Abstract
Serum amyloid A protein (AA) amyloidosis, also known as secondary amyloidosis, is a known consequence of chronic inflammation and results from several conditions including inflammatory arthritis, periodic fever syndromes, and chronic infection. AA amyloidosis can lead to multiorgan dysfunction, including changes in glomerular filtration rate and proteinuria. Definitive diagnosis requires tissue biopsy, and management of AA amyloid kidney disease is primarily focused on treating the underlying inflammatory condition to stabilize glomerular filtration rate, reduce proteinuria, and slow potential progression to kidney failure. In this narrative review, we describe the causes, pathophysiology, presentation, and pathologic diagnosis of AA amyloid kidney disease using an illustrative case of biopsy-proven AA amyloid kidney disease in a patient with long-standing rheumatoid arthritis who had a favorable response to interleukin 6 inhibition. We conclude the review with a description of established and more novel therapies for AA amyloidosis including published cases of use of tocilizumab (an interleukin 6 inhibitor) in biopsy-proven AA amyloid kidney disease.
Index Words: AA amyloidosis, kidney biopsy, nephrotic syndrome, rheumatoid arthritis, serum amyloid A protein, tocilizumab
Introduction
Amyloid A protein (AA) amyloidosis results from chronic inflammation and is characterized by extracellular multiorgan deposition of serum AA protein fibrils leading to organ dysfunction.1 AA amyloid kidney disease is the most common manifestation,2 typically resulting in proteinuria and progressive decreased kidney function. The management of AA amyloidosis focuses on the treatment of the underlying inflammatory conditions, which include inflammatory arthropathies, familial Mediterranean fever and chronic infections.3 Herein, we describe the causes, pathophysiology (including biopsy findings) and diagnosis of AA amyloid kidney disease using an illustrative case in a patient with long-standing rheumatoid arthritis who presented with nephrotic syndrome and acute onset chronic kidney injury. We conclude with a review of current literature on established and emerging therapies for AA amyloidosis, including a specific focus on the use of interleukin 6 (IL-6) inhibitors in biopsy-proven AA amyloid kidney disease.
Clinical Presentation of the Case
Consent was obtained before publishing the results of this case. A 58-year-old woman with a 40-year history of rheumatoid factor and anticyclic citrullinated peptide antibody-positive rheumatoid arthritis was referred to the nephrology clinic with progressive peripheral edema, new-onset dipstick-positive proteinuria (3 g/L, negative 1 year prior) without hematuria, and abnormal kidney function (creatinine increase from 0.89 mg/dL to 1.36 mg/dL over 4 months, corresponding to an estimated glomerular filtration rate [eGFR] of 43 mL/min/1.73 m2). Her disease-modifying antirheumatic regimen consisted of azathioprine and hydroxychloroquine, having previously failed to achieve symptom control with adalimumab. Upon initial assessment, she was otherwise asymptomatic apart from long-standing chronic pain from the arthritis and new pitting edema to the midshin bilaterally over the preceding months. She endorsed long-standing daily use of naproxen for her joint pain (although the dose had been decreased 3 months prior) and no other new prescription or herbal medications. She was normotensive, with no jugular venous distension and had pitting edema in both lower extremities. She had characteristic features of rheumatoid arthritis including metacarpophalangeal joint subluxation and ulnar deviation. The remainder of the physical examination was noncontributory.
Following her visit, the naproxen was immediately discontinued. A work-up for glomerulonephritis including hepatitis B and C, human immunodeficiency virus and syphilis serologies, antinuclear antibody, and vasculitis panels were negative. C3 and C4 were within normal limits. Serum albumin was 2.5 g/dL (normal, 3.4-5.4 g/dL), hemoglobin A1c was 6.1%, and a lipid panel revealed an low-density lipoprotein level of 126.5 mg/dL and high-density lipoprotein level of 75.4 mg/dL. Serum protein electrophoresis demonstrated no evidence of a monoclonal gammopathy. A 24-hour urine output collection confirmed 3.7 g of protein.
What is AA Amyloidosis?
Epidemiology
The history of amyloidosis dates back at least to the 1800s,4 when pathologists noted abnormal infiltrative proteins with an atypical uptake of iodine stain in autopsies of patients with chronic infection. It was not until the 1960s that the concept of “secondary amyloidosis” in patients with chronic inflammatory conditions was introduced and ultimately sequenced as AA protein.5 Since then, determining an accurate prevalence of AA amyloidosis has been difficult, as a tissue diagnosis is not always performed and the management of chronic inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease have evolved. Estimates in European studies describe an incidence of AA amyloidosis of 1 to 2 patients per million life-years.6,7 Initially, AA amyloid was thought to be most prevalent in patients in their 40s; however, more recent studies have identified that the prevalence is highest among those aged 60 years or greater. Not only have the demographics at diagnosis changed, there has also been a relative decrease in the proportion of systemic amyloidosis thought to be secondary to AA amyloid protein, rather than primary or familial amyloidosis.3
Causes
There are a variety of conditions that have been found to be associated with systemic AA amyloidosis. These include chronic inflammatory arthropathies (rheumatoid arthritis being the most widely reported), chronic infections, periodic fever syndromes, inflammatory bowel disease and vasculitis.2 A recent systematic review reported 48 different conditions with strong association to AA amyloidosis and 19 with a weak association.8 There does appear to be some regional variability, with Western regions reporting the highest incidence of AA amyloidosis secondary to rheumatologic disease,9 and Eastern regions showing higher rates of familial Mediterranean fever.10 The proportion of AA amyloidosis caused by specific diseases is difficult to determine given the wide geographic variability; however, chronic inflammatory arthritis has been estimated as the underlying disorder in roughly 60% of cases.2 There is also some suggestion that the predominant cause has changed over time. In a systematic review of infections and AA amyloidosis, the proportion of AA amyloidosis cases fell from >50% before the year 2000 to 20% after. This may reflect changes in the treatment of chronic infections, especially in developed countries where there may be better access to therapeutics.3 Among chronic inflammatory arthropathies, rheumatoid arthritis and juvenile idiopathic arthritis appear to be the most common causes. In terms of AA amyloidosis secondary to infection, mycobacterium is most commonly implicated, especially in regions with higher rates of tuberculosis.3
Clinical Presentation
Systemic AA amyloidosis has been demonstrated to have multiorgan involvement, with biopsy-proven disease in the kidney, liver, gastrointestinal tract, peripheral nerves, lungs and skin, and soft tissue.11 Presentations vary depending on specific organ involvement; however, new-onset proteinuria and progressive decreased kidney function appear to be by far the most commonly involved organ system, in some cases at over 90% of patients at presentation.2 Lung involvement, presenting as a bilateral interstitial pattern and nerve involvement presenting as peripheral neuropathy appears to be less common.2 While cardiac infiltration is well documented in other forms of amyloidosis, both the prevalence and clinical significance appear to be less in AA amyloidosis than amyloid light chain (AL) amyloidosis.12 Other manifestations are much less common; however, diarrhea and hepatomegaly have also been reported in some studies. The highest-yield biopsy targets for diagnosis appear to be kidney, rectum, and abdominal fat pad based on published case reports.13
