Abstract
Background
Autologous platelet‐rich plasma (PRP) is a treatment that contains fibrin and high concentrations of growth factors with the potential to improve the healing of chronic wounds. This is the first update of a review first published in 2012.
Objectives
To determine whether autologous PRP promotes the healing of chronic wounds.
Search methods
In June 2015, for this first update, we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library): Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE; and EBSCO CINAHL. We also searched for ongoing and unpublished clinical trials in the WHO International Clinical Trials Registry Platform (ICTRP) (searched January 2015). We did not impose any restrictions with respect to language, date of publication, or study setting.
Selection criteria
We included randomised controlled trials (RCTs) that compared autologous PRP with placebo or alternative treatments for any type of chronic wound in adults. We did not apply any date or language restrictions.
Data collection and analysis
We used standard Cochrane methodology, including two reviewers independently selecting studies for inclusion, extracting data, and assessing risk of bias.
Main results
The search identified one new RCT, making a total of 10 included RCTs (442 participants, 42% women). The median number of participants per RCT was 29 (range 10 to 117). Four RCTs recruited people with a range of chronic wounds; three RCTs recruited people with venous leg ulcers, and three RCTs considered foot ulcers in people with diabetes. The median length of treatment was 12 weeks (range 8 to 40 weeks).
It is unclear whether autologous PRP improves the healing of chronic wounds generally compared with standard treatment (with or without placebo) (risk ratio (RR) 1.19, 95% confidence interval (CI) 0.95 to 1.50; I2 = 27%, low quality evidence, 8 RCTs, 391 participants). Autologous PRP may increase the healing of foot ulcers in people with diabetes compared with standard care (with or without placebo) (RR 1.22, 95% CI 1.01 to 1.49; I2 = 0%, low quality evidence, 2 RCTs, 189 participants). It is unclear if autologous PRP affects the healing of venous leg ulcers (RR 1.02, 95% CI 0.81 to 1.27; I2 = 0% ). It is unclear if there is a difference in the risk of adverse events in people treated with PRP or standard care (RR 1.05, 95% CI 0.29 to 3.88; I2 = 0%, low quality evidence from 3 trials, 102 participants).
Authors' conclusions
PRP may improve the healing of foot ulcers associated with diabetes, but this conclusion is based on low quality evidence from two small RCTs. It is unclear whether PRP influences the healing of other chronic wounds. The overall quality of evidence of autologous PRP for treating chronic wounds is low. There are very few RCTs evaluating PRP, they are underpowered to detect treatment effects, if they exist, and are generally at high or unclear risk of bias. Well designed and adequately powered clinical trials are needed.
Plain language summary
Autologous platelet‐rich plasma (PRP) for chronic wounds
Review question
What is autologous platelet‐rich plasma and is it useful for treating chronic wounds?
Background
Chronic wounds (or ulcers) are breaks in the skin that do not heal, or require a long time to heal, and frequently recur. Chronic wounds include pressure ulcers, venous leg ulcers, arterial ulcers, neurotrophic ulcers, and foot ulcers in people with diabetes. Autologous platelet‐rich plasma (PRP) is a potential wound healing treatment because it has components such as fibrin (a substance produced in the liver that makes the blood clot) and high concentrations of growth factors that are thought to help healing. We reviewed the evidence on the effect of autologous PRP on wound healing in people aged 18 years or older with chronic wounds from any cause (such as pressure ulcers, arterial ulcers, venous ulcers). We also included patients with wounds of mixed aetiology e.g. mixed arterial‐venous ulcers.
What we found
We included 10 randomised clinical trials, with a total of 442 participants (mean age 61 years and 42% women). Four included studies recruited people with a range of chronic wounds; three studies enrolled people with venous leg ulcers; and the other three studies included people with diabetes who had foot ulcers. The median length of treatment was 12 weeks. All but three trials reported the sources of funding. Four of the studies received financial support from companies manufacturing PRP devices.
The results were non‐conclusive as to whether autologous PRP improves the healing of chronic wounds generally compared with standard treatment. Autologous PRP may increase the healing of foot ulcers in people with diabetes compared with standard care, but it is unclear if autologous PRP has an effect on other types of chronic wound. Three studies reported wound complications such as infection or dermatitis, but results showed no difference in the risk of adverse events in people treated with PRP or standard care. These findings are based on low quality evidence due to the small number of studies and patients included, and their poor methodological quality.
This Plain Language Summary is up to date as of 16 June 2015.
Summary of findings
Summary of findings for the main comparison. Autologous platelet‐rich plasma compared with standard care with/without placebo for chronic wounds.
| Autologous platelet‐rich plasma compared with standard care with/without placebo for chronic wounds | |||||
|
Patient or population: adults with chronic wounds Settings: hospital Intervention: autologous platelet‐rich plasma Comparison: standard treatment with/without placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Standard care with/without placebo | Autologous platelet‐rich plasma | ||||
|
Chronic wounds (all) completely healed Follow‐up: 8‐36 weeks |
Medium risk population |
RR 1.19 (0.95 to 1.50) |
391 (8 studies) | ⊕⊕⊝⊝ low1 |
|
| 514 per 1000 | 611 per 1000 (488 to 771) | ||||
|
Chronic wounds (diabetic foot ulcers) completely healed Follow‐up: 12‐24 weeks |
Medium risk population |
RR 1.22 (1.01 to 1.49) |
189 (2 studies) | ⊕⊕⊝⊝ low2 |
|
| 544 per 1000 | 664 per 1000 (550 to 811) | ||||
|
Chronic wounds (venous leg ulcers) completely healed Follow‐up: 16‐36 weeks |
Medium risk population |
RR 1.02 (0.81 to 1.27) |
101 (2 studies) | ⊕⊕⊝⊝ low3,4 |
|
| 686 per 1000 | 700 per 1000 (556 to 872) | ||||
|
Adverse events Follow‐up: 8‐24 weeks |
Medium risk population |
RR 1.05 (0.29 to 3.88) |
102 (3 studies) | ⊕⊝⊝⊝ very low3,4,5 |
|
| 87 per 1000 | 91 per 1000 (25 to 337) | ||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded two levels due to limitations in the trial execution: three clinical trials presented incomplete outcome data, and one selective reporting. 2 Downgraded two levels due to limitations in the trial execution: one of the trials providing data for this outcome presented incomplete outcome data and selective reporting. 3 Downgraded one level due to limitations in the trial design: the randomisation was unclear. 4 Downgraded one level due to imprecision: the confidence interval was wide. 5 Downgraded one level due to limitations in the trial execution: two clinical trials presented incomplete outcome data, and one selective reporting.
Background
See Glossary of terms for additional explanation of terms (Appendix 1).
Description of the condition
Chronic wounds are breaks in the skin that do not heal, or require a long time to heal, and frequently recur. Chronic wounds include pressure ulcers, venous leg ulcers, arterial ulcers, neurotrophic ulcers, and foot ulcers in people with diabetes.
The normal process of wound healing includes three phases: inflammation, tissue formation, and tissue remodelling. When the normal healing process is disrupted, a wound can become chronic in nature. Risk factors that commonly contribute to poor wound healing are: 1) local causes, such as wound infection, tissue hypoxia, repeated trauma, and presence of debris or necrotic tissue; 2) systemic diseases, such as diabetes mellitus, immunodeficiency, or malnutrition; and 3) certain medications, such as corticosteroids (de la Torre 2015).
Venous ulcers develop when the leg veins become damaged due to injury or disease, causing them to malfunction. Venous ulceration typically develops on either side of the lower leg between the ankle and calf. Venous ulcers have been estimated to affect up to 1% of the population in developed countries (Ebbeskog 1996). The prevalence rates of open ulcers in different studies ranges from 0.12% to 1.1% of the general population, whereas the prevalence rate of open or healed ulcers was reported to be 1.8% (Graham 2003). A study in the UK showed a prevalence of 0.45 per 1000 (Moffat 2004).
A pressure ulcer is an area of tissue breakdown caused by pressure, shear or friction, or a combination of these between a bony prominence and an external surface (Grey 2006). Lack of movement causes compression of the tissues at the point where body and support surface meet. This compression causes impaired blood supply leading to tissue hypoxia and malnutrition. Anatomical sites commonly affected include the skin overlying the sacrum and hips (67%), but other locations commonly affected include heels, ankles, the occipital area, ears, and elbows. Pressure ulcers are relatively common. One epidemiological review reported that the prevalence of pressure ulcers in the UK ranges from 4.4% in a community unit to 37% in palliative care (Kaltenthaler 2001). Prevalence in North America is similar and ranges from 4.7% in hospital patients to 33% in people in the community with spinal cord injuries (Kaltenthaler 2001). Susceptibility is highest in people with neurological or cardiovascular disorders, dehydration, malnutrition, or hypotension, and in those who have undergone prolonged anaesthesia or surgery. Two‐thirds of pressure ulcers occur in people older than 70 years (Barbenel 1977).
Arterial (or ischaemic) ulcers are less common than venous ulcers and account for about 20% of leg ulcers. Atherosclerosis and diabetes are the commonest causes, but thrombotic episodes secondary to vasculitis (thromboangitis), and sickle cell disease can also result in arterial ulcers. Arterial ulceration typically develops on the dorsum of the foot or toes. Pain, with exercise or at night, is one characteristic of arterial ulcers and it is often aggravated by leg elevation.
Neurotrophic ulcers are usually caused by peripheral neuropathy, leading to loss of cutaneous sensitivity. These are often seen over pressure points of the metatarsophalangeal joint.
Diabetes is one frequent pathological condition that can result in an ulcer, with neuropathy and vascular disease being important contributory factors. These factors may lead to a loss of cutaneous sensibility and ischaemia, resulting in the amputation of the toe, foot, or leg (Gonzalez 2000). Approximately 15% to 25% of people with diabetes will develop at least one foot ulcer during their lifetime (Reiber 1996; Lavery 2003; Singh 2005). The annual population‐based incidence ranges from 1% to 4% and the prevalence is 4% to 10% (Reiber 2001; Lavery 2003).
A study from the USA reported that the Medicaid fee‐for‐service system incurred a total cost of approximately USD 11.6 million for the treatment of skin ulcers between 1994 and 1998 (Kumar 2004). Patients with pressure ulcers were older, were more likely to have had surgery, and stayed in hospital longer. Furthermore, pressure ulcers were the most frequent, and also the most costly type of ulcer (Kumar 2004). Another study of home care in Canada presented a similar prevalence of chronic wounds (Rodrigues 2006), and again pressure ulcers were the most common aetiology (37%). A costing study estimated that the cost of pressure ulcers in the UK was 4% of the total National Health Service expenditure for the financial year 1999 to 2000 (Bennett 2004).
Medical management of chronic wounds should, whenever possible, involve treatment of the primary cause. This may be glycaemic control for people with diabetes, or vascular surgery for people with chronic venous disease or ischaemic vascular disease (de la Torre 2015). Other measures thought to be important include the removal of necrotic or infected tissue (Edwards 2002), off‐loading (Spencer 2000), compression therapy (O'Meara 2009a; O'Meara 2009b), maintenance of a moist wound environment, management of wound infection (FDA 2005), wound cleansing (Moore 2005), and diet (Langer 2003; FDA 2005). Despite treatment, many chronic wounds fail to heal, persist for months or years, and/or recur after healing (Rodrigues 2006).
Description of the intervention
Autologous platelet‐rich plasma (PRP) has been under development as a theory since the 1990s (Anitua 2004), and is increasingly used clinically to treat cutaneous chronic wounds (Knighton 1988; Crovetti 2005). There are several techniques used to obtain autologous PRP, although some are not standardised or approved. The most common technique is to obtain a sample of blood from the patients themselves (autologous); this blood is then centrifuged to separate the platelets from red and white blood cells. These platelets rich in growth factors are highly concentrated and suspended in a small volume of plasma. Because most individuals have a baseline blood platelet count of 200,000 (±75,000)/µL, a PRP platelet count of 1 million/µL has been postulated as the ideal therapeutic dose of PRP (Marx 2004).
There are two methods to liberate growth factors from the platelets. The first is to add thrombin or calcium which activates the platelets and release the growth factors (platelet releasate). The second approach is to bring about physical lyses of the platelets (lysate) by freezing (Weed 2004), or by using other methods such as sonication, or to disrupt cell membranes and release cellular content with ultrasounds (Stacey 2000). The final product is applied locally to the wound as a gel or a solution.
How the intervention might work
PRP contains high concentrations of growth factors which are thought to facilitate healing (Marlovits 2004). When these growth factors are released from the platelets they trigger a tissue regeneration process (Knighton 1988; Robinson 1993). One recent study demonstrated that multiple growth factors are increased in the granulation tissue of refractory diabetic dermal ulcers after being treated with PRP (Yuan 2009). PRP, contains intra‐ and extra‐platelet components other than growth factors and these can also contribute to the regeneration of tissue. Fibrinogen, for example, creates the fibrin network necessary for cellular implantation and later multiplication (Munirah 2007). Autologous PRP has the advantage of low or null risk of infection or immune reactions.
Why it is important to do this review
An earlier systematic review about the efficacy of autologous PRP in tissue regeneration forms the basis of this review on chronic wounds (Martinez‐Zapata 2009). The use of autologous PRP is increasing in the clinical setting due to the healthcare and social relevance of chronic wounds and the limited results with current treatments. Clinical trials that evaluate the efficacy of autologous PRP are ongoing, and it is timely to synthesise and evaluate current evidence on this subject.
Objectives
To determine whether autologous PRP promotes the healing of chronic wounds.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared autologous PRP with alternative treatments or placebo for chronic wounds.
Types of participants
We considered trials that included people aged 18 years or older with chronic wounds from any cause (such as pressure ulcers, arterial ulcers, venous ulcers). We also included patients with wounds of mixed aetiology e.g. mixed arterial‐venous ulcers.
Types of interventions
Studies that compared autologous PRP (any method of collection and formulation) with placebo or alternative topical therapies such as standard care or protease‐modulating matrix (Appendix 1).
Types of outcome measures
Primary outcomes
Proportion of chronic wounds completely healed (defined as 100% epithelialisation or skin closure without drainage).
Secondary outcomes
Total area epithelialised at the end of the intervention (measured in cm2).
Percentage of wound area healed.
Time to complete wound healing.
Wound pain (measured by any validated scale).
Wound complications: infection, necrosis.
Quality of life (measured by any validated scale).
Adverse events
Search methods for identification of studies
Electronic searches
For this first update, we searched the following electronic databases to find reports of relevant RCTs:
The Cochrane Wounds Specialised Register (searched 16 June 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2015, Issue 5);
Ovid MEDLINE (1946 to 15 June 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 15 June 2015);
Ovid EMBASE (1974 to 15 June 2015);
EBSCO CINAHL (1982 to 16 June 2015).
The search strategies used for Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Ovid EMBASE, and EBSCO CINAHL can be found in Appendix 2, Appendix 3, Appendix 4, and Appendix 5 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). We combined the Ovid EMBASE and EBSCO CINAHL searches with the randomised controlled trials filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). We did not impose any restrictions with respect to language or date of publication.
We also searched the WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) (searched 30 January 2015), to identify ongoing and unpublished studies.
Searching other resources
We checked the reference lists of all relevant publications retrieved by the database searches to identify further studies. We also contacted trial authors for additional information.
Data collection and analysis
Selection of studies
Two review authors (CZ and MMZ) independently assessed each study identified by the search to check its eligibility. There was agreement between the review authors and it was therefore not necessary to consult a third review author to obtain consensus. Those references which appeared to meet the inclusion criteria were retrieved in full and further assessed independently by the same two review authors (CZ and MMZ).
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and 'Characteristics of excluded studies' table.
Data extraction and management
We extracted details of studies and recorded them using a data extraction sheet. If data were missing from reports, or clarification was needed, we made attempts to contact the trial authors to obtain missing information. We included data from studies published in duplicate only once. Two review authors (CZ and MMZ) independently extracted the data. Any discrepancy was resolved by discussion.
We extracted the following data for each included trial.
Trial characteristics (design, setting, location of care, country, source of funding, if the clinical trial reported the calculation of the sample size, and whether an intention‐to‐treat analysis was performed on the data reported in the published trial).
Participants by treatment group (number, age, sex, type of wound, wound size, length of follow‐up).
Intervention (concurrent interventions, duration of treatment).
Comparison condition.
Outcome measures.
Assessment of risk of bias in included studies
Two review authors (MMZ, and CZ) independently assessed the risk of bias of the eligible trials. There was agreement between the review authors and it was not necessary to consult a third review author (IS) to obtain consensus.
We based our 'Risk of bias' assessment on the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We examined the adequacy of the method used to generate the allocation sequence, the method of allocation concealment and the level of blinding (clinician, participant or outcome assessor). We further examined the presence of incomplete outcome data, and selective reporting (see 'Risk of bias' tables in Characteristics of included studies).
We classified each trial at high, unclear, or low risk of bias. We described the reason for each judgment from details provided in the trial reports or from data sought and provided by trial authors. We considered a trial to be at low risk of bias when it concealed allocation and blinded participants and outcome assessors, if it reported complete outcome data, and where we did not suspect selective outcome reporting (we assessed prespecification of outcomes from methods sections of trial publications). If one or more of these key domains were not met, we considered the trial to be at high risk of bias. If one or more of these key domains were unclear, we considered the trial as 'unclear' with respect to risk of bias (see table 8.7a of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a)).
