Abstract
Arthritis is a common clinical disease that affects millions of people in the world. The most common types of arthritis are osteoarthritis and rheumatoid arthritis. Inflammatory arthritis (IA), a chronic painful disease, is characterized by synovitis and cartilage destruction in the early stages. Pathologically, IA causes inflammatory changes in the joints and eventually leads to joint destruction. Pain is associated with inflammation and abnormal regulation of the nervous system pathways involved in pain promotion and inhibition. In addition, the occurrence of pain is associated with depression and anxiety. We found that there are many factors affecting pain, in addition to inflammatory factors, glutamate receptor may be the possible cause of long-term chronic pain caused by IA. N-methyl-d-aspartate receptor subunit 2B (NR2B) has been reported to involved in IA and nervous system diseases, especially peripheral neuropathic pain. In this review, we summarized the mechanisms of the NR2B subunit of the N-methyl-D-aspartate (NMDA) receptor in peripheral nerve sensitization during IA and chronic pain.
Keywords: inflammatory arthritis, peripheral sensitization, neuropathic pain, inflammatory factors, NR2B subunit
Introduction
Arthritis is a general term for a class of conditions that include aseptic inflammation of the joints and degenerative osteoarthritis caused by immunological diseases.1 Inflammatory arthritis (IA), a form of joint inflammation caused by overacted immune system, is characterized by synovitis and cartilage destruction in the early stages. Rheumatoid arthritis (RA) and osteoarthritis (OA) are the two most common types of arthritis. Often associated with genetic factors, RA is a primarily aggressive joint inflammation. From an immunological perspective, RA is caused by increased inflammation level in the body, which is due to autoimmune dysfunctions and stimulation of inflammatory factors (IFs) in the joints. Clinical incidence of RA is lower than that of OA,2 which mainly occurs in multiple large joints3,4 of the whole body and joints that support relatively lightweight, such as sternoclavicular, acromioclavicular, and wrist joints.5–7 The incidence of OA is significantly associated with age-related synovial degeneration,8 genetic susceptibility and epigenetic regulatory factors.9
Over 20% of patients seek medical assistance because of chronic pain10 that often lasts for more than three months.11 The leading cause of chronic pain is destruction of the articular cartilage and the resulting inflammation from biomechanical damage of joints in IA. When the course of the disease reaches a specific duration, the condition turns to chronic pain,12,13 and the degree of chronic pain is positively correlated with levels of IFs. Wall et al14 reported that pro-inflammatory cytokine signaling between immune cells, glial cells, and nerve cells plays critical role in the development of pathological pain; and modulating pro-inflammatory cytokine communication could offer substantial pain relief. However, even completely regular laboratory examinations such as erythrocyte sedimentation rate and C-reactive protein for expression of inflammatory activities are administrated, patients still show pain symptoms,15,16 which indicates that in addition to IFs, there are other factors that contribute to pain occurrence. When RA patients are in the inactive stage, they still report pain, especially for that linked to the central nervous system (CNS) and neuropathic pain (NP).17,18
Long-term chronic pain is associated with peripheral sensitization that is the key condition for NP. Treatment of chronic pain has gradually shifted towards improving peripheral nerve functions in recent years.19 Peripheral sensitization occurs when immune cells lead to plastic changes in the dorsal root ganglion (DRG) and spinal dorsal horn.20 As a result of ectopic implantation of nerves, peripheral sensitization develops to NP or central pain that could further cause disease complications.21,22 With an incidence rate about 15.7%, NP is a chronic pain syndrome that is caused by peripheral nerve injuries.23 It is also associated with degradation of neurological functions.24 The best treatment strategies for neuralgia involve a multidisciplinary team approach that includes cognitive behavior intervention and rehabilitation for treating psychosomatic complications.25 Although the N-methyl-D-aspartic acid (NMDA) receptor subunit 2B (NR2B) has been postulated as one of the proteins involved in both IA and NP; the role of the NR2B subunit has not been fully examined. Therefore, the purpose of this study is to provide a state-of-the-art review for studies focusing on NR2B subunits in IA and NP; and propose a theoretical signal pathway of NR2B in IA and NP.
Glutamic Acid and Structural Composition of the NR2B Subunit
