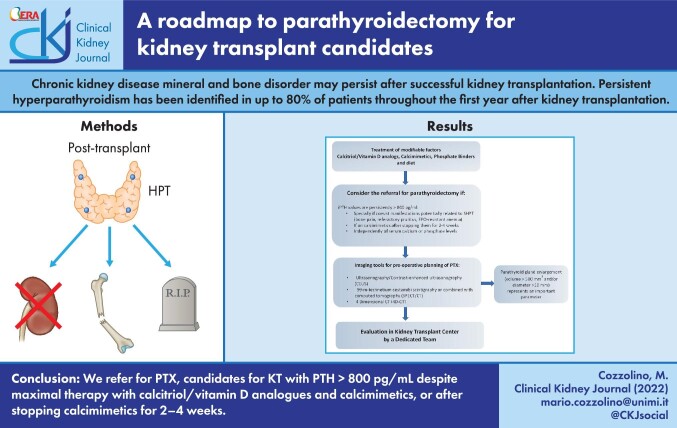
ABSTRACT
Chronic kidney disease mineral and bone disorder may persist after successful kidney transplantation. Persistent hyperparathyroidism has been identified in up to 80% of patients throughout the first year after kidney transplantation. International guidelines lack strict recommendations about the management of persistent hyperparathyroidism. However, it is associated with adverse graft and patient outcomes, including higher fracture risk and an increased risk of all-cause mortality and allograft loss. Secondary hyperparathyroidism may be treated medically (vitamin D, phosphate binders and calcimimetics) or surgically (parathyroidectomy). Guideline recommendations suggest medical therapy first but do not clarify optimal parathyroid hormone targets or indications and timing of parathyroidectomy. There are no clear guidelines or long-term studies about the impact of hyperparathyroidism therapy. Parathyroidectomy is more effective than medical treatment, although it is associated with increased short-term risks. Ideally parathyroidectomy should be performed before kidney transplantation to prevent persistent hyperparathyroidism and improve graft outcomes. We now propose a roadmap for the management of secondary hyperparathyroidism in patients eligible for kidney transplantation that includes the indications and timing (pre- or post-kidney transplantation) of parathyroidectomy, the evaluation of parathyroid gland size and the integration of parathyroid gland size in the decision-making process by a multidisciplinary team of nephrologists, radiologists and surgeons.
Keywords: kidney transplant, parathyroidectomy, secondary hyperparathyroidism
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Kidney transplantation (KT) is the gold standard renal replacement therapy for kidney failure. Kidney transplant recipients (KTRs) have better survival, reduced cardiovascular risk, improved quality of life and reduced health economic costs than dialysis-dependent patients [1–3]. However, KTRs are burdened by the lasting effects of their previous chronic kidney disease (CKD). Although successful KT was deemed to correct CKD–mineral and bone disorder (CKD-MBD) to a large extent, CKD-MBD persists in KTRs, although the phenotype evolves. Post-KT CKD-MBD depends on previous bone damage, persistent secondary hyperparathyroidism (SHPT), de novo CKD-MBD and immunosuppressive therapy. The contribution of each of these components changes over time [4] (Figure 1).
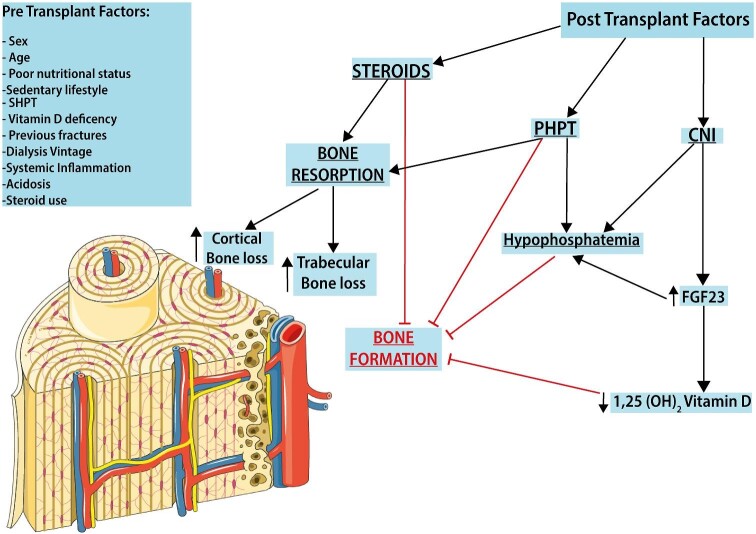
FIGURE 1:
Post-KT CKD-MBD. In KTRs, the progression of bone disease results from the evolution of pre-existing CKD-MBD with several risk factors already present in the pretransplantation period. The successive evolution of post-KT bone disease is conditioned by several posttransplant factors, including the use of immunosuppressive drugs, the degree of graft dysfunction and disturbances in mineral metabolism, including an increased level of FGF23, ongoing SHPT and vitamin D deficiency.
The two factors that mainly impact bone health in the posttransplant phase are steroid therapy and persistent hyperparahtyroidism (PHPT). While PHPT involves mainly cortical bone, in the early posttransplant period, bone loss affects the trabecular side following decreased bone formation as a result of steroid therapy.
Persistent HPT is present in up to 80% of patients throughout the first year after KT and its clinical and biochemical consequences may persist for years if not appropriately treated [5–7]. International guidelines lack strict recommendations about the management of high pretransplant PTH levels, but the negative effects of persistent HPT on graft and patient outcomes make it necessary to define a roadmap in the management of SHPT in patients eligible for KT by analyzing both old and new evidence.
SHPT and tertiary HPT (THPT): pathophysiology
HPT is very common in CKD patients. It is present in ∼50% of patients with stage 3 or 4 CKD and in >90% of those with kidney failure [8]. The knowledge on the pathophysiology of SHPT has greatly expanded in recent decades. Both parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) are key contributors to SHPT that share the same kidney effects on calcium (enhanced reabsorption) and on phosphate (increased excretion) but have an opposite effect on the metabolism of vitamin D [9].
PTH
PTH plays a pivotal role in calcium handling: when the serum calcium level falls below the normal set point, the calcium-sensing receptor (CaSR) is activated to promote release of PTH from the parathyroid glands. PTH increases bone resorption, releasing calcium in the blood; activates the conversion in tubular cells of 25-hydroxyvitamin D to its active form 1,25-dihydroxyvitamin D [1,25(OH)2D] and the reabsorption of calcium; and enhances FGF-23 production by upregulation of nuclear receptor related 1 in osteocytes (Figure 2). In proximal tubules, PTH leads to internalization of the sodium–phosphate cotransporters NPTIIa and NPTIIC, increasing phosphaturia and lowering serum phosphate [10, 11]. The return of serum calcium to normal values silences the CaSRs, normalizing PTH values [12, 13]. These compensation pathways are not effective in CKD because several factors persist as a result of SHPT and CKD progression: hypocalcemia, hyperphosphatemia, low levels of active vitamin D and high FGF-23 levels. The bone response to PTH is markedly deranged in advanced CKD, a phenomenon known as hyporesponsiveness or PTH resistance (Figure 3). The main factors involved in PTH resistance are phosphate loading, calcitriol deficiency, oxidative stress, parathyroid hormone 1 receptor (PTH1R) downregulation and dysfunction, accumulation of PTH fragments, antagonists of the Wnt/β-catenin pathway and uremic toxins. The presence of PTH fragments in association with the desensitization of PTH1R may explain the high prevalence of low-turnover bone disease during SHPT and the consequent persistent hypocalcemia [14].
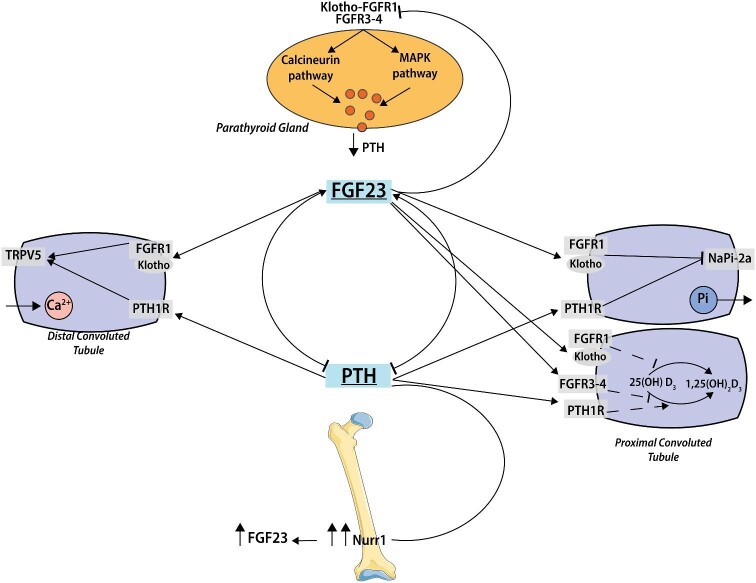
FIGURE 2:
FGF23 and PTH interaction. FGF23 and PTH mutually regulate each other in a negative feedback loop, where PTH stimulates FGF23 production and FGF23 suppresses PTH synthesis. PTH increases FGF23 expression by the nuclear orphan receptor Nurr1. In the parathyroid glands, FGF23 either binds to an FGFR1 and membrane-bound Klotho, to elicit activation of the MAPK pathway, or acts via a calcineurin-dependent signaling pathway. CNI that blocks calcineurin signaling may worsen PHPT in patients with reduced Klotho levels such as in CKD. In the kidney, PTH and FGF23 share the same effect on phosphorus and calcium handling. PTH raises urinary phosphorus excretion, downregulating NPT2a and NPT2c in the kidney. PTH also enhances calcium absorption through the direct effect on bones and kidneys, indirectly upregulating 1,25(OH)2D synthesis by increasing 1α-hydroxylase. FGF-23 inhibits renal phosphorus reabsorption through FGFR1–Klotho signaling on NaPi-2a and 2c at the proximal tubule. FGF-23 suppresses in both a Klotho-dependent pathway (FGFR1) and through FGFR3 and FGFR4 in a Klotho-independent manner 1,25(OH)2D production by decreasing 1α-hydroxylase expression and increases in a Klotho-dependent pathway the expression at distal tubulus of glycosylated TRPV5, leading to increased calcium reabsorption (see text for details). NaPi-2, type II sodium–phosphate cotransporters; TRPV5, transient receptor potential vannilloid-5.