Measures of treatment effect
For binary outcome measures (proportion of wounds completely healed, adverse events), we calculated the risk ratio (RR). For continuous outcomes (total area healed, wound pain, and quality of life), we recorded either mean change from baseline for each group or mean post‐treatment or intervention values and their standard deviation (SD) for each group. We pooled the estimate of treatment effect using the generic inverse method and calculated mean differences (MDs). For all measures, we calculated the 95% confidence interval (95% CI). For time to healing we planned a time to event analysis of survival.
Unit of analysis issues
The unit of analysis was either the participant or the ulcer randomised. We collected and analysed a single measurement for each outcome from each participant or ulcer.
Dealing with missing data
We contacted study authors in an effort to obtain additional information where data were missing or unclear. In order to undertake an intention‐to‐treat analysis, when it was possible, we sought data on the number of participants by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up.
Assessment of heterogeneity
We quantified the impact of statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2011b). Where statistical heterogeneity was high (I2 > 75%) or where there was clinical heterogeneity, we investigated possible causes by exploring the impact of participants' characteristics (e.g. wound aetiology) and the method used to liberate the growth factors. We would not pool studies which had high statistical heterogeneity (I2 > 75%). For levels of I2 less than 50% we applied a fixed‐effect model; for levels of I2 over 50% but less than 75% we used a random‐effects model.
Assessment of reporting biases
We did not assess whether the review was subject to publication bias by using a funnel plot because there were fewer than 10 included studies in our analysis of the main outcome (Sterne 2011).
Data synthesis
We determined the pooled effect estimate for each outcome through a meta‐analysis of the individual effect measures of the studies by means of a random‐effects model when there was clinical heterogeneity (studies with wound ulcers of different aetiologies) (DerSimonian 1986).
When there was neither clinical nor statistical heterogeneity, we used a fixed‐effect model (I2 less than 50%). We included studies that presented results of multiple ulcers on a participant in the analysis, calculating the effective sample size, as per the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). In addition, the intracluster correlation coefficient considered was 0.05 based on published data (Scriven 1998; Vas 2008).
We used the statistical package Review Manager 5, provided by Cochrane (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
Potential sources of clinical heterogeneity are:
wound aetiology (pressure ulcers, diabetes, ischaemia, and venous disease). When the clinical trial included ulcers of different aetiologies, we classified the trial as being of 'mixed chronic wounds';
methods to liberate growth factors from the platelets: lysate and releasate.
For these reasons, we stratified study data by type of chronic wound. Additionally, we performed a prespecified subgroup analysis by the methods used to liberate growth factors from the platelets.
Sensitivity analysis
We prespecified a sensitivity analysis to investigate the effect of excluding studies with high risk of bias (as defined earlier, namely, if one or more of the key domains of concealed allocation, blinded participants, blinded outcome assessors, complete outcome data, and selective reporting were at high risk of bias). We also conducted a sensitivity analysis to examine the effect of excluding from the meta‐analysis studies which either had a total attrition greater than 30%, or differences in attrition between the groups exceeding 10%; we did not prespecify this sensitivity analysis.
'Summary of findings' table
We prepared a 'Summary of findings' table, including assessment of the overall quality of the evidence for the main outcomes using the approach of the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) (Langedam 2013). This approach assesses the quality of the body of evidence per comparison and outcome, taking into account five factors: risk of bias across all studies; indirectness, interventions and outcomes; reporting the outcome; inconsistency amongst studies; imprecision; and publication bias.
Results
Description of studies
Results of the search
The search identified 297 citations. After considering titles and abstracts, we retrieved 66 potentially relevant studies in full‐text. We included 10 studies in qualitative synthesis (Knighton 1990; Krupski 1991; Stacey 2000; Senet 2003; Weed 2004, Driver 2006; Kakagia 2007; Planinsek Rucigaj 2007; Anitua 2008; Li 2012) and nine in quantitative synthesis (Knighton 1990; Krupski 1991; Stacey 2000; Senet 2003; Weed 2004, Driver 2006; Kakagia 2007; Anitua 2008; Li 2012). We also identified nine clinical trials that are ongoing (NCT00658983; ChiCTR‐TRC‐00000325; NCT02213952; IRCT2014060415574N3; ISRCTN84928077; JPRN‐UMIN000004840; NCT02209662; NCT02307448; NCT02312518). A further two studies are awaiting assessment (Obolenskiy 2014; Serra 2014). We excluded the remaining 45 studies, of which 11 are ongoing (Figure 1).
1.
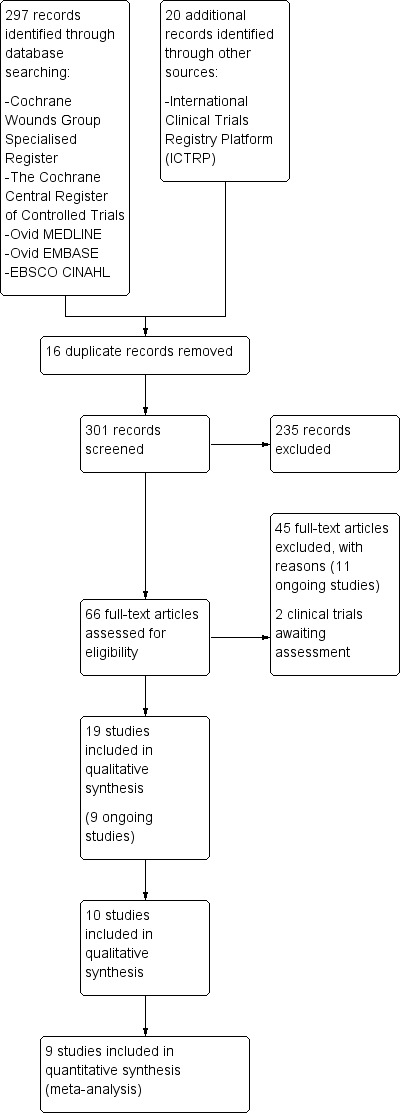
Study flow diagram.
We contacted some authors in an effort to obtain additional information (Tarpila 1998; Stacey 2000; Driver 2006; Planinsek Rucigaj 2007; Saad Setta 2011; NCT00215735). Only one trial author responded and answered our questions (Tarpila 1998).
Included studies
We extracted descriptive data from the ten included trials. Overall, data on 442 participants were included in the review, 228 participants received platelet‐rich plasma (PRP) and 214 received a control treatment. Forty‐two per cent of participants were female and 58% were male; the mean age was 61 years. The median number of participants included per clinical trial was 29 (range 10 to 117).
Four trials treated people with wounds due to different aetiologies ('mixed wounds') (Knighton 1990; Krupski 1991; Weed 2004; Anitua 2008). Two of these included ulcers of more than one aetiology (venous diseases, diabetic disease, occlusive peripheral vascular diseases, vasculitis and/or pressure ulcers) (Knighton 1990; Anitua 2008), another included different ulcers in the trial, but each participant had ulcers due to only one cause (Krupski 1991), and one study included both variations (Weed 2004). Three trials treated venous leg ulcers (Stacey 2000; Senet 2003; Planinsek Rucigaj 2007), and three trials treated foot ulcers in people with diabetes (Driver 2006; Kakagia 2007; Li 2012). The median wound duration at baseline was 49 weeks, with a range from 19 in Kakagia 2007 to 280 weeks in Senet 2003. The median wound size at baseline was 11.2 cm2, ranging from 3.2 cm2 in Weed 2004) to 149 cm2 in Planinsek Rucigaj 2007 (see Table 2).
1. Characteristics of skin ulcer at baseline.
| Study | Ulcer size PRP (cm2) | Ulcer size control (cm2) | Time of ulcer PRP (weeks) | Time of ulcer control (weeks) |
| Anitua 2008 | 5.5 | 27.6 | 68.0 | 110.0 |
| Driver 2006 | 4.0 | 22.0 | ‐ | ‐ |
| Kakagia 2007 | 28.4 | 28.9 | 20.0 | 19.0 |
| Knighton 1990 | 11.6 | 109.7 | 119.0 | 47.0 |
| Krupski 1991 | 13.0 | 10.8 | 22.0 | 24.8 |
| Li 2012 | ‐ | ‐ | 6 (2‐13) | 3 (2‐9) |
| Planinsek Rucigaj 2007 | 148.7 | 4.8 | ‐ | ‐ |
| Senet 2003 | 13.7 | 5.7 | 202.4 | 280.0 |
| Stacey 2000 | 5.1 | 8.9 | 24.0 | 24.0 |
| Weed 2004 | 6.7 | 3.2 | 51.3 | 54.4 |
PRP: platelet‐rich plasma
The methods used to obtain autologous PRP varied between studies, but all used the participants’ own blood and centrifuged this to obtain a concentrate of platelets. The procedure to liberate growth factors from the platelets varied between studies. Four trials applied a platelet lysate (Knighton 1990; Krupski 1991; Stacey 2000; Weed 2004), four used platelet releasate (Driver 2006; Planinsek Rucigaj 2007; Anitua 2008; Li 2012), and in two studies the method used was not clearly reported (Senet 2003; Kakagia 2007).
The four studies that used platelet lysate kept it frozen in the days prior to use. The four studies that used platelet releasate prepared the autologous PRP a few hours before it was administered to the participant (Anitua 2008; Driver 2006; Planinsek Rucigaj 2007; Li 2012). The median duration of treatment was 12 weeks, with a range from eight weeks in Anitua 2008 to 40 weeks in Stacey 2000.
Only three trials specified that they had calculated the required sample size (Stacey 2000; Weed 2004; Driver 2006). Two studies presented the data from more than one ulcer per patient (Knighton 1990; Krupski 1991). One trial did not report a standard deviation and we therefore excluded it from the pooled analyses (Planinsek Rucigaj 2007).
There was imbalance between groups at baseline in seven trials (Knighton 1990; Krupski 1991; Senet 2003; Weed 2004; Driver 2006; Planinsek Rucigaj 2007; Anitua 2008; see Characteristics of included studies). In the Knighton 1990 study, the experimental group had a longer wound duration than the control group (119 weeks versus 47 weeks). In the Krupski 1991 study, the placebo group presented with a larger wound area than the experimental group (29 cm2 versus 13 cm2), the PRP group had more wounds (17 versus 9) and wound duration was longer (6.2 months versus 4.3 months) than in the placebo group. Anitua 2008 reported that participants in the control group were older than those in the experimental group (61 versus 45 years old) and the duration of the ulcer also was longer (110 days versus 68 days) in the control group. In Weed 2004, the experimental group was older than the control group. In the Senet 2003, Driver 2006, and Planinsek Rucigaj 2007 studies, the wound area was significantly different between groups at baseline (see Table 2).
In the Driver 2006 study, only 40 of 72 patients were evaluated due to the high percentage of protocol violations and failure to complete treatment. Weed 2004 experienced difficulty in recruiting patients and the trial authors were unable to achieve the necessary sample size: only 26 of the 80 patients needed were included.
All but three trials reported the sources of funding (Stacey 2000; Weed 2004; Planinsek Rucigaj 2007). Four of the studies received financial support from companies manufacturing PRP devices (Knighton 1990; Krupski 1991; Driver 2006; Anitua 2008).
Excluded studies
We excluded 45 studies for the following reasons (Characteristics of excluded studies):
Twenty‐two studies were not randomised (Knighton 1986; Atri 1990; Köveker 1992; Tarpila 1998; Reutter 1999; Aminian 2000; Margolis 2001; Mazzuco 2004; Saldamalacchia 2004; Sánchez 2007; Aminian 2011; Carter 2011; Jorgensen 2011; Saad Setta 2011; Enriquez‐Vega 2012; NCT00762138; NCT01553955; JPRN‐UMIN000009860; JPRN‐UMIN000015689; NCT02088268; NCT02071979; Morimoto 2015).
Five studies considered acute wounds (Danielsen 2008; Hao 2010; Cervelli 2012; NCT00856934; NCT01639144).
Fifteen studies did not assess autologous PRP (Steed 1992; Holloway 1993; Steed 1993; Steinbaum 1994; Steed 1996; Crovetti 2004; Afshari 2005; Niezgoda 2005; Ma 2007; Chen 2010; Scevola 2010; Jaiswal 2010; Greppi 2011; Soula 2012; Khandelwal 2013).
Two studies were stopped early, one in venous leg ulcers (NCT00273234), and one in diabetic foot ulcers (NCT00338702). The reasons given were lack of financial support in both studies, and the former also had enrolment difficulties due to the stringent patient inclusion criteria.
One study was terminated with inconclusive results and was not published (NCT00215735). We requested more information from the investigators but we have not received a response.
Risk of bias in included studies
Only one study presented low risk of bias across all domains (Krupski 1991). Three studies were at high risk of bias for at least one domain, with the remainder being at overall unclear risk of bias (Figure 2; Figure 3).
2.
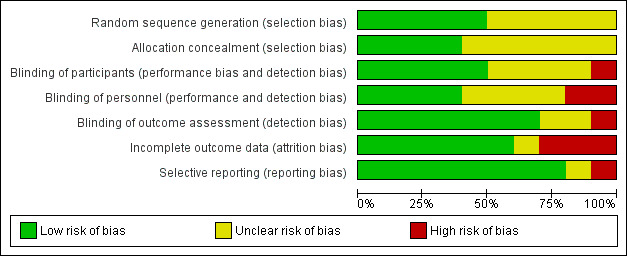
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
3.
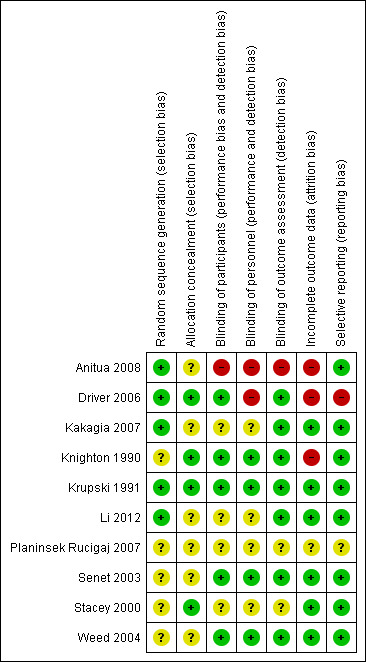
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Allocation
Adequacy of the method used to generate the allocation sequence
Of the ten included studies, only five adequately reported the method used to generate the randomisation sequence (Krupski 1991; Driver 2006; Kakagia 2007; Anitua 2008; Li 2012). The other studies mentioned that the clinical trial was randomised but did not report further details.
Allocation concealment
Four of the ten studies adequately reported allocation concealment (Knighton 1990; Krupski 1991; Stacey 2000; Driver 2006), but in the remaining six studies this was not specified (Senet 2003; Weed 2004; Kakagia 2007; Planinsek Rucigaj 2007; Anitua 2008; Li 2012).
Blinding
Five studies blinded the participants, this was either specifically reported or the control treatment was identical in appearance to the autologous PRP, and we judged that the participants were properly blinded because of this similarity (Knighton 1990; Krupski 1991; Senet 2003; Weed 2004; Driver 2006).
Four studies blinded the caregivers (Knighton 1990; Krupski 1991; Senet 2003; Weed 2004), and seven studies blinded the outcome assessors (Knighton 1990; Krupski 1991; Senet 2003; Weed 2004; Driver 2006; Kakagia 2007; Li 2012).
Anitua 2008 was an open trial and Planinsek Rucigaj 2007 did not mention whether the control group was identical to the experimental group.
Incomplete outcome data
All trials reported if there were any participants lost to follow‐up with the exception of Planinsek Rucigaj 2007. In general terms, the included trials had dropout percentages lower than 30%, with the exception of Driver 2006 and Anitua 2008, with 40% and 44%, respectively. Three studies had no participants lost to follow‐up (Krupski 1991; Weed 2004; Li 2012). Losses to follow‐up were similar between the experimental and control groups in all trials except in the studies by Knighton 1990 and Driver 2006 which had an imbalance in patient loss after randomisation. The losses to follow‐up in Knighton 1990 were three (18.7%) patients in the experimental group and five (31.2%) patients in the control group. The losses to follow‐up in the Driver 2006 study were 21 (52.5%) patients in the experimental group and 11 (34.4%) patients in the control group.
Three trials performed intention‐to‐treat analyses (Stacey 2000; Senet 2003; Driver 2006). Additionally, Driver 2006 performed a per protocol analysis for secondary outcomes because there was a high percentage of protocol violations and failure to complete the treatment.
Selective reporting
One clinical trial presented selective reporting (Driver 2006). The non‐reported results in question referred to the percentage of change in wound area at end‐of‐study visit from baseline, the percentage of change in wound volume at end‐of‐study visit from baseline, and volume closure rate per day at end‐of‐study visit. We did not seek trial protocols but recognise this is something we should consider for the future.
Effects of interventions
See: Table 1
Autologous platelet‐rich plasma (PRP) compared with standard care (with or without placebo)
Primary outcome
Proportion of chronic wounds completely healed
Eight RCTs (391 participants) compared PRP with standard care (with or without placebo), and reported data for the outcome of complete wound healing. Two studies involved 189 participants with diabetic foot ulcers (Driver 2006; Li 2012), two recruited 101 participants with venous leg ulcers (Stacey 2000; Senet 2003), and four studies involved 101 participants with mixed chronic wounds (Knighton 1990; Krupski 1991; Weed 2004; Anitua 2008). Overall, it is unclear whether the addition of autologous PRP to standard treatment affects the risk of chronic wound healing compared with standard treatment alone (low quality evidence, downgraded twice for risk of bias) (RR 1.19, 95% CI 0.95 to 1.50; I2 = 27%).