Activity and expression of protein glutamate receptor play an important role in human CNS diseases. Although NMDAR has no specific cations that can pass through its membrane, it is involved in the transfer of excitatory inputs from primary sensory neurons to the brain via the spinal cord. In chronic inflammatory pain and neuropathic pain, activation of NMDAR promotes central sensitization. For instance, glutamic acid is closely involved in CNS toxicity, Alzheimer’s disease, epilepsy, and other CNS disorders.26–28 There are two types of glutamate receptors: metabolic receptors (mGluRs) that are G protein-coupled metabolic receptors belonging to the 3G protein-coupled receptor (GPCRs) family;29 and ionic receptors (iGluRs) that are composed of four subfamilies, including NMDA receptor (NMDAR), kainic acid receptor (KAR), α-amino-3hydroxy-5methyl-4isoxazole receptor (AMPAR), and δ receptor (GluD).30
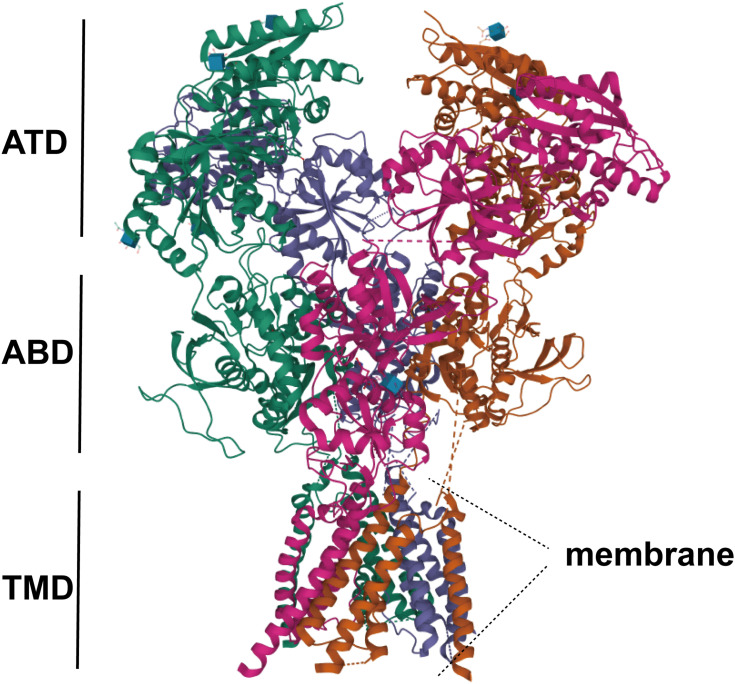
As members of glutamate receptors, NMDARs play a critical role in CNS development and functioning (Figure 1).31 NMDARs, including AMPA and KAR receptors,32 form a tetramer structure consisting of three subfamilies that share substantial homology and are closely related in structure,33 e.g., two GluN1 subunits and two GluN2 or GluN3 subunits. Among them, GLuN1 is the basic unit of NMDA. The NMDA receptor is usually composed of two GLuN1 that is associated with the performance of human mental symptoms. Its auto antibodies often cause autoimmune encephalitis,34 which mainly target the extracellular region of GLuN1, showing a highly specific immune syndrome.35 Different from GLuN1 and 3, GLuN2 consists of four subunits that play related roles in the central nervous system, and binds glutamate.36 Through the c-terminal domain of the GluN2 subunit, NMDA connects to a huge signal transduction and scaffold protein network on the cytoplasmic side.37 The network interaction builds the basis for the downstream signal cascades of NMDA.38 Among them, GluN2A and GluN2B are linked to membrane-associated guanosine kinase (PSD-MAGUKs) of the postsynaptic density family. Ca2+/calmodulin-dependent kinase II (CaMKII) interacts with synuclein messengers and plays a key molecular role in gene transcription.39 Composed of two subunits–GluN3A and GluN3B, GluN3 has multiple roles in general biophysics, and signal transmission,40 and is associated with addiction and neurodegenerative diseases. In the CNS, there are four different GluN2 subunits (NR2A-D), which are categorized by their spatially and developmentally regulated expression in cellular and synaptic specific ways.
Figure 1.
NMDA is composed of a highly homologous extracellular amino-terminal (ATD), a bilobal ligand-binding domain (LBD), three transmembrane regions, a membrane helix (MD) composed of a reentrant ion pore-lining ring, and a more dispersed intracellular carboxy-terminal domain (CTD). Picture excerpt from Vyklicky et al (PDB code 4TLM).
The NR2B subunit, a widely studied NMDAR, is made up of 1456 amino acids with molecular weight of 170–180kDa. Structurally, the extracellular NR2B subunit has an NH2-terminal peptide and four transmembrane sums (M1-M4), of which M1-M3 belong to the transmembrane helix structure, M2 faces the cytoplasm, and the inner membrane is reversely folded to form an ion channel.41 The NR2B cells have the C2-terminal region of the basic structure of GLuN2, and the crucial part of the ligand is spherical, which is divided into two areas, S1 and S2. NMDARs play important roles in neurons and dendrites. For instance, NR2B-related structures are closely associated with NP, and NR2B plays a critical role in formation of dendrogram branches. Ras-specific guanine nucleotide-releasing factor 1 (RasGRF1) is a guanine nucleotide exchange factor that directly interacts with NR2B. The NR2B/RasGRF1 signaling pathway induces superoxide production and promotes dendrite formation.42 Plasticity of the dendritic structure is correlated with nervous system diseases.43 In CNS diseases, such as stroke, Alzheimer’s disease, and epilepsy (Parkinson’s disease, PD), among other conditions, the levels of the NR2B subunit have been shown to be significantly increased.44–46
Activation Mechanisms of the NR2B Subunit in Pain
Under normal physiological conditions, the NR2B subunits are stable and are post-synoptically located. Activation of the NR2B subunit results in separation of ions from NR2B, which alters ion permeability. Persistence of chronic pain induces NR2B subunit activation through presynaptic glutamate release and related excitatory postsynaptic potentials (EPSPs). The increased EPSPs further lead to separation of Mg2+ from the NR2B subunit, elevating EPS level,47 which aggravates chronic pain. The S-nitrosation of Src is caused by the nNOS/nitric oxide signal. Ca2+ influx of the NR2B subunit is excessively activated, promoting tyrosine phosphorylation of Src, which further phosphorylates NR2B.48 Since the NR2B structure is specific for switching ion channels, activation of the NR2B subunit results in changes in concentrations of intracellular and extracellular ions under actions of voltage-gated ion channels,49 leading to pain occurrence. GPCRs stimulation induces NR2B subunit activation and promotes CNS plasticity.