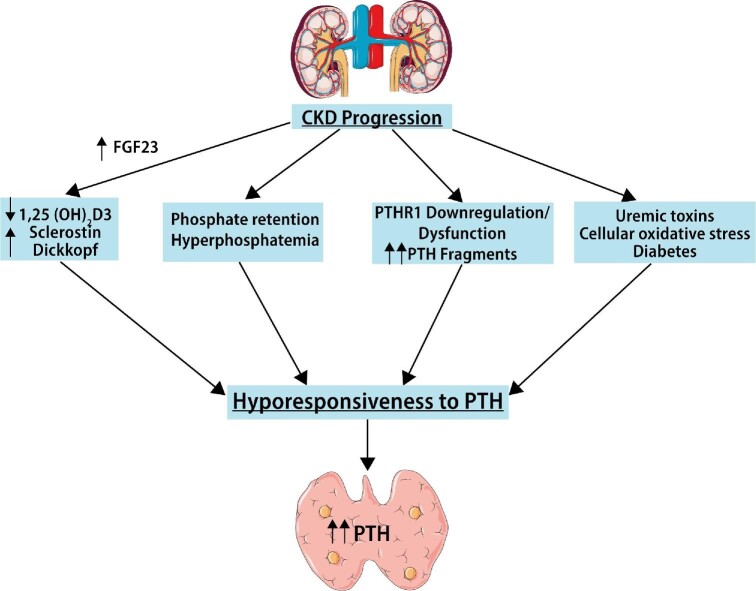
FIGURE 3:
PTH resistance. The target organs’ responses to the action of PTH are progressively impaired in CKD, a condition commonly referred to as PTH resistance. Multiple factors are involved, including phosphate loading, calcitriol deficiency, oxidative stress, PTH1R downregulation and dysfunction, accumulation of PTH fragments, antagonists of the Wnt/β-catenin pathway and uremic toxins and accumulation of PTH fragments and uremic toxins.
FGF-23
FGF-23 increases very early in CKD to prevent phosphate accumulation [13] by compensating for decreased phosphate glomerular filtration. FGFs signal through four different FGF receptors (FGFR1–4), which are all tyrosine kinase receptors. FGFR1c is probably the most important FGFR for FGF-23 signaling, at least under physiological conditions, and requires the presence of coreceptor α-Klotho [15].
In proximal tubular cells, FGF-23 binding to the FGFR1–Klotho complex promotes phosphaturia by decreasing the expression of phosphate transporters NPTIIA and NPTIIC and also decreases gut absorption of phosphate by decreasing CYP27B1 activity [i.e. 1,25(OH)2D generation] [9, 10]. FGF-23 also suppresses 1-α-hydroxylase both in a Klotho-dependent fashion (FGFR1) and through FGFR3 and FGFR4 in a Klotho-independent manner [16].
Decreased 1,25(OH)2D availability in turn, enhances PTH synthesis [17]. Additionally, inflammation, albuminuria [18] and other factors also decrease kidney Klotho expression early in CKD, leading to resistance to FGF-23 [19]. In advanced CKD, the phosphaturic effects of PTH and FGF-23 are no longer evident and high serum phosphate levels also increase PTH levels [20, 21].
FGF-23 activates FGFRs through two main pathways: a Klotho-dependent activation of the mitogen-activated protein kinase (MAPK) cascade and a Klotho-independent cascade characterized by activation of calcineurin–nuclear factor of activated T-cells (NFAT) (Figure 2). Specifically, in parathyroid glands, FGF-23 downregulates PTH secretion principally through classic Klotho-dependent activation or secondarily through the Klotho-independent cascade [22]. In severe SHPT, this inhibitory effect is lacking as a result of the reduced FGF-23–Klotho-dependent receptors in parathyroid glands [23, 24].
Sequential stages in the development of SHPT
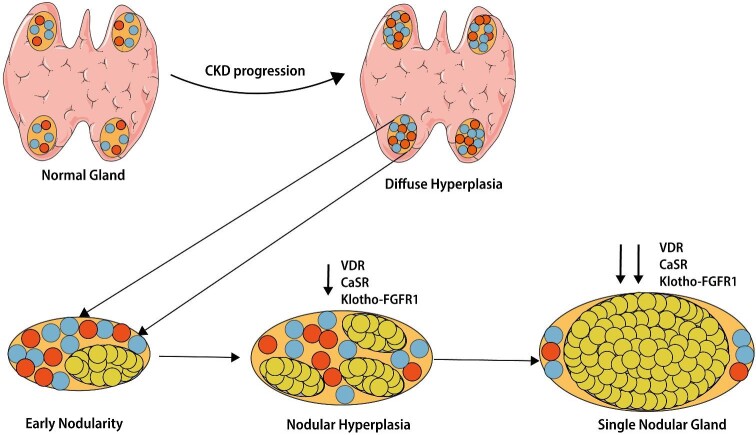
HPT development is characterized by four sequential patterns of parathyroid hyperplasia. The first polyclonal proliferation phase is characterized by diffuse hyperplasia or early nodularity in diffuse hyperplasia. If not appropriately and aggressively managed, parathyroid glands develop progressive monoclonal expansion with nodular hyperplasia or a single nodular gland [23, 25]. Long-lasting and poorly controlled HPT leads progressively to a shift from diffuse to nodular hyperplasia (Figure 4). These nodules usually manifest functional features of autonomous adenomas as in primary HPT. Nodular hyperplasia is characterized by reduced expression of vitamin D receptor, CaSR, FGFR1 and its co-receptor Klotho, making SPHT refractory to medical treatments such as vitamin D agents and calcimimetics. This is the point of no return, termed THPT [25]. THPT is typically found post-KT, although it may be observed in patients on dialysis. In dialysis patients, THPT must be distinguished from SHPT with associated iatrogenic hypercalcemia and/or hyperphosphatemia related to overtreatment, e.g. with vitamin D [23].
FIGURE 4:
Pathophysiology of secondary hyperparathyroidism in CKD. Initially in SHPT, the parathyroid glands grow diffusely with polyclonal parathyroid cell proliferation (diffuse hyperplasia). At this stage, VDRAs activators and calcimimetics are effective in lowering PTH concentrations. Afterward, cells in the nodules are transformed monoclonally and proliferate. In parallel are four patterns of parathyroid hyperplasia: diffuse hyperplasia, early nodularity in diffuse hyperplasia, nodular hyperplasia and single nodular glands. In the advanced stages of SHPT, downregulation of calcium sensing receptor (CaSR), VDR and Klotho-FGFR 1 makes parathyroid cells resistant to the inhibitory effect of calcimimetics, calcitriol and FGF-23.
Post transplant HPT
Successful KT was considered to reduce CKD-MBD to a large extent, but MBDs are common in KTRs, changing only the CKD-MBD phenotype. High PTH levels are observed in up to 80% of patients throughout the first year after KT [26]. Complete resolution of SHPT was observed in only 30% and 57% of recipients within the first and second years post-transplantation, respectively [27]. In 1000 consecutive KTRs, nearly 17% of patients were hypercalcemic at 12 months post-transplant and 10% were hypercalcemic at 48 months. In addition, ∼50% and ∼40% of patients had high PTH levels at months 12 and 48 post transplant, respectively [6]. The main predictive factors of persistent SHPT are dialysis vintage, high pre-KT PTH levels and the size of the parathyroid glands. The rapid growth of kidney failure prevalence is prolonging dialysis vintage before KT and this contributes to increasing the prevalence of persistent HPT in those on the KT waiting list [28–30]. In living donor KT, which is usually characterized by a shorter dialysis vintage prior to KT, mineral metabolism normalizes faster than in deceased donor KT [31]. Differences in prevalence may be explained by different definitions of persistent HPT, the assessment time point as well as the study era [27, 32–35]. In this regard, the ideal range of PTH and calcium levels after transplantation is not yet well defined.
Posttransplant HPT can be differentiated into persistent HPT (maladaptive response) versus de novo HPT (compensatory adaptive response). Persistent HPT results from preexisting CKD-MBD with SHPT, while de novo HPT results from decreasing graft function, leading to elevated PTH levels to maintain normo-phosphatemia and normo-calcemia [31], reproducing the pathophysiology of CKD-MBD as observed in progressive CKD in native kidneys.
PTH levels
There is no consensus about the safe level of PTH, the PTH cut-off level that identifies persistent HPT or the ‘ideal’ timing of PTH assessment after transplantation. Most nephrologists wait up to 12 months after transplantation for PTH to normalize. Beyond 12 months, a PTH level >100 pg/mL [36, 37] or 70 pg/mL [32] is consistent with persistent HPT. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend that KTRs should have the same therapeutic approach for CKD-MBD abnormalities, including PTH, as for patients with CKD stages 3–5. This implies having PTH levels within the reference range defined by each assay [38].
THPT
THPT is a misleading term that originally denoted the occurrence in the same patient of adenoma and hyperplasia in different glands, but classifying a gland as an adenoma in a patient with four enlarged parathyroid glands is questionable [33]. Some authors consider THPT to be a distinct though less frequent (21.5%) phenotype of persistent HPT, detectable in a subset of KTRs with both elevated PTH (>70 pg/mL) and hypercalcemia (serum calcium >10 mg/dL) at 1 year post-KT [32, 39].
Pre-KT PTH levels >300 pg/mL, high serum calcium (before and after KT), the use of calcimimetics, dialysis vintage or enlarged parathyroid glands pre-KT have been associated with the development of persistent HPT and THPT [32, 36, 40–42].
Parathyroid gland size
The proportion of parathyroid tissue that develops nodular hyperplasia increases as gland weight/size increases, despite diffuse hyperplasia often being observed in small glands in patients with SHPT [43, 44]. In enlarged parathyroid glands (volume ≥500 mm3 or diameter ≥10 mm), nodular hyperplastic lesions are likely present and associated with a poor response to treatment with vitamin D receptor activators (VDRAs) and cinacalcet in dialysis-dependent patients [25, 45–52], likely as a consequence of reduced expression of both VDR and CaSR in nodular hyperplastic lesions in the transition from polyclonal to monoclonal proliferation that leads to nodular hyperplasia [23]. Thus long-term drug therapy with VDRA alone or VDRA plus cinacalcet may be helpful in patients with parathyroid glands <10 mm but useless if the size is >10 mm [48, 50]. Few studies have analyzed the predictive value of enlarged parathyroid glands, assessed pre-KT, in the development of persistent HPT after transplantation [42, 53, 54]. Parathyroid glands of patients with persistent HPT after renal transplantation contained more than one nodular hyperplasia and restoration of VDR and CaSR expression after transplantation was observed only in diffuse hyperplasia. Therefore, in KT candidates, one enlarged parathyroid gland likely reflects nodular hyperplasia that is unlikely to regress [55]. Previous studies have demonstrated that therapy for persistent HPT should be started 3 months after KT since the most significant reduction of PTH occurs within the first 3 months after KT and PTH levels are unlikely to return to normal when elevated between 6 months and 1 year post-KT [33, 56–58]. However, the diagnosis is often not established until up to 2 years post-KT, causing increased morbidity from delayed treatment [32].
Persistent HPT and graft and patient outcomes
Persistent HPT contributes to posttransplant complications such as hypercalcemia, hypophosphatemia, high FGF-23 and nephrocalcinosis and is associated with unfavorable graft and patient outcomes [5, 26, 33, 59]. Studies on the impact of persistent HPT on graft and patient outcomes are marred by different definitions of persistent HPT based on PTH thresholds and timing of assessment, with most studies assessing PTH 10 weeks–12 months after transplantation.
