Abstract
Bypass to the peroneal artery has sometimes been effective for pedal gangrene. However, the difficulty of approaching the terminal segment of the peroneal artery because of its anatomic features has been a clinical issue. Surgical access to this area can be achieved via a lateral approach with fibular resection. Although severe complications associated with fibular resection have rarely been reported, a less invasive surgical procedure would enable faster postoperative recovery and reduce the incidence of wound-related complications. We have described our experience with successful terminal peroneal artery bypass via a lateral approach without fibular resection in a 38-year-old male patient with chronic limb-threatening ischemia.
Keywords: Bypass surgery, Chronic limb-threatening ischemia, Fibula resection, Infrapopliteal arterial lesions, Peroneal artery
Surgical access to the peroneal artery (PA) can be achieved via a medial, lateral, or posterior approach.1,2 However, for patients with obesity or a surgical history of lower limb bypass, the lateral approach with fibular resection has been reported to be a better method for exposure of this artery. Although the incidence of severe complications associated with fibular resection has been rare, the benefits of a less invasive procedure have not yet been reported. We have described our method of PA bypass grafting via a lateral approach without fibular resection in a 38-year-old male patient with chronic limb-threatening ischemia (CLTI) and provided technical tips for this procedure. The patient provided written informed consent for the report of his case details and related imaging studies.
Case report
A 38-year-old man had been admitted to our hospital because of intractable gangrene on his right toes that had persisted for 2 months. He was a heavy smoker and obese (body mass index, 27.8 kg/m2), with a medical history of hypertension and diabetes mellitus. His renal function was normal, and he had no history of ischemic heart disease or cerebrovascular disease. The physical examination revealed pulselessness of the right dorsal and posterior tibial arteries and infected necrosis of digits one through three on the right foot (Fig 1, A). His right ankle brachial index was 0.83, and his skin perfusion pressure (SPP) was 21 mm Hg on the plantar surface and 23 mm Hg on the dorsum. The limb was categorized as WIfI (wound, ischemia, foot infection) clinical stage 4 (wound grade 2, ischemia grade 3, foot infection grade 2).3
Fig 1.
Photograph of the patient’s right foot and radiographic findings before revascularization. A, The first to third digits of his right foot were infected with gangrene at admission. B, Enhanced computed tomography (CT) of the right lower limb, with CT angiography showing that the iliac, femoral, and popliteal arteries were not diseased. The infrapopliteal arteries were occluded from the proximal third of the right lower leg to the ankle, except for the distal third of the peroneal artery (PA; white arrow). C, Axial CT scan of the right leg showing the relationship between the PA (white arrow) and leg bones. D,E, Digital subtraction angiography of the lower limb showing severely diseased infrapopliteal and dorsalis pedis arteries with poor outflow, except for the distal segment of the PA (white arrow), which had remained partially patent through the collateral vessels.
Enhanced computed tomography angiography and digital subtraction angiography of the lower limbs revealed occlusion of the right infrapopliteal arteries from their origin to the distal PA, which was patent, filling via collateral vessels, and suitable for distal anastomosis (Fig 1, B-E). The three diseased infrapopliteal arteries were anatomically classified as stage III (femoropopliteal artery, grade 0; infrapopliteal arteries, grade 4 for each) using the GLASS (Global Limb Anatomic Staging System) classification.3 In addition, the right dorsalis pedis was patent; however, the patency of the outflow arteries, including the pedal arches, seemed poor. The inframalleolar/pedal descriptor was P2, defined as the lack of a target artery crossing the ankle and the absence of a suitable pedal or plantar artery target using the GLASS classification. He was ambulatory, and his ipsilateral great saphenous vein (GSV) was available. Thus, the preferred initial revascularization procedure was open bypass surgery because his preoperative risk for surgery was standard.3 The distal segment of the PA was selected as the best distal anastomotic site. Axial computed tomography angiography demonstrated that the segment was easily accessible from the lateral side without excision of the fibula (Fig 1, C).
