Abstract
Geranium wallichianum D. Don ex Sweet is a well-known medicinal plant in Kashmir Himalya. The evidence for its modern medicinal applications remains majorly unexplored. The present study was undertaken to elucidate the detailed antimicrobial promises of different crude extracts (methanolic, ethanolic, petroleum ether, and ethyl acetate) of G. wallichainum against common human bacterial and fungal pathogens in order to scientifically validate its traditional use. The LC–MS analysis of G. wallichainum yielded 141 bioactive compounds with the vast majority of them having therapeutic applications. Determination of minimum inhibitory concentrations (MICs) by broth microdilution method of G. wallichainum was tested against bacterial and fungal pathogens with MICs ranging from 0.39 to 400 µg/mL. Furthermore, virtual ligands screening yielded elatine, kaempferol, and germacrene-A as medicinally most active constituents and the potential inhibitors of penicillin-binding protein (PBP), dihydropteroate synthase (DHPS), elongation factor-Tu (Eu-Tu), ABC transporter, 1,3 beta glycan, and beta-tubulin. The root mean square deviation (RMSD) graphs obtained through the molecular dynamic simulations (MDS) indicated the true bonding interactions which were further validated using root mean square fluctuation (RMSF) graphs which provided a better understanding of the amino acids present in the proteins responsible for the molecular motions and fluctuations. The effective binding of elatine, kaempferol, and germacrene-A with these proteins provides ground for further research to understand the underlying mechanism that ceases the growth of these microbes.
Subject terms: Biochemistry, Biological techniques, Cell biology, Computational biology and bioinformatics, Microbiology
Introduction
Antibiotics are crucial weapons in fighting various microbial infections and have significantly improved human health since their introduction1,2. However, the last few years have witnessed the excessive use of antibiotics cause resistance, leading to hazardous effects on human health2,3. Researchers are trying to develop new drugs with no resistance. As a result, the traditional systems of medicine are gaining enormous popularity since they are more natural, environmentally friendly, and devoid of adverse effects4,5. Thus, despite the numerous advantages of current synthetic medicines, people continue to choose plant-based natural remedies over synthetic medications6–9. The majority of medicinal plants are unique in their potential to treat and cure various human health problems, owing to several essential phytoconstituents in different plant parts10. Numerous bioactive chemicals found in medicinal plants have pharmacological activities like antimicrobial, anticancer, antioxidant and anti-inflammation properties6,10–14. Although many plant species have many biological metabolites, only a limited number have been investigated and confirmed to represent a substantial source of natural compounds. It is essential to create good screening processes to discover new compounds15. The extraction and characterization of a large number of these bioactive chemicals from various medicinal plants have resulted in the administration of specific medications with a high activity profile16,17. The first screening of medicinal plants using chromatographic and spectrometric methods offers essential information on their chemical and pharmacological characteristics, which help to select biologically active plants18.
Liquid chromatography-mass spectrometry (LC–MS) has been mainly used in recent years to detect functional groups and identify a variety of bioactive therapeutic phytocompounds present in medicinal plants19,20. LC–MS is one of the most effective, rapid, and precise method for detecting a wide variety of chemicals, including alkaloids, nitro compounds, asters, alcohols, organic acids, steroids, long-chain hydrocarbons and amino acids21, and utilises a little amount of extracts of plants.
Computer-aided approaches for drug discovery have evolved as improved technologies that can be used to screen for medications derived from phytochemicals present in a variety of medicinal plants. Computational prediction models are critical in guiding the methodology selection process for pharmaceutical and technology research. They have also been used in in silico forecast of pharmacokinetic, pharmacological and toxicological performance22. Presently, molecular docking is an efficient and cost-effective strategy for developing and testing pharmaceuticals. This approach generates data on drug and receptor interactions that may be used to predict the orientation of drug candidates when bound to their target protein23. Additionally, this technique facilitates systemic investigation by non-covalently placing a molecule into the binding site of an object macromolecule, resulting in specific binding at the active sites of every ligand24–26. In this aspect, the present study used the LC–MS method to detect and identify phytochemical components contained in the medicinal plant.
Geranium wallichianum D. Don ex Sweet is a species belonging to the Geraniaceae family27. Geranium L. is a large genus with 325 species found worldwide except in lowland tropical climates27. In India, 27 Geranium species have been recorded, with the most remarkable diversity occurring in the nation's temperate Himalaya and tropical mountainous areas, particularly the Deccan peninsula, Western Ghats, and the northeast region. It is mainly found in high altitude Himalayas of Jammu and Kashmir28.
Polyphenol rich extracts of Geranium L. species as potential natural antioxidant and antimicrobial agents29. Geranium wallichianum D. Don ex Sweet is a well-known traditional plant used by herbalists to treat backache, sexual debility, joint pain, colic, jaundice, and kidney and spleen disorder30. G. wallichianum is usually used as tonic by women especially for physical fitness and other internal body complaints31. In different assay the crude extracts and different fractions of rhizomes and leaves showed varied degree of antimicrobial activities and enzyme inhibitions27,32. It is also rich in phytochemicals such as ursolic acid, β-sitosterol, stigmasterol, β-sitosterolgalactoside herniarin, and 2, 4, 6-trihydroxyethylbenzoate33. Ursolic acid (UA) is a natural terpene compound exhibiting many pharmaceutical properties34. However, there is a significant gap between the paucity of scientific research on G. wallichianum and its value in traditional medicine. So, more research is needed to determine the potential therapeutic efficacy and possible mechanisms of action of G. wallichianum. In response to all of the above, the current study was designed to (1) evaluate the in-vitro antimicrobial activity of the different polarity extracts of G. wallichianum roots, including petroleum ether, ethyl acetate, methanol and ethanol (2) identify the potential bioactive components present in the active extract through the LC/MS technique; and (3) apply an in-silico analysis for the most abundant compounds against the target proteins involved in the life cycle of bacteria and fungi.
Methodology
Collection of plant sample
The plant material of G. wallichianum was collected from different sites of Kashmir valley. The material was recognized and confirmed by Akhtar H. Malik, before its drying in the shade. Voucher specimen number (2954) was kept in the Department of Taxonomy at University of Kashmir (Table 1). The permission for collection of plant material was taken from the concerned authorities.
Table 1.
Details of the locations where plant samples of G. wallichianum were collected from four main sites in India's Jammu and Kashmir union region for ethnopharmacological investigation.
| Name of sampling site | Geographical coordinates | Sample collection date | Sample type | ||
|---|---|---|---|---|---|
| Latitude | Longitude | Altitude (amsl) | |||
| Sadhna Pass | 34.4016°N | 73.9535°E | 3000 | 23-07-20 | Whole plant |
| Kupwara | 34°31′33″N | 74°15′19″E | 3545 | 26-07-20 | Whole plant |
| Sinthantop | 33.5811°N | 75.5102°E | 3784 | 02-08-20 | Whole plant |
| Daksum (Anantnag) | 33.6114°N | 75.4359°E | 2438 | 09-08-20 | Whole plant |
| Uri (Baramullah) | 34.0881°N | 74.0340°E | 1579 | 04-08-20 | Whole plant |
Plant root extract preparation
Petroleum ether, ethyl acetate, ethanol and methanol were chosen as extraction solvents based on their polarity of index. A mechanical grinder is used to powder about 800 g of the roots of the G. wallichianum plant washed with deionized water, shade dried for 10–15 days, pulverized with a mechanical grinder, and stored in an airtight container. Furthermore, 200 g of G. wallichianum powder is mixed in 20 millilitres of Milli Q water and placed for 15 min in a water bath at 55 °C. To obtain the plant extract using the Soxhlet apparatus method, petroleum ether, ethyl acetate, ethyl alcohol, and methyl alcohol solvents were chosen for their polarity index. The extracts were filtered using Whatman No. 1 filter paper, and the extracts were then concentrated using a rotating vacuum evaporator, which were then stored at 4 °C for future purposes4.
Liquid chromatography and mass spectrometry analysis
The LC–MS analysis was specifically carried out using a Nexera UHPLC with quaternary pump, Autosampler, conspicuous degassing unit and DAD unit. It assures high fecundity, increased output, enhanced accuracy, and better results. The solvents used were methanol, ethyl alcohol, ethyl acetate, and petroleum ether, which were eluted at 1 ml/min. All of the solutions were passed through 0.45 mu nylon sheets following ultrasonication. The chromatograms were inspected at 270 nm, and the results were collected using lab-developed software35.
