ABSTRACT
Lung cancer is the leading cause of cancer-related mortality and approximately 5% of non–small-cell lung cancer (NSCLC) patients are positive for anaplastic lymphoma kinase (ALK) gene rearrangement or fusion with echinoderm microtubule-associated protein-like 4. ALK inhibitors are the mainstay treatment for patients with NSCLC harboring a rearrangement of the ALK gene or the ROS1 oncogenes. With the recent publication of pivotal trials leading to the approval of these compounds in different indications, their toxicity profile warrants an update. Several ALK-1 inhibitors are used in clinical practice, including crizotinib, ceritinib and alectinib. According to the package insert and published literature, treatment with several ALK-1 inhibitors appears to be associated with the development of peripheral edema and rare electrolyte disorders, kidney failure, proteinuria and an increased risk for the development and progression of renal cysts. This review introduces the different types of ALK inhibitors, focusing on their detailed kidney-related side effects in clinical practice.
Keywords: ALK-1, anaplastic lymphoma kinase, crizotinib, cyst, onconephrology, serum creatinine
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Non–small-cell lung cancer (NSCLC) is the leading cause of cancer-related deaths worldwide and in the United States [1, 2]. Most patients who have NSCLC present with advanced or incurable disease and cytotoxic chemotherapy generally results in low response rates and only modest improvements in overall survival. Early in the 2000s, investigators in Japan identified anaplastic lymphoma kinase (ALK) as another potential target in NSCLC [3, 4]. In a small subset of NSCLC tumors, a chromosomal inversion event leads to the fusion of a portion of the ALK gene with the echinoderm microtubule-associated protein-like 4 (EML4) gene [3, 4]. The resulting EML4–ALK fusion protein is constitutively activated and transforming, leading to a state of oncogene addiction. EML4–ALK fusion and other ALK rearrangements occur in 3–7% of patients with NSCLC (referred to as ‘ALK-positive’ lung cancer) and are associated with younger age, never-smoking or light-smoking history and adenocarcinoma histology. Patients who have advanced ALK-positive NSCLC are highly responsive to ALK inhibitors [5].
Subsequently, ALK gene fusions, predominantly the NPM1–ALK fusion, have been identified in nearly all pediatric and approximately half of the adult cases of anaplastic large cell lymphoma (ALCL), a rare form of non-Hodgkin lymphoma [6]. Other cancer types in which ALK fusions have been identified include renal, pancreatic, colorectal, breast and thyroid cancers [7]. Neuroblastoma, a childhood cancer arising in immature nerve cells that accounts for ∼10% of pediatric cancer deaths, is characterized by a different kind of oncogenic ALK alteration [8]. In addition to fusions and mutations, ALK gene amplification and copy number gain are observed in many tumor types, including neuroblastoma, rhabdomyosarcoma and esophageal cancer. ALK inhibitors have been used in other cancers, including pancreatic cancer, cancers of unknown origin and thyroid cancer. Data on renal effects in those cancers are not available [7, 9–23].
With the increased use of ALK inhibitors, adverse events have been noted [21–26]. In the US Food and Drug Administration Adverse Event Reporting System analysis [27], 88 cases of renal impairment were noted, consistent with published reports. In addition, we found evidence of electrolyte disorders as well (hyponatremia, 24 cases; hypokalemia, 13 cases). There is no evidence of those disorders reported in the existing literature.
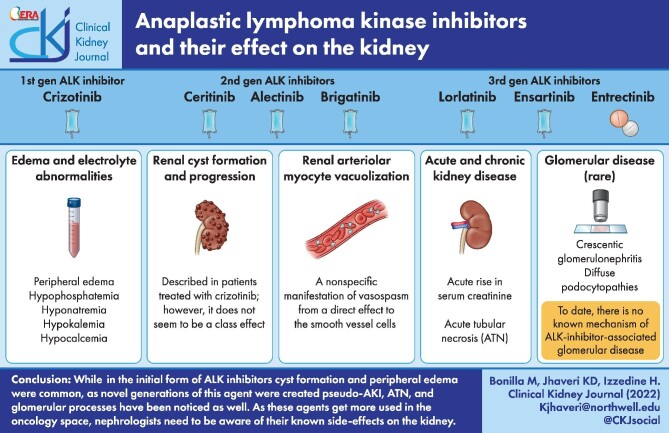
This review focuses on the kidney-related side effects associated with ALK inhibitors (Figure 1) [28, 29].
FIGURE 1:
Summary of the various renal effects of ALK inhibitors. Figure was created using biorender.com.