Bone density and fractures
Persistent HPT in KTRs is associated with decreased bone density and an increased fracture rate [60–62]. Moderate bone minreal density (BMD) losses or BMD changes are observed in the first year after transplantation in the peripheral skeleton but not at the central skeleton. Steroid withdrawal and/or steroid minimization regimens decrease the negative effects of steroid therapy on bone health and persistent HPT is emerging as the main risk factor for bone fragility in KTRs [63, 64]. In particular, persistent HPT and high remodeling rates result in cortical and trabecular bone loss and decreased bone strength at the peripheral skeleton [65, 66].
Graft and patient survival
Studies on the association between persistent HPT and long-term graft and patient survival are limited, and results are contradictory. The pathways linking persistent HPT to graft dysfunction are not well known. Potential mechanisms include excessive vasoconstriction and tubulointerstitial calcification [36, 67].
Studies focused on pre-KT PTH values have reported divergent results. High pre-KT PTH levels have been linked to increased risk of graft failure censored for death [36, 68]. However, a retrospective analysis of >10 000 primary KTRs found no link between pre-KT PTH levels and death or graft loss after transplantation [69].
In 984 KTRs, FGF-23 was a strong and independent risk factor for the composite endpoint of death and graft loss, but the association between PTH and outcome was significant only in univariate analyses and disappeared when adjusting for estimated glomerular filtration rate (eGFR) [70].
The association of persistent HPT, defined as PTH >1.5 times the upper limit of the assay (100 pg/mL) 1 year after KT, with long-term graft outcomes was evaluated in 911 KTRs. Persistent HPT was an independent risk factor for graft loss. Moreover, a PTH level >150 pg/mL at 6 months predicted 1-year persistent HPT with 92% specificity [36].
A similar cut-off value (PTH ≥150 pg/mL) at 3 months after KT was associated with worse allograft function up to 3 years posttransplant and to increased risk for death or death with a functioning graft [71]. In a retrospective analysis of 522 KTRs, intact PTH (iPTH) levels during the early posttransplant period (10 weeks) predicted a composite endpoint of cardiovascular events, graft loss and death. Moreover, patients with the highest levels of iPTH had the highest risk for the composite endpoint, the highest levels of calcium and the lowest levels of phosphate [72].
In a large cohort of 1840 KTRs from the ALERT trial, recruited with a mean of 5.1 years after KT and with a follow-up time of 6–7 years, persistent HPT after KT was significantly associated with all-cause mortality (4% increased risk) and allograft loss (5% increased risk) but not with major cardiovascular events [73]. No significant associations between PTH and serum calcium or phosphate were observed. These results are in agreement with another study showing that the correlation between serum PTH and serum calcium early after transplantation is gradually lost over time [58], but they differ from previous studies showing an association of hypercalcemia with graft loss and nephrocalcinosis [5, 74, 75].
Medical treatment versus parathyroidectomy before or after KT: association with outcomes
Persistent HPT post-KT often requires parathyroidectomy with a prevalence range from 0.6 to 5.6% [76]. However, there are no clear guidelines on how to treat waitlisted dialysis patients to reduce the risk of persistent HPT post-KT [77]. Moreover, the appropriate strategy in KTRs is debated, with particular focus on the risk of worsening of graft function related to medical and surgical therapy [39].
SHPT before KT can be treated by medical (VDRAs, phosphate binders and calcimimetics) or surgical (parathyroidectomy) approaches. Both reduce PTH values, although parathyroidectomy provides better long-term control of calcium and PTH values [77]. Medical treatment is generally the first step.
Calcimimetics
Calcimimetics are positive allosteric modulators of CaSRs that increase the sensitivity of the parathyroid glands to circulating calcium and VDR expression [23]. Randomized studies on cinacalcet have demonstrated its efficacy in the control of SHPT in patients on dialysis compared with vitamin D analogs and placebo [78, 79]. The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events study had a primary composite end point of time to death, myocardial infarction, hospitalization for unstable angina, heart failure and peripheral vascular events. The unadjusted intention-to-treat analysis of the 3883 dialysis patients treated with cinacalcet and placebo did not demonstrate differences in the primary endpoint (48.2% of patients treated with cinacalcet and 49.2% of patients treated with placebo) [80]. A prespecified analysis by age categories (≥65 years and <65 years) showed a reduction in major cardiovascular events {hazard ratio [HR] 0.70 [95% confidence interval (CI) 0.60–0.81], P ≤ .001} and all-cause mortality [HR 0.68 (95% CI 0.58–0.81), P ≤ .001] in older patients [81]. Further analysis did not disclose differences in the risk of fractures, although after adjusting for baseline characteristics, multiple fractures and discontinuation of therapy, cinacalcet reduced the frequency of clinically evident fractures by 16–29% [82].
The use of calcimimetics pre-KT is frequently associated with the onset of mild hypercalcemia after KT, although this is present in 30% of KTRs (treated in dialysis with cinacalcet or not) and is potentially related with rebound of SHPT and nephrocalcinosis may develop months after KT [83]. In dialysis patients with PTH levels well controlled by cinacalcet, there is a direct relationship between cinacalcet dosage and the development of hypercalcemia after KT [83].
Etelcalcetide is an intravenous direct CaSR agonist to treat SHPT in hemodialysis patients. In two recent cases, severe hypercalcemia developed in KT patients who had been on etelcalcetide in dialysis, requiring parathyroidectomy about 1 month after KT [84].
Parathyroidectomy
There are no clear guidelines on parathyroidectomy for patients on dialysis suffering from SHPT, beyond the notion that it is reserved for patients not responding to medical therapy [23]. Thus there are no clear indications on the optimal PTH targets to be achieved or the timing of parathyroidectomy. In the USA, between 2004 and 2016, the number of parathyroidectomies performed for SHPT in patients with kidney failure decreased by 40% [85]. In both KTRs and kidney failure patients, parathyroidectomy decreased from 7.9 to 5.4 per 1000 patients between 2002 and 2011, a major reduction being observed in 2004 (3.3 per 1000 patients), the year of cinacalcet release [85]. Evidence on the timing of parathyroidectomy in transplant candidates is also limited.
Post-KT parathyroidectomy and graft function
Post-KT parathyroidectomy has been associated with worse graft function outcomes than pre-KT parathyroidectomy. In a retrospective single-center study of 123 patients (67 pre-KT parathyroidectomy and 56 post-KT parathyroidectomy), parathyroidectomy after KT was associated with a decrease in eGFR following surgery. Post-KT parathyroidectomy was an independent risk factor for recipient graft function worsening. The risk decreased when parathyroidectomy was performed 1 year post-KT or, even better, before KT [86]. Similarly, graft function decreased in transplant patients who underwent post-KT parathyroidectomy, but the lipid profile and blood pressure control improved [87]. The negative impact of parathyroidectomy performed <1 year post-KT on renal function persisted for 5 years after transplantation when compared with parathyroidectomy performed pre-KT or 1–5 years post-KT [88]. In 76 KT patients who underwent parathyroidectomy, worsening of renal function was related to the change in PTH decline before and after surgery: a reduction in PTH >80% was followed by an important reduction of creatinine clearance. A retrospective study of 108 patients did not observe differences in graft survival between pre-KT and post-KT parathyroidectomy. However, post-KT parathyroidectomy was an independent risk factor for persistent hypocalcemia, due to hungry bone disease or hypoparathyroidism, and for the reduction of eGFR by 20% 12–36 months after transplantation (P = .029) [67].
In other studies, renal function in patients undergoing post-KT parathyroidectomy stabilized after an initial worsening [87, 89]. In patients who underwent post-KT parathyroidectomy [median time from KT 11 months (range 1–167)], serum creatinine increased in the first month post-parathyroidectomy compared with patients transplanted in the same period who did not require parathyroidectomy (1.91 ± 0.72 versus 1.76 ± 0.63 mg/dL, P < .01). In the long term, however, renal function stabilized and no differences in graft survival were observed [87]. Similar results were obtained when comparing 102 patients who underwent pre-KT parathyroidectomy with 83 patients who underwent post-KT parathyroidectomy [89].
The reason for the deterioration of graft function is unclear, but a hemodynamic mechanism has been suggested [87]. Thus, PTH induces afferent arteriole vasodilatation and efferent vasoconstriction. This would result in glomerular hyperfiltration and the sudden removal of PTH may decrease GFR [59]. In this case, however, parathyroidectomy would also be expected to be protective in the long term, as is the case for nephroprotective interventions that decrease glomerular hyperfiltration.
Cinacalcet versus post-KT parathyroidectomy
To our knowledge, only a study compared cinacalcet and parathyroidectomy in KT patients. A 12-month prospective, multicenter, open-label, randomized study compared subtotal parathyroidectomy (n = 15) and cinacalcet (n = 15) in KT patients with hypercalcemia and HPT. The primary outcome was the normalization of calcium values, achieved in 100% of patients undergoing parathyroidectomy and in 67% of patients treated with cinacalcet (P = .04). Secondary outcomes included normalization of PTH values, which was achieved in 10 of 15 patients undergoing parathyroidectomy (P = .002) and in none of those receiving cinacalcet. Cinacalcet decreased PTH values, but they remained above the normal range: this was hypothesized to result from an increase in calcitonin with consequent hypocalcemia and persistence of HPT. BMD did not improve in patients treated with cinacalcet but increased in the femoral neck in patients undergoing parathyroidectomy (P = .01): BMD improvement was associated with the normalization of PTH and bone turnover marker values and the administration of calcium and vitamin D to avoid hungry bone syndrome. eGFR decreased in both groups and neither treatment decreased vascular calcifications [90]. In a 5-year extension study, parathyroidectomy had a lower risk of recurrence of THPT [91].
In a retrospective study in 92 patients treated with parathyroidectomy or cinacalcet pre-KT, parathyroidectomy resulted in better control of PTH and calcium values after transplantation (P < .01), although no statistically significant differences were observed in serum calcium, phosphate and graft and overall survival at 10 years [92].
Safety
In studies that compared cinacalcet and parathyroidectomy, the most frequent side effect of cinacalcet was gastric intolerance, which may have limited dosing and compliance [88]. Complications after parathyroidectomy include surgical wound infection, temporary or permanent recurrent laryngeal nerve palsy and transient or permanent hypocalcemia, the most frequent being transient hypocalcemia related to hungry bone syndrome [93, 94]. Other parathyroidectomy complications include mortality during hospitalization or within 1 month of parathyroidectomy (2%), rehospitalization (24% required), the need for intensive care (29%) and a 39% increase in 1-year hospitalizations, according to United States Renal Data System information on parathyroidectomies performed from 2007 to 2009. The most frequent disorders were hypocalcemia, myocardial infarction and arrhythmias. The number of adverse events varied significantly based on the patient's clinical history [93].
