Abstract
Purpose
Craniopharyngiomas are nonmalignant sellar and parasellar tumors exhibiting a bimodal age distribution. While the outcomes following treatment in patients with childhood-onset craniopharyngiomas are well characterized, similar information in adult-onset craniopharyngiomas is limited. We aimed to describe the long-term outcomes (weight and metabolic parameters, mortality) in patients with adult-onset craniopharyngioma following treatment.
Methods
Patients with adult-onset craniopharyngioma with initial treatment (1993–2017) and >6 months of follow-up at our institution were retrospectively identified. Body mass index (BMI) categories included obese (BMI ≥ 30 kg/m2), overweight (BMI 25–29.9 kg/m2), and normal weight (BMI < 25 kg/m2).
Results
For the 91 patients with adult-onset craniopharyngioma (44% women, mean diagnosis age 48.2 ± 18 years) over a mean follow-up of 100.3 ± 69.5 months, weight at last follow-up was significantly higher than before surgery (mean difference 9.5 ± 14.8 kg, P < 0.001) with a higher percentage increase in weight seen in those with lower preoperative BMI (normal weight (20.7 ± 18%) vs. overweight (13.3 ± 18.0%) vs. obese (6.4 ± 15%), P = 0.012). At last follow-up, the prevalence of obesity (62 vs. 40.5%, P = 0.0042) and impaired glucose metabolism (17.4% vs. 34%, P = 0.017) increased significantly. All-cause mortality was 12%, with the average age of death 71.9 ± 19.7 years (average U.S. life expectancy 77.7 years, CDC 2020).
Conclusion
Patients with adult-onset craniopharyngioma following treatment may experience weight gain, increased prevalence of obesity, impaired glucose metabolism, and early mortality. Lower preoperative BMI is associated with a greater percentage increase in postoperative weight.
Keywords: Weight gain, Obesity, Body mass index, Metabolic comorbidities, Hypothalamic injury
Introduction
Craniopharyngiomas are rare epithelial tumors of the sellar, para- and suprasellar region, arising from the embryonic remnants of Rathke’s pouch. Though benign by histopathology, the clinical course of these tumors has the potential to be devastating due to their proximity to critical intracranial structures (the pituitary gland, hypothalamus, third ventricle, intracranial nerves, and blood vessels). Furthermore, treatment including surgery, radiation, and, more recently, targeted medical therapy, can be associated with complications [1, 2]. Consequently, both craniopharyngioma and its treatment can contribute to significant morbidity, and poor quality of life [3–8].
Craniopharyngiomas can present at any age but usually exhibit a bimodal age distribution, the first peak occurring in childhood between 4–15 years and the second peak during adulthood between 40–79 years [9–11]. Several cohorts assessing long-term outcomes of childhood-onset craniopharyngioma (COC) [12–17] or mixed cohorts of adult-onset craniopharyngioma (AOC) and COC [18–22] have been reported. In patients with COC, postoperative weight gain and the development of metabolic syndrome thought to be primarily related to hypothalamic damage has been described [14–17, 23]. Furthermore, metabolic syndrome a significant risk factor for cardiovascular disease and diabetes, has been reported in up to 80% of patients with COP [20]. Although the 5- and 10-year survival rate of patients with craniopharyngioma is high (85–95%), the aforementioned complications are essential to recognize and treat as premature mortality is seen in patients with craniopharyngioma in whom the most common cause of death is cardiovascular in nature [19, 21, 22, 24].
On the other hand, only a few studies with small sample sizes and variable follow-up durations have explicitly described the postoperative weight changes in patients with AOC [25–28]. In addition, reports on predictive factors of these are limited and somewhat conflicting [25–27, 29]. Literature on metabolic comorbidities in patients with AOC is even further limited with most of the studies representing mixed patient cohorts of COC and AOC [5, 20, 30]. To the best of our knowledge, only one study to date has compared the prevalence of metabolic comorbidities (hypertension, type 2 diabetes mellitus, hyperlipidemia, cardiovascular disease, obstructive sleep apnea) before and after surgery over a follow-up period of 2-years in patients with AOC and found no significant increase [25].
To help address the limitations of currently available information, we conducted a single-center retrospective study on patients with AOC. Our objectives were to (i) describe changes in weight and body mass index (BMI) following initial neurosurgical treatment; (ii) identify factors associated weight gain of ≥5% and postoperative obesity; (iii) describe the prevalence of long-term metabolic outcomes [hypertension, dysglycemia, dyslipidemia, non-alcoholic fatty liver disease (NAFLD)]; and (iv) to estimate all-cause mortality in a large cohort of patients with AOC over a longer follow-up duration.
Methods
Patient cohort
Mayo Clinic Institutional Review Board approved the study protocol. All adult patients (≥18 years old) diagnosed with a craniopharyngioma between 1993–2017 who received their initial neurosurgical treatment at Mayo Clinic, Rochester MN were identified from the institutional database. The initial search resulted in 204 patients. Of these, 113 patients did not meet the inclusion criteria, as 17 had another histopathological diagnosis, 16 patients had COC, 54 patients with AOC had initial surgery at another facility, 6 patients presented with recurrent disease, and lastly, 20 patients had less than six months of follow-up. The final cohort included 91 patients with AOC with initial neurosurgical treatment and >6 months of postoperative follow-up at our institute.
Data collection
Medical records of final cohort were retrospectively reviewed for demographic information and clinical presentation at baseline as well as anthropometric measurements (weight, height, BMI), pituitary hormone evaluation, and metabolic parameters (hypertension, impaired glucose metabolism, dyslipidemia, NAFLD) at baseline and last follow-up. Impaired glucose metabolism was defined as diabetes mellitus, prediabetes, impaired fasting glucose, or impaired glucose tolerance. Diagnosis of hypertension was based on medical treatment with 1 or more antihypertensive medications. Statin therapy was used as a surrogate for dyslipidemia as a lipid panel was missing in a significant proportion of patients. Diagnosis of NAFLD was based on radiographic evidence on abdominal ultrasound (US), computed tomography (CT), or magnetic resonance imaging (MRI). Secondary adrenal insufficiency (AI) was defined by a low morning serum cortisol with a concurrently low or inappropriately normal corticotropin and need for glucocorticoid replacement. Secondary hypothyroidism was defined by low free thyroxine levels with low or inappropriately normal thyroid-stimulating hormone and need for thyroid hormone replacement. Hyperprolactinemia was defined by serum prolactin levels above the upper limit of reference range. Growth hormone (GH) stimulation test was not performed in most; therefore, GH deficiency was defined by a low insulin- like growth factor (IGF)-1 for age and gender normative data. Patients were considered to have persistent diabetes insipidus (DI) if they had polyuria and polydipsia at presentation or post-operatively in setting of hypernatremia and/or hyperosmolality of serum with hypotonic urine and were prescribed desmopressin at last follow-up. Based on BMI, patients were categorized as obese (BMI ≥ 30 Kg/m2), overweight (BMI 25–29.9 Kg/m2), and normal weight (BMI < 25 Kg/m2).