Microbial strains and culture
Council of Scientific and Industrial Research, Institute of Microbial Technology (CSIR-IMTech) in Chandigarh, Punjab India furnished the microbial strains for the study. Six of the nine microbial species designated for the experiment were bacterium strains, while three were fungal strains that were evaluated for antifungal activity. Escherchia coli, Mycobacterium luteus, Streptococcus pneumoniae, Klebsiella pneumoniae, Neisseria mucosa, Haemophilus influenzae, Candida albicans, Candida paropsilosis and Candida glabrata were used for the current research. Subcultures of bacterial strains were performed on Muller Hinton Agar [MHA] media. They were grown for 24 h at 37 °C on an agar medium until visible colonies emerged on the plate. Subcultures of fungal strains were grown in YPED broth on agar medium until sporulation occurred, which typically took 5 days. Bacteria colonies and spores of fungal strains were obtained in Muller Hinton Agar [MHA] and YEPD Broth, respectively, until the late log growth phase. The bacterial and fungal strains were kept at −70 °C in 1 ml glycerol stocks.
Minimum inhibitory concentration (MIC) through broth dilution method
Minimum Inhibitory Concentration (MIC) of various plant extracts of G. wallichianum were measured using the Micro Dilution Method with slight modifications in 96 well plates (Corning; polystyrene; Flat Bottom). The different plant extracts concentrations ranged from .39 to 400 μg/ml, and ciprofloxacin was taken as a positive antibacterial agent (0.039–20 μg/ml). 50 μl of exponentially grown bacterial cultures were inoculated on plates, and the final volume was maintained as 200 μl. In 96 well plates, drug-free growth and drug-free medium control are included. The MIC values of plates were measured after 24 h of incubation at 37 °C. MIC was referred to as the lowest antimicrobial concentrations capable of suppressing detectable bacterial growth. Clinical and Laboratory Standards Institute (CLSI) criteria was used to determine the antifungal activity of plant extracts. Amphotericin-B was taken as a positive antifungal agent. MIC reading for antifungal activity was measured after 24, 48 and 72 h of incubation at 35 °C36.
Protein preparation
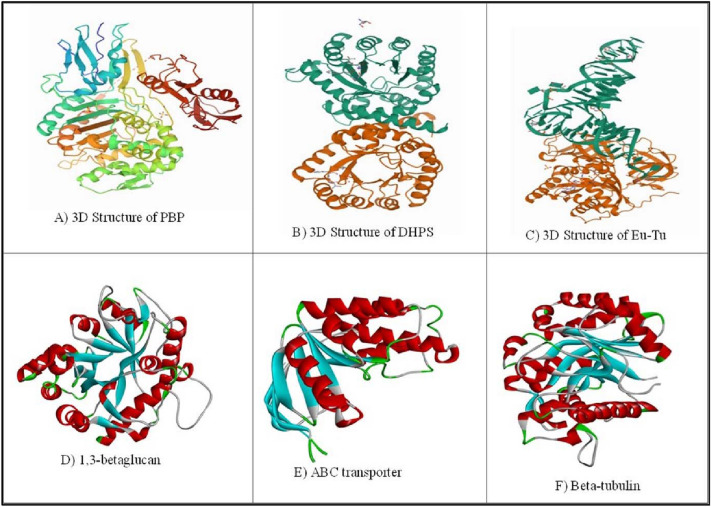
In the present study, we have selected various bacterial and fungal target proteins such as penicillin-binding protein (PBP), dihydropteroate synthase (DHPS), elongation factor-Tu (Eu-Tu), ABC transporter, 1,3 β-glycan and beta-tubulin. All six proteins play an important role in the life cycle of bacteria and fungi. Based on the role performed by these proteins, we have selected these protein targets. The targeted macromolecules such as penicillin-binding protein, dihydropteroate synthase, elongation factor-Tu, beta-tubulin, ABC transporter and 1,3-Betaglycan were obtained from the RCSB PDB database37 and extracted as a PDB file (Fig. 1). After that, these biomolecules were individually entered into the molecular docking software AutoDock25. To begin, the proteins were further trimmed by extracting the cocrystallized ligands using Biovia Software. The protein was then processed by eliminating water molecules, removing superfluous chains or heteroatoms, introducing hydrogen, estimating charges (Kollman charges), and converting it to a pdbqt file. Ultimately, the cocrystallized ligands were placed in the centre of the grid box. Possible active sites of each target were determined using CASTp web server38. For docking with Autodock Vina25, the grid box measurements were recorded in a config.txt format. The co-crystallized ligands were then deleted from the resulting protein pdbqt files.
Figure 1.
3D structure of different microbial target proteins.
Ligand preparation
The bioactive ligand molecules, Elatine, Kaempferol, and Germacrene A, were downloaded from the PubChem39 directory as 3D Standard Data Format (3D SDF) format. PyMol40 was used to translate the ligands from 3D SDF files to Protein Data Bank (PDB) format. These ligand molecules were independently uploaded into the AutoDock Tools during ligand preparation. Gasteiger charges were introduced to the compounds. Additionally, non-polar hydrogen atoms were combined, and rotational interactions were identified and altered.
Purification and refinement of proteins and ligands
Unwanted interactions, disparate bindings, ligand compounds, molecules of water, and other impurities were removed from the macromolecule using Dassault Systems Biovia Discovery Studio Visualizer. To facilitate better interactions, only polar hydrogens were introduced to the protein during the preparation, following the addition of Kollman charges. After protein fabrication, all peptides and motifs were evaluated in Discovery studio for effectual active binding site prediction and saved in pdb format. The 3-dimensional and 2-dimensional structures of the ligands Elatine, Kaempferol, and Germacren A, were obtained using the PubChem39 repository. The 3D structures of Microbial Target Proteins were extracted from the RCSB PDB37 libraries and downloaded as pdf files.
Molecular docking analysis
The molecular docking analysis of all the selected phytocompounds were subjected to AutoDock Vina 4.025 using the script standard method. Both target proteins and selected compounds were then saved in pdbqt format after combining non-polar hydrogens. Molecular docking was performed within a grid box dimension 22 × 26 × 21 Å. It was necessary to design grid boxes with particular dimensions and 0.3 Å spacing. Docking studies of the protein–ligand complex were carried out in accordance with the Lamarckian Genetic Algorithm (LGA)40. All the binding affinities were measured and considered for dynamic simulation studies. Biovia Discovery studio40 was used to investigate the interacted amino acids and docked poses of the complex structures.
Molecular dynamic simulation
The docking calculation were performed by Desmond Schrodinger v3.841 with the best binding affinity compound. The current study utilized the NPT ensemble with the 300 K temperature and 1 bar pressure in all runs for nanoseconds. During dynamic simulation, the OPLS_2005 force field was employed for the hit compound, followed by the electrostatic charges were analyzed through the Ewald method. All the possible trajectories were considered at 4.0 picosecond intervals for better accuracy. Ligand and protein behaviour were analyzed using the simulation interaction tool implemented in the Desmond package tool41 as well as the stability of the complex was monitored by showing the Root Mean Square of Deviation (RMSD) and Root Mean Square of Deviation (RMSF) of the complex.
Plant material collection statement
The permission for collection of plant material (G. wallichainum D. Don ex sweet) was taken from the concerned authorities. Further, all local, national or international guidelines and legislation were adhered to in the production of this study.
Results
Preliminary phytochemical screening
The phytochemical study of various extracts from the roots of G. wallichianum revealed a variety of phytochemicals, such as flavonoids, phenolics, terpenoids, saponins and tannins present in the plant extracts as represented in Table 2.
Table 2.