ALK inhibitor molecules
First-generation ALK inhibitors
Crizotinib
Crizotinib (Xalkori) is a first-generation ALK inhibitor approved for ALK-positive NSCLC [30]. It has activity against EML4–ALK, mesenchymal–epithelial transition factor (c-MET) and ROS1 tyrosine kinases [31–34]. It is approved for ALK-positive, locally advanced and metastatic NSCLC [35].
Second-generation ALK inhibitors
Ceritinib
Ceritinib (Zykadia) is a potent ALK inhibitor compared with crizotinib [36–38]. Ceritinib was approved for the treatment of relapsed or refractory NSCLC after crizotinib [39].
Alectinib
Alectinib (Alecensa) is a potent and highly selective inhibitor of ALK tyrosine kinase and has activity against L1196M, one of the commonly seen secondary mutations in the ALK gene leading to resistance to crizotinib. Grade 3 adverse events were reported in 26% of patients (n = 12) and included elevated creatinine phosphokinase and neutropenia [40].
Brigatinib (AP26113)
Brigatinib is another second-generation ALK inhibitor. It is a potent dual inhibitor of ALK and epidermal growth factor receptor (EGFR), including ALK L1196M and EGFR T790M mutants, shown in preclinical and first-in-human studies [41–43]. The most common treatment-emergent adverse events included nausea, diarrhea, fatigue, cough and headache. Early-onset pulmonary events were observed less frequently with the 90 mg starting dose compared with higher doses.
Third-generation ALK inhibitors
Lorlatinib
Lorlatinib (PF-06 463 922) is a novel, reversible, potent adenosine triphosphate (ATP)-competitive small-molecule inhibitor of ALK and ROS1. This third-generation inhibitor is effective against all known resistant mutants [44–46]. Lorlatinib combined with PI3K pathway inhibitors, such as PF-05212384 (PI3K/mTOR), GDC0941 (pan-PI3K) or GDC0032 (beta-sparing), is used to overcome ALK mutations and ALK inhibitor resistance [47].
Ensartinib
Ensartinib (X-396) is an aminopyridazine-based potent ALK–tyrosine kinase inhibitor with high activity against both wild-type ALK and all evaluated ALK variants (F1174, C1156Y, L1196M, S1206R, T1151 and G1202R mutants) and brain metastases; it also potently inhibits TPM3-TRKA, TRKC, ROS1, EphA2, EphA1, EphB1 and c-MET [48].
Preclinical data demonstrated increased potency of the drug compared with crizotinib and other second-generation tyrosine kinase inhibitors [49]. The most common treatment-related adverse events were rash [89 (56%)], increased alanine aminotransferase concentrations [74 (46%)] and increased aspartate aminotransferase concentrations [65 (41%)].
Entrectinib (RXDX-101)
Entrectinib is a potent, orally available ATP-competitive inhibitor of the ALK, ROS1 and tropomyosin receptor kinase (TRK) family rearrangements. In vitro and in vivo models of entrectinib showed activity against ALK-rearranged NSCLC with strong intracranial activity. It showed good antiproliferative activity with high activity against the G1269A mutation, slight loss of potency in the presence of C1156Y and L1196M ALK-resistance mutations and minimal activity on G1202R mutation [50].
In two phase I/II trials (Alka-372-001 trial and the STARTRK-1), no significant safety issues were reported, with majority of the adverse events being of grade 1 or 2 [51–53].
Kidney effects of ALK inhibitors
Edema and electrolyte abnormalities
Peripheral edema appears to be the most common side effect from ALK-inhibitor therapy, with up to 50% of patients affected when treated with the first-generation crizotinib and the third-generation lorlatinib [54]. The exact mechanism is unknown, but a postulated explanation is inhibition of the c-MET pathway. This adverse event is generally graded 1 or 2 (Table 1). Compression stockings are used for the management of these patients. For more resistant cases, diuretic use might be appropriate. Peripheral edema appears late in the treatment with ALK inhibitors and seems to be a cumulative effect of these drugs.
Table 1.