Specific to AA amyloid kidney disease, proteinuria is a common initial finding. Although many patients may have subnephrotic proteinuria on presentation, hypoalbuminemia and the nephrotic syndrome as presenting diagnoses are often reported.14 In a prospective cohort of 374 patients with AA amyloidosis who were referred to a National Amyloidosis Center, median proteinuria was 3.9 g/day (interquartile range of 0-26.0), and 97% of patients had >500 mg/day of protein excretion, acknowledging that many of these patients were likely referred later in the course of their disease. The location of protein deposition varies in AA amyloid kidney disease and may dictate clinical presentation and course. Amyloid deposition isolated to the tubulointerstitium tends to present with less severe proteinuria, whereas glomerular involvement results in nephrotic-range proteinuria and more rapid decline in eGFR. One study reported progression to kidney failure in up to 85% in those with biopsy-proven glomerular disease, compared with 0% in those with vascular or tubular AA deposition.15 Similar to proteinuria, changes in eGFR depend largely on the timing of the diagnosis. In the abovementioned prospective cohort study, the median creatinine clearance was 41 mL/min at presentation, and 59 out of 257 patients with a creatinine clearance >20 mL/min at baseline progressed to kidney failure. Specific to rheumatoid arthritis, mesangial proliferative glomerulonephritis, membranous kidney disease, thin basement membrane disease, and interstitial nephritis can occur.16 Crescentic glomerulonephritis has also been reported but appears to be much more rare.17 Although most cases of AA amyloidosis appear to have a more indolent presentation, a subset of patients with familial Mediterranean fever present with acute illness, massive proteinuria, elevated inflammatory markers, and rapid progression to kidney failure within weeks. These rare cases have been described as “amyloid storm” and are thought be precipitated by superimposed infections.18
Pathophysiology
The pathophysiology of AA-amyloid kidney disease involves the deposition of amyloid protein in the kidney parenchyma, resulting in proteinuria and a decline in glomerular filtration. Mechanistically there appears to be a few reasons for proteinuria and the decline in kidney function.14 The first involves physical disruption of tissue architecture from protein deposition, due to amyloid fibril self-aggregation. There is also evidence of direct tissue toxicity by amyloid precursor proteins supported by in vitro studies19 and detection of protein aggregates in the absence of amyloid deposition. Serum AA, the precursor protein of AA amyloidosis, is an acute phase reactant and plays a role as an opsonin in bacterial phagocytosis. Its production by hepatocytes is mediated by tumor necrosis factor (TNF), IL-1, and IL-6. Serum AA circulates on plasma high-density lipoprotein and is cleaved to AA by macrophages.20 Human serum AA genes have multiple alleles with polymorphisms, and it is this variability along with significant heterogeneity in the composition of high-density lipoprotein that likely explains the large spectrum of clinical presentations for AA amyloidosis.21 Although the exact mechanism of serum AA cleavage leading to nephron AA deposition is unclear, there does appear to be correlation with serum AA levels. Gillmore et al22 demonstrated that amyloid deposits, measured with amyloid P scintigraphy, regressed with improvement in serum AA levels. In addition, end organ dysfunction was more severe in those with serum AA concentrations >50 mg/L, and serum AA levels <10 mg/L were associated with almost 90% ten-year survival. In cases of AA amyloid kidney disease, serum AA levels were the strongest risk factor for the development of kidney failure. Fortunately, with effective treatment of systemic inflammation, serum AA levels do appear to decrease, and this, in turn, is also associated with improved long-term prognosis.13
Diagnosis and Pathologic Findings
The diagnosis of AA amyloid kidney disease should be suspected in any patient presenting with new proteinuria associated with a decline in kidney function, in the context of an underlying chronic inflammatory condition. Some have suggested (perhaps most importantly in rheumatologic disease, which is the most common underlying condition) that even the presence of >500 mg of proteinuria should prompt consideration of a kidney biopsy, although this approach is not substantiated by evidence. Although serum AA levels have shown correlation with severity of AA amyloidosis and may play a role in monitoring therapeutic response, they do not always correlate with the presence of amyloid deposits.23 In addition, serum AA levels are often elevated in the presence of rheumatologic disease, therefore they are not sufficient to diagnose AA amyloid kidney disease in the absence of kidney biopsy.24 Finally, assays for serum AA levels are not widely available, limiting their use for frequent testing or monitoring. 123I-labeled serum amyloid P component scintigraphy has shown more promise as a noninvasive diagnostic test. Although the diagnostic sensitivity has been shown to be 90% for both AA and AL amyloidosis,25 the degree of uptake may not correlate with the severity of clinical symptoms. The main barrier to routine diagnostic use of serum amyloid P scintigraphy appears to be access, as the required iodine isotypes are expensive and not readily available at all centers. Serum amyloid P scintigraphy has also been shown to be useful for monitoring therapeutic response26 and as a result has been used in evaluating novel therapeutics for AA amyloidosis.
Biopsy remains the gold standard diagnostic test for AA amyloid kidney disease. Pathologically, amyloid protein is an amorphous extracellular material that is lightly eosinophilic, and sections with amyloid deposition will classically have affinity for Congo red stain.14 Findings vary depending on histologic technique. Under light microscopy, findings of amyloid deposition are identical regardless of protein subtype. These include accumulation of acellular amorphous eosinophilic material on hematoxylin and eosin stain that are pale on periodic acid–Schiff stain. A nodular appearance of acellular amorphous expansion in the mesangial matrix is more commonly associated with AA amyloidosis but is not always seen.27 Congo red stain can produce false-positives with overstaining; therefore it is recommended that results be confirmed under polarized light identifying green birefringence.28 Electron microscopy may also be used as a confirmatory test for amyloid deposition; however, it is not able to differentiate between subtypes. Given the difficulty of distinguishing amyloid subtypes despite immunofluorescence, further analysis with mass spectrometry is recommended for definitive diagnosis. Gilbertson et al29 demonstrated tandem mass spectrometry in addition to immunofluorescence increased the identification of specific amyloid protein in 94% of biopsies, with an 18% increase in pick-up rate compared with immunofluorescence alone. As a result, mass spectrometry is the preferred method for confirmation of AA amyloid deposition.