Phosphorylated expressions of the NR2B subunits are also involved in pain occurrence. The binding of NMDA receptors and transmitters induces intracellular cascade reactions, triggering a series of biochemical reactions, leading to changes in synaptic structures and functions. These synaptic changes enhance excitatory synaptic transmissions, resulting in chronic pain. Studies of the CNS have shown that the NR2B subunit is the most important tyrosine phosphorylated protein, and calcium influx is the main outcome of NR2B phosphorylation.50 In addition, tyrosine phosphorylation (ThetyrosinephosphorylationofNR2B, NR2B-pTyr) of NR2B is involved in pain occurrence through central sensitization caused by synaptic plasticity. During phosphorylation, expressions of pNR2B-Y1472 and pNR2B-Y1252 as well as synaptic-related proteins (PSD95, SYP and SYT-1) are significantly increased. Thus, phosphorylation of NR2B subunits plays an important role in the occurrence of central nervous system pain.51 Post-synaptic activation of NMDA leads to calcium influx, which causes activation of Ca(2+)/calmodulin-dependent protein kinase (CaMKII) and expressions on NR2B subunits. In vivo, the main binding site of CaMKII on NR2B undergoes Ser1303 phosphorylation, which enables NR2B subunits to distinguish between Ca(2+)/calmodulin activated forms and self-activated thr286-CaMKII autophosphorylation forms. The intracellular NR2B non-mutant binds calcium ions, promoting calcium influx and neuropathic pain occurrence. NMDAR is controlled by GPCR to regulate NMDA-mediated current effects, which may provide a new therapeutic target for chronic pain.52
NMDA and NR2B Receptor Antagonists
Mechanical ectopic pain related to oxaliplatin is a common side effect. Applications of the NR2B antagonist, Ro25-6981, and fendil can significantly reduce oxaliplatin-induced pain, and the effect is more noticeable when nitric oxide synthase (NOS) inhibitor is used as the downstream target of NR2B.53 Pain occurrence is positively correlated with oxaliplatin dose. Oral administration of the traditional Chinese medicine (Kei-kyoh-zoh-soh-oh-shin-bu-toh, KSOT) reduced the number of astrocytes and the level of inhibited NR2B.54 For cancer-induced neuropathic pain, especially bone cancer pain (BCP), in addition to the above mechanisms, NR2B is present in astrocytic cells. Interactions between Cx43 and neuronal NMDARs play important roles in astrocyte-neuronal signaling in the spinal cord.55 For instance, when phosphorylated NR2B (p-NR2B) activities are high, the Cx43/p-NR2B signaling pathway affects BNP occurrence. In BCP treatment, etanercept has a good therapeutic effect on bone cancer-induced mechanical ectopic pain,56 by reducing the expressions of NR2B through the MrgC-NR2B signaling pathway and relieving neuralgia for bone cancer pain. Dopamine D1 and D2 receptors eliminate glutamate receptor-mediated spinal cord neuronal activation via Src kinase in a g α Q protein-dependent manner, resulting in suppressed NR2B activities.52 In a study involving chronic contractile injury animal models (CCI), the number of NR2B immune reactive cells are significantly increased with low-frequency acupoint electro acupuncture stimulation.57 In Spared Nerve Injury (SNI) animal models,58 two types of NP models, tibial-SNI variant and sural-SNI, were used. Animal behavior tests and immune studies revealed that elevated NR2BmRNA levels are associated with persistent hypersensitivity in sural-SNI. Increased suppression of NR2BmRNA and retention of NR2B proteins around the somatic nucleus of neurons is associated with the recovery of tibial-SNI hypersensitivity.
The radiofrequency technique (radiofrequency ablation, RFA) around the knee joint is used to treat knee joint OA and RA pain.59 Peripheral nerves of knee joints mainly include superior medial knee nerves (superior medial genicular nerve, SMGN), superior lateral knee nerve (superior lateral genicular nerve, SLGN), intermediate knee nerve (middle genicular nerve, MGN), inferior lateral knee nerve (inferior lateral genicular nerve, ILGN), lateral retinaculum nerve (lateral retinacular nerve, LRN), medial inferior knee nerve inferior medial genicular nerve (IMGN), and posterior genicular nerve plexus (PGN). PGNs are involved in the occurrence of knee joint pain. Treatment of these nerves has been shown to have satisfactory clinical effect for relieving knee joint pain, without changing the internal structures of joint cavities, synovium degeneration and cartilage injury degree.60 However, the mechanisms involved in this treatment have not been elucidated, which may relate to changes of gated ion channels caused by NMDAR activation due to long-term pain. We postulated that because activation and expression of the NR2B subunit play an important role in peripheral nerve sensitization of the knee joint; thus, the radiofrequency technique that reduces nerve sensitivity and kills painful nerve fibers such as the C fiber can effectively reduce the pain. Therefore, the successful relief of knee joint pain provides a new piece of evidence supporting theoretical hypothesis of knee joint radio frequency.
In clinical applications of NMDA antagonists, Berman et al61 tested the effect of low-dose ketamine, which is a rapid and continuous antidepressant, and found that repeated heavy use of ketamine cause certain neurotoxicity that leads to cognitive and memory impairments. Due to these side effects, extensive research efforts have been devoted to developing safe and effective drugs in clinical studies. For example, a NR2B selective, moderate NMDA receptor antagonist–Neu2000, which is a molecule obtained from derivatives of sulfasalazine and aspirin, shows good, cell membrane permeability, spin capture antioxidation effects,62 and achieves promising efficacies in human Phase 1 studies and non-clinical trials. The NMDA receptor antagonist EVT101 and the NR2B receptor antagonists TXT-0300 (TraxionTreeutics and MK-0657) are also used to treat central nervous system diseases, mental diseases and pain diseases. However, clinical trials of the other related drugs have been sparse.63 In contrast, the NR2B receptor antagonists have been widely evaluated in animal experiments. Applications of NR2B receptor antagonists in dyskinesia models (levodopa-induced dyskinesia, LID) revealed that CP101, 606 can effectively suppress pNR2B-Tyr1472 and its interactions with Fyn, as well as inhibit phosphorylations and pCaMKII-Thr286 levels,64 which echoes the mechanisms of pain caused by phosphorylated expressions of NR2B. In addition, previous study reported that in trathecal injections of If enprodil and Ro25-6981 could effectively suppress NR2B concentrations and reduce the threshold of mechanical pain, but have no effect on motor nerves.65 These results indicated that the NMDA receptor NR2B subunit plays a key role in development and maintenance of chronic pain, which could be a good target point in pain control therapy. The NR2B receptor antagonists also play an important role in neuralgia. A study of neuronal pain in the central amygdale oid nucleus (central nucleus of the amygdala, CEA), found that the NR2B receptor antagonist (Ro-256981) effectively inhibits pain occurrence in CEA neuronal pain in joint harmless and harmful stimulations, which offers the potential for inducing neuralgia in arthritis models. Studies have also reported that along with other factors NMDA receptors containing NR2B subunits contribute to pain-related changes of CEA neurons.66
Relationship Between the NR2B Subunit and IFs
Biological Ifs such as interleukin (IL), tumor necrosis factor (TNF), substance P, and prostaglandin E2 (PGE2) are positively correlated with pain occurrence.14 Elevated IFs levels are significantly associated with aggravated pain. In active periods of disease, increase in expressions of Ifs is coupled with escalation of NR2B levels.67 Moreover, the levels of IFs such as TNF-α, NR1 and NR2B were significantly raised in induced neuroinflammation; while NR2A levels were suppressed,67 suggesting that inflammation plays a role in regulating the functional activities of the NR2B subunit. In a whole-cell patch-clamp experiment, IL-2 reduces the peak amplitude of NMDAR-mediated current containing NR2A and NR2B by 54 ± 5% and 30 ± 4%, respectively,68 indicating that NR2B subunit levels vary with IL levels. A study on spinal cord injury (SCI) found that TNF-α and NR2B subunit levels that are related to Ifs in neuralgia are significantly increased in the second stage of inflammation of SCI.69 Similar findings were also reported in IA.70
The above findings suggested that Ifs are closely associated with NR2B levels, and inhibition of IF expressions reduces the expressions of the NR2B subunit, which further alleviates pain. The NR2B subunit plays a key role in pain occurrence. For instance, consistent with findings reported byAl-Khrasani,71 during inflammation process, a glutamatergic neurotransmitter may be transferred to the NR2B subunit to exert its effects, causing pain occurrence.
