ABSTRACT
Background
Erythropoiesis-stimulating agents (ESAs) revolutionized the management of anaemia in chronic kidney disease (CKD) when introduced in the late 1980s. A range of ESA types, preparations and administration modalities now exist, with newer agents requiring less frequent administration. Although systematic reviews and meta-analyses have been published in adults, no systematic review has been conducted investigating ESAs in children.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses statement for the conduct of systematic reviews was used. All available literature on outcomes relating to ESAs in children with CKD was sought. A search of the MEDLINE, CINAHL and Embase databases was conducted by two independent reviewers. Inclusion criteria were published trials in English, children with chronic and end-stage kidney disease and use of any ESA studied against any outcome measure. An assessment of risk of bias was carried out in all included randomized trials using the criteria from the Cochrane Handbook for Systematic Reviews of Interventions. Two tables were used for data extraction for randomized and observational studies. Study type, participants, inclusion criteria, case characteristics, follow-up duration, ESA type and dosage, interventions and outcomes were extracted by one author.
Results
Of 965 identified articles, 58 were included covering 54 cohorts. Six were randomized trials and 48 were observational studies. A total of 38 studies assessed the efficacy of recombinant human erythropoietin (rHuEPO), 11 of darbepoetin alpha (DA) and 3 of continuous erythropoietin receptor activator (CERA), with 6 studies appraising secondary outcome measures exclusively. Recruitment to studies was a consistent challenge. The most common adverse effect was hypertension, although confounding effects often limited direct correlation. Two large cohort studies demonstrated a greater hazard of death independently associated with high ESA dose. Secondary outcome measures included quality of life measures, growth and nutrition, exercise capacity, injection site pain, cardiovascular function, intelligent quotient, evoked potentials and platelet function.
Conclusions
All ESA preparations and modes of administration were efficacious, with evidence of harm at higher doses. Evidence supports individualizing treatments, with strong consideration given to alternate treatments in patients who appear resistant to ESA therapy. Further research should focus on randomized trials comparing the efficacy of different preparations, treatment options in apparently ESA-resistant cohorts and clarification of meaningful secondary outcomes to consolidate patient-relevant indices.
Keywords: chronic renal failure, ESA, haemoglobin, paediatrics, systematic review
INTRODUCTION
Chronic kidney disease (CKD) is a substantial global health burden, with mortality rates for children with end-stage kidney disease (ESKD) 55 times higher than the general paediatric population [1]. Anaemia is a common complication observed in up to 73% of children with CKD stage 3 and 93% in stages 4 and 5 [2, 3].
The primary cause of this anaemia is a deficiency of erythropoietin (EPO). EPO is a 30.4-kDa glycoprotein that stimulates red cell production, differentiation and survival [4]. EPO gene expression is upregulated by hypoxia-inducible transcription factor (HIF), although in CKD the response to hypoxia is deranged, resulting in impaired production and reduced HIF-binding capacity [5–7].
Erythropoiesis-stimulating agents (ESAs) replicate EPO. A recombinant human erythropoietin (rHuEPO) was synthesized in 1985 [8], trialled in 25 adults in 1987, with demonstrated efficacy [9].
The short half-life of rHuEPO necessitates administration three times per week [10]. In the late 1990s, darbepoetin alpha (DA) was synthesized through ‘glycoengineering’ amino acid changes to rHuEPO, extending its half-life to allow once- or twice-weekly dosing [11]. In 2007, continuous erythropoietin receptor activator (CERA) usage was approved, with the addition of a methoxy-polyethylene glycol polymer further prolonging the half-life to permit fortnightly or monthly dosing [12].
In adults, ESA therapy is associated with hypertension, stroke, vascular access thrombosis and overall mortality when higher haemoglobin (Hb) levels (>12.5 g/dL) are targeted [13, 14]. In children this association is less clear—one large retrospective cohort study of 1569 children found no relationship [15]. The Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines recommend modest Hb targets of 11.0–12.0 g/dL with initial doses of 60–150 IU/kg/week for rHuEPO and 0.45 µg/kg/week for DA [16]. There also appears to be an independent association with mortality when high ESA doses are administered [17, 18], therefore KDIGO specifically cautions against dose escalation in failed responders [16].
