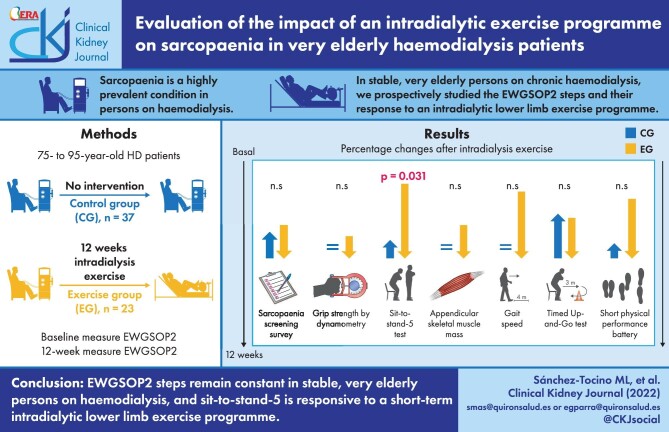
ABSTRACT
Sarcopaenia is a highly prevalent condition in persons on haemodialysis (HD). In stable very elderly (75–95 years old) persons on chronic HD, we prospectively studied the European Working Group on Sarcopaenia in Older People (EWGSOP2) steps stability over time in 37 controls and their response to a 12-week intradialytic lower limb exercise programme in 23 persons. Overall dropout was 15% and the main cause for dropout was death (8%). Thus 33 controls and 18 exercise participants were evaluated at 12 weeks. In controls, comorbidity, nutrition, dependency and frailty scales, anthropometric assessments, EWGSOP2 step values and the prevalence of suspected, confirmed and severe sarcopaenia as assessed by EWGSOP2 remained stable. In contrast, in persons who completed the exercise programme, a significant improvement in the five times sit-to-stand (STS-5) test was noted at the end of the 12-week exercise programme (19.2 ± 4.9–15.9 ± 5.9 seconds; P = .001), consistent with the lower limb nature of the exercise programme, that persisted 12 weeks after completion of the programme. Exercise also improved the Fried frailty scale (1.7 ± 1.0–1.1 ± 0.6; P = .004). In conclusion, EWGSOP2 steps remain stable in stable very elderly persons on HD and STS-5 is responsive to a short-term intradialytic lower limb exercise programme. These results may help define EWGSOP2-based primary endpoints in future large-scale clinical trials assessing exercise interventions.
Keywords: elderly, exercise, frailty, haemodialysis, sarcopaenia
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Sarcopaenia is a clinical condition characterized by the loss of muscle mass and strength in the context of ageing with a negative impact on quality of life and care [1]. The loss of muscle mass starts from the age of 40 years and progresses at a rate of 8% per decade from that age and accelerates to 5% per decade from the age of 70 years. It is estimated that 50% of people >80 years of age have sarcopaenia [2]. The diagnosis of sarcopaenia is challenging in clinical practice, as loss of muscle mass and muscle strength do not necessarily correspond to each other [3, 4].
Muscle strength, power and performance result from multiple components of skeletal muscle, including size, fibre type, quality and innervation. Therefore, even in patients with muscle mass within normal limits, weakness may be apparent in functional performance or activities of daily living [5]. Diagnosis of sarcopaenia should focus on the identification of a loss of muscle strength regardless of muscle size. Recently the European Working Group on Sarcopenia in Older People (EWGSOP2) [6] established new diagnostic steps for sarcopaenia. The first step is a novel case-finding test, called SARC-F (strength, assistance in walking, rise from a chair, climb stairs, falls), composed of five easy-to-answer questions [7]. The second step establishes the diagnosis of probable sarcopaenia through the assessment of muscle strength and the third step confirms the diagnosis by measuring muscle mass. The final step assesses severity by the ability to perform certain physical tests [6].
Sarcopaenia is common in adults with chronic kidney disease (CKD) and its prevalence increases markedly with declining kidney function [8]. In persons on haemodialysis (HD), the prevalence is highly variable depending on the assessment method, ranging from 4 to 64% [4, 9, 10]. Bioelectrical impedance analysis (BIA) and anthropometric predictive equations estimate whole body skeletal muscle mass (SMM) and appendicular skeletal muscle mass (ASM) [11], but not strength. However, persons with CKD may have poor muscle function despite an acceptable muscle mass [12].
There is much debate about the best therapeutic approach to sarcopaenia in HD. Dietary interventions [13, 14] and dialysis optimization have been proposed [15]. However, physical activity is the most effective and cost-effective therapy to counteract the changes in muscle mass and strength caused by age and chronic diseases [16]. The Sarcopaenia, Cachexia and Wasting Disorders Society recommends aerobic and muscular endurance exercises for 20–30 minutes three times a week [17]. The implementation of exercise programmes within HDs sessions has been proposed [18], including low-intensity exercises, adapted to older population groups [19]. These programmes are frequently limited in time, there is little information on their longer-term impact and it is unknown whether and how they impact the EWGSOP2 steps.
The aim of the present study was to assess changes over time in sarcopaenia assessed by the new EWGSOP2 diagnostic tool in very elderly (75–95 years) persons on HD under control conditions or following an intradialytic physical exercise programme.
MATERIALS AND METHODS
This was a prospective, non-randomized, interventional study. Participants were clinically stable persons with kidney failure on chronic HD at three outpatient centres and a hospital dialysis unit of the Fundación Renal Íñigo Álvarez de Toledo (FRIAT). The study was approved by the ethics committee of the Hospital Universitario Fundación Jiménez Díaz (number 03/19) and complied with the standards recognized by the Declaration of Helsinki of the World Medical Association as well as by the Standards of Good Clinical Practice, in addition to compliance with Spanish legislation on biomedical research (14/2007 Law). All participants signed informed consent forms. The study period was from February to November 2019. The intervention consisted of an intradialytic exercise programme for 12 weeks.
