Abstract
Eleven Sinorhizobium meliloti 1021 loci whose expression was induced under low oxygen concentrations were identified in a collection of 5,000 strains carrying Tn5-1063 (luxAB) transcriptional reporter gene fusions. The 11 Tn5-1063-tagged loci were cloned and characterized. The dependence of the expression of the tagged loci on the FixL/FixJ oxygen-sensing two-component regulatory system was examined. Three of the loci were found to be dependent upon fixL and fixJ for their expression, while one locus showed a partial dependence. The remaining seven loci showed fixL- and fixJ-independent induction of expression in response to oxygen limitation. This suggests that in S. meliloti, additional regulatory system(s) exist that respond either directly or indirectly to oxygen limitation conditions.
Bacteria in soil environments are continuously exposed to changing environmental conditions, including oxygen limitation (23). Oxygen levels in the soil fluctuate with time, often resulting in microaerobic and anaerobic microsites (15). In the rhizosphere, the soil adjacent to and influenced by the plant root system, root and microbial respiration can result in oxygen levels lower than those found in bulk soil (4). Microorganisms have developed mechanisms to sense and adapt to environmental changes, such as oxygen limitation. These responses may be important for the persistence of and competition between microorganisms in the soil.
The aerobic soil bacterium Sinorhizobium meliloti encounters oxygen limitation conditions in two different ecological niches: while in a free-living state in the soil or when in symbiotic association with the legume alfalfa (Medicago sativa) inside microaerobic nitrogen-fixing root nodules. Microaerobic conditions inside legume nodules are necessary to maintain nitrogen fixation activity due to the oxygen sensitivity of the enzyme nitrogenase. S. meliloti coordinates the expression of genes required for nitrogen fixation and for respiration inside nodules via a two-component regulatory system, FixL/FixJ, that senses microaerobic conditions and controls target genes accordingly (3, 6).
Towards the goal of examining the importance of the oxygen limitation response for S. meliloti survival in the soil environment, we have initiated a characterization of the molecular response of free-living S. meliloti to oxygen limitation using a gene reporter system. The involvement of FixL/FixJ in the regulation of the oxygen limitation response was also examined.
Isolation of S. meliloti loci expressed in response to oxygen limitation.
A previously described (16) collection of 5,000 S. meliloti 1021 strains containing transposon Tn5–1063 insertions was screened for strains that luminesced when oxygen levels became limiting. Tn5–1063 functions as a transcriptional reporter system as it contains promoterless luxA luxB genes which encode the enzyme luciferase (24). The collection was screened by first spotting the strains onto duplicate plates containing solid GTS medium (12) lined with a filter membrane as previously described (16). The duplicate plates' contents were incubated at 28°C for 36 h followed by the incubation (28°C) of one plate's contents with atmospheric oxygen concentrations (∼21%) and the incubation of the second plate's contents in an airtight jar under continuous flushing with an ∼1% oxygen gas mixture. The gas mixture was generated by mixing nitrogen and compressed air using a Multigas System (Hotpack, Philadelphia, Pa.) and was monitored periodically using an oxygen electrode (Microelectrodes Inc., Londonderry, N.H.). After 6 to 8 h of incubation, the strains were examined for luciferase activity using a photonic camera system as described previously (16). A total of 11 strains containing Tn5-1063 fusions that showed significantly increased luminescence after incubation under 1% oxygen, compared to incubation under 21% oxygen, were identified (Fig. 1). The loci tagged in these strains have been designated loe (low-oxygen expressed).
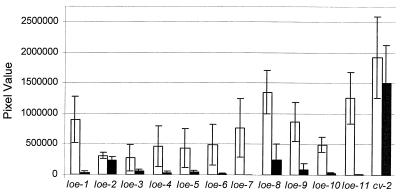
FIG. 1.
Induction of expression of the loe fusions after 6 h of incubation under decreased oxygen concentrations. Shown are the average levels of luminescence generated by the loe fusion strains on solid medium when incubated for 6 h with 1% oxygen (white bars) or 21% oxygen (black bars). The cv-2::Tn5-1063 fusion is expressed at both oxygen concentrations; therefore, the strain harboring the cv-2 fusion was included to serve as a positive control in these studies. Shown atop each bar is the standard deviation observed in four or five independent trials. The pixel values correlate to the level of luminescence observed for each strain and were obtained using the University of Texas Health Science Center, San Antonio, Image Tool program. A standard t test of the data indicated that the difference observed between the two conditions for each loe fusion strain was significant.