Looking separately at the two studies in people with diabetic foot ulcers (189 participants), there is some low quality evidence (downgraded twice for risk of bias) that autologous PRP may increase the risk of complete healing in people with diabetic foot ulcers (RR 1.22, 95% CI 1.01 to 1.49). However, both these studies used a different method of harvesting the PRP from all but one of the other studies. It is unclear whether PRP affects the healing of venous leg ulcers (RR 1.02, 95% CI 0.81 to 1.27; I2 = 0%, low quality evidence), or mixed chronic wounds (RR 1.85, 95% CI 0.76 to 4.51; I2 = 42%) (Analysis 1.1). In each case, we downgraded the quality of the evidence for imprecision (the confidence interval was wide) and risk of bias (usually incomplete outcome data, selective reporting, or the randomisation process was unclear) (Table 1).
1.1. Analysis.
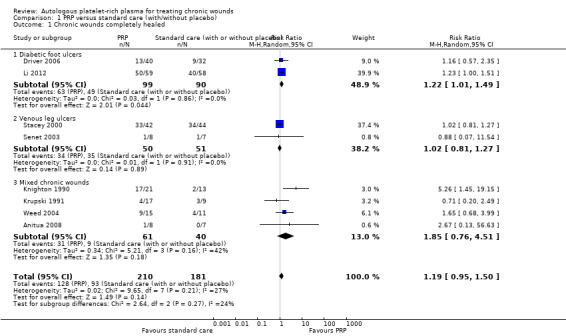
Comparison 1 PRP versus standard care (with/without placebo), Outcome 1 Chronic wounds completely healed.
We conducted a subgroup analysis to investigate whether different methods of liberating growth factors from the platelets resulted in different clinical effects, however, this comparison was confounded by wound type (most of the trials using PRP releasate involve people with diabetic foot ulcers). The studies which used PRP releasate had a pooled RR of complete healing of 1.23, 95% CI 1.01 to 1.49; I2 = 0% (Driver 2006; Anitua 2008; Li 2012), and those for PRP lysate had a pooled RR of 1.45, 95% CI 0.67 to 3.13 (I2 = 70%) (Knighton 1990; Krupski 1991; Stacey 2000; Weed 2004) (Analysis 2.1). Consequently it is unclear whether the method of harvesting PRP influences any clinical effect.
2.1. Analysis.
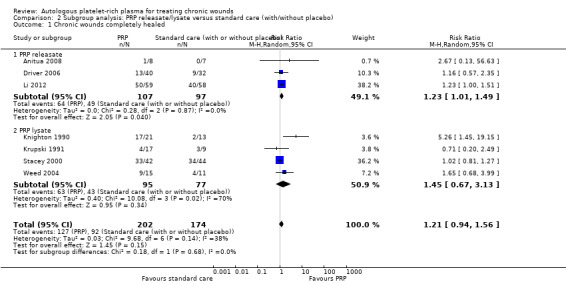
Comparison 2 Subgroup analysis: PRP releasate/lysate versus standard care (with/without placebo), Outcome 1 Chronic wounds completely healed.
We did not perform the prespecified sensitivity analysis because the quality of evidence was low, principally due to risk of bias of studies.
Secondary outcomes
Total area epithelialised
Three trials of mixed chronic wounds (66 participants) reported data for this outcome (Krupski 1991; Weed 2004; Anitua 2008). There was no clear evidence of a difference between the groups (pooled MD ‐2.78 cm2, 95% CI ‐8.67 to 3.11; I2 = 47%) (Analysis 1.2).
1.2. Analysis.
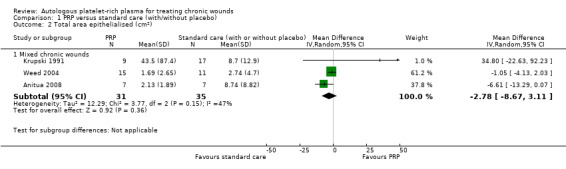
Comparison 1 PRP versus standard care (with/without placebo), Outcome 2 Total area epithelialised (cm2).
Percentage of wound area healed
One trial reported the average reduction in ulcer size after two days of treatment (5.42 cm2 in the experimental group and 0.8 cm2 in the control group). However, the standard deviations of these measures were not reported and we could not analyse the results (Planinsek Rucigaj 2007).
Two small trials of mixed chronic wounds (47 participants) reported data on percentage of wound area healed and we pooled these data (Anitua 2008; Knighton 1990). Although a greater area was healed with PRP than control (MD 51.78%, 95% CI 32.70 to 70.86; I2 = 0%; Analysis 1.3), these data are at high risk of bias: Anitua 2008 due to unblinded outcome assessment and attrition, and Knighton 1990 due to attrition; and this must be taken into consideration when interpreting the finding.
1.3. Analysis.
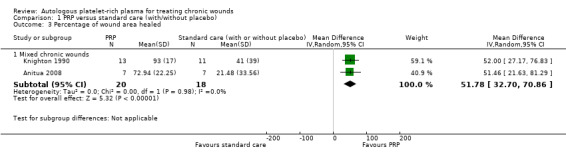
Comparison 1 PRP versus standard care (with/without placebo), Outcome 3 Percentage of wound area healed.
Time to complete wound healing
Two RCTs reported data on this outcome (Stacey 2000; Driver 2006). Neither trial reported sufficient information to replicate the analysis.
Wound pain
Not reported in any trial.
Wound complications
Three trials (117 participants) reported wound complications such as infection (Senet 2003; Anitua 2008), or dermatitis (Senet 2003; Driver 2006). Overall it was not clear whether there was a difference in rates of wound complication between PRP and standard care. Two small trials (30 participants) reported data for wound infection (Senet 2003; Anitua 2008), and there was no clear difference (RR 0.80, 95% CI 0.14 to 4.73; I2 = 0%). Senet 2003 and Driver 2006 (87 participants) reported dermatitis, and there was no clear difference (RR 1.31, 95% CI 0.18 to 9.69; I2 = 0%) (Analysis 1.4).
1.4. Analysis.
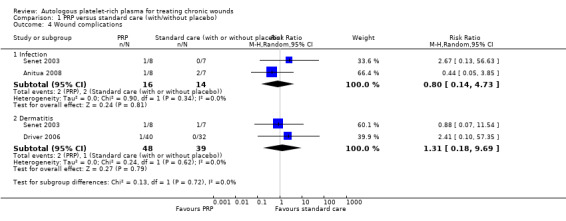
Comparison 1 PRP versus standard care (with/without placebo), Outcome 4 Wound complications.
Quality of life
Not reported in any trial.
Adverse events
Six out of nine trials reported information on adverse events (Krupski 1991; Stacey 2000; Senet 2003; Weed 2004; Driver 2006; Anitua 2008), but only three trials presented them (Senet 2003; Driver 2006; Anitua 2008). Overall, 5/56 participants (8.9%) in the PRP group experienced an adverse event compared with 4/46 (8.6%) in the control group. It is unclear whether there was a difference in the risk of adverse events between PRP and standard care (very low quality evidence) (RR 1.05, 95% CI 0.29 to 3.88; I2 = 0%) (Analysis 1.5). We downgraded the quality of evidence for risk of bias and imprecision (Table 1). Knighton 1990, Planinsek Rucigaj 2007, and Li 2012 did not report data on adverse events.
1.5. Analysis.
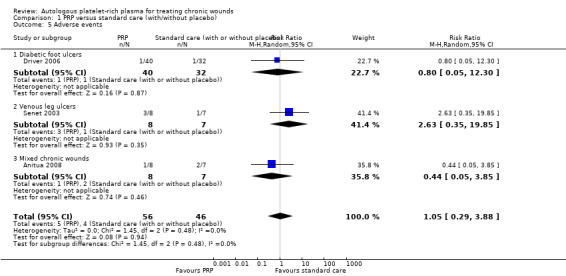
Comparison 1 PRP versus standard care (with/without placebo), Outcome 5 Adverse events.
Autologous PRP plus protease‐modulating matrix compared with protease‐modulating matrix alone
Primary outcomes
Proportion of chronic wounds completely healed
We identified one trial with three treatment groups that compared protease‐modulating matrix alone, PRP alone, and PRP and protease‐modulating matrix in combination (total of 51 participants, 17 in each group) in people with diabetes and a foot ulcer (Kakagia 2007). We only extracted data for the comparison of protease‐modulating matrix with and without PRP as the only systematic difference between groups was the presence/absence of PRP. There was no difference in the risk of complete ulcer healing with and without PRP in this context (2 participants in each group completely healed; RR 1.00, 95% CI 0.16 to 6.30). There was very low quality evidence for this outcome (downgraded for risk of (attrition) bias and imprecision) (Analysis 3.1).
3.1. Analysis.
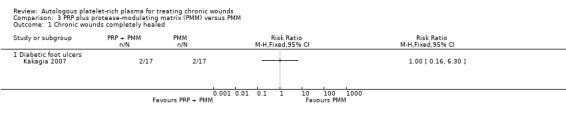
Comparison 3 PRP plus protease‐modulating matrix (PMM) versus PMM, Outcome 1 Chronic wounds completely healed.
Secondary outcomes
Wound pain or quality of life
Not reported in Kakagia 2007.
Adverse events
Not reported in Kakagia 2007.
Discussion
Summary of main results
Our aim was to evaluate the effectiveness and safety of autologous platelet‐rich plasma (PRP) in healing chronic wounds. This is the first update of a review published in 2012 (Martinez‐Zapata 2012), and we have included one new randomised controlled trial (RCT) and nine ongoing RCTs, bringing the total number of included studies to 10.
We observed substantial variations within trials regarding eligible participants, wound aetiologies, and other design and conduct features. Four trials treated people with mixed aetiology chronic wounds (there were participants with wounds caused by more than one aetiology and participants who had wounds of several aetiologies in the same trial) (Knighton 1990; Krupski 1991; Anitua 2008; Weed 2004); three treated people with venous leg ulcers (Stacey 2000; Senet 2003; Planinsek Rucigaj 2007), and three treated people with diabetes and foot ulcers (Driver 2006; Kakagia 2007; Li 2012). Nine out of 10 studies compared PRP plus standard care with standard care alone (with or without placebo). One study in people with diabetes evaluated PRP in the context of protease‐modulating matrix (Kakagia 2007).
The process used to 'harvest' autologous PRP varied between studies, however, it was impossible to draw conclusions about any differences in the effects of PRP harvested in different ways since these studies were also different in the types of patients included.
We analysed the overall effect of PRP on complete wound healing with data from eight RCTs and there was uncertainty as to whether PRP affects the risk of complete healing (low quality evidence). Although there is a possible beneficial effect of PRP on complete wound healing when the studies confined to diabetic foot ulcers are examined, this is low quality evidence and these studies also harvested the PRP in a way that was different to most of the other studies (PRP releasate rather than lysate). There were no data reported on quality of life.
There is great uncertainty in terms of whether there are differential effects of PRP and standard care in terms of safety (adverse events). Pooling the data from three trials showed no clear evidence of a difference, however, this comparison is very underpowered.
Overall completeness and applicability of evidence
There is increasing interest in using autologous PRP, as evidenced by the large number of ongoing trials that we have identified in this review. Autologous PRP is used because it contains growth factors which are thought to aid wound repair, however, the current evidence is very sparse and of low quality, therefore, we do not know whether PRP speeds wound healing in people with chronic wounds such as foot ulcers in people with diabetes and venous leg ulcers.
Quality of the evidence
Evidence concerning the efficacy of autologous PRP in chronic wounds is low or very low quality; typically due to various risks of bias and imprecision (due to small sample sizes and lack of statistical power).
Most studies were very small, did not report an a priori sample size calculation, and would have been underpowered to detect anything but very large treatment effects. In the Weed 2004 study a target sample size was calculated but there were difficulties in achieving full recruitment, and the final sample size was smaller than that planned. The lack of statistical power limits the adequate evaluation of autologous PRP efficacy. In seven of the included studies there was an apparent imbalance at baseline for important characteristics, probably chance imbalances due to small sample sizes (Knighton 1990; Krupski 1991; Senet 2003; Weed 2004; Driver 2006; Planinsek Rucigaj 2007; Anitua 2008).
Most studies were at high or unclear risk of bias due to poor reporting (Figure 2; Figure 3).
These limitations (inadequate simple size, unclear randomisation sequence, and allocation concealment) could explain that in seven trials there was an imbalance between groups of baseline characteristics.
Potential biases in the review process
Our assessment of risk of bias was hampered by the poor reporting of the included studies. We requested information from six authors (Tarpila 1998; Stacey 2000; NCT00215735; Driver 2006; Planinsek Rucigaj 2007; Saad Setta 2011), but we only received one response (Tarpila 1998).
Agreements and disagreements with other studies or reviews
We previously published a systematic review concerning the effectiveness of autologous PRP for tissue regeneration (search date February 2006), which included seven RCTs of chronic wounds (Martinez‐Zapata 2009). Our overall conclusion is unchanged, namely that we are unclear whether PRP influences the healing rate of chronic wounds.
There are three other published systematic reviews that assess efficacy of PRP in wound care (Lacci 2010; Mao 2010; Villela 2010). The reviews by Lacci 2010 and Mao 2010 were narrative reviews (no meta‐analysis) and concluded that more rigorous trials were needed before the clinical use of PRP could be recommended. The review by Villela 2010 focused on PRP for diabetic foot ulcers and had more liberal inclusion criteria (any clinical trial design and homologous or autologous PRP). The meta‐analysis combined the results of four RCTs. Two studies assessed homologous PRP (Steed 1992; Holloway 1993), and the other two studies, autologous PRP (Knighton 1990; Driver 2006). The meta‐analysis indicated that PRP significantly improved the healing of diabetic foot ulcers, however, this finding was heavily influenced by the Knighton 1990 trial which arguably should have been excluded because the participants had ulcers of different aetiologies.
This current review is an update of a previously published version (Martinez‐Zapata 2012), and offers a more rigorous 'Risk of bias' assessment, a more recent search, and an evaluation of the quality of evidence. We found some low quality evidence of a possible effect of autologous PRP on the healing of diabetic foot ulcers.
Authors' conclusions
Implications for practice.
It is unclear whether autologous platelet‐rich plasma (PRP) influences the healing of chronic wounds, as the existing evidence is sparse and of low or very low quality.
Implications for research.
Well designed, adequately powered RCTs are needed to determine whether using PRP confers any benefit in terms of more rapid or increased wound healing. Nine trials assessing the efficacy of autologous PRP in chronic wounds are ongoing and their results will provide further, valuable evidence (NCT00658983; ChiCTR‐TRC‐00000325; NCT02213952; IRCT2014060415574N3; ISRCTN84928077; JPRN‐UMIN000004840; NCT02209662; NCT02307448; NCT02312518).
What's new
| Date | Event | Description |
|---|---|---|
| 23 May 2016 | New search has been performed | First update. New search. |
| 3 March 2016 | New citation required and conclusions have changed | This is the first update of this review. A new search resulted in the inclusion of one additional study; the conclusions of the review have been changed |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 2 April 2008 | Amended | Converted to new review format |
| 10 October 2007 | New citation required and major changes | Substantive amendment |
Acknowledgements
The authors would like to acknowledge the contribution of peer referees (David Armstrong, Robert Ashford, Rachel Richardson) and editors of Cochrane Wounds (David Margolis, Andrea Nelson, Gill Worthy) for their comments on the protocol and review. Thanks also to copy‐editors Heather Maxwell and Clare Dooley and to Carolyn Newey for help in editing the first version of the manuscript.
Appendices
Appendix 1. Glossary of terms
Granulation is fibrous connective tissue that replaces a fibrin clot in healing wounds. Fibrin is a fibrous non‐globular protein involved in the clotting of blood. Haemostasis is a process which causes bleeding to stop. Lysate refers to the breaking down of a cell. Platelet lysate is to break the plaquetar membrane by physical methods such as freezing or sonication. Platelet releasate is to activate the platelet by chemical methods with thrombin or calcium to liberate the contents. Sonication is the process that disrupts cell membranes and releases cellular content using ultrasound. Synonyms of autologous platelet‐rich plasma (PRP): Autologous platelet gel, plasma‐rich growth factors (PRGFs), autologous platelet concentrate. Thromboangitis is a thrombotic episode secondary to vasculitis. Protease‐modulating matrix is a natural or synthetic substance used in medicine and introduced into the body in order to support or replace a natural function.
