ABSTRACT
Background
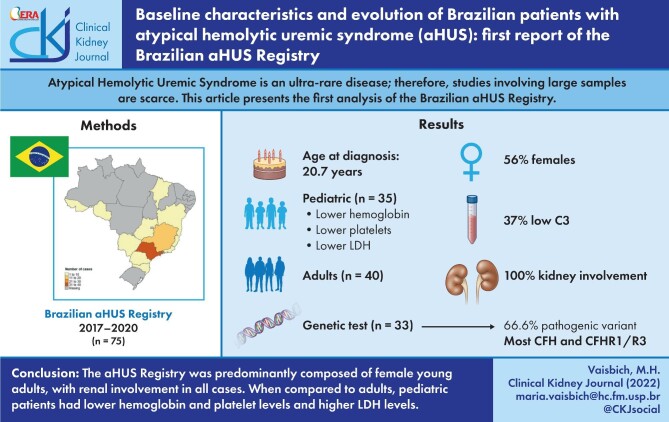
Atypical hemolytic uremic syndrome (aHUS) is an ultra-rare disease. Therefore, studies involving large samples are scarce, making registries powerful tools to evaluate cases. We present herein the first analysis of the Brazilian aHUS Registry (BRaHUS).
Methods
Analysis of clinical, laboratory, genetic and treatment data from patients inserted in the BRaHUS, from 2017 to 2020, as an initiative of the Rare Diseases Committee of the Brazilian Society of Nephrology.
Results
The cohort consisted of 75 patients (40 adults and 35 pediatric). There was a predominance of women (56%), median age at diagnosis of 20.7 years and a positive family history in 8% of cases. Renal involvement was observed in all cases and 37% had low C3 levels. In the <2 years of age group, males were predominant. Children presented lower levels of hemoglobin (P = .01) and platelets (P = .003), and higher levels of lactate dehydrogenase (LDH) (P = .004) than adults. Genetic analysis performed in 44% of patients revealed pathogenic variants in 66.6% of them, mainly in CFH and the CFHR1-3 deletion. Plasmapheresis was performed more often in adults (P = .005) and 97.3% of patients were treated with eculizumab and its earlier administration was associated with dialysis-free after 3 months (P = .08).
Conclusions
The cohort of BRaHUS was predominantly composed of female young adults, with renal involvement in all cases. Pediatric patients had lower hemoglobin and platelet levels and higher LDH levels than adults, and the most common genetic variants were identified in CFH and the CFHR1-3 deletion with no preference of age, a peculiar pattern of Brazilian patients.
Keywords: atypical hemolytic uremic syndrome, Brazil, eculizumab, genetic, rare diseases
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Atypical hemolytic uremic syndrome (aHUS) is a thrombotic microangiopathy caused by the inability to self-regulate the alternative complement pathway. As consequence of this pathway imbalance, a massive membrane attack complex (MAC, C5b-9) production occurs causing severe damage to endothelial cells throughout the body [1].
There is a well-known genetic basis for nearly two-thirds of cases of aHUS, most related to inactivating mutations in genes codifying inhibiting proteins of the alternative pathway: Factor H (CFH), Factor I (CFI), membrane cofactor protein (MCP or CD46), thrombomodulin (THBD), large deletions or insertions in Factor H-related proteins 1 to 5 (CFHR1 to 5) or gain-of-function mutations in genes codifying activating factors of this complement pathway (C3 or Factor B) [2–4].
aHUS is a rare genetic disease and the knowledge of its epidemiological data, natural history, genetic profile and pathophysiology have been increasing over recent years [5]. However, reports from low-middle income countries' populations of aHUS are restricted to a few cohort series [6–12]. The availability of data from these countries can broaden the spectrum of genotype according to the region.
In rare diseases, studies enrolling a large population are difficult to achieve and registries are powerful tools to overcome this obstacle. Registry data of rare diseases are important in understanding and providing clinical insights and are essential for strategic planning in structuring support and allocation of healthcare resources. In addition, rare diseases registries can provide research opportunities and solve issues related to scientific studies. They can facilitate patient's recruitment for clinical trials as well as providing historical controls data [13].
The Brazilian aHUS Registry (http://comdora-sbn.org.br/registros) is an observational, non-interventional, industry-independent and multicenter registry of patients with aHUS. The aims of the Registry are to assess the clinical and epidemiological characteristics, genetic profile as well as long-term outcomes of aHUS patients in Brazil.
The Brazilian aHUS Registry was an initiative of the Rare Diseases Committee of the Brazilian Society of Nephrology, named COMDORA, which is in charge of scientific oversight, governance and coordination of all COMDORA's registries. COMDORA is formed by expert physicians in the diagnosis and management of aHUS patients (e.g. adult and pediatric nephrologists). These members are responsible for validating the aHUS diagnosis of each registered case, and for contacting the physician who registered the patient, in case of doubts. The registry recommends a clinical update at 6 months and then annually.
The aim of this study was to describe the epidemiological and clinical characteristics, genetic profile and evolution of Brazilian aHUS patients.
MATERIALS AND METHODS
Study population
Eligible patients included individuals of all ages with a clinical diagnosis of aHUS as determined by the treating clinicians at each site in Brazil, with or without an identified complement regulatory factor genetic abnormality. This first report is related to data from July 2017 (first data inclusion) to 31 December 2020.
Patient data were collected following a research protocol based mainly on the choice of alternatives related to clinical data but with space for remarks that the attending physician reported spontaneously.
All procedures were performed in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines and the Declaration of Helsinki. The study was approved by the Research Ethics Committee of the Faculty of Medicine of Botucatu-UNESP (# 09 831 719.7.0000.5411). Informed consent was available on the platform and was presented to the patient/parent/guardian by the attending clinician. Patients were identified by encrypted codes in the datasheets, hosted on the Brazilian Society of Nephrology website and in full compliance with Brazilian data protection law.
Inclusion criteria
Male or female patients of any age who have been diagnosed with aHUS.
Patients with or without an identified complement pathogenic variant or anti-complement factor antibody.
Exclusion criteria
Secondary causes of TMA, in the setting of drug use, infections, cobalamin metabolism defects, neoplasia, scleroderma, antiphospholipid antibody syndrome and others.