Results of preliminary tests of G. wallichianum.
| Tests | Inference | Methanol | Ethanol | Ethyl acetate | Petroleum ether |
|---|---|---|---|---|---|
| Carbohydrates | |||||
| Molisch’s test | Violet ring | + | + | + | + |
| Fehling’s test | Formation of yellow pot | + | − | + | − |
| Benedict’s test | Red precipitate | + | + | + | + |
| Anthraquinone glycosides | |||||
| Anthraquinone glycosides | The ammoniacal layer turns pink | − | + | + | + |
| Saponin glycosides | |||||
| Foam test | Persistent foam | + | + | + | + |
| Flavonoids | |||||
| Shinoda test | Pink color appears | + | + | + | + |
| Alkaline Reagent test | Concentrated yellow color | + | − | + | + |
| Tannins and phenolics | |||||
| FeCl3 test | Black color | + | + | + | + |
| Lead acetate test | White precipitate | + | + | + | − |
| Steroids | |||||
| Salkowski reaction | Chloroform layer appears red | + | − | + | + |
| Alkaloids | |||||
| Mayer’s test | Formation of precipitate | − | − | + | + |
| Dragendroff’s test | Organic precipitate | + | + | + | + |
| Wagner’s test | Formation of radish brown precipitate | + | + | − | − |
| Terpenoids | |||||
| Terpenoid test | Grey color | + | + | + | + |
Liquid chromatography-mass spectrometry (LC–MS) analysis of plant extracts
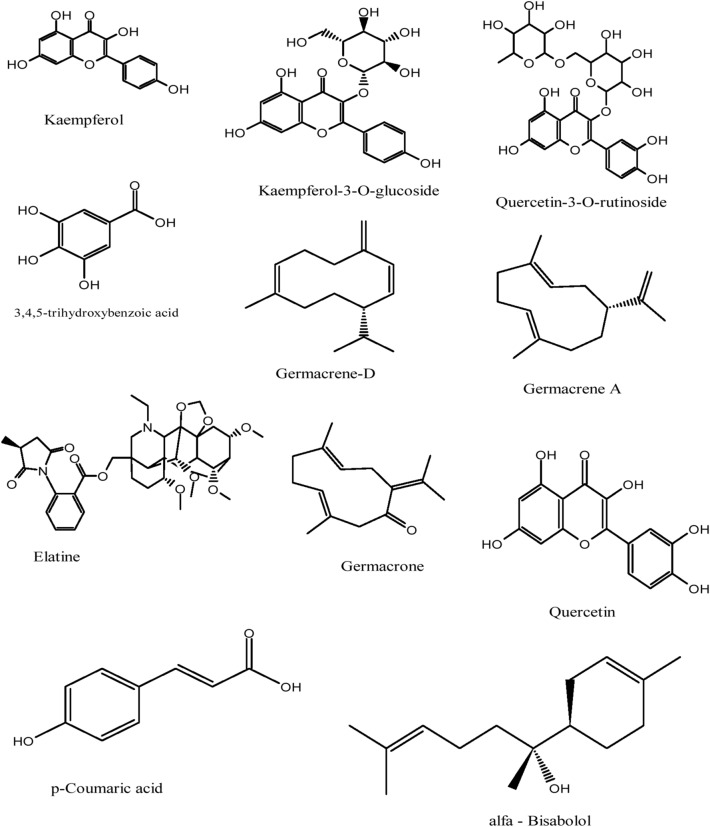
Quantitative and qualitative analysis of the various extracts of G. wallichianum were determined by LC–MS. The LC–MS total ion chromatograms of various G. wallichianum extracts are shown in (Fig. 2). The solvent extraction was carried out by Soxhlet extraction and was subjected to LC–MS analysis to obtain 141 important bioactive phytocompounds through in-depth research. Some of the important ones are shown in (Table 3, Fig. 3). The maximum number of phytocompounds were obtained using the methanolic extract (40) followed by ethyl acetate (36), ethanol (33) and petroleum ether extracts (32) (Supplementary file). The identified compounds belong to the various secondary metabolites like terpenoids, alkaloids, aliphatic compounds, and phenolics. Some of the examples are Kaempferol, Quercetin, Kaempferol-3-O-glucoside, Quercetin-3-O-rutinoside, Gallic acid, Germacrene-D, Germacrene A, Elatine, Germacrone, α- Bisabolol and p-Coumaric acid (Table 3).
Figure 2.
LC–MS-ESI–MS chromatograms of reference compounds using Nexera in Methanolic extract.
Table 3.
The major components found in G. wallichianum based on LC–MS analysis.
| Compound name | IUPAC name | Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| Kaempferol | 3,5,7-Trihydroxy-2-(4-hydroxyphenyl) chromen-4-one | C15H10O6 | 286.24 |
| Kaempferol-3-O-glucoside | 5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl] oxychromen-4-one | C21H20O11 | 448.4 |
| Quercetin-3-O rutinoside | 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-[3,4,5-trihydroxy-6-[(3,4,5-trihydroxy-6-methyloxan-2-yl) oxymethyl] oxan-2-yl] oxychromen-4-one | C27H30O16 | 610.5 |
| Gallic acid | 3,4,5-Trihydroxybenzoic acid | C7H6O5 | 170.12 |
| Germacrene D | (1Z,6Z,8S)-1-methyl-5-methylidene-8-propan-2-ylcyclodeca-1,6-diene | C15H24 | 204.35 |
| Germacrene A | (1E,5E,8R)-1,5-dimethyl-8-prop-1-en-2-ylcyclodeca-1,5-diene | C15H24 | 204.35 |
| Elatine | [(4S,6S,19R,21R)-14-ethyl-4,6,19,21-tetramethoxy-9,11-dioxa-14 azaheptacyclo [10.7.2.12,5.01,13.03,8.08,12.016,20] docosan-16-yl] methyl 2-[(3S)-3-methyl-2,5-dioxopyrrolidin-1-yl] benzoate | C38H50N2O10 | 694.8 |
| Germacrone | (3E,7E)-3,7-dimethyl-10-propan-2-ylidenecyclodeca-3,7-dien-1-one | C15H22O | 218.33 |
| Quercetin | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one | C15H10O7 | 302.23 |
| p- Coumaric acid | (2R)-6-methyl-2-[(1R)-4-methylcyclohex-3-en-1-yl] hept-5-en-2-ol | C15H26O | 222.37 |
| Alfa Bisabolol | (E)-3-(4-hydroxyphenyl) prop-2-enoic acid | C9H8O3 | 164.16 |
Figure 3.
Structures of the compounds identified based on LC–MS from G. wallichainum.
Antimicrobial activity
Research studies have revealed that G. wallichianum possess antimicrobial potential against various strains of bacteria and fungi32. Regardless of the conducted research, it is complicated to compare the results, mainly due to differences in composition and origin of the plant, employed extraction techniques, the concentration of the obtained extracts, tested microorganisms, and so forth. Toward this end, it was necessary to evaluate the antimicrobial properties of the G. wallichainum dry extracts (ethyl acetate, Petroleum ether, ethanol and methanol) obtained in the present study. The minimum inhibitory concentration (MIC) was determined for all tested microorganisms. The antimicrobial activity of various extracts of G. wallichianum had showed strong antimicrobial potential against the selected microorganisms. MICs of standard antimicrobial drug targets such as ciprofloxacin and amphotericin B through broth dilution are shown in Table 4. Ethyl acetate had showed strong antimicrobial activity as compared to all other extracts. The MIC values of ethyl acetate extracts of G. wallichianum against M. luteus, H. influenzae, S. pneumonia, K. pneumoniae, N. mucosa and E. coli were 3.12, 6.25, 12.5, 25, 25, and 100 μg/mL, respectively. Plant extracts had shown less antimicrobial activity against the fungal strains viz, C. albicans, C. glabrata and C. paropsilosis compared to the bacterial strains. The antimicrobial potential of G. wallichianum extracts against selected bacterial and fungal strains observed by the MIC method is presented in Table 4.
Table 4.
Invitro antimicrobial activity of different extracts of G. wallichianum.
| Strain | MIC (µg/mL)* | ||||
|---|---|---|---|---|---|
| ME | ET | EA | PE | CIP/AMF-B | |
| E. coli (MTCC 443) | 100 | 50 | 100 | 100 | 0.625 |
| M. luteus (10240) | 6.25 | 3.12 | 3.12 | 1.56 | 1.25 |
| K. pneumoniae (MTCC 19) | 6.25 | 25 | 25 | 25 | 0.039 |
| S. pneumoniae (MTCC 655) | 25 | 25 | 12.5 | 12.5 | 0.625 |
| H. influenzae (MTCC 3826) | 25 | 25 | 6.25 | 25 | 1.25 |
| N. mucosa (MTCC 1772) | 25 | 25 | 25 | 25 | 03.12 |
| C. albicans (ATCC 24433) | 200 | 6.25 | > 400 | > 400 | 1.25 |
| C. glabrata (ATCC2001) | > 400 | > 400 | > 400 | > 400 | 2 .5 |
| C. Paropsilosis (ATCCC90018) | > 400 | > 400 | > 400 | > 400 | 2 .5 |
*Results are the average of the triplicate readings. Where CIP: Ciprofloxacin (Positive antibacterial agent), AMF-B: Amphotericin-B (Positive antifungal agent), PE: Petroleum Ether, ET: Ethanol, ME: Methanol, EA: Ethyl Acetate.