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Platelet‐Derived Growth Factor explode all trees #2 (platelet‐derived NEXT growth NEXT factor*) or PDGF #3 MeSH descriptor Blood Platelets explode all trees #4 (platelet NEXT rich NEXT plasma) or (platelet‐rich NEXT plasma) or PRP or (platelet gel*) #5 MeSH descriptor Platelet Activation explode all trees #6 platelet* NEXT activat* #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 MeSH descriptor Wound Healing explode all trees #9 MeSH descriptor Skin Ulcer explode all trees #10 MeSH descriptor Diabetic Foot explode all trees #11 (skin NEXT ulcer*) or (foot NEXT ulcer*) or (diabetic NEXT foot) or (leg NEXT ulcer*) or (varicose NEXT ulcer*) or (venous NEXT ulcer*) or (stasis NEXT ulcer*) or (arterial NEXT ulcer*) #12 ((ischaemic or ischemic) NEXT (wound* or ulcer*)) #13 (bed NEXT sore*) or (pressure NEXT sore*) or (pressure NEXT ulcer*) or (decubitus NEXT ulcer*) #14 chronic NEXT wound* #15 chronic NEXT ulcer* #16 (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) #17 (#7 AND #16)
Appendix 3. Ovid MEDLINE search strategy
1 exp Platelet‐Derived Growth Factor/ 2 (platelet‐derived growth factors or PDGF).mp. 3 exp Blood Platelets/ 4 (platelet rich plasma or platelet‐rich plasma or PRP or platelet gel$).mp. 5 exp Platelet Activation/ 6 (platelet$ adj activat$).mp. 7 or/1‐6 8 exp Wound Healing/ 9 exp Skin Ulcer/ 10 exp Diabetic Foot/ 11 (skin ulcer$ or foot ulcer$ or diabetic foot or diabetic feet or leg ulcer$ or varicose ulcer$ or venous ulcer$ or stasis ulcer$ or arterial ulcer$ or neuropathic ulcer$).mp. 12 ((ischaemic or ischemic) adj (wound$ or ulcer$)).mp. 13 (bed sore$ or pressure sore$ or pressure ulcer$ or decubitus ulcer$).mp. 14 (chronic adj wound$).mp. 15 (chronic adj ulcer$).mp. 16 or/8‐15 17 7 and 16
Appendix 4. Ovid EMBASE search strategy
1 exp Platelet Derived Growth Factor/ 2 (platelet‐derived growth factors or PDGF).mp. 3 exp Thrombocyte/ 4 exp Thrombocyte Rich Plasma/ 5 (platelet rich plasma or platelet‐rich plasma or PRP or platelet gel$).mp. 6 exp Thrombocyte Activation/ 7 (platelet$ adj activat$).mp. 8 or/1‐7 9 exp Wound Healing/ 10 exp Skin Ulcer/ 11 exp Diabetic Foot/ 12 (skin ulcer$ or foot ulcer$ or diabetic foot or diabetic feet or leg ulcer$ or varicose ulcer$ or venous ulcer$ or stasis ulcer$ or arterial ulcer$ or neuropathic ulcer$).mp. 13 ((ischaemic or ischemic) adj (wound$ or ulcer$)).mp. 14 (bed sore$ or pressure sore$ or pressure ulcer$ or decubitus ulcer$).mp. 15 (chronic adj wound$).mp. 16 (chronic adj ulcer$).mp. 17 or/9‐16 18 8 and 17
Appendix 5. EBSCO CINAHL search strategy
S17 S7 and S16 S16 (S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 S15 TI ( chronic wound* or chronic ulcer* ) or AB ( chronic wound* or chronic ulcer*) S14 TI ( bed sore* or pressure sore* or pressure ulcer* or decubitus ulcer* ) or AB ( bed sore* or pressure sore* or pressure ulcer* or decubitus ulcer* ) S13 TI ( bed sore* or pressure sore* or pressure ulcer* or decubitus ulcer* ) or AB ( bed sore* or pressure sore* or pressure ulcer* or decubitus ulcer* ) S12 TI ( ischaemic ulcer* or ischemic ulcer* or ischaemic wound* or ischemic wound* ) or AB ( ischaemic ulcer* or ischemic ulcer* or ischaemic wound* or ischemic wound* ) S11 TI ( skin ulcer* or foot ulcer* or diabetic foot or diabetic feet or leg ulcer* or varicose ulcer* or venous ulcer* or stasis ulcer* or arterial ulcer* or neuropathic ulcer* ) or AB ( skin ulcer* or foot ulcer* or diabetic foot or diabetic feet or leg ulcer* or varicose ulcer* or venous ulcer* or stasis ulcer* or arterial ulcer* or neuropathic ulcer* ) S10 (MH "Diabetic Foot") S9 (MH "Skin Ulcer+") S8 (MH "Wound Healing+") S7 S1 or S2 or S3 or S4 or S5 or S6 S6 TI platelet* activat* or AB platelet* activat* S5 (MH "Platelet Activation+") S4 TI ( platelet rich plasma or platelet‐rich plasma or PRP or platelet gel* ) or TI (platelet rich plasma or platelet‐rich plasma or PRP or platelet gel* ) S3 (MH "Blood Platelets") S2 TI ( platelet‐derived growth factor* or PDGF ) or AB ( platelet‐derived growth factor* or PDGF ) S1 (MH "Platelet‐Derived Growth Factor")
Data and analyses
Comparison 1. PRP versus standard care (with/without placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chronic wounds completely healed | 8 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.95, 1.50] |
| 1.1 Diabetic foot ulcers | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.01, 1.49] |
| 1.2 Venous leg ulcers | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.81, 1.27] |
| 1.3 Mixed chronic wounds | 4 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.76, 4.51] |
| 2 Total area epithelialised (cm2) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Mixed chronic wounds | 3 | 66 | Mean Difference (IV, Random, 95% CI) | ‐2.78 [‐8.67, 3.11] |
| 3 Percentage of wound area healed | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Mixed chronic wounds | 2 | 38 | Mean Difference (IV, Random, 95% CI) | 51.78 [32.70, 70.86] |
| 4 Wound complications | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infection | 2 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.14, 4.73] |
| 4.2 Dermatitis | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.18, 9.69] |
| 5 Adverse events | 3 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.29, 3.88] |
| 5.1 Diabetic foot ulcers | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.05, 12.30] |
| 5.2 Venous leg ulcers | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.35, 19.85] |
| 5.3 Mixed chronic wounds | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.05, 3.85] |
Comparison 2. Subgroup analysis: PRP releasate/lysate versus standard care (with/without placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chronic wounds completely healed | 7 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.94, 1.56] |
| 1.1 PRP releasate | 3 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.01, 1.49] |
| 1.2 PRP lysate | 4 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.67, 3.13] |
Comparison 3. PRP plus protease‐modulating matrix (PMM) versus PMM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chronic wounds completely healed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Diabetic foot ulcers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anitua 2008.
| Methods | Design: Randomised open‐label controlled pilot trial Number of participant centres: 1 Setting: Hospital Country: Spain Unit of randomisation: the patient Unit of analysis: the patient Follow‐up: 8 weeks |
|
| Participants | Number randomised (patients): 15 (Group 1 experimental: 8 and Group 2 control: 7) Number ulcers assessed: 15 Wound aetiology: mixed 10, venous ulcers 4, pressure ulcers and 1 other Age (mean and SD): Group 1: 45 (20) Group 2: 61 (16) Sex: 7 F/8 M Inclusion criteria: Adults of both sexes with chronic ( > 4 weeks) skin ulcers of less than 12 cm in diameter or Wagner grade II/III Exclusion criteria: ulcer of arterial origin; infection; insulin‐dependent diabetes mellitus; vasculitis; lupus; cryoglobulinemia; haematological abnormality; epilepsy; solid tumour; anticoagulants; immunosuppressant drugs; anaemia; pregnant women or inadequate birth control | |
| Interventions | At baseline all patients received conventional treatment (cleansing, debridement, and wet cure with physiological saline and sterile gauzes) After randomisation it was not reported if the participants randomised to the experimental group continued to receive the conventional treatment in addition to the weekly treatment of autologous PRGF Experimental group: Autologous PRGF Control group: Conventional treatment Length of treatment: 8 weeks |
|
| Outcomes | Mean percentage of surface healed* Lesion area* Adverse events *Measures were made from photographic records using Mouseyes software | |
| Notes | Funding: The Biotechnology Institute provided the PRGF System® device. Baseline characteristics were not similar between groups. Patients in the control group were older, had longer ulcer duration and larger wound sizes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned according to a computer generated randomisation table to wound care with PRGF (experimental group) or standard wound care (control group)" Comments: randomisation sequence was generated by computer |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias and detection bias) | High risk | Quote: "...open‐label, standard care‐controlled pilot clinical trial" Comments: The clinical trial was open. No masking of participants |
| Blinding of personnel (performance and detection bias) | High risk | Quote: "...open‐label, standard care‐controlled pilot clinical trial" Comments: The clinical trial was open. No masking of care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "...open‐label, standard care‐controlled pilot clinical trial" Comments: The clinical trial was open. No masking of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40% of patients were lost to follow‐up; Group 1: 3/8 (37.5%) Group 2: 3/7 (42.8%) Comments: this represents a high level of loss (over 30%) |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes prespecified in the methods of the trial report were presented. The trial protocol was not sought |
Driver 2006.
| Methods | Design: Randomised double‐blind placebo controlled trial Number of participant centres: 14 Setting: wound care physicians’ and podiatrists’ offices, outpatient wound care centres, a university‐based college of podiatric medicine clinic, Veteran’s Administration wound care clinics, and an Army hospital limb preservation programme Country: USA Unit of randomisation: the patient Unit of analysis: the patient Follow‐up: 24 weeks |
|
| Participants | Number randomised (patients): 72 (40 treatment arm and 32 control arm) Wound aetiology: Diabetic foot ulcers Age, mean (SD) years: 56.4 (10.2) treatment arm and 57.5 (9.1) control arm Sex: 59 M/13 F Inclusion criteria: Adults of both sexes with diabetes mellitus 1 or 2, and a chronic skin ulcer with evolution of at least 4 weeks. Hb A1c < 12; foot ulcer; wound area measurement between 0.5 cm2 and 20 cm2, inclusive. Ulcer had to be clinically non‐infected Exclusion criteria: Patient currently enrolled in another clinical trial. Non‐diabetic ulcers. Ulcer had exposed tendons, ligaments, muscle, or bone. Gangrene or osteomyelitis. Acute Charcot foot. Patient currently receiving or having received radiation, chemotherapy, IV antibiotic/antimicrobial agents, or growth factor therapy. Serum albumin level < 2.5 g/dL, Hb < 10.5 mg/dL, or platelet count < 100 x 109/L. Renal dialysis, immune insufficiency, platelet disorders, eating/ nutritional, haematological, collagen vascular disease, rheumatic disease, or bleeding disorders. History of peripheral vascular repair. Surgical correction (other than debridement) required for ulcer to heal. Any situation that could interfere with compliance of the study |
|
| Interventions | Experimental group: To obtain the PRP, 20 mL of blood was extracted from the patient. This blood was centrifuged to separate PRP and was administered as gel (AutoloGel®, Cytomedix, Inc, Rockville, Md) in the treatment group Control group: Wounds in the control group were treated with a saline gel (placebo) (Normlgel®,Mölnlycke Health Care, Norcross, Ga). In both groups the gel was covered with a contact layer dressing, followed by the non‐absorbent side of a foam dressing, and finally, the absorbent side of a foam dressing. Frequency of administration: twice weekly at 3‐ or 4‐day intervals. Length of treatment: 12 weeks or until the ulcer was healed. Follow‐up post‐treatment: 12 weeks |
|
| Outcomes | Primary outcome: Healing (100% epithelisation) at the end of study Secondary outcomes: Time to healing; percent change in wound area at end‐of‐study visit from baseline; percent change in wound volume at end‐of‐study visit from baseline; area closure rate per day at end‐of‐study visit; volume closure rate per day at end‐of‐study visit | |
| Notes | Funding: Not specified, but Cytomedix Inc participated and provided the machine used to centrifuge the blood for PRP preparation. Basal characteristics of the patients were similar between groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"The randomization schedule was electronically generated, blocked per investigational center, ...". "Each eligible study participant was assigned to one of two treatment groups, PRP or control, and received the next available consecutive randomization number" Comments: The randomisation schedule was electronically generated and blocked per investigational centre |
| Allocation concealment (selection bias) | Low risk | Quote:"The randomization schedule was electronically generated, blocked per investigational center, and provided to the site by the contract research organization (CRO)" Comments: The randomisation schedule was provided to the site by a contact with a contract research organisation and this was judged to be an adequate form of concealment |
| Blinding of participants (performance bias and detection bias) | Low risk | Quote: "A strategically placed drape prohibited the patient from seeing which treatment was applied to the wound. Blood was drawn from both the treatment and control patients to maintain blinding" Comments: The participants were blinded |
| Blinding of personnel (performance and detection bias) | High risk | Quote: "Each site had one designated “unblinded” person to treat the patient (also blinded) and maintain documents in a secure private area to maintain blinding of the investigator, investigative site staff, patient, sponsor, and CRO staff and monitor. This person did not participate in any other aspect of the patient’s care" Comments: No masking of care provider. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The blinded investigators and staff measured the wounds; performed all tests, assessments, and debridement; and determined wound closure" Comments: The outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total losses were 44.4%, 21 (52.5%) patients in experimental group and 11 (34.4%) patients in control group Comments: this represents a high level of loss (over 30%) |
| Selective reporting (reporting bias) | High risk | The results of prespecified outcomes in methods were not reported as "percent change in wound area at the end‐of‐study visit (EOSV) from baseline", "percent change in wound volume at the EOSV from baseline"; and "volume closure rate per day at the EOSV". The trial protocol was not sought |
Kakagia 2007.
| Methods | Design: Randomised controlled trial Number of participant centres: 1 Setting: Hospital Country: Greece Unit of randomisation: the patient Unit of analysis: the patient Follow‐up: 8 weeks |
|
| Participants | Number randomised (patients): 51 Wound aetiology: Diabetic Age: 58‐61 years Sex: 29 F/22 M Inclusion criteria: Diabetic patients with skin wounds that had been present for at least 3 months, > 2.5 cm2 of area after debridement. Exclusion criteria: Previous treatment with vacuum, hyperbaric oxygen immunosuppressive agents, radiation or growth factors; anaemia; cellulitis; venous stasis; pulses < 40; osteomyelitis; malignancy in the wound; difficult to follow‐up |
|
| Interventions | Group I: Protease‐modulating matrix (Promogran®); Group II: PRP; Group III: protease‐modulating matrix plus PRP. The PRP was prepared by the Gravitational Platelet Separation System. All ulcers were sharply debrided prior to the application of study treatment and covered by vapour permeable film (Tegaderm® 3M) after the application of study treatment. The ulcers were assessed weekly. Length of treatment: not specified | |
| Outcomes | Primary outcomes: Percentage change in ulcer dimensions (length, width and depth) with respect to baseline Secondary outcomes: Complete healing at 8 weeks | |
| Notes | We extracted only the data for the groups receiving protease‐modulating matrix alone (Group I) and protease‐modulating matrix with PRP (Group III). The change in wound dimension data were wrongly analysed (they did not account for baseline values; baseline values of length, width and depth were not presented). Consequently we did not analyse these data Funding was not specified. At baseline the comparison groups had similar characteristics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At enrolment, patients were randomly assigned by the use of a random number generator to receive treatment for 8 weeks" Comments: The random sequence was generated by a computer |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias and detection bias) | Unclear risk | Not specified |
| Blinding of personnel (performance and detection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Wound dimensions were calculated in a blinded fashion and analyzed" Comments: The outcome assessor was blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients were selected but finally did not participate. Loss to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes were presented. The trial protocol was not sought |
Knighton 1990.
| Methods | Design: Randomised double‐blind placebo controlled cross‐over trial Number of participant centres: 1 Setting: Wound Healing Clínic, Department of Surgery Country: USA Unit of randomisation: the patient Unit of analysis: the ulcer Follow‐up: 16 weeks. |
|
| Participants | Number randomised (patients): 32 (16 in each group). Number of patients analysed: 13 in PRP group and 11 in control group Number of patients by wound aetiology: 10 venous diseases, 9 diabetic, 4 occlusive peripheral vascular diseases, and 1 vasculitis. One ulcer in each group affected the bone Number of ulcers assessed: 21 in the experimental group and 13 in the control group Age (mean and SD): 64 (±8) years treatment group and 62 (±10) years control group Sex: Both Inclusion criteria: Adults with a chronic skin leg ulcer, an evolution of at least 8 weeks and a normal platelet number count Exclusion criteria: Failure to follow‐up the protocol, amputation of the extremity, and extensive surgical intervention needed |
|
| Interventions | Experimental group: Autologous platelet‐derived wound healing formula added to mycrocrystalline collagen (Avitene®) Control group: placebo (platelet buffer solution added to mycrocrystalline collagen) All ulcers were sharply debrided prior to the application of study treatment. The patient applied the treatment and used a twice‐daily wound dressing protocol. The experimental treatment or placebo was applied and covered by one layer petrolatum‐impregnated gauze, followed by sized gauze sponges for 12 hours. Sulfadiazine was then applied for the next 12 hours. Length of treatment: 8 weeks |
|
| Outcomes | Total epithelialisation of the wound. Time to 100% of epithelialisation | |
| Notes | At baseline the experimental group had longer wound duration than the control group (119 weeks versus 47 weeks). The sample size was not specified in the paper. There was no mention of the effect of cluster when the unit of analysis was the ulcer and not the patient. Analysis was per protocol This clinical trial was supported by a grant from the Veterans Administration and from Cura Tech Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients were randomised by the laboratory personnel who prepared the PDWHF, using a blinded card selection process" Comments: How randomisation was generated was not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "All patients were randomised by the laboratory personnel who prepared the PDWHF, using a blinded card selection process" Comments: The allocation was centralised using a blinded card selection process and this was judged to be adequate |
| Blinding of participants (performance bias and detection bias) | Low risk | Quote: "Patients randomised to the treatment group received PDWHF added to microcrystalline collagen (Avitene®). Those randomised to the non‐treatment group received an equivalent amount of platelet buffer solution added to the same amount of microcrystalline collagen. Both preparations were identical in colour, consistency and smell" Comments: Participants were blinded |
| Blinding of personnel (performance and detection bias) | Low risk | Quote: "Patients randomised to the treatment group received PDWHF added to microcrystalline collagen (Avitene®). Those randomised to the non‐treatment group received an equivalent amount of platelet buffer solution added to the same amount of microcrystalline collagen. Both preparations were identical in colour, consistency and smell" Comments: Personnel was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients randomised to the treatment group received PDWHF added to microcrystalline collagen (Avitene®). Those randomised to the non‐treatment group received an equivalent amount of platelet buffer solution added to the same amount of microcrystalline collagen. Both preparations were identical in colour, consistency and smell" Comments: Personnel that assessed outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 25% of participants lost, 3 (18.7%) patients in the experimental group and 5 (31.2%) patients in the control group. Analysis was per protocol Comments: this represents a high level of loss (over 30%) |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes specified in methods were presented. The trial protocol was not sought |
Krupski 1991.