Thrombotic thrombocytopenic purpura (TTP): TMA resulting from severe ADAMTS13 deficiency. TTP was defined by a severe deficiency of ADAMTS13 (activity <10%).
Shiga toxin-mediated hemolytic uremic syndrome (ST-HUS): related to Shiga toxin. Shiga toxins are produced by Shigella dysenteriae and some serotypes of Escherichia coli, such as O157:H7 and O104:H4.
Diagnosis of aHUS
The diagnosis of TMA was performed using the clinical history and laboratory exams compatible with TMA [microangiopathic hemolytic anemia, increased lactate dehydrogenase (LDH) >1.5 upper normal limit, thrombocytopenia and kidney injury] after exclusion of other causes of TMA [4].
The authors checked the accuracy of aHUS diagnosis of all included patients based on history and baseline exams. The presence of genetic analysis was not necessary to diagnose aHUS. All patients should have an ADAMTS13 activity measurement performed with a result higher than 10% before receiving plasma therapy, if applicable. All patients with the presence of diarrhea should have a negative Shiga Toxin PCR and/or negative stool culture. In case of concomitant infection, it should be resolved before the establishment of aHUS diagnosis. In patients using known TMA-inducing medications, the diagnosis of aHUS was established if TMA persisted for 1 week after discontinuation of the putative drug. The TMA-inducing medications list included cyclosporine, tacrolimus, rifampicin, cisplatin, bleomycin, mitomycin, bevacizumab, clopidogrel and ticlopidine.
Genetic analysis
Genetic analysis was performed according to the indication of each center. The most common test employed was an aHUS panel, which comprised the PCR amplification and target sequencing (next-generation sequencing) of complete regions of genes encoding at least the following genes according to KDIGO recommendations [4]: CFH, CD46, CFI, C3, CFB, THBD, CFHR1, CFHR5 and DGKE and including 10 base pairs next to exons. However, in some cases, more extended panels were performed.
Data collection
Demographic data included gender, age at presentation and diagnosis, family history of kidney diseases, comorbidities and clinical presentation (kidney, cardiovascular, neurological, gastrointestinal and pulmonary involvements). We evaluated all investigational diagnostic tests and exams at diagnosis. The reported exams were the most recent prior to aHUS diagnosis and included hemoglobin, platelets, LDH, haptoglobin, direct Coombs Test, presence of schistocytes in peripheral blood smears, serum creatinine, urinary protein/creatinine ratio, serum complement fractions C3 and C4, SHIGA-toxin PCR, stool culture, serum ADAMTS-13 activity, antinuclear factor test, anti-DNA test and serum homocysteine. The glomerular filtration rate (eGFR) was estimated by Chronic Kidney Disease Epidemiology Collaboration equation [14] for patients older than 18 years and the Schwartz Modified equation for patients younger than 18 years [15], using serum creatinine at presentation. Renal biopsy results were also analyzed when available.
Groups
Patients were divided into three groups according to the age at diagnosis: under 2, between 2 and 18, and older than 18 years of age. Demographic data, baseline exams, outcome and genetic tests were analyzed.
Outcomes
The primary outcome was change in eGFR and need for dialysis within 3 months of first aHUS presentation.
The secondary outcomes were:
Need for plasma exchange, blood, platelets or plasma transfusions within the first 3 months.
Time between aHUS diagnosis and eculizumab administration, if applicable.
Correlation between time from aHUS diagnosis to first eculizumab dose with long-term dialysis need.
Statistical analysis
The distribution of variables was assessed with the Shapiro–Wilk test. Qualitative variables were expressed as proportions and compared among each other via the chi-squared test or Fisher's exact test. Variables following a parametric distribution were expressed as mean ± standard error and compared among each other with ANOVA test. Variables with non-parametric distributions were expressed as median (percentiles 25 and 75) and compared among each other with the Kruskal–Wallis test. We provided the number of missing values in the tables. For statistical analysis, the R program was used (https://www.r-project.org/). Statistical significance was assigned to P < .05.
RESULTS
In the first report of Brazilian aHUS registry, most cases were from the Southeast region of Brazil (74.6%), with the state of São Paulo contributing 49.3% of the total sample (Supplementary data, Fig. S1). During the selected period of this report, 75 cases were registered, 35 of which (46.6%) were pediatric patients (17 cases <2 years of age) and 40 (53.4%) were adults. The median age at diagnosis was 20.7 years (percentiles 2.4–30.3, range 3 months to 54 years of age) and there was a predominance of females (56%). However, in patients under 2 years of age, male gender was predominant, approximately 82% of cases (14/17 patients) (Table 1). For the majority of the patients (76%), the diagnosis of aHUS was made in the first episode of TMA. Family history was reported in only 8% of cases (6/75).
Table 1.
Baseline characteristics on Brazilian aHUS Registry patients divided by age: <2 years old, between 2 and 18 years old and >18 years old
| <2 years old (n = 17) | 2–18 years old (n = 18) | >18 years old (n = 40) | Total (n = 75) | P-value | |
|---|---|---|---|---|---|
| Female (n/%) | 3 (17.6%) | 13 (72.2%) | 26 (65%) | 42 (56%) | .001 |
| Age (years) | 0.81 (0.7–1.2) | 8.84 (6.5–14.8) | 29.7 (25.95–34.5) | 20.7 (2.4–30.3) | <.001 |
| Family history (n/%) | 1 (6.2%) | 1 (5.6%) | 4 (10%) | 6 (8.1%) | .809 |
| Previous hypertension (n/%) | 3 (30%) | 7 (50%) | 31 (93.7%) | 41 (71.9%) | <.001 |
| Kidney transplant (n/%) | 1 (5.8%) | 3 (16.6%) | 18 (45%) | 22 (29%) | <.001 |
| Clinical presentation (n/%) | |||||
| Hypertension | 14 (87.5%) | 11 (64.7%) | 31 (77.5%) | 56 (76.7%) | .516 |
| Diarrhea | 3 (17.6%) | 2 (11.8%) | 5 (12.5%) | 10 (19.6%) | .831 |
| Dyspnea | 3 (30%) | 1 (12.5%) | 8 (32%) | 12 (27.9%) | .556 |
| Fatigue | 3 (27.3%) | 8 (72.7%) | 30 (85.7%) | 41 (71.9%) | <.001 |
| Elevated creatinine | 16 (94.1%) | 16 (94.1%) | 37 (94.9%) | 69 (94.5%) | .990 |
Continuous variables were expressed as median and percentiles 25 and 75.