Incidence of common renal adverse effects of ALK inhibitors based on Kassem et al. and Costa et al. [28, 29]
| Author/study | Study type | Patients, n | Edema, grade 1–2/≥3, n (%) | Electrolyte abnormalities and/or HT, grade 1–2/≥3, n (%) | Increased SCr, grade 1–2/≥3, n (%) |
|---|---|---|---|---|---|
| ALK inhibitor, first generation | |||||
| Crizotinib | |||||
| PROFILE 1001 | IB | 149 | 44 (39)/0 | Hypophosphatemia 2 (4)/5 (10) | |
| PROFILE 1007 | III | 343 | 54 (31)/0 | ||
| PROFILE 1014 | III | 172 | 83 (49)/1(1) | ||
| J-Alec 2017 | III | 104 | NR | 9 (9)/0 | |
| Alex trial | III | 151 | 42 (28)/1 (1) | ||
| Camidge 2018 | III | 138 (Crizo arm) | 6 (4)/1 (1) | HT 31 (23)/13 (10) | 3 (2)/0 |
| Shaw 2020 | III | 142 (Crizo arm) | 54 (38)/18 (12) | HT 3 (2)/0 | |
| ALK inhibitor, second generation | |||||
| Alectinib | |||||
| AF-001JP | I/II | 46 | NR | 12 (26)/0 | |
| AF-001JP, third year | I/II | 58 | NR | 19 (32.8)/0 | |
| NP28673, Global | II | 138 | 34 (25)/0 | ||
| NP28761, North America | II | 87 | 20 (23)/0 | Hypophosphatemia 2 (2.3)/2 (2.3) Hypocalcemia 2 (2.3)/1 (1.1) Hyponatremia 2 (2.3)/1 (1.1) Hypokalemia 6 (6.9)/2 (2.3) |
|
| J-Alec 2017 | I/II | 103 | NR | 11 (11)/0 | |
| J-Alec 2017 | III | 103 | 9 (9)/0 | ||
| Alex trial | III | 152 | 26 (17)/0 | ||
| Ceritinib | |||||
| ASCEND-1 2627 | IB | 246 | NR | Hypophosphatemia 8 (3.3)/8 (3.3) Hyponatremia 8 (3.3)/11 (4.5) Hypokalemia 17 (6.9)/11 (4.5) |
42 (17.1)/0 |
| ASCEND-2 | II | 140 | NR | Hypophosphatemia 7 (5.0)/3 (2.1) Hypokalemia 8 (5.7)/4 (4.9) |
|
| ASCEND-4 | III | 189 | NR | 44 (22)/4 (2) | |
| ASCEND-5 | III | 115 | NR | 22 (19)/0 | |
| ASCEND-6 | I/II | 103 | NR | Hypokalemia 11 (10.7)/8 (7.8) | 34 (33)/0 |
| Real life | 208 | NR | 14 (6.7)/8 (3.8) | ||
| Brigatinib | |||||
| Camidge 2018 | III | 137 (Briga arm) | 53 (39)/1(1) | HT 10 (7)/4 (3) | 19 (14)/1 (1) |
| Gettinger 2016 | I/II | 137 | Dose independent (33%) | HT dose dependent: 6–27% and 0–7% | |
| Kim 2017 | II | 222 | NR | HT dose dependent: 11–21% and 6% | |
| ALK inhibitor, third generation | |||||
| Lorlatinib | |||||
| Bauer | I | 295 | 151 (51.2)/7(2.4) | ||
| Shaw 2017 | I | 54 | 21 (39)/0 | ||
| Solomon 2018 | II | 276 | 113 (41)/6 (2) | Hypophosphatemia 2 (1)/2 (1) Hyponatremia 1 (<1)/1 (<1) HT 4(1)/4(1) |
|
| Shaw 2019 | I/II | 346 | 27 (39)/1(1) | Hypophosphatemia 2 (3)/4 (6) Hyponatremia 0/1 (1) |
|
| Shaw 2020 | I | 149 (Lorla arm) | 76 (51)/6 (4) | HT 12 (8)/15 (10) | |
| Ensartinib | |||||
| Horn 2018 | I/II | 97 | 15 (15)/1 (1) | ||
| Yang 2019 | II | 156 | 14 (9)/2 (1) | 30 (19)/0 | |
| Entrectinib | |||||
| Drillon 2019 | I/II | 53 | 22 (16)/0 | Hypophosphatemia 2 (1)/1 (<1) Hyperuricemia 11 (8)/1 (<1) HT 0/1 (<1) |
17 (13)/1(<1) |
| Doebele 2019 | I/II | 355 | 49 (14)/1 (<1) | Hypophosphatemia 6 (2)/4 (1) Hyperuricemia 13 (4)/5 (1) Hyponatremia 3 (1)/2 (1) HT 0/1 (<1) |
52 (15)/2 (1) |
NR, not reported; HT, hypertension; SCr, serum creatinine.
Electrolyte abnormalities have been described with the use of ALK inhibitors, and though uncommon, hypophosphatemia has been most recognized. Hypophosphatemia during the treatment with crizotinib was reported in ∼15% of subjects as a severe adverse event [31]. With the second-generation ALK inhibitors alectinib and ceritinib, hypophosphatemia was reported as a severe event (grade 3–4) in ∼2–4% of subjects [55, 56], and with the third-generation ALK inhibitors, hypophosphatemia as a severe adverse event was reported in 1% of patients. In a review by Adhikari et al. [57], the authors attributed hypophosphatemia in patients treated with crizotinib to inhibition of the insulin (IGF-1) receptor located in the proximal tubules blocking the reabsorption of phosphate and thus producing phosphaturia.