Molecular characterization of the loe strains.
A Southern analysis using pRL1063a (24) as probe revealed that the 11 loe strains each contained a single Tn5–1063 fusion and that the location of each fusion was distinct (data not shown).
The 11 Tn5–1063 fusions were excised from genomic DNA and cloned, and at least 200 bp of the DNA sequence flanking each end of the Tn5–1063 insertions was determined as described previously (5). The sequence obtained for each locus was assembled using the program Sequencher (Gene Code Corporation, Ann Arbor, Mich.) and compared to GenBank databases using the gapped BLASTX and BLASTN programs (2). The results are summarized in Table 1.
TABLE 1.
Sequence similarities for loe loci
| Locus | Protein similaritya | Proposed function | % Identity/BLAST score | E valueb |
|---|---|---|---|---|
| loe-1 | FixN | High-affinity oxidase (cbb3) subunit | 100/362 | 2.0 × 10−99 |
| loe-2 | HI1351 | Hypothetical protein | 28/42 | 0.006 |
| loe-3 | APE0766 | Hypothetical protein | 27/55 | 6.0 × 10−7 |
| loe-4 | AsnB | Asparagine synthesis | 35/68 | 7.0 × 10−11 |
| loe-5 | NS | |||
| loe-6 | CphA | Cyanophycin synthesis | 30/76 | 4.0 × 10−13 |
| loe-7 | NS | |||
| loe-8/loe-9 | CyoC | Ubiquinol oxidase subunit | 59/246 | 2.0 × 10−64 |
| loe-10 | ORF278 | Unknown | 32/40 | 0.014 |
| loe-11 | ORF278 | Unknown | 27/45 | 5.0 × 10−4 |
GenBank matches identified at the protein level (BLASTX). NS, no significant similarity. Origin of similar proteins: FixN, S. meliloti; HI1351, Haemophilus influenzae; APE0766, Aeropyrum pernix; AsnB, B. subtilis; CphA, Anabaena variabilis; CyoC, E. coli; and ORF278, P. denitrificans.
Significance of similarity at protein level, indicated by the E value.
The loe-1 fusion was shown to be located within the fixN gene of S. meliloti. fixN, the first gene of the fixNOQP operon, encodes a subunit of the cbb3-type cytochrome c oxidase that has been shown in Bradyrhizobium japonicum to be required for bacterial respiration inside soybean nodules (17, 18). The induction of fixN gene expression from S. meliloti in free-living microaerobic cultures had been demonstrated previously (6). Therefore, the identification of this locus in our screen indicated that our protocol was suitable for the isolation of loci expressed in response to oxygen limitation.
The loe-8 and loe-9 fusions were found to be within the same locus 474 bp apart. The predicted protein encoded by this locus was similar to subunit III of a family of ubiquinone oxidases conserved in a variety of microorganisms. A protein sequence alignment of representatives from this family was generated using the program Pile-up (Genetics Computer Group, Madison, Wis.) and is shown in Fig. 2. The S. meliloti sequence demonstrated 68, 61, 59, 51, and 47% amino acid identity to oxidases from Paracoccus denitrificans, Pseudomonas putida IH-2000, Escherichia coli, Acetobacter aceti, and B. japonicum, respectively. Based on the observed degree of amino acid sequence similarity and the fact that the regions of identity span the entire length of the proteins, it is likely that we have identified an ubiquinol oxidase subunit III protein from S. meliloti.
FIG. 2.
Amino acid sequence comparison among ubiquinol oxidase subunit III homologs. The amino acid sequences aligned with the predicted polypeptide encoded by the loe-8 and loe-9 loci from S. meliloti (sino) include QoxC from P. denitrificans (para), CyoC from P. putida IH-2000 (pseu); CyoC from E. coli (esch); ubiquinol oxidase subunit III precursor from A. aceti (acet); and bo-type ubiquinol oxidase chain III from B. japonicum (brad). Sequence accession numbers for the homologs are C54759, BAA76358, C42226, BAA02482, and JC5901, respectively. Shaded regions correspond to amino acids identical to those found in the S. meliloti polypeptide. Boxed regions correspond to amino acid similarities and were determined using the PAM250 matrix. Dashes indicate gaps introduced by the computer program to maximize the alignment.