Information was collected on initial and subsequent treatments (surgery and radiation therapy), and the development of tumor recurrence. To define the extent of resection (complete versus partial), the operative and radiological reports within six months of surgery were reviewed. Tumor size was obtained from preoperative radiology reports (MRI or CT). Preoperative and postoperative MRI at 3–6 months were assessed by J.J.V.G. for hypothalamic involvement or damage using the grading system described by de Vile et al. [16]—Grade 0 (no discernible damage to the hypothalamus), Grade 1 (abnormality of the floor of the third ventricle and/or a breach in the tuber cinereuum), Grade 2 (floor of the third ventricle completely deficient or extensively breached by the residual tumor). Data on the histological subtype (adamantinomatous or papillary) was obtained from pathology reports, and if not specified, the pathology slides were reviewed by a senior neuropathologist (C.G.) for subtyping.
Statistical Analysis
Categorical variables are described as numbers and percentages, and continuous variables as mean and standard deviation. Comparison of proportions was assessed using the using the Chi-squared test or Fischer’s exact test when the expected values were small. Comparison of continuous variable was performed using the student’s T-test. To assess difference in means between baseline to last follow-up a paired T-test was performed. All analyses were performed using JMP Pro 16® (JMP, SAS Institute Inc, Cary, NC) statistical package. Differences with a level P < 0.05 were considered to be statistically significant.
Results
Baseline patient characteristics
For the 91 patients assessed, the mean age at diagnosis was 48.2 ± 18 years and included 40 (44%) females. The average duration of symptoms prior to diagnosis was 19.2 ± 31.2 months. On presentation, 12/76 patients tested (16%) had secondary AI, 18/79 (23%) had secondary hypothyroidism, 31/66 (47%) had secondary hypogonadism, 33/78 (42%) had hyperprolactinemia, and 15/52 (29%) were GH deficient. Of 67 patients who had preoperative assessment of at least 3 or more pituitary hormone axes, 9 (13%) had ≥3 deficiencies. At presentation, the mean weight (n = 90) and BMI (n = 89) were 87.5 ± 22.6 Kg and 29.4 ± 5.8 Kg/m2, respectively. Based on BMI, 36 (41%) patients were obese, 35 (39%) were overweight, and 18 (20%) were in the normal weight category. Hypertension was present in 25 (28%) patients, glycemic disturbances in 16 (17%), and 27 (30%) patients were on statin therapy (Table 1).
Table 1.
Characteristics at baseline and follow-up
| Adult (n = 91) | Male (n = 51) | Female (n = 40) | P-value | |
|---|---|---|---|---|
| Mean age at diagnosis (SD), years | 48.2 ± 18.0 | 48.8 ± 17.3 | 47.4 ± 19.1 | 0.71 |
| Race, N (%) | ||||
| White | 78 (86) | 46 (90) | 32 (80) | |
| Asian | 6 (7) | 2 (4) | 4 (10) | |
| American Indian, Alaska native | 2 (2) | 0 (0) | 2 (5) | |
| Hawaiian/ Pacific Islander | 1 (1) | 1 (2) | 0 (0) | |
| Not reported | 4 (4) | 2 (4) | 2 (5) | |
| Symptoms at presentation, N (%) | ||||
| Headache | 51 (56) | 24 (47) | 27 (68) | 0.06 |
| Visual abnormality | 67 (74) | 39 (75) | 29(73) | 0.83 |
| Altered Mental Status | 9 (10) | 7 (13) | 2 (5) | 0.16 |
| Polyuria/polydipsia | 13 (14) | 6 (12) | 7 (18) | 0.56 |
| Mean duration of symptoms prior to presentation (SD), months | 19.2 ± 31.2 | 1.8 ± 0.4 | 1.5 ± 0.4 | 0.57 |
| Mean duration of follow-up after initial surgery (SD), months | 100.3 ± 69.5 | 98.1 ± 70.6 | 103.2 ± 68.9 | 0.7 |
| Tumour characteristics | ||||
| Mean tumor size (SD), cm | 2.6 ± 1.0 | 2.8 ± 1.1 | 2.4 ± 0.9 | 0.09 |
| Histological subtype, N (%) | ||||
| Adamantinomatous | 74 (81) | 41 (80) | 33 (83) | 0.54 |
| Papillary | 13 (14) | 9 (18) | 4 (10) | |
| Unknown | 4 (4) | 1 (2) | 3 (7) | |
| Surgical procedure performed, N (%) | ||||
| Gross total resection | 44 (49) | 24 (48) | 20 (50) | 0.6^ |
| Partial resection | 45 (50) | 26 (52) | 19 (48) | |
| P32 injection | 1(1) | 0 | 1 (2) | |
| Use of radiotherapy, N (%) | 43 (47) | 25 (49) | 18 (45) | 0.7 |
| Grade of hypothalamic injury pre-operative, N (%) | ||||
| 0 | 27 (30) | 12 (24) | 15 (38) | |
| 1 | 16 (17) | 9 (17) | 7 (17) | 0.5 |
| 2 | 29 (32) | 18 (35) | 11 (27) | |
| NA | 19 (21) | 12 (24) | 7 (18) | |
| Grade of hypothalamic injury post-operative, N (%) | ||||
| 0 | 44 (48) | 21 (41) | 23 (57) | |
| 1 | 22 (24) | 14 (27) | 8 (20) | |
| 2 | 21 (23) | 15 (29) | 6 (15) | 0.15^ |
| NA | 4 (4) | 1 (2) | 3 (8) | |
| Tumor recurred, N (%) | 24 (26) | 16 (30) | 8 (20) | 0.22 |
| Hormonal evaluation | ||||
| Anterior Pituitary Hormone at diagnosis, N (%) | ||||
| ACTH deficiency | 12/76 (16) | 8/42 (19) | 4/34 (12) | |
| TSH deficiency | 18/79 (23) | 11/45 (24) | 7/34 (21) | |
| Gonadotropin deficiency | 31/66 (47) | 20/42 (48) | 11/24 (46) | |
| Hyperprolactinemia | 33/78 (42) | 13/43 (30) | 20/35 (57) | |