Pathologic Findings of the Case
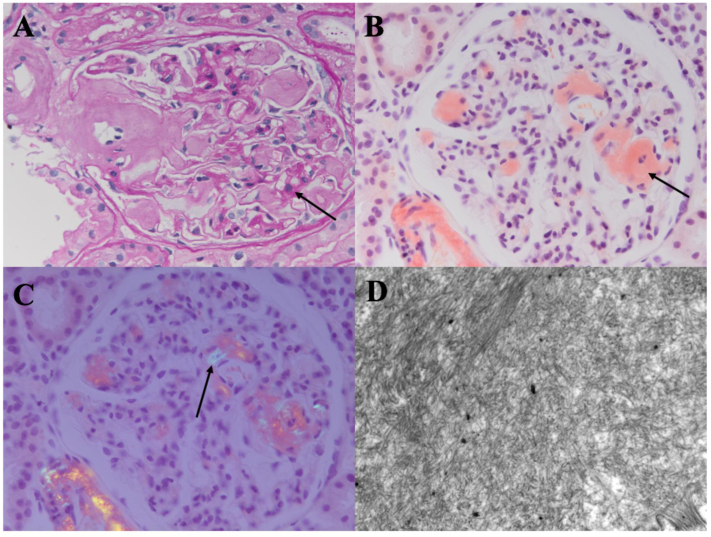
Our patient underwent a kidney biopsy which revealed prominent expansion of the glomerular mesangium with homogeneous eosinophilic material (Fig 1A). The arteries showed foci of expansion by similar homogeneous eosinophilic material. These expanded areas stained salmon red with Congo red (Fig 1B) and showed apple green birefringence on polarization (Fig 1C). Four of 18 glomeruli were globally sclerosed. There was mild tubular atrophy with interstitial fibrosis, and mild arterial sclerosis. Electron microscopy showed glomerular basement membrane, mesangial and arteriolar expansion by randomly arranged fibrils, approximately 10 nm in diameter, consistent with amyloid (Fig 1D). Foot process effacement was noted in the examined glomerulus. Immunofluorescence showed 1+ mesangial IgM κ and λ and trace IgG and C3. Amyloid subtyping by liquid chromatography tandem mass spectrometry (Mayo Clinic) was consistent with AA amyloidosis.
Figure 1.
Kidney biopsy findings (clinical case). (A) Homogeneous weak material (arrow) in glomerular mesangium and arteriole. Periodic acid–Schiff stain. Original magnification, ×400. (B) Salmon red staining. Congo red stain (arrow). Original magnification, ×400. (C) Apple green birefringence (arrow). Congo red stain viewed under polarized light. Original magnification, ×400. (D) Randomly arranged fibrils, ∼10 nm in diameter, in glomerular mesangium. Electron microscopy. Original magnification, ×10,000.
Management and Emerging Therapies
General Approach
The management of AA amyloid kidney disease involves suppression of systemic inflammation, in addition to pharmacologic treatment generally aimed at treating proteinuric kidney disease. Although formal studies are lacking, intuitively, the latter would include renin-angiotensin-aldosterone system inhibitors, and sodium/glucose cotransporter 2 inhibition. Furthermore, management of hyperlipidemia and edema may be required if the patient has high-grade proteinuria. Therapies targeted to decrease inflammation (and serum AA levels) should be tailored to a patient’s underlying disease process. Examples of these strategies are outlined below.
Disease Specific-Approach
Rheumatoid and Inflammatory Arthritis
Treatment for AA amyloidosis in patients with rheumatoid arthritis has evolved with the increased use of biologics over the past 20 years. Initially, cyclophosphamide in combination with prednisolone had the best evidence for AA amyloidosis in patients with rheumatoid arthritis refractory to initial disease-modifying antirheumatic drug therapy.30 Cyclophosphamide gradually became replaced by anti-TNF agents, especially after anticytokine therapy was shown to be better tolerated and associated with improved survival. As the European Alliance of Associations for Rheumatology guidelines adopted anti-TNF agents as first-line therapy for rheumatoid arthritis,31 anticytokine therapy as a treatment for AA amyloidosis was studied. When compared directly, etanercept was shown to be superior to cyclophosphamide in both preservation of eGFR and reduction in proteinuria.32 In addition to etanercept, both infliximab33 and adalimumab34 have been used to treat AA amyloidosis, although there have been no head-to-head comparisons of anti-TNF agents. Beyond rheumatoid arthritis, anti-TNF agents have also been shown to be effective and well-tolerated during long-term follow-up in both inflammatory bowel disease and other inflammatory arthropathies as well.35
Familial Mediterranean Fever
As the mainstay of treatment, colchicine’s impact on AA amyloid kidney disease secondary to underlying familial Mediterranean fever has been well documented.36 Colchicine has been shown to reduce proteinuria and preserve kidney function. In one study, this effect did appear to be contingent on reaching a therapeutic dose of >1.5 mg per day and beginning treatment in patients with serum creatinine <1.5 mg/dL.37 The presumed mechanism of action for colchicine’s impact on amyloid deposition and subsequent kidney disease is suppression of cytokines involved in serum AA production that are also targeted by other therapies. Colchicine inhibits neutrophil production, and therefore production of IL-1 and IL-8. In addition, it reduces the protein expression of TNF.38
Emerging Therapies
Tocilizumab
The management of AA amyloidosis continues to evolve as alternative cytokines involved in serum AA production are targeted. IL-6 is a proinflammatory cytokine that induces serum AA production via interaction with the signal transducer and activator of transcription protein.39 Tocilizumab is a recombinant monoclonal antibody that blocks IL-6 signal transduction by targeting IL-6 receptor complex formation and has been shown clinically and in vitro to decrease AA production.40 IL-6 inhibitors have gradually become more widely used in the treatment of inflammatory arthropathies and as a result have been used in the treatment of AA amyloid kidney disease with a goal of reducing proteinuria and stabilizing kidney function.41
Outcomes of these cases vary; however, tocilizumab has been shown to decrease serum AA, as well as improve proteinuria and GFR, most notably in AA amyloidosis associated with inflammatory arthritis.
The earliest reports of tocilizumab for systemic AA amyloidosis appear to date back to 2006, when Okuda et al42 reported improvements in serum AA amyloid levels, improvements in proteinuria, and histologic improvement in a 26-year-old woman with juvenile idiopathic arthritis. However, it was not until 2011 that case reports of biopsy-proven AA amyloid kidney disease responding to tocilizumab were published. The first involved a patient presenting with nephrotic syndrome that was found to have biopsy-proven AA amyloid kidney disease in addition to underlying latent tuberculosis.43 Subsequent treatment with tocilizumab lead to rapid improvement in proteinuria over a 9-week follow-up period; however, the patient experienced GFR decline because of gastrointestinal illness, ultimately leading to the need for hemodialysis. Since then, there have been several case reports regarding the efficacy of tocilizumab in chronic diseases, including Behcet disease, polyarteritis nodosa, psoriatic arthritis, and rheumatoid arthritis (Table 144, 45, 46, 47, 48, 49, 50).