In conclusion, there are no long-term studies on the impact of parathyroidectomy or medical therapy for HPT, especially in kidney failure patients awaiting KT. However, available studies show that parathyroidectomy is more effective in controlling HPT. Pre-KT parathyroidectomy is preferable to decrease the risk of persistent HPT and protect graft outcomes [86].
International guidelines
Table 1 summarizes the main guidelines since 2001 that address or should have addressed parathyroidectomy in KTRs or in patients waitlisted for KT.
Table 1.
Main guidelines since 2001 for parathyroidectomy in kidney transplant candidates
| Guidelines | Year | Indication | Grade of evidence |
|---|---|---|---|
| The Evaluation of Renal Transplantation Candidates: Clinical Practice Guidelines | 2001 | Calcium, phosphorous, and PTH should be measured as part of the pretransplant evaluation and should be repeated periodically while patients are on the transplant waiting list. | A |
| Parathyroidectomy should be considered for renal transplant candidates who have failed medical management and/or have severe, persistent, complications of hyperparathyroidism (e.g., refractory hypercalcemia, refractory hyperphosphatemia, severe intractable pruritus, serum calcium–phosphorus products that persistently exceed 70–80 mg/dL with progressive extra skeletal calcifications, and calciphylaxis). | B | ||
| Kidney Transplant Working Group of the Canadian Society of Transplantation. Canadian Society of Transplantation: Consensus Guidelines on Eligibility for Kidney Transplantation | 2005 | Calcium, phosphorus and PTH levels should be measured as part of the pretransplant evaluation. | A |
| Parathyroidectomy should be considered for those in whom medical management has not worked or those with severe, persistent, complications of hyperparathyroidism. | B | ||
| European Renal Best Practice Guideline on Kidney Donor and Recipient Evaluation and Perioperative Care | 2015 | We recommend not refusing a cadaveric graft only because of uncontrolled hyperparathyroidism in the recipient. | 1D |
| However, for patients on the waiting list, efforts should be made to comply with existing CKD–metabolic bone disease guidelines, including parathyroidectomy, when indicated. | Ungraded statement | ||
| KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation | 2020 | Measure serum PTH at the time of transplant evaluation. | Not graded |
| We suggest not transplanting patients with severe hyperparathyroidism until they are adequately treated (medically or surgically), as per the KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) guideline. | 2D | ||
| BMD should not be measured as part of the transplant evaluation. | Not graded |
There are no clear guidelines on the indication and timing of parathyroidectomy versus pharmacological treatment, therapeutic targets or primary outcomes [95].
In the 2001 American Society of Transplantation Guideline, serum calcium, phosphate and PTH should be evaluated periodically in patients waitlisted for KT and parathyroidectomy should be considered when medical management fails and/or patients have severe, persistent, complications of HPT [96]. Similarly, the 2005 Canadian Society of Transplantation Consensus Guidelines on eligibility for KT suggested assessing calcium, phosphate and PTH levels as part of pre-KT evaluation (Grade A) and parathyroidectomy for patients not responding to medical therapy or those with severe, persistent, complications of HPT (Grade B) [97].
The 2006 Caring for Australians and New Zealanders with Kidney Impairment guidelines on management of CKD-MBD suggest considering parathyroidectomy for patients in whom cinacalcet does not achieve target levels of calcium, phosphate and PTH (<800 pg/mL) without mentioning patients waitlisted for KT [98]. Instead, more recent 2011 UK Renal Association and British Transplant Society Guidelines on KTRs give no advice about CKD-MBD and parathyroidectomy during evaluation for KT eligibility [99], as well as 2013 Kidney Health Australia-Caring for Australians and New Zealanders with Kidney Impairment Guidelines [100].
In 2015, the European Renal Best Practice Guideline on kidney donor and recipient evaluation recommends not refusing a cadaveric graft only because of uncontrolled HPT in the recipient (1D). However, it also recommends that for patients on the waiting list, efforts should be made to comply with existing CKD-MBD guidelines, including parathyroidectomy, when indicated (ungraded statement) [101].
The 2017 KDIGO CKD-MBD guidelines advise parathyroidectomy in patients with G3a–G5D with severe HPT who fail to respond to medical or pharmacological therapy (2B) [102]. The UK Renal Association commentary on the KDIGO 2017 CKD-MBD guidelines agrees on the indication of parathyroidectomy but does not mention preparation for KT [103].
Only the 2019 Chinese guidelines for CKD-MBD considered radiological parathyroid gland enlargement as a parameter to be considered before parathyroidectomy, although no suggestion is made for KTRs despite dealing with transplant bone disease [104].
The 2020 KDIGO clinical practice guidelines on evaluation of candidates for KT contains a chapter dedicated to the preparation of the KT candidate with CKD-MBD. The rationale is that severe HPT needs to be treated before KT, and if medical therapy fails, pre-KT parathyroidectomy is indicated. These new guidelines suggest measuring serum PTH at the time of transplant evaluation and not transplanting patients with severe SHPT until they are adequately treated (medically or surgically) as per the 2017 KDIGO CKD-MBD guidelines (2D) [38]. Nevertheless, in the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) US Commentary on the 2020 KDIGO guidelines on evaluation of candidates for KT, there is no comment on management of pre-KT parathyroidectomy [105].
From the analysis of different guidelines emerges the need for new guidelines that clarify the indications for pre-KT parathyroidectomy and its role in the prevention of graft failure and transplant bone disease.
Preoperative imaging
Although the diagnosis of SHPT is biochemical and does not require imaging confirmation, in the era of minimally invasive parathyroid surgery, preoperative detection of pathological parathyroid glands is essential for a targeted surgical approach [106, 107]. Diagnostic imaging techniques routinely used include ultrasound, scintigraphy, computed tomography (CT) and magnetic resonance imaging (MRI). No studies have evaluated the newer 4-dimensional CT (4D-CT) [108] (Figure 5).
FIGURE 5:
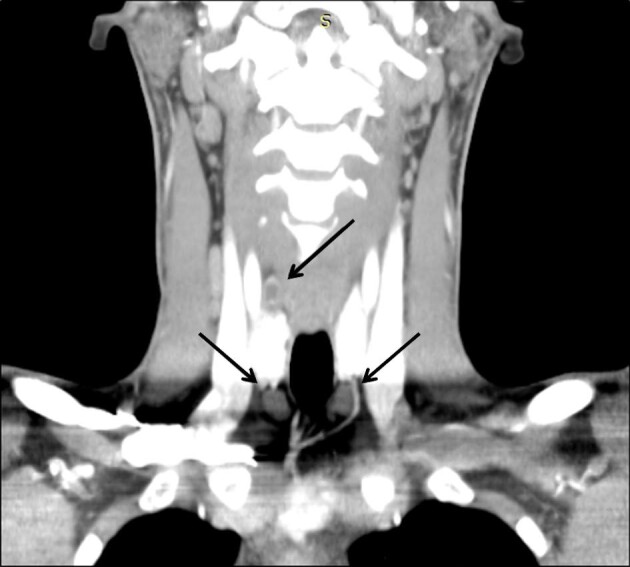
Four-dimensional parathyroid CT scan of a patient with SHPT before parathyroidectomy. The image in the coronal planes shows three hyperplastic parathyroid glands (black arrows).
Ultrasound and scintigraphy are two of the imaging techniques most widely used to locate pathological parathyroid tissue, and they are first-line localization methods [109]. Their diagnostic accuracy is similar or even superior to second-line localization methods like CT or MRI [110–112]. Parathyroid scintigraphy uses technetium (99mTc) setamibi (MIBI) as radiopharmaceutical to evaluate enlarged or ectopic parathyroid glands and its combination with CT (SPECT/CT) guarantees more sensitivity in parathyroid evaluation during SHPT [108, 113].
Scintigraphy has greater sensitivity than ultrasound in locating enlarged parathyroid glands, while ultrasound has higher specificity. The combination of scintigraphy and ultrasound allows 95% specificity in the diagnosis of enlarged parathyroid glands. Thus, scintigraphy and ultrasonography are complementary and should be used together [114].
Ultrasound identifies parathyroid tumors and potential concomitant thyroid disease and locates suspected parathyroid glands [115, 116]. In earlier studies, the sensitivity of parathyroid ultrasound ranged from 20 to 79% [117, 118], but in a recent review the sensitivity and positive predictive value (PPV) of ultrasound ranged from 51 to 96% and from 50 to 100%, respectively [119]. In this regard, ultrasound devices keep evolving and providing better quality imaging. Factors that decrease the sensitivity and PPV of ultrasound include concomitant thyroid nodules and multiple parathyroid adenomas or parathyroid hyperplasia.
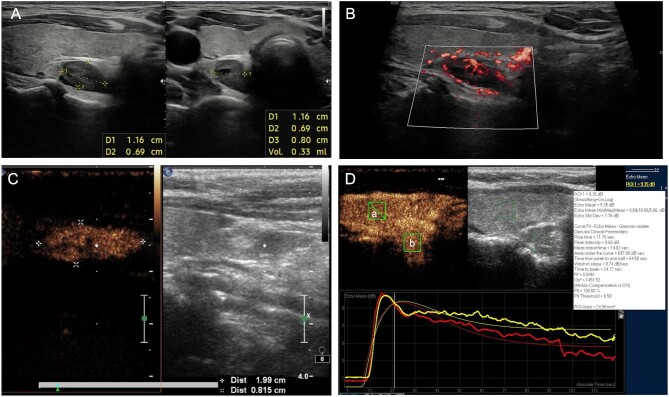
Parathyroid ultrasound is performed with high-frequency linear transducers (9–15 MHz) and the entire anterior cervical region should be examined. Parathyroid tissue is usually found along the posterior margin of the thyroid lobes or near their lower poles. Normal parathyroid glands are small and isoechoic relative to the thyroid parenchyma and cannot be visualized. The pathological parathyroid tissue (or more simply ‘adenoma’) becomes visible due to its increased size and altered echogenicity. Parathyroid adenomas appear as well-circumscribed, oval masses whose echogenicity is clearly inferior to the adjacent thyroid gland (Figure 6A). Larger parathyroid adenomas sometimes present partially liquid echo structures that reflect areas of cystic involution. Colour Doppler imaging reveals vascular patterns (Figure 6B) that can be helpful for differentiating parathyroid adenomas from enlarged lymph nodes and for identifying parathyroid adenomas within the thyroid gland.
