Abstract
Floret opening and closure are critical for rice to complete reproductive development. To further understand the molecular mechanism of floret opening and closure in rice, RNA-seq was performed on the floret of Indica and Japonica rice in the state of 1-h before floret opening, at the opening and closure, respectively. Our results show that many differentially expressed genes are produced throughout the floret opening and closure of both Indica and Japonica rice. Differentially expressed genes shared between Indica and Japonica rice at floret opening were involved in seven metabolic pathways, including plant hormone signal transduction, MAPK signaling pathway-plant, starch and sucrose metabolism, alpha-Linolenic acid metabolism, plant–pathogen interaction, diterpenoid biosynthesis, glucuronate interconversions, phenylpropanoid biosynthesis, compared to 1 h before floret opening. In addition, the expression patterns of some genes, OsJAZ13, OsJAZ11 and OsCML1 which the above metabolic pathways, were different between Indica and Japonica rice. Compared to the floret opening, the differentially expressed genes at floret closure were mainly involved in the following three metabolic pathways: Circadian rhythm-plant, sesquiterpenoid and triterpenoid biosynthesis, starch and sucrose metabolism, and thiamine metabolism. This study provides insights into revealing the molecular mechanism of floret opening and closure in rice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03226-y.
Keywords: Indica and Japonica rice, Comparative transcriptome, Floret opening, Floret closure
Introduction
The floret opening and closure in rice, caused by water absorption and shrinkage of the lodicule, is a key process in the reproductive development of rice and an important factor in heterosis utilization in rice (Huang and Zeng 2021). Floret opening and closure is a complex biological process regulated by environmental factors, endogenous hormones and multiple genes. Fu et al. (2016) reported that the expression levels of genes related to carbon metabolism, cellular energy metabolism, phytohormone metabolism and signal transduction were significantly altered 1 h before floret opening in Indica rice. Ma et al. (2011) reported that raising the temperature promoted rice floret opening. Humidity usually interacts with temperature to influence rice floret opening, and the sensitivity of floret opening to temperature and humidity response differs between indica and japonica rice (Ma et al. 2001). High levels of CO2 weaken the stomatal conductance of leaves which causes an increase in spike temperature and thus promotes floret opening in rice (Kobayasi et al. 2019). Strong light radiation or rice transfer from light to dark environments promotes floret opening in rice (Ko Ba Yasi et al. 2010). Soluble sugars are the main osmoregulatory substances of the lodicule cells. Increased soluble sugar content increases the osmotic potential of the lodicule cells, promoting floret opening in rice (Wang 1991; Zeng et al. 2004) The endogenous hormone jasmonic acid (JA) plays an important role in regulating floret opening in rice. Expression levels of genes involved in JA biosynthesis and its signal transduction pathway were significantly up-regulated at floret opening and the content of endogenous JA was significantly increased (Huang et al. 2015; He et al. 2018b). OsJAR and OG1 are JA biosynthetic genes whose mutations cause floret opening dispersion in rice (Xiao et al. 2014; Li et al. 2017), Mutations in SH1, which is involved in JA signaling, cause the floret to fail close (Pan et al. 2019). Zeng et al. (1999) first reported that methyl jasmonate (MeJA) could promote floret opening in rice and that salicylic acid could inhibit the flowering-promoting effect of MeJA. ABA promoted the closure of rice floret regardless of the varieties (Huang et al. 2018). Rice floret closure is associated with programmed cell death of the lodicule cells. At 5–20 min after flowering, the plasma cells begin to enter a programmed death process in which free Ca2+ is deposited in the canopy, the nucleoplasm condenses, the nucleolus gradually fades and the cell matrix is gradually digested, leading to the death of the entire cell and the closure of the rice (Liu 2008). Next-generation sequencing (NGS) has the characteristics of high flux and wide coverage, which can provide strong technical support for analyzing the regulation mechanism of rice floret opening and closure (He et al. 2018a). Indica and japonica rice are two subspecies of cultivated rice in Asia with different flowering habits and different sensitivities to external environmental factors (Peng et al. 1994) and exogenous chemically regulated substances (Yan et al. 2014, 2015; Xia et al. 2019) in response to their flowering habits. Previous studies have mostly investigated the temporal changes in gene expression profiles before and at floret opening in Indica rice, thus failing to address the universality of the floret opening regulatory pathways in the Indica–Japonica subspecies and to reveal the regulatory pathways for floret closure. Therefore, it is critical to conduct a transcriptomic analysis of the entire process of floret opening and closure in both Indica and Japonica rice to investigate the similarities and differences in the pathways of floret opening and closure in rice subspecies, to lay the foundation for revealing the molecular mechanism of floret opening and closure regulation in rice and provide a theoretical basis for the differences in the flowering habits of Indica and Japonica subspecies. Herein, we applied the Illumina Novaseq™ 6000 high-throughput sequencing platform to compare the transcriptomic differences between before floret opening, at the opening and closure of Indica and Japonica rice, thus providing a theoretical basis for unraveling the molecular mechanism of rice floret opening and closure.
Materials and methods
Planting materials and sampling methods
Four Indica–Japonica rice varieties were used as test material, including two indica varieties, Hefengsimaio (PI1) and Kasalath (PI2), and two japonica varieties, Jingdao 104 (PJ1) and Jigeng 88 (PJ2). In 2020, the trial material was planted in Guiyang, located in southwest China at an altitude of 1,100 m with a humid subtropical climate. Each variety is planted in two rows of 10 plants per row with a field planting size of 30 cm × 16 cm. Furrow irrigation was adopted. The trial material, planted at Guiyang, was sown on 21 April and transplanted on 1 June. After transplanting, shallow irrigation is the mainstay. After the seedlings return to green, intermittent irrigation is adopted, with a shallow irrigation of 3–5 cm shallow irrigation. At the heading stage, florets that have flowered are removed the day before sampling. On the second day, the florets were sampled for transcriptome sequencing with scissors 1 h before floret opening, at the opening and closure. Samples were immediately placed in liquid nitrogen and stored in an ultra-low temperature refrigerator. Each sample was collected in three biological replicates. The sampling is listed in Table 1.
Table 1.