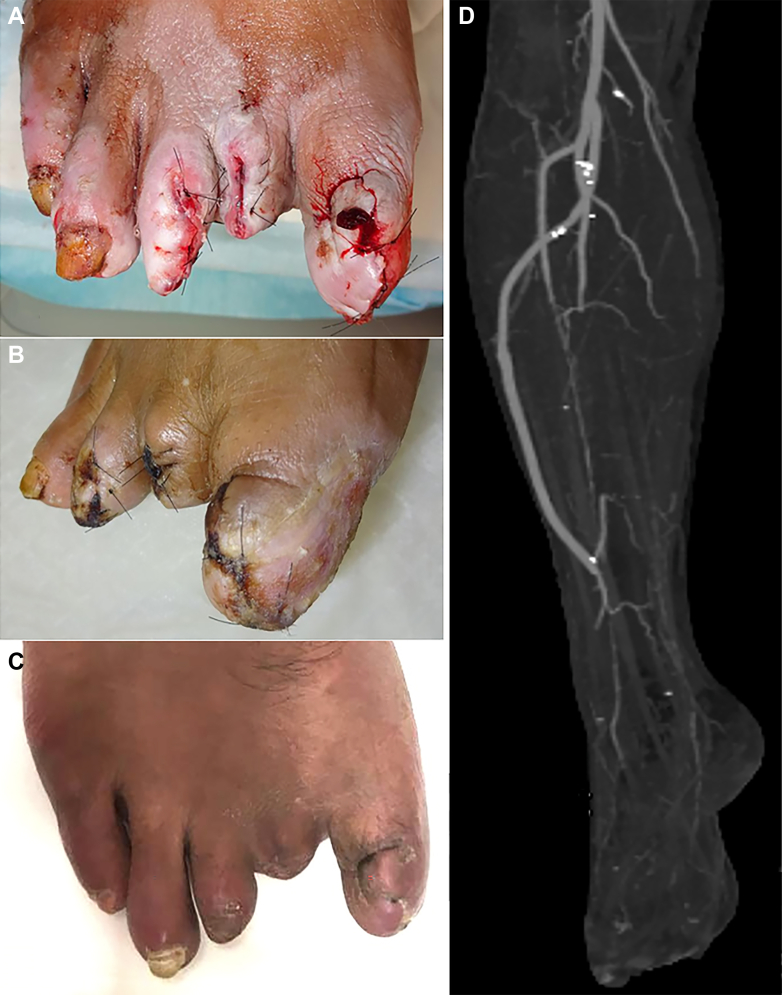
Before revascularization, a minor amputation of the infected digits one to three was performed to prevent progression of infection to the proximal foot segment. Two days later, bypass surgery from the right below-the-knee popliteal artery (BKPA) to the terminal PA was performed. The patient was placed in the supine position with the knee flexed to ∼60° under general anesthesia. A 6-cm-long incision was made 1 cm parallel and anterior to the distal third of the fibula immediately proximal to the ankle (Fig 2, A). The incision was deepened through the subcutaneous tissue and fascial plane. The dissection then proceeded between the extensor halluces longus and fibula to reach the deep posterior compartment. The vascular bundle was found just anterior and medial to the fibula in the intermuscular groove (Fig 2, B). Next, we performed lower limb bypass from the BKPA to the PA. The ipsilateral GSV was placed in a nonreversed translocation fashion using a LeMaitre valvulotome (LeMaitre Vascular, Burlington, MA). The vein graft was passed through an interosseous membrane between the tibia and fibula near the proximal anastomosis. Open revascularization was successfully performed (Fig 2, C-E). The patient was discharged from our hospital 10 days postoperatively without any complications. The wounds had completely healed by 2 months after surgery (Fig 3, A-C), with no recurrence observed. The postoperative SPP had improved to 60 mm Hg on the plantar surface and 50 mm Hg on the dorsum. At 2 years postoperatively, the vein graft remained patent without any reintervention required (Fig 3, D).
Fig 2.
Images of revascularization. A, Preoperative marking was performed on the lateral side of the right ankle, with the skin incision line indicated by arrowheads. B, The extensor halluces longus (arrowhead) was retracted toward the upper side of this view and just anterior and medial to the fibula (black arrow) and the peroneal artery (PA; white arrows) was exposed. C, The inside of the PA was found to be normal after arterial incision. D, A vein graft was anastomosed to the PA. E, Angiography was performed after PA bypass grafting. The distal anastomosis site is indicated by the white arrow.
Fig 3.
Postoperative photographs and computed tomography angiography findings of the right foot. A, Wounds after minor amputation and surgical revascularization. B, Photograph showing wounds at 10 days after revascularization. C, Photograph showing complete wound healing at 2 months after revascularization. D, Postoperative computed tomography angiogram showing good patency of the vein graft.
Discussion
Surgical revascularization still plays an important role in treating CLTI, in accordance with the Global Vascular Guidelines and the SPINACH (surgical reconstruction versus peripheral intervention in patients with critical limb ischemia) study, representing a Japanese multicenter CLTI registry. Open surgical bypass is preferred for cases of severe limb disease, especially cases with high wound and foot infection grades and severely diseased lower extremity arteries.3,4 Although the surgical risk should be considered, the patient’s ambulation status and GSV availability are also significant factors for determining a revascularization strategy for patients with CLTI. In the present case, we considered bypass to be the most durable revascularization for our young patient. Bypass grafting from the BKPA to the distal PA was the best option for revascularization owing to the presence of a reliable inflow artery and suitable outflow artery.