The most frequent clinical characteristic was hypertension (76.8% of all cases), regardless of the age at diagnosis, followed by fatigue in patients older than 2 years of age (Table 1). Neurological manifestations were more frequent in patients <18 years of age than in adults (42.5% versus 32.5%). Drowsiness and seizures were the most frequent neurological findings in both groups. Gastrointestinal manifestations were also more often observed in children than adults (45.5% versus 30%), and nausea and vomiting were most frequently reported. Among the 75 cases, 22 cases (29%) were kidney-transplanted recipients, 18 of whom (81.8%) were older than 18 years of age (Table 1).
Among the 26 adult female patients, five (19.2%) were diagnosed at pregnancy. A history of concomitant infectious disease was detected in 16.2% of the total population. History of drug use was present in 20% of the cases—all of them kidney-transplanted patients—tacrolimus (11 cases), everolimus (2 patients), cyclosporine (1 case) and sirolimus (1 case) (Table 2). There was no patient with cobalamin metabolism defect.
Table 2.
Concomitant conditions on Brazilian aHUS Registry patients divided by age: <2 years old, between 2 and 18 years old and >18 years old
| <2 years old (n = 17) | 2–18 years old (n = 18) | >18 years old (n = 40) | Total (N = 75) | P-value | |
|---|---|---|---|---|---|
| Cobalamin defect | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.0 |
| Malignant HTN | 1 (5.9%) | 1 (5.9%) | 3 (8.6%) | 5 (7.2%) | .911 |
| Pregnancy | 0 (0.0%) | 0 (0.0%) | 5 (13.5%) | 5 (7.0%) | .084 |
| SLE | 0 (0.0%) | 0 (0.0%) | 1 (2.8%) | 1 (1.4%) | .619 |
| Scleroderma | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.0 |
| APS | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.0 |
| Infection | 0 (0.0%) | 5 (33.3%) | 2 (12.5%) | 4 (10.8%) | .122 |
| Neoplasia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.0 |
| Medicationsa | 0 (0.0%) | 0 (0.0%) | 15 (39.5%) | 15 (20.8%) | <.001 |
Abbreviations: APS, antiphospholipid syndrome; HTN, hypertension; SLE, systemic lupus erythematous. aTMA-inducing medications.
The most common aHUS-associated condition in the age group <2 years was malignant hypertension, present in 5.9% of total cases; the infection was most associated with aHUS in the age group between 2 and 18 years (33.3%), and in age >18 years the principal conditions associated with aHUS were medications (39.5%) followed by infections (10.8%).
Hematological exams at baseline
Anemia, negative direct Coombs Test, platelet consumption, presence of schistocytes and high levels of LDH were reported in all age groups. The levels of hemoglobin (P = .01) and platelets (P = .003) were significantly lower and LDH levels were significantly higher (P = .004) in children compared with adult patients (Table 3; Supplementary data, Fig. S2). The haptoglobin was reduced in the three groups and serum C3 complement was reduced in 26.7% of total cases (Table 3).
Table 3.
Baseline laboratory exams at diagnosis onset in Brazilian aHUS registry patients divided by age: <2 years old, between 2 and 18 years old and >18 years old
| <2 years old (n = 17) | 2–18 years old (n = 18) | >18 years old (n = 40) | Total (n = 75) | P-value | |
|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 6.0 (5.3–6.9) | 6.5 (6.0–7.4) | 7.7 (6.2–9.4) | 7.0 (6.0–8.6) | .012 |
| Coombs Test positive | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Platelets (×103/mm3) | 53 (34–55) | 55 (35.7–88.7) | 89.5 (53–126.7) | 65 (40–107) | .003 |
| LDH (U/dl) | 1855 (1484–3408) | 2097 (1186–2625) | 1000 (677–1567) | 1400 (850–2344) | .004 |
| Schistocyte | 14 (100.0%) | 13 (86.7%) | 25 (71.4%) | 52 (81.2%) | .150 |
| Not performed | 0 (0.0%) | 2 (13.3%) | 6 (17.1%) | 8 (12.5%) | |
| Haptoglobin (mg/dL) | 12.5 (10–26) | 12 (6–16) | 20 (6–37) | 13 (7–30) | .388 |
| Proteinuria | .493 | ||||
| Absent | 1 (7.7%) | 1 (6.7%) | 3 (11.1%) | 5 (9.1%) | |
| nephrotic | 5 (38.5%) | 2 (13.3%) | 6 (22.2%) | 13 (23.6%) | |
| Not nephrotic | 6 (46.2%) | 11 (73.3%) | 15 (55.6%) | 32 (58.2%) | |
| albuminuria | 0 (0.0%) | 1 (6.7%) | 0 (0.0%) | 1 (1.8%) | |
| Not performed | 1 (7.7%) | 0 (0.0%) | 3 (11.1%) | 4 (7.3%) | |
| Creatinine (mg/dL) | 1.9 (1.5–2.1) | 4.8 (2.8–9.4) | 4.6 (2.7–7.6) | 3.9 (1.9–7) | .003 |
| eGFR (mL/min) | 14.2 (7–15.6) | 14.6 (9.4–37) | 12.6 (7.7–26) | 14.2 (8.1–23.3) | .715 |
| ADAMTS-13 activity (%) | 93 (40–100) | 87 (85–100) | 79 (70–98) | 85 (68–100) | .424 |
| Shiga Toxin PCR | .007 | ||||
| Negative | 5 (33.3%) | 5 (33.3%) | 2 (5.4%) | 12 (17.9%) | |
| Positive | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Not performed | 10 (66.7%) | 10 (66.7%) | 35 (94.6%) | 55 (82.1%) | |
| Stool culture | .199 | ||||
| Negative | 5 (38.5%) | 7 (43.8%) | 7 (20.6%) | 19 (30.2%) | |
| Positive | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Not performed | 8 (61.5%) | 9 (56.2%) | 27 (79.4%) | 44 (69.8%) | |
| Antinuclear factor test | .016 | ||||
| Negative | 10 (71.4%) | 16 (100.0%) | 28 (75.7%) | 54 (80.6%) | |
| Positive | 0 (0.0%) | 0 (0.0%) | 6 (16.2%) | 6 (9.0%) | |
| Not performed | 4 (28.6%) | 0 (0.0%) | 3 (8.1%) | 7 (10.4%) | |
| Complement C3 serum | .927 | ||||
| Normal Reduced |
8 (72.7%) 3 (27.3%) |
9 (69.2%) 4 (30.8%) |
21 (75.0%) 7 (25.0%) |
38 (73.1%) 14 (26.9%) |
|
| Complement C4 serum | .314 | ||||
| Normal Reduced |
9 (81.8%) 2 (18.2%) |
13 (92.9%) 1 (7.1%) |
26 (96.3%) 1 (3.7%) |
48 (92.3%) 4 (7.7%) |
|
| Kidney biopsy | 6 (42.9%) | 10 (58.8%) | 28 (80.0%) | 44 (58.6%) | .033 |
Continuous variables were expressed as median and percentiles 25 and 75. LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate.