Sample list
| Sample | 1-h before-floret opening | Floret opening | Floret closure |
|---|---|---|---|
| Hefengsimaio | PI1-1 | PI1-2 | PI1-3 |
| Kasalath | PI2-1 | PI2-2 | PI2-3 |
| Jingdao104 | PJ1-1 | PJ1-2 | PJ1-3 |
| Jigeng88 | PJ2-1 | PJ2-2 | PJ2-3 |
Measurement of heading date, floret opening time, and floret closure time
The time when 50% of the rice panicles are heading is used as the heading date. The manual observation was conducted to evaluate the floret opening and closure time in rice. When the first floret on the rice panicle opens is taken as the time of initiation and when all the floret close is taken as the time of final closure.
RNA extraction library construction and sequencing
Total RNA was extracted using Trizol reagent (Thermo Fischer, 15596018) following the manufacturer’s procedure. The total RNA quantity and purity were analyzed by of Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Agilent, CA, USA, 5067–1511), and high-quality RNA samples with RIN number > 7.0 were used to construct the sequencing library. After total RNA was extracted, mRNA was purified from total RNA (5 μg) using Dynabeads Oligo (dT) (Thermo Fisher, CA, USA) with two rounds of purification. Following purification, the mRNA was fragmented into short fragments using divalent cations under elevated temperature (Magnesium RNA Fragmentation Module (NEB, cat. e6150, USA) under 94 °C 5–7 min). Then, the cleaved RNA fragments were reverse-transcribed to create the cDNA by SuperScript™ II Reverse Transcriptase (Invitrogen, cat.1896649, USA), which were next used to synthesize U-labeled second-stranded DNAs with E. coli DNA polymerase I (NEB, cat.m0209, USA), RNase H (NEB, cat.m0297, USA) and dUTP Solution (Thermo Fisher, cat.R0133, USA). Then, A-base was added to the blunt ends of each strand, preparing them for ligation to the indexed adapters. Each adapter contained a T-base overhang for ligating the adapter to the A-tailed fragmented DNA. Dual-index adapters were ligated to the fragments, and size selection was performed with AMPureXP beads. After the heat-labile UDG enzyme (NEB, cat.m0280, USA) treatment of the U-labeled second-stranded DNAs, the ligated products were amplified with PCR by the following conditions: initial denaturation at 95 °C for 3 min; 8 cycles of denaturation at 98 °C for 15 s, annealing at 60 °C for 15 s, and extension at 72 °C for 30 s; and then final extension at 72 °C for 5 min. The average insert size for the final cDNA libraries was 300 ± 50 bp. Finally, the 2 × 150 bp paired-end sequencing (PE150) was performed on an Illumina Novaseq™ 6000 (LC-Bio Technology CO., Ltd., Hangzhou, China) following the vendor’s recommended protocol.
Data quality control and filtering
Reads obtained from the sequencing machines include raw reads containing adapters or low-quality bases, affecting the following assembly and analysis. Thus, to get high-quality clean reads, reads were further filtered by Cutadapt. Remove reads with joints, remove reads with polyA, polyG, remove reads containing more than 5% N (N means base information cannot be determined), and remove low quality reads (the number of bases with quality Q less than 10 accounts for more than 20% of the entire reads) (https://cutadapt.readthedocs.io/en/stable/,version:cutadapt-1.9) (Martin 2011).
Alignment with reference genome and differentially expressed genes analysis
Cutadapt software (1.9) was used to remove the reads that contained adaptor contamination. After removing the low-quality bases and undetermined bases, the reads of all samples were aligned to the reference genome of Oryza sativa (JGI v7.0). using HISAT2. (https://daehwankimlab.github.io/hisat2/,version:hisat2-2.0.4) package. HISAT2 allows multiple alignments per reading (up to 20 by default) and a maximum of two mismatches when mapping the reads to the reference. HISAT2 builds a database of potential splice junctions and confirms these by comparing the previously unmapped reads against the database of putative junctions (Kim et al. 2015, 2019). The mapped reads of each sample were assembled using StringTie (1.3.4d) with default parameters.
Genes differential expression analysis was performed using DESeq2 software between two different groups (and by edgeR between two samples). The differentially expressed mRNAs were selected with fold change > 2 or fold change < 0.5 and p value < 0.05 by the R package. Differentially expressed genes were then subjected to enrichment analysis of GO functions and the KEGG pathway (Love et al. 2014). Firstly, all differentially expressed genes (DEGs) were mapped to GO terms in the Gene Ontology database (http://www.geneontology.org/) by perl script, gene numbers were calculated for every term, and significantly enriched GO terms in DEGs compared to the genome background were defined by hypergeometric test.
Quantitative real-time PCR analysis
A total of three genes, with different expression patterns between indica and japonica subspecies from floret opening to closure, were verified by real time RT-PCR. Primers were designed by Beacon Designer 7.9. GAPDH was used as a reference gene. Primer information for the candidate genes as well as the reference genes is given in Table S1. Real time RT-PCR was performed using qTOWER 2.0/2.2 Quantitative Real-Time PCR Thermal Cyclers (Germany). Reaction conditions were as follows: Step1: 95 °C for 3 Min, Step2: 95 °C for 10 s, Step3: 58 °C for 30 s + plate read, Step4: 39 cycles of 95 °C for 10 s, Step5: Melt curve analysis (60–95 °C, + 1 °C/cycle, holding time 4 s). Relative expression was calculated using 2−ΔΔCT method, the formula was as follows: ΔCT (test) = CT (target gene) – CT (GAPDH), ΔCT (calibrator) = CT (target, calibrator) – CT (GAPDH, calibrator), ΔΔCT = CT (test) – CT (calibrator), Expression values = 2–ΔΔCT.
Results
Investigation of heading date and flowering time
As listed in Table 2, the heading date of Hefengsimiao (PI1) and Kasalath (PI2) was on August 10 and August 12, 2020, respectively, while those of Jingdao104 (PJ1) and Jigeng88 (PJ2) were on August 9. Compared to Hefengsimiao (PI1) and Kasalath (PI2), Jingdao104 (PJ1) and Jigeng88 (PJ2) had later floret opening time and closure time and shorter duration from floret opening to closure. The Hefengsimiao (PI1) and Kasalath (PI2) florets open at 11:00 and close at 13:00, duration from floret opening to closure of 120 min while Jingdao 104 (PJ1) and Jigeng 88 (PJ2) open at 11:50 and close at 13:00 with a duration of 100 min.
Table 2.