The loe-10 and loe-11 fusions were positioned within two different loci whose predicted partial amino acid sequences showed weak similarity to a hypothetical protein of unknown function, ORF278 from P. denitrificans (23 and 28% identity for loe-10 and loe-11, respectively) (8), and to each other (21% amino acid identity). The deduced protein sequences were also weakly similar to that for ORF277 from B. japonicum (27% amino acid identity) (17) and to that for ORF277 from Rhodobacter capsulatus (28% amino acid identity), respectively (13).
The loe-4 fusion was found to be in a locus whose predicted amino acid sequence was similar to that of asparagine synthetase from Bacillus subtilis (25). This family of asparagine synthetases uses preferably l-glutamine or alternatively ammonia as the amino group donor to generate l-asparagine from l-aspartate (11, 25).
The loe-6 fusion was in a locus encoding a protein sharing similarity to cyanophycin synthetase from a number of cyanobacteria. Cyanophycin synthetase catalyzes the ATP-dependent polymerization of arginine and aspartate, generating cyanophycin (multi-l-arginyl-poly-l-aspartate) (20, 21). Cyanophycin is believed to function as a nitrogen reserve polymer (20) and has been shown to accumulate in the presence of a source of nitrogen under a variety of different growth conditions in cyanobacteria (1, 14, 22). The synthesis of cyanophycin under microaerobic and anaerobic growth conditions has also been suggested (1).
The loci identified by the loe-5 and loe-7 fusions encoded putative polypeptides lacking significant similarity with proteins in the databases, indicating that they are novel. The proteins predicted to be encoded by the loe-2 and loe-3 loci were found to share a low level of similarity to hypothetical proteins of unknown function.
Regulation of the loe fusions by fixL and fixJ.
An important objective of this research was to determine if regulatory systems in addition to FixL/FixJ exist that help to mediate the physiological response of S. meliloti to changes in oxygen availability. Towards this goal, the luciferase expression patterns of the strains carrying the loe fusions were examined in genetic backgrounds where the oxygen regulatory genes fixL and fixJ were inactivated.
To generate loe::Tn5–1063 fixL or fixJ double mutants, it was necessary to first generate insertions within fixL and fixJ that contained a selectable marker different from the markers carried by Tn5–1063. Therefore, we obtained S. meliloti strains GMI5705 (fixL2.66::Tn5) and GMI5704 (fixJ2.3::Tn5) (6) and replaced the Tn5 insertions in these strains with Tn5-233 via homologous recombination using the method described by De Vos et al. (9). The exact replacement of Tn5 with Tn5-233 within fixL and fixJ in the resulting strains (FdB3463 and FdB3464) was verified by Southern analysis (data not shown.)
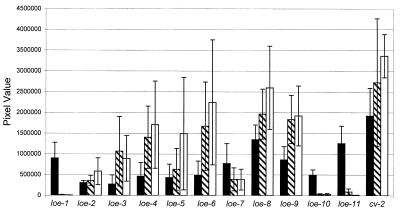
By using protocols described in reference 10, phage φ M12 lysates of FdB3463 and FdB3464 were generated and used to transfer the fixL::Tn5–233 and the fixJ::Tn5–233 insertion mutations into the 11 loe fusion strains. The resulting double mutants were examined by Southern analysis of genomic DNA to ensure that the positions of the Tn5–1063 and Tn5–233 markers within the genome had been maintained (data not shown). The double mutants were then examined for changes in the expression of the loe fusions relative to the parent strains after a switch to oxygen limitation conditions. The expression of the loe-1, loe-10, and loe-11 fusions was found to be dependent upon both fixL and fixJ, while loe-7 fusion expression was found to be partially dependent upon fixL and fixJ (Fig. 3). fixL- and fixJ-dependent expression of loe-1 (fixN) was the expected result, as it had been demonstrated previously in S. meliloti that fixN expression is controlled via the FixL/FixJ regulatory system (6). The remaining loe fusions demonstrated increased expression in a background of fixL and fixJ. This increase may have been an artifact of the luciferase reporter system and assay, since the positive control fusion, cv-2, also showed this increase. It was clear, however, that the induction of the expression of the remaining loe fusions (loe-2, loe-3, loe-4, loe-5, loe-6, loe-8, and loe-9) was independent of fixL and fixJ. These results support the idea that at least one additional regulatory system exists in S. meliloti that responds to low-oxygen conditions. Whether the regulator(s) responds to oxygen levels directly or indirectly (e.g., a change in redox or growth rate caused by oxygen depletion) remains to be determined.