Renal biopsy
Kidney biopsy reports were described for 44 (58.7%) of patients and it was more frequently performed in adults when compared with pediatric patients (80% versus 58.8% and 42.9%, P = .033) (Table 3). Although there are specific blanks for filling with the description of light microscopy, immunofluorescence microscopy and electron microscopy reports, most physicians reported only the diagnosis of TMA (Supplementary data, Table S02).
Evolution of kidney function
Among the 75 patients enrolled in the registry, 45% were on dialysis 3 months after diagnosis, ranging from 42.5% (≥18 years of age) to 50% (between 2 and 18 years of age) (Table 4). Most patients were classified as CKD stage 5 at 3 months (46.2% of the total cases) (Table 4). Evolution of kidney function in patients grouped <18 years and >18 years are provided in Supplementary data, Table S01.
Table 4.
Clinical evolution of Brazilian aHUS Registry patients divided by age: <2 years old, between 2 and 18 years old and >18 years old
| <2 years old (n = 17) | 2–18 years old (n = 18) | >18 years old (n = 40) | Total (n = 75) | P-value | |
|---|---|---|---|---|---|
| Renal injury within 3 months (n/%) | |||||
| No kidney damage | 2 (14.4%) | 1 (7.7%) | 4 (16%) | 7 (13.5%) | .334 |
| Chronic kidney disease stage 1 | 1 (7.1%) | 2 (23.1%) | 1 (4%) | 5 (9.6%) | |
| Chronic kidney disease stage 2 | 1 (7.1%) | 2 (15.4%) | 2 (8%) | 5 (9.6%) | |
| Chronic kidney disease stage 3 | 2 (14.3%) | 1 (7.7%) | 6 (24%) | 9 (17.3%) | |
| Chronic kidney disease stage 4 | 2 (14.3%) | 0 | 0 | 2 (3.8%) | |
| Chronic kidney disease stage 5 | 6 (42.9%) | 6 (46.2%) | 12 (48%) | 24 (46.2%) | |
| Dialysis need | 8 (47%) | 9 (50%) | 17 (42.5%) | 34 (45%) | .956 |
| Treatment within 3 months (n/%) | |||||
| Red blood cells transfusion | 15 (93.8%) | 14 (82.4%) | 22 (59.5%) | 51 (72.9%) | .095 |
| Platelet transfusion | 10 (66.7%) | 4 (23.5%) | 8 (21.1%) | 22 (31.4%) | .019 |
| Plasma transfusion | 6 (42.9%) | 7 (43.8%) | 12 (32.4%) | 25 (37.3%) | .891 |
| Plasma exchange | 0 | 3 (20%) | 16 (42.1%) | 19 (27.5%) | .005 |
| Treatment ≥3 months (n/%) | |||||
| Eculizumab use | 16 (94.1%) | 16 (94.1%) | 39 (100%) | 71 (97.3%) | .307 |
| Time eculizumab infusion (days) | 15 (14–25) | 30 (14–44) | 45 (6–260) | 25 (7–118) | .600 |
Treatment
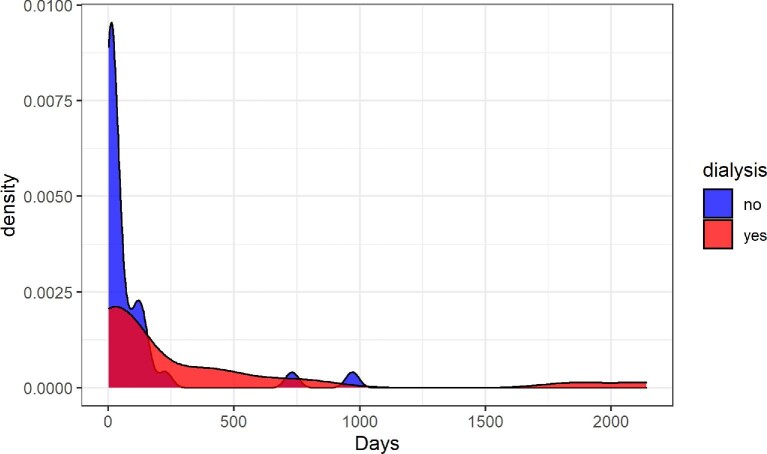
Plasma exchange was more frequently performed in adults (42.1%) than children (20%), P = .005. There was a high frequency of eculizumab treatment in all age groups, reaching 97.3% of the total population (Tables 5 and 6). The median time to eculizumab administration after aHUS diagnosis was 25 (7–188) days with no significant difference among the three groups. In dialysis-free patients, the median time to eculizumab administration was 16 days (6–87) compared with 34 (15–372) days in ongoing-dialysis cases at 3 months of follow-up, P = .081 (Fig. 1).
Table 5.
Genetic variants in aHUS Brazilian Registry divided by age ≤18 years old and >18 years old
| ≤18 years (n = 35) | >18 years (n = 40) | Total (n = 75) | |
|---|---|---|---|
| Genetic test performed | 14 (40%) | 19 (47.5%) | 33 (44%) |
| Patients where genetic test were performed | |||
| Negative genetics (n/%) | 6/43 | 5/26 | 11/33,5 |
| CFH (n/%) | 2/14 | 5/26 | 7/21 |
| CFHR1/R3 deletion | 2/14 | 5/26 | 7/21 |
| Other CFHR (n/%) | 3/21 | 3/16 | 6/18 |
| CFI (n/%) | 1 (VUS)/7 | 3/16 | 4/12 |
| C3 (n/%) | 0/0 | 3/16 | 3/9 |
| CFB (n/%) | 0/0 | 1/5 | 1/3 |
| CD46 (n/%) | 0/0 | 1/5 | 1/3 |
| Not specified (n/%) | 1/7 | 1/5 | 0/0 |
CFHR, CFH-Related protein; VUS, variant of unknown significance.
