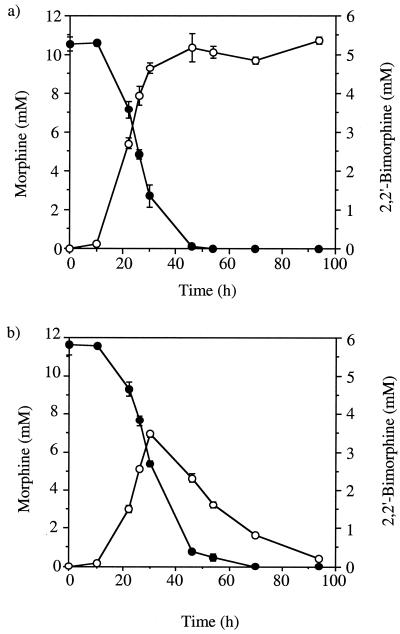
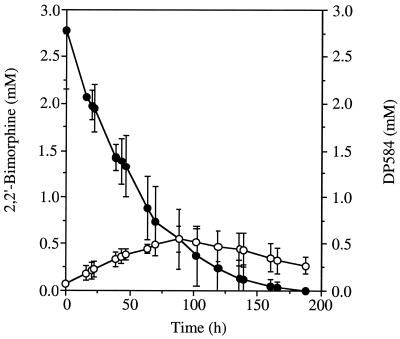
The effect of pH on the transformation of morphine by mycelia was also tested. The morphine transformation at pH 4.0 proved to be similar to that observed previously at pH 7.0, with similar morphine depletion rates and initial 2,2′-bimorphine accumulation rates. However, whereas at pH 7.0 accumulation of 2,2′-bimorphine continued with no sign of further transformation, after incubation for 30 h at pH 4.0, the 2,2′-bimorphine concentration was observed to decrease (Fig. 1). The use of an acidic medium was clearly necessary for further transformation of 2,2′-bimorphine. Consequently, all subsequent whole-cell incubations were carried out at pH 4.0 in mineral medium buffered with 20 mM 2,2-dimethyl succinic acid (S medium). When cyclohexamide (0.5 mg/ml) was added to whole-cell preparations containing mycelia that had not been challenged previously with morphine, no transformation of morphine or 2,2′-bimorphine was observed. Including cyclohexamide in whole-cell preparations with mycelia that had been previously challenged with morphine did not prevent transformation of either compound. This indicated that the activities responsible for transforming both morphine and 2,2′-bimorphine were induced by morphine. HPLC, thin-layer chromatography (TLC), and spectrophotometric analyses indicated that two significant metabolites were produced during whole-cell incubation with 2,2′-bimorphine at pH 4.0. One of these metabolites (DP584) appeared in culture media prior to the other (DP600), and both were eventually depleted during extended incubation. The maximum levels of DP584 observed were significantly higher than the levels of DP600. The levels of DP600 observed proved to be insufficient for effective quantitation, whereas the DP584 levels could be monitored by HPLC (Fig. 2). Although the conversion of morphine to 2,2′-bimorphine is stoichiometric (19), accumulation of DP584 and accumulation of DP600 appeared to be transient. This indicates that these oxidation products are themselves transformed prior to depletion of 2,2′-bimorphine and that although no other metabolites of 2,2′-bimorphine were detected, other transformation activities may occur.
FIG. 1.
Transformation of morphine and 2,2′-bimorphine by C. didymum at pH 7.0 and 4.0. Whole-cell incubation with C. didymum mycelia in mineral media was carried out with morphine (10 mM) at pH 7.0 (a) and pH 4.0 (b). Samples were assayed in triplicate for morphine concentration (●) and 2,2′-bimorphine concentration (○) by HPLC. Error bars indicate ±1 standard deviation.
FIG. 2.