Investigation of heading date and flowering time
| Cultivar Name | Subsp. Indica/Subsp. Japonica | Heading date (month-date-year) | Floret opening time (hour-minute) | Floret closure time (hour-minute) | Duration from floret opening to closure (minute) |
|---|---|---|---|---|---|
| Hefengsimaio (PI1) | Subsp. Indica | August 10, 2020 | 11:00 | 13:00 | 120 |
| Kasalath (PI2) | Subsp. Indica | August 12, 2020 | 11:00 | 13:00 | 120 |
| Jingdao104 (PJ1) | Subsp. Japonica | August 9, 2020 | 11:50 | 13:30 | 100 |
| Jigeng88 (PJ2) | Subsp. Japonica | August 9, 2020 | 11:50 | 13:30 | 100 |
Mapping reads to reference
A total of 36 cDNA libraries were constructed in the trial (Table 3). Each library has more than 36 million valid Reads. The percentage of reads with Q20% sequencing quality is above 99% and those with Q30% sequencing quality are above 96%. When the clean reads were compared to the entire reference genome sequences, we found that at least 88.48% of each sample could be mapped to the genome and more than 52.07% of the clean reads for each sample could be mapped to the genome uniquely. These results indicated that the sequencing quality was good for further analysis.
Table 3.
Summary of the sequencing data
| Sample | Raw data (Read) | Valid data (Read) | Valid ratio (reads) | Q20% | Q30% | Mapping ratio (%) | Uniquely mapping ratio (%) |
|---|---|---|---|---|---|---|---|
| PI1_1_1 | 46,672,000 | 43,777,082 | 93.80 | 99.90 | 98.16 | 91.19 | 71.60 |
| PI1_1_2 | 55,303,680 | 54,438,122 | 98.43 | 99.96 | 97.62 | 90.26 | 62.78 |
| PI1_1_3 | 52,495,452 | 51,658,394 | 98.41 | 99.95 | 97.69 | 90.33 | 61.21 |
| PI1_2_1 | 46,231,246 | 42,962,038 | 92.93 | 99.90 | 96.56 | 88.59 | 61.36 |
| PI1_2_2 | 54,278,584 | 53,514,012 | 98.59 | 99.95 | 97.73 | 91.96 | 65.06 |
| PI1_2_3 | 54,769,968 | 53,925,824 | 98.46 | 99.94 | 97.75 | 88.72 | 61.88 |
| PI1_3_1 | 51,166,820 | 49,164,564 | 96.09 | 99.93 | 98.45 | 92.64 | 72.30 |
| PI1_3_2 | 52,532,212 | 51,697,290 | 98.41 | 99.95 | 97.78 | 91.22 | 64.04 |
| PI1_3_3 | 45,986,064 | 45,183,688 | 98.26 | 99.96 | 97.83 | 90.88 | 61.80 |
| PI2_1_1 | 46,882,160 | 38,554,402 | 82.24 | 99.88 | 97.54 | 91.54 | 71.47 |
| PI2_1_2 | 45,124,654 | 44,266,558 | 98.10 | 99.95 | 97.91 | 92.45 | 65.21 |
| PI2_1_3 | 45,916,574 | 45,092,032 | 98.20 | 99.95 | 97.86 | 92.13 | 63.57 |
| PI2_2_1 | 54,499,658 | 40,992,052 | 75.22 | 99.88 | 97.65 | 92.20 | 71.92 |
| PI2_2_2 | 50,182,808 | 49,245,826 | 98.13 | 99.96 | 97.81 | 90.65 | 52.07 |
| PI2_2_3 | 47,783,578 | 46,862,666 | 98.07 | 99.95 | 97.95 | 91.80 | 59.52 |
| PI2_3_1 | 46,391,044 | 44,477,492 | 95.88 | 99.92 | 97.79 | 92.40 | 70.11 |
| PI2_3_2 | 54,313,700 | 53,199,290 | 97.95 | 99.95 | 97.69 | 90.69 | 53.20 |
| PI2_3_3 | 48,570,786 | 47,771,482 | 98.35 | 99.97 | 98.02 | 92.36 | 65.40 |
| PJ1_1_1 | 53,199,986 | 51,407,886 | 96.63 | 99.92 | 98.41 | 96.31 | 82.11 |
| PJ1_1_2 | 44,622,192 | 43,962,360 | 98.52 | 99.97 | 98.00 | 96.12 | 76.47 |
| PJ1_1_3 | 43,855,496 | 43,082,982 | 98.24 | 99.95 | 98.08 | 93.66 | 70.54 |
| PJ1_2_1 | 55,741,922 | 53,605,796 | 96.17 | 99.92 | 98.29 | 93.95 | 79.29 |
| PJ1_2_2 | 37,248,018 | 36,505,478 | 98.01 | 99.95 | 97.98 | 88.48 | 63.04 |
| PJ1_2_3 | 37,286,646 | 36,660,902 | 98.32 | 99.95 | 97.96 | 91.68 | 65.34 |
| PJ1_3_1 | 52,609,210 | 49,952,606 | 94.95 | 99.91 | 98.08 | 95.27 | 81.31 |
| PJ1_3_2 | 49,341,424 | 48,357,314 | 98.01 | 99.94 | 97.90 | 90.58 | 64.46 |
| PJ1_3_3 | 48,576,570 | 47,660,482 | 98.11 | 99.94 | 97.87 | 94.03 | 67.23 |
| PJ2_1_1 | 49,395,192 | 47,433,530 | 96.03 | 99.93 | 98.48 | 96.06 | 82.40 |
| PJ2_1_2 | 46,207,324 | 45,412,494 | 98.28 | 99.94 | 98.03 | 95.40 | 72.43 |
| PJ2_1_3 | 44,442,954 | 43,744,244 | 98.43 | 99.94 | 98.14 | 96.61 | 78.43 |
| PJ2_2_1 | 47,629,448 | 45,549,538 | 95.63 | 99.92 | 97.92 | 95.75 | 81.61 |
| PJ2_2_2 | 40,919,178 | 40,166,796 | 98.16 | 99.94 | 97.94 | 91.75 | 66.12 |
| PJ2_2_3 | 43,186,432 | 42,432,400 | 98.25 | 99.94 | 97.62 | 92.91 | 60.36 |
| PJ2_3_1 | 52,876,728 | 50,297,096 | 95.12 | 99.93 | 98.38 | 96.21 | 81.99 |
| PJ2_3_2 | 45,110,644 | 44,285,424 | 98.17 | 99.94 | 97.71 | 91.91 | 62.55 |
| PJ2_3_3 | 44,588,938 | 43,769,630 | 98.16 | 99.94 | 97.68 | 92.42 | 56.99 |
Correlation and PCA analysis
The correlation analysis (Fig. S1) and PCA (Fig. S2) results showed high correlation coefficients between the sample replicates and good clustering of the samples.