Inclusion criteria were age 75–95 years, capability to perform physical fitness assessment tests or dynamometry, an HD vintage >3 months and signing the consent form. Exclusion criteria were the presence of intra-dialysis instability, comorbid conditions that contraindicated exercise or dementia preventing signing of the consent form.
This was a pilot study and no formal sample size calculations were made. A total of 60 patients participated in two non-randomized groups, the control group (n = 37), who did not perform physical activity during HD sessions, and the exercise group (n = 23), who performed the physical exercise programme during HD.
All subjects meeting the inclusion criteria in one of the dialysis centres were assigned by convenience to the exercise group, given the need for the presence of specialized personnel, while patients from the other two participating centres were assigned to the control group. Of 25 patients meeting the inclusion criteria for the exercise group, 23 agreed to perform the exercise programme and 2 refused but agreed to follow-up evaluations and were included in the control group. All patients who offered to participate in the control group accepted. Except for the exercise programme, individuals received routine clinical care.
Intradialytic exercise programme
The intradialytic programme began with warm-up respiratory and joint mobility exercises and was followed by four groups of lower limb strength exercises: hip flexion, hip/knee extension, hip abduction and adduction and ankle flexion–extension/abduction–adduction. Elastic bands, weighted ankle braces, foam balls and Pilates rings were used to perform the exercises. A foot peddler was used as aerobic resistance work, progressively adapting the intensity and duration, with a maximum of 30 minutes at an intensity corresponding to 12–14 points on the Borg 6–20 scale. The exercise was supervised by two physical activity and sports science professionals and four trainess who personalized the most appropriate programme for each participant following evaluation of their capacities, dependence and comorbidities. Exercise was carried out in the three weekly HD sessions for 12 weeks. In each session, exercise started 30 minutes after the initiation of the HD session. Each session duration was 60 minutes. The physical exercise programme involved the lower limbs. Additionally, to ensure vascular access stability during the programme, needle clamping in the arteriovenous fistulas was reinforced.
Vital signs (blood pressure, temperature, heart rate and glycaemia in diabetics) were recorded before and after the start of the activity. Adverse effects during dialysis sessions, such as vascular access incidents, hypotension, headache, cramps and pain, and, if necessary, the cause of abandonment of the programme were recorded.
Study variables
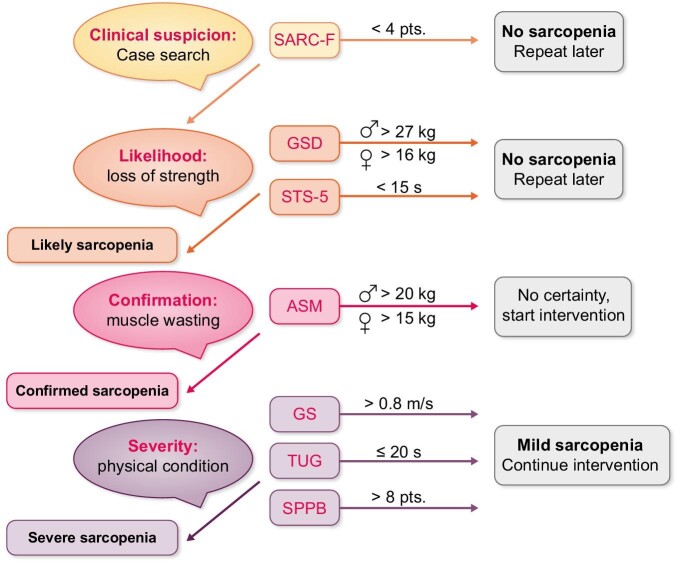
Study variables were assessed at baseline and at the end of the 12-week programme. In addition, in the exercise group, they were assessed again 12 weeks after completing the exercise programme, i.e. 24 weeks since the start of the study. The observer-dependent variables were obtained by the two physical activity and sports science professionals. The primary outcome variables were those used to diagnose sarcopaenia according to the EWGSOP2 steps (Figure 1) [6].
FIGURE 1:
Sarcopaenia diagnostic tree according to EWGSOP2. Diagnostic variables and sarcopaenia cut-off points are shown.
Find. Clinical suspicion/case finding is defined by the SARC-F survey score. It is a simple scale that identifies suspected sarcopaenia, composed of five questions scored differently according to their intensity (strength, assistance in walking, rise from a chair, climb stairs, falls) [7].
Assess. Loss of strength is defined by the following upper limb and lower limb variables:
Grip strength by dynamometry (GSD). A CAMRY EH101 electric dynamometer was used to measure handgrip strength. Participants were standing, arm extended and parallel to the body and without supporting or moving the wrist. The maximum grip strength was analysed for 3 seconds, with a 1-minute rest between repetitions, performing two attempts in both arms. The best score of the dominant arm (the arm with the greater strength) was used for the study.
Five times sit-to-stand (STS-5). The STS-5 evaluates the time required by patients to get up from a chair without any support, performing this movement five consecutive times [20].
Confirm sarcopaenia. The amount of muscle mass was defined by the variable appendicular skeletal muscle mass (ASM) assessed by a BioScan touch i8 BIA device (Maltron, Essex, UK). Assessments were performed in the second session of the week, between the first and second hour of dialysis, given that the device allows measurements during the HD session.
Severity of sarcopaenia. Physical condition was assessed by the following:
Gait speed (GS) measures the time required to walk 4 m [also included as the second test of the Short Physical Performance Battery (SPPB)]. The readout is the walking speed in meters per second, considering the need for assistance (cane, walker, another person, etc.) to maintain balance during the walk. To increase test reliability, 1 m in front and 1 m behind these 4 m were not considered, so that results are not influenced by acceleration or deceleration [21].
The Timed Up and Go test (TUG) assesses agility and dynamic balance. Subjects must get up from a chair, walk for 3 m, turn around a cone, and sit down again. The test is performed at the maximum speed at which persons can walk, three times, and the result is the fastest time [22].