Molecular docking analysis
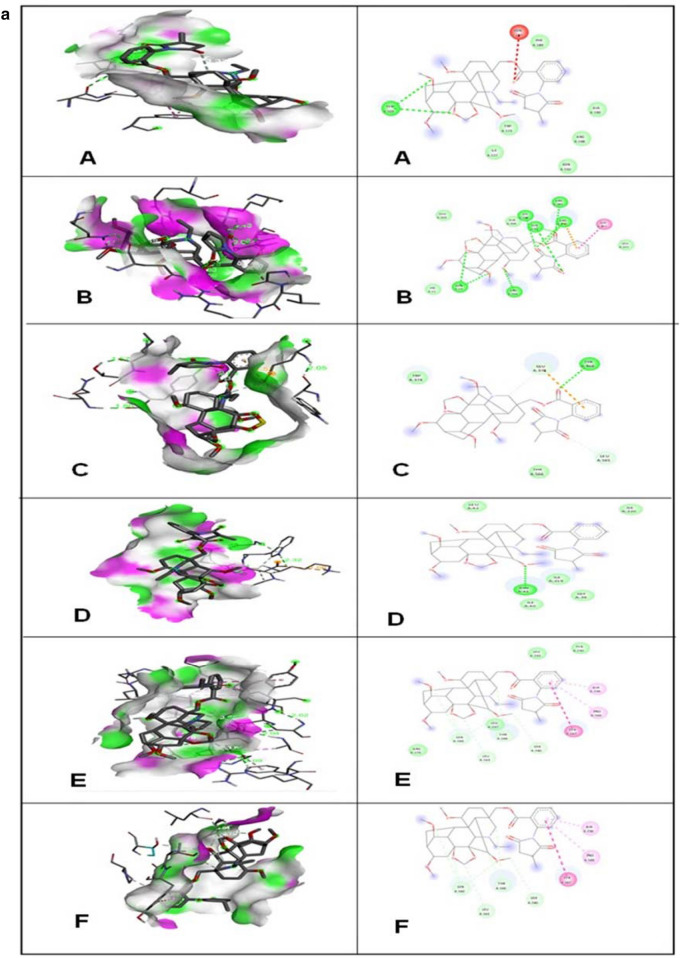
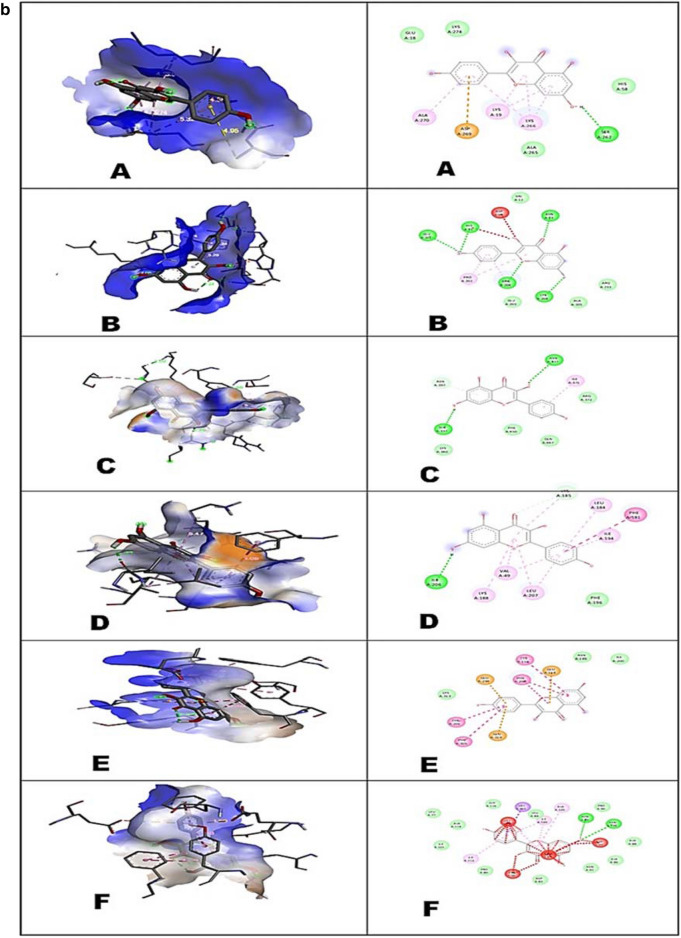
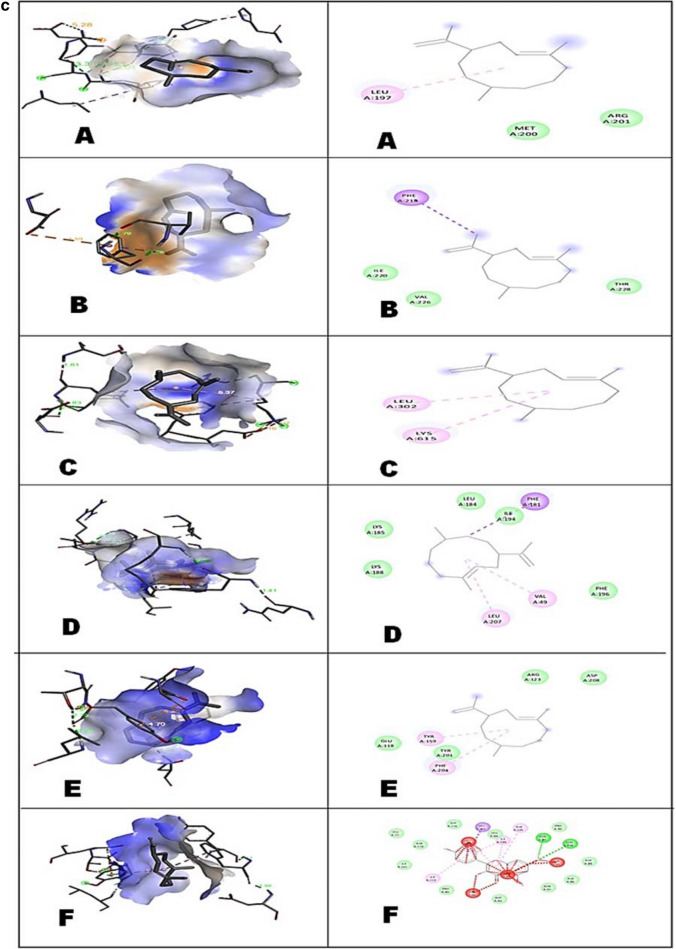
In docking results, the binding affinity (Docking Free energy) and amino acid interactions of the compounds; Kaempferol, Germacrene A and Elatine with selected bacterial drug targets are shown in (Table 5, Fig. 4 a-c). Highest docked score of − 9.2 kcal/mol was showed by elatine against Penicillin Binding Protein (PBP) and the lowest docked score of − 8.2 kcal/mol against the Elongation factor (EF-Tu). The docked structure was imaged to illustrate the ligand (Elatine) interactions with significant amino acids such as Tryptophan (TRP-374), Glutamate (GLU-378), Tyrosine (TYR-568), Threonine (THR-566) and Leucine (LEU-565) of Penicillin Binding Protein (PBP) through Vander Waal forces as well as hydrogen bonding. Ligand (Kaempferol) interacts with significant amino acids such as Phenylalanine (PHE-450), Isoleucine (ILE-371), Asparagine (ASN-377), Serine (SER-337), Lysine (LYS-340) and Arginine (ARG-372), Glutamine (GLN-447) of Penicillin Binding Protein (PBP). The best pose for each molecule was considered to investigate the intramolecular correlations. The docking of ligand elatine with Elongation Factor EF-Tu indicated the binding interactions with significant and functionally relevant amino acids such as Arginine (ARG-204), Alanine (ALA-205), Glutamate (GLU-203), Asparagine (ASN-13), Glycine (GLY-371), Glutamine (GLN-97) and Lysine (LYS-208). The docking studies of kaempferol ligand with elongation factor EF-Tu indicated the binding interactions with significant and functionally relevant amino acids such as Aspartate (ASP-99), Histidine (HIS-11), Glutamate (GLU-201), Asparagine (ASP-13), Proline (PRO-202), Arginine (ARG-204) and Lysine (LYS-208). The docked interaction studies of kaempferol with the dihydropteroate synthase (DHPS) displayed additional pi-cation/anion/alkyl binding, which established interaction with primary amino acids such as Asparagine (ASP-269), Alanine (ALA-270), Lysine (LYS-19) and Serine (SER-262), Histidine (HIS-58) and Glutamate (GLU-18). Furthermore, three targeted fungal proteins were also docked with Elatine, Kaempferol and Germacrene A, and binding affinity results are shown in Table 5. Elatine had showed the highest binding affinity against beta-tubulin with a docked score of − 8.8 kcal/mol followed by ABC transporters (− 6.9 kcal/mol) and 1,3-beta glycan (− 6.7 kcal/mol). The number of hydrogen bonds and amino acid residues involved in the best compound's ligand–protein interaction with three distinct fungal targets is given in (Fig. 4a–c). The docking of Elatine with ABC transporters had showed interaction with amino acids like Leucine (LEU-42), Glycine (GLY-39) and Isoleucine (ILE-40). The docking of kaempferol with ABC transporters had shown interaction with amino acids like Lysine (LLY-188), Leucine (LEU-184), Valine (VAL-49), Phenylalanine (PHE-196) and Isoleucine (ILE-206). Docked interactions of ligand (Elatine) with Beta-Tubulin had shown the interactions with amino acids such as Serine (SER-160), Leucine (LEU-163), Threonine (THR-166), Tyrosine (TYR-167), Proline (PRO-169) and Alanine (ALA-236). Docked interactions of ligand (Kaempferol) with beta- tubulin had shown the interactions with amino acids such as Valine (VAL-79), Tryptophan (TRP-96), Luecine (LEU-87), Alanine (ALA-93 and Isoleucine (113). Elatine interacts with target protein beta-glycan through various amino acids such as Serine (SER-160), Arginine (ARG-175), Tyrosine (TYR-167), Threonine (THR-167), Leucine (LEU-237) and Proline (PRO-169). Kaempferol interacts with target protein beta- glycan through various amino acids such as Lysine (LYS-313), Glutamate (GLU-259), Tyrosine (TYR-159) and Phenylalanine (PHE-305). Previous reports also revealed excellent interaction of naturally isolated compounds from endophytic Penicillium setosum against various microbial drug target proteins42. The details regarding the number of hydrogen bonds shared with the amino acid/nucleotide residues at the active site regions of target proteins are represented in Table 6.