Table 1.
Summary of Published Cases of Biopsy-Confirmed AA-Amyloid Kidney Disease in Patients With Rheumatoid Arthritis Treated With Tocilizumab and Reported Outcomes
| Reference | Age (y) | Sex | Presentation | Treatment Dose (mg/kg/mo) | Treatment Duration (mo) | Concurrent Treatment | Response |
|---|---|---|---|---|---|---|---|
| Vinicki et al (2013)45 | 48 | F | 5.0 g/24 h proteinuria | 8 | 24 | None | 0.5 g proteinuria/24 h |
| Matsui et al (2014)46 | 60 | F | Progressive kidney dysfunction | 8 | 36 | None | Persistent deposition on biopsy, progression to kidney failure |
| Yamada et al (2014)47 | 71 | F | Nephrotic syndrome, 4.3 g/24 h proteinuria | 8 | 10 | Losartan | Resolved proteinuria at 3 mo |
| Courties et al (2015)44 | 52 | F | 9.0 g/24 h proteinuria | 8 | 8 | Glucocorticoids | Progression to kidney failure within 20 mo |
| 70 | F | 8.4 g/24 h proteinuria | 8 | 3 | Glucocorticoids | Stable eGFR, 0.5 g/24 h proteinuria | |
| 47 | M | 10.0 g/24 h proteinuria | 8 | 6 | Colchicine | Progression to kidney failure within 6 mo | |
| 80 | F | 4.0 g/24 h proteinuria | 8 | 34 | Glucocorticoids | Improved eGFR, progression to 6.0 g/24 h proteinuria | |
| 80 | F | 2.1 g/24 h proteinuria | 8 | 32 | Glucocorticoids | Stable eGFR, 0.09 g/24 h proteinuria | |
| 73 | M | 8.0 g/24 h proteinuria | 8 | 6 | Glucocorticoids | Stable eGFR, 4.7 g/24 h proteinuria | |
| Iijima et al (2015)48 | 51 | F | Nephrotic syndrome, 5.2 g/24 h proteinuria and RPGN | 8 | 18 | None | Improved eGFR, 1.2 g/24 h proteinuria |
| Yamagata et al (2017)49 | 67 | F | Urinary protein excretion of 7.5 g per gram urinary creatinine | Not reported | 10 | None | 0.5 g/24 h proteinuria |
| Fukuda et al (2020)50 | 59 | F | Nephrotic syndrome, 6.5 g/24 h proteinuria | 8 | 24 | None | 1.1g/24 h proteinuria |
| 71 | M | 0.06 g/24 h proteinuria | 8 | 60 | None | Glomerular amyloid deposits unchanged on repeat biopsy (at 2 y) |
Abbreviations: AA, serum amyloid A protein; eGFR, estimated glomerular filtration rate; F, female; M, male; RPGN, rapidly progressive glomerulonephritis.
Specific to rheumatologic disease, Okuda et al51 compared the effectiveness of tocilizumab to anti-TNF agents in 42 patients with AA amyloidosis. Patients in the tocilizumab group showed greater reduction in serum AA protein levels as well as improvements in kidney function and clinical disease activity. Notably, all patients had biopsy-proven gastrointestinal AA amyloid involvement, whereas only 3 patients in the study had biopsy-diagnosed AA amyloid kidney disease. Shortly after, a 2015 series of 6 cases of biopsy-proven AA amyloid kidney disease44 also demonstrated the efficacy of tocilizumab in stabilizing GFR, reducing proteinuria, and improving inflammatory markers. Additional case series have focused on amyloid load measured with serum amyloid P scintigraphy in addition to patient quality of life and have demonstrated rapid reduction in amyloid deposition within 10 days that was sustained over almost 2 years of follow-up.52
Tocilizumab has also shown benefit in treating AA amyloidosis in a variety of other underlying conditions, including familial Mediterranean fever (Table 2)53,54, multicentric Castleman disease and viral hepatitis (Table 3).55, 56, 57, 58, 59, 60
Table 2.
Summary of Published Cases of Biopsy-Confirmed AA-Amyloid Kidney Disease in Patients With Familial Mediterranean Fever Treated With Tocilizumab and Reported Outcomes
| Reference | Age (y) | Sex | Presentation | Tocilizumab Dose (mg/kg/mo) | Treatment Duration (mo) | Concurrent Treatment | Response |
|---|---|---|---|---|---|---|---|
| Serelis et al (2015)53 | 32 | F | Nephrotic syndrome and 9.0 g/24 h proteinuria | 8 | 2 | Colchicine 1 mg, lisinopril 5 mg twice a day | Resolution of nephrotic syndrome, 3.0 g/24 h proteinuria |
| Ugurlu et al (2017)54 | 36 | M | 12 g/24 h proteinuria | 8 | 6 | None | Resolved nephrotic syndrome, 2.1 g/24 h proteinuria |
| 44 | M | Baseline Cr 2.58 mg/dL, 23.7 g/24 h proteinuria | 8 | 5 | None | Cr 1.85 mg/dL and 14.9 g/24 h proteinuria | |
| 45 | M | Baseline Cr 1.28 mg/dL, 4.7 g/24 h proteinuria | 8 | 31 | None | Cr 1.12 mg/dL and 4.4 g/24 h proteinuria | |
| 47 | F | Baseline Cr 0.79 mg/dL, 2.1 g/24 h proteinuria | 8 | 31 | None | Cr 0.83 mg/dL and 1.4 g/24 h proteinuria | |
| 23 | M | Baseline Cr 0.69 mg/dL, 3 g/24 h proteinuria | 8 | 4 | None | Cr 0.71 mg/dL and 1.4 g/24 h proteinuria | |
| 35 | M | Baseline Cr 1.18 mg/dL, 1.7 g/24 h proteinuria | 8 | 28 | None | Cr 1.39 mg/dL and 2.7 g/24 h proteinuria | |
| 41 | F | Baseline Cr 0.71 mg/dL, 3 g/24 h proteinuria | 8 | 13 | None | Cr 0.64 mg/dL and 1.9 g/24 h proteinuria | |
| 39 | F | Baseline Cr 0.43 mg/dL, 1.6 g/24 h proteinuria | 8 | 32 | None | Cr 0.54 mg/dL and 0.7 g/24 h proteinuria | |
| 24 | F | Baseline Cr 0.4 mg/dL, 6 g/24 h proteinuria | 8 | 4 | None | Cr 0.35 mg/dL and 4.7 g/24 h proteinuria | |
| 22 | F | Baseline Cr 0.38 mg/dL, 1.8 g/24 h proteinuria | 8 | 20 | None | Cr 0.5 mg/dL and 0.1 g/24 h proteinuria | |
| 45 | F | Baseline Cr 0.79 mg/dL, 7.1 g/24 h proteinuria | 8 | 6 | None | Cr 0.93 mg/dL and 5.7 g/24 h proteinuria | |
| 21 | M | Baseline Cr 0.83 mg/dL, 11.7 g/24 h proteinuria | 8 | 4 | None | Cr 0.85 mg/dL and 16.7 g/24 h proteinuria |
Abbreviations: AA, serum amyloid protein; Cr, creatinine; eGFR, estimated glomerular filtration rate; F, female, M, male.