| Methods | Design: Randomised double‐blind placebo controlled trial Number of participant centres: 1 Setting: San Francisco Department of Veterans Affairs Medical Center Country: USA. Unit of randomisation: the patient Unit of analysis: the ulcer Follow‐up: 12 weeks |
|
| Participants | Number randomised (patients): 18 (10 in experimental group and 8 in control group) Number ulcers: 26 Wound aetiology: Mixed 78% diabetic, 72% occlusive peripheral vascular disease, and 28% venous disease. Age (mean and SD): 66 (± 5) years treatment group and 67 (± 4.5) years control group Sex: Both Inclusion criteria: Adult men, with a chronic skin leg ulcer and an evolution of at least 8 weeks Exclusion criteria: Platelet count < 100.000/mm3; tcPO2 < 20 mmHg; infection; inability to remain non‐weight‐bearing; terminal disease; > 100 cm2 of area wound or > 50,000 mm3 in volume wound; > 3 chronic wounds; allergy; non‐compliance with the protocol |
|
| Interventions | Experimental group: PRP topical solution Control group: saline solution (placebo) every 12 hours All ulcers were sharply debrided prior to the application of study treatment. The treatment was applied every 12 hours. The experimental treatment or placebo was applied and covered by a 4 x 4 gauze and a petrolatum‐impregnated gauze, followed by a gauze wrap dressing Length of treatment: 12 weeks |
|
| Outcomes | Total epithelialisation of the wound, total wound area, wound volume, rate of healing | |
| Notes | The sample size was not specified in the paper. There was no mention of the effect of cluster when the unit of analysis was the ulcer and not the patient. Analysis was per protocol. At baseline, the wound area was larger in the placebo group than in the experimental group. The PRP group had a greater number (17 versus 9) of wounds and wound duration was longer (6.2 versus 4.3 month) than in the placebo group Supported by the Department of Veterans Affairs Research Service and a grant of Curative Technologies Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“... patients were randomized to control (placebo) or treatment (PDWHF) groups by use of a blinded card process by means of computer‐generated random numbers” Comments: The random sequence was generated by a computer |
| Allocation concealment (selection bias) | Low risk | Quote:“...patients were randomized to control (placebo) or treatment (PDWHF) groups by use of a blinded card process by means of computer‐generated random numbers” Comments: Patients were allocated to PRP or control by a "blinded card process" and this was judged to be adequate |
| Blinding of participants (performance bias and detection bias) | Low risk | Quote: "Neither patients nor care‐providers knew whether the topical solution was placebo or PDWHF until the study was terminated and participants were told which solution was used". "Placebo solution consisted of physiologic saline. The two solutions were identical in colour, consistency, and smell" Comment: Patients did not know whether the topical solution was placebo or PDWHF until the study was terminated |
| Blinding of personnel (performance and detection bias) | Low risk | Quote: "Neither patients nor care‐providers knew whether the topical solution was placebo or PDWHF until the study was terminated and participants were told which solution was used" "Placebo solution consisted of physiologic saline. The two solutions were identical in colour, consistency, and smell" Comment: Care‐providers did not know whether the topical solution was placebo or PDWHF until the study was terminated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Placebo solution consisted of physiologic saline. The two solutions were identical in colour, consistency, and smell." Comment: The outcome assessors did not know whether the topical solution was placebo or PDWHF until the study was terminated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes were reported. The trial protocol was not sought |
Li 2012.
| Methods | Design: Prospective randomised controlled trial Number of participant centres: 1 Setting: Department of Diabetic Ulcers Centre in West China Hospital of Sichuan University Country: China Unit of randomisation: the patient Unit of analysis: the ulcer Follow‐up: 12 weeks |
|
| Participants | Number randomised (patients): 117 (59 in experimental group and 58 in control group) Number ulcers: 117 Wound aetiology: diabetic ulcer Age: not reported Sex: Both (75 men/42 women) Inclusion criteria: Duration of ulcers before been admitted to hospital > 2 weeks After 2 weeks treatment in hospital, including blood sugar/blood pressure control; anticoagulation; anti‐infection; standard usual care for ulcers (debridement, drainage, pressure reduce, dressing change), there is no improvement – defined as “non‐healing diabetic ulcers” Written consent from patients/patients family |
|
| Interventions | Experimental group: Autologous platelet gel + usual care Autologous platelet gel was prepared by applying two‐level manual differential centrifugation and is mixed with thrombin‐calcium at 10:1 ratio. Autologous platelet gel treatment every 2 weeks Suile Wound Dressing changes every 3 days and photographed Control group: Usual care (debridement; a Suile Wound Dressing (Hedonist Biochemical Technologies Co. Ltd, USA); a secondary dressing. The Suile Wound Dressing was changed every 3 days and photographed) Length of treatment: 12 weeks or until ulcer healed |
|
| Outcomes | Complete healing Length of healing Expenditure Length of stay in the hospital |
|
| Notes | This paper is in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The random sequence was generated using a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not stated |
| Blinding of participants (performance bias and detection bias) | Unclear risk | Comment: Not stated |
| Blinding of personnel (performance and detection bias) | Unclear risk | Comment: Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “after the following‐up of the study, a researcher who is responsible for collecting the data (the photos) of the study did the analysis and measurements of the ulcers, using software Image J 1.46 h” Comment: The assessor was blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The data of all included patients were reported |
| Selective reporting (reporting bias) | Low risk | Comment: There were results of all variables listed in methods |
Planinsek Rucigaj 2007.
| Methods | Design: Randomised controlled trial Number of participant centres: 1 Setting: Dermatology department Country: Slovenia Unit of randomisation: the patient Unit of analysis: the patient Follow‐up: Not specified |
|
| Participants | Number randomised (patients): 10 (5 patients in each group) Number ulcers: 22 Wound aetiology: chronic venous insufficiency Age: Not reported Sex: Not reported Inclusion criteria: Patients with a venous leg ulcer Exclusion criteria: Acute wound infection and > 30 cm2 of ulcer area |
|
| Interventions | Experimental group: Autologous platelet releasate obtained from 80 mL of the patient's own blood and prepared by the GPSTM System. The product was mixed with thrombin and 1M Cl2Ca. The PRP gel was applied to the ulcer that was covered with antibiotic and collagen dressing every 2 days Control group: the ulcer was treated with antibiotic and collagen dressing with 1M Cl2Ca every 2 days All patients received compression therapy with long‐stretch therapy. Length of treatment: not specified |
|
| Outcomes | Reduction in ulcer size | |
| Notes | This is an abstract. There were no data with SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In a small randomised study..." Comments: The paper states this was randomised but gives no further detail |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants (performance bias and detection bias) | Unclear risk | There was no information in the abstract to allow a judgement to be made for this domain |
| Blinding of personnel (performance and detection bias) | Unclear risk | There was no information in the abstract to allow a judgement to be made for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information in the abstract to allow a judgement to be made for this domain |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no information in the abstract to allow a judgement to be made for this domain |
| Selective reporting (reporting bias) | Unclear risk | Outcome results were incomplete because the SD was not reported. The trial protocol was not sought |
Senet 2003.
| Methods | Design: Randomised double‐blind placebo controlled trial Number of participating centres: 1 Setting: Hospital Country: France Unit of randomisation: the patient Unit of analysis: the patient Follow‐up: 16 weeks |
|
| Participants | Number randomised (patients): 15 (8 in the experimental group and 7 in the control group) Number ulcers: 1 per patient Wound aetiology: Chronic venous leg ulcer Age (mean): 72.3 years Sex: 6 F/7 M Inclusion criteria: Adults of both sexes with chronic skin venous leg ulcers of at least 2 months duration; ulcer size between 3 and 50 cm2; established venous disease; homolateral ankle‐brachial index > 0.8 or peripheral pulses present; normal platelet count, Hb > 11g/dL and albumin > 35 g/L Exclusion criteria: pregnancy; allergy to hydrocolloid dressing; systemic diseases; treatment with cytostatics or corticosteroids; ulcers with exposed tendons or bones; infected ulcers; poor compliance with compression therapy; positive serology to lues, Hepatitis B, Hepatitis C, HIV, Human T Lymphocyte virus I and II. Diabetes if the concentration of blood glucose was > 2 g/L |
|
| Interventions | Experimental group: topical use of frozen autologous platelet suspension in saline solution Control group: saline solution (placebo) Patients received standard topical and pressure treatment. The frequency of treatment was 3 times/week at hospital. Length of treatment: 12 weeks |
|
| Outcomes | Ulcer healing, rate of ulcer healing and adverse effects. Other outcomes: local expression of the vascular endothelial growth factor; local expression of the keratocytes growth factor; local expression of the interleukin‐8; local expression of the metalloproteinase‐1 tissular inhibitor | |
| Notes | Supported by the Institut National de la Santé et de la Reserche Medicale and Coloplast. Both groups were homogeneous at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Immediately after collection and preparation of platelets, patients were randomized to receive either placebo or platelets" Comments: The paper states this was randomised but gives no further detail |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias and detection bias) | Low risk | Quote: "After the wound was cleansed with normal saline solution, the appropriate volume of either FAP or placebo was applied to the wound surface with a syringe. FAP and placebo appeared identical" Comments: The treatments (experimental and control) were of similar appearance and therefore it was judged that the participant was properly blinded |
| Blinding of personnel (performance and detection bias) | Low risk | Quote: "After the wound was cleansed with normal saline solution, the appropriate volume of either FAP or placebo was applied to the wound surface with a syringe. FAP and placebo appeared identical" Comments: The treatments (experimental and control) were of similar appearance and therefore it was judged that the care giver was properly blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After the wound was cleansed with normal saline solution, the appropriate volume of either FAP or placebo was applied to the wound surface with a syringe. FAP and placebo appeared identical" Comments: The treatments (experimental and control) were of similar appearance. The outcome assessor was judged to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of total losses was low (13.3%), 1(12.5%) patient in experimental group and 1(14.3%) patient in control group |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes were reported. The trial protocol was not sought |
Stacey 2000.
| Methods | Design: Randomised double‐blind placebo controlled trial Number of participant centres: 1 Setting: Departament of Surgery Country: Australia Unit of randomisation: the patient Unit of analysis: the patient Follow‐up: 9 months |
|
| Participants | Number randomised (patients): 86 (42 in the experimental group and 44 in the control group) Number ulcers: 1 per patient Wound aetiology: Chronic venous leg ulcer Age (median): 70 years Sex: 50 F/36 M Inclusion criteria: Adults of both sexes with demonstrated chronic venous ulcer Exclusion criteria: Any patient who did not meet the inclusion criteria |
|
| Interventions | Experimental group: growth factors obtained from autologous platelet lysate Control group: placebo Topical application 2 times a week associated with gauze and pressure dressing. Length of treatment: until ulcer healing or for a 9‐month period |
|
| Outcomes | Ulcer healing. Time to ulcer healing. Platelet growing factor and epidermic growing factor concentrations in the platelet lysate. Mitogenic ability of the platelet lysate in a fibroblast culture | |
| Notes | Both groups were homogeneous at baseline. The study was supported by The Medical Research Foundation of Western of Australia and Beiersdorf A.G. (Germany) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was by a sealed envelope system which was opened after all entry criteria were fulfilled and the patient had given informed consent" Comments: How the randomisation sequence was generated was not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was by a sealed envelope system which was opened after all entry criteria were fulfilled and the patient had given informed consent" Comments: The allocation concealment was by sealed envelope, although this was not described as an opaque and sequentially sealed envelope we have judged this to be adequate |
| Blinding of participants (performance bias and detection bias) | Unclear risk | Quote: "The aim of this study was to undertake a double blind placebo‐controlled trial...." Comment: The process of intervention concealment to participants was not specified |
| Blinding of personnel (performance and detection bias) | Unclear risk | Quote: "The patients were attended twice weekly for application of either platelet lysate or placebo and for replacement of dressings and bandages" Comment: The process of intervention concealment was not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All patients had their leg ulcers treated at the leg ulcer clinic at Fremantle Hospital. They attended twice weekly for application of either platelet lysate or placebo and for replacement of dressings and bandages" Comment: It is unknown if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses in both groups were 12.8%, 5 (11.9%) patients in the experimental group and 6 (13.6%) in the control group |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes specified in methods are given. The trial protocol was not sought |
Weed 2004.
| Methods | Design: Randomised double‐blind placebo controlled trial Number of participant centres: 1 Setting: Dermatology department Country: USA Unit of randomisation: the patient Unit of analysis: the patient Follow‐up: 24 weeks |
|
| Participants | Number randomised (patients): 26 (15 experimental group and 11 control group) Number of ulcers assessed: 1 per patient Wound aetiology: Mixed 9; multifactorial 7; neurotrophic 5; venous ulcers 3; traumatic 1; idiopathic and 1 pressure ulcer Age (mean and SD): 67.6 (11.9) years (experimental group) and 57.8 (18.2) years (placebo group) Sex: 11 F/15 M Inclusion criteria: Adults of both sexes with a chronic skin leg ulcer and an evolution of at least 8 weeks. Arterial, venous, neuropathic or vascular (small‐vessel) ulcers. Hb > 9.0g/dL and a platelet count > 100 x 109/L Exclusion criteria: Angina pectoris; symptomatic hypotension; myocardial infarction; class III or IV congestive heart failure; clotting function disorders; or a platelet count < 100 x 109/L; osteomyelitis; wounds caused by burns or irradiation; wounds > 100 cm2; and pregnancy or lactation |
|
| Interventions | Experimental group: Autologous platelet lysate combined with collagen Control group: Platelet‐poor plasma combined with collagen (placebo group) for the first 12 weeks of therapy. After 12 weeks, there was a washout period of two weeks. During this washout period, patients applied only normal saline‐moistened gauze twice‐daily to their ulcerations. Patients whose ulcers had not healed were then assigned to receive whichever treatment they had not received in the previous 12 weeks. Patients were instructed to apply the product in a thin layer over the entire surface of the wound. Xeroform gauze was applied in a double layer over the platelet product, and a sterile gauze dressing was placed over this. The entire wound site was covered with a gauze wrap Length of treatment: Twice a day for 12 weeks |
|
| Outcomes | Complete healing (100% epithelialisation). Rate of wound healing (ulcer surface depending on the duration of the treatment) | |
| Notes | Funding was not specified. Originally, this study was designed to include a higher number of patients: "This study was originally designed to accrue 40 patients in each group; the actual number of patients enrolled in the study was small and the study was not powered to detect significance. The study had to be terminated prematurely because of the difficulty of enrolling patients." At baseline, the experimental group was older than the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to receive either platelet lysate product mixed with collagen (the treatment group) or platelet‐poor plasma mixed with collagen (the placebo group) for the first 12 weeks of therapy" Comments: How the allocation sequence was generated is not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias and detection bias) | Low risk | Quote: "Plasma and platelet lysate products were indistinguishable in physical appearance (straw‐coloured material)." "The placebo product was composed of platelet‐poor plasma added to collagen. This placebo product looked, smelled, and behaved like the autologous platelet lysate product. Both products were packaged and prepared identically (i.e. freezing technique)" Comments: It was judged that the participants were likely blinded to the intervention because both products were similar |
| Blinding of personnel (performance and detection bias) | Low risk | Quote: "Plasma and platelet lysate products were indistinguishable in physical appearance (straw‐coloured material)." "The placebo product was composed of platelet‐poor plasma added to collagen. This placebo product looked, smelled, and behaved like the autologous platelet lysate product. Both products were packaged and prepared identically (i.e. freezing technique)" Comments: It was judged that the personnel were likely blinded to the intervention because both products were similar |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Plasma and platelet lysate products were indistinguishable in physical appearance (straw‐coloured material)." "The placebo product was composed of platelet‐poor plasma added to collagen. This placebo product looked, smelled, and behaved like the autologous platelet lysate product. Both products were packaged and prepared identically (i.e. freezing technique)" Comments: It was judged that the outcome assessors were likely blinded to the intervention because both products were similar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no exclusions after randomisation |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes were presented. The trial protocol was not sought |
F: female M: male Hb: haemoglobin IV: intravenous PDWHF: platelet‐derived wound healing formula PRGF: plasma‐rich growth factor PRP: platelet‐rich plasma SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afshari 2005 | Randomised clinical trial that assessed recombinant epidermal growth factor compared with placebo in diabetic foot ulcers |
| Aminian 2000 | Not randomised. Patient allocation was by alternation |
| Aminian 2011 | Not randomised. Patient allocation was by alternation, except in cases of two or more ulcers, in which case allocation was by lot |
| Atri 1990 | Not randomised, sequential clinical trial (a three‐month treatment period first, followed by a three‐month period with experimental treatment) |
| Carter 2011 | Observational study in chronic wounds |
| Cervelli 2012 | PRP for cosmetic or functional improvement of traumatic scars (acute wounds) |
| Chen 2010 | PRP used was allogeneic, not autologous. Not a randomised clinical trial |
| Crovetti 2004 | Not a randomised clinical trial. Three of 24 patients received autologous PRP and the others homologous PRP |
| Danielsen 2008 | The primary outcome was healing quality of the donor site in patients with a skin graft |
| Enriquez‐Vega 2012 | It is a before‐after study to assess efficacy of a single dose of 20mL to 30 mL autologous platelet‐rich plasma in ulcers of diabetic patients |
| Greppi 2011 | Allogeneic platelet gel in recalcitrant ulcers. Not controlled clinical trial |
| Hao 2010 | This non‐controlled study assessed the efficacy of PRP in healing deep II degree burns (acute wounds) |
| Holloway 1993 | This randomised clinical trial assessed an activated platelet supernatant, topical CT‐102. PRP used was homologous, not autologous |
| Jaiswal 2010 | PRP used was recombinant, not autologous |
| Jorgensen 2011 | Uncontrolled pilot study in recalcitrant chronic wounds |
| JPRN‐UMIN000009860 | This is an ongoing non‐randomised clinical trial |
| JPRN‐UMIN000015689 | This is an ongoing single‐arm non‐randomised clinical trial |
| Khandelwal 2013 | This is a randomised three‐arm study that assess recombinant PRP (rhPDGF) |
| Knighton 1986 | Not randomised clinical trial |
| Köveker 1992 | Not randomised clinical trial; compared two platelet‐derived wound healing factors (one was autologous and other was a commercial preparation) |
| Ma 2007 | This clinical trial assessed a topical recombinant human acidic fibroblast growth factor |
| Margolis 2001 | Not randomised. Retrospective case series |
| Mazzuco 2004 | Not randomised. Clinical trial controlled with a retrospective cohort of patients |
| Morimoto 2015 | This is an open‐label, non‐randomised controlled, ongoing clinical trial. Both arms will include treatment with PRP. Patients will be randomised to the gelatin sheet or the hydrocolloid dressing |
| NCT00215735 | This was a single, blind multicentric, randomised controlled trial that assessed the effect of platelet concentrate in the treatment of diabetic ulcers. This study was terminated; the results were inconclusive and not published |
| NCT00273234 | This study was stopped prior to recruitment. The reasons were the lack of financial support and that study criteria severely limited enrolment |
| NCT00338702 | This study was stopped prior to recruitment. Industry support and funding not forthcoming |
| NCT00762138 | Not randomised. Prospective, open‐label, patient registry. The primary objective is safety. The study will evaluate the incidence of hematologic and immunologic adverse events, including coagulopathies in patients with wounds to which AutoloGel TM® was applied. These adverse events can be associated with bovine thrombin when used in the preparation of PRP |
| NCT00856934 | This study assessed the efficacy of PRP in healing a skin graft donor site acute wound |
| NCT01553955 | This is an ongoing non‐randomised clinical trial, with a single group |
| NCT01639144 | This is an ongoing randomised clinical trial in acute wounds |
| NCT02071979 | This is an observational controlled study that will include 1500 patients with diabetic foot ulcers, venous ulcers, or pressure ulcers and will compare PRP with standard wound care |
| NCT02088268 | Not randomised clinical trial |
| Niezgoda 2005 | It was about Regranex (Becaplermin), a synthetic product |
| Reutter 1999 | Not randomised clinical trial |
| Saad Setta 2011 | The method of randomisation was not correct: "This study was performed on 24 patients. They were systematically randomised into two groups. The odd‐numbered patients had PRP (group I) and the even‐numbered patients had PPP (group II)" |
| Saldamalacchia 2004 | Not randomised clinical trial |
| Scevola 2010 | PRP used was allogeneic, not autologous |
| Soula 2012 | This is a phase I/II clinical trial to compare the efficacy and safety of BioChaperone PDGF‐BB (a growth factor) versus becaplermin gel (synthetic product) administered once a day in patients with diabetic foot ulcer |
| Steed 1996 | PRP used was homologous, not autologous |
| Steed 1992 | Randomised clinical trial. PRP used was homologous, not autologous |
| Steed 1993 | PRP used was homologous, not autologous |
| Steinbaum 1994 | The experimental group was treated with autologous fibronectin cryoprecipitate |
| Sánchez 2007 | Not randomised. Case‐control study. Application of autologous platelet‐rich during Achilles tendon surgery |
| Tarpila 1998 | Not randomised clinical trial |
PRP: platelet‐rich plasma
Characteristics of studies awaiting assessment [ordered by study ID]
Obolenskiy 2014.