Expressions of the NR2B Subunit in IA
Except for a few, previous studies on NMDA in articular cartilage are limited. In IA pathogenesis, NMDA stimulates NMDAR in chondrocytes. As a non-selective cation channel, NMDAR increases calcium influx and membrane depolarization, and provides a corresponding pathway for the influx of potassium, sodium, calcium and other gated ion channels, facilitating transmission of the glutamate signal. Moreover, measurements of calcium influx and cell membrane potential in OA chondrocytes evidenced that NMDAR provides an influx pathway for calcium ions and induces depolarization.72
NR2B has been closely linked to IA. Previous study reported that significant increase in NR2B levels is detected in IA, while NR2B is involved in IA pathogenesis as an essential protein substance.73 Kalev-Zylinska73 analyzed the articular chondrocyte clock gene and the phenotype of chondrocytes using real-time quantitative PCR, and found that when NR2B subunit levels are decreased due to conditional knockout, period2 and BMAL1 expressions return to a level similar to that of normal chondrocytes, suggesting that NR2B has a significant impact on IA pathogenesis. Suppression of NR2B levels contributes to a favorable IA transformation. Previous studies on temporomandibular arthritis (TMJ) found that magnesium chloride inhibits NR2B mRNA levels and induces the rearrangement of NMDAR subunits,74 thereby it suppresses joint inflammation-mediated facial pain. In immune-mediated arthritis, a large number of pro-inflammatory cytokines are found in the synovial fluid of RA patients, and up-regulated expressions of Ifs enhance the activation of the NR2B receptor, promoting the occurrence of immune-mediated arthritis.75 In general, NR2B does not exist in normal cartilages. The NR2B subunit induces pathological changes in chondrocytes and leads to chondrocyte apoptosis, thereby accelerating IA progression. The NR2B subunit also induces peripheral sensitization in long-term chronic pain.76 These findings indicated that NR2B is a novel research target for IA pathogenesis and pain management.
Expressions of the NR2B Subunit in NP
Apart from its high level of expression in CNS diseases, NR2B has significant activities in NP.77 Studies on mechanisms of NR2B against NP have shown that various signaling pathways affect changes in NR2B levels, causing NP occurrence.42,78 Dendritic structural plasticity has a clear correlation with NP,79 and the NR2B/RasGRF1 signaling pathway induces the generation of superoxide and promotes the formation of dendrites,42 leading to changes in neural structures. NR2B is located in layer II of the spinal dorsal horn; and the level of NR2B in DRG significantly increases during NP. Studies using animal models of mechanical ectopic pain have reported that the cyclic adenosine monophosphate (cAMP) signal plays a vital role in regulating pain sensitivity through the exchange protein directly activated by cAMP (Epac), which significantly increases NR2B protein levels in ipsilateral DRG.80 NR2B was found to be an essential downstream expression factor in Epac1‑Piezo2 signaling pathway for long-term mechanical ectopic pain induced by plantar injection of the Epac agonist, 8-CPT. This indicated that the NR2B subunit is strongly associated with signaling pathways during NP, in which inhibits the conduction of signaling pathways and reduces the expressions of NR2B, as one of the possible mechanisms that curb further NP development.
The pathogenic role of NR2B in NP has also been investigated in literature. When low-frequency acupoint electroacupuncture stimulation was applied in animal models for chronic contractile injury (CCI), the number of NR2B subunits and that of immune reactive cells were significantly increased.57 In spared nerve injury (SNI) animal models of NP,58 two variants of SNI–tibial-SNI and sural-SNI were studied. Animal behavior tests and immune studies showed that elevated NR2B mRNA levels are associated with the occurrence of sural-SNI continuous hypersensitivity. Suppression of NR2B mRNA and retention of NR2B protein levels in perinuclear regions of the neuronal somata are coupled with recovery of tibial-SNI hypersensitivity. The NR2B subunit is involved in various aspects of nervous system diseases. In CNS diseases or peripheral NP, elevated NR2B levels produce different degrees of influence on the structure and functions of neurons, leading to the occurrence of nervous system diseases.