Table 3.
Summary of Published Cases of Biopsy-Confirmed AA-Amyloid Kidney Disease in Patients Without Rheumatoid Arthritis or Familial Mediterranean Fever Treated With Tocilizumab and Reported Outcomes
| Reference | Age (y) | Sex | Associated Condition | Presentation | Tocilizumab Dose | Duration (mo) | Concurrent Treatment | Response |
|---|---|---|---|---|---|---|---|---|
| Magro-Checa et al (2011)43 | 25 | M | Latent tuberculosis | Nephrotic syndrome (18 g/24 h proteinuria) | 8 mg/kg/mo | 12 | Isoniazid 300 mg | Improved proteinuria to 1.7 g/24 h. Unchanged colonic amyloid deposition on repeat biopsy (12 mo) |
| De La Torre et al (2011)55 | 14 | F | Juvenile idiopathic arthritis | Nephrotic syndrome (7 g/24 h proteinuria) | 8 mg/kg/2 wk | 12 | None | Improved proteinuria and kidney function |
| Hočevar et al (2013)56 | 33 | M | Polyarteritis nodosa | 3.6 g/24 h proteinuria | 8 mg/kg/mo | 10 | Methylprednisolone 4 mg | Improvement in proteinuria to 1.0 g/24 h, regression of glomerular amyloid depositions on repeat biopsy at 6 mo |
| Redondo- Pachón et al (2013)57 | 51 | F | Bechets | Nephrotic syndrome (9.5 g/24 h proteinuria) | 8 mg/kg/mo | 12 | Colchicine 1 mg/d + isoniazid | Resolved nephrotic syndrome, improvement in proteinuria to 1.7 g/24 h |
| Pelegrin et al (2016)58 | 58 | F | Seronegative polyarthritis | Nephrotic syndrome (11 g/24 h proteinuria) | 480 mg/mo | 60 | None | Resolved nephrotic syndrome, stabilized eGFR |
| 46 | M | Chronic osteomyelitis | Nephrotic syndrome (6 g/24 h proteinuria) | 8 mg/kg/mo | 8 | None | Progression to kidney failure, increase to 11 g/24 h proteinuria | |
| 56 | F | Systemic sclerosis | Nephrotic syndrome (3 g/24 h proteinuria) | 8 mg/kg/mo | 5 | None | Stable eGFR, progression to 6 g/24 h proteinuria | |
| Ugurlu et al (2017)54 | 25 | M | Latent tuberculosis | Nephrotic syndrome (18 g/24 h proteinuria) | 8 mg/kg/mo | 12 | Isoniazid 300 mg | Improved proteinuria to 1.7 g/24 h, resolved nephrotic syndrome |
| Eriksson et al (2021)59 | 60 | M | AS | 0.52 g/24 h proteinuria | 8 mg/kg/mo | 52 | None | Stability |
| 69 | M | AS | urinary albumin-creatinine ratio 913 g/mol | 8 mg/kg/mo | 18 | None | Stability, decreased protein | |
| Giannese et al (2021)60 | 63 | M | Sweet syndrome | 10 g/24 h proteinuria | 8 mg/kg/mo | 23 | None | Complete resolution of nephrotic syndrome, 1.5 g/24 h proteinuria |
Abbreviations: AA, serum amyloid A protein; AS, ankylosing spondylitis; eGFR, estimated glomerular filtration rate; F, female; M, male.
Other Treatments
Eprodisate, thought to prevent amyloid deposition by directly targeting glycosaminoglycan-amyloid fibril complexes,61 initially showed promise after demonstrating superiority to placebo in AA-amyloid kidney disease in a composite outcome of kidney function and/or death.62 Unfortunately, a follow-up phase 3 clinical trial63 did not meet its primary outcome of preserved kidney function, and further studies targeting this pathway are ongoing. In addition, efforts to target amyloidogenic precursor proteins (specifically their interaction with glycosaminoglycans)64 are also in development. These treatments are not yet approved for use outside clinical trials. Anti-AA amyloid-specific monoclonal antibodies have also been developed and have been shown to be effective in specifically targeting amyloid deposition in small animal studies.65 Although the hope is that fibril-specific monoclonal antibodies can remove pathologic amyloid deposits, this has yet to be demonstrated clinically. The role of IL-6 in systemic inflammation and AA amyloidosis has been targeted via a different route, namely an IL-6 binding protein that has shown sustained antagonism of the receptor in vivo.66 Similar binding proteins have been used in ongoing studies for patients with hereditary amyloidosis as potential therapeutic options.67
Management and Outcome of the Case
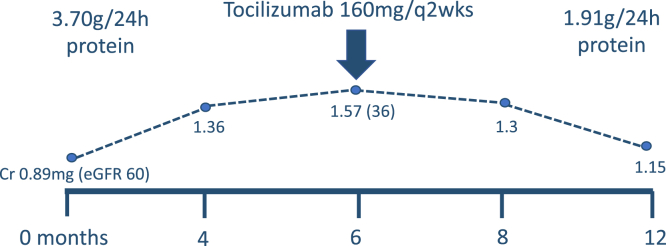
At the time of her presentation, she was on lisinopril 5 mg daily. This was changed following the initial 24-hour urine output collection to candesartan because of cough (which resolved) but could not be up-titrated due to hypotension. In collaboration with the referring rheumatologist, in addition to conservative treatment with dietary salt reduction, she was prescribed tocilizumab (160 mg subcutaneously every 2 weeks). Before initiating treatment, serum creatinine peaked at 1.57 mg/dL (eGFR 36 mL/min/1.73 m2). There was no evidence of cardiac amyloidosis on transthoracic echocardiography. Follow-up 6 months after starting anti-IL-6 therapy revealed a reduction in proteinuria to 1.91 g on a 24-hour collection improvement in serum creatinine to 1.15 mg/dL (eGFR 52 mL/min/1.73 m2) and a fall in serum C-reactive protein from 15 mg/L at the time of diagnosis to <5 mg/L (Fig 2).