Table 6.
Detailed genetic variants in aHUS Brazilian Registry
| Variant | Number of cases | Female/male | Age at diagnosis |
|---|---|---|---|
| CFH | 5 | 3/2 | 4 mo; 1y; 23y; 29y; 32y |
| del CFHR1/R3 | 5 | 4/1 | 1.5y; 2.2y; 8y; 20y; 29y |
| CFHR1 | 1 | 1/0 | 28y |
| C3 | 2 | 1/1 | 29y; 32y |
| CD46 | 1 | 1/0 | 31y |
| CFHR2 | 1 | 0/1 | 20y |
| CFHR3 | 2 | 1/1 | 17y; 44y |
| CFHR5 | 2 | 2/0 | 3 mo; 17y |
| CFH + del CFHR1/R3 | 1 | 0/1 | 20y |
| CFH + CFI (VUS) | 1 | 1/0 | 16y |
| CFI + CFB | 1 | 0/1 | 34y |
| CFI + C3 | 1 | 1/0 | 22y |
| CFI + del CFHR1/R3 | 1 | 0/1 | 38y |
| Heterozygous variant in ADAMTS13 | 1 | 0/1 | 49y |
| PLAT | 1 | 0/1 | 26y |
y, years; mo, months of age; VUS, variant of unknown significance.
FIGURE 1:
Density distribution of the time between aHUS diagnosis and eculizumab infusion in days. Patients are divided in two groups by dialysis need: the blue color refers to dialysis independent and red color to dialysis dependent.
Genetics
Genetic analysis was performed in 33/75 cases (44%). Overall, the most frequent variants identified were in CFH (7 patients) and the CFHR1-3 deletion (7 patients) (Table 5). Other genetic variants were identified in other CFHR (CFH-Related proteins) (18% of patients), CFI (12%), C3 (9%), CFB (3%) and CD46 (3%) (Table 5). Table 6 shows detailed genetic results by patient and age of manifestation, including 5 patients with combined genetic abnormalities identified: CFH + CFHR1/R3 del (n = 1), CFH + CFI (VUS) (n = 1), CFI + CFB (n = 1), CFI + C3 (n = 1), CFI + CFHR1/R3 del (n = 1) (Table 6). Negative genetic tests were found in 33.5% of the cohort. The genetic profile was similar between pediatric and adult patients (Table 5).
Summary of worldwide registries or case series
Supplementary data S2 highlights a summary of cohort data from other registries or significant case series around the world that we selected to be compared with this current Brazilian Registry. In this table, we performed a review of the clinical, laboratory and genetic data, response to treatment and mortality of aHUS patients, from those pediatric and adult cohorts.
DISCUSSION
aHUS is a rare disease and registries are useful to evaluate the natural history and progression of the disease as well as to address some questions related to diagnosis and treatment. Although investigators are aware about the influence of ethnic background on genetic abnormalities and characteristics of the disease, there are a few registries of patients with different ethnic backgrounds [8, 10, 11, 12, 16–18]. In this first Brazilian aHUS Registry report, demographic, clinical, laboratory and genetic data were analyzed. Patients were divided in three age groups based on observations from previous studies regarding differences among different ages such as gender, genetic findings, triggers and outcomes [8, 16, 19, 20]. aHUS is a disease that can affect adults and children. There was a slightly higher prevalence in adults aged >18 years (53.4%) as reported by others [16, 17, 20, 21].
In the pediatric group (<18 years of age), the frequency of aHUS in patients <2 years of age was 48.5%. This finding was similar to what was observed in the global and French registries (43.9% and 56%, respectively) [16, 19] yet, in the Turkish Pediatric Registry the percentage of cases <2 years of age was lower, around 36% [8].
The female gender was predominant in all ages except in the very young group. The female predominance in adults was previously described [12, 16–20] as was the highest rate of males in the <2 years of age group [16]. However, the Pediatric Turkish Registry revealed a female prevalence in children (57.6%) [8] even in those <2 years of age (57%) [11]. In addition, Lee et al. have reported the same proportion of male and female patients from a pediatric Korean cohort [10].
In this Brazilian Registry, family history was reported in only 8% of cases, much lower when compared with the aHUS Global Registry (20.4%) [20], but in accordance with the Canadian and Australian cohorts of the Global Registry, 5.4% and 10%, respectively [17, 18], and with the Turkish Pediatric Registry, in which only 4.8% of cases had a positive family history (despite a high consanguinity rate) [8]. A higher rate of positive family history has been found in children compared with adult patients in some cohorts [16, 20, 21].
The diagnosis of aHUS demands tailored steps and, not infrequently, aHUS is considered a diagnosis of exclusion [4, 22]. Traditionally, the causes of TMA are divided into primary and secondary. Primary TMA is designated when the endothelial injury mechanism is known. Classically, this group encompasses thrombotic TTP, Shiga Toxin uremic hemolytic syndrome (STEC-HUS) and aHUS. Secondary TMA usually occurs in the context of other diseases, frequently systemic and TMA tends to resolve with treatment or removal of the underlying cause [23].
In the Global aHUS Registry, diagnosis accuracy is not checked for each new entry case [20]. In this Brazilian Registry, for every patient TTP and STEC-HUS was excluded. Also, physicians were cautious with secondary causes, excluding cobalamin metabolism defects, neoplasia, scleroderma, antiphospholipid syndrome and other causes (infection and drugs) before designating a patient with aHUS. The diagnosis of aHUS must be established after the resolution of the infection and withdrawal of TMA-inducing medications by a minimum of 1 week. These steps were also part of the Brazilian Registry database, which directly instructed physicians during data entry through alerts and notes. For instance, if a value of ADAMTS-13 activity <10% was inputted, the system showed a red alert informing that the aHUS diagnosis must be revised. In cases of drug-induced TMA, there was an extensive checkbox list with all possible medications. Additionally, there was a note that guided the physician to suspend the medication for a minimum of 1 week to validate the aHUS diagnosis if TMA persisted.