Table 5.
The binding affinity of selected compounds against microbial proteins.
| Ligands | Dihydropteroate synthase (kcal/mol) | Penicillin binding protein (kcal/mol) | Elongation factor EF-Tu (kcal/mol) |
|---|---|---|---|
| Kaempferol | − 8.0 | − 7.9 | − 6.1 |
| Germacrene A | − 7.2 | − 7.1 | − 6.2 |
| Elatine | − 8.5 | − 9.2 | − 8.2 |
| Antifungal proteins | |||
|---|---|---|---|
| 1,3 β-glycan (kcal/mol) | ABC transporter (kcal/mol) | Beta-tubulin (kcal/mol) | |
| Kaempferol | − 6.7 | − 7.3 | − 7.1 |
| Germacrene A | − 7.7 | − 8.7 | − 8.1 |
| Elatine | − 6.7 | − 6.9 | − 8.8 |
Figure 4.
(a) 3D interactions of Ligands with (A) Dihydropteroate synthase (B) Elongation factor Tu and (C) Penicillin Binding Protein (D) ABC transporter (E) 1,3-Betaglycan (F) Beta-tubulin with Elatine and 2D structure of ligands interacted with respective amino acids. Read the text for further information. (b) 3D interactions of Ligands with (A) Dihydropteroate synthase (B) Elongation factor Tu and (C) Penicillin Binding Protein (D) ABC transporter (E) 1,3-Betaglycan (F) Beta-tubulin with Kaempherol and 2D structure of ligands interacted with respective amino acids. Read the text for further information. (c). 3D interactions of Ligands with (A) Dihydropteroate synthase (B) Elongation factor Tu and (C) Penicillin Binding Protein (D) ABC transporter (E) 1,3-Betaglycan (F) Beta-tubulin with Germacrene A and 2D structure of ligands interacted with respective amino acids. Read the text for further information.
Table 6.
Lists the interacting amino acid residues involved in the ligand–protein interaction of the selected compounds against six different targets and bond lengths between the amino acid of the target protein and ligand.
| Ligand | Target proteins | Bond lengths | Interacting amino acids |
|---|---|---|---|
| Kaempferol | Penicillin-binding protein (PBP) | 3.40, 2.02, 2.70, 1.94, 1.88, 2.53 | ILE-371, PHE-450, ASN-377, SER-337, LYS-340, ARG-372 and GLN-447 |
| Kaempferol | Elongation factor Tu (ETU) | 5.29, 2.29, 2.05, 2.89, 2.90, 2.14 | ASP-99, HIS-11, GLU-201, ASP-13, PRO-202, ARG-204 and LYS-208 |
| Kaempferol | Dihydropteroate synthase (DHPS) | 2.40, 2.13, 3.74, 4.10, 5.32, 4.55, 4.58 | ASP-269, ALA-270, LYS-19, SER-262, HIS-58 and GLU-18 |
| Kaempferol | ABC transporter | 5.47, 2.23, 5.05, 4.90, 5.48 | LEU-184, ILE-206, VAL-49, PHE-196 and LYS-188 |
| Kaempferol | 1,3 β-glycan | 2.58, 4.44, 5.28, 3.56, 4.66, 4.69, 5.50 | ASN-199, GLU-259, LYS-313, PHE-305, ASN-159 and ILE-200 |
| Kaempferol | Beta-tubulin | 3.64, 3.46, 2.17, 2.56, 4.44 | VAL-79, TRP-96, LEU-87 and ALA-93 |
| Germacrene A | Penicillin-binding protein (PBP) | 4.35, 4.75, 2.05 | LEU-302 and LYS-615 |
| Germacrene A | Dihydropteroate synthase (DHPS) | 4.77, 5.65, 4,19, 3.01 | MET-200, ARG-201 and LEU-197 |
| Germacrene A | Elongation factor Tu (ETU) | 3.70, 4.86, 0.58, 3.91 | PHE-218, ILE-220, VAL-226 and THR-228 |
| Germacrene A | ABC transporter | 2.04, 5.02, 4.54, 5.16, 5.84 | LEU-184, ILE-194, VAL-49, PHE-181 and GLN-185 |
| Germacrene A | 1,3 β-glycan, | 1.45, 2.35, 5.06, 4.33, 5.01 | PHE-205, ARG-123, ASP-208, GLU-118, TYR-159 and TYR-201 |
| Germacrene A | Beta-tubulin | 2.14, 5.30, 5.39, 1.44, 5.20, 1.91, 3.88 | VAL-79, LEU-87, TRP-96, ALA-93 and ILE-113 |
| Elatine | Penicillin-binding protein (PBP) | 1.80, 1.57, 2.05, 2.88, 3.58 | TRP-374, GLU-378, TYR-568, THR-566 and LEU-565 |
| Elatine | Elongation factor Tu (ETU) | 2.43, 2.79, 2.59, 3.46, 2.65, 1.95 | GLU-203, ASN-13, ARG-204, ALA-205, LYS-208, GLN-97 and GLY-371 |
| Elatine | Dihydropteroate synthase (DHPS) | 2.78, 3.33, 5.09, 3.17 | TYR-103, ASN-147, ILE-122, PHE-123, TRP-189, ILE-150, ARG-148 and ALA-190 |
| Elatine | ABC transporter | 3.32, 4.66, 2.39, 2.90, 2.14, 3.87 | LEU-42, ILE-219, ASN-41 and GLY-39 |
| Elatine | 1,3 β-glycan | 2.02, 2.04, 3.25, 3.30, 3.87, 4.99 | LEU-237, ARG-175, SER-160, THR-166, TYR-167, PRO-169 and ALA-236 |
| Elatine | Beta-tubulin | 3.54, 3.79, 3.38, 3.26, 5.35, 5.36 | SER-160, LEU-163, TYR-167, THR-166, PRO-169 and ALA-236 |
Molecular dynamic simulation
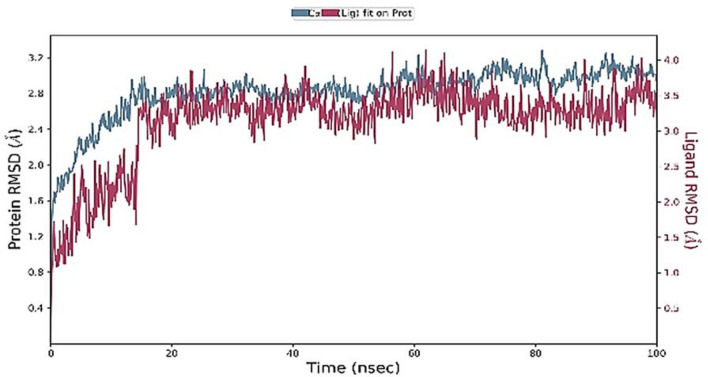
The docking interpretation was validated using the dynamic simulation Desmond Schrodinger tool. MD simulation was carried out to investigate the stability of elatine in the active pocket of beta-tubulin. MD simulation determines the stability and convergence between ligand and target protein. Two independent MD simulation of 100 ns each was run. The root mean square deviation (RMSD), root mean square fluctuations (RMSF), radius of gyration (Rg) and hydrogen bond distances were analysed to establish the related stability (Supplementary file). The RMSD provides information on the stability of the complex. The RMSD of the ligand obtained after least square fit shows few fluctuations during the first 10 ns, and then remained stable until 100 ns time Fig. 5. The internal motion and fluctuations of the residues were analysed by calculating the RMSF. Higher fluctuations were observed to residues forming the loop 1 (Fig. 6) at the region 45 to 50. Other region observed to have large fluctuations is 270 to 290.