Figure 2.
Treatment of biopsy-proven AA-amyloid kidney disease in a patient with rheumatoid arthritis using tocilizumab and subsequent response.
Conclusion
In conclusion, in cases of kidney disease in patients with long-standing underlying autoimmune conditions, chronic infection, or periodic fever syndromes, the diagnosis of AA-amyloid kidney disease should be considered. AA-amyloid kidney disease is associated most strongly with inflammatory arthropathies and familial Mediterranean fever and most commonly presents with proteinuria. Definitive diagnosis requires biopsy with additional mass spectrometry analysis. Treatment consists primarily of targeting the inflammatory condition. In addition, more contemporary therapies including IL-6 inhibitors have been shown to be effective at reducing serum AA production and improving/stabilizing proteinuria and eGFR.
Article Information
Authors’ Full Names and Academic Degrees
Jordan Thorne, MD, David Clark, MD, Laurette Geldenhuys, MD, Keigan More, MD, Amanda Vinson, MD, and Karthik Tennankore, MD
Support
None
Financial Disclosure
Dr Vinson has received consultancy and ad board fees from Paladin Labs Inc as well as fellowship grant funding. Dr Clark has received advisory board or consultancy fees from Baxter; received unrestricted grant funding from Baxter (for fellowship program) and Otsuka (for investigator-initiated research). Dr Tennankore has received advisory board or consultancy fees from Baxter, Bayer, AstraZeneca, Otsuka and GSK. He has received unrestricted grant funding from Baxter (for fellowship program) and Otsuka (for investigator-initiated research). The remaining authors declare that they have no relevant financial interests.
Peer Review
Received January 25, 2022 as a submission to the expedited consideration track with 3 external peer reviews. Direct editorial input from the Editor-in-Chief. Accepted in revised form April 29, 2022.
Footnotes
Complete author and article information provided before references.
References
- 1.Khalighi M.A., Dean Wallace W., Palma-Diaz M.F. Amyloid nephropathy. Clin Kidney J. 2014;7(2):97–106. doi: 10.1093/ckj/sfu02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lachmann H.J., Goodman H.J., Gilbertson J.A., et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356(23):2361–2371. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 3.Real de Asúa D., Costa R., Galván J.M., Filigheddu M.T., Trujillo D., Cadiñanos J. Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clin Epidemiol. 2014;6:369–377. doi: 10.2147/CLEP.S39981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle R.A. Amyloidosis: a convoluted story. Br J Haematol. 2001;114(3):529–538. doi: 10.1046/j.1365-2141.2001.02999.x. [DOI] [PubMed] [Google Scholar]
- 5.Harrison J.S., Cohen Y., Ioffe I., Bulvik S. In: Amyloidosis –History and Perspectives. Harrison J.S., editor. IntechOpen; 2021. An historical overview of the amyloidoses. [DOI] [Google Scholar]
- 6.Hemminki K., Li X., Försti A., Sundquist J., Sundquist K. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health. 2012;12:974. doi: 10.1186/1471-2458-12-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinney J.H., Smith C.J., Taube J.B., et al. Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161(4):525–532. doi: 10.1111/bjh.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunger A.F., Nienhuis H.L.A., Bijzet J., Hazenberg B.P.C. Causes of AA amyloidosis: a systematic review. Amyloid. 2020;27(1):1–12. doi: 10.1080/13506129.2019.1693359. [DOI] [PubMed] [Google Scholar]
- 9.Bunker D., Gorevic P. AA amyloidosis: Mount Sinai experience, 1997-2012. Mt Sinai J Med. 2012;79(6):749–756. doi: 10.1002/msj.21342. [DOI] [PubMed] [Google Scholar]
- 10.Ensari C., Ensari A., Tümer N., Ertug E. Clinicopathological and epidemiological analysis of amyloidosis in Turkish patients. Nephrol Dial Transplant. 2005;20(8):1721–1725. doi: 10.1093/ndt/gfh890. [DOI] [PubMed] [Google Scholar]
- 11.Deshayes S., Aouba A., Grateau G., Georgin-Lavialle S. Infections and AA amyloidosis: an overview. Int J Clin Pract. 2021;75(6) doi: 10.1111/ijcp.13966. [DOI] [PubMed] [Google Scholar]
- 12.Shah K.B., Inoue Y., Mehra M.R. Amyloidosis and the heart: a comprehensive review. Arch Intern Med. 2006;166(17):1805–1813. doi: 10.1001/archinte.166.17.1805. [DOI] [PubMed] [Google Scholar]
- 13.Wisniowski B., Wechalekar A. Confirming the diagnosis of amyloidosis. Acta Haematol. 2020;143(4):312–321. doi: 10.1159/000508022. [DOI] [PubMed] [Google Scholar]
- 14.Dember L.M. Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;17(12):3458–3471. doi: 10.1681/ASN.2006050460. [DOI] [PubMed] [Google Scholar]
- 15.Uda H., Yokota A., Kobayashi K., et al. Two distinct clinical courses of renal involvement in rheumatoid patients with AA amyloidosis. J Rheumatol. 2006;33(8):1482–1487. [PubMed] [Google Scholar]
- 16.Kuroda T., Wada Y., Nakano M. In: Amyloidosis. IntechOpen. Feng D., editor. 2013. Diagnosis and treatment of AA amyloidosis with rheumatoid arthritis: state of the art. [DOI] [Google Scholar]
- 17.Nagata M., Shimokama T., Harada A., Koyama A., Watanabe T. Glomerular crescents in renal amyloidosis: an epiphenomenon or distinct pathology? Pathol Int. 2001;51(3):179–186. doi: 10.1046/j.1440-1827.2001.01188.x. [DOI] [PubMed] [Google Scholar]
- 18.Kukuy O.L., Beckerman P., Dinour D., Ben-Zvi I., Livneh A. Amyloid storm: acute kidney injury and massive proteinuria, rapidly progressing to end-stage kidney disease in AA amyloidosis of familial Mediterranean fever. Rheumatology (Oxford) 2021;60(7):3235–3242. doi: 10.1093/rheumatology/keaa772. [DOI] [PubMed] [Google Scholar]
- 19.Keeling J., Teng J., Herrera G.A. AL-amyloidosis and light-chain deposition disease light chains induce divergent phenotypic transformations of human mesangial cells. Lab Invest. 2004;84(10):1322–1338. doi: 10.1038/labinvest.3700161. [DOI] [PubMed] [Google Scholar]