| GH deficiency | 15/52 (29) | 10/29 (35) | 5/23 (22) | |
| ≥3 Anterior Pituitary Deficiencies | 9/67 (13) | 7/40 (18) | 2/27 (7) | |
| Anterior Pituitary Hormone at last follow-up, N (%) | ||||
| ACTH deficiency | 68/90 (76) | 37/50 (74) | 31/40 (78) | |
| TSH deficiency | 72/90 (80) | 40/50 (80) | 32/40 (80) | |
| Gonadotropin deficiency | 65/78 (83) | 40/49 (82) | 25/29 (86) | |
| Hyperprolactinemia | 30/61 (49) | 13/33 (39) | 17/28 (61) | |
| GH deficiency | 25/42 (60) | 10/19 (53) | 15/23 (65) | |
| ≥3 Anterior Pituitary Deficiencies | 58/80 (73) | 34/48 (71) | 24/32 (75) | |
| Diabetes insipidus at last follow-up, N (%), n = 91 | 57/91 (63) | 30 (60) | 26 (65) | 0.7 |
| Anthropometric measurements | ||||
| Mean height at presentation (SD), cm (n = 90) | 172.1 ± 10.9 | 178.7 ± 7.1 | 163.8 ± 9.0 | <0.001 |
| Mean weight at presentation (SD), kg (n = 90) | 87.5 ± 22.6 | 97.2 ± 21.7 | 75.5 ± 17.6 | <0.001 |
| Mean BMI at presentation (SD), kg/m2 (n = 89) | 29.4 ± 5.8 | 30.5 ± 6 | 28.0 ± 5.3 | 0.03 |
| BMI category at presentation, N (%) (n = 89) | ||||
| Obese | 36 (41) | 23 (46) | 13 (32) | 0.2 |
| Overweight | 35 (39) | 19 (38) | 16 (40) | |
| Normal weight | 18 (20) | 7 (14) | 11 (28) | |
| Mean weight at last follow-up (SD), kg (n = 87) | 96.9 ± 24.6 | 105.6 ± 26.1 | 86.0 ± 18.2 | <0.001 |
| Mean BMI at last follow-up (SD), kg/m2 (n = 86) | 32.7 ± 6.5 | 33.1 ± 7.3 | 32.2 ± 5.5 | 0.5 |
| BMI category at last follow-up, N (%) (n = 86) | ||||
| Obese | 54 (63) | 28 (59) | 26 (66) | 0.4 |
| Overweight | 21 (24) | 14 (30) | 7 (18) | |
| Normal weight | 11 (13) | 5 (11) | 6 (15) | |
| Mean difference in weight at baseline and last follow-up (SD), kg (n = 86) | 9.5 ± 14.8# | 8.4 ± 17.4 | 10.3 ± 10.9 | 0.64 |
| Mean difference in BMI at baseline and last follow-up (SD), kg/m2, (n = 85) | 3.4 ± 4.8# | 2.6 ± 5.3 | 4.2 ± 0.6 | 0.11 |
| ≥5% weight gain from baseline, N (%) (n = 86) | 55 (64) | 29 | 26 | 0.67 |
| ≥5% weight gain from baseline based on preop BMI category, N (%), (n = 85) | ||||
| Obese | 20/35 (57) | 14 (50) | 6 (23) | 0.1 |
| Overweight | 21/32 (66) | 19 (32) | 12 (46) | |
| Normal weight | 13/18 (72) | 5 (18) | 8 (31) | |
| Mean percent weight gain (%) based on preop BMI category (SD), (n = 85)* | ||||
| Obese | 6.4 ± 15 | 7.4 ± 16.7 | 4.8 ± 10.3 | 0.18 |
| Overweight | 13.3 ± 18 | 10.5 ± 21.3 | 16.4 ± 11.6 | |
| Normal weight | 20.7 ± 18 | 12.9 ± 10.3 | 25.6 ± 19.2 | |
| Metabolic parameters | ||||
| At diagnosis, N (%) | ||||
| Hypertension | 25 (28) | 17 (35) | 8 (20) | 0.16 |
| Glycemic disturbance | 16 (17) | 10 (20) | 6 (15) | 0.8 |
| Dyslipidemia | 27 (30) | 15 (29) | 12 (30) | 1 |
| At last follow-up, N (%) | ||||
| Hypertension | 36/91 (40) | 23 (46) | 13 (32) | 0.3 |
| Glycemic disturbance | 31/91 (34) | 19 (937) | 12 (30) | 0.5 |
| Dyslipidemia | 39/91 (43) | 22 (43) | 17 (43) | 1 |
| NAFLD | 11/21 (52) | x | x | |
| Mortality | ||||
| Deaths during follow-up, N (%)## | 11 (12) | 3 (8) | 8 (16) | 0.2 |
| Average age of death (SD), years## | 71.9 ± 19.7 | 82 ± 3.8 | 68 ± 22.1 | |
ACTH adrenocorticotrophic hormone, BMI body mass index, GH growth hormone, TSH thyroid-stimulating hormone, NAFLD nonalcoholic fatty liver disease, SD standard deviation
#Difference statistically significantly at follow-up than prior to surgery, P = < 0.001
*Lower preoperative BMI had significantly greater percentage increase in weight, P = 0.012
##Numbers are too small to rely on the P-value
^Fischer’s exact test
Tumor characteristics
The mean maximum tumor diameter was 2.6 ± 1.0 cm. The histological subtype was adamantinomatous in 74 (81%) patients and papillary in 13 (14%). The subtype could not be identified in 3 patients due to severe necrosis or xanthogranulomatous reaction and in 1 patient treated with a P-32 injection. The proportion of patients with grade of pre- and postoperative hypothalamic injury are presented in (Table 1). Initial procedure entailed gross total resection in 44 (49%) patients, partial resection in 45 (50%), and P32 injection in 1 (1%). Tumor recurrence occurred in 45 (50%) patients. Radiotherapy was administered to 43 (47%) patients either as adjuvant treatment following surgery or primary treatment for recurrent disease (Table 1).
The baseline patient and tumor-related characteristics did not differ between males and females, except for mean height (178.7 ± 7.1 vs. 163.8 ± 9.0 cm, P < 0.001) and weight (97.2 ± 21.7 vs. 75.5 ± 17.6 kg, P < 0.001) (Table 1).
Follow-up
During a mean follow-up of 100.3 ± 69.5 months, 68/90 (76%) patients tested had secondary AI, 72/90 (80%) had secondary hypothyroidism, 65/78 (83%) had secondary hypogonadism, 30/61 (49%) hyperprolactinemia, and 25/42 (60%) had GH deficiency. Overall, 58 (72%) of 81 patients tested had ≥ 3 anterior pituitary hormone deficiencies, and 57 (63%) of 91 patients had DI (Table 1).