Analysis of Differently expressed genes
As shown in Fig. 1, the number of genes significantly up-regulated and down-regulated in Hefengsimiao at floret opening was 1874 and 2151 (PI1-1_VS_PI1-2), 1421 and 1704 in Ksalath (PI2-1_VS_PI2-2), 1239 and 912 in Jingdao104 (PJ1-1_VS_PJ1-2),1502 and 1017 in Jigeng88 (PJ2-1_VS_PJ2-2) respectively, compared to 1 h before floret opening. The number of genes significantly up-regulated and down-regulated in Hefengsimiao at floret opening was 308 and 192 (PI1-2_VS_PI1-3), 1266 and 880 in Ksalath (PI2-2_VS_PI2-3),1726 and 1611 in Jingdao104 (PJ1-2_VS_PJ1-3),878 and 1610 in Jigeng88 (PJ2-2_VS_PJ2-3) respectively, compared to floret opening.
Fig. 1.
Distribution of DEGs between every two samples
DEGS Functional classification using the KEGG pathway between 1 h before floret opening and floret opening
There were 482 significantly differentially expressed genes common to the four varieties at floret opening, compared to 1 h before floret opening, including 326 up-regulated genes (Fig. 2a) and 156 down-regulated expressed genes (Fig. 2b). The 326 up-regulated genes contained 93 genes with KEGG annotations and the 156 down-regulated genes contained 53 genes with KEGG annotations.
Fig. 2.
Venn diagram exhibiting the DEGs distribution in four comparison groups. A Venn diagram exhibiting the up-regulated DEGs in four comparison groups. B Venn diagram exhibiting the down-regulated DEGs in four comparison groups. PI1-1_VS_PI1-2, PI2-1_VS_PI2-2, PJ1-1_VS_PJ1-2, PJ2-1_VS_PJ2-2: the comparisons of DEGs between 1 h before floret opening and floret opening in four rice varieties
As shown in Fig. 3a, the up-regulated genes can be classified into five main classes: cellular processes, environmental information processing, genetic information processing, metabolism, and organismal systems. In environmental information processing, enriched genes are located in signal transduction. In metabolism, enriched genes are located in lipid metabolism and carbohydrate metabolism. In organismal systems, enriched genes are located in environmental adaptation.
Fig. 3.
KEGG enrichment analysis of up-regulated DEGs shared by four rice varieties between 1 h before floret opening and at floret opening. A Up-regulated distribution of DEGs in the KEGG main class and subclass pathways; B Up-regulated distribution of DEGs in the KEGG sub-subclass pathways. The p value represents significance, with 0.05 as the threshold
As shown in Fig. 3b, plant hormone signal transduction and MAPK signaling pathway—plant were gene enriched and significant subclasses in the signal transduction. In the lipid metabolism, alpha-Linolenic acid metabolism was gene enriched and significant subclasses. Starch and sucrose metabolism was gene enriched and significant subclasses in carbohydrate metabolism. In the environmental adaptation, Plant-pathogen interaction was gene enriched and significant subclasses.
As shown in Fig. 4a, the down-regulated genes were also classified into the same five main classes as Fig. 3a. In metabolism, the enriched genes can be found in the metabolism of terpenoids and polyketides, where diterpenoid biosynthesis, pentose and glucuronate interconversions and phenylpropanoid biosynthesis were gene enriched and significant metabolic pathway (Fig. 4b).
Fig. 4.
KEGG enrichment analysis of down-regulated DEGs shared by four rice varieties between 1 h before floret opening and at floret opening. A Down-regulated distribution of DEGs in the KEGG main class and subclass pathways; B down-regulated distribution of DEGs in the KEGG sub-subclass pathways. The p value represents significance, with 0.05 as the threshold
Express patterns of up-regulated DEGS between 1 h before floret opening and floret opening
As shown in Fig. 5a genes in the Plant hormone signal transduction. OsJAZ8, OsJAZ12, OsJAZ13, OsJAZ9, OsJAZ7, OsJAZ5, OsJAZ6, OsJAZ10 and OsJAZ11 belong to the JAZs while JAZ are repressors of the JA signaling pathway (Fig. 6a). LOC_Os09g37480, LOC_Os09g37470, LOC_Os01g56240, LOC_Os02g42990 belong to the SAURs, SAUR is involved in auxin signaling (Fig. 6a). In japonica rice, the expression levels of these 14 genes were up-regulated at floret opening and down-regulated at floret closure significantly except for LOC_Os49010. The expression patterns of OsSAUR53, OsSAUR52, OsSAUR11and LOC_Os05g49010 in indica followed the same trend as that in japonica rice. However, the expression patterns of OsJAZ13 and OsJAZ11 in indica rice differed from those in japonica rice, where expression levels were up-regulated at floret opening and again at floret closure. Expression patterns of OsJAZ13 and OsJAZ11 were verified using qRT-PCR and the results were found to be in line with transcriptome sequencing (Fig. S3).
Fig. 5.
Express patterns of up-regulated DEGs shared by four rice varieties between 1 h before floret opening and floret opening. Gene expression levels were computed using the FPKM. Different colours indicated different levels of gene expression, with colours ranging from blue through white to red indicating low to high levels of expression. A Heatmap of plant hormone signal transduction; B Heatmap of MAPK signaling pathway-plant; C heatmap of alpha-Linolenic acid metabolism; D heatmap of Starch and sucrose metabolism; E heatmap of plant–pathogen interaction
Fig. 6.