Hyponatremia as a grade ≥3 event was reported in up to 5% of patients treated with ceritinib (second-generation ALK inhibitor), a tyrosine kinase inhibitor that selectively and potently inhibits ALK. Several reports have associated hyponatremia in patients treated with tyrosine kinase inhibitors with inadequate secretion of antidiuretic hormone [58]. Fewer adverse events associated with hyponatremia were reported with alectinib (1.1%) and third-generation ALK inhibitors (≤1%). Hypokalemia and a few cases of hypocalcemia have also been reported (Table 1) [28, 29]. Currently, no specific etiology has been described with ALK inhibitors.
Acute and chronic kidney disease
ALK inhibitors have been associated with acute and chronic kidney disease. Initially described with the first-generation crizotinib, an acute increase in serum creatinine might not be a reflection of a true kidney injury but a pseudo kidney injury from inhibition of a creatinine transporter, thus interfering with the secretion of creatinine in the proximal tubule [59]. Second-generation ALK inhibitors are more frequently associated with elevated serum creatinine after starting treatment. Alectinib is associated with increased serum creatinine in up to 26% of patients [40], whereas ceritinib is associated in up to 33% of patients [60]. Multiple case reports have described the association of starting alectinib with an increase in serum creatinine and improvement in creatinine level upon withdrawal of alectinib [61, 62], but the exact underlying mechanism is yet to be discovered. Management of an acute increase in serum creatinine levels has been managed with withdrawal of the offending drug, but this becomes challenging in patients with a reduction in the progression of cancer with these therapies; possibly changing to a third-generation ALK inhibitor like lorlatinib may be beneficial. Regardless, any patient treated with ALK inhibitors who develops creatinine elevation should have a cystatin C level and/or iothalamate study to assess for an actual kidney injury. Patients with underlying chronic kidney disease, previous chemotherapy treatments and volume depletion could be predisposed to true kidney injury [63].
In addition, it is essential to keep in mind that diarrhea and vomiting are highly prevalent with this class of medications [29] and can lead to prerenal azotemia.
Gastaud et al. [64] described a case of kidney biopsy–proven acute tubular necrosis (ATN) in a patient with locally advanced NSCLC treated with crizotinib for targeted therapy of EML4–ALK. Another case reported an acute kidney injury (AKI) with the second-generation ALK inhibitor alectinib; the authors described a euvolemic patient with no previous exposure to nephrotoxic agents who developed AKI within 6 weeks of treatment with alectinib, requiring emergent hemodialysis. Due to the temporal association between initiation of ALK inhibitor and the absence of other causes of AKI, the authors hypothesized that AKI was likely secondary to ATN, even though no kidney biopsy was performed in this patient. Kidney recovery was achieved after discontinuation of alectinib [61].
Renal arteriolar myocyte vacuolization
Mainly described as vascular toxicity from calcineurin inhibitors (CNIs), arteriolar myocyte vacuolization is a nonspecific manifestation of vasospasm from a direct effect of CNI on smooth vessels cells [65]. It has been described as well with amphotericin B [66].
To our knowledge, there is one case reporting renal arteriolar myocyte vacuolization associated with the first-generation ALK inhibitor crizotinib [67]. Nephrologists should be aware of these effects from ALK inhibitors; however, it should only be attributed to ALK inhibitors after excluding other causes of vascular injury.
Renal cyst formation and progression
The development of renal cysts has been described in patients treated with crizotinib; however, it does not seem to be a class effect (Table 1). In a study by Lin et al. [68], investigators enrolled patients who participated and received crizotinib in three prospective clinical trials. They found that 23 patients had renal cysts before initiation of crizotinib treatment. Seven patients demonstrated a change in their previous renal cysts during treatment with crizotinib. New complex cysts were identified in four patients. After discontinuation of crizotinib, complex renal cysts regressed [68]. A blinded, retrospective independent radiologic review performed by Schnell et al. [69] reviewed scans from patients in three clinical trials (PROFILE 1001, PROFILE 1005 and PROFILE 1007). They describe that of 255 patients treated with crizotinib, 22% had a pre-existing simple cyst, 3% had a complex cyst and 2% had both. Patients were assessed at 6 months and 9% of all patients had acquired new cysts. Two percent of patients with preexisting cysts had developed new cysts and enlargements of simple cysts [69]. Close monitoring of patients on treatment with crizotinib who have preexisting cysts is recommended.