Conclusions
During clinical diagnosis and treatment, there are many OA or RA patients who complained of pain, but little evidence of inflammatory-related causes, such as ESR and C-reactive protein can be found. Therefore, it is worthy to explore the reasons behind it. Clinical practice showed that the pain symptoms of patients with knee osteoarthritis are significantly relieved after the treatment of peripheral nerve, suggesting a potential link between arthralgia and neuralgia. Previous studies had reported that NR2B subunit is one of the key reasons for connecting neuralgia and arthralgia, and inflammatory arthritis may lead to chronic pain under the influence of early inflammatory factors. Long lasting inflammatory factors may result in changes at the level of ionic glutamate, which could in turn develop into NP.
Given the high prevalence of chronic pain in the general population, chronic pain management poses a major clinical challenge for medical delivery system. Therefore, understanding the fundamental causes of chronic pain syndromes and develop appropriate treatment would provide substantial assistance to alleviate the problem.
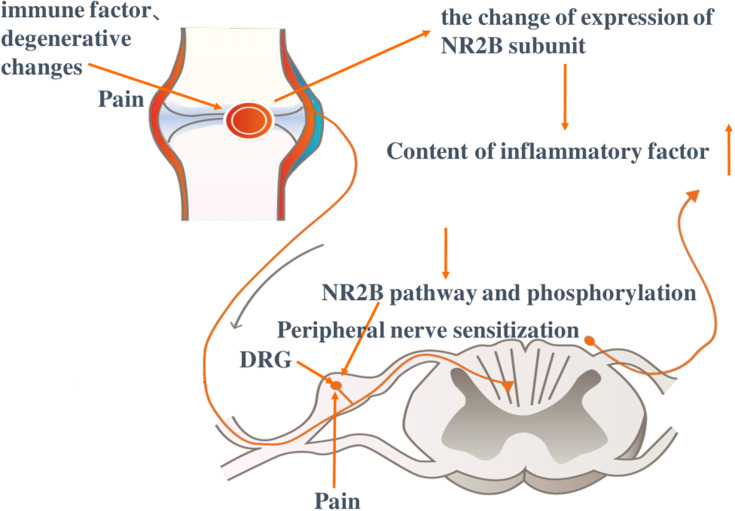
Positively associated with levels of IFs including TNF, IL, and P, IA and NP are two common diseases that cause chronic pain. As an essential pathogenic factor, NR2B subunit is associated with Ifs and is involved in both IA and NP development. Although there is a lack of evidence that up-regulation of Ifs in IA and NP always induces to chronic pain, it is related to nerve sensitization processes caused by long-term pain. Thus, IA may be initiated by peripheral sensitization, which finally leads to NP transformation (Figure 2).
Figure 2.
Induction of immune destruction and degeneration leads to occurrence of inflammatory arthritis and pain. The NR2B subunit is activated and its content as well as phosphorylation levels increase, which is associated with the expressions of inflammatory factors. Dorsal root ganglion (DRG) cells exhibit a sensitization reaction under the influence of the NR2B signal pathway and phosphorylation. Taking the NR2B subunit as the target, reactivity in DRG causes changes in inflammatory factors and gated ion channels in neurons and Ca2+ influx, resulting in peripheral sensitization and long-term chronic pain. Picture excerpt from Buch et al.81
Therefore, treatment of IA cannot be achieved without considering NP. The NR2B subunit might be an essential therapeutic factor. However, the exact mechanisms are still unclear, which requires further studies.
Funding Statement
This study is funded by Gansu Province Natural Science Foundation Project (ID: 20JR10RA736).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Udomsinprasert W, Jittikoon J, Honsawek S. Interleukin-34 as a promising clinical biomarker and therapeutic target for inflammatory arthritis. Cytokine Growth Factor Rev. 2019;47:43–53. doi: 10.1016/j.cytogfr.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 2.Letarouilly JG, Salmon JH, Flipo RM. Factors affecting persistence with biologic treatments in patients with rheumatoid arthritis: a systematic literature review. Expert Opin Drug Saf. 2021;20(9):1087–1094. doi: 10.1080/14740338.2021.1924146 [DOI] [PubMed] [Google Scholar]
- 3.Sharma L, Solomon CG. Osteoarthritis of the knee. N Engl J Med. 2021;384(1):51–59. doi: 10.1056/NEJMcp1903768 [DOI] [PubMed] [Google Scholar]
- 4.Rees HW. Management of osteoarthritis of the hip. J Am Acad Orthop Surg. 2020;28(7):e288–e291. doi: 10.5435/JAAOS-D-19-00416 [DOI] [PubMed] [Google Scholar]
- 5.Martetschläger F, Warth RJ, Millett PJ. Instability and degenerative arthritis of the sternoclavicular joint: a current concepts review. Am J Sports Med. 2014;42(4):999–1007. doi: 10.1177/0363546513498990 [DOI] [PubMed] [Google Scholar]
- 6.Menge TJ, Boykin RE, Bushnell BD, et al. Acromioclavicular osteoarthritis: a common cause of shoulder pain. South Med J. 2014;107(5):324–329. doi: 10.1097/SMJ.0000000000000101 [DOI] [PubMed] [Google Scholar]
- 7.Wu JC, Calandruccio JH. Evaluation and management of scaphoid-trapezium-trapezoid joint arthritis. Orthop Clin North Am. 2019;50(4):497–508. doi: 10.1016/j.ocl.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 8.Sacitharan PK. Ageing and osteoarthritis. Subcell Biochem. 2019;91:123–159. doi: 10.1007/978-981-13-3681-2_6 [DOI] [PubMed] [Google Scholar]
- 9.Geyer M, Schönfeld C. Novel insights into the pathogenesis of osteoarthritis. Curr Rheumatol Rev. 2018;14(2):98–107. doi: 10.2174/1573397113666170807122312 [DOI] [PubMed] [Google Scholar]
- 10.Pak DJ, Yong RJ, Kaye AD, et al. Chronification of pain: mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep. 2018;22(2):9. doi: 10.1007/s11916-018-0666-8 [DOI] [PubMed] [Google Scholar]
- 11.Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37(1):29–42. doi: 10.1007/s00296-016-3481-8 [DOI] [PubMed] [Google Scholar]
- 12.Bhatia A, Hoydonckx Y, Peng P, et al. Radiofrequency procedures to relieve chronic hip pain: an evidence-based narrative review. Reg Anesth Pain Med. 2018;43(1):72–83. doi: 10.1097/AAP.0000000000000694 [DOI] [PubMed] [Google Scholar]
- 13.Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96(10):656–663. [PubMed] [Google Scholar]