There are several large randomized controlled trials (RCTs) [19–21] feeding into systematic reviews appraising the efficacy of ESAs in adults [13, 22–24]. Although review articles exist [25–27], there are currently no systematic reviews regarding ESA use in children.
This systematic review will appraise studies assessing the efficacy of ESAs in children with CKD. It will also appraise the extent to which a safety profile has been established, while outlining all other secondary outcomes explored.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for the conduct of systematic reviews was used.
Eligibility criteria
Published studies in English were included that investigated children with CKD using any ESA. Any outcomes were considered. Studies examining single-dose pharmacokinetics were excluded.
Outcome measures
Primary outcome measures included any measure of red blood cell quantity and function. Secondary outcome measures included adverse effects and any other measure of physiological function or patient satisfaction.
Search strategy
A computerized search was undertaken using MEDLINE, Embase and CINAHL through February 2021 by two independent reviewers (see Figure 1). Years included in each search were 1946–2021, 1974–2021 and 1961–2021, respectively.
Figure 1.
Literature search strategy.
Study selection
Both reviewers independently conducted a manual search. Titles and abstracts were assessed against inclusion criteria with duplicates and non-relevant studies removed. The remaining studies were reviewed in full. Studies pertaining to the same patient cohorts were collated.
Assessment of risk of bias
An assessment of risk of bias was carried out on all included randomized trials using the criteria from the Cochrane Handbook for Systematic Reviews of Interventions [27].
Data extraction
Two tables were used for data extraction for randomized and observational studies. Study type, participants, inclusion criteria, case characteristics, follow-up duration, ESA type and dosage, interventions and outcomes were extracted.
RESULTS
The search identified 965 articles and 3 duplicates. A total of 898 articles were initially excluded and 65 studies were reviewed in full. A total of 7 studies were then excluded, leaving 58 studies included in the final review (see Figure 2). Collating studies with secondary analysis of identical cohorts resulted in 54 studies: 6 randomized trials and 48 observational studies. A total of 3895 children were included.
Figure 2.
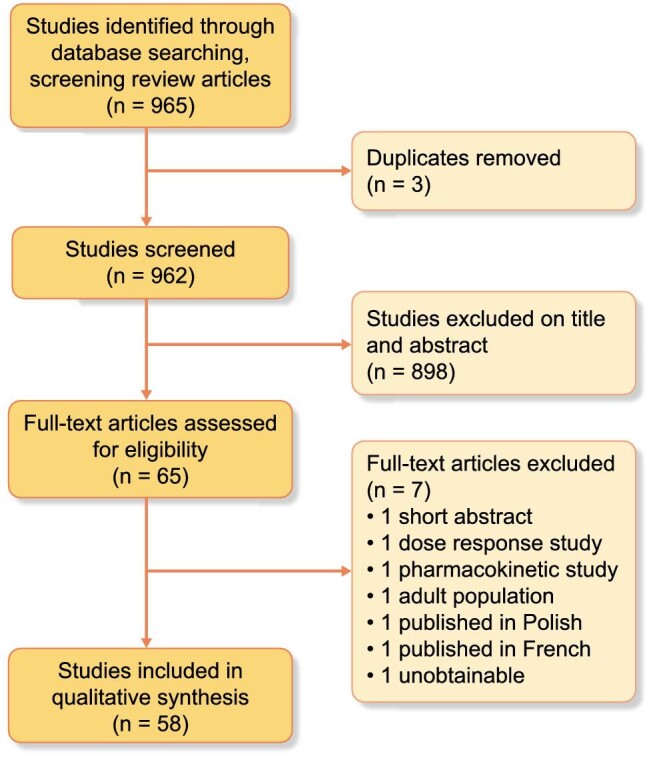
PRISMA flow chart.