KEGG pathway analysis of up-regulated DEGs shared by four rice varieties between 1 h before floret opening and floret opening. Red represents differentially expressed genes annotated to a ko node and up-regulated, green represents differentially expressed genes annotated to a ko node and down-regulated. The 4 digits in the box indicated the EC number of the enzyme. Solid arrows indicated the direction of biochemical reactions. dashed arrows connected other related metabolic pathways. A Plant hormone signal transduction; B MAPK signaling pathway-plant; C alpha-Linolenic acid metabolism; D plant–pathogen interaction; E starch and sucrose metabolism
As shown in Fig. 5b, nine genes in the MAPK signaling pathway-plant pathway, including five cloned genes, namely OsCML1, OsWRKY53, OsWRKY8, OsMKK62, OsWRKY86. OsCML1 is a calmodulin involved in the wounding response, while other genes were involved in signal transduction for biotic or abiotic stresses, respectively (Fig. 5b). The expression pattern of the nine genes in japonica rice was up-regulated at floret opening and down-regulated at floret closure. In indica rice, the expression patterns of six genes, LOC_Os05g49010, LOC_Os01g50370, OsWRKY53, OsMKK62, OsWRKY86, LOC_Os02g21700, were the same in indica rice as in japonica rice. However, the expression patterns of OsCML1 and OsWRKY8 in indica rice differed from those in japonica rice, in that expression levels were up-regulated at floret opening and again at floret closure (Fig. 5b). Transcriptome sequencing results showed that the expression levels of Oscml1 in the four rice varieties PI1, PI2, PI3 and PI4 significantly increased from 1.97 to 2.36, 1.80 to 2.56, 2.08 to 2.44, 2.24 to 2.63, respectively, at floret opening, while the expression levels of OsCML1 changed from 2.36 to 2.79 significantly, 2.56 to 2.66, 2.44 to 2.23, and 2.63 to 2.41, respectively, at floret closure (Fig. 5b). The expression pattern of the OsCML1 derived from qRT-PCR was consistent with the transcriptome sequencing. qRT-PCR revealed that the expression levels of Oscml1 in the four rice varieties PI1, PI2, PI3 and PI4 increased from 0.43 to 0.49, 0.14 to 0.32 significantly, 0.28 to 0.60 significantly and 0.20 to 0.64, respectively, at floret opening, while the expression levels of OsCML1 significantly changed from0.49 to 0.78, 0.32 to 0.65, 0.60 to 0.24 and 0.64 to 0.44 (Fig. S3).
As shown in Fig. 5c, five genes were targeted in the alpha-Linolenic acid metabolism pathway, and these five genes were involved in the biosynthesis of JA. The hydroperoxide dehydratase (EC:4.2.1.92) encoded by OsAOS1 and the allene-oxide cyclase (EC:5.3.99.6) encoded by OsAOC were key intermediate enzymes in JA biosynthesis. They were involved in the reactions of 13 (S)—HpOTrE to 12,13-EOTrE and 12,13-EOTrE to 12-OPDA, respectively. Phospholipase A1 (EC:3.1.1.32) encoded by LOC_Os02g43700 is involved in the reaction of Phosphatidylcholine toα-Linolenic acid. Jasmonate O-methyltransferase (EC:2.1.1.141) encoded by LOC_Os05g01140 was in the reaction of JA to MeJA (Fig. 6c). LOC_Os02g43700, OsAOS1, LOC_Os05g01140 showed the same expression pattern in indica and japonica rice, with up-regulated expression levels at floret opening and down-regulated expression levels at floret closure (Fig. 5c).
As shown in Fig. 5d, the starch and sucrose metabolism pathway contains a total of six genes. The 1,3-β-D-glucan endoglucanase (EC:3.2.1.39), encoded by genes LOC_Os01g64170 and LOC_Os05g41610, is involved in the conversion of 1,3-β-glucan to D-glucose. OsTPS1 can encode Alpha-trehalose-phosphate synthase (EC:2.4.1.15), involved in the transformation of Trehalose-6P from UDP-glucose and D-Glucose-6P, in addition OsTPS1 can encode trehalose-phosphatase (EC:3.1.3.12), involved in the generation of Trehalose from Trehalose-6P. Trehalose-phosphatase (EC:3.1.3.12), encoded by LOC_Os02g44230 and LOC_Os08g31630, engaged in the conversion of Trehalose-6P to Trehalose. Glycogen phosphorylase (EC:2.4.1.1) encoded by LOC_Os10g40640 was responsible for converting UDP-glucose to amylose (Fig. 6d). In japonica rice, all six genes significantly were expressed at up-regulated levels at floret opening and down-regulated levels at floret closure. The expression pattern of LOC_Os08g31630 among the six genes was consistent in the following four rice varieties: PI1, PI2, PI3 and PI4. The transcriptome sequencing showed that the expression levels of LOC_Os08g31630 increased from 1.57 to 2.19 significantly, 1.71–2.12, 1.59–2.26 significantly and 1.82–2.30, respectively, at floret opening, while the expression levels of LOC_Os08g31630 decreased from 2.19 to 2.09, 2.12 to 2.10, 2.26 to 1.87 significantly and 2.30 to 1.87 significantly, respectively, at floret closure (Fig. 5d).
As shown in Fig. 5e, In the plan–pathogen interaction pathway 19 genes were involved in the defense response of bacteria or fungi (Fig. 6e). Seventeen genes showed an up-regulated expression pattern at floret opening and down-regulated expression at floret closure in japonica rice significantly except for OsCML1 and OsWRKY8. The expression patterns of 12 of the 19 genes, including OsPAD4, LOC_Os02g43700, OsWRKY53, OsWRKY71, OsJMT1, OsWRKY86, OsWRKY7, OsCPK20, OsAOS1, OsWRKY74 and OsCML31, followed the same trend between indica and japonica rice (Fig. 5e).
Express patterns of down-regulated DEGS between 1 h before floret opening and floret opening
As shown in Fig. 7a genes were present in the diterpenoid biosynthesis pathway. Ent-copalyl diphosphate synthase (EC:5.5.1.13) and gibberellin 3beta-dioxygenase (EC:1.14.11.15) encoded by OsCPS2 and OsGA3ox1, respectively, was part of the biosynthetic process of GAs (Fig. 8a). OsCyc1 expression patterns differed between indica and japonica rice, with OsCyc1 expression levels down-regulated at floret opening and up-regulated at floret closure in indica rice; however, in japonica rice, OsCyc1 expression levels were down-regulated at floret opening and again at floret closure (Fig. 7a).
Fig. 7.
Express patterns of down-regulated DEGs shared by four varietiess between 1 h before floret opening and floret opening. Gene expression levels were computed using the FPKM. Different colours indicated different levels of gene expression, with colours ranging from blue through white to red indicating low to high levels of expression. A Heatmap of Diterpenoid biosynthesis.; B heatmap of Pentose and glucuronate interconversions; C Heatmap of Phenylpropanoid biosynthesis
Fig. 8.
KEGG pathway analysis of down-regulated DEGs shared by four rice varieties between 1 h before floret opening and floret opening. Red represents differentially expressed genes annotated to a ko node and up-regulated, and the green represents differentially expressed genes annotated to a ko node and down-regulated. The 4 digits in the box indicated the EC number of the enzyme. Solid arrows indicated the direction of biochemical reactions. Dashed arrows connected other related metabolic pathways. A Diterpenoid biosynthesis; B pentose and glucuronate interconversions; C Phenylpropanoid biosynthesis
As shown in Fig. 7b, five genes were in the pentose and glucuronate interconversions pathway.