Figure 5.
Protein–ligand RMSD plot.
Figure 6.
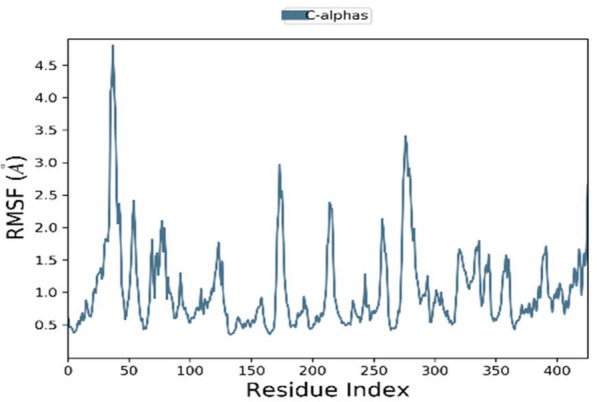
Protein–RMSF plot.
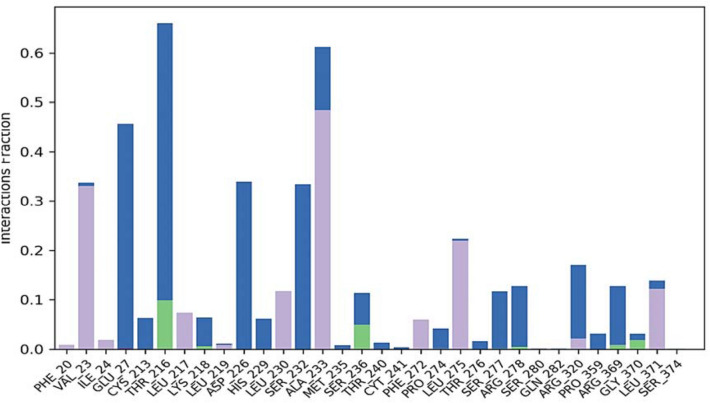
The ligand exposed high water bridges, hydrogen bonding and hydrophobic interactions with the amino acid residue of Threonine 216, Alanine 233, Glutamate 27 as shown in Fig. 7. The protein–ligand contact of amino acid residues of ligand–protein made hydrogen bond contacts with the ligands throughout the simulation time. The overall results of the molecular dynamics showed that elatine compound was stable and interacted with the protein during the simulation period. These results were very well correlated with the results of the molecular docking.
Figure 7.
Hydrogen bond contact analysis of lead compound and elatine–protein complexes. Various intermolecular interactions made by elatine–protein amino acid residues with lead ligand during molecular dynamics simulations. Bar colors: Hydrogen bond (Green), Hydrophobic (Purple), Water bridge (Blue).
Discussion
Phytochemical analysis of G. wallichainum extracts had shown presence of various secondary metabolites such as alkaloid, phenolic, flavonoids, glycosides, saponins and tannins, as depicted in Table 2. Main phytochemicals such as alkaloids have antimicrobial activity and analgesic; tannins and flavonoids contribute as antibacterial and antioxidant agents43; saponins have anticancer, antibacterial, anti-diabetic and anti-inflammatory activities44. The abundance of these phytochemicals in the G. wallichianum extracts might contribute to its therapeutic potential. Several research studies have revealed the antimicrobial activities of the genus Geranium45–47. In this respect, it was essential to investigate the antimicrobial potential of the G. wallichianum, a critical medicinal species of the genus Geranium. The antimicrobial activity may be caused by bioactive compounds, namely phenolics, flavonoids and alkaloids compounds48. Tentative identification of the various extracts by LC–MS investigated many compounds with antimicrobial potential, namely kaempferol49, quercetin50, germacrene D51, caffeic acid52,53 and p-coumaric acid54. The minimum inhibitory concentration (MIC) performed the preliminary screening of antimicrobial activity. Based on the obtained results (Table 4), the highest antimicrobial activity was observed in the case of ethyl acetate extract against various bacterial strains compared to methanol, ethanol and petroleum ether. Plant extracts had showed less sensitivity against three fungal strains. In general, extracts of ethyl acetate had shown the most promising antibacterial potential against M. luteus with MIC value of 3.5 μg/ml followed by H. influenzae, S. pneumoniae, K. pneumoniae, N. mucosa and E. coli (MIC values: 6.25, 12.5, 25, 25, and 100 μg/mL respectively), whereas Candida species were less sensitive to ethyl extract with MIC values of 400 μg/ml respectively. Results of methanol and ethanol extracts were moderately effective against the different microbial strains. Petroleum ether extracts had showed maximum antimicrobial activity against the M. luteus with MIC value of 1.56 μg/ml and the least antimicrobial potential against the E. coli with an MIC value of 100 μg/ml. However, all the four plants extract of G. wallichianum had showed significant antimicrobial activity against the selected microbial pathogens. The antimicrobial potential of various extracts of G. wallichianum might be attributed to the presence of phytocompounds; flavonoids, phenolic acids, alkaloids and diterpenoids. Hence, the results of antimicrobial activity obtained in the present study of four G. wallichianum extracts were correlated with their total polyphenolic contents. Besides, the research conducted confirmed that phenolics were the most significant active compounds against bacterial infection. Previous reports on antimicrobial activities of various species of Geranium genus had also revealed excellent results on various bacterial and fungal strains. In silico investigations have been effectively used to predict the theoretical ligand and target interactions for more complete understanding of the molecular basis of natural product biological activity.
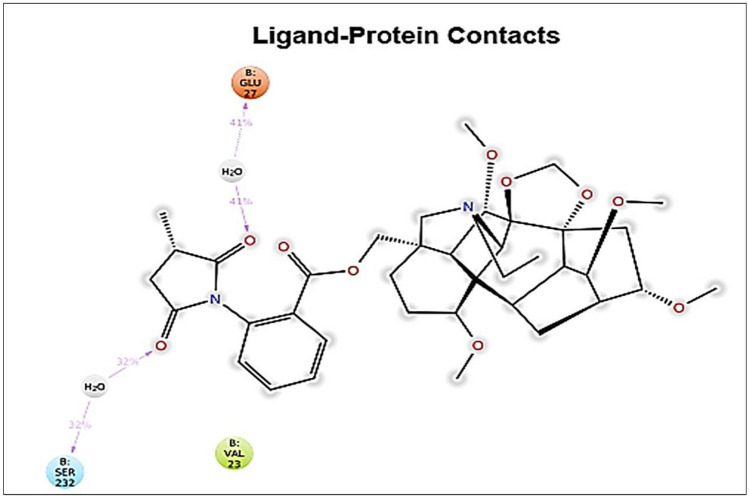
Based on the virtual screening, three compounds viz. Kaempferol, Germacrene A and Elatine were obtained through LC–MS analysis. Ligands identified through LC–MS from G. wallichainum were docked to the active sites of various microbial drug target proteins using AutoDock4.255. Further detailed investigation on interaction of the obtained compounds against different targets involved in various biochemical processes of microbial growth were evaluated using the in-silico approach. Binding energy is a function of the stability of the complex formed between ligand and target protein. It also optimizes new bonds that in turn may affect the biological activity of the resulting complex. To further display various interactions involved between ligands and target protein at the active site, the docked complexes were visualized through Discovery Studio Visualizer56. Among the three compounds, elatine showed highest binding affinity with pencillin binding protein (PBP) followed by kaempherol towards the selected drug targets of bacteria and fungi. There are many different targets through which an antibacterial compound can inhibit cell wall synthesis. Such mechanisms have been regarded as important antibacterial targets for years57. In bacterial cells, penicillin-binding proteins (PBPs) polymerize and modify peptidoglycan, the stress-bearing component of the bacterial cell wall. As part of this process, the PBPs help to create the morphology of the peptidoglycan exoskeleton together with cytoskeleton proteins that regulate septum formation and cell shape. Many natural compounds were reported to inhibit the synthesis of PBP58,59. Interestingly, elatine and kaempherol showed highest binding affinities as compared to already known natural inhibitors against PBP. The highest binding affinity of kaempherol was also supported by molecular simulation study on the interaction between tyrosinase and flavonoids from Sea Buckthorn60,61. Another well-known antimicrobial target is (DHPS) is an essential enzyme in the biosynthesis of dihydrofolate in microorganisms. DHPS is an important target for selective antimicrobial agents. Sulfonamides are the oldest synthetic, effective antimicrobial agents and they target DHPS in bacteria. Other inhibitors, such as diaminodiphenylsulfone (dapsone) or para-aminosalicylic acid (PAS), also inhibit DHPS and are effective against certain mycobacteria (M. tuberculosis, M. leprae). Both kaempherol (flavonoid) and elatine showed significant binding affinities with DHPS. Many known antibacterial drugs interfere with protein synthesis by binding with specific sites on ribosomes, which can also be considered as another important target62. Elongation factor Tu (EF-Tu) is responsible for attachment of aminoacylated tRNA to 16S rRNA A site of 30S rRNA, hence binding of antibacterial compounds to EF-Tu as well 16S rRNA A site leads to translational errors63. In the docking study, it was also found that elatine exhibits significant binding affinity with EF-Tu. The components identified from the antimicrobial active fraction are all reported as plant secondary metabolites. Many plants derived flavonoids have been reported for their broad-spectrum antibacterial action, but few reports are available on the identification of fungal derived antibacterial flavonoids together with detailed aspect of their mechanisms. The docking results obtained with the test ligands were compared with ciprofloxacin, a commercially available antibacterial drug. The docking of the ciprofloxacin against PBP showed a binding energy of − 8.04 kcal/mol. This interaction