Glomerular disease
ALK inhibitor is rarely associated with glomerular diseases. To our knowledge, there are two case reports of minimal change disease or diffuse podocytopathies and one case of crescentic glomerulonephritis associated with ALK inhibitor treatment. Betton et al. [70] described a case of an elderly woman who developed nephrotic syndrome 1 month after initiation of lorlatinib for lung adenocarcinoma. A kidney biopsy showed normal cortical structures under light microscopy and diffuse podocyte foot process effacement under electron microscopy. Lorlatinib was discontinued and within 2 weeks the edema resolved, proteinuria decreased to 0.2 g/g and the level of serum albumin increased. The patient was rechallenged with lorlatinib due to disease progression and subsequent proteinuria quantification increased to 3.6 g/g within 3 days of drug initiation [70]. McGee et al. [71] reported a female patient who developed hyperlipidemia while on lorlatinib and was found to have minimal change disease on kidney biopsy. Another case by Lee et al. [72] reported lorlatinib-induced proteinuria in NSCLC. This was not biopsy-proven but was presumed to be podocytopathy. A dose reduction by 50% led to improvement of the proteinuria [72].
A case of rapid progressive glomerulonephritis was described by Nagai et al. [73], associated with alectinib (second-generation ALK inhibitor). They reported a 68-year-old woman with advanced NSCLC on alectinib who developed rapidly progressive glomerulonephritis within 1 year of starting therapy. Kidney biopsy demonstrated light microscopy, interstitial nephritis with tubular vacuolization and fibrocellular crescent formations in several glomeruli. The immunofluorescence study was negative and electron microscopy showed diffuse foot process effacement. The patient was treated with pulse corticosteroids and corticosteroid taper. The ALK inhibitor was discontinued and kidney function remained stable [73]. To date, there is no known mechanism of ALK inhibitor–associated glomerular disease. Physicians should be aware of this association and monitor kidney function closely while patients receive therapy with these medications. However, it appears that the second-generation ALK inhibitors may lead to podocytopathies. Table 2 summarizes the clinical approach to the kidney adverse events associated with ALK inhibitors.
Table 2.
Clinical approach to renal adverse effects of ALK inhibitors
| Adverse event | Mechanism | Management |
|---|---|---|
| Renal cyst | Unknown | Self-limiting, not to be confused with tumor progression |
| Peripheral edema | Inhibition of c-MET | Diuretics only to be used if severe edema. Be mindful of electrolyte imbalances |
| Elevated serum creatinine | Pseudo AKI, ATN | Check Cystatin Cbased glomerular filtration rate to rule out pseudo-AKI. If ATN, drug needs to be withheld |
| Proteinuria | Likely podocytopathies | Consider holding or decreasing dose |
CONCLUSION
ALK inhibitors have now been approved and are being used for not just NSCLC, but other hematologic and solid tumors. We reviewed the several renal effects of these agents. While in the initial form of ALK inhibitors, cyst formation and peripheral edema were common, as novel generations of these agents have been created, we are noticing pseudo-AKI, ATN and glomerular processes as well. As these agents are used more often in oncology, nephrologists need to be aware of their known side effects on the kidney.
Acknowledgments
Thanks to Dr Edgar Lerma for creating the visual abstract.
Contributor Information
Marco Bonilla, Division of Kidney Diseases and Hypertension, Donald and Barbara Zucker School of Medicine at Hofstra Northwell, Great Neck, NY, USA.
Kenar D Jhaveri, Division of Kidney Diseases and Hypertension, Donald and Barbara Zucker School of Medicine at Hofstra Northwell, Great Neck, NY, USA.
Hassan Izzedine, Department of Nephrology, Peupliers Private Hospital, Ramsay Générale de Santé, Paris, France.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no relevant financial interests. K.D.J. is a consultant for Astex Pharmaceuticals, Natera, GlaxoSmithKline, ChemoCentryx and Chinook and George Clinicals. He is a paid contributor to Uptodate.com and receives honorarium from the International Society of Nephrology and American Society of Nephrology. K.D.J. servers as co-president of the American Society of Onconephrology. M.B. and H.I. have nothing to disclose.