Risk of bias summary
Risk of bias was conducted for six randomized trials (see Table 1). Randomization was low risk in one trial [28]. The other five were unclear risk, with missing details on randomization or concealment [29, 30]. Blinding was unclear risk in one study [30].
Table 1.
Analysis of risk of bias
| Risk of bias summary for randomized trials | |||||||
|---|---|---|---|---|---|---|---|
| Study | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other bias | Justification |
| Warady et al. [29] | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | No details of randomization method or concealment |
| Schmitt et al. [58] | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | No details of randomization method or concealment |
| Warady et al. [28] | Low risk | Unclear risk | High risk | Unclear risk | Low risk | Unclear risk | No detail of concealment |
| Brandt et al. [30] | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Unclear risk | No details of randomization method or concealment No discussion around absence of blinding |
| Morris et al. [44, 45] | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk | No details of randomization method or concealment Single blinded but unlikely to make difference to outcome |
| Yalçınkaya et al. [43] | Unclear risk | High risk | High risk | Low risk | Unclear risk | Unclear risk | No details of randomization method |
Characteristics of included trials
All trials had differences in study design, size, populations studied, interventions, outcomes and ESA investigated. Table 2 details six randomized trials. Table 3 details 48 observational studies.
Table 2.
Characteristics of included randomized trials
| Characteristics of included trials—randomized trials | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study design | Participants | Population | Inclusion criteria | ESA evaluated | Intervention | Control | Follow-up duration | Outcomes | Results |
| Warady et al. [29] | Prospective double-blinded RCT | 114: 59 intervention, 57 control |
43 centres: USA, European Union, Mexico | Age 1–18 years CKD CMT Hb <10 g/dL ESA naïve |
DA sc/iv |
Weekly (QW) dosing 0.45 µg/kg Adjustment increment not specified |
Fortnightly (Q2W) dosing 0.75 µg/kg Adjustment increment not specified |
24 weeks | Percent achieving target Hb (10–12 g/dL) Median time to target Hb QoL (PedsQL) |
QW: 98% (>80% P < .001) Q2W: 84% (>80% P = .293) QW: 24 days Q2W: 22 days QW: 61.1 → 68.1 Q2W: 62.6 → 67.2 |
| Schmitt et al. [58] | Prospective double-blinded RCT | 13 | Single-centre Germany |
Age 3.7–22 years (mean 13.6) 10/13 PD 3/13 HD |
DA sc 0.21–1.35 µg/kg/week rHuEPO Epoetin-beta sc 42–271 U/kg/week |
DA then epoetin-beta injections | Epoetin-beta then DA injections | 12 weeks | Mean pain perception (VAS 0–10) | DA Patient: 5.4 ± 1 Parent: 5.3 ± 1 Nurse: 4.4 ± 1 Epoetin-beta Patient: 2.3 ± 0.6 Parent: 2.0 ± 0.9 Nurse: 2.2 ± 0.6 P < .05 for all comparisons |
| Warady et al. [28] | Prospective randomized open- label non-inferiority trial | 124: 82 intervention, 42 control |
Multicentre (NOS) USA |