- 20.Muchtar E., Dispenzieri A., Magen H., et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021;289(3):268–292. doi: 10.1111/joim.13169. [DOI] [PubMed] [Google Scholar]
- 21.Westermark G.T., Fändrich M., Westermark P. AA amyloidosis: pathogenesis and targeted therapy. Annu Rev Pathol. 2015;10:321–344. doi: 10.1146/annurev-pathol-020712-163913. [DOI] [PubMed] [Google Scholar]
- 22.Gillmore J.D., Lovat L.B., Persey M.R., Pepys M.B., Hawkins P.N. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet. 2001;358(9275):24–29. doi: 10.1016/S0140-6736(00)05252-1. [DOI] [PubMed] [Google Scholar]
- 23.Migita K., Eguchi K., Tsukada T., et al. Increased circulating serum amyloid A protein derivatives in rheumatoid arthritis patients with secondary amyloidosis. Lab Invest. 1996;75(3):371–375. [PubMed] [Google Scholar]
- 24.Sorić Hosman I., Kos I., Lamot L. Serum amyloid A in inflammatory rheumatic diseases: a compendious review of a renowned biomarker. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.631299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazenberg B.P., van Rijswijk M.H., Piers D.A., et al. Diagnostic performance of 123I-labeled serum amyloid P component scintigraphy in patients with amyloidosis. Am J Med. 2006;119(4):355.e15–355.e24. doi: 10.1016/j.amjmed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 26.Sachchithanantham S., Wechalekar A.D. Imaging in systemic amyloidosis. Br Med Bull. 2013;107:41–56. doi: 10.1093/bmb/ldt021. [DOI] [PubMed] [Google Scholar]
- 27.Dikman S.H., Churg J., Kahn T. Morphologic and clinical correlates in renal amyloidosis. Hum Pathol. 1981;12(2):160–169. doi: 10.1016/s0046-8177(81)80103-7. [DOI] [PubMed] [Google Scholar]
- 28.Paueksakon P. In: Exploring New Findings on Amyloidosis. Fernandez-Escamilla A., editor. IntechOpen; 2016. Amyloid nephropathy: a practical diagnostic approach and review on pathogenesis. [DOI] [Google Scholar]
- 29.Gilbertson J.A., Theis J.D., Vrana J.A. A comparison of immunohistochemistry and mass spectrometry for determining the amyloid fibril protein from formalin-fixed biopsy tissue. J Clin Pathol. 2015;68(4):314–317. doi: 10.1136/jclinpath-2014-202722. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T., Yamamura Y., Tomoda K., Tsukano M., Shono M., Baba S. Efficacy of cyclophosphamide combined with prednisolone in patients with AA amyloidosis secondary to rheumatoid arthritis. Clin Rheumatol. 2003;22(6):371–375. doi: 10.1007/s10067-003-0763-9. [DOI] [PubMed] [Google Scholar]
- 31.Smolen J.S., Landew R.B.M., Bijlsma J.W.J., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T., Higashi S., Tomoda K., Tsukano M., Shono M. Effectiveness of etanercept vs cyclophosphamide as treatment for patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Rheumatology (Oxford) 2012;51(11):2064–2069. doi: 10.1093/rheumatology/kes190. [DOI] [PubMed] [Google Scholar]
- 33.Kuroda T., Otaki Y., Sato H., et al. A case of AA amyloidosis associated with rheumatoid arthritis effectively treated with infliximab. Rheumatol Int. 2008;28(11):1155–1159. doi: 10.1007/s00296-008-0590-z. [DOI] [PubMed] [Google Scholar]
- 34.Fikri-Benbrahim O., Rivera-Hernández F., Martínez-Calero A., Cazalla-Cadenas F., García-Agudo R., Mancha-Ramos J. Treatment with adalimumab in amyloidosis secondary to rheumatoid arthritis: two case reports. Nefrologia. 2013;33(3):404–409. doi: 10.3265/Nefrologia.pre2012.Aug.11552. [DOI] [PubMed] [Google Scholar]
- 35.Esatoglu S.N., Hatemi G., Ugurlu S., Gokturk A., Tascilar K., Ozdogan H. Long-term follow-up of secondary amyloidosis patients treated with tumor necrosis factor inhibitor therapy: a STROBE-compliant observational study. Medicine (Baltimore) 2017;96(34) doi: 10.1097/MD.0000000000007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Chetrit E. Familial Mediterranean fever (FMF) and renal AA amyloidosis--phenotype-genotype correlation, treatment and prognosis. J Nephrol. 2003;16(3):431–434. [PubMed] [Google Scholar]
- 37.Andronesi A.G., Ismail G., Gherghiceanu M., Mitroi G., Hârza M.C. Familial Mediterranean fever-associated renal amyloidosis: case report and review of the literature. Rom J Morphol Embryol. 2019;60(4):1299–1303. [PubMed] [Google Scholar]
- 38.Livneh A., Zemer D., Langevitz P., Laor A., Sohar E., Pras M. Colchicine treatment of AA amyloidosis of familial Mediterranean fever. An analysis of factors affecting outcome. Arthritis Rheum. 1994;37(12):1804–1811. doi: 10.1002/art.1780371215. [DOI] [PubMed] [Google Scholar]
- 39.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine--update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito S., Suzuki K., Yoshimoto K., et al. A new bioassay for measuring the strength of IL-6/STAT3 signal inhibition by tocilizumab in patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):231. doi: 10.1186/s13075-017-1434-6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane T., Gillmore J.D., Wechalekar A.D., Hawkins P.N., Lachmann H.J. Therapeutic blockade of interleukin-6 by tocilizumab in the management of AA amyloidosis and chronic inflammatory disorders: a case series and review of the literature. Clin Exp Rheumatol. 2015;33(6 suppl 94):S46–S53. [PubMed] [Google Scholar]
- 42.Okuda Y., Takasugi K. Successful use of a humanized anti-interleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis. Arthritis Rheum. 2006;54(9):2997–3000. doi: 10.1002/art.22118. [DOI] [PubMed] [Google Scholar]
- 43.Magro-Checa C., Navas-Parejo Casado A., Borrego-García E., et al. Successful use of tocilizumab in a patient with nephrotic syndrome due to a rapidly progressing AA amyloidosis secondary to latent tuberculosis. Amyloid. 2011;18(4):235–239. doi: 10.3109/13506129.2011.613962. [DOI] [PubMed] [Google Scholar]
- 44.Courties A., Grateau G., Philippe P., et al. AA amyloidosis treated with tocilizumab: case series and updated literature review. Amyloid. 2015;22(2):84–92. doi: 10.3109/13506129.2014.1002031. [DOI] [PubMed] [Google Scholar]