REFERENCES
- 1. American Cancer Society . Cancer Facts & Figures 2015. Atlanta: American Cancer Society, 2015 [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit Ret al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386 [DOI] [PubMed] [Google Scholar]
- 3. Soda M, Choi YL, Enomoto Met al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561–566 [DOI] [PubMed] [Google Scholar]
- 4. Lin JJ, Riely GJ, Shaw AT.. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov 2017; 7: 137–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salgia SK, Govindarajan A, Salgia Ret al. ALK-directed therapy in non-NSCLC malignancies: are we ready? JCO Precis Oncol 2021; 767–770 [DOI] [PubMed] [Google Scholar]
- 6. Fukano R, Mori T, Sekimizu Met al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: an open-label phase II trial. Cancer Sci 2020; 111: 4540–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mossé YP, Lim MS, Voss SDet al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol 2013; 14: 472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Umapathy G, Mendoza-Garcia P, Hallberg Bet al. Targeting anaplastic lymphoma kinase in neuroblastoma. APMIS 2019; 127: 288–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulte JH, Eggert A.. ALK inhibitors in neuroblastoma: a sprint from bench to bedside. Clin Cancer Res 2021; 27: 3507–3509 [DOI] [PubMed] [Google Scholar]
- 10. Pezeshki PS, Moeinafshar A, Ghaemdoust Fet al. Advances in pharmacotherapy for neuroblastoma. Expert Opin Pharmacother 2021; 22: 2383–2404 [DOI] [PubMed] [Google Scholar]
- 11. Brunac AC, Laprie A, Castex MPet al. The combination of radiotherapy and ALK inhibitors is effective in the treatment of intraosseous rhabdomyosarcoma with FUS-TFCP2 fusion transcript. Pediatr Blood Cancer 2020; 67: e28185. [DOI] [PubMed] [Google Scholar]
- 12. Pacenta HL, Macy ME.. Entrectinib and other ALK/TRK inhibitors for the treatment of neuroblastoma. Drug Des Devel Ther 2018; 12: 3549–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh RR, O'Reilly EM.. New treatment strategies for metastatic pancreatic ductal adenocarcinoma. Drugs 2020; 80: 647–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ou K, Liu X, Li Wet al. ALK rearrangement-positive pancreatic cancer with brain metastasis has remarkable response to ALK inhibitors: a case report. Front Oncol 2021; 11: 724815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao P, Peng L, Wu Wet al. Carcinoma of unknown primary with EML4-ALK fusion response to ALK inhibitors. Oncologist 2019; 24: 449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsiao SY, He HL, Weng TSet al. Colorectal cancer with EML4-ALK fusion gene response to alectinib: a case report and review of the literature. Case Rep Oncol 2021; 14: 232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amatu A, Somaschini A, Cerea Get al. Novel CAD-ALK gene rearrangement is drugable by entrectinib in colorectal cancer. Br J Cancer 2015; 113: 1730–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aydemirli MD, van Eendenburg JDH, van Wezel Tet al. Targeting EML4-ALK gene fusion variant 3 in thyroid cancer. Endocr Relat Cancer 2021; 28: 377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boulos JC, Saeed MEM, Chatterjee Met al. Repurposing of the ALK inhibitor crizotinib for acute leukemia and multiple myeloma cells. Pharmaceuticals (Basel) 2021; 14: 1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moharram SA, Shah K, Khanum Fet al. The ALK inhibitor AZD3463 effectively inhibits growth of sorafenib-resistant acute myeloid leukemia. Blood Cancer J 2019; 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schöffski P, Sufliarsky J, Gelderblom Het al. Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European Organisation for Research and Treatment of Cancer 90101 CREATE): a multicentre, single-drug, prospective, non-randomised phase 2 trial. Lancet Respir Med 2018; 6: 431–441 [DOI] [PubMed] [Google Scholar]
- 22. Gambacorti-Passerini C, Orlov S, Zhang Let al. Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): a phase 1b open-label study. Am J Hematol 2018; 93: 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He X, Jiao XD, Liu Ket al. Clinical responses to crizotinib, alectinib, and lorlatinib in a metastatic colorectal carcinoma patient with ALK gene rearrangement: a case report. JCO Precis Oncol 2021; 5: PO.20.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takeyasu Y, Okuma HS, Kojima Yet al. Impact of ALK inhibitors in patients with ALK-rearranged nonlung solid tumors. JCO Precis Oncol 2021; 756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drilon AE, Liu H, Wu Fet al. Tumor-agnostic precision immuno-oncology and somatic targeting rationale for you (TAPISTRY): a novel platform umbrella trial. J Clin Oncol 2021; 39: TPS3154 [Google Scholar]