The SPPB, modified by Pavasini et al. [23], consists of three tests. The first is an adaptation of the Romberg tests for balance. Persons are asked to stand with their feet together, then are instructed to place one foot next to the other, with the heel of one foot halfway to the other foot, then place one foot in front of the other, resting the heel in front of the toes. In all three instances, balance preservation is evaluated. The second test of this battery is GS over 4 m, as described above. The third test is the STS-5.
The cut-off points for sarcopaenia markers for each variable are described in Table 1.
Table 1.
Cut-off points for sarcopaenia markers included in EWGSOP2
Other variables
Anthropometric variables included body mass index (BMI), arm perimeter and waist–hip index (WHI) and analytical variables were albumin, haemoglobin, C-reactive protein (CRP), 25-hydroxy vitamin D and Daugirdas, Kt/Vurea. Additionally, scales of malnutrition [malnutrition-inflammation score (MIS)] [31], comorbidity (Charlson Comorbidity Index) [32], dependence (Barthel Index) [33], frailty (Fried frailty scale) [34] and physical activity [Physical Activity Scale for the Elderly (PASE)] [35] were used. Demographic variables such as sex and age, cause of kidney disease and dialysis vintage were collected. Anthropometric measurements were made by the two physical activity and sports science professionals.
Statistical analysis
SPSS Statistics version 20 (IBM, Armonk, NY, USA) was used for statistical analyses. Quantitative variables were presented as mean and standard deviation (SD) and qualitative variables as absolute numbers and percentages. The chi-squared test was used to evaluate the homogeneity of the groups under study. To analyse the impact of exercise on the variables under study, the Student's t-test and McNemar's test were used. The level of statistical significance was set at P ≤ .05 and when multiple testing was performed, modified as per the Bonferroni's correction and the P-value threshold for statistical significance in multiple testing corresponding to an α level equal to 0.05 in single testing is indicated in a footnote in each table.
RESULTS
Study design and patient population
A cohort study was conducted in three FRIAT dialysis centres and a total of 60 persons participated in the study, 37 (61.7%) controls and 23 (38.3%) in the exercise group. Of these, 41 (68%) were men, mean age was 81.85 ± 5.58 years and HD vintage was 49.88 ± 40.29 months. The causes of kidney disease were diabetes mellitus (28%), unknown (22%), hypertension (20%), interstitial nephritis (7%), glomerulonephritis (5%) and other (8%).
At 12 weeks, 51/60 (85%) participants remained in the study: 33/37 (89%) in the control group and 18/23 (78%) in the exercise group. Reasons for not completing the study were death [5/60; 3 in the control group and 2 in the exercise group (8.3%)], hospital admission [2/60 (3.3%)], holidays [1/60 (1.6%)] and stopping the exercise programme [1/60 (1.6%) of the full cohort and 1/23 (4.3%) of those in the exercise programme].
Table 2 describes baseline demographic characteristics, scales, laboratory analytical data and dialysis adequacy for participants who completed the protocol. Baseline significant differences were only observed between the groups for the malnutrition inflammation score (MIS) malnutrition scale, serum albumin and Kt/Vurea that were no longer observed after applying Bonferroni's correction for multiple testing. Additionally, no significant differences were observed in the mean baseline values for the components of the EWGSOP2 steps (Table 3).
Table 2.
Demographic, scales, anthropometric and analytical data and dialysis parameters at baseline
| Characteristics | Control group (n = 37) | Exercise group (n = 23) | P-value |
|---|---|---|---|
| Demographic data | |||
| Gender (male), % (n) | 75.8 (25) | 61.1 (11) | .218 |
| Age (years) | 81.7 ± 5.3 | 82.0 ± 5.8 | .854 |
| HD vintage (months) | 52.5 ± 45.8 | 50.4 ± 35.9 | .867 |
| Comorbidity, nutrition, dependency, frailty and physical activity scales | |||
| Charlson Comorbidity Index (points) | 10.1 ± 2.2 | 9.5 ± 1.8 | .358 |
| MIS nutrition (points) | 6.9 ± 4.3 | 3.9 ± 1.6 | .006 |
| Barthel dependency (points) | 88 ± 18.9 | 93.6 ± 11.7 | .263 |
| Fried frailty (points) | 2.2 ± 1.3 | 1.7 ± 1.0 | .140 |
| PASE physical activity (points) | 20.4 ± 28 | 31.3 ± 34.9 | .230 |
| Anthropometric data | |||
| BMI (kg/m2) | 25.2 ± 3.8 | 25.3 ± 3.7 | .948 |
| Arm perimeter (cm) | 26.7 ± 2.6 | 26.3 ± 2.9 | .825 |
| Waist–hip index (WHI) | 0.92 ± 0.1 | 0.95 ± 0.1 | .303 |
| Analytical data | |||
| Albumin (g/dL) | 3.6 ± 0.4 | 3.9 ± 0.2 | .022 |
| Haemoglobin (g/dL) | 11.2 ± 1 | 11.7 ± 0.8 | .133 |
| C-reactive protein (mg/L) | 0.91 ± 1.1 | 1.75 ± 2.3 | .084 |
| 25-hydroxy vitamin D (ng/mL) | 22.5 ± 12.6 | 20.3 ± 14.5 | .584 |
| Dialysis adequacy | |||
| Kt/Vurea | 1.9 ± 0.4 | 1.6 ± 0.3 | .012 |
Values presented as mean ± SD unless stated otherwise. Application of Bonferroni's correction to this data set would result in a P-value threshold of .0031 for statistical significance corresponding to an α level of .05 in single testing.
Table 3.