- 45.Vinicki J.P., De Rosa G., Laborde H.A. Renal amyloidosis secondary to rheumatoid arthritis: remission of proteinuria and renal function improvement with tocilizumab. J Clin Rheumatol. 2013;19(4):211–213. doi: 10.1097/RHU.0b013e318293793c. [DOI] [PubMed] [Google Scholar]
- 46.Matsui M., Okayama S., Tsushima H., et al. Therapeutic benefits of tocilizumab vary in different organs of a patient with AA amyloidosis. Case Rep Nephrol. 2014;2014 doi: 10.1155/2014/823093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada S., Tsuchimoto A., Kaizu Y., et al. Tocilizumab-induced remission of nephrotic syndrome accompanied by secondary amyloidosis and glomerulonephritis in a patient with rheumatoid arthritis. CEN Case Rep. 2014;3(2):237–243. doi: 10.1007/s13730-014-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iijima T., Suwabe T., Sumida K., et al. Tocilizumab improves systemic rheumatoid vasculitis with necrotizing crescentic glomerulonephritis. Mod Rheumatol. 2015;25(1):138–142. doi: 10.3109/14397595.2013.874748. [DOI] [PubMed] [Google Scholar]
- 49.Yamagata A., Uchida T., Yamada Y., et al. Rapid clinical improvement of amyloid A amyloidosis following treatment with tocilizumab despite persisting amyloid deposition: a case report. BMC Nephrol. 2017;18(1):377. doi: 10.1186/s12882-017-0799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukuda M., Sawa N., Hoshino J., Ohashi K., Motoaki M., Ubara Y. Tocilizumab preserves renal function in rheumatoid arthritis with AA amyloidosis and end-stage kidney disease: two case reports. Clin Nephrol. 2021;95(1):54–61. doi: 10.5414/CN109971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okuda Y., Ohnishi M., Matoba K., et al. Comparison of the clinical utility of tocilizumab and anti-TNF therapy in AA amyloidosis complicating rheumatic diseases. Mod Rheumatol. 2014;24(1):137–143. doi: 10.3109/14397595.2013.854048. [DOI] [PubMed] [Google Scholar]
- 52.Okuda Y. AA amyloidosis - benefits and prospects of IL-6 inhibitors. Mod Rheumatol. 2019;29(2):268–274. doi: 10.1080/14397595.2018.1515145. [DOI] [PubMed] [Google Scholar]
- 53.Serelis J., Christaki S., Skopouli F.N. Remission of nephrotic syndrome due to AA-amyloidosis, complicating familiar Mediterranean fever, with tocilizumab. Clin Exp Rheumatol. 2015;33(6 suppl 94):S169. [PubMed] [Google Scholar]
- 54.Ugurlu S., Hacioglu A., Adibnia Y., Hamuryudan V., Ozdogan H. Tocilizumab in the treatment of twelve cases with aa amyloidosis secondary to familial Mediterranean fever. Orphanet J Rare Dis. 2017;12(1):105. doi: 10.1186/s13023-017-0642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De La Torre M., Arboleya L., Pozo S., Pinto J., Velasco J. Rapid and sustained response to tocilizumab, anti-interleukin-6 receptor antibody, in a patient with nephrotic syndrome secondary to systemic juvenile idiopathic arthritis-related amyloidosis. NDT Plus. 2011;4(3):178–180. doi: 10.1093/ndtplus/sfr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hočevar A., Lestan B., Šemrl S.S., et al. AA amyloidosis in a polyarteritis nodosa patient treated with tocilizumab. Amyloid. 2013;20(4):275–276. doi: 10.3109/13506129.2013.838947. [DOI] [PubMed] [Google Scholar]
- 57.Redondo-Pachón M.D., Enríquez R., Sirvent A.E., et al. Tocilizumab treatment for nephrotic syndrome due to amyloidosis in Behcet’s disease. Ren Fail. 2013;35(4):547–550. doi: 10.3109/0886022X.2013.773913. [DOI] [PubMed] [Google Scholar]
- 58.Cabello Pelegrin S., Uriol Rivera M.G., Fernandez Melón J., Rascón Risco J. MP172 Tocilizumab treatment for nephrotic syndrome due to secondary amyloidosis in rheumatic and inflammatory diseases. Nephrol Dial Transplant. 2016;31(suppl 1):i398. doi: 10.1093/ndt/gfw185.63. [DOI] [Google Scholar]
- 59.Eriksson P., Mölne J., Wirestam L., Sjöwall C. Successful treatment of AA amyloidosis in ankylosing spondylitis using tocilizumab: report of two cases and review of the literature. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.661101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giannese D., Ferro F., Moriconi D., et al. Use of tocilizumab in amyloid a nephropathy associated with Sweet syndrome: a case report and literature review. CEN Case Rep. 2021;10(1):23–29. doi: 10.1007/s13730-020-00507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dember L.M., Hawkins P.N., Hazenberg B.P., et al. Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med. 2007;356(23):2349–2360. doi: 10.1056/NEJMoa065644. [DOI] [PubMed] [Google Scholar]
- 62.Rumjon A., Coats T., Javaid M.M. Review of eprodisate for the treatment of renal disease in AA amyloidosis. Int J Nephrol Renovasc Dis. 2012;5:37–43. doi: 10.2147/IJNRD.S19165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Efficacy and safety study of KIACTA in preventing renal function decline in AA amyloidosis ClinicalTrials.gov identifier: NCT01215747. https://clinicaltrials.gov/ct2/show/NCT01215747 Updated March 10, 2016.
- 64.Study of the safety and efficacy of NC-503 in secondary (AA) amyloidosis ClinicalTrials.gov identifier: NCT00035334. https://www.clinicaltrials.gov/ct2/show/NCT00035334
- 65.Wall J.S., Kennel S.J., Richey T., et al. Generation and characterization of anti-AA amyloid-specific monoclonal antibodies. Front Immunol. 2011;2:32. doi: 10.3389/fimmu.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranganath S., Bhandari A., Avitahl-Curtis N., et al. Discovery and characterization of a potent interleukin-6 binding peptide with neutralizing activity in vivo. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0141330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schonhoft J.D., Monteiro C., Plate L., et al. Peptide probes detect misfolded transthyretin oligomers in plasma of hereditary amyloidosis patients. Sci Transl Med. 2017;9(407) doi: 10.1126/scitranslmed.aam7621. [DOI] [PMC free article] [PubMed] [Google Scholar]