FIGURE 6:
(A) Ultrasound of the neck showing inferior parathyroid adenomas (roughly 1.16 × 0.69 cm) located behind the thyroid. The parathyroid adenoma appears as a well-circumscribed, oval mass, hypoechoic in comparison to the adjacent thyroid gland. Longitudinal (left) and transvers (right) scans. (B) An inferior parathyroid adenoma located behind the thyroid with color Doppler imaging (longitudinal scan). (C) Longitudinal scan demonstrating an inferior parathyroid adenoma located near the inferior pole of the thyroid lobe (left). The CEUS examination is characterized by a strong early contrast enhancement in the arterial phase beginning 15 s after the bolus injection (right). (D) CEUS time–intensity curves: the wash-in (arterial phase in the figure, 0–30 s after application of contrast agent) and wash out (venous phase, 30–120 s) of the adenomas (a), represented in the graph by the red line, compared with the thyroid gland (b), represented by the yellow line.
The role of contrast-enhanced ultrasonography (CEUS) prior to surgery in patients with primary HPT is still debated [120, 121]. CEUS for parathyroid adenomas and hyperplasia is characterized by a strong early contrast enhancement beginning 12 s after bolus injection at the margin of parathyroid glands in the arterial phase, a short enhancement of the noncystic parenchyma followed by an early wash out beginning at 50 s (Figure 6C). Sensitivity was ∽96–97% while specificity and PPV were ∽98% [121–123].
Conventional ultrasound has low sensitivity in assessing the severity of SHPT because it cannot display the dynamic perfusion characteristics. CEUS could address this deficiency and detect nodular hyperplasic parathyroid glands before SHPT becomes resistant to medical therapy through time–intensity curves that illustrate the dynamic features of tissue vascularization and blood flow (wash-in time, intensity and wash-out time) [124] (Figure 6D).
Ultrasound performed by experts should be sufficient in most cases to discover abnormal parathyroid glands. The high PPV of CEUS is helpful in doubtful cases for the differential diagnosis between multiple findings.
Planning parathyroidectomy in SHPT
Parathyroidectomy is suggested for patients with SHPT refractory to medical therapy [112].
Surgical approaches
There are three surgical approaches: total parathyroidectomy with or without thymectomy, subtotal parathyroidectomy (3/4 glands or 7/8 glands), and total parathyroidectomy with heterotopic autotransplantation [89, 125]. Usually, in SHPT, all four glands are enlarged and total parathyroidectomy decreases the risk of relapse, but increases the risk of permanent hypocalcemia and hungry bone syndrome [108]. Thus, to balance the risks of persistent or recurrent disease versus permanent hypoparathyroidism with hypocalcemia, most centers have opted in recent years for subtotal parathyroidectomy or total parathyroidectomy + autotransplantation to preserve mineral homeostasis by leaving a small residue of parathyroid tissue [23].
There is no consensus on the indications for total versus subtotal parathyroidectomy, and on the amount of remnant parathyroid tissue in subtotal parathyroidectomy. In 34 consecutive kidney failure patients with SHPT, 3/4 subtotal parathyroidectomy seemed effective and safer than 7/8 subtotal parathyroidectomy, as assessed by lower rates of irreversible hypoparathyroidism and shorter hospital stays [126]. The prevalence variability between parathyroidectomy and subtotal parathyroidectomy reflects, in part, different approaches in dialysis practices [127, 128].
Postoperative morbidity and mortality do not differ significantly between total parathyroidectomy + autotransplantation and subtotal parathyroidectomy [129]. However, reoperations for recurrent HPT after subtotal parathyroidectomy entail more potential complications given the need of a neck reexploration under general anesthesia, whereas after total parathyroidectomy + autotransplantation, patients with parathyroid graft-dependent recurrent disease undergo resection of the autograft under local anesthesia only [129].
Parathyroid gland autotransplantation
There are different protocols for parathyroid gland autotransplantation. Usually the parathyroid tissue is fragmented and then autotransplanted by injection into the arm. However, since autotransplanted parathyroid tissue will not be functional until it has an adequate blood supply by neovascularization, some surgeons mobilize a portion of one parathyroid gland and place the tissue anteriorly, superficial to the strap muscles. The blood supply in this case is guaranteed and the gland is easily accessed in case of reoperation by placing a surgical clip easily identified on X-ray [130].
Autotransplantation may be immediate or delayed. Immediate autotransplantation allows for maintenance of PTH levels following parathyroidectomy. However, not all patients will develop hypoparathyroidism. This implies that some patients will undergo unnecessary autotransplantation with the risk of persistent HPT. The alternative is parathyroid cryopreservation [131]. Cryopreservation preserves cellular function (up to 5 years) and allows the storage of parathyroid tissue for potential reimplantation in patients who develop hypoparathyroidism [132].
Assessing effectiveness
The effectiveness of parathyroidectomy is usually determined by a rapid decrease in PTH values, although there is no clear numerical definition. Rapid intraoperative PTH (ioPTH) testing can guide an adequate resection of parathyroid tissue [133]. A reduction of 60–70% of the pre-excision ioPTH value 10–30 minutes after excision was strongly correlated with resolution of HPT, while the Miami criteria (50% reduction in PTH compared with the highest pre-excision value 10 min after excision) were not predictive [108]. However, the ioPTH value after excision does not predict the risk of postoperative hypoparathyroidism. Severe hypoparathyroidism and hypocalcemia have been observed even if patients have a PTH >2-fold above the upper limit of normal 15–20 min after excision [108, 134].
A roadmap for parathyroidectomy in patients waitlisted for KT: why not?
After successful KT, some CKD-MBD indexes [calcium, phosphate, 1,25(OH)2D] normalize or improve in most KTRs, although persistently high levels of PTH and FGF-23 can worsen bone and mineral homeostasis and negatively impact graft and patient outcomes. Once graft function has recovered, parathyroid gland hyperplasia and SHPT improve over 6–12 months, thus most nephrologists consider it safe to wait 12 months post-KT before considering parathyroidectomy. However, SHPT may not completely resolve in ∽70% and 40% of KTRs within the first and second years posttransplantation, respectively [27].
The KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation suggests not transplanting patients with severe HPT until they are adequately treated (medically or surgically) as per the KDIGO CKD-MBD guidelines [38]. Indeed, patients who fail to respond to medical therapy or have severe, persistent complications of HPT [38] should undergo parathyroidectomy before transplantation. Beyond the generic indications of the KDIGO guidelines, there is no agreement on the definition of adequacy of SHPT control in patients waitlisted for KT. Indeed, the KDIGO transplantation guidelines identify the need for studies addressing the association between pre-KT PTH levels and clinically important posttransplant outcomes [35].
Indications of pre-KT parathyroidectomy
Persistent HPT is usually associated with pre-KT PTH levels above the target of KDOQI guidelines (PTH >300 pg/mL, which is well below the KDIGO target), the use of calcimimetics while on dialysis and the presence of enlarged parathyroid glands [32, 36, 40–42]. We propose that pre-KT parathyroidectomy should be preferred in KT candidates to prevent persistent HPT and to protect graft function. In KT candidates, similar to patients with stage 5D CKD, parathyroidectomy should be considered when PTH levels are >800 pg/mL for >6 months despite maximized medical therapy, especially if associated with persistent hypercalcemia or hyperphosphatemia, tissue or vascular calcification including calciphylaxis and/or worsening osteodystrophy.
Timing of pre-KT PTH assessment
While calcimimetics reduce PTH, they also lower serum calcium and thus can mask THPT. There is debate as to how to measure PTH in patients waitlisted for KT. Coyne and Delos Santos [135] suggest that calcimimetics should be stopped for 2–4 weeks before measuring PTH in KT candidates as part of the assessment for transplantation. If the PTH is >800 pg/mL, the patient's risk of persistent HPT appears to be high. A cut-off for PTH of 1000 pg/mL without calcimimetic use or 500 pg/mL with calcimimetic use has also been proposed: higher values would suggest enlarged parathyroid glands, making improvement post-KT unlikely and, thus, making pre-KT parathyroidectomy preferable [37].
Parathyroid gland size
In our opinion, pre-KT determination of PTH levels is not the optimal tool for decision-making about parathyroidectomy for KT candidates, as parathyroid gland size can not be assessed. Enlarged parathyroid glands (volume ≥500 mm3 or diameter ≥10 mm) frequently present nodular hyperplastic lesions that are unlikely to regress.
Assessment of parathyroid gland size requires imaging tools that additionally inform of parathyroid gland localization, which is especially relevant for ectopic glands. Imaging tools include ultrasound and scintigraphy, often combined with CT (i.e. SPECT/CT) [108]. CEUS allows estimation of parathyroid gland size and a differential diagnosis through the interpretation of dynamic microvascular features.
Multidisciplinary teams
Given the high expertise required from each specialist involved, it is desirable to create multidisciplinary teams composed of nephrologists, radiologists and surgeons for the optimal management of pretransplant SHPT in KT candidates, as well as of persistent/tertiary HPT in KTRs.
A roadmap for parathyroidectomy in patients waitlisted for KT
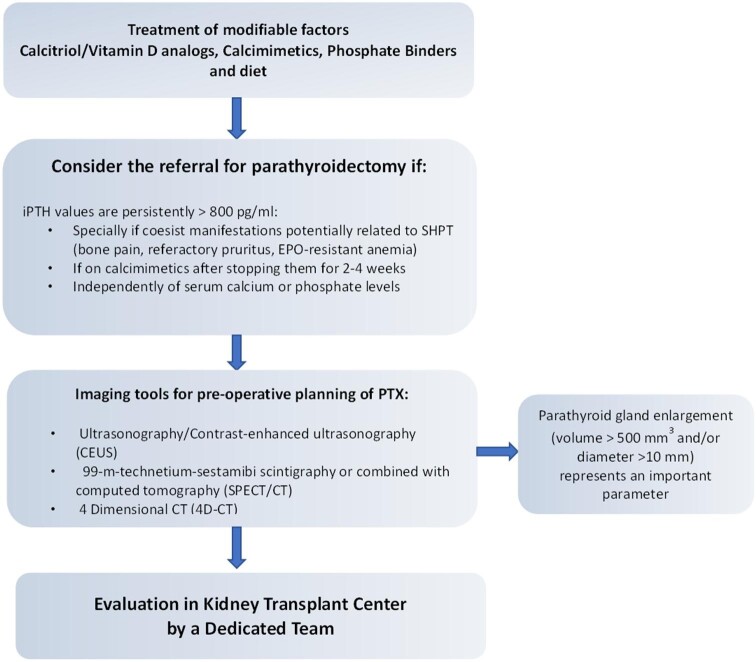
We refer for parathyroidectomy (preferably subtotal parathyroidectomy) candidates for KT with PTH >800 pg/mL despite maximal therapy with calcitriol/vitamin D analogs and calcimimetics or after stopping calcimimetics for 2–4 weeks. Both these conditions have to be associated with enlarged parathyroid glands (diameter >10 mm), independent from the presence or absence of hypercalcemia or hyperphosphatemia (Figure 7). A further item in the decision-making process by a multidisciplinary team is the number of prior KTs. Thus patients waitlisted for a second or more KT are usually burdened by longer-lasting CKD and dialysis vintage and may be highly sensitized with multiple preformed anti-human leukocyte antigen antibodies and therefore may also remain longer on the waitlist compared with unsensitized transplant candidates, in particular those with a greater number of HLA mismatches in prior grafts [136].