EWGSOP2 components at baseline
| Components | Control group (n = 37) | Exercise group (n = 23) | P-value |
|---|---|---|---|
| Find: clinical suspicion | |||
| SARC-F(points) | 2.5 ± 2.3 | 2.3 ± 2.1 | .861 |
| Assess: loss of strength | |||
| GSD (kg) | 19.6 ± 6.1 | 20.9 ± 6.3 | .416 |
| STS-5 (s) | 21.3 ± 7.4 | 19.2 ± 4.9 | .266 |
| Confirm: muscle wasting | |||
| ASM (kg) | 19.5 ± 3.8 | 18.9 ± 3.8 | .553 |
| Severity: physical condition | |||
| GS (m/s) | 0.76 ± 0.2 | 0.75 ± 0.2 | .458 |
| TUG (s) | 16.2 ± 5.1 | 16 ± 6.2 | .338 |
| SPPB (points) | 6.4 ± 2.4 | 7.2 ± 2.9 | .307 |
Values are presented as mean ± SD.
Impact of an intradialytic physical exercise programme on scales, adverse effects, hospital admissions, anthropometry and analytical and dialysis variables
The impact of the intradialytic physical exercise programme on scales, anthropometry and analytical and dialysis variables was assessed at 12 weeks, i.e. at the end of the exercise programme for participants exercising during the HD sessions and at the same time point in controls who did not exercise. The exercise programme was associated with a significant improvement in the frailty score from baseline to the end of the exercise programme (12 weeks) (Table 4). No other significant differences were observed after applying Bonferroni’s correction. The exercise was well tolerated, with no differences in headache, cramps and pain. Hypotension was observed in 24/33 (73%) patients in the control group and 9/18 (50%) in the exercise group (P = ns) during the 12-week exercise (or control) period. In the 12-week follow-up period, hypotension was observed in 13/18 (72%) patients in the exercise group and 22/33 (67%) in the control group (P = ns). No vascular access incidents were recorded.
Table 4.
Scales, anthropometry, analytical and dialysis variables after 12 weeks
| Control group (n = 33) | Exercise group (n = 18) | |||||
|---|---|---|---|---|---|---|
| Baseline | 12 weeks | P-value | Baseline | 12 weeks | P-value | |
| Comorbidity, nutrition, dependency, frailty and physical activity scales | ||||||
| MIS (nutrition) | 6.9 ± 4.3 | 6.8 ± 4.2 | .363 | 3.9 ± 1.6 | 3.8 ± 1.2 | .816 |
| Barthel Index (dependency) | 88 ± 18.9 | 86.5 ± 20.1 | .150 | 93.6 ± 11.7 | 94 ± 10.2 | .830 |
| Fried scale (frailty) | 2.2 ± 1.3 | 2.2 ± 1.4 | .786 | 1.7 ± 1.0 | 1.1 ± 0.6 | .004* |
| PASE physical activity (points) | 20.4 ± 28 | 19.4 ± 21.8 | .152 | 31.3 ± 34.9 | 45.6 ± 39.1 | .085 |
| Anthropometric data | ||||||
| BMI (kg/m2) | 25.2 ± 3.8 | 25.3 ± 3.9 | .297 | 25.3 ± 3.7 | 25.3 ± 3.5 | .713 |
| Arm perimeter (cm) | 26.7 ± 2.6 | 27.02 ± 3.1 | .358 | 26.3 ± 2.9 | 27.3 ± 3.4 | .024 |
| Waist–hip index | 0.92 ± 0.1 | 0.93 ± 0.1 | .304 | 0.95 ± 0.1 | 0.94 ± 0.1 | .121 |
| Serum biochemistry | ||||||
| Albumin (g/dL) | 3.6 ± 0.4 | 3.7 ± 0.4 | .100 | 3.9 ± 0.2 | 4.0 ± 0.3 | .034 |
| Hemoglobin (g/dL) | 11.2 ± 1.03 | 10.8 ± 1.2 | .099 | 11.7 ± 0.8 | 11.7 ± 1.1 | .899 |
| CRP (mg/L) | 0.91 ± 1.1 | 1.46 ± 2.1 | .107 | 1.75 ± 2.3 | 0.88 ± 0.8 | .117 |
| 25-hydroxy vitamin D (ng/mL) | 22.5 ± 12.6 | 28.1 ± 16.8 | .009* | 20.3 ± 14.5 | 21.5 ± 13.9 | .635 |
| Dialysis adequacy | ||||||
| Kt/Vurea | 1.9 ± 0.4 | 1.9 ± 0.4 | .657 | 1.6 ± 0.3 | 1.7 ± 0.3 | .020 |
Values presented as mean ± SD. Statistically significant after Bonferroni correction. Application of Bonferroni's correction to this data set would result in a P-value threshold of .00426 for statistical significance corresponding to an α level of .05 in single testing.
During the 12 weeks of the study there were 12 admissions involving eight patients in the control group, with a mean hospital stay of 9.1 ± 5 days, while in the exercise group there were no admissions. In the following 12 weeks, five patients in the control group and two in the exercise group were hospitalized, with hospital stays of 5.6 ± 2.6 and 7.5 ± 7.8 days (P = ns).
Impact of an intradialytic physical exercise programme on values for the individual EWGSOP2 steps components
The impact of the intradialytic physical exercise programme on EWGSOP2 steps was assessed at 12 weeks, i.e. at the end of the exercise programme for participants exercising during the HD sessions and at the same time point in controls, as well as at 24 weeks, i.e. 12 weeks after the end of the exercise programme for programme participants (Table 5). At the end of the intradialytic physical exercise programme (12 weeks), only the STS-5 values in the EWGSOP2 steps had significantly improved in the exercise group, after Bonferroni’s correction. This is consistent with a lower limb exercise programme. Moreover, the improved STS-5 persisted 12 weeks after the end of the exercise programme (the 24 week time point), as P-values versus 12 weeks remained >.05. In contrast, no significant changes were observed in the control group.
Table 5.