- 14.Vanderwall AG, Milligan ED. Cytokines in pain: harnessing endogenous anti-inflammatory signaling for improved pain management. Front Immunol. 2019;10:3009. doi: 10.3389/fimmu.2019.03009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McWilliams DF, Walsh DA. Factors predicting pain and early discontinuation of tumour necrosis factor-α-inhibitors in people with rheumatoid arthritis: results from the British society for rheumatology biologics register. BMC Musculoskelet Disord. 2016;17:337. doi: 10.1186/s12891-016-1192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salaffi F, Giacobazzi G, Di Carlo M. Chronic pain in inflammatory arthritis: mechanisms, metrology, and emerging targets-A focus on the JAK-STAT pathway. Pain Res Manag. 2018;2018:8564215. doi: 10.1155/2018/8564215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou YQ, Liu Z, Liu ZH, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13(1):141. doi: 10.1186/s12974-016-0607-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu LW, Cheng KI, Chen JY, et al. Loganin prevents chronic constriction injury-provoked neuropathic pain by reducing TNF-α/IL-1β-mediated NF-κB activation and Schwann cell demyelination. Phytomedicine. 2020;67:153166. doi: 10.1016/j.phymed.2019.153166 [DOI] [PubMed] [Google Scholar]
- 19.Berta T, Qadri Y, Tan PH, et al. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. 2017;21(7):695–703. doi: 10.1080/14728222.2017.1328057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malcangio M. Role of the immune system in neuropathic pain. Scand J Pain. 2019;20(1):33–37. doi: 10.1515/sjpain-2019-0138 [DOI] [PubMed] [Google Scholar]
- 21.Bjurström MF, Bodelsson M, Montgomery A, et al. Differential expression of cerebrospinal fluid neuroinflammatory mediators depending on osteoarthritis pain phenotype. Pain. 2020;161(9):2142–2154. doi: 10.1097/j.pain.0000000000001903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meacham K, Shepherd A, Mohapatra DP, et al. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21(6):28. doi: 10.1007/s11916-017-0629-5 [DOI] [PubMed] [Google Scholar]
- 23.DiBonaventura MD, Sadosky A, Concialdi K, et al. The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res. 2017;10:2525–2538. doi: 10.2147/JPR.S127014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schain M, Kreisl WC. Neuroinflammation in neurodegenerative disorders-a review. Curr Neurol Neurosci Rep. 2017;17(3):25. doi: 10.1007/s11910-017-0733-2 [DOI] [PubMed] [Google Scholar]
- 25.Zilliox LA. Neuropathic pain. Continuum. 2017;23(2):512–532. doi: 10.1212/CON.0000000000000462 [DOI] [PubMed] [Google Scholar]
- 26.Kwon OY, Lee SH. Ishige okamurae suppresses trimethyltin-induced neurodegeneration and glutamate-mediated excitotoxicity by regulating MAPKs/Nrf2/HO-1 antioxidant pathways. Antioxidants. 2021;10(3):440. doi: 10.3390/antiox10030440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CH, Lin CH, Lane HY. d-glutamate and gut microbiota in Alzheimer’s disease. Int J Mol Sci. 2020;21(8):2676. doi: 10.3390/ijms21082676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabezudo-García P, Mena-Vázquez N, Ciano-Petersen NL, et al. Prevalence of neural autoantibodies in epilepsy of unknown etiology: systematic review and meta-analysis. Brain Sci. 2021;11(3):392. doi: 10.3390/brainsci11030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolber BJ. mGluRs head to toe in pain. Prog Mol Biol Transl Sci. 2015;131:281–324. doi: 10.1016/bs.pmbts.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 30.Fossati M, Charrier C. Trans-synaptic interactions of ionotropic glutamate receptors. Curr Opin Neurobiol. 2021;66:85–92. doi: 10.1016/j.conb.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 31.Grand T, Abi Gerges S, David M, Diana MA, Paoletti P. Unmasking GluN1/GluN3A excitatory glycine NMDA receptors. Nat Commun. 2018;9(1):4769. doi: 10.1038/s41467-018-07236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGee MA, Abdel-Rahman AA. N-Methyl-D-aspartate receptor signaling and function in cardiovascular tissues. J Cardiovasc Pharmacol. 2016;68(2):97–105. doi: 10.1097/FJC.0000000000000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vyklicky V, Korinek M, Smejkalova T, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191–S203. doi: 10.33549/physiolres.932678 [DOI] [PubMed] [Google Scholar]
- 34.Reddy MSS, Thippeswamy H, Ganjekar S, et al. Anti-NMDA receptor encephalitis presenting as postpartum psychosis-a clinical description and review. Arch Womens Ment Health. 2018;21(4):465–469. doi: 10.1007/s00737-018-0816-3 [DOI] [PubMed] [Google Scholar]
- 35.Guasp M, Dalmau J. Encephalitis associated with antibodies against the NMDA receptor. Encefalitis por anticuerpos contra el receptor de NMDA. Med Clin (Barc). 2018;151(2):71–79. doi: 10.1016/j.medcli.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 36.Vieira M, Yong XLH, Roche KW, Anggono V. Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J Neurochem. 2020;154(2):121–143. doi: 10.1111/jnc.14970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardingham G. NMDA receptor C-terminal signaling in development, plasticity, and disease. F1000Res. 2019;8:F1000Faculty Rev–1547. doi: 10.12688/f1000research.19925.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyllie DJ, Livesey MR, Hardingham GE. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology. 2013;74:4–17. doi: 10.1016/j.neuropharm.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardoni F, Di Luca M. Protein-protein interactions at the NMDA receptor complex: from synaptic retention to synaptonuclear protein messengers. Neuropharmacology. 2021;190:108551. doi: 10.1016/j.neuropharm.2021.108551 [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Otaño I, Larsen RS, Wesseling JF. Emerging roles of GluN3-containing NMDA receptors in the CNS. Nat Rev Neurosci. 2016;17(10):623–635. doi: 10.1038/nrn.2016.92 [DOI] [PubMed] [Google Scholar]