Age 1–18 years CKD CMT Stable on rHuEpo >8 weeks Diastolic BP <95th centile |
DA 100 U rHuEpo to 0.42 µg DA | DA (QW or Q2W) Adjustment increment not specified |
rHuEPO Adjustment increment not specified |
28 weeks | Adjusted mean ΔHb Mean % Hb values in target (10–12.5 g/dL) Change in dosing over time Safety |
rHuEPO: −0.16 g/dL (95% CI −0.77–0.45) DA: 0.15 g/dL (95% CI −0.30–0.60) Difference: 0.22 g/dL (95% CI −0.47–0.92) rHuEPO: 73% DA: 75% rHuEPO: 55.9% required dose increase DA: 37.3% required dose increase rHuEPO:14% treatment-related adverse events DA: 20% treatment-related adverse events |
| Brandt et al. [30] | Prospective randomized open-label multiple-dose study | 44 22 intervention 22 control |
3 centres USA |
Age <21 years CKD CMT 25/44 HD 9/44 PD ESA naïve Hb <−2SD for age BP <95th centile |
rHuEPO iv 3/week |
Low dose: 150 U/kg/week for 12 weeks or until target achieved |
High dose: 450 U/kg/week for 12 weeks or until target achieved |
12 weeks initial phase Up to 81 weeks dose adjustment (median 37 weeks) |
% at target Hb (10–12 g/dL) in initial phase Mean time to target Mean rHuEPO dose Adverse events |
High dose: 95% Low dose: 66% High dose: 5 weeks Low dose: 13 weeks 157 ± 107 U/kg per week HTN: 30% [high dose: 38% high dose, low dose 21% (P = .17)] Iron deficiency: 30% |
| Yalçınkaya et al. [43] | Prospective open-label multiple-dose study | 20 | Single centre Turkey |
Age 5–16 years (mean 10) CAPD ESA naïve |
rHuEPO sc 1–3/week |
Low dose: 50 U/kg/week |
High dose: 150 U/kg/week |
6 months | Mean ΔHCT Mean ΔBP (MAP) |
High dose: 0.19 → 0.32 L/L (P < .001) Low dose: 0.19 → 0.30 L/L (P < .001) High dose: 85 → 101 mmHg (P < .05) Low dose: 83 → 87 mmHg |
| Morris et al. [44, 45] | Prospective single-blind crossover trial | 11 | Single centre UK |
Age 2.3–12.3 years (median 6.7) 1/11 HD 9/11 CAPD 1/11 CKD CMT ESA naïve |
rHuEPO sc 1–2/week 50 U/kg/week then adjusted |
Phase 1: rHuEPO Phase 2: Placebo |
Phase 1: Placebo 3 Phase 2: rHuEPO |
24 weeks per phase | Median ΔHb post-rHuEPO Dietary intake Anthropometric measures Exercise Tolerance (2 m walking distance) QoL Echocardiography |
7.3 → 11.2 g/dL (P < .001) No significant changes No significant changes Increase (NOS) (P = .06) Improvement in two domains (self-created study) Reduced cardiac index (P = .01) |
Table 3.
Characteristics of included observational studies
| Characteristics of included trials—observational studies, case reports and series | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Study design | n | Population | Case characteristics | ESA | Duration | Outcomes measured | Results |
| Fischbach et al. [60] | Phase II, open-label, prospective, multiple-dose study | 64 | Multiple centres 10 countries, unspecified |
Age 5–17 years (mean 12.6) CKD CMT Hb 10–12 g/dL On stable dose of rHuEPO or DA |
MPG-epo beta
(Mircera) iv 4 weekly Group 1 (16/64): intermediate conversion factor Group 2 (48/64): high conversion factor |
20-week core phase 16-week dose adjustment 25% increments 1-year safety extension |