- 26. Kurzrock R, MacKenzie AR, Jurdi AAet al. Alpha-T: an innovative decentralized (home-based) phase 2 trial of alectinib in ALK-positive (ALK+) solid tumors in a histology-agnostic setting. J Clin Oncol 2021; 39: TPS3155 [Google Scholar]
- 27. Jhaveri KD, Wanchoo R, Sakhiya Vet al. Adverse renal effects of novel molecular oncologic targeted therapies: a narrative review. Kidney Int Rep 2016; 2: 108–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kassem L, Shohdy KS, Lasheen Set al. Safety issues with the ALK inhibitors in the treatment of NSCLC: a systematic review. Crit Rev Oncol Hematol 2019; 134: 56–64 [DOI] [PubMed] [Google Scholar]
- 29. Costa RB, Costa RLB, Talamantes SMet al. Systematic review and meta-analysis of selected toxicities of approved ALK inhibitors in metastatic non-small cell lung cancer. Oncotarget 2018; 9: 22137–22146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwak EL, Bang YJ, Camidge DRet al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010; 363: 1693–16703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaw AT, Ou SH, Bang YJet al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014; 371: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui JJ, Tran-Dube M, Shen Het al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 2011; 54: 6342–6363 [DOI] [PubMed] [Google Scholar]
- 33. Yasuda H, de Figueiredo-Pontes LL, Kobayashi Set al. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol 2012; 7: 1086–1090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zou HY, Li Q, Lee JHet al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 2007; 67: 4408–4417 [DOI] [PubMed] [Google Scholar]
- 35. Camidge DR, Bang YJ, Kwak ELet al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012; 13: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friboulet L, Li N, Katayama Ret al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014; 4: 662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marsilje TH, Pei W, Chen Bet al. Synthesis, structure activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013; 56: 5675–5690 [DOI] [PubMed] [Google Scholar]
- 38. Rolfo C, Passiglia F, Russo Aet al. Looking for a new panacea in ALK rearranged NSCLC: may be ceritinib? Expert Opin Ther Targets 2014; 18: 983–985 [DOI] [PubMed] [Google Scholar]
- 39. Chabner BA. Approval after phase I: ceritinib runs the three-minute mile. Oncologist 2014; 19: 577–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seto T, Kiura K, Nishio Met al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced nonsmall-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol 2013; 14: 590–598 [DOI] [PubMed] [Google Scholar]
- 41. Camidge DR, Bazhenova L, Salgia Ret al. First-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies: updated results. J Clin Oncol 2013; 31: 8031 [Google Scholar]
- 42. Huang W-S, Li F, Cai Let al. Abstract 2827: discovery of AP26113, a potent, orally active inhibitor of anaplastic lymphoma kinase and clinically relevant mutants. Cancer Res 2015; 75: 2827 [Google Scholar]
- 43. Zhang S, Nadworny S, Wardwell SDet al. Abstract 781: the potent ALK inhibitor AP26113 can overcome mechanisms of resistance to first- and second-generation ALK TKIs in preclinical models. Cancer Res 2015; 75: 781. [DOI] [PubMed] [Google Scholar]
- 44. Zou HY, Friboulet L, Kodack DPet al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell 2015; 28: 70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tucker ER, Danielson LS, Innocenti Pet al. Tackling crizotinib resistance: the pathway from drug discovery to the pediatric clinic. Cancer Res 2015; 75: 2770–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Infarinato NR, Park JH, Krytska Ket al. The ALK/ROS1 inhibitor PF-06463922 overcomes primary resistance to crizotinib in ALK-driven neuroblastoma. Cancer Discov 2016; 6: 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei P, Qiu M, Lee Net al. Abstract 764: rational combination of PF-06463922 (next-generation ALK inhibitor) with PI3K pathway inhibitors overcomes ALKi resistance in EML4-ALK+ NSCLC models. Cancer Res 2015; 75: 764 [Google Scholar]
- 48. Li T, Ma W, Tian EC.. Ensartinib (X-396): what does it add for patients with ALK-rearranged NSCLC. Chin Clin Oncol 2019; 8(Suppl 1): S4 . [DOI] [PubMed] [Google Scholar]
- 49. Singhi EK, Horn L.. Background and rationale of the eXalt3 trial investigating X-396 in the treatment of ALK+ non-small-cell lung cancer. Future Oncol 2018; 14: 1781–1787 [DOI] [PubMed] [Google Scholar]