FIGURE 7:
Roadmap to parathyroidectomy for kidney failure patients with SHPT candidates to KT. The first step to reduce the risk of persistent HPT in KT candidates is the correction of modifiable factors: hypocalcemia, hyperphosphatemia, hyperparathyroidism (PTH target values 2–9 times the upper limit of normal in dialysis patients). If PTH levels are >800 pg/mL with or without hypercalcemia and/or hyperphosphatemia and symptoms or complications coexist related to SPHT, referral for parathyroidectomy should be considered. Imaging is necessary for preoperative parathyroidectomy planning, aimed to localize and define the size of parathyroid glands. Enlarged parathyroid glands (volume >500 mm3 and/or diameter >10 mm) represent a further parameter, in addition to high PTH levels, in the decision-making regarding parathyroidectomy. In our centers, a multidisciplinary team (nephrologists, otolaryngologists, radiologists) decides on the indication of parathyroidectomy based on preoperative findings. In all cases, CEUS is performed a few days before surgery by an expert and dedicated sonographer in the presence of the nephrologist and surgeon to verify helpful landmarks (skin, vascular axis, trachea, esophagus, upper and lower thyroid poles) for surgery.
EPO, erythropoietin; 4D-CT, 4-dimensional computed tomography.
Contributor Information
Giuseppe Cianciolo, Nephrology, Dialysis and Renal Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Italy.
Francesco Tondolo, Nephrology, Dialysis and Renal Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Italy.
Simona Barbuto, Nephrology, Dialysis and Renal Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Italy.
Andrea Angelini, Nephrology, Dialysis and Renal Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Italy.
Francesca Ferrara, Nephrology, Dialysis and Renal Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Italy.
Francesca Iacovella, Nephrology, Dialysis and Renal Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Italy.
Concettina Raimondi, Nephrology, Dialysis and Renal Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Italy.
Gaetano La Manna, Nephrology, Dialysis and Renal Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Italy.
Carla Serra, Interventional, Diagnostic and Therapeutic Ultrasound Unit, Department of Medical and Surgical Sciences, IRCCS Azienda Ospedaliero-Universitaria Sant’Orsola Malpighi Hospital, Bologna, Italy.
Chiara De Molo, Interventional, Diagnostic and Therapeutic Ultrasound Unit, Department of Medical and Surgical Sciences, IRCCS Azienda Ospedaliero-Universitaria Sant’Orsola Malpighi Hospital, Bologna, Italy.
Ottavio Cavicchi, Department of Otolaryngology Head and Neck Surgery, IRCSS Azienda Ospedaliero Universitaria di Bologna, Policlinico Sant'Orsola, Bologna, Italy.
Ottavio Piccin, Department of Otolaryngology Head and Neck Surgery, IRCSS Azienda Ospedaliero Universitaria di Bologna, Policlinico Sant'Orsola, Bologna, Italy.
Pasquale D'Alessio, Department of Otolaryngology Head and Neck Surgery, IRCSS Azienda Ospedaliero Universitaria di Bologna, Policlinico Sant'Orsola, Bologna, Italy.
Loredana De Pasquale, Department of Otolaryngology, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy.
Giovanni Felisati, Department of Otolaryngology, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy.
Paola Ciceri, Renal Division, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy.
Andrea Galassi, Renal Division, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy.
Mario Cozzolino, Renal Division, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy.
CONFLICT OF INTEREST STATEMENT
M.C. is a member of the CKJ editorial board.
REFERENCES
- 1. Wang JH, Skeans MA, Israni AK. Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis 2016; 23: 281–286 [DOI] [PubMed] [Google Scholar]
- 2. Wolfe RA, Ashby VB, Milford ELet al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 3. Tonelli M, Wiebe N, Knoll Get al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes: systematic review of kidney transplantation. Am J Transplant 2011; 11: 2093–2109 [DOI] [PubMed] [Google Scholar]
- 4. Khairallah P, Nickolas TL. Bone and mineral disease in kidney transplant recipients. Clin J Am Soc Nephrol 2022; 17: 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Messa P, Cafforio C, Alfieri C. Clinical impact of hypercalcemia in kidney transplant. Int J Nephrol 2011; 2011: 906832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muirhead N, Zaltman JS, Gill JSet al. Hypercalcemia in renal transplant patients: prevalence and management in Canadian transplant practice. Clin Transplant 2014; 28: 161–165 [DOI] [PubMed] [Google Scholar]
- 7. Mazzaferro S, Pasquali M, Taggi Fet al. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin J Am Soc Nephrol 2009; 4: 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin A, Bakris GL, Molitch Met al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71: 31–38 [DOI] [PubMed] [Google Scholar]
- 9. Erben RG, Andrukhova O. FGF23-Klotho signaling axis in the kidney. Bone 2017; 100: 62–68 [DOI] [PubMed] [Google Scholar]
- 10. Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 2010; 61: 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meir T, Durlacher K, Pan Zet al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int 2014; 86: 1106–1115 [DOI] [PubMed] [Google Scholar]
- 12. Portillo MR, Rodríguez-Ortiz ME. Secondary hyperparthyroidism: pathogenesis, diagnosis, preventive and therapeutic strategies. Rev Endocr Metab Disord 2017; 18: 79–95 [DOI] [PubMed] [Google Scholar]
- 13. Rodríguez-Ortiz ME, Rodríguez M. Recent advances in understanding and managing secondary hyperparathyroidism in chronic kidney disease. F1000Research 2020; 9: 1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evenepoel P, Bover J, Torres. PU. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int 2016; 90: 1184–1190 [DOI] [PubMed] [Google Scholar]
- 15. Erben RG. Physiological actions of fibroblast growth factor-23. Front Endocrinol 2018; 9: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho BB, Bergwitz C. FGF23 signalling and physiology. J Mol Endocrinol 2021; 66: R23–R32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isakova T, Wahl P, Vargas GSet al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011; 79: 1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernández-Fernández B, Valiño-Rivas L, Sánchez-Niño MDet al. Albuminuria downregulation of the anti-aging factor Klotho: the missing link potentially explaining the association of pathological albuminuria with premature death. Adv Ther 2020; 37: 62–72 [DOI] [PubMed] [Google Scholar]
- 19. Sakan H, Nakatani K, Asai Oet al. Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One 2014; 9: e86301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Komaba H, Goto S, Fujii Het al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 2010; 77: 232–238 [DOI] [PubMed] [Google Scholar]
- 21. Goto S, Komaba H, Fukagawa M. Pathophysiology of parathyroid hyperplasia in chronic kidney disease: preclinical and clinical basis for parathyroid intervention. Clin Kidney J 2008; 1: iii2–iii8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cianciolo G, Galassi A, Capelli Iet al. Klotho-FGF23, cardiovascular disease, and vascular calcification: black or white? Curr Vasc Pharmacol 2018; 16: 143–156 [DOI] [PubMed] [Google Scholar]
- 23. Lau WL, Obi Y, Kalantar-Zadeh K. Parathyroidectomy in the management of secondary hyperparathyroidism. Clin J Am Soc Nephrol 2018; 13: 952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olauson H, Vervloet MG, Cozzolino Met al. New insights into the FGF23-Klotho axis. Semin Nephrol 2014; 34: 586–597 [DOI] [PubMed] [Google Scholar]
- 25. Jäger MD, Serttas M, Beneke Jet al. Risk-factors for nodular hyperplasia of parathyroid glands in sHPT patients. PLoS One 2017; 12: e0186093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolf M, Weir MR, Kopyt Net al. A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation 2016; 100: 184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lou I, Foley D, Odorico SKet al. How well does renal transplantation cure hyperparathyroidism? Ann Surg 2015; 262: 653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCormick F, Held PJ, Chertow GM. The terrible toll of the kidney shortage. J Am Soc Nephrol 2018; 29: 2775–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bastani B. The present and future of transplant organ shortage: some potential remedies. J Nephrol 2020; 33: 277–288 [DOI] [PubMed] [Google Scholar]
- 30. Israni AK, Zaun D, Rosendale JDet al. OPTN/SRTR 2017 annual data report: deceased organ donation. Am J Transplant 2019; 19: 485–516 [DOI] [PubMed] [Google Scholar]
- 31. Cianciolo G, Cozzolino M. FGF23 in kidney transplant: the strange case of Doctor Jekyll and Mister Hyde. Clin Kidney J 2016; 9: 665–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sutton W, Chen X, Patel Pet al. Prevalence and risk factors for tertiary hyperparathyroidism in kidney transplant recipients. Surgery 2022; 171: 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evenepoel P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin Nephrol 2013; 33: 191–203 [DOI] [PubMed] [Google Scholar]
- 34. Dulfer RR, Franssen GJH, Hesselink DAet al. Systematic review of surgical and medical treatment for tertiary hyperparathyroidism. Br J Surg 2017; 104: 804–813 [DOI] [PubMed] [Google Scholar]
- 35. Tseng P-Y, Yang W-C, Yang C-Yet al. Long-term outcomes of parathyroidectomy in kidney transplant recipients with persistent hyperparathyroidism. Kidney Blood Press Res 2015; 40: 386–394 [DOI] [PubMed] [Google Scholar]
- 36. Araujo MJCLN, Ramalho JAM, Elias RMet al. Persistent hyperparathyroidism as a risk factor for long-term graft failure: the need to discuss indication for parathyroidectomy. Surgery 2018; 163: 1144–1150 [DOI] [PubMed] [Google Scholar]
- 37. Delos Santos R, Rossi A, Coyne Det al. Management of post-transplant hyperparathyroidism and bone disease. Drugs 2019; 79: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chadban SJ, Ahn C, Axelrod DAet al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 2020; 104 (4 Suppl 1): S11–S103 [DOI] [PubMed] [Google Scholar]
- 39. Yang RL, Freeman K, Reinke CEet al. Tertiary hyperparathyroidism in kidney transplant recipients: characteristics of patients selected for different treatment strategies. Transplantation 2012; 94: 70–76 [DOI] [PubMed] [Google Scholar]