A kidney manifestation was almost universally present in all age groups (elevated serum creatinine, low creatinine clearance and/or proteinuria). Importantly, hypertension was the most frequent manifestation and occurred in 86.2% of the total cohort with no difference according to the age group. Yun et al. have also reported a high percentage of hypertension (64%) in aHUS adults (Korean TTP and TMA Registry) [12]; however, Lee et al. found only 47% of hypertension in the Pediatric Korean cohort [10].
In this Brazilian aHUS Registry, neurological and gastrointestinal manifestations were more frequently in pediatric patients than adults. Those manifestations have been evaluated in other registries and case series with great variability [8, 11, 20, 22, 23]. In our registry, fatigue was a frequent finding and it is a very important patient-reported symptom that has been studied by Greenbaum et al. in patients from the Global Registry of aHUS. The recovering of fatigue remained over time with continuous treatment with eculizumab [24].
Laboratory exams at diagnosis showed that pediatric patients had a different profile, presenting with lower levels of hemoglobin and platelets compared with adults as well as higher levels of LDH. These could suggest that children have a more pronounced hemolytic effect compared with adults. To the best of our knowledge, these aHUS laboratory patterns were rarely described earlier. Frémeaux-Bacchi et al. have already observed lower hemoglobin and platelet levels in children compared with adults, but no mention was made of higher LDH levels in their paper [16]. In addition, a high proportion of patients evolved with dialysis dependence in the first 3 months (45%), regardless of age, and a very high percentage of the cohort was treated with eculizumab (97.3%).
Among patients with genetic analysis, we found 33.5% negative compared with 40% in the Global aHUS Registry [20] and compared with 78.4% in the Canadian cohort of the Global aHUS Registry [17]. In the Pediatric Turkish Registry, 81% of patients had at least one mutation [8]; however, Çakar et al. studying the <2 years of age group from the same population, detected only 14/53 (26%) of positive mutation rate [11]. In addition, Yun et al. found a higher rate of positivity when the number of genes analyzed was increased [12].
The genetic findings in the Brazilian Registry are in agreement with those from the Global Registry in which CFH mutations were prevalent regardless of age group, in addition to which CFI variants were not identified in pediatric patients [20]. In 66 adults diagnosed with aHUS from the Korean TTP and TMA Registry, CFH mutations were prevalent (20%) followed by THBD mutations (14%), but a recurrent missense variant was observed in THBD, Asp486Tyr [12]. Yet, in a Pediatric Korean cohort, there was a predominance of AntiCFH antibodies (29%) [10]. In the Pediatric Turkish Registry, MCP variants were the most frequent, followed by C3 mutations [8].
We identified a higher proportion of variants in genes encoding Factor H-related proteins (CFRH) compared with the Italian and French cohorts [25, 26]. We detected the CFHR1-3 del in a high proportion of patients and it is important to emphasize that the presence of this deletion is related to presence of Anti-CFH antibodies [8], which were not evaluated in this current Brazilian Registry report.
All these findings taken together show that the rate of positivity as well as the spectrum of mutations can vary with the region and the genes analyzed (Tables 5 and 6). The Brazilian population has particularities such as the high rate of miscegenation and several ethnic origins. These factors can also determine different genetic and clinical characteristics of this disease in this population. More studies are needed to explore the potential differences [27–29].
Eculizumab was administered to 97.3% of the patients compared with 68% of the Australian cohort Registry [18] and superior to aHUS Global Registry (59.1%) [19]. This could be explained because the Brazilian aHUS Registry is relatively recent (it was created in 2017) combined with strictly aHUS criteria to enter data in the study. In Brazil, eculizumab has been available since 2011 with a progressive rise in aHUS therapy since then [30].
We also showed that patients with lower time between diagnosis and eculizumab infusion had a lower probability of being on dialysis at the 3-month follow-up (Fig. 1), which was similar to previous reports [31]. A more recent publication from the Global Registry compared Eculizumab-treated and untreated patients and showed that treated patients presented more severe clinical picture, but with low mortality rate [21]. Data on kidney or transplant loss or actual graft function are under analysis.
Among the strengths of this registry, we highlight the verification of the accuracy of aHUS diagnosis by the Committee members, as well as the fact that data were imputed by physicians. These actions have been recommended by Licht et al. [19] to improve the quality of the aHUS Global Registry.
Additionally, we provide details regarding clinical, laboratory and treatment data for these patients which have been rarely reported. We also provide data about laboratory diagnosis with missing data lower than 30%, except for haptoglobin, complement C3 and C4 values. Missing data report is a quality control tool and in this Brazilian aHUS Registry these data were reported [32].
The study has several limitations. Information regarding discontinuation of eculizumab and long-term renal outcomes in patients as well as allograft loss in kidney transplant recipients were not available. Additionally, we could not retrieve mortality data. Also, we were not able to check the pathogenicity of the variants and we had a lack of uniformity in the aHUS panel among centers.
In conclusion, we reported a cohort of aHUS Brazilian patients who were predominantly female young adults. aHUS patients had a high rate of renal involvement (100%) and the laboratory profile showed that pediatric patients had lower hemoglobin and platelet levels compared with adult patients, especially those <2 years of age. To the best of our knowledge, significant higher serum LDH levels in children is described for the first time in the current registry. The most common genetic variants were identified in CFH and the CFHR1-3 deletion. We showed a high rate of eculizumab use, and the probability of dialysis-free evolution was correlated with shorter time between diagnosis and first infusion.
aHUS, as a genetic disease that can be influenced by precipitating factors, including some external ones, can vary among regions of the globe and populations [1, 3, 4]. Therefore, knowledge from different parts of the world is needed to complete the spectrum of genetic and clinical characteristics of this disease. This is an important contribution of the current Brazilian aHUS Registry.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
FUNDING
Brazilian Society of Nephrology.