- 41.Li J, Zhang J, Tang W, et al. De novo GRIN variants in NMDA receptor M2 channel pore-forming loop are associated with neurological diseases. Hum Mutat. 2019;40(12):2393–2413. doi: 10.1002/humu.23895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abarzúa S, Ampuero E, van Zundert B. Superoxide generation via the NR2B-NMDAR/RasGRF1/NOX2 pathway promotes dendritogenesis. J Cell Physiol. 2019;234(12):22985–22995. doi: 10.1002/jcp.28859 [DOI] [PubMed] [Google Scholar]
- 43.Forrest MP, Parnell E, Penzes P. Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci. 2018;19(4):215–234. doi: 10.1038/nrn.2018.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tameh AA, Karimian M, Zare-Dehghanani Z, Aftabi Y, Beyer C. Role of steroid therapy after ischemic stroke by n-Methyl-d-aspartate receptor gene regulation. J Stroke Cerebrovasc Dis. 2018;27(11):3066–3075. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.041 [DOI] [PubMed] [Google Scholar]
- 45.Song Y, Zhao X, Wang D, et al. Inhibition of LPS-induced brain injury by NR2B antagonists through reducing assembly of NR2B-CaMKII-PSD95 signal module. Immunopharmacol Immunotoxicol. 2019;41(1):86–94. doi: 10.1080/08923973.2018.1549566 [DOI] [PubMed] [Google Scholar]
- 46.Hu W, He J, Wang Y, et al. Protective effect of Achyranthes bidentata polypeptides on NMDA-mediated injury is developmentally regulated via modulating NR2A and NR2B differentially. Ann Transl Med. 2021;9(3):248. doi: 10.21037/atm-20-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogden KK, Chen W, Swanger SA, et al. Molecular mechanism of disease-associated mutations in the Pre-M1 helix of NMDA receptors and potential rescue pharmacology. PLoS Genet. 2017;13(1):e1006536. doi: 10.1371/journal.pgen.1006536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ba M, Ding W, Guan L, Lv Y, Kong M. S-nitrosylation of Src by NR2B-nNOS signal causes Src activation and NR2B tyrosine phosphorylation in levodopa-induced dyskinetic rat model. Hum Exp Toxicol. 2019;38(3):303–310. doi: 10.1177/0960327118806633 [DOI] [PubMed] [Google Scholar]
- 49.Song X, Jensen MØ, Jogini V, et al. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nature. 2018;556(7702):515–519. doi: 10.1038/s41586-018-0039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Cao J, Yang X, et al. NR2B phosphorylation at tyrosine 1472 in spinal dorsal horn contributed to N-methyl-D-aspartate-induced pain hypersensitivity in mice. J Neurosci Res. 2011;89(11):1869–1876. doi: 10.1002/jnr.22719 [DOI] [PubMed] [Google Scholar]
- 51.Wang XY, Zhou HR, Wang S, et al. NR2B-Tyr phosphorylation regulates synaptic plasticity in central sensitization in a chronic migraine rat model. J Headache Pain. 2018;19(1):102. doi: 10.1186/s10194-018-0935-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai WL, Bao YN, Fan JF, et al. Blockade of spinal dopamine D1/D2 receptor suppresses activation of NMDA receptor through Gαq and Src kinase to attenuate chronic bone cancer pain. J Adv Res. 2020;28:139–148. doi: 10.1016/j.jare.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mihara Y, Egashira N, Sada H, et al. Involvement of spinal NR2B-containing NMDA receptors in oxaliplatin-induced mechanical allodynia in rats. Mol Pain. 2011;7:8. doi: 10.1186/1744-8069-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andoh T, Fukutomi D, Uta D, et al. Prophylactic repetitive treatment with the herbal medicine Kei-kyoh-zoh-soh-oh-shin-bu-toh attenuates oxaliplatin-induced mechanical allodynia by decreasing spinal astrocytes. Evid Based Complement Alternat Med. 2019;2019:4029694. doi: 10.1155/2019/4029694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Yan H, Li X, et al. Inhibition of connexin 43 and phosphorylated NR2B in spinal astrocytes attenuates bone cancer pain in mice. Front Cell Neurosci. 2018;12:129. doi: 10.3389/fncel.2018.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao Y, Xu M. Efficacy and mechanism of action of etanercept in bone cancer pain. Pharmazie. 2017;72(4):219–222. doi: 10.1691/ph.2017.6901 [DOI] [PubMed] [Google Scholar]
- 57.Zhao WS, Jiang ZN, Shi H, et al. Low-frequency electroacupuncture alleviates chronic constrictive injury-induced mechanical allodynia by inhibiting NR2B upregulation in ipsilateral spinal dorsal horn in rats. Chin J Integr Med. 2019;25(6):462–467. doi: 10.1007/s11655-018-3057-4 [DOI] [PubMed] [Google Scholar]
- 58.Norcini M, Sideris A, Adler SM, et al. NR2B expression in rat DRG is differentially regulated following peripheral nerve injuries that lead to transient or sustained stimuli-evoked hypersensitivity. Front Mol Neurosci. 2016;9:100. doi: 10.3389/fnmol.2016.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed A, Arora D. Ultrasound-guided radiofrequency ablation of genicular nerves of knee for relief of intractable pain from knee osteoarthritis: a case series. Br J Pain. 2018;12(3):145–154. doi: 10.1177/2049463717730433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta A, Huettner DP, Dukewich M. Comparative effectiveness review of cooled versus pulsed radiofrequency ablation for the treatment of knee osteoarthritis: a systematic review. Pain Physician. 2017;20(3):155–171. doi: 10.36076/ppj.2017.171 [DOI] [PubMed] [Google Scholar]