Mean ∆Hb during evaluation phase (target 10–12 g/dL) % maintaining target Hb during evaluation phase Adverse effects |
Group 1: 11.30 → 10.36 g/dL (95% CI 9.98–11.14) Group 2: 11.10 → 11.01 g/dL (95% CI 10.65–11.36) Group 1: 75% Group 2: 81% 7 worsening HTN 1 new HTN 4 vascular access thrombosis |
| Libudzic-Nowak et al. [56] | Retrospective case series | 3 | Single centre Switzerland |
Age 1–7 months (mean 4 months) CKD CMT Hb 7.7–10.7 ESA naïve |
DA
sc fortnightly 0.27–0.5 µg/kg/week Adjusted in 25% increments |
18–41 months | Mean ∆Hb (target 10.7–12 g/dL) |
Target Hb achieved at 11–22 weeks |
| Gaydarova et al. [52] | Prospective case series | 7 | Single centre Hungary |
Age 3–16 years CKD CMT 5/7 ESA naïve 2/7 rHuEPO |
DA
sc fortnightly 0.36 µg/kg/week Adjusted in 25–50% increments |
5–34 months | % maintaining Hb (target >11.8 g/dL) | 86% |
| Schaefer et al. [62] | Phase IV, prospective observational study | 319 | Multiple centres 13 EU countries |
Age <16 years (mean 9.1) |
DA
Variable regimens |
2 years | Adverse drug reactions Mean DA dose Mean baseline Hb Transfusion rate |
Six events in four patients (1.3%) 1.4–2.0 µg/kg/month 11.1 g/dL 15% received one or more transfusions |
| Lestz et al. [18] | Retrospective cohort study | 829 | Multiple centres USA |
Age <18 years (mean 12.9) HD/PD |
rHuEPO/DA
variable regimens |
N/A | Adverse effects in relation to dose | Increased hazard of death in highest dose regimen versus reference [HR 3.37 (95% CI 1.37–8.26), P = .01] (EPO 100 to <200 units/kg/week, DA 0.49 to <1 µg/kg/week) |
| Borzych-Duzalka et al. [63] | Prospective cohort study | 1394 | Multiple centres Worldwide |
Age 1 month–20 years (median 10.2) PD |
rHuEPO/DA
variable regimens |
Up to 48 months Median 0.8 months |
Adverse effects in relation to dose | Increased HR per 1000 IU/m2 per week, 1.33; P = .01 |
| Can et al. [48] | Prospective case–control study | 34 | Multiple centres Turkey |
Age 4–18 years (mean 11.4) Any renal disease including HD and PD On rHuEPO or DA for >6 months |
Group A: rHuEPO
IV/sc 50–150 U/kg 2–3/week Group B: DA alpha IV/sc 0.5 µg/kg weekly Adjusted in 25% increments |
6 months | Mean ∆Hb (target 11–12 g/dL) Rate of change of Hb Injection site pain Adverse effects |
Group A: 9.56 → 10.67 g/dL (P = 0.01) Group B: 9.19 → 10.35 g/dL (P = .02) No difference between Group A and B No difference between Group A and B Systolic HTN in one DA patient No significant difference in BP between groups |
| Hattori et al. [89] | Prospective case series | 25 | Single centre Japan |
Age 1–18 years (mean 11.2) PD Stable on rHuEPO >8 weeks |
DA
iv 2–4 weekly 1 µg DA to 200 IU rHuEPO Adjustment increment not specified |
28 weeks | Mean ∆Hb (Target 11–13 g/dL) % achieving target Hb |
9.9 ± 1.0 → 11.1 ± 1.0 g/dL 88% (15 patients changed from 2 to 4 weekly dosing) |
| Jander et al. [59] | Cross-sectional study | 117 | Multiple centres Poland |
Age 8–16 years (mean 13.8) HD and PD |
MPG-epo beta (7%)
DA (19%) rHu EPO (74%) |
6 months | Mean EPO dose Mean Hb during observation period % with Hb >11 g/dL |
99 U/kg/week 10.91 ± 1.18 g/dL 48% |