EWGSOP2 at baseline, at the end of the intradialytic exercise programme (12 weeks) and 12 weeks after completing the intradialytic exercise programme (24 weeks)
| Control group (n = 33) | Exercise group (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | P-value | Baseline | 12 weeks | P-value | 24 weeks | P-value 24 versus 12 weeks | |
| Find: clinical suspicion | ||||||||
| SARC-F (points) | 2.5 ± 2.3 | 2.6 ± 2.3 | .103 | 2.3 ± 2.1 | 1.9 ± 2.00 | .110 | 2.5 ± 2.2 | .056 |
| Assess: loss of strength | ||||||||
| GSD (kg) | 19.6 ± 6.1 | 19.7 ± 6.6 | .855 | 20.9 ± 6.3 | 22.5 ± 6.8 | .019 | 22.9 ± 6.4 | .237 |
| STS-5 (s) | 21.3 ± 7.4 | 23 ± 11.5 | .398 | 19.2 ± 4.9 | 15.9 ± 5.9 | .001* | 14.4 ± 2.9 | .707 |
| Confirm: muscle wasting | ||||||||
| ASM (kg) | 19.5 ± 3.8 | 19.5 ± 3.8 | .795 | 18.9 ± 3.8 | 19.5 ± 3.9 | .010 | 19.2 ± 3.5 | .560 |
| Severity: physical condition | ||||||||
| GS (m/s) | 0.76 ± 0.2 | 0.77 ± 0.3 | .801 | 0.75 ± 0.2 | 0.92 ± 0.3 | .013 | 0.99 ± 0.3 | .267 |
| TUG (s) | 16.2 ± 5.1 | 15.3 ± 5.3 | .392 | 16.02 ± 6.2 | 13.6 ± 7.2 | .041 | 13.5 ± 6.4 | .623 |
| SPPB (points) | 6.4 ± 2.4 | 6.3 ± 2.8 | .732 | 7.2 ± 2.9 | 8.6 ± 2.8 | .027 | 9.6 ± 1.9 | .165 |
Values presented as mean ± SD.
*Statistically significant after Bonferroni correction. Application of Bonferroni's correction to this data set would result in a P-value threshold of .0071 for statistical significance corresponding to an α level of .05 in single testing.
Impact of an intradialytic physical exercise programme on EWGSOP2 steps
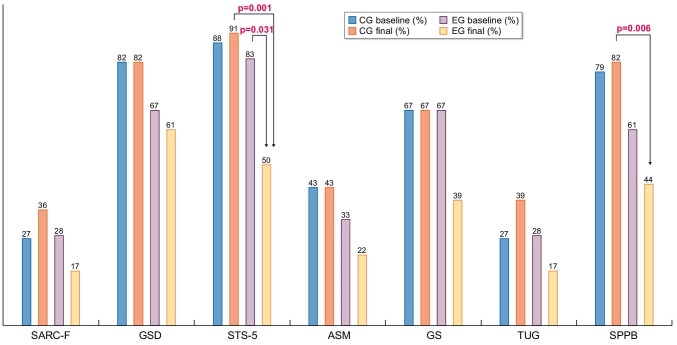
We next assessed the impact of an intradialytic physical exercise programme on EWGSOP2 steps (Figure 2). That is, we assessed the percentage of patients who would fit into the sarcopaenic category for each EWGSOP2 step. In the exercise group, numerical decreases in the percentage of individuals meeting each sarcopaenia step criterion were observed for all EWGSOP2 steps. However, these changes only reached statistical significance for STS-5, which assesses lower limb strength. The percentage of sarcopaenic individuals among participants in the exercise programme, as assessed by this criterion, decreased from 86 to 53% (P = .031). These results are consistent with the exercise programme being focussed on the lower limbs. In contrast, no significant changes were observed in the control group.
FIGURE 2:
Percentage of participants that met different EWGSOP2 steps associated with sarcopaenia at 12 weeks. Control baseline in blue and 12 weeks in dark orange, exercise baseline in grey and 12 weeks in light orange. CG, control group; EG, exercise group.
Impact of an intradialytic physical exercise programme on the prevalence of sarcopaenia as assessed by EWGSOP2
Supplementary data, Table S1 shows the percentage of participants meeting each step of the EWGSOP2 sarcopaenia diagnosis algorithm before and after exercise. Each probability step criterion and each severity step criterion were analysed independently. Probable sarcopaenia at baseline was found in 94% of the exercise group participants and 97% of controls. At 12 weeks, no statistically significant differences from baseline were observed in either group.
DISCUSSION
The present study explored the changes over time of components of the EWGSOP2 steps to diagnose sarcopaenia in very elderly persons who were stable on chronic HD, both under control conditions and following a 12-week programme of intradialytic lower limb exercise. The main findings were that over a 12-week follow-up, values for the individual components of the EWGSOP2 steps as well as the prevalence of probable and confirmed sarcopaenia and the severity of sarcopaenia remained stable under control conditions. Exercise improved physical frailty as measured by the Fried scale as well as the lower limb parameter of the STS-5. Lower limb frailty is related to adverse outcomes in HD patients [36]. A positive trend was also observed for the mean values for almost all components of the EWGSOP2 steps, which was evident both at the end of the programme and persisted 12 weeks after the end of the programme. Thus the exercise programme achieved a persistent improvement of sarcopaenia-related parameters assessed by the EWGSOP2 steps and the EWGSOP2 steps were responsive to the exercise programme. Despite numerical differences in the prevalence of probable sarcopaenia, confirmed sarcopaenia and severe sarcopaenia, differences were not statistically significant, likely because the study was rendered underpowered by a high dropout rate.