- 61.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- 62.Cho SI, Park UJ, Chung JM, Gwag BJ. Neu2000, an NR2B-selective, moderate NMDA receptor antagonist and potent spin trapping molecule for stroke. Drug News Perspect. 2010;23(9):549–556. doi: 10.1358/dnp.2010.23.9.1513493 [DOI] [PubMed] [Google Scholar]
- 63.Briefs B. NR2B antagonist pursued for treatment-resistant depression. Nat Rev Drug Discov. 2009;8(5):349. doi: 10.1038/nrd2880 [DOI] [PubMed] [Google Scholar]
- 64.Kong M, Ba M, Liu C, Zhang Y, Zhang H, Qiu H. NR2B antagonist CP-101,606 inhibits NR2B phosphorylation at tyrosine-1472 and its interactions with Fyn in levodopa-induced dyskinesia rat model. Behav Brain Res. 2015;282:46–53. doi: 10.1016/j.bbr.2014.12.059 [DOI] [PubMed] [Google Scholar]
- 65.Kim Y, Cho HY, Ahn YJ, et al. Effect of NMDA NR2B antagonist on neuropathic pain in two spinal cord injury models. Pain. 2012;153(5):1022–1029. doi: 10.1016/j.pain.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 66.Ji G, Horváth C, Neugebauer V. NR2B receptor blockade inhibits pain-related sensitization of amygdala neurons. Mol Pain. 2009;5:21. doi: 10.1186/1744-8069-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra SK, Hidau M, Rai S. Memantine and Ibuprofen pretreatment exerts anti-inflammatory effect against streptozotocin-induced astroglial inflammation via modulation of NMDA receptor-associated downstream calcium ion signaling. Inflammopharmacology. 2021;29(1):183–192. doi: 10.1007/s10787-020-00760-0 [DOI] [PubMed] [Google Scholar]
- 68.Shen Y, Zhu LJ, Liu SS, Zhou SY, Luo JH. Interleukin-2 inhibits NMDA receptor-mediated currents directly and may differentially affect subtypes. Biochem Biophys Res Commun. 2006;351(2):449–454. doi: 10.1016/j.bbrc.2006.10.047 [DOI] [PubMed] [Google Scholar]
- 69.Castany S, Codony X, Zamanillo D, et al. Repeated sigma-1 receptor antagonist MR309 administration modulates central neuropathic pain development after spinal cord injury in mice. Front Pharmacol. 2019;10:222. doi: 10.3389/fphar.2019.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S, Han J, Wang DS, et al. Sinomenine attenuates chronic inflammatory pain in mice. Metab Brain Dis. 2017;32(1):211–219. doi: 10.1007/s11011-016-9889-8 [DOI] [PubMed] [Google Scholar]
- 71.Al-Khrasani M, Mohammadzadeh A, Balogh M, et al. Glycine transporter inhibitors: a new avenue for managing neuropathic pain. Brain Res Bull. 2019;152:143–158. doi: 10.1016/j.brainresbull.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 72.Ramage L, Martel MA, Hardingham GE, et al. NMDA receptor expression and activity in osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage. 2008;16(12):1576–1584. doi: 10.1016/j.joca.2008.04.023 [DOI] [PubMed] [Google Scholar]
- 73.Kalev-Zylinska ML, Hearn JI, Rong J, et al. Altered N-methyl D-aspartate receptor subunit expression causes changes to the circadian clock and cell phenotype in osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2018;26(11):1518–1530. doi: 10.1016/j.joca.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 74.Cavalcante AL, Siqueira RM, Araujo JC, et al. Role of NMDA receptors in the trigeminal pathway, and the modulatory effect of magnesium in a model of rat temporomandibular joint arthritis. Eur J Oral Sci. 2013;121(6):573–583. doi: 10.1111/eos.12093 [DOI] [PubMed] [Google Scholar]
- 75.Noh ASM, Ismail CAN. A review on chronic pain in rheumatoid arthritis: a focus on activation of NR2B subunit of N-Methyl-D-aspartate receptors. Malays J Med Sci. 2020;27(1):6–21. doi: 10.21315/mjms2020.27.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Y, Zhang K, Miao J, et al. The spinal NR2BR/ERK2 pathway as a target for the central sensitization of collagen-induced arthritis pain. PLoS One. 2018;13(7):e0201021. doi: 10.1371/journal.pone.0201021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang Y, Ma Y, Wang J, et al. Leptin contributes to neuropathic pain via extrasynaptic NMDAR-nNOS activation. Mol Neurobiol. 2021;58(3):1185–1195. doi: 10.1007/s12035-020-02180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li CD, Zhao JY, Chen JL, et al. Mechanism of the JAK2/STAT3-CAV-1-NR2B signaling pathway in painful diabetic neuropathy. Endocrine. 2019;64(1):55–66. doi: 10.1007/s12020-019-01880-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nanou E, Catterall WA. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron. 2018;98(3):466–481. doi: 10.1016/j.neuron.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 80.Ni K, Zhang W, Ni Y, et al. Dorsal root ganglia NR2B-mediated Epac1-Piezo2 signaling pathway contributes to mechanical allodynia of bone cancer pain. Oncol Lett. 2021;21(4):338. doi: 10.3892/ol.2021.12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buch MH, Eyre S, McGonagle D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol. 2021;17(1):17–33. doi: 10.1038/s41584-020-00541-7 [DOI] [PubMed] [Google Scholar]