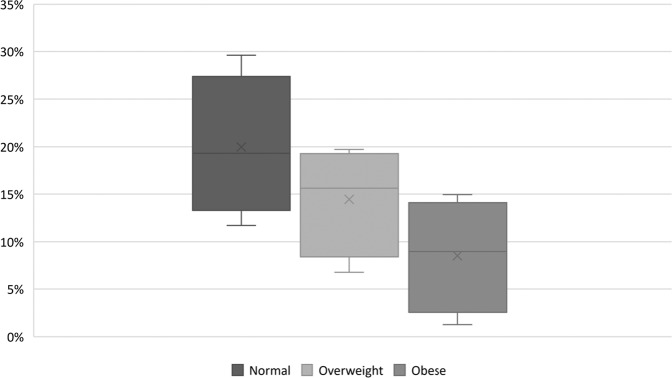
Among the 85 patients with follow-up weight available, weight was significantly higher at the last follow-up than weight at presentation (96.9 ± 24.6 vs. 87.5 ± 22.6 kg, mean difference 9.4 ± 14.8 kg, P < 0.001). In our study, the mean total weight gain was not associated with preoperative BMI category (P = 0.3) or gender (P = 0.69). However, the mean percent weight gain was higher in those with lower preoperative BMI [normal weight (20.7 ± 18%) vs. overweight (13.3 ± 18.0 %) vs. obese (6.4 ± 15%), P = 0.012] (Table 1, Fig. 1). Weight gain of ≥5% by the last follow-up occurred in 55 (65%) patients with a mean increase of 17.6 ± 10.6 kg. Weight gain of ≥5% was not associated with gender, preoperative BMI category, tumor size, histological subtype, gross total resection, postoperative grade of hypothalamic injury, radiotherapy use, and type or number of pituitary hormone deficiencies at last follow-up (Table 2, Fig. 1).
Fig. 1.
Box-whisker plot showing postoperative percentage increase in weight based on preoperative body-mass index. X represents mean value. Horizontal line represents median value. Categories based on body mass index (BMI): obese (BMI ≥ 30 kg/m2), overweight (BMI 25–29.9 kg/m2), and normal weight (BMI < 25 kg/m2). Mean percentage increase in weight based on preoperative BMI: normal weight (20.7 ± 18%) vs. overweight (13.3 ± 18.0 %) vs. obese (6.4 ± 15%), P = 0.012
Table 2.
Subgroup analysis based on postoperative weight gain of <5% vs. ≥5%
| Weight gain < 5% (n = 31) | Weight gain ≥ 5% (n = 55) | P -value | |
|---|---|---|---|
| BMI category at presentation, N (%) n = 85 | |||
| Obese | 15 (48) | 20 (37) | 0.5 |
| Overweight | 11 (34) | 21 (39) | |
| Normal weight | 5 (16) | 13 (24) | |
| Mean tumor size (SD), cm n = 82 | 2.4 ± 0.2 (n = 30) | 2.8 ± 0.1 (n = 52) | 0.5 |
| Histological subtype, N (%) n = 83 | |||
| Adamantinomatous | 24 (83) | 47 (87) | 0.6 |
| Papillary | 5 (17) | 7 (13) | |
| Gross total resection, N (%), (n = 31) | 17 (57) | 24 (43) | 0.6^ |
| Use of radiotherapy, N (%) n = 86 | 12 (28) | 31 (72) | 0.1 |
| Grade of hypothalamic injury postoperative, N (%) n = 82 | |||
| Grade 0 | 17 (59) | 25 (47) | 0.5 |
| Grade 1 | 5 (17) | 15 (28) | |
| Grade 2 | 7 (24) | 13 (25) | |
| Anterior Pituitary Hormone at last follow-up, N (%) | |||
| ACTH deficiency (n = 85) | 25 (81) | 40 (74) | 0.5 |
| TSH deficiency (n = 85) | 26 (84) | 43 (80) | 0.6 |
| Gonadotropin deficiency n = 74 | 22 (81) | 39 (83) | 0.9 |
| Hyperprolactinemia n = 59 | 11 (55) | 18 (46) | 0.5 |
| GH deficiency n = 41 | 11 (65) | 14 (58) | 0.7 |
| ≥3 Anterior Pituitary Deficiencies n = 77 | 22 (79) | 33 (67) | 0.3 |
| GH deficiency, not on replacement at last follow-up, N (%) n = 25 | 6 (55) | 5 (36) | 0.3 |
| Diabetes insipidus at last follow-up, N (%) n = 85 | 22 (71) | 32 (60) | 0.3 |
| Metabolic parameters at last follow-up, N (%) | |||
| Hypertension | 11 (35) | 22 (40) | 0.6 |
| Glycemic disturbance n = 86 | 11 (35) | 18 (33) | 0.8 |
| Dyslipidemia, n = 86 | 18 (58) | 19 (35) | 0 |
ACTH adrenocorticotrophic hormone, BMI body mass index, GH growth hormone, TSH thyroid-stimulating hormone, SD standard deviation
^Fischer’s exact test
BMI was significantly higher at the last follow-up than at presentation (32.7 ± 6.5 vs. 29.4 ± 5.8 kg/m2, mean difference of 3.3 ± 4.8 kg/m2, P < 0.001), resulting in a higher prevalence of obesity compared to prior to initial treatment (62% vs. 40.5%, P = 0.0042) (Table 1, Fig. 2). Patients with DI at last follow-up had a higher BMI compared to those without DI (33 ± 6.6 vs. 30 ± 6.2 kg/m2, P = 0.049), otherwise BMI at last follow-up did not differ between those with ≥3 anterior pituitary hormone deficiencies compared to those with <3 (32.8 ± 7.2 vs 32.1 ± 5.6 kg/m2, p = 0.7). Postoperative obesity (BMI ≥ 30 kg/m2 at last follow-up) was not associated with tumor size, histological subtype, gross total resection, postoperative grade of hypothalamic injury, radiotherapy use, and type or number of pituitary hormone deficiencies at last follow-up (Table 3).
Fig. 2.
Body-mass index distribution before surgery and at last follow-up. Categories based on body mass index (BMI): obese (BMI ≥ 30 kg/m2), overweight (BMI 25–29.9 kg/m2), and normal weight (BMI < 25 kg/m2)
Table 3.