Based on the angiosome concept, the anterior and posterior tibial arteries will be preferred for use as outflow vessels because they lead directly to the plantar arch. However, the PA will often be the only patent or least diseased outflow vessel in the lower leg. In contrast to the tibial artery, the PA will have often been spared the ravages of atherosclerotic lesions. Although distal peroneal bypass will usually only provide indirect revascularization according to the angiosome concept, the efficacy of peroneal bypass has been well reported in previous studies.5,6 Wound healing was achieved well in the present patient (Fig 3). An increase in the postoperative SPP was also observed.
With the spread of endovascular therapy for CLTI, vascular surgeons will be required to manage ever more challenging cases. The diseased anterior tibial artery and PA in the present patient could have been treated endovascularly. However, bypass surgery was selected as the preferred revascularization method because of its long-term durability owing to the patient’s age, ambulatory status, and GSV availability. Various approaches to the infrapopliteal arteries should be considered. In particular, dissection of the PA can be difficult because the PA runs parallel to the inside of the fibula, and a lateral approach with fibular resection will often be the best method to expose this artery, especially for patients with obesity or a history of limb surgery. In 1945, Elkin and Kelly1 were the first to describe a lateral approach with fibular resection for exposure of the proximal PA. In 1974, Dardik et al2 reported that this approach can also be useful to achieve good exposure of the distal PA. Partial fibulectomy has also been used for mandibular or extremity reconstruction, and few complications associated with fibular resection have been reported with each surgery.7 However, one study reported the occurrence of a tibial fracture after PA bypass with fibular resection, which included two patients with osteoporosis.8 Because distal bypass surgery is performed with the aim of salvaging the limb and improving the activities of daily living, a minimally invasive approach might possibly be better for CLTI patients. For the present patient, peroneal bypass surgery was performed without fibular resection to maintain the young man’s ability to perform activities of daily living. The preoperative axial computed tomography scan helped us to proceed with this surgical procedure by revealing the anatomy of the muscles and the relationships between the muscles, bones, and arteries (Fig 1, C). Only one previous report has described a lateral approach without fibular resection.9 In contrast to the present study, they had conducted this approach from the posterior side of the fibula, suggesting that with the use of both anterior and posterior approaches, many patients could receive treatment with distal peroneal bypass without fibular resection.
Conclusions
We have reported a less invasive procedure for PA bypass without resection of the fibula. Additional studies are needed to validate the approach we have described in the present case report.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Elkin D.C., Kelly R.P. Arteriovenous aneurysm: exposure of the tibial and peroneal vessels by resection of the fibula. Ann Surg. 1945;122:529–545. doi: 10.1097/00000658-194510000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dardik H., Dardik I., Veith F.J. Exposure of the tibial-peroneal arteries by a single lateral approach. Surgery. 1974;75:377–382. [PubMed] [Google Scholar]
- 3.Conte M.S., Bradbury A.W., Kolh P., White J.V., Dick F., Fitridge R., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iida O., Takahara M., Soga Y., Kodama A., Terashi H., Azuma N. Three-year outcomes of surgical versus endovascular revascularization for critical limb ischemia: the SPINACH study (surgical reconstruction versus peripheral intervention in patients with critical limb ischemia) Circ Cardiovasc Interv. 2017;10:e005531. doi: 10.1161/CIRCINTERVENTIONS.117.005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricco J.B., Gargiulo M., Stella A., Abualhin M., Gallitto E., Desvergnes M., et al. Impact of angiosome- and nonangiosome-targeted peroneal bypass on limb salvage and healing in patients with chronic limb-threatening ischemia. J Vasc Surg. 2017;66:1479–1487. doi: 10.1016/j.jvs.2017.04.074. [DOI] [PubMed] [Google Scholar]
- 6.Azuma N., Uchida H., Kokubo T., Koya A., Akasaka N., Sasajima T. Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery? Eur J Vasc Endovasc Surg. 2012;43:322–328. doi: 10.1016/j.ejvs.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Sieg P., Taner C., Hakim S.G., Jacobsen H.C. Long-term evaluation of donor site morbidity after free fibula transfer. Br J Oral Maxillofac Surg. 2010;48:267–270. doi: 10.1016/j.bjoms.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Kahn M.B., Profeta B., Hume E., Leichter R., Carabasi R.A., DiMuzio P.J. Tibia fracture after fibula resection for distal peroneal bypass. J Vasc Surg. 2001;34:979–982. doi: 10.1067/mva.2001.119891. [DOI] [PubMed] [Google Scholar]
- 9.De Luccia N., Queiroz A.B., Mulatti G.C., Ferreira Espírito Santo F.R., Sassaki Neto P.I., Schneidwind K.D. Lateral approach to the peroneal artery without resection of the fibula for lower limb revascularization. J Vasc Surg. 2014;59:857–859. doi: 10.1016/j.jvs.2013.09.008. [DOI] [PubMed] [Google Scholar]