- 40. Dewberry LC, Tata S, Graves Set al. Predictors of tertiary hyperparathyroidism: who will benefit from parathyroidectomy? Surgery 2014; 156: 1631–1637 [DOI] [PubMed] [Google Scholar]
- 41. Kirnap NG, Kirnap M, Sayin Bet al. Risk factors and treatment options for persistent hyperparathyroidism after kidney transplantation. Transplant Proc 2020; 52: 157–161 [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto T, Tominaga Y, Okada Met al. Characteristics of persistent hyperparathyroidism after renal transplantation. World J Surg 2016; 40: 600–606 [DOI] [PubMed] [Google Scholar]
- 43. Tominaga Y, Tanaka Y, Sato Ket al. Histopathology, pathophysiology, and indications for surgical treatment of renal hyperparathyroidism. Semin Surg Oncol 1997; 13: 78–86 [DOI] [PubMed] [Google Scholar]
- 44. Tominaga Y. Surgical management of secondary hyperparathyroidism in uremia. Am J Med Sci 1999; 317: 390–397 [DOI] [PubMed] [Google Scholar]
- 45. Hirai T, Nakashima A, Takasugi Net al. Response of secondary hyperparathyroidism to cinacalcet depends on parathyroid size. Nephron Clin Pract 2010; 114: c187–c193 [DOI] [PubMed] [Google Scholar]
- 46. Hirai T, Nakashima A, Takasugi Net al. Association of nodular hyperplasia with resistance to cinacalcet therapy for secondary hyperparathyroidism in hemodialysis patients: cinacalcet and nodular hyperplasia. Ther Apher Dial 2010; 14: 577–582 [DOI] [PubMed] [Google Scholar]
- 47. Vulpio C, Bossola M, Magalini SCet al. Parathyroid-gland ultrasonography in clinical and therapeutic evaluation of renal secondary hyperparathyroidism. Radiol Med (Torino) 2013; 118: 707–722 [DOI] [PubMed] [Google Scholar]
- 48. Vulpio C, Bossola M, De Gaetano Aet al. Parathyroid gland ultrasound patterns and biochemical findings after one-year cinacalcet treatment for advanced secondary hyperparathyroidism. Ther Apher Dial 2010; 14: 178–185 [DOI] [PubMed] [Google Scholar]
- 49. Yamamoto M, Ogata H, Mizobuchi Met al. Number of enlarged parathyroid glands might be a predictor of cinacalcet response in advanced secondary hyperparathyroidism. Clin Exp Nephrol 2012; 16: 292–299 [DOI] [PubMed] [Google Scholar]
- 50. Yamada S, Tokumoto M, Taniguchi Met al. Two years of cinacalcet hydrochloride treatment decreased parathyroid gland volume and serum parathyroid hormone level in hemodialysis patients with advanced secondary hyperparathyroidism: cinacalcet reduces PTH and PTG volume. Ther Apher Dial 2015; 19: 367–377 [DOI] [PubMed] [Google Scholar]
- 51. Hong YA, Cho YS, Kim SWet al. Diameter of parathyroid glands measured by computed tomography as a predictive indicator for response to cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Blood Press Res 2015; 40: 277–287 [DOI] [PubMed] [Google Scholar]
- 52. Matsuoka S, Tominaga Y, Sato Tet al. Relationship between the dimension of parathyroid glands estimated by ultrasonography and the hyperplastic pattern in patients with renal hyperparathyroidism: PT gland size vs hyperplastic pattern. Ther Apher Dial 2008; 12: 391–395 [DOI] [PubMed] [Google Scholar]
- 53. Nakai K, Fujii H, Ishimura Tet al. Incidence and risk factors of persistent hyperparathyroidism after kidney transplantation. Transplant Proc 2017; 49: 53–56 [DOI] [PubMed] [Google Scholar]
- 54. Nakamura M, Tanaka K, Marui Yet al. Clinicopathological analysis of persistent hypercalcemia and hyperparathyroidism after kidney transplantation in long-term dialysis patients: hyperparathyroidism in long-term dialysis recipients. Ther Apher Dial 2013; 17: 551–556 [DOI] [PubMed] [Google Scholar]
- 55. Taniguchi M, Tokumoto M, Matsuo Det al. Persistent hyperparathyroidism in renal allograft recipients: vitamin D receptor, calcium-sensing receptor, and apoptosis. Kidney Int 2006; 70: 363–370 [DOI] [PubMed] [Google Scholar]
- 56. Copley JB, Wüthrich RP. Therapeutic management of post-kidney transplant hyperparathyroidism: managing post-kidney transplant HPT. Clin Transplant 2011; 25: 24–39 [DOI] [PubMed] [Google Scholar]
- 57. Pitt SC, Sippel RS, Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am 2009; 89: 1227–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Evenepoel P, Claes K, Kuypers Det al. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant 2004; 19: 1281–1287 [DOI] [PubMed] [Google Scholar]
- 59. Schwarz A, Mengel M, Gwinner Wet al. Risk factors for chronic allograft nephropathy after renal transplantation: a protocol biopsy study. Kidney Int 2005; 67: 341–348 [DOI] [PubMed] [Google Scholar]
- 60. Heaf J, Tvedegaard E, Kanstrup I-Let al. Hyperparathyroidism and long-term bone loss after renal transplantation: hyperparathyroidism and bone loss. Clin Transplant 2003; 17: 268–274 [DOI] [PubMed] [Google Scholar]
- 61. Perrin P, Caillard S, Javier RMet al. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation: fractures after kidney transplantation. Am J Transplant 2013; 13: 2653–2663 [DOI] [PubMed] [Google Scholar]
- 62. Giannini S, Sella S, Silva-Netto Fet al. Persistent secondary hyperparathyroidism and vertebral fractures in kidney transplantation: role of calcium-sensing receptor polymorphisms and vitamin D deficiency. J Bone Miner Res 2009; 25: 841–848 [DOI] [PubMed] [Google Scholar]
- 63. Nikkel LE, Mohan S, Zhang Aet al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant: fewer fractures with ECSW after transplant. Am J Transplant 2012; 12: 649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Iseri K, Carrero JJ, Evans Met al. Fractures after kidney transplantation: incidence, predictors, and association with mortality. Bone 2020; 140: 115554. [DOI] [PubMed] [Google Scholar]
- 65. Iyer SP, Nikkel LE, Nishiyama KKet al. Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol 2014; 25: 1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Evenepoel P, Claes K, Meijers Bet al. Natural history of mineral metabolism, bone turnover and bone mineral density in de novo renal transplant recipients treated with a steroid minimization immunosuppressive protocol. Nephrol Dial Transplant 2020; 35: 697–705 [DOI] [PubMed] [Google Scholar]
- 67. Okada M, Hiramitsu T, Ichimori Tet al. Comparison of pre- and post-transplant parathyroidectomy in renal transplant recipients and the impact of parathyroidectomy timing on calcium metabolism and renal allograft function: a retrospective single-center analysis. World J Surg 2020; 44: 498–507 [DOI] [PubMed] [Google Scholar]
- 68. Roodnat JI, van Gurp EAFJ, Mulder PGHet al. High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation 2006; 82: 362–367 [DOI] [PubMed] [Google Scholar]
- 69. Molnar MZ, Kovesdy CP, Mucsi Iet al. Association of pre-kidney transplant markers of mineral and bone disorder with post-transplant outcomes. Clin J Am Soc Nephrol 2012; 7: 1859–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wolf M, Molnar MZ, Amaral APet al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 2011; 22: 956–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Isakov O, Ghinea R, Beckerman Pet al. Early persistent hyperparathyroidism post-renal transplantation as a predictor of worse graft function and mortality after transplantation. Clin Transplant 2020; 34: e14085. [DOI] [PubMed] [Google Scholar]
- 72. Bleskestad IH, Bergrem H, Leivestad Tet al. Parathyroid hormone and clinical outcome in kidney transplant patients with optimal transplant function. Clin Transplant 2014; 28: 479–486 [DOI] [PubMed] [Google Scholar]
- 73. Pihlstrøm H, Dahle DO, Mjøen Get al. Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation 2015; 99: 351–359 [DOI] [PubMed] [Google Scholar]
- 74. Egbuna OI, Taylor JG, Bushinsky DAet al. Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure. Clin Transplant 2007; 21: 558–566 [DOI] [PubMed] [Google Scholar]
- 75. Gwinner W, Suppa S, Mengel Met al. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant 2005; 5: 1934–1941 [DOI] [PubMed] [Google Scholar]
- 76. Evenepoel P, Claes K, Kuypers DRet al. Parathyroidectomy after successful kidney transplantation: a single centre study. Nephrol Dial Transplant 2007; 22: 1730–1737 [DOI] [PubMed] [Google Scholar]
- 77. Mathur A, Sutton W, Ahn JBet al. Association between treatment of secondary hyperparathyroidism and posttransplant outcomes. Transplantation 2021; 105: e366–e374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wetmore JB, Gurevich K, Sprague Set al. A randomized trial of cinacalcet versus vitamin D analogs as monotherapy in secondary hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol 2015; 10: 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Charytan C, Coburn JW, Chonchol Met al. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis 2005; 46: 58–67 [DOI] [PubMed] [Google Scholar]
- 80. EVOLVE Trial Investigators . Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012; 367: 2482–2494 [DOI] [PubMed] [Google Scholar]
- 81. Parfrey PS, Drüeke TB, Block GAet al. The effects of cinacalcet in older and younger patients on hemodialysis: the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Clin J Am Soc Nephrol 2015; 10: 791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moe SM, Abdalla S, Chertow GMet al. Effects of cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE trial. J Am Soc Nephrol 2015; 26: 1466–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Evenepoel P, Sprangers B, Lerut Eet al. Mineral metabolism in renal transplant recipients discontinuing cinacalcet at the time of transplantation: a prospective observational study: cinacalcet discontinuation at transplantation. Clin Transplant 2012; 26: 393–402 [DOI] [PubMed] [Google Scholar]
- 84. Dachy G, Pochet J-M, Labriola Let al. Severe hypercalcaemia early after kidney transplantation in two patients with severe secondary hyperparathyroidism previously treated with etelcalcetide. Clin Kidney J 2021; 14: 1977–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim SM, Long J, Montez-Rath MEet al. Rates and outcomes of parathyroidectomy for secondary hyperparathyroidism in the United States. Clin J Am Soc Nephrol 2016; 11: 1260–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Littbarski SA, Kaltenborn A, Gwiasda Jet al. Timing of parathyroidectomy in kidney transplant candidates with secondary hyperparathryroidism: effect of pretransplant versus early or late post-transplant parathyroidectomy. Surgery 2018; 163: 373–380 [DOI] [PubMed] [Google Scholar]
- 87. Evenepoel P, Claes K, Kuypers Det al. Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: a single centre study. Nephrol Dial Transplant 2005; 20: 1714–1720 [DOI] [PubMed] [Google Scholar]