FIG. 3.
The effect of fixL and fixJ mutations on the induction of expression of the loe fusions upon oxygen limitation. Shown are the average luminescence levels (pixel values) observed for each loe fusion strain (black bars) and its fixL (hatched white bars) and fixJ (white bars) derivatives when incubated under 1% oxygen. The standard of deviation for four or five independent trials is shown for each strain.
The symbiotic phenotype of the loe fusion strains.
Because the physiological environment inside alfalfa nodules is microaerobic (0.03 μM oxygen) (3), we hypothesized that the loci identified in this study may have roles important in the formation of functional nitrogen-fixing nodules on alfalfa. To test this hypothesis, the 11 loe fusion strains were inoculated onto alfalfa seedlings and examined for nodule formation and for nitrogenase activity (via acetylene reduction assays) as described previously (16). All 11 loe fusion strains generated nodules with nitrogenase activity (data not shown) comparable to those for the reference strain S. meliloti 1021 (data not shown), suggesting that the loci disrupted by Tn5–1063 in these strains were not essential for symbiotic nitrogen fixation. Although not essential, the loe loci may still be involved in symbiotic nitrogen fixation, since our assay may not have been sensitive enough to detect small decreases in nitrogenase activity. Lack of a nitrogen fixation phenotype may also be due to the presence of additional copies of the loci within the genome. For example, the finding that the strain carrying loe-1(Tn5-1063 insertion within fixN) was symbiotically proficient was expected, as it had been noted in the literature that a second functional copy of fixN exists within the genome of S. meliloti (7, 19).
In conclusion, the approach presented here has clearly permitted the identification of loci affected by oxygen availability. In addition we have provided evidence for the existence of additional regulatory systems in S. meliloti that are responsive either directly or indirectly to oxygen limitation conditions. Future studies in our laboratory will focus on isolating the genes involved in the regulation of the FixL/FixJ-independent loe loci and on examining a possible role for the regulatory genes in microbial persistence in the soil environment.
Nucleotide sequence accession numbers.
The GenBank nucleotide sequence accession numbers for the loe loci are AF336863 (loe-2), AF336864 (loe-3), AF336865 (loe-4), AF336866 (loe-5), AF336867 (loe-6), AF336868 (loe-7), AF336869 (loe-8, loe-9), AF336870 (loe-10), and AF336871 (loe-11).
Acknowledgments
We thank G. Walker and P. Boistard for strains and U. Rossbach for computer support. We also thank C. P. Wolk and de Bruijn lab members for helpful suggestions and discussions.
This work was supported by grant DE-FG02–91ER20021 from the U.S. Department of Energy. J. Trzebiatowski was supported by National Research Service Award 5-F32-GM19412–03 from the National Institute of Health.
REFERENCES
- 1.Allen M M, Hutchison F, Weathers P J. Cyanophycin granule polypeptide formation and degradation in the cyanobacterium Aphanocapsa6308. J Bacteriol. 1980;141:687–693. doi: 10.1128/jb.141.2.687-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zheng Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batut J, Boistard P. Oxygen control in Rhizobium. Antonie Leeuwenhoek. 1994;66:129–150. doi: 10.1007/BF00871636. [DOI] [PubMed] [Google Scholar]
- 4.Bolton H Jr, Fredrickson J, Elliott L, editors. Microbial ecology of the rhizosphere. New York, N.Y: Marcel Dekker, Inc; 1993. [Google Scholar]
- 5.Davey M E, de Bruijn F J. A homologue of the tryptophan-rich sensory protein TspO and FixL regulate a novel nutrient deprivation-induced Sinorhizobium melilotilocus. Appl Environ Microbiol. 2000;66:5353–5359. doi: 10.1128/aem.66.12.5353-5359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David M, Daveran M L, Batut J, Dedieu A, Domergue O, Ghai J, Hertig C, Boistard P, Kahn D. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988;54:671–683. doi: 10.1016/s0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- 7.David M, Domergue O, Pognonec P, Kahn D. Transcription patterns of Rhizobium meliloti symbiotic plasmid pSym: identification of nifA-independent fixgenes. J Bacteriol. 