| Methods | Design: Controlled clinical trial |
| Participants | Number included patients: 81 (Group 1 experimental: 44 and Group 2 control: 37) Wound aetiology: chronic wounds of various aetiologies Age (mean and SD): adults Inclusion criteria: Adults of both sexes with chronic wounds of various aetiologies |
| Interventions | Experimental group: Autologous platelet gel Control group: Conventional treatment |
| Outcomes | Time to complete wound re‐epithelialisation Mean inpatient hospital duration Direct cost of treatments |
| Notes |
Serra 2014.
| Methods | Design: Randomised controlled trial Unit of randomisation: the patient |
| Participants | Number randomised (patients): 162 (Group 1 experimental: 87 and Group 2 control: 75) Wound aetiology: lower limbs ulcers (venous, arterial, diabetic) Age (mean and SD): adults Sex: 93 F/69 M Inclusion criteria: Adults of both sexes with chronic with lower limbs ulcers (venous, arterial, diabetic) with a duration of more than six weeks |
| Interventions | All patients undergo autologous skin grafting procedure Experimental group: Autologous platelet gel Control group: Conventional treatment |
| Outcomes | Healing time after skin grafting procedure |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐00000325.
| Trial name or title | A prospective, randomised, controlled trial of autologous platelet‐rich plasma gel to treat refractory dermal ulcer |
| Methods | Randomised controlled study |
| Participants | Country: China. Number randomised (patients): 100 Wound aetiology: Mixed Age: 18 years and older Sex: male and female Inclusion criteria:18 years or older. Standard therapy of dermal ulcer for 2 to 6 weeks, was ineffective. ABI < 0.6 or good blood supply to the ulcer. Fasting blood glucose < 8 mmol/L. Postprandial blood glucose < 11 mmol/L. Infection or osteomyelitis well controlled. Bood pressure < 160/90 mmHg. No use of immunosuppressor or its dosage maintained in recent three weeks. No severe heart, lung, liver or renal dysfunctions, blood or psychological disease. The patient accepts to participate and sign the information consent form Exclusion criteria: Diabetic acute complications. Severe infection or uncontrolled osteomyelitis. Pregnant and lactating women. Allergic history to several drugs. Myocardial infarction, arrhythmia or cerebral infarction in recent 3 to 6 months, cardiac dysfunction, COPD, or hepatocirrhosis. Ulcer caused by malignant tumour. The patient received radiotherapy, chemotherapy, immunosuppressant or overdose of glucocorticoid therapy in recent 3 weeks. Anaemia (Hb < 90 g/L), thrombocytopenia, platelet count < 100×10^9/L, leukaemia; psychological disease. Poor compliance. The patient took part in other new drug clinical trials in recent 3 months |
| Interventions | Experimental: PRP gel Comparator: Standard treatment consists of daily topical washing, cleaning, debridement and dressing changing of the wounds |
| Outcomes | Primary outcome: Variation of ulcer area and volume Secondary outcomes: Blood and urine routine examination, blood glucose levels, lipid profiles, bacterial culture on ulcer, and HbA1c |
| Starting date | January 2007 |
| Contact information | Wang Chun; Department of Endocrinology and Metabolism, West China Hospital, Guoxue Lane 37#, Chengdu, Sichuan; snoopywc@163.com |
| Notes |
IRCT2014060415574N3.
| Trial name or title | Effect of platelet dressing on the healing of diabetic foot ulcers compared with routine dressing in patients with diabetic foot ulcers Shahid Beheshti Hospital |
| Methods | Open‐label, parallel‐group, randomised study |
| Participants | Country: Iran Number randomised (patients): 60 Wound aetiology: diabetic foot ulcers Age: 18 year and older Sex: male and female Inclusion criteria: Diabetic patients with foot ulcers who entered is stage 1 or 2 foot ulcer. Platelet count equal to or greater than 100,000. Haemoglobin equal or greater 10 g/dL Exclusion criteria: Use of medications that suppress the immune system. Clotting problems. Signs of sepsis |
| Interventions | Experimental: After preparing the PRP, 5 cc of it will be used for wound dressing using a 5 cc syringe we pour the PRP on the sterile gauze ant put it on the wound covering with sterile gauze. This PRP dressing will remain for 3 days on the wound Comparator: After debridement, the wound will be irrigated with normal saline and sterile gauze dressing. Dressing change daily Wounds will be evaluated on days 7, 14 and 21 by a digital camera that gets pictures from the wounds from 30 cm distance |
| Outcomes | Primary outcome: Platelet dressing Secondary outcomes: Depth of wound. Duration of wound healing. Wound area. Wound healing Timepoint of all measures: Days 1, 7, 14 and 21. Method of measurement: by checklist |
| Starting date | 19/11/2014 |
| Contact information | Mohammad Afshar; afshar_m_1343@yahoo.com; mohammad.afshar@modares.ac.ir |
| Notes | Founding: Vice chancellor for research, Kashan University of Medical Sciences |
ISRCTN84928077.
| Trial name or title | Randomised controlled trial of platelet rich plasma biotherapies in the management of adult patients with recalcitrant and slow healing wounds following major trauma |
| Methods | Randomised multicentre controlled study |
| Participants | Country: UK Number randomised (patients): 100 Wound aetiology: recalcitrant and slow healing wounds following major trauma Age: 18 years and older Sex: Male and female Inclusion criteria: Adults (male and female patients) over 18 years of age. Patients with slow healing wounds and patients with wounds that have not healed within 28 days of the initial injury Exclusion criteria: Patients do not consent to participation or refuse to donate blood for the PRP gel treatment |
| Interventions | Experimental: 1. Autologel autologous PRP gel plus bovine thrombin until > 90% wound closure is achieved 2. Angel autologous PRP gel plus autologous thrombin until > 90% wound closure is achieved Total anticipated duration of the PRP treatments will be 10 weeks. However the Standard Advanced Wound Care may very well exceed this Comparator: Standard Advanced Wound Care Follow‐up for all treatment will be the same and is as per the standard care pathway for complex wounds involving a multicentre approach |
| Outcomes | Primary outcome: Time to 90% wound closure as measured by 3D photography. The wounds will be monitored on a weekly basis Secondary outcomes: 1. Quality of life using the SF‐36 health survey (this is the key secondary outcome) 2. Number of treatment 'deferrals' (i.e. temporary rejections) of donors due to low haemoglobin and other factors 3. Markers of platelet concentration, leucocyte levels within the PRP Biotherapies 4. Cognitive ability (reasoning, attention and memory) 5. Levels of physical activity 6. Cost‐effectiveness 7. Donor attitudes, beliefs and values The wounds will be monitored on a weekly basis using 3D photographic measurement. Secondary outcomes regarding wound infection and antibiotic usage will be monitored on a monthly basis |
| Starting date | 31/10/2013 |
| Contact information | Steven Jeffery. The Queen Elizabeth Hospital Mindelsohn Way Edgbaston, B15 2TH, Birmingham, United Kingdom |
| Notes | Founding: National Institute for Health Research (NIHR) (UK) ‐ Efficacy and Mechanism Evaluation (EME) Programme, Ref 13/55/99 |
JPRN‐UMIN000004840.
| Trial name or title | Clinical study for the treatment of chronic wounds using platelet‐rich plasma |
| Methods | Randomised parallel controlled study |
| Participants | Country: Japan Number randomised (patients): 20 Wound aetiology: Pressure ulcer, foot ulcer Age: 20 years and older Sex: Male and female Inclusion criteria: Chronic or unstable skin ulcer. Written informed consent by patient themselves Exclusion criteria: presence of anaemia, thrombocytopenia or local infection |
| Interventions | Experimental: Local injection of PRP together with standard therapy using ointment and dressing materials Comparator: Standard therapy using ointment and dressing materials |
| Outcomes | Primary outcome: Period for complete wound closure Secondary outcomes: If complete wound closure is unsuccessful, other evaluation will be: wound condition, wound area, formation of granulation tissue, epithelialisation of wound margin, etc |
| Starting date | 1/1/2011 |
| Contact information | Jiro Maegawa; Kazunori Yasumura, burngdy@hotmail.com |
| Notes | Funding: Yokohama City University Hospital (Japan) |
NCT00658983.
| Trial name or title | Autologous platelet enriched gel versus metalloproteinase inhibitor in the healing of chronic lower leg ulcers |
| Methods | Randomised, open‐label, parallel assignment, efficacy study |
| Participants | Country: Belgium. Number randomised (patients): 20 Wound aetiology: Mixed. Age: 18 years and older. Sex: male and female Inclusion criteria: 18 years or older. A non‐healing chronic lower leg ulcer. Platelet ranges of 150,000 per mL circulating blood Exclusion Criteria: Presence of a tumour or metastatic disease. Hypersensitive to collagen regenerated cellulose. Hemodynamic unstable patient. Hypercoagulability. Heart decompensation or angina pectoris |
| Interventions | Experimental: Autologous Platelet Enriched Gel Active Comparator: Metalloproteinase Inhibitor (Promogran) |
| Outcomes | Wound healing (time frame: 6 weeks) |
| Starting date | April 2008 |
| Contact information | Wim Bongaerts, MD; University Hospital Ghent Willem.bongaerts@ugent.be |
| Notes | Colaborators: Medtronic and Johnson & Johnson |
NCT02209662.
| Trial name or title | Safety and efficacy study of APIC‐PRP in non‐healing diabetic foot ulcers |
| Methods | Multicentre, randomised, double‐blind (subject, caregiver, investigator, outcomes assessor) controlled study |
| Participants | Country: USA Number randomised (patients): 274 Wound aetiology: Diabetic food ulcer Age: 18 year and older. Sex: Male and female. Inclusion criteria: Age > 18 years old at the time the informed consent is signed. Diabetes type I or II). Subjects will have only one diabetic foot ulcer on the target limb (referred to as the index ulcer). Debrided ulcer size between 1 cm2 and 4 cm2. Ulcer duration ≥ 1 month at first visit and free of clinical signs of infection. Subject has adequate circulation to the study foot as evidenced by a Doppler measured ABI of ≥ 0.7 after 10 minutes of rest Exclusion criteria: Hemoglobin of less than 12 g/dL. Ulcer has increased in size by > 50% or ulcer healed by 25% or more during the run‐in screening period. History of bleeding disorder. Any malignancy other than non‐melanoma skin cancer requiring treatment with immunosuppressive or chemotherapeutic agents, radiotherapy or corticosteroids less than 30 days before enrolment. Subject has gangrene present on any part of the affected limb. Ulcer is over a Charcot deformity of the mid‐foot or over the tarsal bones‐talus, distal calcaneus, navicular, and cuboid. Severe malnutrition or with Acquired Immunodeficiency Syndrome (AIDS), liver disease, aplastic anaemia, scleroderma, malignancy, cellulitis, suspected osteomyelitis or other evidence of systemic infection, or is Human Immunodeficiency Virus (HIV)‐positive. Subject is on dialysis |
| Interventions | Experimental: Cytonics Autologous Platelet Integrated Concentration (APIC‐PRP) plus standard of care Comparator: Placebo (saline) plus standard of care |
| Outcomes | Primary outcome: Complete wound closure within 12 weeks Secondary outcomes: Improvement in wound healing trajectory of diabetic food ulcer over the 12‐week treatment period between the APIC‐PRP + standard of care groups and standard of care alone groups |
| Starting date | October 2014 |
| Contact information | Gaetano J Scuderi. Cytonics Corporation |
| Notes | Funding: Cytonics Corporation |
NCT02213952.
| Trial name or title | Feasibility, potential efficacy and safety of autologous platelet‐rich plasma in the treatment of vascular venous ulcers in primary care (phase I and II pilot study) ‐ PRP in vascular ulcers in primary care |
| Methods | Phase III, open‐label, parallel‐group, multicentre, randomised study |
| Participants | Country: Spain Number randomised (patients): 150 Wound aetiology: Vascular venous ulcer Age: 18 years and older Sex: Male and female Inclusion criteria: Between 40 and 100 years of age with an at least 2‐month history of a vascular venous ulcer Exclusion criteria: ABI below 0.8 or above 1.5. Chronic use of immunosuppressants or antiretroviral drugs. Patients with syphilis, Hepatitis B, Hepatitis C and HIV. Active infection or fever at the beginning of the study. Clotting disorders. Chronic infectious diseases. History of cancer. Treatment with radiotherapy or chemotherapy |
| Interventions | Experimental: Autologous PRP; one heal per week during the two months of treatment Comparator: Current treatment |
| Outcomes | Primaries outcomes: Reduction of the ulcer at baseline and 5 and 9 weeks after starting the treatment. The area of the wound will be compared between baseline, after a month and two months of treatment, measuring the area in cm2 by image processing of photographs of the ulcer using ImageJ software Secondary outcomes: Reduction in pain, percentage of infected ulcers and wound edge. Quality of live using the CIVIQ |
| Starting date | 17 December 2012 |
| Contact information | Natalia Burgos Alonso. Unidad de investigacion de atencion Primaria de Bizakaia. Spain natalia.burgosalonso@osakidetza.net |
| Notes | Founding. Spanish Carlos III Health Institute and Department of Health and Consumer Affairs of the Government of the Basque Country This clinical trial is also registered in clinicaltrial.gov (NCT02213952 Title: PRP ULCERAS: Clinical Trial Phase III (PRPULCERAS)) |
NCT02307448.