Sarcopaenia detection is necessary to design therapeutic plans that may involve correction of comorbidities, an exercise plan, improved nutritional support and optimized dialysis itself [14]. This pilot study identified potential endpoints for clinical trials aimed at improving sarcopaenia in very elderly persons on HD but also illustrated the difficulty of performing interventional studies on sarcopaenia in very elderly HD patients, as the dropout rate was 15% in 12 weeks. Extrapolation to an annual dropout would balloon to a whopping 60%. While some may consider this too high, we should remember that the expected remaining lifetime of prevalent dialysis patients in this age range is much closer to that of the general population than that of HD patients in their 20s [37].
Intradialytic exercise is prescribed to improve sarcopaenia in persons with kidney disease [38–41]. It increased muscle strength, especially of the lower extremities and manual dexterity [18]. A 2019 systematic review and meta-analysis of 27 RCTs involving 1215 subjects concluded that intradialytic exercise resulted in benefits in terms of improving HD adequacy, exercise capacity, depression and quality of life [38]. However, the mean age of participants in the trials ranged from 34 to 69.7 years, well below the 82 years mean age among participants in the present study and only three trials enrolled participants with a mean age ≥65 years. Moreover, none of the trials explored the impact on sarcopaenia as assessed by EWGSOP2, which was published in 2019. More recent reviews have emphasized the benefits of exercise training, although they were not limited to intradialytic exercise [40, 41]. Again, the age range of the studies was either not mentioned [40] or ranged from 36 to 71 years [41]. Thus, although the authors concluded that there is convincing evidence that exercise training improves physical function measured as aerobic capacity, muscle endurance strength and balance at all ages and all stages of CKD [41], in fact, the evidence so far is thin regarding the age range of participants in the present study. A more recent exploratory trial showed that intra dialytic cycle ergometry reduced cardiac stunning in 20 HD patients with a mean age of 59 years [42]. An ongoing large (n = 335) pragmatic randomized controlled trial is assessing the impact of intradialytic cycle exercise training on quality of life, but again, the median age of participants is 59 years [39, 43].
The concept of dynapaenia refers to the loss of muscle strength, differentiating it from low muscle mass, although both are components of sarcopaenia. It may be argued that functionally dynapaenia is an important concept for patient well-being. Exercise improves physical performance tests, and hence muscle strength or dynapaenia, in both legs and arms [44]. Lower extremity resistance exercise during HD was previously shown to increase muscle strength but not muscle mass [12, 45, 46]. We observed that the 3-month exercise programme did impact generic tests such as those for frailty but not those for dependence or MIS. A novelty of the present report is the analysis of the impact of exercise on EWGSOP2 parameters. Indeed, all EWGSOP2 parameters assessed improved, while they remained stable in the control group. The only exception was the case detection survey score. SARC-F is highly sensitive and thus is a good screening tool, but it may be non-specific. Other authors [19] have found an improvement after exercise in some of these tests but did not systematically analyse all EWGSOP2 steps.
The main and only significant change after applying Bonferroni’s correction was the mean STS-5 decrease from 19.2 to 15.9 s, close to the sarcopaenia cut-off point (15 s) [47]. For the severity step, the GS EWGSOP2 cut-off point of 0.8 m/s has been questioned as too demanding for the elderly [48]. In our study, GS reached 0.92 m/s. GS, TUG and STS-5 are simpler to perform than the SPPB test and would allow assessing changes in strength and functionality in dialysis units. Overall, individual EWGSOP2 step parameters were responsive to an exercise programme in very elderly patients on dialysis, suggesting that EWGSOP2 represents an appropriate tool to assess progress and may be used as a primary endpoint in interventional studies. At 6 months after exercise initiation, the achieved improvement remained stable. This suggests that in a resource-limited environment, intermittent periods of exercise programmes may be tested to improve and maintain muscle mass and strength, but continuous exercise programmes are needed if continuous improvement is the aim.
The present data also suggest that muscle functionality is lost before muscle mass in very elderly persons on HD. The first muscle groups to be affected by sarcopaenia are those of the lower limbs, but they are also the first to recover with exercise [49]. We hypothesize that in dialysis patients it is preferable to use functional variables for the detection of sarcopaenia rather than muscle mass measured by BIA, which may be influenced by hydration status [50], as pointed out by EWGSOP2. Indeed, considering muscle mass measured by ASM reduced the number of severe sarcopaenia cases, in our opinion, in uraemic patients, muscle mass and strength can be dissociated and ASM should not be taken into account in assessing the severity of sarcopaenia. The EWGSOP 2 steps allow observation of the impact of exercise on sarcopaenia, although the requirement of simultaneous use of muscle mass and strength should be re-evaluated in a routine clinical setting in this population. Despite this, ASM by BIA remains a key predictor of mortality outcomes that should be monitored in clinical practice [51]. The exercise was not associated with adverse effects and it did not modify hypotension episodes when compared with controls [19, 52].
Some limitations should be acknowledged. This was a non-randomized and non-blinded study, which may have resulted in bias. Thus EWGSOP2 identified severe sarcopaenia in 42% of controls and 22% of exercise participants, although there were no statistically significant differences. In this regard, the results obtained in control and exercise patients cannot be directly compared. Follow-up was for 12 weeks for both groups but was only prolonged 12 more weeks in the exercise group. In addition, comorbidities may limit the ability to exercise for many very elderly dialysis patients and thus the results regarding the efficacy of exercise cannot be extrapolated to the wider very elderly population in HD. BIA is not considered a gold-standard method to assess sarcopaenia. However, magnetic resonance imaging or computed tomography are either not accessible for routine clinical care assessment of sarcopaenia or marred by radiation. Dropout was higher than initially expected and the clinical impact of the intervention on falls or quality of life was not assessed. Finally, patient allocation was pragmatic but not optimal, which may also have resulted in bias. Despite these limitations, the present study provides valuable insights for the design of clinical trials aimed at improving sarcopaenia in very elderly persons on HD, both from the point of view of sample size calculations and regarding the selection of specific primary endpoints.