Transformation 2,2′-bimorphine and transient accumulation of DP584. C. didymum mycelia were challenged with morphine in mineral medium buffered at pH 4.0 with 2,2-dimethyl succinic acid (20 mM) for 24 h. The mycelia were washed and used for whole-cell incubation in mineral media with 2,2′-bimorphine (2.5 mM). Samples were assayed in triplicate for 2,2′-bimorphine concentration (●) and DP584 concentration (○) by HPLC. Error bars indicate ±1 standard deviation.
Identification of 10-α-S-monohydroxy-2,2′-bimorphine and 10,10′-α,α′-S,S′-dihydroxy-2,2′-bimorphine.
Whole-cell incubation mixtures after 100 h were acidified to pH 3.5 with 1.0 M HCl in order to dissolve any insoluble metabolites. Fungal mycelia were then removed by filtration through Whatman no. 1 filter paper in vacuo. The pH values of the supernatants were raised to neutral with 1.0 M NaOH, which resulted in reprecipitation of insoluble metabolites. These metabolites were collected by centrifugation at 6,000 × g for 10 min at 4°C, dissolved in ammonia (specific gravity, 0.88; typically at a concentration of 50 mg/ml), and purified by preparative TLC. For preparative TLC we used silica gel glass-backed plates (20 by 20 cm; silica gel thickness, 250 μm) treated to fluoresce (K6F Silica Gel 60 A; Whatman Laboratory Division, Clifton, N.J.) and a chloroform-methanol-ammonia (specific gravity, 0.88) (6:3:1, by volume) solvent system. The relevant bands were visualized by fluorescence quenching at 254 nm and removed from the plates. DP584 and DP600 were eluted from the silica with ammonia (specific gravity, 0.88; 3 to 4 ml), and the silica was removed by centrifugation. Samples were freeze-dried after the volume of solvent was reduced to 1 ml under a stream of nitrogen.
Positive fast atom bombardment mass spectra of the samples were obtained with a model MF890 mass spectrometer. The spectra of DP584 and DP600 showed molecular ions of m/z 585.26 and m/z 601.25, respectively. This indicated that DP584 and DP600 were oxidation products of 2,2′-bimorphine, with DP584 containing one additional oxygen atom compared to 2,2′-bimorphine and DP600 containing two additional oxygen atoms.
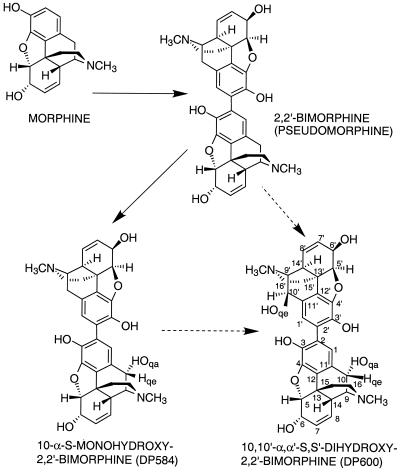
1H nuclear magnetic resonance spectra of the metabolites DP564 and DP600 were obtained by using a Brucker AM-400 spectrometer, D-6 dimethyl sulfoxide solvent, and tetramethylsilane as an internal standard. The following signals were obtained for DP584: H-6.64 (H, d, 1-H); 6.32 (H, d, 1′-H); 5.56 (H, m, J 10.0, 7′-H); 5.46 (H, m, J 10.0, 7-H); 5.41 (H, m, J 10.0, 8-H); 5.24 (H, m, J 10.0, 8′-H); 4.71 (H, broad 6-OH); 4.68 (H, dd, J 4.8, 5′-H); 4.60 (2H, 10qe-H & 5-H); 4.07 (H, dd, J 5.25, 2.74, 6-H); 4.05 (H, dd, J 5.25, 2.74, 6′-H); 3.24 (H, dd, J 5.94, 3.2, 9′-H); 3.11 (H, d, J 2.74, 9-H); 2.90 (H, d, J 18.27, 10qe′-H); 2.52 (H, m, J 2.51 14′-H); 2.48 (2H, m, H-16eq and H-16eq′ overlapping); 2.44 (3H, s, N-Me′); 2.42 (1H, m, 14-H); 2.35 (H, m, H-16ax′); 2.31 (3H, s, N-Me); 2.22 (2H, m, 10qa′ and 16ax overlapping); 1.97 (H, m, H-15eq′); 1.92 (H, m, H-15eq); 1.66 (H, m, H-15ax′); 1.64 (H, m, H-15ax). The following signals were obtained for DP600: δ H-6.73 (2H, s, 1-H & 1′-H); 5.49 (2H, m, J 10.0, 7-H & 7′-H); 5.46 (2H, m, J 10.0, 8-H & 8′-H); 4.71 (2H, dd, J 5.96, 1.38, 5-H & 5′-H); 4.65 (2H, s, 10qe-H & 10qe′-H); 4.10 (2H, m, 6-H & 6′-H); 3.18 (2H, d, 2.98, 9-H & 9′-H); 2.49 (10 H, N-Me & N-Me′, H-14 & H-14′, H-16eq, and H-16eq′ overlapping); 2.27 (2H, ddd, J 24.27, 12.14, 3.44, H-16ax & H-16ax′); 1.99 (2H, ddd, J 24.95, 12.59, 5.27, H-15eq & H-15eq′); 1.72 (2H, m, J 14.19, 1.37). For comparison, the signals for 2,2′-bimorphine are H 6.31 (2H, s, 1-H & 1′-H); 5.58 (2H, dd, J 9.6 and 2.5, 7-H & 7′-H); 5.26 (2H, d, J 9.6, 8-H & 8′-H); 4.70 (2H, d, J 5.7, 5-H & 5′-H); 4.10 (2H, dd, J 5.7 and 2.5, 6-H & 6′-H); 3.29 (2H, dd, J 6.2 and 2.6, 9-H & 9′-H); 2.91 (2H, d, J 18.6, 10-H & 10′-H); 2.57 (2H, d, J 2.6, 14-H & 14′-H); 2.50 (2H, dd, J 12.5 and 3.5, 16-H & 16′-H); 2.32 (6H, s, N-Me & N-Me′); 2.28 (2H, d, J 12.5, 16-H & 16′-H); 2.23 (2H, dd, J 18.6 and 6.2, 10-H & 10′-H); 1.99 (2H, dd, J 11.4 and 3.5, 15-H & 15′-H); 1.68 (2H, d, J 11.4, 15-H & 15′-H) (19). Structures and numbers are shown in Fig. 3. The data for DP600, comprising a single set of signals that integrate doubly, clearly indicate that the compound is a dimer of equivalent monomers. The data for DP584 show that there are two sets of signals with values integrating singly, which are equivalent to a dimer comprising one monomer identical to the monomers forming 2,2′-bimorphine and one monomer identical to the monomers forming DP600. The C-10(10′) quasi-equatorial signal obtained for the nonsubstituted monomers of 2,2′-bimorphine, occurring at 2.90 ppm, was absent from DP600 and occurred singly for DP584. The spectrum for DP600 has the appearance of a double proton signal at 4.65 ppm, and the spectrum for DP584 has the appearance of a single proton signal at 4.68 ppm; both spectra are consistent with hydroxymethine protons. The combination of these spectra and the mass spectrometric data identified DP584 and DP600 as 10-α-S-monohydroxy-2,2′-bimorphine and 10,10′-α,α′-S,S′-dihydroxy-2,2′-bimorphine, respectively.
FIG. 3.
Transformation of morphine by C. didymum.
Hydroxy functionalization of codeine, morphine, and bimorphine.