Subgroup analysis based on BMI at last follow-up of <30 vs. ≥30 kg/m2
| BMI < 30 kg/m2 at last follow-up (n = 32) | BMI ≥ 30 kg/m2 at last follow-up (n = 54) | P-value | |
|---|---|---|---|
| BMI category at presentation, N (%) n = 85 | |||
| Obese | 5 (15) | 30 (57) | <0.001 |
| Overweight | 13 (41) | 19 (36) | |
| Normal weight | 14 (44) | 4 (7) | |
| Mean tumor size (SD), cm | 2.5 ± 0.98 | 2.7 ± 1 | 0.5 |
| Histological subtype, N (%), n = 83 | |||
| Adamantinomatous | 24 (83) | 47 (87) | 0.5 |
| Papillary | 5 (17) | 7 (13) | |
| Gross total resection, N (%) | 14 (45) | 28 (52) | 0.4^ |
| Use of radiotherapy, N (%) n = 86 | 16 (50) | 26 (48) | 0.86 |
| Grade of hypothalamic injury postoperative, N (%), n = 75 | |||
| Grade 0 | 12 (58) | 23 (45) | 0.5 |
| Grade 1 | 7 (23) | 12 (24) | |
| Grade 2 | 6 (19) | 15 (29) | |
| Anterior Pituitary Hormone at last follow-up, N (%) | |||
| ACTH deficiency (n = 86) | 26 (81) | 39 (72) | 0.4 |
| TSH deficiency (n = 86) | 27 (84) | 42 (78) | 0.5 |
| Gonadotropin deficiency (n = 75) | 25 (83) | 37 (82) | 1 |
| Hyperprolactinemia (n = 60) | 9 (42) | 20 (51) | 0.6 |
| GH deficiency n = 42 | 11 (58) | 14 (61) | 1 |
| ≥3 Anterior Pituitary Deficiencies (n = 78) | 23 (77) | 32 (67) | 0.4 |
| GH deficiency, not on replacement at last follow-up, N (%) n = 27 | 6 (46) | 5 (36) | 0.7 |
| Diabetes insipidus at last follow-up, N (%) (n = 85) | 20 (63) | 34 (64) | 1 |
| Metabolic parameters last follow-up, N (%) | |||
| Hypertension | 8 (26) | 26 (48) | 0.43 |
| Glycemic disturbance | 5 (15) | 24 (44) | 0.0063 |
| Dyslipidemia | 12 (38) | 26 (48) | 0.33 |
ACTH adrenocorticotrophic hormone BMI body mass index, GH growth hormone, TSH thyroid-stimulating hormone, SD standard deviation
^Fischer’s exact test
On follow-up, the proportion of patients who developed hypertension (28 vs. 40% P = 0.09) or required statin therapy (30 vs. 43%, P = 0.06) increased; however, this difference did not reach statistical significance. Whereas the proportion of patients with glycemic disturbances increased significantly by the last follow-up (17.4% vs. 34%, P = 0.017), (Table 1). The prevalence of hypertension, glycemic disturbances, and dyslipidemia at the last follow-up did not differ between subgroups based on postoperative BMI ≥ 30 kg/m2 or >5% weight gain. Of the 21 patients who had abdominal imaging, 11 had radiographic evidence of fatty liver disease (Table 1).
At last follow-up, all-cause mortality was 12%, with an average age of death of 71.9 ± 19.7 years. There were no differences in survival based on BMI ≥ 30 (P = 0.83), ≥3 anterior pituitary hormone deficiencies (P = 0.77), and desmopressin use (P = 0.37). For the 12% with mortality (n = 11), the cause of death was not available in 5 patients, 2 patients died from cardiovascular causes, 2 due to sepsis, 1 due to COVID-19 infection, and 1 due to failure to thrive. Importantly as all patients with less than 6 months of follow-up were excluded, there were no documented deaths secondary to tumor progression.
Discussion
The current study illustrates the weight changes, metabolic comorbidities, and all-cause mortality in a large cohort of patients with AOC over a mean duration of 8.3 years following initial treatment. Patients with AOC experienced a significant increase in weight (9.5 ± 14.8 kg) and BMI (3.4 ± 4.8 kg/m2) on follow-up with almost two-thirds gaining ≥5% of their baseline weight with a mean increase of 17.6 kg. By the last follow-up, the prevalence of obesity increased from 40.5% to 62%, and glycemic disturbances increased from 17% to 34%. Furthermore, 73% had ≥3 anterior pituitary deficits and 63% required long-term desmopressin on follow-up. The overall survival at 8.3 years was 88%; however, the average age of death for the 12% with mortality was 71.9 years, lower than the average life expectancy in the US (77.0 years, CDC 2020) [31].
Hypothalamic obesity (HO) is a recognized complication of craniopharyngioma and has been extensively reported in patients with COC [14–17, 32, 33]. The hypothalamus, through its complex neurohormonal signaling, coordinates the regulation of energy intake and expenditure, thus playing a vital role in the body’s energy homeostasis. Injury to the hypothalamus (especially the ventromedial and arcuate nuclei) alters this homeostasis, causing impaired regulation of hunger, satiety, and energy expenditure, eventually resulting in substantial weight gain [14, 32, 34]. Although HO is a well-defined phenomenon in patients with COC [14–17, 32, 33], limited studies exist specifically assessing the prevalence and risk factors for development of HO among patients with AOC.
The baseline prevalence of obesity in our cohort matched that of the general population of the U.S. (42.4%, CDC 2020) [35] but increased significantly by the last follow-up (62%). A similar baseline prevalence of obesity (37.8%) among a smaller group of patients with AOC was observed in a recent U.S. study by Duan et al. who also noted a significant increase on follow up, but at a slightly lower prevalence (55.6%) over a shorter follow up period of 2.2 years [25]. A large study from China which included 120 patients with AOC, noted a significant increase in HO (≥28 kg/m2) after a median follow-up of 12 months, however the reported prevalence of obesity was much lower at baseline (19.2%) and at follow-up (29.2%) [26] perhaps reflecting differences in geography, ethnicity, and dietary habits. Yet, despite these differences, the development of obesity in patients with AOC is a real concern.
Gradual weight gain over time in the general population has been observed in multiple epidemiological studies [36–38], however, weight gain in patients with AOC is accelerated. In the epidemiological data provided by Chen et al. on 33,890 U.S. participants (mean age 47 years, women 52.9%), the mean weight gain over a 10-year duration of 4.4 kg [37] is significantly lower than the weight gain of 9.5 kg over an 8-year period in our cohort with AOC (mean age 48.2 years, women 43.9%).
Previous studies with a follow-up duration of 3 months to 2 years have reported weight gain of 3.0 to 3.9 kg [25, 26, 39] among patients with AOC, which is lower than our cohort who had a weight gain of 9.5 kg over 8-years. It is possible that the difference in the total weight gain is related to the duration of follow-up. In the study by Karavitaki et al. of 121 patients with craniopharyngioma (COC and AOC), the cumulative probability of hyperphagia or excessive weight gain increased from 10% at baseline to 20% at 5 years, 39% at 10 years and 67% at 20 years [18]. However, studies assessing weight gain in COC have reported that the greatest change in weight occurs within the 1st year following surgery suggesting hypothalamic damage as a cause for such accelerated weight gain [32, 34, 40]. We were unable to assess the pattern of weight gain as follow-up intervals were not consistent in our retrospective cohort.