AUTHORS’ CONTRIBUTIONS
M.H.V., C.A.B.S., L.C.S., G.B., V.S.P.V., P.M.F., V.S.C., J.G.G., A.F.P.L., L.C.S., P.G.M.M., and O.M.V.-N. designed the Registry. M.H.V., L.G.M.A., L.M.P.P., M.C.R.C., M.I.N.H.B., M.G.G.P., O.A.F.N., R.M.L.S., S.M.C.M., H.M.T., C.R., R.M.S., C.A.A.C., D.J.B.M., A.M.S.T.S., A.R.S., E.R.R., F.H.S.B., J.C.L.N., L.S.S.O., L.C.S., R.W., and S.O.N. provided patient data. M.H.V., L.G.M.A., P.D.M.M.N., L.M.P.P., M.C.R.C., C.A.B.S., M.I.N.H.B., M.G.M.G.P., O.A.F.N., R.M.L.S. and S.M.C.M. provided intellectual content to the manuscript. M.H.V., L.G.M.A. and P.D.M.M.N. designed the study and were responsible for data analysis. M.H.V., L.G.M.A., P.D.M.M.N., L.M.P.P. and M.C.R.C. drafted and revised the article. All the authors approved the final version of the manuscript.
CONFLICT OF INTEREST STRATEMENT
M.H.V. reports lecture fees from Alexion Pharmaceuticals and grants from Roche. L.G.M.A. reports lecture fees from Alexion Pharmaceuticals, Takeda and Sanofi. L.M.P.P. reports lecture fees from Alexion Pharmaceuticals. M.C.R.C. reports lecture fees from Alexion Pharmaceuticals. M.I.N.H.B. reports lecture fees from Alexion Pharmaceuticals. The other authors declare that they have no conflict of interest. The results presented in this article have not been published previously in whole or part.
Supplementary Material
Contributor Information
Maria Helena Vaisbich, Pediatric Nephrology Service, Child Institute, University of São Paulo, São Paulo, Brazil.
Luís Gustavo Modelli de Andrade, Nephrology Division, Department of Internal Medicine, Universidade Estadual Paulista (UNESP), Botucatu, Brazil.
Precil Diego Miranda de Menezes Neves, Division of Nephrology, University of São Paulo School of Medicine, São Paulo, Brazil; Nephrology and Dialysis Center, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil.
Lílian Monteiro Pereira Palma, Pediatric Nephrology Service, State University of Campinas, Campinas, Brazil.
Maria Cristina Ribeiro de Castro, Renal Transplant Unit, University of São Paulo School of Medicine, São Paulo, Brazil.
Cassiano Augusto Braga Silva, Nephrology Department, Grupo CSB, Feira de Santana, Brazil.
Maria Izabel Neves de Holanda Barbosa, Nephrology and Transplant Center, Federal Hospital of Bonsucesso, Rio de Janeiro, Brazil.
Maria Goretti Moreira Guimarães Penido, Pediatric Nephrology Unit – Nephrology Center, Santa Casa de Belo Horizonte, Belo Horizonte, Brazil.
Oreste Ângelo Ferra Neto, Pediatric Nephrology Service, Federal University of Mato Grosso do Sul, Campo Grande, Brazil.
Roberta Mendes Lima Sobral, Pediatric Nephrology Service, Federal University of Bahia, Salvador, Brazil.
Silvana Maria Carvalho Miranda, Nephrology Center, Santa Casa de Belo Horizonte, Belo Horizonte, Brazil.
Stanley de Almeida Araújo, Nephropathology Institute, Belo Horizonte, Brazil.
Igor Gouveia Pietrobom, Nephrology Discipline, Federal University of São Paulo, São Paulo, Brazil.
Henrique Mochida Takase, Pediatric Nephrology Service, Universidade Estadual Paulista (UNESP), Botucatu, Brazil.
Cláudia Ribeiro, Nephrology Center, Santa Casa de Belo Horizonte, Belo Horizonte, Brazil.
Rafael Marques da Silva, Pró-Rim Foundation, Joinvile, Brazil.
César Augusto Almeida de Carvalho, Santa Casa de Franca, Franca, Brazil.
David José Barros Machado, Renal Transplant Unit, University of São Paulo School of Medicine, São Paulo, Brazil.
Ana Mateus Simões Teixeira e Silva, Clinical Hospital, Federal University of Goiás, Goiania, Brazil.
Andreia Ribeiro da Silva, INEFRO – Nephrology Institute / DAVITA, São José dos Campos, Brazil.
Enzo Ricardo Russo, Nephrology Service, Sinhá Junqueira Hospital, Ribeirão Preto, Brazil.
Flávio Henrique Soares Barros, Nephrology Service, Presidente Dutra Hospital, Presidente Dutra, Brazil.
Jarinne Camilo Landim Nasserala, Nephrology Service, State Hospital of Acre Foundation, Rio Branco, Brazil.
Luciana Schmitt Cardon de Oliveira, Pró-renal Foundation, Curitiba, Brazil.
Lucimary de Castro Sylvestre, Pediatric Nephrology Service, Pequeno Príncipe Hospital, Curitiba, Brazil.
Rafael Weissheimer, Nephrology Service, Marcelino Champagnat Hospital, Curitiba, Brazil.
Sueli Oliveira Nascimento, NEFRON – Nephrology Service, Porto Velho, Brazil.
Gilson Bianchini, Nephrology Service, Federal University of Paraná, Curitiba, Brazil.
Fellype de Carvalho Barreto, Nephrology Service, Federal University of Paraná, Curitiba, Brazil.
Valéria Soares Pigozzi Veloso, Clinical Hospital, Federal University of Goiás, Goiania, Brazil.
Patrícia Marques Fortes, Pediatric Nephrology Service, Federal University of Goiás, Goiânia, Brazil.
Vinicius Sardão Colares, Nephrology Service, Santa Casa de Juíz de Fora, Juíz de Fora, Brazil.
Jaelson Guilhem Gomes, Hemodialysis Institute of Sorocaba, Sorocaba, Brazil.
André Falcão Pedrosa Leite, Nephrology Division, Universidade Estadual de Ciencias da Saúde de Alagoas, Maceio, Brazil.
Pablo Girardelli Mendonça Mesquita, Clinical Hospital Samuel Libânio, Pouso Alegre, Brazil.
Osvaldo Merege Vieira-Neto, Nephrology Discipline, Ribeirão Preto Medical School – University of São Paulo, Ribeirão Preto, Brazil.