Although C-14 hydroxylation has been described relatively frequently, reports of hydroxylation at the C-10 position of the morphinan structure, under any circumstances, are scarce. Consequently, the effects on the properties of morphine that such a change has have not been determined. 10-Hydroxymorphine is known to be a product of morphine oxidation and has been identified in mixtures of morphine oxidation products (17) and as an active impurity in morphine (6). An adduct of morphine, 10-α-S-glutathionyl morphine, has been reported in rat hepatic microsomal studies. It was suggested that the reaction of morphine with cytochrome P-450 generated a reactive intermediate, 10-α-S-hydroxymorphine, which rapidly and spontaneously reacted with the thiol groups of proteins and amino acids. The postulated 10-α-S-hydroxymorphine intermediate was not isolated and characterized (4, 5, 12). In vivo studies with human patients located a morphine metabolite thought to be 10-α-S-hydroxymorphine but did not locate any thiolated products (13). The possibility that 2,2′-bimorphine, DP584, and DP600 could form thiol adducts with glutathione in acidic media was tested by using conditions described previously (4, 5, 12), but no such adducts were detected. To the best of our knowledge, hydroxylation of 2,2′-bimorphine has not been described previously.
The potential applications of the novel compounds described here, whether as pharmaceuticals themselves or as intermediates in the synthesis of other novel alkaloids, remain to be investigated.
Acknowledgments
This work was supported by the Biotechnology and Biological Science Research Council.
We acknowledge the comments and advice of Adam Walker with respect to nuclear magnetic resonance spectrometry.
REFERENCES
- 1.Bruce N C, Caswell D A, French C E, Hailes A M, Long M T, Willey D L. Towards engineering pathways for the synthesis of analgesics and antitussives. Ann N Y Acad Sci. 1994;721:85–99. doi: 10.1111/j.1749-6632.1994.tb47380.x. [DOI] [PubMed] [Google Scholar]
- 2.Bruce N C, Wilmot C J, Jordan K N, Gray Stephens L D, Lowe C R. Microbial degradation of the morphine alkaloids. Purification and characterization of morphine dehydrogenase from Pseudomonas putida M10. Biochem J. 1991;274:875–880. doi: 10.1042/bj2740875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce N C, Wilmot C J, Jordan K N, Trebilcock A E, Gray Stephens L D, Lowe C R. Microbial degradation of the morphine alkaloids: identification of morphinone as an intermediate in the metabolism of morphine by Pseudomonas putida M10. Arch Microbiol. 1990;154:465–470. doi: 10.1007/BF00245229. [DOI] [PubMed] [Google Scholar]
- 4.Correia M A, Krowech G, Caldera-Munoz P, Yee S L, Straub K, Castagnoli N. Morphine metabolism revisited. 2. Isolation and chemical characterization of a glutathionylmorphine adduct from rat-liver microsomal preparations. Chem-Biol Interact. 1984;51:13–24. doi: 10.1016/0009-2797(84)90016-4. [DOI] [PubMed] [Google Scholar]
- 5.Correia M A, Wong J S, Soliven E. Morphine metabolism revisited. 1. Metabolic-activation of morphine to a reactive species in rats. Chem-Biol Interact. 1984;49:255–268. doi: 10.1016/0009-2797(84)90101-7. [DOI] [PubMed] [Google Scholar]
- 6.Farsam H, Eiger S, Lameh J, Rezvani A, Gibson B W, Sadee W. Morphine impurity with opioid activity is identified as 10α-hydroxymorphine. Pharm Res. 1990;7:1205–1207. doi: 10.1023/a:1015957031449. [DOI] [PubMed] [Google Scholar]