- 88. Jeon HJ, Kim YJ, Kwon HYet al. Impact of parathyroidectomy on allograft outcomes in kidney transplantation: impact of parathyroidectomy on kidney transplantation. Transpl Int 2012; 25: 1248–1256 [DOI] [PubMed] [Google Scholar]
- 89. van der Plas WY, Noltes ME, van Ginhoven TMet al. Secondary and tertiary hyperparathyroidism: a narrative review. Scand J Surg 2020; 109: 271–278 [DOI] [PubMed] [Google Scholar]
- 90. Cruzado JM, Moreno P, Torregrosa JVet al. A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol 2016; 27: 2487–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Moreno P, Coloma A, Torregrosa JVet al. Long-term results of a randomized study comparing parathyroidectomy with cinacalcet for treating tertiary hyperparathyroidism. Clin Transplant 2020; 34: e13988. [DOI] [PubMed] [Google Scholar]
- 92. Koh EY, van der Plas WY, Dulfer RRet al. Correction to: outcomes of parathyroidectomy versus calcimimetics for secondary hyperparathyroidism and kidney transplantation: a propensity-matched analysis. Langenbecks Arch Surg 2021; doi: 10.1007/s00423-020-02046-z [DOI] [PubMed] [Google Scholar]
- 93. Piccin O, D'Alessio P, Cioccoloni Eet al. Pre-operative imaging workup for surgical intervention in primary hyperparathyroidism: a tertiary referral center experience. Am J Otolaryngol 2021; 42: 102819. [DOI] [PubMed] [Google Scholar]
- 94. Schneider R, Slater EP, Karakas Eet al. Initial parathyroid surgery in 606 patients with renal hyperparathyroidism. World J Surg 2012; 36: 318–326 [DOI] [PubMed] [Google Scholar]
- 95. Zhang JLH, Appelman-Dijkstra NM, Fu ELet al. Practice variation in the treatment of patients with renal hyperparathyroidism: a survey-based study in the Netherlands. BMC Nephrol 2021; 22: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kasiske BL, Cangro CB, Hariharan Set al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant 2001; 1(Suppl 2): 3–95 [PubMed] [Google Scholar]
- 97. Knoll G. Canadian Society of Transplantation: consensus guidelines on eligibility for kidney transplantation. CMAJ 2005; 173: S1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Elder G, Faull R, Branley Pet al. Management of bone disease, calcium, phosphate and parathyroid hormone. Nephrology 2006; 11(Suppl 1): S230–S261 [DOI] [PubMed] [Google Scholar]
- 99. Dudley C, Harden P. Renal Association clinical practice guideline on the assessment of the potential kidney transplant recipient. Nephron Clin Pract 2011; 118: c209–c224 [DOI] [PubMed] [Google Scholar]
- 100. Campbell S, Pilmore H, Gracey Det al. KHA-CARI guideline: recipient assessment for transplantation: transplant recipient assessment guidelines. Nephrology 2013; 18: 455–462 [DOI] [PubMed] [Google Scholar]
- 101. Abramowicz D, Cochat P, Claas FHJet al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant 2015; 30: 1790–1797 [DOI] [PubMed] [Google Scholar]
- 102. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Burton JO, Goldsmith DJ, Ruddock Net al. Renal Association commentary on the KDIGO (2017) clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of CKD-MBD. BMC Nephrol 2018; 19: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu Z-H, Li G, Zhang Let al. Executive summary: clinical practice guideline of chronic kidney disease–mineral and bone disorder (CKD-MBD) in China. Kidney Dis 2019; 5: 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Puttarajappa CM, Schinstock CA, Wu CMet al. KDOQI US commentary on the 2020 KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Am J Kidney Dis 2021; 77: 833–856 [DOI] [PubMed] [Google Scholar]
- 106. Bunch PM, Kelly HR. Preoperative imaging techniques in primary hyperparathyroidism: a review. JAMA Otolaryngol Neck Surg 2018; 144: 929. [DOI] [PubMed] [Google Scholar]
- 107. Jiang S-Q, Yang T, Zou Qet al. The role of 99mTc-MIBI SPECT/CT in patients with secondary hyperparathyroidism: comparison with 99mTc-MIBI planar scintigraphy and ultrasonography. BMC Med Imaging 2020; 20: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Steinl GK, Kuo JH. Surgical management of secondary hyperparathyroidism. Kidney Int Rep 2021; 6: 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Uruno T, Kebebew E. How to localize parathyroid tumors in primary hyperparathyroidism? J Endocrinol Invest 2006; 29: 840–847 [DOI] [PubMed] [Google Scholar]
- 110. Scheiner JD, Dupuy DE, Monchik JMet al. Pre-operative localization of parathyroid adenomas: a comparison of power and colour Doppler ultrasonography with nuclear medicine scintigraphy. Clin Radiol 2001; 56: 984–988 [DOI] [PubMed] [Google Scholar]
- 111. De Feo ML, Colagrande S, Biagini Cet al. Parathyroid glands: combination of 99mTc MIBI scintigraphy and US for demonstration of parathyroid glands and nodules. Radiology 2000; 214: 393–402 [DOI] [PubMed] [Google Scholar]
- 112. Kidney Disease: Improving Global Outcomes Transplant Work Group . KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl 3): S1–155 [DOI] [PubMed] [Google Scholar]
- 113. Zhang R, Zhang Z, Huang Pet al. Diagnostic performance of ultrasonography, dual-phase 99mTc-MIBI scintigraphy, early and delayed 99mTc-MIBI SPECT/CT in preoperative parathyroid gland localization in secondary hyperparathyroidism. BMC Med Imaging 2020; 20: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kobylecka M, Płazińska MT, Chudziński Wet al. Comparison of scintigraphy and ultrasound imaging in patients with primary, secondary and tertiary hyperparathyroidism—own experience. J Ultrason 2017; 17: 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Milas M, Stephen A, Berber Eet al. Ultrasonography for the endocrine surgeon: a valuable clinical tool that enhances diagnostic and therapeutic outcomes. Surgery 2005; 138: 1193–1201 [DOI] [PubMed] [Google Scholar]
- 116. Milas M, Mensah A, Alghoul Met al. The impact of office neck ultrasonography on reducing unnecessary thyroid surgery in patients undergoing parathyroidectomy. Thyroid 2005; 15: 1055–1059 [DOI] [PubMed] [Google Scholar]
- 117. Lloyd MNH, Lees WR, Milroy EJG. Pre-operative localisation in primary hyperparathyroidism. Clin Radiol 1990; 41: 239–243 [DOI] [PubMed] [Google Scholar]
- 118. Purcell GP. Parathyroid localization with high-resolution ultrasound and technetium Tc 99m sestamibi. Arch Surg 1999; 134: 824–830 [DOI] [PubMed] [Google Scholar]
- 119. Mihai R, Simon D, Hellman P. Imaging for primary hyperparathyroidism—an evidence-based analysis. Langenbecks Arch Surg 2009; 394: 765–784 [DOI] [PubMed] [Google Scholar]
- 120. Karakas E, Kann S, Höffken Het al. Does contrast-enhanced cervical ultrasonography improve preoperative localization results in patients with sporadic primary hyperparathyroidism? J Clin Imaging Sci 2012; 2: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Agha A, Hornung M, Schlitt HJet al. The role of contrast-enhanced ultrasonography (CEUS) in comparison with 99mTechnetium-sestamibi scintigraphy for localization diagnostic of primary hyperparathyroidism. Clin Hemorheol Microcirc 2014; 58: 515–520 [DOI] [PubMed] [Google Scholar]
- 122. Agha A, Hornung M, Rennert Jet al. Contrast-enhanced ultrasonography for localization of pathologic glands in patients with primary hyperparathyroidism. Surgery 2012; 151: 580–586 [DOI] [PubMed] [Google Scholar]
- 123. Agha A, Hornung M, Stroszczynski Cet al. Highly efficient localization of pathological glands in primary hyperparathyroidism using contrast-enhanced ultrasonography (CEUS) in comparison with conventional ultrasonography. J Clin Endocrinol Metab 2013; 98: 2019–2025 [DOI] [PubMed] [Google Scholar]
- 124. Liang X, Li F, Gao Fet al. The value of the model and quantitative parameters of contrast-enhanced ultrasound in judging the severity of SHPT. BioMed Res Int 2016; 2016: 6064526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Madorin C, Owen RP, Fraser WDet al. The surgical management of renal hyperparathyroidism. Eur Arch Otorhinolaryngol 2012; 269: 1565–1576 [DOI] [PubMed] [Google Scholar]
- 126. Veyrat M, Fessi H, Haymann J-Pet al. Conservative three-quarter versus subtotal seven-eighths parathyroidectomy in secondary hyperparathyroidism. Eur Ann Otorhinolaryngol Head Neck Dis 2019; 136: 63–68 [DOI] [PubMed] [Google Scholar]
- 127. Anderson K, Ruel E, Adam MAet al. Subtotal vs. total parathyroidectomy with autotransplantation for patients with renal hyperparathyroidism have similar outcomes. Am J Surg 2017; 214: 914–919 [DOI] [PubMed] [Google Scholar]
- 128. Tominaga Y, Kakuta T, Yasunaga Cet al. Evaluation of parathyroidectomy for secondary and tertiary hyperparathyroidism by the Parathyroid Surgeons’ Society of Japan. Ther Apher Dial 2016; 20: 6–11 [DOI] [PubMed] [Google Scholar]
- 129. Chen J, Jia X, Kong Xet al. Total parathyroidectomy with autotransplantation versus subtotal parathyroidectomy for renal hyperparathyroidism: a systematic review and meta-analysis: parathyroidectomy for renal hyperparathyroidism. Nephrology 2017; 22: 388–396. [DOI] [PubMed] [Google Scholar]
- 130. Shokri T, Lew SQ, Sadeghi N. Pedicled parathyroid gland autotransposition in secondary and tertiary hyperparathyroidism: parathyroid gland autotransposition. Laryngoscope 2015; 125: 894–897 [DOI] [PubMed] [Google Scholar]
- 131. Schneider R, Ramaswamy A, Slater EPet al. Cryopreservation of parathyroid tissue after parathyroid surgery for renal hyperparathyroidism: does it really make sense? World J Surg 2012; 36: 2598–2604 [DOI] [PubMed] [Google Scholar]
- 132. Aiti A, Rossi M, Alviano Fet al. Parathyroid tissue cryopreservation: does the storage time affect viability and functionality? Biopreserv Biobank 2019; 17: 418–424 [DOI] [PubMed] [Google Scholar]
- 133. El-Husseini A, Wang K, Edon Aet al. Value of intraoperative parathyroid hormone assay during parathyroidectomy in dialysis and renal transplant patients with secondary and tertiary hyperparathyroidism. Nephron 2018; 138: 119–128 [DOI] [PubMed] [Google Scholar]
- 134. Miccoli P. Intraoperative parathyroid hormone assay during surgery for secondary hyperparathyroidism: is it time to give up the chase at the hormone? Endocrine 2012; 42: 459–460 [DOI] [PubMed] [Google Scholar]
- 135. Coyne DW, Delos Santos R. Evaluating the safety and rationale for cinacalcet posttransplant hyperparathyroidism and hypercalcemia. Am J Transplant 2014; 14: 2446–2447 [DOI] [PubMed] [Google Scholar]
- 136. Wong G, Chua S, Chadban SJet al. Waiting time between failure of first graft and second kidney transplant and graft and patient survival. Transplantation 2016; 100: 1767–1775 [DOI] [PubMed] [Google Scholar]