| Wedekin et al. [49] | Prospective case–control | 12 | Single centre Germany |
Age 6–17 year (median 15.2) Post-renal transplant eGFR 17–73 mL/min/1.73 m2 7/12 ESA naïve: cases 5/12 on DA: controls |
MPG-epo beta
iv 4 weekly 2.3 µg/kg/dose Adjustment increment not specified |
6 months | Mean ∆Hb (target 11–12 g/dL) % achieving target Hb |
Cases: 9.9 → 11.2 g/dL (P = .004) Controls: 10.3 → 11.6 g/dL (P = .39) 9/12 (75%) |
| Cano et al. [61] | Prospective case series | 16 | Single centre Chile |
Age 2–14 (mean 9.7) Hb >10 g/dL for >4 weeks PD On rHuEPO |
MPG-EPO beta
(Mircera) sc 2 weekly 0.5 µg/kg/dose Adjusted in 25/50% increments |
6 months | Mean ∆Hb (target >11 g/dL) Dosing profile over time BP profile |
Hb 11.12 → 12.2 g/dL 5/16 Hb >13 g/dL at end 3/16 switched to HD 2/16 transplanted 2/16 switched to once a month dosing Mean 57th centile (unchanged) |
| Andre et al. [51] | Prospective case–control | 39 | 12 centres France |
Age 11–18 (mean 15.2) CKD CMT HD/PD Pre-transplant 10/39 ESA naïve (cases) 29/39 on rHuEPO (controls) |
DA
sc 1–2 weekly 0.45 µg/kg/week 1 µg DA to 200 IU r-HuEPO Adjusted in 7%–24% increments |
6 months | Mean ∆Hb (target 11–13 g/dL) % achieving target Hb Mean maintenance dose at end Adverse effects |
Cases: 9.5 →11.7 g/dL Controls: 11.1 → 11.5 g/dL 66.7% (26/39) Cases: 0.34 µg/kg/week Controls: 0.73 µg/kg/week One vascular access thrombosis One abdominal pain |
| Rijk et al. [54] | Retrospective case–control | 19 | Two centres Netherlands |
Age 0–17 years (mean 6.8) NIPD 11/19 ESA naïve (cases) 8/19 on ip rHuEPO (controls) % |
DA
ip 0.45 µg/kg/week 1 µg DA to 200 IU r-HuEPO Adjustment increment not specified |
31.5 months (median) |
Mean ∆Hb (target 10.9–12.8 g/dL) Median maintenance dose Peritonitis incidence Adverse effects |
10.9 → 11.4 g/dL (cases + controls) 0.79 µg/kg/week (cases + controls) One episode every 25.1 months Three worsening HTNs |
| Boehm et al. [46] | Retrospective cohort study | 47 | Single centre Austria |
Age 0.8–11.2 years (mean 6.0) | Not stated | 2.5 years (median) | Likelihood for catch up growth observed >6 months (Odds Ratio) after EPO commenced | 6.67 (P < .05) |
| Durkan et al. [55] | Retrospective case series | 6 | Single centre US |
Age <1 year Weight <8 kg CKD CMT ESA naïve |
DA
iv weekly 0.45 µg/kg/week Adjusted in 25% increments |
20 weeks | Mean ∆Hb (target 10–11 g/dL) % achieving target Hb Adverse effects |
9.0 → 11.0 g/dL 50% (3/6) One pain at injection site |
| Geary et al. [53] | Prospective/retrospective case series | 33 | Single centre Canada |
Age 1–18 years CKD CMT HD and PD ESA naïve/ on rHuEPO (not further specified) |
DA
sc weekly 0.45 µg/kg Adjusted in 30%–50% increments |
28 weeks | Mean ∆Hb (target > 10 g/dL) % achieving target Hb Adverse effects |
ESA naïve–9.0→ 11.6 g/dL Switched–10.5 →11.1 g/dL Combined: 10.2 →11.4 g/dL (P <.0001) 91% One new HTN DA more painful than rHuEPO in 57% |
| De Palo et al. [50] | Prospective case series | Seven | Single centre Italy |
Age 7–15 years (mean 11.5) HD On rHuEPO (EPO alpha) |
DA