Pectinesterase (EC:3.1.1.11) encoded by LOC_Os11g45730 and LOC_Os07g41650 participated in the conversion of poly (1,4-a-d-galacturonide) to poly (1,4-a-d-galacturonate) (n). Pectate lyase (EC:4.2.2.2) encoded by LOC_Os06g38510 was involved in the conversion of poly (1,4-a-d-galacturonate) (n) to Unsaturated digalacturonate. Galacturonan 1,4-alpha-galacturonidase (EC:3.2.1.67) encoded by LOC_Os06g35320 and LOC_Os06g35370 was involved in the conversion of Digalacturonate to d-Galacturonate (Fig. 8b). Three of the five genes, LOC_Os06g35320, LOC_Os06g35370 and LOC_Os06g38510, showed the same expression pattern between indica and japonica rice, with down-regulated expression levels at floret opening and up-regulated expression levels at floret closure (Fig. 7a).
As shown in Fig. 7c, there were five genes in the Phenylpropanoid biosynthesis pathway, including one cloned gene, OsALDH2C1. Beta-glucosidase (EC:3.2.1.21) encoded by LOC_Os04g43360 converted B-d-Glucosyl-2-coumarinate to coumarinate. Coniferyl-aldehyde dehydrogenase (EC:1.2.1.68) encoded by LOC_Os01g40870 participated in two conversion processes, coniferyl-aldehyde to Ferulic acid and inapaldehyde to Sinapic acid. Peroxidase (EC:1.11.1.7) encoded by LOC_Os05g42000, LOC_Os07g02440 and LOC_Os09g29490 were involved in the following four reactions: p-coumaryl alcohol to p-Hydroxy-phenyl lignin, coniferyl-alcohol to Guaiacyl lignin, 5_Hydroxy-conifery alcohol to 5_Hydroxy-guaiacyl lignin, Sinapyl alcohol to Sinapyl lignin (Fig. 8c). Among the five genes, only two genes, expression pattern of LOC_Os07g02440 and LOC_Os05g42000, showed the same trend both in indica-japonica rice, with expression levels down-regulated at floret opening and up-regulated at floret closure (Fig. 7c).
DEGS Functional classification using the KEGG pathway between floret opening and floret closure
A total of 16 significantly differentially expressed genes were shared by the four rice varieties (10 were down-regulated (Fig. 9a) and 6 were up-regulated (Fig. 9b)) at floret closure compared to floret opening. Only 2 of the 6 up-regulated genes had KEGG annotations, and 3 of the 10 down-regulated genes had KEGG annotations.
Fig. 9.
Venn diagram exhibiting the DEGs distribution in four comparison groups. A Venn diagram exhibiting the up-regulated DEGs in four comparison groups. B Venn diagram exhibiting the down-regulated DEGs in four comparison groups
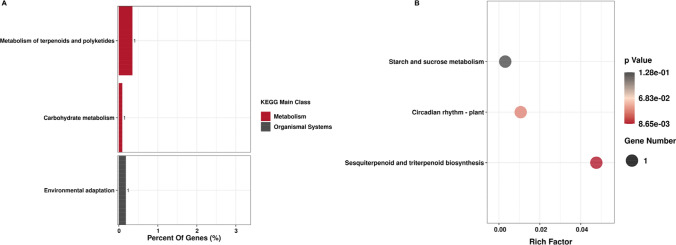
As shown in Fig. 10, down-regulated genes were mainly involved in the following metabolic pathways: Metabolism → Metabolism of terpenoids and polyketides → Sesquiterpenoid and triterpenoid biosynthesis, Metabolism → Carbohydrate metabolism → Starch and sucrose metabolism, Organismal Systems → Environmental adaptation → Circadian rhythm-plant while up-regulated genes were mainly involved in Metabolism → Metabolism of cofactors and vitamins → Thiamine metabolism (Fig. 11).
Fig. 10.
KEGG enrichment analysis of down-regulated DEGs shared by four rice varieties between floret opening and floret closure. A Down-regulated distribution of DEGs in the KEGG main class and subclass pathways; B down-regulated distribution of DEGs in the KEGG sub-subclass pathways. The p value represents significance, with 0.05 as the threshold
Fig. 11.
KEGG enrichment analysis of up-regulated DEGs shared by four rice varieties between floret opening and floret closure. A Up-regulated distribution of DEGs in the KEGG main class and subclass pathways; B Up-regulated distribution of DEGs in the KEGG sub-subclass pathways. The p value represents significance, with 0.05 as the threshold
Express patterns of down-regulated DEGS between floret opening and floret closure
As shown in Fig. 12a, OsLHY was the core component of Circadian rhythm. (−)-Germacrene D synthase (EC:4.2.3.75) encoded by LOC_Os04g2767 participated in the synthesis of Germacrene D (Fig. 12b). Glucan endo-1,3-beta-d-glucosidase (EC:3.2.1.39) encoded by LOC_Os05g31140 involved in the conversion of 1,3-β-Glucan to d- Glucose (Fig. 12c). Eight of the ten genes, LOC_Os04g27670, LOC_Os01g74040, OsDREB1B, LOC_Os10g41330, LOC_Os08g08850, LOC_Os12g32610, OsDREB1BG, LOC_Os04g39350, had the same expression pattern between indica and japonica rice, with expression levels up-regulated at floret opening and down-regulated at floret closure (Fig. 13).
Fig. 12.
KEGG pathway analysis of down-regulated DEGs shared by four rice varieties between floret opening and floret closure. A Circadian rhythm-plant; B sesquiterpenoid and triterpenoid biosynthesis; C starch and sucrose metabolism
Fig. 13.
Express patterns of up-regulated DEGs shared by four varieties between floret opening and floret closure. Gene expression levels were computed using the FPKM. Different colours indicated different gene expression levels, with colours ranging from blue through white to red indicating low to high levels of expression
Express patterns of up-regulated DEGS between floret opening and floret closing
As shown in Fig. 14, there were two genes in the Thiamine metabolism pathway. Cysteine-dependent adenosine diphosphate thiazole synthase (EC:2.4.2.60) encoded by OsXNP was involved in the conversion of Glycine to ADP-5-ethyl-4-methyl-thiazole-2-carboxuylate.2-methylisocitrate dehydratase (EC:4.1.99.170) encoded by LOC_Os03g4761 participated in the Biosynthesis of 1- (5′-Phospho-ribosyl)-5-aminoimidazole (Fig. 15). Among the six genes, only two genes, LOC_Os01g65630 and LOC_Os09g233002, showed consistent expression patterns both in Indica and Japonica rice, with expression levels down-regulated at floret opening and up-regulated at floret closure (Fig. 14).