There is growing albeit conflicting literature on risk factors of HO in patients with AOC. In a 2010 study from our institute, we reported hypothalamic involvement on preoperative MRI was predictive of postoperative weight gain but found no association with preoperative BMI in a small subset [27]. More recently, Duan et al. found lower preoperative BMI and ≥3 anterior pituitary hormonal deficiencies as risk factors for postoperative weight gain but reported no association with tumor subtype or hypothalamic injury [25]. Wu et al. reported that lower pre-operative BMI and adamantinomatous subtype were predictive of postoperative weight gain of ≥5% but found no association with ≥3 anterior pituitary hormonal deficiencies or hypothalamic injury [26]. Though Hong et al. noted greater postoperative gain and steroid use in patients with AOC than those with nonfunctioning pituitary adenoma, the weight gain in patients with AOC remained significantly higher despite adjusting for daily steroid use dose [39]. In our study, we found that patients with lower preoperative BMI had the greatest percentage increase in weight, and the presence of obesity at baseline was predictive of postoperative obesity at the last follow-up, implying that most patients with obesity at baseline continue to be obese following craniopharyngioma treatment. We also found that in patients requiring long-term postoperative desmopressin compared to those who did not, the mean BMI was higher, but no difference was seen in the prevalence of obesity (Table 4).
Table 4.
Comparison table of different studies on adult-onset craniopharyngioma and weight-related outcomes
| Author, year | Country of the study | Type of study | N | Follow-up duration | Postoperative weight change | Obesity prevalence, preoperative vs. last follow-up | Risk Factors |
|---|---|---|---|---|---|---|---|
| Van Gompel, 2010 | United States | Retrospective | 28 | – | +10.1% | – | Preoperative hypothalamic injury on MRI |
| Duan, 2021 | United States | Retrospective | 45 | Median 26 months (range 3–124) | +3.4 kg | 37.8% vs. 55.6% | Lower preoperative BMI; ≥3 pituitary hormonal deficiencies |
| Wu, 2021 | China | Retrospective | 120 | Median 12 months (range 4–14) | +3.9 kg | 19.2% vs. 29.2% | Lower preoperative BMI; adamantinomatous subtype |
| Hong, 2022 | South Korea | Retrospective | 48 | Mean 90.9 ± 16.6 days | +3.0 kg | 45.8% vs. 62.5% | Possibly hypothalamic injury and steroid use |
| Current study, 2022 | United States | Retrospective | 91 | Mean 100.3 ± 69.5 months | +9.5 kg | 40.5% vs. 62% | Lower preoperative BMI associated with higher percentage of weight gain |
Variable follow-up durations, populations with different ethnicities and dietary habits, and grading systems for assessing hypothalamic injury, including interobserver variability, potentially explain the disparate results in the published literature. Studies comparing weight gain in patients with craniopharyngioma, and nonfunctioning pituitary adenomas report greater postoperative weight gain in patients with craniopharyngioma, thus, making it conceivable that hypothalamic injury resulting in weight gain may not be completely evident in the current imaging studies [27, 39]. Furthermore, it is possible that rather than a single factor, it is a constellation of multiple factors including hormonal milieu predisposing patients with craniopharyngioma to postoperative weight gain.
At last follow-up, a strikingly higher number had pituitary hormonal deficiencies with ≥3 anterior pituitary hormonal deficiencies present in 73% of patients compared to only 13% at presentation, and desmopressin use in 63% while only 14% complained of polyuria and polydipsia at presentation. As discussed above, postoperative weight gain and obesity were not associated with the type or number of pituitary hormonal deficiencies in our study. However, similar to the published literature, we noted a low testing rate for GH deficiency and GH replacement [25, 26]. Adult-onset GH deficiency is associated with alteration in body composition, hyperlipidemia, and hyperinsulinemia, thereby predisposing to metabolic syndrome and increased risk for cardiovascular disease [41]. GH replacement positively influences body composition, lipid profile, and insulin sensitivity [42]; however, limited data suggest that patients with craniopharyngioma are less likely to experience these beneficial effects [43, 44]. Additionally, the role of oxytocin and thereof dysregulation is not possible to assess currently in routine clinical practice, but some data suggest that it might play an important role in appetite and weight regulation [45, 46].
There was a trend toward the increased prevalence of hypertension, glycemic disturbances, and dyslipidemia by the last follow-up; however, the difference was significant only for glycemic disturbances. Whether this increase in glycemic disturbances is related to the natural aging process or a consequence of excessive weight gain is unclear. Only in a small subset of patients we were able to evaluate for the presence of NAFLD, and in those tested, the prevalence was 52%. A growing body of literature suggests an association between NAFLD and growth hormone deficiency [47–50], with limited data reporting on the effects of GH replacement [51]. Our results, however, should be interpreted with caution as this prevalence is representative of a highly select group of patients. Additionally, NAFLD based on imaging (CT, MRI or US) may not be the optimal diagnostic modality.
Current literature on the mortality rate in patients with AOC is limited to a handful of studies reporting on mixed cohorts of AOC and COC[18, 52]. Karavitaki et al. reported no difference in the overall survival between patients with COC and AOC [18], while Lehrich et al., in their population-based study, observed a better survival in those with COC [52]. Our study’s 88% overall survival at 8-years of is similar to that reported by Karavitaki et al. (91% at 5-year; 90% at 10-year) [18] but much higher than Lehrich et al. (77.9% at 5-year; 64.4% at 10-year) [52].
The strengths of our study include a large cohort and a long duration of follow-up. Limitations include the retrospective design of the study and information bias. However, considering the rarity of craniopharyngiomas, a prospective study with an adequate sample size may not be practically feasible. As weight was not recorded at established time intervals, this consequently limited our ability to assess the trajectory of weight gain. Furthermore, we could not assess for changes in satiety, daily activity, or the degree of hyperphagia as these symptoms were not consistently recorded. Additionally, in patients with diabetes insipidus, significant changes in fluid status can occur which can impact weight and therefore BMI.
In conclusion, after treatment, patients with AOC experience significant weight gain and increased prevalence of obesity, predisposing them to increased risk of metabolic complications. Though weight gain occurs regardless of preoperative weight status, the percentage increase in weight is higher in those with lower preoperative BMI. From a practical perspective, we propose that all patients with AOC be seen by a nutritionist preoperatively, and regularly thereafter for counselling regarding dietary and lifestyle interventions given the propensity for continued weight gain. Future studies would be of interest to assess the role of appetite suppressants and weight loss medications during early postoperative periods. Additionally, increased awareness about growth hormone deficiency and replacement is needed. Physicians caring for patients with AOC should periodically and frequently test for metabolic abnormalities, including the presence of NAFLD.