was achieved by van der Waals forces, pi-pi stacking, pi-alkyl, and alkyl interactions, which probably helped loperamide to intercalate at the binding site of PBP. But these are weaker interactions in comparison to the hydrogen bonds64. In fact, amongst all the intermolecular non-covalent interactions, hydrogen bonds play a central role in the binding of a ligand to the active site of the protein. In the MD simulation, the stable complex system was analyzed for the type of protein ligand interaction in 100 ns of simulation. The interaction with GLU-27, SER-232, and VAL-23 was the most frequent interaction that could be maintained during the simulation. GLU-27 was found to form hydrogen bonds with two hydroxyl groups ofElatine, 41% and 41%, over the 100 ns of simulation time. SER-232 was also found to interact by hydrogen bonding for 32% and 32%% through two hydroxyl groups of Elatine (Fig. 8). These could be considered key residues for the interactions. Conclusively, the in silico molecular docking results describe the interaction of Kaempferol, Germacrene A and Elatine with the penicillin binding protein (PBP), dihydropteroate synthase (DHPS), elongation factor-Tu (Eu-Tu), 1,3 β-glycan, ABC transporter, and beta-tubulin confirm our finding of the plant extract possess antimicrobial activity.
Figure 8.
A schematic of detailed ligand atom interactions with the protein residues. Interactions that occur more than 30.0% of the simulation time in the selected trajectory (0.00 through 100.00 nsec), are shown.
Conclusion
The present study reflects that G. wallichianum has significant antimicrobial activity against various microbial strains. Molecular binding interaction of in-silico data demonstrated that elatine, kaempferol, and germacrene A have more specificity towards the penicillin binding protein (PBP) and beta tubulin binding sites. They could be compounds with a potent antimicrobial activity. This can be further exploited to provide insights into the mechanism of action of potential antimicrobial drugs for resistant bacterial and fungal strains.
Supplementary Information
Author contributions
W.R.M and B.A.B. conducted the experiments, analysed the data, wrote the manuscript; M.A.M conceptualization, designed research work, interpreted the data; M.A.M., B.A.B, W.R.M, M.A.R, S.M, A.A, and S.M.B.A reviewed, edited the manuscript and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by Science and Engineering Research Board, Department of Science and Technology (SERB-DST) Govt. Of India New Delhi; vide Project No: TAR/001213/2018. We highly appreciate SERB-DST for financial assistance. Mustfa F. Alkhanani would like to express his gratitude to Almaarefa University Riyadh, KSA for providing funding support (TUMA-2021-53) to the study.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wajahat Rashid Mir and Basharat Ahmad Bhat.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-16102-9.
References
- 1.Hutchings MI, Truman AW, Wilkinson B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Qadri H, Shah AH, Mir M. Novel strategies to combat the emerging drug resistance in human pathogenic microbes. Curr. Drug Targets. 2021;22:1424–1436. doi: 10.2174/1389450121666201228123212. [DOI] [PubMed] [Google Scholar]
- 3.Sheikh BA, Bhat BA, Ahmad Z, Mir MA. Strategies employed to evade the host immune response and the mechanism of drug resistance in Mycobacterium tuberculosis: In search of finding new targets. Curr. Pharm. Biotechnol. 2021 doi: 10.2174/1389201023666211222164938. [DOI] [PubMed] [Google Scholar]
- 4.Bhat BA, et al. In vitro and in silico evaluation of antimicrobial properties of Delphinium cashmerianum L., a medicinal herb growing in Kashmir, India. J. Ethnopharmacol. 2022;291:115046. doi: 10.1016/j.jep.2022.115046. [DOI] [PubMed] [Google Scholar]
- 5.Sahoo N, Manchikanti P. Herbal drug regulation and commercialization: An Indian industry perspective. J. Altern. Complement. Med. 2013;19:957–963. doi: 10.1089/acm.2012.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheikh BA, et al. Development of new therapeutics to meet the current challenge of drug resistant tuberculosis. Curr. Pharm. Biotechnol. 2021;22:480–500. doi: 10.2174/1389201021666200628021702. [DOI] [PubMed] [Google Scholar]
- 8.Sheikh BA, et al. Nano-drug delivery systems: Possible end to the rising threats of tuberculosis. J. Biomed. Nanotechnol. 2021;17:2298–2318. doi: 10.1166/jbn.2021.3201. [DOI] [PubMed] [Google Scholar]
- 9.Mir MA, Hamdani SS, Sheikh BA, Mehraj U. Recent advances in metabolites from medicinal plants in cancer prevention and treatment. Curr. Immunol. Rev. 2019;15:185–201. doi: 10.2174/1573395515666191102094330. [DOI] [Google Scholar]
- 10.Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9:258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussein RA, El-Anssary AA. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med. 2019;1:13. [Google Scholar]
- 12.Mehraj U, Dar AH, Wani NA, Mir MA. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother. Pharmacol. 2021;87:147–158. doi: 10.1007/s00280-020-04222-w. [DOI] [PubMed] [Google Scholar]
- 13.Bhat BA, Nisar S, Sheikh BA, Mir WR, Mir MA. Antioxidants (Natural and Synthetic) Screening Assays: An Overview. In: Kaur P, Mehta RG, Robin X, Thind TS, Arora S, editors. Bentham Briefs in Biomedicine and Pharmacotherapy Oxidative Stress and Natural Antioxidants. Bentham Science Publishers; 2021. p. 105. [Google Scholar]
- 14.Mir WR, Bhat BA, Almilaibary A, Asdaq SMB, Mir MA. Evaluation of the in vitro antimicrobial activities of Delphinium roylei: An insight from molecular docking and MD-simulation studies. Med. Chem. (Shariqah (United Arab Emirates)) 2022 doi: 10.2174/1573406418666220429093956. [DOI] [PubMed] [Google Scholar]
- 15.Keskes H, et al. LC–MS–MS and GC–MS analyses of biologically active extracts and fractions from Tunisian juniperus phoenice leaves. Pharm. Biol. 2017;55:88–95. doi: 10.1080/13880209.2016.1230139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav R, Khare RK, Singhal A. Qualitative phytochemical screening of some selected medicinal plants of Shivpuri district (MP) Int. J. Life. Sci. Sci. Res. 2017;3:844–847. [Google Scholar]
- 17.Mir MA, Mehraj U, Sheikh BA. Recent advances in chemotherapeutic implications of deguelin: A plant-derived retinoid. Nat. Prod. J. 2021;11:169–181. [Google Scholar]
- 18.Juszczak AM, Zovko-Končić M, Tomczyk M. Recent trends in the application of chromatographic techniques in the analysis of luteolin and its derivatives. Biomolecules. 2019;9:731. doi: 10.3390/biom9110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satapute P, Paidi MK, Kurjogi M, Jogaiah S. Physiological adaptation and spectral annotation of Arsenic and Cadmium heavy metal-resistant and susceptible strain Pseudomonas taiwanensis. Environ. Pollut. 2019;251:555–563. doi: 10.1016/j.envpol.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 20.Fan S, Chang J, Zong Y, Hu G, Jia J. GC-MS analysis of the composition of the essential oil from Dendranthema indicum Var. Aromaticum using three extraction methods and two columns. Molecules. 2018;23:576. doi: 10.3390/molecules23030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes AS, Cruz ECS, Sussulini A, Klassen A. Metabolomic Strategies Involving Mass Spectrometry Combined with Liquid and Gas Chromatography. In: Sussulini A, editor. Metabolomics: From Fundamentals to Clinical Applications. Springer; 2017. pp. 77–98. [DOI] [PubMed] [Google Scholar]