- 7.French C E, Bruce N C. Purification and characterization of morphinone reductase from Pseudomonas putida M10. Biochem J. 1994;301:97–103. doi: 10.1042/bj3010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French C E, Hailes A M, Rathbone D A, Bruce N C. Morphinone reductase. Characterization, cloning and application to biocatalytic hydromorphone production. Ann N Y Acad Sci. 1996;799:97–101. doi: 10.1111/j.1749-6632.1996.tb33185.x. [DOI] [PubMed] [Google Scholar]
- 9.Hailes A M, Bruce N C. Biological synthesis of the analgesic hydromorphone, an intermediate in the metabolism of morphine, by Pseudomonas putida M10. Appl Environ Microbiol. 1993;59:2166–2170. doi: 10.1128/aem.59.7.2166-2170.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iizuka K, Okuda S, Aida K, Asai T, Tsuda K, Yamada M, Seki I. Mikrobioloogische Verwandlung von Thebainein das 14-Hydroxycodeinon mittels einiger Pilze. Chem Pharm Bull (Tokyo) 1960;8:1056–1057. [Google Scholar]
- 11.Iizuka K, Yamada M, Suzuki J, Seki I, Aida K, Okuda S, Asai T, Tsuda K. Untersuchungen auf dem Gebiet der mikrobiologischen Umsetzung. XVI. Umwandlung von Alkaloiden in der Morphin-Reihe durch Trametes sanguinea (1) Chem Pharm Bull (Tokyo) 1962;10:67–70. doi: 10.1248/cpb.10.67. [DOI] [PubMed] [Google Scholar]
- 12.Krowech G, Caldera-Munoz P S, Straub K, Castagnoli N, Correia M A. Morphine metabolism revisited. 3. Confirmation of a novel metabolic pathway. Chem-Biol Interact. 1986;58:29–40. doi: 10.1016/s0009-2797(86)80084-9. [DOI] [PubMed] [Google Scholar]
- 13.Levi V, Scott J C, White P F, Sadee W. Improved radioreceptor assay of opiate narcotics in human serum: application to fentanyl and morphine metabolism. Pharm Res. 1987;4:46–49. doi: 10.1023/a:1016429927467. [DOI] [PubMed] [Google Scholar]
- 14.Liras P, Kasparian S S, Umbrett W W. Enzymatic transformation of morphine by hydroxysteroid dehydrogenase from Pseudomonas testosteroni. Appl Microbiol. 1975;30:650–656. doi: 10.1128/am.30.4.650-656.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liras P, Umbrett W W. Transformation of morphine by resting cells and cell free systems of Arthrobacter sp. Appl Microbiol. 1975;30:262–266. doi: 10.1128/am.30.2.262-266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long M T, Hailes A M, Kirby G W, Bruce N C. Transformations of morphine alkaloids by Pseudomonas putida M10. Appl Environ Microbiol. 1995;61:3645–3649. doi: 10.1128/aem.61.10.3645-3649.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proksa B. Separation of morphine and its oxidation products by capillary zone electrophoresis. J Pharm Biomed Anal. 1999;20:179–183. doi: 10.1016/s0731-7085(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberger R F, Elsdon S R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960;22:716–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- 19.Stabler P J, Bruce N C. Oxidation of morphine to 2,2′-bimorphine by Cylindrocarpon didymum. Appl Environ Microbiol. 1998;64:4106–4108. doi: 10.1128/aem.64.10.4106-4108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willey D L, Caswell D A, Lowe C R, Bruce N C. Nucleotide sequence and over-expression of morphine dehydrogenase, a plasmid-encoded gene in Pseudomonas putida M10. Biochem J. 1993;290:539–544. doi: 10.1042/bj2900539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada M, Iisuka K, Okuda S, Asai T, Tsuda K. Untersuchungen auf dem Gebiet der mikrobiologischen Umsetzung. XVII. Umwandlung von Alkaloiden in der Morphin-Reihe durch Trametes sanguinea (2) Chem Pharm Bull (Tokyo) 1962;10:981–984. [Google Scholar]
- 22.Yamada M, Iisuka K, Okuda S, Asai T, Tsuda K. Untersuchungen auf dem Gebiet der mikrobiologischen Umsetzung. XVIII. Umwandlung von Alkaloiden in der Morphin-Reihe durch Trametes sanguinea (3) Chem. Pharm Bull (Tokyo) 1963;11:206–208. [Google Scholar]