- 50. Ardini E, Menichincheri M, Banfi Pet al. Entrectinib, a Pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther 2016; 15: 628–639 [DOI] [PubMed] [Google Scholar]
- 51. De Braud F, Niger M, Damian Set al. Alka-372-001: First-in-human, phase I study of entrectinib—an oral pantrk, ROS1, and ALK inhibitor—in patients with advanced solid tumors with relevant molecular alterations. J Clin Oncol 2015; 33: abstract 2517 [Google Scholar]
- 52. Patel MR, Bauer TM, Liu SVet al. STARTRK-1: Phase 1/2a study of entrectinib, an oral Pan-Trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations. J Clin Oncol 2015; 33: abstract 2596 [Google Scholar]
- 53. Drilon A, Siena S, Ou Set al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov 2017; 7: 400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shaw AT, Bauer TM, de Marinis Fet al. First-line lorlatinib or crizotinib in advanced ALK-Positive lung cancer. N Engl J Med 2020; 383: 2018–2029 [DOI] [PubMed] [Google Scholar]
- 55. Gadgeel SM, Gandhi L, Riely GJet al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014; 15: 1119–1128 [DOI] [PubMed] [Google Scholar]
- 56. Crinò L, Ahn MJ, De Marinis Fet al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol 2016; 34: 2866–2878 [DOI] [PubMed] [Google Scholar]
- 57. Adhikari S, Mamlouk O, Rondon-Berrios Het al. Hypophosphatemia in cancer patients. Clin Kidney J 2021; 14: 2304–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hill J, Shields J, Passero V.. Tyrosine kinase inhibitor-associated syndrome of inappropriate secretion of anti-diuretic hormone. J Oncol Pharm Pract 2016; 22: 729–732 [DOI] [PubMed] [Google Scholar]
- 59. Izzedine H, El-Fekih RK, Perazella MA.. The renal effects of ALK inhibitors. Invest New Drugs 2016; 34: 643–649 [DOI] [PubMed] [Google Scholar]
- 60. Wu YL, Shi Y, Tan DSet al. Phase 1/2 study of ceritinib in Chinese patients with advanced anaplastic lymphoma kinase-rearranged non–small cell lung cancer previously treated with crizotinib: results from ASCEND-6. Lung Cancer 2020; 150: 240–246 [DOI] [PubMed] [Google Scholar]
- 61. Ramachandran P, Morcus R, Tahir Met al. Alectinib (Alecensa)-induced reversible grade IV nephrotoxicity: a case report and review of the literature. J Med Case Rep 2018; 12: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shimada M, Fukuda M, Fukuda Met al. Adverse renal effects of anaplastic lymphoma kinase inhibitors and the response to alectinib of an ALK+ lung cancer patient with renal dysfunction. OncoTargets Ther 2017; 10: 3211–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Perazella MA, Izzedine H.. Crizotinib: renal safety evaluation. J Onco-Nephrol 2017; 1: 49–56 [Google Scholar]
- 64. Gastaud L, Ambrosetti D, Otto Jet al. Acute kidney injury following crizotinib administration for non-small-cell lung carcinoma. Lung Cancer 2013; 82: 362–364 [DOI] [PubMed] [Google Scholar]
- 65. Randhawa PS, Starzl TE, Demetris AJ.. Tacrolimus (FK506)-associated renal pathology. Adv Anat Pathol 1997; 4: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bullock WE, Luke RG, Nuttall CEet al. Can mannitol reduce amphotericin B nephrotoxicity? Double-blind study and description of a new vascular lesion in kidneys. Antimicrob Agents Chemother 1976; 10: 555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Izzedine H, Brocheriou I, Amoura Zet al. Acute tubular injury and renal arterial myocyte vacuolization following crizotinib administration. Kidney Int Rep 2021; 6: 526–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lin YT, Wang YF, Yang JCet al. Development of renal cysts after crizotinib treatment in advanced ALK-positive non–small-cell lung cancer. J Thorac Oncol 2014; 9: 1720–1725 [DOI] [PubMed] [Google Scholar]
- 69. Schnell P, Bartlett CH, Solomon BJet al. Complex renal cysts associated with crizotinib treatment. Cancer Med 2015; 4: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Betton M, Gounant V, Sannier Aet al. Minimal change disease induced by lorlatinib. J Thorac Oncol 2018; 13: e154–e156 [DOI] [PubMed] [Google Scholar]
- 71. McGee K, Stone NJ, Wadhwani Set al. A possible mechanism of hyperlipidemia in a patient with metastatic non–small cell lung cancer on lorlatinib therapy. J Oncol Pharm Pract 2021; 27: 2010–2013 [DOI] [PubMed] [Google Scholar]
- 72. Lee CS, Wanchoo R, Seetharamu N.. Lorlatinib induced proteinuria: a case report. J Oncol Pharm Pract 2021; 27: 1037–1039 [DOI] [PubMed] [Google Scholar]
- 73. Nagai K, Ono H, Matsuura Met al. Progressive renal insufficiency related to ALK inhibitor, alectinib. Oxf Med Case Rep 2018; 2018: omy009. [DOI] [PMC free article] [PubMed] [Google Scholar]