1987;169:2239–2244. doi: 10.1128/jb.169.5.2239-2244.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Gier J W, Schepper M, Reijnders W N, van Dyck S J, Slotboom D J, Warne A, Saraste M, Krab K, Finel M, Stouthamer A H, van Spanning R J, van der Oost J. Structural and functional analysis of aa3-type and cbb3-type cytochrome c oxidases of Paracoccus denitrificansreveals significant differences in proton-pump design. Mol Microbiol. 1996;20:1247–1260. doi: 10.1111/j.1365-2958.1996.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 9.De Vos G, Walker G, Signer E. Genetic manipulations in Rhizobium meliloti utilizing two new transposon Tn5derivatives. Mol Gen Genet. 1986;204:485–491. doi: 10.1007/BF00331029. [DOI] [PubMed] [Google Scholar]
- 10.Finan T M, Hartwieg E, LeMieux K, Bergman K, Walker G C, Signer E. General transduction in Rhizobium meliloti. J Bacteriol. 1984;159:120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humbert R, Simoni R. Genetic and biochemical studies demonstrating a second gene coding for asparagine synthetase in Escherichia coli. J Bacteriol. 1980;142:212–220. doi: 10.1128/jb.142.1.212-220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiss G B, Vincze É, Kálmán Z, Forrai T, Kondorosi Á. Genetic and biochemical analysis of mutants affected in nitrate reduction in Rhizobium meliloti. J Gen Microbiol. 1979;113:105–118. [Google Scholar]
- 13.Koch H-G, Hwang O, Daldal F. Isolation and characterization of Rhodobacter capsulatus mutants affected in cytochrome cbb3oxidase activity. J Bacteriol. 1998;180:969–978. doi: 10.1128/jb.180.4.969-978.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawry N H, Simon R D. The normal and induced occurrence of cyanophycin inclusion bodies in several blue-green algae. J Phycol. 1982;18:391–399. [Google Scholar]
- 15.Metting F B Jr, editor. Structure and physiological ecology of soil microbial communities. New York, N.Y: Marcel Dekker, Inc; 1993. [Google Scholar]
- 16.Milcamps A, Ragatz D M, Lim P, Berger K A, de Bruijn F J. Isolation of carbon- and nitrogen deprivation-induced loci of Sinorhizobium meliloti 1021 by Tn5-luxABmutagenesis. Microbiology. 1998;144:3205–3218. doi: 10.1099/00221287-144-11-3205. [DOI] [PubMed] [Google Scholar]
- 17.Preisig O, Anthamatten D, Hennecke H. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicumare essential for a nitrogen-fixing endosymbiosis. Proc Natl Acad Sci USA. 1993;90:3309–3313. doi: 10.1073/pnas.90.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preisig O, Zufferey R, Thöny-Meyer L, Appleby C A, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renalier M, Batut J, Ghai J, Terzaghi B, Gherardi M, David M, Garnerone A, Vasse J, Truchet G, Huguet T, Boistard P. A new symbiotic cluster on the pSym megaplasmid of Rhizobium meliloti 2011 carries a functional fix gene repeat and a nodlocus. J Bacteriol. 1987;169:2231–2238. doi: 10.1128/jb.169.5.2231-2238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon R. Cyanophycin granules from the blue-green alga Anabaena cylindrica: a reserve material consisting of copolymers of aspartic acid and arginine. Proc Natl Acad Sci USA. 1971;68:265–267. doi: 10.1073/pnas.68.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon R D. The biosynthesis of multi-l-arginyl-poly(l-aspartic acid) in the filamentous cyanobacterium Anabaena cylindrica. Biochim Biophys Acta. 1976;422:407–418. doi: 10.1016/0005-2744(76)90151-0. [DOI] [PubMed] [Google Scholar]
- 22.Simon R D. The effect of chloramphenicol on the production of cyanophycin granule polypeptide in the blue-green alga Anabaena cylindrica. Arch Mikrobiol. 1973;92:115–122. doi: 10.1007/BF00425009. [DOI] [PubMed] [Google Scholar]
- 23.van Elsas J D, van Overbeek L S, editors. Bacterial responses to soil stimuli. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 24.Wolk P C, Cai Y, Panoff J. Use of a transposon with luciferase as a reporter to identify environmentally responsive genes in cyanobacterium. Proc Natl Acad Sci USA. 1991;88:5355–5359. doi: 10.1073/pnas.88.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida K, Fujita Y, Ehrlich S. Three asparagine synthetase genes of Bacillus subtilis. J Bacteriol. 1999;181:6081–6091. doi: 10.1128/jb.181.19.6081-6091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]