Fig. 14.
Express patterns of down-regulated DEGs shared by four varieties between floret opening and floret closure. Gene expression n levels were computed using the FPKM. Different colours indicated different levels of gene expression levels, with colours ranging from blue through white to red indicating low to high levels of expression
Fig. 15.
KEGG pathway analysis of up-regulated DEGs shared by four rice varieties between floret opening and floret closure
Discussion
Floret opening and closure is an important process in the reproductive development of rice, which is related to the genetic characteristics of rice and is also regulated by a combination of environmental factors, endogenous hormones and multiple genes. In this study, RNA-seq was applied to compare the transcriptomic differences between 1 h before floret opening and floret opening and between floret opening and floret closure to provide a theoretical basis for further analysis of the regulatory mechanism of floret opening and closure in rice.
Compared to 1 h before flowering, significant up-regulation of genes common to all four rice cultivars was observed at floret opening in plant hormone signal transduction, MAPK signaling pathway-plant, alpha-Linolenic acid metabolism, Starch and sucrose metabolism, plant–pathogen interaction pathway, while significantly down-regulated genes were involved in the diterpenoid biosynthesis, pentose and glucuronide interconversion and phenylpropanoid biosynthesis pathways.
The expression levels of OsJAZ5, OsJAZ6, OsJAZ7, OsJAZ9, OsJAZ11, OsJAZ12, OsJAZ8, OsJAZ10 and OsJAZ13, involved in JA signaling in the plant hormone signal transduction pathway, were significantly up-regulated at floret opening compared to 1 h before floret opening. He et al. (2018b) reported that OsJAZ5, OsJAZ6, OsJAZ7, OsJAZ9, OsJAZ11 and OsJAZ12 were significantly up-regulated at 1 h before floret opening compared to 12 h before floret opening, and the results of this study corroborated with the results of the present study.
Starch and sucrose metabolism pathways are important for floret opening in rice. Soluble sugars are the main osmoregulatory substance in the lodicule cells, and an increase in soluble sugars in the lodicule cells will promote water absorption and swelling of the lodicule, thus enabling the floret to open (Wang 1991; Zeng et al. 2004). Mukherjee et al. reported that alterations in the expression pattern of genes involved in glycolysis/gluconeogenesis resulted in earlier flowering in late-maturing indica rice (Mukherjee et al. 2012). OsAOS1 and OsAOC are key enzyme genes in JA biosynthesis in the alpha-Linolenic acid metabolism pathway. This study shows that the expression levels of OsAOS1 and OsAOC were significantly higher at floret opening compared to 1 h before floret opening. He et al. (Yong-Ming et al. 2012) reported that JA played an important role in regulating floret opening in rice, with JA levels being stable before floret opening; however, JA levels increased sharply at floret opening. They decreased after floret closure, and the expression patterns of OsAOC and OsAOS1 during floret opening to closure were consistent with the changes in JA levels; the results of this study were consistent with our findings.
There are two subspecies of Asian cultivated rice, indica and japonica. Exploiting the hybrid advantage between indica and japonica subspecies is important for ensuring food security. In general, indica rice has an early floret opening time and a long duration, while japonica rice has a late floret opening time and a short duration. Differences in floret opening time between indica and japonica rice limit the use of hybrid advantage and seed production. This study showed that OsJAZ11, OsJAZ13, and OsCML are involved in the regulating of rice floret opening. Both transcriptome sequencing and qRT-PCR experiments showed different expression patterns of OsJAZ11, OsJAZ13 and OsCML between subsp. indica and subsp. japonica rice throughout the process of floret opening and closure. In subsp. indica rice, the expression levels of OsJAZ13, OsJAZ11 and OsCML1 were up-regulated or significantly up-regulated at floret opening and again significantly up-regulated or not significantly changed at floret closure, whereas in subsp. japonica rice, the expression levels were significantly up-regulated at floret opening and significantly down-regulated at floret closure. The above results showed that three genes, including OsJAZ13, OsJAZ11 and OsCML1 were more consistently expressed in indica rice than in japonica rice during the process from floret opening to closure. Differences in the persistence of expression of these genes between Indica and Japonica subspecies may be the underlying reason for the difference in duration of floret opening. Next, overexpression vectors would be constructed for floret opening and closure regulatory genes with different expression persistence, such as OsJAZ13, OsJAZ11 and OsCMLS in this study, and transformed in indica and japonica rice, respectively, to investigate whether the persistent expression of these genes could make the flowering habit of indica and japonica rice consistent.
In summary, floret opening involves multiple metabolic pathways such as Plant hormone signal transduction, MAPK signaling pathway-plant, starch and sucrose metabolism, alpha-Linolenic acid metabolism, plant-pathogen interaction, diterpenoid biosynthesis, glucuronate interconversions, and phenylpropanoid biosynthesis. Among the above metabolic pathways involved in floret opening, many genes had different expression patterns between indica and japonica rice. Fewer significantly differentially expressed genes were more common to the four indica and japonica varieties at floret closure than at floret opening.
Few significantly differentially expressed genes were common to the four indica and japonica varieties at floret closure than at floret opening. These genes were mainly involved in metabolic pathways such as circadian rhythm-plant sesquiterpenoid and triterpenoid biosynthesis, Starch and sucrose metabolism, and Thiamine metabolism. The present study may provide a theoretical basis for uncovering the molecular mechanisms underlying the regulation of floret opening and closure in rice.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1 The correlation analysis of samples (PDF 2791 kb)
Fig. S2 PCA analysis of samples (PDF 2494 kb)
Fig. S3 OsJAZ13, OsJAZ11, and OsCML1 gene expression levels (PDF 4565 kb)
Acknowledgements
This research was funded by Science and Technology Fundation of Guizhou Province (QKHJC-ZK[2021]YB132), Guizhou Academy of Agricultural Sciences Youth Fund [2019] 02. Guizhou Academy of Agricultural Sciences Youth Fund [2020] 20, Science and Technology Fundation of Guizhou Province (QKHJC- [2020]1Y100).