In conclusion, assessment of the EWGSOP2 steps is stable over time in stable very elderly persons on HD and STS-5 is responsive to a 12-week lower limb exercise programme. Furthermore, assessment of the EWGSOP2 step STS-5 shows persistent benefit 3 months after completing the exercise programme. Thus it represents a feasible primary endpoint to assess the efficacy of exercise programmes or other interventions to improve sarcopaenia in very elderly HD patients. Furthermore, based on the present results, we anticipate that numerical changes in specific parameters within EWGSOP2 steps may be appropriate primary endpoints for small-sized trials. However, a primary endpoint of change in sarcopaenia diagnosis (either probable or confirmed) or of an impact on the severity of sarcopaenia according to EWGSOP2 steps will require a larger sample size that should consider the potentially high dropout rate, which may reach 60% annually.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staff of the centres where the study was performed, especially Dr Roberto Martín, Mónica Pereira, Silvia Villoria and Dr Victor Lorenzo Sellares, for their invaluable assistance in the successful completion of this study.
Contributor Information
Maria Luz Sánchez-Tocino, Fundación Renal Íñigo Álvarez de Toledo. Salamanca, Spain.
Emilio González-Parra, Servicio de Nefrología e Hipertensión. Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Blanca Miranda Serrano, Fundación Renal Íñigo Álvarez de Toledo. Salamanca, Spain.
Carolina Gracia-Iguacel, Servicio de Nefrología e Hipertensión. Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Ana María de-Alba-Peñaranda, Fundación Renal Íñigo Álvarez de Toledo, Madrid, Spain.
Antonio López-González, Complejo Hospitalario Universitario A Coruña, A Coruña, Spain.
Marcos García Olegario, Fundación Renal Íñigo Álvarez de Toledo, Madrid, Spain.
Alberto Ortíz, Servicio de Nefrología e Hipertensión. Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Sebastian Mas-Fontao, Laboratorio de patología renal y diabetes, CIBERDEM. IIS-Fundación Jiménez Díaz UAM, Madrid, Spain.
FUNDING
The authors would like to thank FRIAT for its support to the present study. The research groups of E.G.P., S.M.F. and A.O. are funded by the Ministerio de Economia, Industria y competitividad: FIS/Fondos FEDER (PI16/01298, PI18/01386, PI19/00588, PI19/00815, PI20/00487, PI21/01240, DTS18/00032), ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/0009) and Sociedad Española de Nefrología, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM.
AUTHORS’ CONTRIBUTIONS
M.L.S.T. and B.M.S. were responsible for subject recruitment. M.L.S.T., A.M.P.A., A.L.G. and M.G.O. were responsible for physical intervention and assessment. M.L.S.T., E.G.P., C.G.I., B.M.S. and S.M.F. were responsible for the interpretation of results. M.L.S.T. and A.L.G. were responsible for the statistical methods and analysis. M.L.S.T., E.G.P., C.G.I., A.O. and S.M.F. wrote the manuscript.
CONFLICT OF INTEREST STATEMENT
A.O. is the Editor-in-Chief of CKJ. None of the other authors have any conflicts of interest to declare.
DATA AVAILABILITY STATEMENT
A spreadsheet with all clinical data collected from patients (whose information is anonymized) is available upon request.
REFERENCES
- 1. Epidemiologic and methodologic problems in determining nutritional status of older persons . Proceedings of a conference. Albuquerque, New Mexico, October 19–21, 1988. Am J Clin Nutr 1989; 50: 1121–1235 [PubMed] [Google Scholar]
- 2. Baumgartner RN, Koehler KM, Gallagher Det al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763 [DOI] [PubMed] [Google Scholar]
- 3. Ren H, Gong D, Jia Fet al. Sarcopenia in patients undergoing maintenance hemodialysis: incidence rate, risk factors and its effect on survival risk. Ren Fail 2016; 38: 364–371 [DOI] [PubMed] [Google Scholar]
- 4. Lamarca F, Carrero JJ, Rodrigues JCDet al. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. J Nutr Health Aging 2014; 18: 710–717 [DOI] [PubMed] [Google Scholar]
- 5. Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens 2017; 26: 219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz-Jentoft AJ, Bahat G, Bauer Jet al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malmstrom TK, Miller DK, Simonsick EMet al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016; 7: 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foley RN, Wang C, Ishani Aet al. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol 2007; 27: 279–286 [DOI] [PubMed] [Google Scholar]
- 9. Isoyama N, Qureshi AR, Avesani CMet al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014; 9: 1720–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JK, Choi SR, Choi MJet al. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin Nutr 2014; 33: 64–68 [DOI] [PubMed] [Google Scholar]
- 11. Barreto Silva MI, Menna Barreto APM, Pontes KS da Set al. Accuracy of surrogate methods to estimate skeletal muscle mass in non-dialysis dependent patients with chronic kidney disease and in kidney transplant recipients. Clin Nutr 2021; 40: 303–312 [DOI] [PubMed] [Google Scholar]
- 12. Carrero JJ, Johansen KL, Lindholm Bet al. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int 2016; 90: 53–66 [DOI] [PubMed] [Google Scholar]
- 13. Bauer J, Biolo G, Cederholm Tet al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013; 14: 542–559 [DOI] [PubMed] [Google Scholar]
- 14. Nishi H, Takemura K, Higashihara Tet al. Uremic sarcopenia: clinical evidence and basic experimental approach. Nutrients 2020; 12: 1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gamboa JL, Billings FT, Bojanowski MTet al. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol Rep 2016; 4: e12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ortiz A, Sanchez-Niño MD. Sarcopenia in CKD: a roadmap from basic pathogenetic mechanisms to clinical trials. Clin Kidney J 2019; 12: 110–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morley JE, Abbatecola AM, Argiles JMet al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011; 12: 403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Vilsteren MCBA, de Greef MHG, Huisman RM.. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in the Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant 2005; 20: 141–146 [DOI] [PubMed] [Google Scholar]