| Trial name or title | Effectiveness of autologous platelet rich plasma in the treatment of chronic non‐healing wounds |
| Methods | Randomised single‐blind (outcomes assessor) multicentre study |
| Participants | Country: USA Number randomised (patients): 1500 Wound aetiology: Diabetic foot ulcers, venous ulcers, or pressure ulcers Age: 18 years and older Sex: Male and female Inclusion criteria: Medicare eligible. Written informed consent. Male or female ≥ 18 years of age. Duration of diabetic foot ulcers, venous ulcers, or pressure ulcers is greater than 30 days at first visit/patient screening. Classified as Wagner 1‐3 on the Wagner classification system. The ulcer must be clinically non‐infected Exclusion Criteria: Patients with known sensitivity to components of the PRP kit. Presence of non‐treated osteomyelitis. Received systemic corticosteroids or immunosuppressive agents, electrostimulation, growth factors, or any cell or tissue‐derived products for wounds during the 30 days preceding the screening visit Received radiation therapy or chemotherapy within previous 3 months. Charcot foot. Patients with thrombocytopenia < 50,000 platelets/µL. Wounds smaller than 2 cm will be excluded. Minimum Hgb/HCT level: Hgb 9 g/dL Hct 27%. Min/max ABIs: Min = 0.7, No maximum |
| Interventions | Experimental: PRPa. Patients will receive weekly PRP treatments with standard of care Comparator: Standard of care. Patients will receive weekly standard of care |
| Outcomes | Primary outcome: The primary objective of this trial is to evaluate increasing the proportion of wounds with complete closure within 20 weeks of initial treatment |
| Starting date | January 2015 |
| Contact information | Study Director: Todd Shaffett, APRN, FNP‐C |
| Notes | Sponsor:ACR Biologics, LLC |
NCT02312518.
| Trial name or title | Clinical Trial of ECLIPSE PRP™ Wound Biomatrix in chronic non‐healing venous leg ulcers |
| Methods | Randomised single‐blinded (outcomes assessor) multicentre study |
| Participants | Country: USA Number randomised (patients): 250 Wound aetiology: Venous leg ulcers Age: 18 years and older Sex: Male and female Inclusion criteria: subjects ≥ 18 years of age with chronic non‐healing venous leg ulcers (greater than 4 weeks duration). Three or fewer ulcers that are separated by > 3.0 cm distance. Post‐debridement, the ulcer size must be between 2 cm2 and 200 cm2. Demonstrated adequate compression regimen. Able and willing to attend scheduled follow‐up visits and study related exams. Able and willing to provide a voluntary written informed consent Exclusion criteria: No venous ulcers. Greater than 30% reduction in wound size during the first two weeks of observation and treatment by the investigator. Gross clinical infection at the study ulcer site. Known allergy tor sensitivity to Eclipse PRP kit components. Plasma platelet count of less than 100 x 109/L, or Hemoglobin of less than 10.5 g/dL, known renal failure, rheumatoid arthritis, vasculitis, sickle cell disease, HIV severe liver disease, presence of additional abnormal lab values, radiation therapy, chemotherapy, immunosuppressive therapy or chronic steroid use within 30 days of enrolment |
| Interventions | Experimental: Eclipse PRP™ Wound Biomatrix Comparator: usual and customary practice |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | January 2015 |
| Contact information | Damon Keeky: dk@hemoconcepts.com |
| Notes | Sponsor:PRP Concepts, LLC |
ABI: ankle‐brachial index COPD: Chronic obstructive pulmonary disease Hb: haemoglobin PRP: platelet‐rich plasma
Differences between protocol and review
In the protocol, we stated that we would consider risk of bias based on sequence generation, allocation concealment, blinding (participants, clinicians, outcome assessors), and withdrawals. However, in the review we have reflected the current guidance in the Cochrane Handbook for Systematic Reviews of Interventions and have added 'incomplete outcome data' and 'selective reporting' to reflect this (Higgins 2011a).
We included the percentage of wound area healed, a secondary outcome that we did not prespecify in the protocol, as we judged it to be clinically important.
We excluded the secondary outcome percentage of change in healed wound width, length or depth. The reason of this exclusion has been to avoid multiple testing of healing outcomes.
In addition to the searches planned in the protocol, we also searched for ongoing studies in the database, International Clinical Trials Registry Platform (ICTRP): http://www.who.int/ictrp/network/en/, that includes clinicaltrial.gov and other clinical trial registry databases.
In this up‐to‐date review we have added the quality of evidence and a 'Summary of findings' table with GRADE ratings.
Contributions of authors
Maria Jose Martinez‐Zapata: Conceived, designed and co‐ordinated the review. Selected the studies, extracted data and checked the quality of data extraction, undertook quality assessment, analysed or interpreted data, checked quality assessment, performed part of data analysis or interpretation, and performed statistical analysis. Completed the first draft, performed previous work that was the foundation of the current review, performed part of writing or editing, made an intellectual contribution to, and advised on the review, secured funding, and wrote to study author/experts/companies. Approved final review prior to submission and acted as guarantor of the review.
Arturo Marti‐Carvajal: Designed the review, extracted data, checked the quality of data extraction, undertook quality assessment, analysed or interpreted data, checked quality assessment, performed part of data analysis or interpretation, checked the quality of statistical analysis, and performed part of writing or editing of the review. Made an intellectual contribution, performed previous work that was the foundation of the current review, wrote to study author/experts/companies, advised on, and approved the final review prior to submission.
Carlos Zaror selected the studies, extracted data, and undertook quality assessment in the current update.
Jose‐Angel Exposito: Performed part of writing or editing, advised on part of the review, and approved the final review prior to submission.
Ivan Sola: Advised on part of the review and approved final review prior to submission.
Luciano Rodriguez: Performed part of writing or editing, advised on part of the review, and approved the final review prior to submission. Joan Garcia: Performed part of writing or editing, advised on part of the review, and approved the final review prior to submission.
Ignasi Bolibar: Advised on part of the review and approved final review prior to submission.
Contributions of editorial base
For the first version of this review:
Nicky Cullum: Edited the review, advised on methodology, interpretation, and review content. Approved the final review prior to publication.
Sally Bell‐Syer: Co‐ordinated the editorial process. Advised on methodology, interpretation, and content. Edited and copy‐edited the review.
Ruth Foxlee: Designed the search strategy, ran the searches, and edited the search methods section.
For this update:
Nicky Cullum: Edited the review, advised on methodology, interpretation, and review content. Approved the final review prior to publication.
Gill Rizzello: Co‐ordinated the editorial process. Edited the review.
Rocio Rodriguez‐Lopez: Ran the searches.
Reetu Child: Checked the search strategies and methods section.
Sources of support
Internal sources
Iberoamerican Cochrane Center (Barcelona). CIBER de Epidemiología y Salud Pública (CIBERESP), Spain.
External sources
-
PI0590173, Spain.
Spanish Agency of Health Technology Assessment. Institute of Health Carlos III
-
National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health
-
CONICYT, Chile.
- Higher educational program. Government of Chile, Project number 80140042. 2014‐15
-
Instituto de Salud Carlos III, Spain.
Dr. Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP15/00116).
Declarations of interest
Maria José Martinez‐Zapata: None.
Arturo J Martí‐Carvajal: Arturo J Martí‐Carvajal was employed in by Eli Lilley in 2004 and Merck in 2007 to run workshops on the critical appraisal of clinical trials. This activity was not related to his work with Cochrane or any Cochrane review.
Ivan Solà: None.
José Angel Expósito: None.
Ignasi Bolíbar: None.
Luciano Rodríguez: None.
Joan Garcia: None.
Carlos Zaro: None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anitua 2008 {published data only}
- Aguirre JJ, Francisco S, Cabezas A, Calleja A, Ayerdi E, Pena MA, et al. Randomized open‐label controlled pilot trial to evaluate the effectiveness of PRGF in the treatment of chronic cutaneous ulcers: results at 4 weeks of follow‐up. [Ensayo clínico piloto, aleatorizado, en grupos paralelos, ciego y controlado con tratamiento convencional para evaluar la eficacia de plasma rico en factores de crecimiento en el tratamiento de las úlceras cutáneas: resultados a las 4 semanas de seguimiento]. XIX Congreso de la Sociedad Española de Farmacología Clínica 2004:Abstract 19.
- Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. Journal of Biomedical Materials Research ‐ Part B Applied Biomaterials 2008;84(2):415‐21. [DOI] [PubMed] [Google Scholar]
Driver 2006 {published data only}
- Driver VR, Hanft J, Fylling CP, Beriou JM, Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet‐rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Management 2006;52(6):68‐87. [PubMed] [Google Scholar]
Kakagia 2007 {published data only}
- Kakagia D, Kazakos K, Xarchas K, Karanikas M, Georgiadis G, Tripsiannis G, et al. Synergistic action of protease‐modulating matrix and autologous growth factors in healing diabetic foot ulcers. A prospective randomized trial. Journal of Diabetes and its Complications 2007;21(6):387‐91. [DOI] [PubMed] [Google Scholar]
Knighton 1990 {published data only}
- Knighton DR, Ciresi K, Fiegel VD, Schumerth S, Butler E, Cerra F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet‐derived wound healing formula. Surgery, Gynecology and Obstetrics 1990;170(1):56‐60. [PubMed] [Google Scholar]
- Knighton DR, Doucette M, Fiegel VD, Ciresi K, Butler E, Austin L. The use of platelet derived wound healing formula in human clinical trials. Progress in Clinical and Biological Research 1988;266:319‐29. [PubMed] [Google Scholar]
Krupski 1991 {published data only}
- Krupski WC, Reilly LM, Perez S, Moss KM, Crombleholme PA, Rapp JH. A prospective randomized trial of autologous platelet‐derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. Journal of Vascular Surgery 1991;14(4):526‐32. [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li L, Wang C, Wang Y, He LP, Yang YZ, Chen LH, et al. Impact of topical application of autologous platelet‐rich gel on medical expenditure and length of stay in hospitals in diabetic patients with refractory cutaneous ulcers. Sichuan Da Xue Xue Bao Yi Xue Ban 2012;43(5):762‐5. [PubMed] [Google Scholar]
Planinsek Rucigaj 2007 {published data only}
- Planinsek Rucigaj T, Lunder T. Stimulation of venous leg ulcers with thrombocytic growth factors: A randomised study. Proceedings of the 17th Conference of the European Wound Management Association. Glasgow, UK: European Wound Healing Association, 2007:Abstract 93.
Senet 2003 {published data only}
- Senet P, Bon FX, Benbunan M, Bussel A, Traineau R, Calvo F. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. Journal of Vascular Surgery 2003;38(6):1342‐8. [DOI] [PubMed] [Google Scholar]
Stacey 2000 {published data only}
- Stacey MC, Mata SD, Trengove NJ, Mather CA. Randomised double‐blind placebo controlled trial of topical autologous platelet lysate in venous ulcer healing. European Journal of Vascular and Endovascular Surgery 2000;20(3):296‐301. [DOI] [PubMed] [Google Scholar]
Weed 2004 {published data only}
- Weed B, Davis MDP, Felty CL, Liedl DA, Pineda AA, Moore SB, et al. Autologous platelet lysate product versus placebo in patients with chronic leg ulcerations: a pilot study using a randomized, double‐blind, placebo‐controlled trial. Wounds: A Compendium of Clinical Research and Practice 2004;16(9):273‐82. [Google Scholar]
References to studies excluded from this review
Afshari 2005 {published data only}
- Afshari M, Larijani B, Fadayee M, Darvishzadeh F, Ghahary A, Pajoui M, et al. Efficacy of topical epidermal growth factor in healing diabetic foot ulcers. Therapy 2005;2(5):759‐65. [Google Scholar]
Aminian 2000 {published data only}
- Aminian B, Shams M, Soveyd M, Omrani GR. Topical autologous platelet‐derived growth factors in the treatment of chronic diabetic ulcers. Archives of Iranian Medicine 2000;3(2):55‐9. [Google Scholar]
Aminian 2011 {published data only}
- Aminian B, Shams M, Karim‐Aghaee B, Soveyd M, Omrani GR. Tha role of autologous derived growth factor in the management of decubitus ulcers. www.ams.ac.ir/AIM/9922/aminian9922.html (accessed 2 March 2016).
Atri 1990 {published data only}
- Atri SC, Misra J, Bisht D, Misra K. Use of homologous platelet factors in achieves total healing of recalcitrant skin ulcers. Surgery 1990;108(3):508‐12. [PubMed] [Google Scholar]
Carter 2011 {published data only}
- Carter MJ, Fylling CP, Li WW, Leon J, Driver VR, Serena TE, et al. Analysis of run‐in and treatment data in a wound outcomes registry: Clinical impact of topical platelet‐rich plasma gel on healing trajectory. International Wound Journal 2011;8 (6 ):638‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cervelli 2012 {published data only}
- Cervelli V, Nicoli F, Spallone D, Verardi S, Sorge R, Nicoli M, et al. Treatment of traumatic scars using fat grafts mixed with platelet‐rich plasma, and resurfacing of skin with the 1540 nm nonablative laser. Clinical and Experimental Dermatology 2012 ;37 (1 ):55‐61. [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen TM, Tsai J‐C, Burnouf T. A novel technique combining platelet gel, skin graft, and fibrin glue for healing recalcitrant lower extremity ulcers. Dermatologic Surgery 2010;36(4):453‐60. [DOI] [PubMed] [Google Scholar]
Crovetti 2004 {published data only}
- Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, et al. Platelet gel for healing cutaneous chronic wounds. Transfusion and Apheresis Science 2004;30:145–51. [DOI] [PubMed] [Google Scholar]
Danielsen 2008 {published data only}
- Danielsen P, Jorgensen B, Karlsmark T, Jorgensen LN, Agren MS. Effect of topical autologous platelet‐rich fibrin versus no intervention on epithelialization of donor sites and meshed split‐thickness skin autografts: a randomized clinical trial. Plastic and Reconstructive Surgery 2008;122(5):1431‐40. [DOI] [PubMed] [Google Scholar]
- Danielsen P, Jorgensen B, Karlsmark T, Jorgensen LN, Agren MS. Effects of locally applied autologous platelet‐rich fibrin on split thickness skin graft donor sites. Proceedings of the 16th Conference of the European Wound Management Association; Prague, Czech Republic; 18‐20 May, 2006. Poster 083. Prague.
Enriquez‐Vega 2012 {published data only}
- Enriquez‐Vega ME, Bobadilla‐Flores NO, Rodriguez‐Jimenez OA, Guerra‐Marquez A, Carrasco‐Nava L, Varela‐Silva J. Platelet rich plasma for the treatment of ischemic ulcers in diabetic patients. Revista Mexicana de Angiologia 2012;40(2):51‐6. [Google Scholar]
Greppi 2011 {published data only}
- Greppi N, Mazzucco L, Galetti G, Bona F, Petrillo E, Smacchia C, et al. Treatment of recalcitrant ulcers with allogeneic platelet gel from pooled platelets in aged hypomobile patients. Biologicals 2011;39 (2):73‐80. [DOI] [PubMed] [Google Scholar]
Hao 2010 {published data only}
- Hao T, Zhu J, Hu W, Zhang H, Gao Z, Wen X, et al. Autogenous platelet‐rich plasma gel with acellular xenogeneic dermal matrix for treatment of deep II degree burns. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2010;24(6):647‐9. [PubMed] [Google Scholar]
Holloway 1993 {published data only}
- Holloway GA, Steed DL, DeMarco MJ, Masumoto T, Moosa HH, Webster MW, et al. A randomized,controlled dose response trial of activated platelet supernatant, topical CT‐102 in chronic, nonhealing diabetic wounds. Wounds 1993;5(4):198–206. [Google Scholar]
Jaiswal 2010 {published data only}
- Jaiswal SS, Gambhir RPS, Agrawal A, Harish S. Efficacy of topical recombinant human platelet derived growth factor on wound healing in patients with chronic diabetic lower limb ulcers. Indian Journal of Surgery 2010;72(1):31‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorgensen 2011 {published data only}
- Jorgensen B, Karlsmark T, Vogensen H, Haase L, Lundquist R. A pilot study to evaluate the safety and clinical performance of Leucopatch, an autologous, additive‐free, platelet‐rich fibrin for the treatment of recalcitrant chronic wounds. International Journal of Lower Extremity Wounds 2011;10(4):218‐23. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN000009860 {published data only}
- JPRN‐UMIN000009860. Treatment of cutaneous ulcer using platelet‐rich plasma. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000009860 (accessed 2 March 2016).
JPRN‐UMIN000015689 {published data only}
- JPRN‐UMIN000015689. An exploratory clinical trial using platelet‐rich plasma in patients with chronic skin ulcers. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000015689 (accessed 2 March 2016).
Khandelwal 2013 {published data only}
- Khandelwal S, Chaudhary P, Poddar DD, Saxena N, Singh RA, Biswal UC. Comparative study of different treatment options of grade III and IV diabetic foot ulcers to reduce the incidence of amputations. Clinical Practice 2013;3(1):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knighton 1986 {published data only}
- Knighton DR, Fiegel VD, Austin LL, Butler EL. Classification and treatment of chronic non‐healing wounds. Annals of Surgery 1986;204(3):322‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Köveker 1992 {published data only}
- Köveker G, Coerper S, Tolksdorf A, Becker HD. Clinical experience with platelet‐derived wound healing factors (PDWHF) in venous leg ulcers. Proceedings of the 5th Annual Symposium on Advanced Wound Care; 1992, 23‐25 April; New Orleans, Lousiana. 1992:63.