Acknowledgments
Author contributions
D.E. and D.M.D. contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by P.D. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
J.J.V.G. is a named inventor for intellectual property licensed to Cadence Neuroscience Inc, which is co-owned by Mayo Clinic. J.J.V.G is an investigator for the Medtronic EPAS trial and Mayo Clinic Medtronic NIH Public Private Partnership (UH3-NS95495). J.J.V.G. has stock ownership and consults for Neuro-One Inc. None of these financial disclosures are relevant to this paper. D.E. reported consulting fees from Clarivate, not related to the current research topic. Other authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Consent to participate
Patient consent was waived due to the retrospective nature of the study.
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Mayo Clinic, Rochester, Minnesota, United States
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Diane M. Donegan, Dana Erickson
References
- 1.Muller HL. The diagnosis and treatment of craniopharyngioma. Neuroendocrinology. 2020;110(9–10):753–766. doi: 10.1159/000504512. [DOI] [PubMed] [Google Scholar]
- 2.Alexandraki KI, Kaltsas GA, Karavitaki N, Grossman AB. The medical therapy of craniopharyngiomas: the way ahead. J. Clin. Endocrinol. Metab. 2019;104(12):5751–5764. doi: 10.1210/jc.2019-01299. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M, Guger S, Hamilton J. Long term sequelae of pediatric craniopharyngioma - literature review and 20 years of experience. Front Endocrinol. 2011;2:81. doi: 10.3389/fendo.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dekkers OM, et al. Quality of life in treated adult craniopharyngioma patients. Eur. J. Endocrinol. 2006;154(3):483–489. doi: 10.1530/eje.1.02114. [DOI] [PubMed] [Google Scholar]
- 5.Wijnen M, et al. The metabolic syndrome and its components in 178 patients treated for craniopharyngioma after 16 years of follow-up. Eur. J. Endocrinol. 2018;178(1):11–22. doi: 10.1530/EJE-17-0387. [DOI] [PubMed] [Google Scholar]
- 6.Wijnen M, et al. Very long-term sequelae of craniopharyngioma. Eur. J. Endocrinol. 2017;176(6):755–767. doi: 10.1530/EJE-17-0044. [DOI] [PubMed] [Google Scholar]
- 7.Erfurth EM, Holmer H, Fjalldal SB. Mortality and morbidity in adult craniopharyngioma. Pituitary. 2013;16(1):46–55. doi: 10.1007/s11102-012-0428-2. [DOI] [PubMed] [Google Scholar]
- 8.K.C. Mende et al., Clinical situation, therapy, and follow-up of adult craniopharyngioma. J. Clin. Endocrinol. Metab. 105, 1, 2020. [DOI] [PubMed]
- 9.Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J. Neurosurg. 1998;89(4):547–551. doi: 10.3171/jns.1998.89.4.0547. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen EH, et al. “Incidence of craniopharyngioma in Denmark (n = 189) and estimated world incidence of craniopharyngioma in children and adults,”. J. Neurooncol. 2011;104(3):755–763. doi: 10.1007/s11060-011-0540-6. [DOI] [PubMed] [Google Scholar]
- 11.Zacharia BE, Bruce SS, Goldstein H, Malone HR, Neugut AI, Bruce JN. “Incidence, treatment and survival of patients with craniopharyngioma in the surveillance, epidemiology and end results program,”. Neuro Oncol. 2012;14(8):1070–1078. doi: 10.1093/neuonc/nos142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.V.M. Ravindra et al., Comparison of multimodal surgical and radiation treatment methods for pediatric craniopharyngioma: long-term analysis of progression-free survival and morbidity. J. Neurosurg. Pediatr. 28,1–8 (2021). [DOI] [PubMed]
- 13.Zucchini S, et al. Management of childhood-onset craniopharyngioma in Italy: a multicenter, 7-year follow-up study of 145 patients. J. Clin. Endocrinol. Metab. 2022;107(3):e1020–e1031. doi: 10.1210/clinem/dgab784. [DOI] [PubMed] [Google Scholar]
- 14.Sterkenburg AS, Hoffmann A, Gebhardt U, Warmuth-Metz M, Daubenbuchel AM, Muller HL. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro Oncol. 2015;17(7):1029–1038. doi: 10.1093/neuonc/nov044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller HL, et al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur. J. Endocrinol. 2011;165(1):17–24. doi: 10.1530/EJE-11-0158. [DOI] [PubMed] [Google Scholar]
- 16.de Vile CJ, Grant DB, Hayward RD, Kendall BE, Neville BG, Stanhope R. Obesity in childhood craniopharyngioma: relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J. Clin. Endocrinol. Metab. 1996;81(7):2734–2737. doi: 10.1210/jcem.81.7.8675604. [DOI] [PubMed] [Google Scholar]
- 17.Elowe-Gruau E, et al. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J. Clin. Endocrinol. Metab. 2013;98(6):2376–2382. doi: 10.1210/jc.2012-3928. [DOI] [PubMed] [Google Scholar]
- 18.Karavitaki N, et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. (Oxf.) 2005;62(4):397–409. doi: 10.1111/j.1365-2265.2005.02231.x. [DOI] [PubMed] [Google Scholar]
- 19.Pereira AM, et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin. Endocrinol. 2005;62(2):197–204. doi: 10.1111/j.1365-2265.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 20.Jazbinsek S, et al. Prevalence of endocrine and metabolic comorbidities in a national cohort of patients with craniopharyngioma. Horm. Res Paediatr. 2020;93(1):46–57. doi: 10.1159/000507702. [DOI] [PubMed] [Google Scholar]
- 21.Olsson DS, Andersson E, Bryngelsson IL, Nilsson AG, Johannsson G. Excess mortality and morbidity in patients with craniopharyngioma, especially in patients with childhood onset: a population-based study in Sweden. J. Clin. Endocrinol. Metab. 2015;100(2):467–474. doi: 10.1210/jc.2014-3525. [DOI] [PubMed] [Google Scholar]
- 22.Van Effenterre R, Boch AL. Craniopharyngioma in adults and children: a study of 122 surgical cases. J. Neurosurg. 2002;97(1):3–11. doi: 10.3171/jns.2002.97.1.0003. [DOI] [PubMed] [Google Scholar]
- 23.Muller HL, et al. Hypothalamic syndrome. Nat. Rev. Dis. Prim. 2022;8(1):24. doi: 10.1038/s41572-022-00351-z. [DOI] [PubMed] [Google Scholar]
- 24.Bulow B, Attewell R, Hagmar L, Malmstrom P, Nordstrom CH, Erfurth EM. Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J. Clin. Endocrinol. Metab. 1998;83(11):3897–3904. doi: 10.1210/jcem.83.11.5240. [DOI] [PubMed] [Google Scholar]