- 22.Loza-Mejía MA, Salazar JR, Sánchez-Tejeda JF. In Silico studies on compounds derived from Calceolaria: Phenylethanoid glycosides as potential multitarget inhibitors for the development of pesticides. Biomolecules. 2018;8:121. doi: 10.3390/biom8040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, Kim D. In-silico molecular binding prediction for human drug targets using deep neural multi-task learning. Genes. 2019;10:906. doi: 10.3390/genes10110906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharathi A, et al. In silico molecular docking and in vitro antidiabetic studies of dihydropyrimido [4, 5-a] acridin-2-amines. BioMed Res. Int. 2014;2014:10. doi: 10.1155/2014/971569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeliger D, de Groot BL. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010;24:417–422. doi: 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaheen S, Bibi M, Hussain H, Iqbal Saira I, Safdar Laraib S. A review on Geranium wallichianum D-Don ex-sweet: An endangered medicinal herb from Himalaya Region. Med. Aromat. Plants (Los Angl.) 2017;6:1000288. [Google Scholar]
- 28.Morgan WTW. Ethnobotany of the Turkana: Use of plants by a pastoral people and their livestock in Kenya. Econ. Bot. 1981;35:96–130. doi: 10.1007/BF02859220. [DOI] [Google Scholar]
- 29.Ilić M, et al. Polyphenol rich extracts of Geranium L. species as potential natural antioxidant and antimicrobial agents. Eur. Rev. Med. Pharmacol. Sci. 2021;25:6283–6294. doi: 10.26355/eurrev_202110_26998. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad M, et al. Use of chemotaxonomic markers for misidentified medicinal plants used in traditional medicines. J. Med. Plants Res. 2010;4:1244–1252. [Google Scholar]
- 31.Qureshi RA, et al. Indigenous medicinal plants used by local women in southern Himalayan regions of Pakistan. Pak. J. Bot. 2009;41:19–25. [Google Scholar]
- 32.Ismail M, et al. Antibacterial, antifungal, cytotoxic, phytotoxic, insecticidal, and enzyme inhibitory activities of Geranium wallichianum. Evid.-Based Complement. Altern. Med. 2012;2012:8. doi: 10.1155/2012/305906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ismail M, et al. Chemical constituents and antioxidant activity of Geranium wallichianum. Rec. Nat. Prod. 2009;3:193. [Google Scholar]
- 34.Woźniak Ł, Skąpska S, Marszałek K. Ursolic acid—A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules. 2015;20:20614–20641. doi: 10.3390/molecules201119721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maillard MP, Wolfender J-L, Hostettmann K. Use of liquid chromatography—Thermospray mass spectrometry in phytochemical analysis of crude plant extracts. J. Chromatogr. A. 1993;647:147–154. doi: 10.1016/0021-9673(93)83334-O. [DOI] [Google Scholar]
- 36.Andrews JM. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 37.Berman HM, et al. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian W, Chen C, Lei X, Zhao J, Liang J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018;46:W363–W367. doi: 10.1093/nar/gky473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, et al. PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009;37:W623–W633. doi: 10.1093/nar/gkp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrodinger, L. L. C. The PyMOL molecular graphics system. Version1, 0 (2010).
- 41.Bowers, K. J. et al. 43–43 (IEEE).
- 42.George TK, Joy A, Divya K, Jisha MS. In vitro and in silico docking studies of antibacterial compounds derived from endophytic Penicillium setosum. Microb. Pathog. 2019;131:87–97. doi: 10.1016/j.micpath.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Yan Y, et al. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics. 2021;10:318. doi: 10.3390/antibiotics10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou Y. Naturally occurring steroidal saponins as potential anticancer agents: Current developments and mechanisms of action. Curr. Top. Med. Chem. 2022 doi: 10.2174/1568026622666220330011047. [DOI] [PubMed] [Google Scholar]
- 45.Bigos M, Wasiela M, Kalemba D, Sienkiewicz M. Antimicrobial activity of geranium oil against clinical strains of Staphylococcus aureus. Molecules. 2012;17:10276–10291. doi: 10.3390/molecules170910276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wafa N, Sofiane G, Ouarda D. Antioxidant, antimicrobial and anti-inflammatory activities valorisation of methanol extract of two Geranium species growth in Setif Algeria. Int. J. Pharma Res. Health Sci. 2017;5:1698–1702. doi: 10.21276/ijprhs.2017.03.03. [DOI] [Google Scholar]
- 47.Renda G, Celik G, Korkmaz B, Karaoglu SA, Yayli N. Antimicrobial activity and analyses of six Geranium L species with headspace SPME and hydrodistillation. J. Essent. Oil Bear. Plants. 2016;19:2003–2016. doi: 10.1080/0972060X.2016.1235995. [DOI] [Google Scholar]
- 48.Kazłowska K, Hsu T, Hou C-C, Yang W-C, Tsai G-J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010;128:123–130. doi: 10.1016/j.jep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 49.Calderon-Montano JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 50.Maalik A, et al. Pharmacological applications of quercetin and its derivatives: A short review. Trop. J. Pharm. Res. 2014;13:1561–1566. doi: 10.4314/tjpr.v13i9.26. [DOI] [Google Scholar]
- 51.Belmekki N, Bendimerad N, Bekhechi C. Chemical analysis and antimicrobial activity of Teucrium polium L. essential oil from Western Algeria. J. Med. Plants Res. 2013;7:897–902. [Google Scholar]
- 52.Chong KP, Rossall S, Atong M. In vitro antimicrobial activity and fungitoxicity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against Ganoderma boninense. J. Agric. Sci. 2009;1:15. [Google Scholar]
- 53.Magnani C, Isaac VLB, Correa MA, Salgado HRN. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods. 2014;6:3203–3210. doi: 10.1039/C3AY41807C. [DOI] [Google Scholar]
- 54.Boz H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015;50:2323–2328. doi: 10.1111/ijfs.12898. [DOI] [Google Scholar]
- 55.Morris GM, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- 56.Karadayi FZ, Yaman M, Kisla MM, Konu O, Ates-Alagoz Z. Design, synthesis, anticancer activity, molecular docking and ADME studies of novel methylsulfonyl indole-benzimidazoles in comparison with ethylsulfonyl counterparts. New J. Chem. 2021;45:9010–9019. doi: 10.1039/D1NJ01019K. [DOI] [Google Scholar]
- 57.Bruning JB, Murillo AC, Chacon O, Barletta RG, Sacchettini JC. Structure of the Mycobacterium tuberculosis D-alanine: D-alanine ligase, a target of the antituberculosis drug D-cycloserine. Antimicrob. Agents Chemother. 2011;55:291–301. doi: 10.1128/AAC.00558-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Daniel PI, et al. Discovery of a new class of non-β-lactam inhibitors of penicillin-binding proteins with gram-positive antibacterial activity. J. Am. Chem. Soc. 2014;136:3664–3672. doi: 10.1021/ja500053x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newman H, et al. High-throughput crystallography reveals boron-containing inhibitors of a penicillin-binding protein with di-and tricovalent binding modes. J. Med. Chem. 2021;64:11379–11394. doi: 10.1021/acs.jmedchem.1c00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, et al. Molecular simulation study on the interaction between tyrosinase and flavonoids from sea buckthorn. ACS Omega. 2021;6:21579–21585. doi: 10.1021/acsomega.1c02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh K, Coopoosamy RM, Gumede NJ, Sabiu S. Computational insights and in vitro validation of antibacterial potential of Shikimate pathway-derived phenolic acids as NorA efflux pump inhibitors. Molecules. 2022;27:2601. doi: 10.3390/molecules27082601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brodersen DE, et al. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell. 2000;103:1143–1154. doi: 10.1016/S0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 63.Hong W, Zeng J, Xie J. Antibiotic drugs targeting bacterial RNAs. Acta Pharm. Sin. B. 2014;4:258–265. doi: 10.1016/j.apsb.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berg, L. Exploring non-covalent interactions between drug-like molecules and the protein acetylcholinesterase. (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].