Ma 2007 {published data only}
- Ma B, Cheng DS, Xia ZF, Ben DF, Lu W, Cao ZF, et al. Randomized, multicenter, double‐blind, and placebo‐controlled trial using topical recombinant human acidic fibroblast growth factor for deep partial‐thickness burns and skin graft donor site. Wound Repair and Regeneration 2007;15(6):795–9. [DOI] [PubMed] [Google Scholar]
Margolis 2001 {published data only}
- Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001;24(3):483‐8. [DOI] [PubMed] [Google Scholar]
Mazzuco 2004 {published data only}
- Mazzucco L, Medici D, Serra M, Panizza R, Rivara G, Orecchia S. The use of autologous platelet gel to treat difficult‐to‐heal wounds: a pilot study. Transfusion 2004;44(7):1013‐8. [DOI] [PubMed] [Google Scholar]
Morimoto 2015 {published data only}
- Morimoto N, Kakudo N, Matsui M, Ogura T, Hara T, Suzuki K, et al. Exploratory clinical trial of combination wound therapy with a gelatin sheet and platelet‐rich plasma in patients with chronic skin ulcers: study protocol. BMJ Open 2015;5(5):e007733. [DOI: 10.1136/bmjopen-2015-007733] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00215735 {published data only}
- NCT00215735. Effect of platelet concentrate in treatment of diabetic ulcers. clinicaltrials.gov/ct2/show/NCT00215735 (accessed 2 March 2016).
NCT00273234 {published data only}
- NCT00273234. Clinical effectiveness of topical autologous platelet gel for the treatment of venous ulcers. clinicaltrials.gov/ct2/show/NCT00273234 (accessed 2 March 2016).
NCT00338702 {published data only}
- NCT00338702. A randomized, controlled trial of autologous platelet gel treatment in diabetic foot ulcers. clinicaltrials.gov/ct2/show/NCT00338702 (accessed 2 March 2016).
NCT00762138 {published data only}
- NCT00762138. The AutoloGel™ Post‐Market Surveillance (TAPS) Program. clinicaltrials.gov/ct2/show/NCT00762138 (accessed 2 March 2016).
NCT00856934 {published data only}
- NCT00856934. Effect of platelet rich plasma and keratinocyte suspensions on wound healing. clinicaltrials.gov/ct2/show/NCT00856934 (accessed 2 March 2016).
NCT01553955 {published data only}
- NCT01553955. Efficacy of training in preparation of platelet rich plasma using a standard centrifuge and manual pipetting methods. clinicaltrials.gov/show/NCT01553955 (accessed 2 March 2016).
NCT01639144 {published data only}
- NCT01639144. Use of platelet‐rich plasma (PRP) and platelet‐poor plasma (PPP) to prevent infection and delayed wound healing after elective foot and ankle surgery in healthy adults. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT016391 (accessed 2 March 2016).
NCT02071979 {published data only}
- NCT02071979. Registry trial of the effectiveness of platelet rich plasma for chronic non‐healing wounds (CMS). www.clinicaltrials.gov/ct2/show/NCT02071979?term=NCT02071979&rank=1 (accessed 2 March 2016).
NCT02088268 {published data only}
- NCT02088268. Treatment of PRP on diabetes wound. www.clinicaltrials.gov/ct2/show/NCT02088268?term=NCT02088268&rank=1 (accessed 2 March 2016).
Niezgoda 2005 {published data only}
- Niezgoda JA, Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS wound matrix to Regranex gel for diabetic ulcers. Advances in Skin and Wound Care 2005;18(5):258‐66. [DOI] [PubMed] [Google Scholar]
Reutter 1999 {published data only}
- Reutter H, Bort S, Jung MF, Klyscz T, Schippert W, Zuder D, et al. Questionable effectiveness of autologous platelet growth factors (PDWHF) in treatment of venous ulcers of the leg. Hautarzt 1999;50(12):859‐65. [DOI] [PubMed] [Google Scholar]
Saad Setta 2011 {published data only}
- Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet‐rich plasma versus platelet‐poor plasma in the management of chronic diabetic foot ulcers: a comparative study. International Wound Journal 2011;8(3):307‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saldamalacchia 2004 {published data only}
- Saldalamacchia G, Lapice E, Cuomo V, Feo E, D'Agostino E, Rivellese AA, et al. A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutrition, Metabolism, and Cardiovascular Disease 2004;14(6):395‐6. [DOI] [PubMed] [Google Scholar]
Sánchez 2007 {published data only}
- Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet‐rich fibrin matrices. American Journal of Sports Medicine 2007;35(2):245‐51. [DOI] [PubMed] [Google Scholar]
Scevola 2010 {published data only}
- Scevola S, Nicoletti G, Brenta F, Isernia P, Maestri M, Faga A. Allogenic platelet gel in the treatment of pressure sores: A pilot study. International Wound Journal 2010;7(3):184‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soula 2012 {published data only}
- Soula O, Seroussi C, Desort‐Henin V, Correia J, Soula R, Soula G, et al. Phase I/II results of innovative PDGF‐BB formulation with improved compliance at low‐cost for the treatment of diabetic foot ulcer. EWMA Journal 2012;12(2):91. [Google Scholar]
Steed 1992 {published data only}
- Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double‐blind trial in healing chronic diabetic foot ulcers. CT‐102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992;15(11):1598‐604. [DOI] [PubMed] [Google Scholar]
Steed 1993 {published data only}
- Steed DL. Growth factors in the treatment of diabetic foot ulcers. Wounds: A Compendium of Clinical Research and Practice 1993;5(2):80‐3. [Google Scholar]
Steed 1996 {published data only}
- Steed DL, Edington HD, Webster MW. Recurrence rate of diabetic neurotrophic foot ulcers healed using topical application of growth factors released from platelets. Wound Repair and Regeneration 1996;4(2):230‐3. [DOI] [PubMed] [Google Scholar]
Steinbaum 1994 {published data only}
- Steinbaum SS, Cucinell S, Hathaway TK. Effects of cryoprecipitate on the healing of chronic wounds. Military Medicine 1994;159(2):105‐8. [PubMed] [Google Scholar]
Tarpila 1998 {published data only}
- Tarpila E, Jergovic D, Sjoberg F, Bjornsson A, Abildgard L, Rosseau A. Autologous platelet‐derived wound healing factors in the treatment of leg ulcers with split‐skin grafts. European Wound Management Association Conference 1998. Harrogate, UK: European Wound Management Association, 1998:81‐5.
References to studies awaiting assessment
Obolenskiy 2014 {published data only}
- Obolenskiy VN, Ermolova A, Laberko A, Semenova TV. Efficacy of platelet‐rich plasma for the treatment of chronic wounds. European Wound Management Association Journal 2014;14(1):37‐41. [Google Scholar]
Serra 2014 {published data only}
- Serra R, Grande R, Butrico L, Montemurro R, Caridi G, Fugetto F, et al. Skin grafting and topical application of platelet gel in the treatment of vascular lower extremity ulcers. Acta Phlebologica 2014;15(3):129‐36. [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐00000325 {published data only}
- ChiCTR‐TRC‐00000325. A prospective, randomized, controlled trial of autologous platelet‐rich plasma gel to treat refractory dermal ulcer. www.chictr.org/en/proj/show.aspx?proj=1072 (accessed 2 March 2016).
IRCT2014060415574N3 {published data only}
- IRCT2014060415574N3. Effect of platelet dressing on the healing of diabetic foot ulcers compared with routine dressing in patients with diabetic foot ulcers ‐ Shahid Beheshti Hospital. www.irct.ir/searchresult.php?id=15574&number=3 (accessed 2 March 2016).
ISRCTN84928077 {published data only}
- ISRCTN84928077. Randomised controlled trial of platelet rich plasma biotherapies in the management of adult patients with recalcitrant and slow healing wounds following major trauma. isrctn.com/ISRCTN84928077 (accessed 2 March 2016).
JPRN‐UMIN000004840 {published data only}
- JPRN‐UMIN000004840. Clinical study for the treatment of chronic wounds using Platelet‐rich Plasma. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000004840 (accessed 2 March 2016).
NCT00658983 {published data only}
- NCT00658983. Autologous platelet enriched gel versus metalloproteinase inhibitor in the healing of chronic lower leg ulcers. clinicaltrials.gov/show/NCT00658983 (accessed 2 March 2016).
NCT02209662 {published data only}
- NCT02209662. Safety and efficacy study of APIC‐PRP in non‐healing diabetic foot ulcers. www.clinicaltrials.gov/ct2/show/NCT02209662?term=NCT02209662&rank=1 (accessed 2 March 2016).
NCT02213952 {published data only}
- NCT02213952. PRP ULCERAS: Clinical trial phase III (PRPULCERAS). clinicaltrials.gov/ct2/show/NCT02213952 (accessed 2 March 2016).
- San Sebastian KM, Lobato I, Hernández I, Burgos‐Alonso N, Gomez‐Fernandez M, López J, et al. Efficacy and safety of autologous platelet rich plasma for the treatment of vascular ulcers in primary care: Phase III study. BMC Family Practice 2014;15:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02307448 {published data only}
- NCT02307448. Effectiveness of autologous platelet rich plasma in the treatment of chronic non‐healing wounds. www.clinicaltrials.gov/ct2/show/NCT02307448?term=NCT02307448&rank=1 (accessed 2 March 2016).
NCT02312518 {published data only}
- NCT02312518. Clinical trial of ECLIPSE PRP™ Wound Biomatrix in chronic non‐healing venous leg ulcers. www.clinicaltrials.gov/ct2/show/NCT02312518?term=NCT02312518&rank=1 (accessed 2 March 2016).
Additional references
Anitua 2004
- Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thrombosis and Haemostasis 2004;91(1):4‐15. [DOI] [PubMed] [Google Scholar]
Barbenel 1977
- Barbenel JC, Jordan MM, Nicol SM, Clark MO. Incidence of pressure‐sores in the Greater Glasgow Health Board area. Lancet 1977;2(8037):548‐50. [DOI] [PubMed] [Google Scholar]
Bennett 2004
- Bennett G, Dealey C, Posnett J. The cost of pressure ulcers in the UK. Age and Ageing 2004;33(3):230‐5. [DOI] [PubMed] [Google Scholar]
Crovetti 2005
- Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, et al. Autologous platelet gel applications during cardiovascular surgery: effect on wound healing. Journal of Extra‐Corporeal Technology 2005;37(2):148‐52. [PMC free article] [PubMed] [Google Scholar]
de la Torre 2015
- Torre J. Chronic wounds. emedicine.medscape.com/article/1298452‐overview#a1 (accessed 2 March 2016).
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Ebbeskog 1996
- Ebbeskog B, Lindholm C, Ohman S. Leg and foot ulcer patients. Epidemiology and nursing care in an urban population in south Stockholm, Sweden. Scandinavian Journal of Primary Health Care 1996;14(4):238‐43. [DOI] [PubMed] [Google Scholar]
Edwards 2002
- Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003556.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2005
- US Dept of Health and Human Services, Food, Drug Administration (FDA). Guidance for industry: chronic cutaneous ulcer and burn wounds‐developing products for treatment. www.fda.gov/cber/guidelines.htm (accessed 2 March 2016).
Gonzalez 2000
- Gonzalez ER, Oley MA. The management of lower‐extremity diabetic ulcers. Managed Care Interface 2000;13(11):80‐7. [PubMed] [Google Scholar]
Graham 2003
- Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower‐limb ulceration: a systematic review of prevalence studies. Advances in Skin and Wound Care 2003;16(6):305‐16. [DOI] [PubMed] [Google Scholar]
Grey 2006
- Grey JE, Harding KG, Enoch S. Pressure ulcers. BMJ 2006;332(7539):472‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kaltenthaler 2001
- Kaltenhaler E, Whitfield, Walters SJ, Akehurst RL, Paisley S. UK, USA and Canada: how do their pressure ulcer prevalence and incidence data compare?. Journal of Wound Care 2001;10(1):530‐5. [DOI] [PubMed] [Google Scholar]
Knighton 1988
- Knighton DR, Doucette M, Fiegel VD, Ciresi K, Butler E, Austin L. The use of platelet derived wound healing formula in human clinical trials. Progress in Clinical and Biological Research 1988;266:319‐29. [PubMed] [Google Scholar]
Kumar 2004
- Kumar RN, Gupchup GV, Dodd MA, Shah B, Iskedjian M, Einarson TR, et al. Direct health care costs of 4 common skin ulcers in New Mexico Medicaid fee‐for‐service patients. Advances in Skin and Wound Care 2004;17(3):143‐9. [DOI] [PubMed] [Google Scholar]
Lacci 2010
- Lacci KM, Dardik A. Platelet‐rich plasma: support for its use in wound healing. Yale Journal of Biology and Medicine 2010;83(1):1‐9. [PMC free article] [PubMed] [Google Scholar]
Langedam 2013
- Langendam MW, Aki EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2003
- Langer G, Schloemer G, Knerr A, Kuss O, Behrens J. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003216] [DOI] [PubMed] [Google Scholar]
Lavery 2003
- Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non‐Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003;26(5):1435‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mao 2010
- Mao CL, Rivet AJ, Sidora T, Pasko MT. Update on pressure ulcer management and deep tissue injury. Annals of Pharmacotherapy 2010;44(2):325‐32. [DOI] [PubMed] [Google Scholar]
Marlovits 2004
- Marlovits S, Mousavi M, Gäbler C, Erdös J, Vécsei V. A new simplified technique for producing platelet‐rich plasma: A short technical note. European Spine Journal 2004;13(Suppl 1):102‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez‐Zapata 2009
- Martínez‐Zapata MJ, Martí‐Carvajal A, Solà I, Bolibar I, Angel Expósito J, Rodriguez L, et al. Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: a systematic review. Transfusion 2009;49(1):44‐56. [DOI] [PubMed] [Google Scholar]
Marx 2004
- Marx RE. Platelet‐rich plasma: evidence to support its use. Journal of Oral and Maxillofacial Surgery 2004;62(4):489‐96. [DOI] [PubMed] [Google Scholar]
Moffat 2004
- Moffat CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulcers in a London population. QJM 2004;97(7):431‐7. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
Moore 2005
- Moore ZE, Cowman S. Wound cleansing for pressure ulcers. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004983.pub2] [DOI] [PubMed] [Google Scholar]
Munirah 2007
- Munirah S, Samsudin OC, Chen HC, Sharifah Salmah SH, Aminuddin BS, Ruszymah BHI. Articular cartilage restoration in load‐bearing osteochondral defects by implantation of autologous chondrocyte‐fibrin constructs. Journal of Bone and Joint Surgery 2007;89(8):1099‐109. [DOI] [PubMed] [Google Scholar]
O'Meara 2009a
- O'Meara S, Tierney J, Cullum N, Bland JM, Franks PJ, Mole T, et al. Four layer bandage compared with short stretch bandage for venous leg ulcers: systematic review and meta‐analysis of randomised controlled trials with data from individual patients. BMJ 2009;338:b1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Meara 2009b
- O'Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000265.pub2] [DOI] [PubMed] [Google Scholar]
Reiber 1996
- Reiber GE. The epidemiology of diabetic foot problems. Diabetic Medicine 1996;13(Suppl 1):6‐11. [PubMed] [Google Scholar]
Reiber 2001
- Reiber GE. Epidemiology of foot ulcers and amputations in the diabetic foot. In: Bowker JH, Pfeifer MA editor(s). The Diabetic Foot. St Louis, Mo: Mosby, 2001:13‐32. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1993
- Robinson CJ. Growth factors: therapeutic advances in wound healing. Annals of Medicine 1993;25:535‐8. [PubMed] [Google Scholar]
Rodrigues 2006
- Rodrigues I, Mégie MF. Prevalence of chronic wounds in Quebec home care: an exploratory study. Ostomy/Wound Management 2006;52(5):46‐8, 50, 52‐7. [PubMed] [Google Scholar]
Scriven 1998
- Scriven JM, Taylor LE, Wood AJ, Bell PRF, Naylor AR, London NJM . A prospective randomised trial of four‐layer versus short stretch compression bandages for the treatment of venous leg ulcers . Annals of the Royal College of Surgeons of England 1998;80(3):215‐20. [PMC free article] [PubMed] [Google Scholar]
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed 23 May 2015).
Singh 2005
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217‐28. [DOI] [PubMed] [Google Scholar]
Spencer 2000
- Spencer S. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002302] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting bias. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Available from www.cochrane‐handbook.org.
Vas 2008
- Vas J, Modesto M, Mendez C, Perea‐Milla E, Aguilar I, Carrasco‐Lozano JM, et al. Effectiveness of acupuncture, special dressings and simple, low‐adherence dressings for healing venous leg ulcers in primary healthcare: study protocol for a cluster‐randomized open‐labeled trial. BMC Complementary and Alternative Medicine 2008;8:29. [DOI: 10.1186/1472-6882-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Villela 2010
- Villela DL, Santos VL. On the use of platelet‐rich plasma for diabetic ulcer: a systematic review. Growth Factors 2010;28(2):111‐6. [DOI] [PubMed] [Google Scholar]
Yuan 2009
- Yuan NB, Long Y, Zhang XX, Ran XW. Study on the mechanism of autologous platelet‐rich gel to treat the refractory diabetic dermal. Sichuan Da Xue Xue Bao Yi Xue Ban 2009;40(2):292‐4. [PubMed] [Google Scholar]
References to other published versions of this review
Martinez‐Zapata 2008
- Martinez‐Zapata MJ, Martí‐Carvajal AJ, Solà I, Exposito JA, Bolivar I, Rodríguez L, et al. Autologous platelet‐rich plasma for treating chronic wounds. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006899.pub2] [DOI] [PubMed] [Google Scholar]
Martinez‐Zapata 2012
- Martinez‐Zapata MJ, Martí‐Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L, et al. Autologous platelet‐rich plasma for treating chronic wounds. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD006899.pub2] [DOI] [PubMed] [Google Scholar]