REFERENCES
- 1. Noris M, Remuzzi G.. Atypical hemolytic-uremic syndrome. N Engl J Med 2009; 361: 1676–1687 [DOI] [PubMed] [Google Scholar]
- 2. Dragon-Durey MA, Loirat C, Cloarec Set al. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 2005; 16: 555–563 [DOI] [PubMed] [Google Scholar]
- 3. Campistol JM, Arias M, Ariceta Get al. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia 2015; 35: 421–447 [DOI] [PubMed] [Google Scholar]
- 4. Goodship TH, Cook HT, Fakhouri Fet al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney disease: improving global outcomes” (KDIGO) controversies conference. Kidney Int 2017; 91: 539–551 [DOI] [PubMed] [Google Scholar]
- 5. Zuber J, Fakhouri F, Roumenina LTet al. French study group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 2012; 8: 643–657 [DOI] [PubMed] [Google Scholar]
- 6. Nga HS, Palma LMP, Ernandes Neto Met al. Thrombotic microangiopathy after kidney transplantation: analysis of the Brazilian atypical hemolytic uremic syndrome cohort. PLoS One 2021; 16: e0258319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Andrade LGM, Contti MM, Nga HSet al. Long-term outcomes of the atypical hemolytic uremic syndrome after kidney transplantation treated with eculizumab as first choice. PLoS One 2017; 12: e0188155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Besbas N, Gulhan B, Soylemezoglu Oet al. Turkish pediatric atypical hemolytic uremic syndrome registry: initial analysis of 146 patients. BMC Nephrol 2017; 18: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palma LMP, Eick RG, Dantas GCet al. Brazilian thrombotic microangiopathy and atypical hemolytic uremic syndrome study group (aHUS Brazil). Atypical hemolytic uremic syndrome in Brazil: clinical presentation, genetic findings and outcomes of a case series in adults and children treated with eculizumab. Clin Kidney J 2020; 14: 1126–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JM, Park YS, Lee JHet al. Atypical hemolytic uremic syndrome: Korean pediatric series. Pediatr Int 2015; 57: 431–438 [DOI] [PubMed] [Google Scholar]
- 11. Çakar N, Ozcakar ZB, Ozaltin Fet al. Atypical hemolytic uremic syndrome in children aged <2 years. Nephron 2018; 139: 211–218 [DOI] [PubMed] [Google Scholar]
- 12. Yun JW, Oh J, Lee KOet al. Distinct genetic profile with recurrent population-specific missense variants in Korean adult atypical hemolytic uremic syndrome. Thromb Res 2020; 194: 45–53 [DOI] [PubMed] [Google Scholar]
- 13. Kodra Y, Weinbach J, Posada-de-la-Paz Met al. Recommendations for improving the quality of rare disease registries. Int J Environ Res Public Health 2018; 15: 1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CHet al. CKD-EPI (Chronic kidney disease epidemiology collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwartz GJ, Work DF.. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009; 4: 1832–1843 [DOI] [PubMed] [Google Scholar]
- 16. Fremeaux-Bacchi V, Fakhouri F, Garnier Aet al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 2013; 8: 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lapeyraque AL, Bitzan M, Al-Dakkak Iet al. Clinical characteristics and outcome of canadian patients diagnosed with atypical hemolytic uremic syndrome. Can J Kidney Health Dis 2020; 7: 2054358119897229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soraru J, Isbel N, Wong Get al. Baseline characteristics of patients with atypical haemolytic uraemic syndrome (aHUS): the Australian cohort in a global aHUS registry. Nephrology (Carlton) 2020; 25: 683–690 [DOI] [PubMed] [Google Scholar]
- 19. Licht C, Ardissino G, Ariceta Get al. The global aHUS registry: methodology and initial patient characteristics. BMC Nephrol 2015; 16: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaefer F, Ardissino G, Ariceta Get al. Global aHUS registry. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int 2018; 94: 408–418 [DOI] [PubMed] [Google Scholar]
- 21. Rondeau E, Cataland SR, Al-Dakkak Iet al. Eculizumab safety: five-year experience from the global atypical hemolytic uremic syndrome registry. Kidney Int Rep 2019; 4: 1568–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loirat C, Frémeaux-Bacchi V.. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis 2011; 6: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fox LC, Cohney SJ, Kausman JYet al. Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Nephrology (Carlton) 2018; 23: 507–517 [DOI] [PubMed] [Google Scholar]
- 24. Greenbaum LA, Licht C, Nikolaou Vet al. Functional assessment of fatigue and other patient-reported outcomes in patients enrolled in the global aHUS registry. Kidney Int Rep 2020; 5: 1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ardissino G, Testa S, Possenti Iet al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 2014; 64: 633–637 [DOI] [PubMed] [Google Scholar]
- 26. Fakhouri F, Fila M, Provôt Fet al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 2017; 12: 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moura RR, Coelho AV, Balbino VQet al. Meta-analysis of Brazilian genetic admixture and comparison with other Latin America countries. Am J Hum Biol 2015; 27: 674–680 [DOI] [PubMed] [Google Scholar]
- 28. Pena SDJ, Santos FR, Tarazona-Santos E.. Genetic admixture in Brazil. Am J Med Genet C Semin Med Genet 2020; 184: 928–938 [DOI] [PubMed] [Google Scholar]
- 29. Kehdy FS, Gouveia MH, Machado Met al. Brazilian EPIGEN project consortium. Origin and dynamics of admixture in Brazilians and its effect on the pattern of deleterious mutations. Proc Natl Acad Sci USA 2015; 112: 8696–8701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neto ME, de Moraes Soler L, Vasconcelos HVGet al. Eculizumab interruption in atypical hemolytic uremic syndrome due to shortage: analysis of a Brazilian cohort. J Nephrol 2021; 34: 1373–1380 [DOI] [PubMed] [Google Scholar]
- 31. Siedlecki AM, Isbel N, Vande Walle Jet al. Global aHUS registry. Eculizumab use for kidney transplantation in patients with a diagnosis of atypical hemolytic uremic syndrome. Kidney Int Rep 2018; 4: 434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lazem M, Sheikhtaheri A, Hooman N.. Lessons learned from hemolytic uremic syndrome registries: recommendations for implementation. Orphanet J Rare Dis 2021; 16: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.