- 19. Simo VE, Jiménez AJ, Guzmán FMet al. Benefits of a low intensity exercise programme during haemodialysis sessions in elderly patients. Nefrologia 2015; 35: 385–394 [DOI] [PubMed] [Google Scholar]
- 20. Giannaki CD, Sakkas GK, Karatzaferi Cet al. Evidence of increased muscle atrophy and impaired quality of life parameters in patients with uremic restless legs syndrome. PLoS One 2011; 6: e25180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abellan Van Kan G, Rolland Y, Andrieu Set al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13: 881–889 [DOI] [PubMed] [Google Scholar]
- 22. Martinez BP, Gomes IB, de Oliveira CSet al. Accuracy of the timed up and go test for predicting sarcopenia in elderly hospitalized patients. Clinics 2015; 70: 369–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pavasini R, Guralnik J, Brown JCet al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016; 14: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dodds RM, Syddall HE, Cooper Ret al. Grip strength across the life course: normative data from twelve British studies. PLoS One 2014; 9: e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cesari M, Kritchevsky SB, Newman ABet al. Added value of physical performance measures in predicting adverse health-related events: results from the health, aging, and body composition study. J Am Geriatr Soc 2009; 57: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Studenski SA, Peters KW, DE Alleyet al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69: 547–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Studenski S, Perera S, Patel Ket al. Gait speed and survival in older adults. JAMA 2011; 305: 50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bischoff HA, Stähelin HB, Monsch AUet al. Identifying a cut-off point for normal mobility: a comparison of the timed “up and go” test in community-dwelling and institutionalised elderly women. Age Ageing 2003; 32: 315–320 [DOI] [PubMed] [Google Scholar]
- 29. Pavasini R, Guralnik J, Brown JCet al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016; 14: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guralnik JM, Ferrucci L, Simonsick EMet al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995; 332: 556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalantar-Zadeh K, Kopple JD, Humphreys MHet al. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant 2004; 19: 1507–1519 [DOI] [PubMed] [Google Scholar]
- 32. Charlson ME, Pompei P, Ales KLet al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383 [DOI] [PubMed] [Google Scholar]
- 33. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 1989; 42: 703–709 [DOI] [PubMed] [Google Scholar]
- 34. Fried LP, Tangen CM, Walston Jet al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M157 [DOI] [PubMed] [Google Scholar]
- 35. Johansen KL, Painter P, Kent-Braun JAet al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int 2001; 59: 1121–1127 [DOI] [PubMed] [Google Scholar]
- 36. Anderson BM, Qasim M, Correa Get al. Correlations, agreement and utility of frailty instruments in prevalent haemodialysis patients: baseline cohort data from the FITNESS study. Clin Kidney J 2021; 15: 145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kramer A, Pippias M, Noordzij Met al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary. Clin Kidney J 2019; 12: 702–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pu J, Jiang Z, Wu Wet al. Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open 2019; 9: e020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graham-Brown MPM, Herrington WG, Burton JO. Spinning the legs and blood: should intradialytic exercise be routinely offered during maintenance haemodialysis? Clin Kidney J 2021; 14: 1297–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deligiannis A, D'Alessandro C, Cupisti A. Exercise training in dialysis patients: impact on cardiovascular and skeletal muscle health. Clin Kidney J 2021; 14: ii25–ii33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clyne N, Anding-Rost K. Exercise training in chronic kidney disease–effects, expectations and adherence. Clin Kidney J 2021; 14(Suppl 2): ii3–ii14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGuire S, Horton EJ, Renshaw Det al. Cardiac stunning during haemodialysis: the therapeutic effect of intra-dialytic exercise. Clin Kidney J 2021; 14: 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greenwood SA, Koufaki P, Macdonald Jet al. The PrEscription of intraDialytic exercise to improve quAlity of Life in patients with chronic kidney disease trial: study design and baseline data for a multicentre randomized controlled trial. Clin Kidney J 2021; 14: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Segura-Ortí E, Kouidi E, Lisón JF. Effect of resistance exercise during hemodialysis on physical function and quality of life: randomized controlled trial. Clin Nephrol 2009; 71: 527–537 [DOI] [PubMed] [Google Scholar]
- 45. Groussard C, Rouchon-Isnard M, Coutard Cet al. Beneficial effects of an intradialytic cycling training program in patients with end-stage kidney disease. Appl Physiol Nutr Metab 2015; 40: 550–556 [DOI] [PubMed] [Google Scholar]
- 46. Johansen KL, Painter PL, Sakkas GKet al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol 2006; 17: 2307–2314 [DOI] [PubMed] [Google Scholar]
- 47. Guralnik JM, Simonsick EM, Ferrucci Let al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–M94 [DOI] [PubMed] [Google Scholar]
- 48. Rosas-Estrada GM, Yarce Pinzón E, Paredes-Arturo YVet al. Velocidad de la marcha en ancianos de la comunidad de la ciudad de pasto. Rev UNIMAR 2015; 33: 191–199 [Google Scholar]
- 49. Serra Rexach JA. Consecuencias clínicas de la sarcopenia. Nutr Hosp 2006; 21: 46–50 [PubMed] [Google Scholar]
- 50. Chamney PW, Wabel P, Moissl UMet al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 2007; 85: 80–89 [DOI] [PubMed] [Google Scholar]
- 51. Gracia-Iguacel C, González-Parra E, Pérez-Gómez MVet al. Prevalencia del síndrome de desgaste proteico-energético y su asociación con mortalidad en pacientes en hemodiálisis en un centro en españa. Nefrologia 2013; 33: 495–50523897181 [Google Scholar]
- 52. Cheema B, Abas H, Smith Bet al. Progressive Exercise for Anabolism in Kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol 2007; 18: 1594–1601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A spreadsheet with all clinical data collected from patients (whose information is anonymized) is available upon request.