- 25.Duan D, et al. Preoperative BMI predicts postoperative weight gain in adult-onset craniopharyngioma. J. Clin. Endocrinol. Metab. 2021;106(4):e1603–e1617. doi: 10.1210/clinem/dgaa985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, et al. Risk factors for hypothalamic obesity in patients with adult-onset craniopharyngioma: a consecutive series of 120 cases. Front Endocrinol. 2021;12:694213. doi: 10.3389/fendo.2021.694213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Gompel JJ, Nippoldt TB, Higgins DM, Meyer FB. Magnetic resonance imaging-graded hypothalamic compression in surgically treated adult craniopharyngiomas determining postoperative obesity. Neurosurg. Focus. 2010;28(4):E3. doi: 10.3171/2010.1.FOCUS09303. [DOI] [PubMed] [Google Scholar]
- 28.Steele CA, et al. Hypothalamic obesity: prevalence, associations and longitudinal trends in weight in a specialist adult neuroendocrine clinic. Eur. J. Endocrinol. 2013;168(4):501–507. doi: 10.1530/EJE-12-0792. [DOI] [PubMed] [Google Scholar]
- 29.Andereggen L, et al. A ten-year follow-up study of treatment outcome of craniopharyngiomas. Swiss Med. Wkly. 2018;148:w14521. doi: 10.4414/smw.2018.14521. [DOI] [PubMed] [Google Scholar]
- 30.Wijnen M, et al. Excess morbidity and mortality in patients with craniopharyngioma: a hospital-based retrospective cohort study. Eur. J. Endocrinol. 2018;178(1):93–102. doi: 10.1530/EJE-17-0707. [DOI] [PubMed] [Google Scholar]
- 31.Murphy SL, Kochanek KD, Xu J, Arias E. Mortality in the United States, 2020”. NCHS Data Brief. 2021;427:1–8. [PubMed] [Google Scholar]
- 32.Roth CL, et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity. 2015;23(6):1226–1233. doi: 10.1002/oby.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmer H, et al. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J. Clin. Endocrinol. Metab. 2010;95(12):5395–5402. doi: 10.1210/jc.2010-0993. [DOI] [PubMed] [Google Scholar]
- 34.Muller HL, et al. Obesity after childhood craniopharyngioma–German multicenter study on pre-operative risk factors and quality of life. Klin. Padiatr. 2001;213(4):244–249. doi: 10.1055/s-2001-16855. [DOI] [PubMed] [Google Scholar]
- 35.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 36.Zheng Y, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318(3):255–269. doi: 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C, Ye Y, Zhang Y, Pan XF, Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ. 2019;367:l5584. doi: 10.1136/bmj.l5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nooyens AC, et al. Age, period and cohort effects on body weight and body mass index in adults: The Doetinchem Cohort Study. Public Health Nutr. 2009;12(6):862–870. doi: 10.1017/S1368980008003091. [DOI] [PubMed] [Google Scholar]
- 39.A.R. Hong et al., Determinants of short-term weight gain following surgical treatment for craniopharyngioma in adults. J. Korean Neurosurg. Soc. 65(3), 439–448 (2022). [DOI] [PMC free article] [PubMed]
- 40.Ahmet A, Blaser S, Stephens D, Guger S, Rutkas JT, Hamilton J. Weight gain in craniopharyngioma–a model for hypothalamic obesity. J. Pediatr. Endocrinol. Metab. 2006;19(2):121–127. doi: 10.1515/JPEM.2006.19.2.121. [DOI] [PubMed] [Google Scholar]
- 41.Melmed S. Pathogenesis and diagnosis of growth hormone deficiency in adults. N. Engl. J. Med. 2019;380(26):2551–2562. doi: 10.1056/NEJMra1817346. [DOI] [PubMed] [Google Scholar]
- 42.Hazem A, et al. Body composition and quality of life in adults treated with GH therapy: a systematic review and meta-analysis. Eur. J. Endocrinol. 2012;166(1):13–20. doi: 10.1530/EJE-11-0558. [DOI] [PubMed] [Google Scholar]
- 43.Profka E, et al. Analysis of short- and long-term metabolic effects of growth hormone replacement therapy in adult patients with craniopharyngioma and non-functioning pituitary adenoma. J. Endocrinol. Invest. 2015;38(4):413–420. doi: 10.1007/s40618-014-0196-0. [DOI] [PubMed] [Google Scholar]
- 44.Attanasio AF, et al. Prevalence of metabolic syndrome in adult hypopituitary growth hormone (GH)-deficient patients before and after GH replacement. J. Clin. Endocrinol. Metab. 2010;95(1):74–81. doi: 10.1210/jc.2009-1326. [DOI] [PubMed] [Google Scholar]
- 45.Daubenbuchel AM, et al. Oxytocin in survivors of childhood-onset craniopharyngioma. Endocrine. 2016;54(2):524–531. doi: 10.1007/s12020-016-1084-5. [DOI] [PubMed] [Google Scholar]
- 46.Hsu EA, Miller JL, Perez FA, Roth CL. Oxytocin and naltrexone successfully treat hypothalamic obesity in a boy post-craniopharyngioma resection”. J. Clin. Endocrinol. Metab. 2018;103(2):370–375. doi: 10.1210/jc.2017-02080. [DOI] [PubMed] [Google Scholar]
- 47.Nishizawa H, et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur. J. Endocrinol. 2012;167(1):67–74. doi: 10.1530/EJE-12-0252. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Qi ZR, Zhang TT, Kang YJ, Wang X. Rapidly progressive non-alcoholic fatty liver disease due to hypopituitarism. Rep. 5 cases,” Neuro Endocrinol. Lett. 2018;39(2):99–104. [PubMed] [Google Scholar]
- 49.Xu L, et al. Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS One. 2012;7(8):e44136. doi: 10.1371/journal.pone.0044136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffmann A, Bootsveld K, Gebhardt U, Daubenbuchel AM, Sterkenburg AS, Muller HL. Nonalcoholic fatty liver disease and fatigue in long-term survivors of childhood-onset craniopharyngioma. Eur. J. Endocrinol. 2015;173(3):389–397. doi: 10.1530/EJE-15-0422. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto R, et al. Long-term effects of growth hormone replacement therapy on liver function in adult patients with growth hormone deficiency. Growth Horm. IGF Res. 2014;24(5):174–179. doi: 10.1016/j.ghir.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Lehrich BM, Goshtasbi K, Hsu FPK, Kuan EC. Characteristics and overall survival in pediatric versus adult craniopharyngioma: a population-based study. Childs Nerv. Syst. 2021;37(5):1535–1545. doi: 10.1007/s00381-021-05094-y. [DOI] [PubMed] [Google Scholar]