Author contributions
Idea of the paper, ZY, DR and ZS; writing, YZ and ZH; Sampling, YZ, DR, and LJ; administration, ZS, YZ.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
The ethical appoval
Not applicable.
Footnotes
Zhiqiang Yan and Ruyue Deng contributed equally to this work.
Contributor Information
Zhiqiang Yan, Email: 2414904364@qq.com.
Susong Zhu, Email: 13984033281@139.com.
References
- Fu Y, Xiang M, Jiang H, He Y, Zeng X. Transcriptome profiling of lodicules before floret opening in Oryza sativa L. Scientia Agricultura Sinica. 2016;49(06):1017–1033. [Google Scholar]
- He B, Hu Z, Ma L, Li H, Ai M, Han J, Zeng B. Transcriptome analysis of different growth stages of Aspergillus oryzae reveals dynamic changes of distinct classes of genes during growth. BMC Microbiol. 2018;18(1):1–11. doi: 10.1186/s12866-018-1158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Liu S, Lei S. The dynamic changes in jasmonate levels and expression of its pathway-related genes in anthers before dehiscence in rice. Acta Agric Univ Jiangxiensis. 2018;40(03):429–434. [Google Scholar]
- Huang Y, Zeng X. Effects of environmental factors and spikelet architecture on rice spikelet closure. Jiangsu Agric Sci. 2021;49(19):94–100. [Google Scholar]
- Huang J, He Y, Zeng X, Xiangm M-l, Fu Y. Changes of JA levels in foral organs and expression analysis of JA signaling genes in lodicules before floret opening in rice. Sci Agric Sinica. 2015;48(06):1219–1227. [Google Scholar]
- Huang Y, Zeng X, Cao H. Hormonal regulation of floret closure of rice (Oryza sativa) PLoS ONE. 2018;13(6):e0198828. doi: 10.1371/journal.pone.0198828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL (2015) HISAT: A fast spliced aligner with low memory requirements. Nat Methods 12(4) [DOI] [PMC free article] [PubMed]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):1. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Ba Yasi K, Matsui T, Yoshimoto M, Hasegawa T. Effects of temperature, solar radiation, and vapor-pressure deficit on flower opening time in rice. Plant Product Sci. 2010;13(1):21–28. doi: 10.1626/pps.13.21. [DOI] [Google Scholar]
- Kobayasi K, Sakai H, Tokida T, Nakamura H, Usui Y, Yoshimoto M, Hasegawa T (2019) Effects of free-air CO2 enrichment on flower opening time in rice. Plant Production Science:1–7
- Li X, Wang Y, Duan E, Qi Q, Zhou K, Lin Q, Wang D, Wang Y, Long W, Zhao Z. OPEN GLUME1: a key enzyme reducing the precursor of JA, participates in carbohydrate transport of lodicules during anthesis in rice. Plant Cell Rep. 2017;37(2):329–346. doi: 10.1007/s00299-017-2232-y. [DOI] [PubMed] [Google Scholar]
- Liu W (2008) Study on cytology bas ic of rice anthesis and lodicule programmed cell death. Master Yangzhou University
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Hu D, Wang W. Optimum temperature and humidity ratio for hybrid rice seed production at flowering stag. Chin J Rice Sci. 2001;01:42–46. doi: 10.16819/j.1001-7216.2001.01.008. [DOI] [Google Scholar]
- Ma Z, Zahn Z, Cheng X, Gao J, He G, Liu D, Xu H, Xu Z. Flowering time in filial generations of cross between Iindica andjaponica rice and its response to external environment. Hybrid Rice. 2011;26(05):70–76. doi: 10.16267/j.cnki.1005-3956.2011.05.025. [DOI] [Google Scholar]
- Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17(1)
- Mukherjee R, Gayen S, Chakraborty A, Bhattacharyya J, Maiti MK, Basu A, Sen SK. Double-stranded RNA-mediated downregulation of pdhk gene expression to shorten maturation time of a late maturing native indica rice cultivar, badshahbhog. Crop Sci. 2012;52(4):1743–1753. doi: 10.2135/cropsci2011.07.0352. [DOI] [Google Scholar]
- Pan X, Liu W, Li Y, Xiong H, Xinnian S, Duan Y, Yu Y, Zhao W, Wei X, Li X. Identification and genetic analysis of split husk mutant sh1 in rice. Chin J Rice Sci. 2019;33(04):323–330. [Google Scholar]
- Peng J, Mo H, D H (1994) The relationship between flowering time of rice and weather factor. Chinese J Rice Sci (03):75
- Wang Z (1991) Studies on the mechanism of the anthesis of rice III. Structure of the lodicule and changes of its contents during flowering. Acta Agron Sinica
- Xia Y, Du Z, Yang Y, Gong Y, Yan Z, H X (2019) Effects of Epi-Brassinolide treatments on floret opening time of indica and japonica rice. Crops (04):139–147
- Xiao Y, Yi C, Charnikhova T, Mulder P, Ouwerkerk P. OsJAR1 is required for JA-regulated floret opening and anther dehiscence in rice. Plant Mol Biol. 2014;86(1–2):19–33. doi: 10.1007/s11103-014-0212-y. [DOI] [PubMed] [Google Scholar]
- Yan Z, Xu H, Ma Z, Gao D, Z X (2014) Differential Response offloret opening to exo-methyl jasmonate between subsp. Indica and subsp. Japonica in rice. Sci Agric Sinica 47(13):2529–2540
- Yan Z, Xu H, Gong H, Xia Y, Ma Z, Z X (2015) Difference of floret opening time between subsp. indica and subsp. Japonica rice in response to ethephon. J Shenyang Agric Univ 46(06):641–647
- Yong-Ming H, Yong-Jun L, Xiao-Chun Z. Dynamic changes of jasmonic acid biosynthesis in rice florets during natural anthesis. Acta Agrono Sinica. 2012;38(10):1891–1899. [Google Scholar]
- Zeng XC, Zhou X, Zhang W, Murofushi N, Kitahara T, Kamuro Y. Opening of rice floret in rapid response to methyl jasmonate. J Plant Growth Regul. 1999;18(4):153. doi: 10.1007/PL00007063. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhou X, Wu X. Advances in study of opening mechanism in rice Florets. Sci Agric Sinica. 2004;02:188–195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 The correlation analysis of samples (PDF 2791 kb)
Fig. S2 PCA analysis of samples (PDF 2494 kb)
Fig. S3 OsJAZ13, OsJAZ11, and OsCML1 gene expression levels (PDF 4565 kb)