IV weekly 1.59 ±1.19 µg/kg/week (dose based on rHuEPO dose) Adjustment increment not specified |
6 months | Mean ∆Hb (target 11–13 g/dL) Mean DA dose change over time to maintain target Hb Adverse effects |
11.04 ± 1.53 → 11.44 ±1.14 g/dL 1.59 (SD ±1.19) → 0.55 (SD ± 0.14) µg/kg/dose (baseline–6 months) (P < .05) Suggested long-term dose 0.25–0.75 µg/kg/dose Two severe new HTNs One persistent elevation in platelets |
| Rusthoven et al. [39] | Prospective case series | 20 | Single centre Netherlands |
Age 0.9–14 years (mean 3.8) CCPD ESA naïve |
rHuEPO
ip 3/week 200 units/kg/week 50 mL dialysis bag |
12 months | Median ∆Hb (target 10.4–11.2 g/dL) Median dose to maintain target Hb Incidence of peritonitis Adverse effects |
9.4 → 11.0 g/dL (range 8.96–13.1) 200 → 179 U/kg/week 1/11.2 person months None reported |
| Kausz et al. [38] | Prospective case series | 14 | Single centre US |
Age 0.9–18 years (mean 7.9) CCPD On sc rHuEPO |
rHuEPO
ip 3/week 300 U/kg/week 50 mL dialysis bag |
12 weeks | Mean ∆HCT Mean EPO dose sc versus ip Patient satisfaction Incidence of peritonitis Adverse effects |
0.34 → 0.33 L/L (P > .05) sc: 279 ±126 U/kg/week ip: 290 ± 194 U/kg/week All patients preferred ip administration 1/32.5 person months [RR versus centre rates: 3.1 (95% CI 0.92–6.3)] One HTN |
| Port et al. [90] | Prospective case series | 8 | Single centre | Age 7–15 6/8 PD 2/8 CKD CMT ESA naïve |
rHuEPO
sc 1/week 100–170 U/kg/week |
4–38 months | Increase in Hb (before treatment—end of monitoring) | 2.5 → 5.6 g/dL (median 3.7) |
| Sieniawska and Roszkowska-Blaim [91] | Prospective case series | 19 | Single centre Poland |
Age 4–17.5 years (mean 11.8) 11/19 on HD 8/19 on CAPD Off ESA for >8 weeks |
rHuEPO
sc weekly 50 U/kg/week For 12 weeks, then dose adjusted |
24 weeks | Mean ∆Hb (target > 10 g/dL) % reaching target Hb at 12 weeks |
CAPD: 7.7 ± 0.2 → 11.2 ± 0.6 g/dL (P < .001) HD: 7.7 ± 0.6 → 9.3 ± 0.8 g/dL (P < .001) CAPD: 100% HD: 64% |
| Reddingius et al. [42] | Prospective case series | 10 | Single centre Netherlands |
Age 4.1–15.2 years (median 7.8) 8/9 CAPD 1/9 NIPD Group A: ESA naïve, 4/10 Group B: ip EPO, in 250-mL dialysis bag |
rHuEPO
ip 3/week 50 mL bag Group A: 300 U/kg/week Group B: Previous dose |
6 months | Median ∆Hb (target 10.4–11.2 g/dL) Change in mean EPO dose to maintain Hb |
Group A: 8.5 → 10.6 g/dL Group B: 10.9 → 10.9 g/dL Group A: 262 → 194 U/kg/week Group B: 266 → 234 U/kg/week |
| Steele and Vigneux [40] | Prospective case series | 3 | Single centre Canada |
Age 11 months–11 years Two CCPD One CAPD On sc rHuEPO |
rHuEPO
ip 100–150 U/kg/week CCPD: 2/week direct injections with 20 mL dialysate CAPD: 2/week in 300-mL dialysate bag |
6 months | Mean ∆Hb (target unspecified) Adverse effects |
9.2–10.4 g/dL One incident of peritonitis |
| Burke [73] | Prospective case series | 22 | Multicentre Australia | Age 4 months–16 years (mean 9 years) 9/22 CKD CMT, 10/22 CAPD 1/22 CCPD 2/22 HD Hb <8 g/dL ESA Naïve |
rHuEPO alpha
sc 3/week Initial 100 U/kg/week, increased 50 U/kg/week each month if needed |
12 months | Mean ∆Hb (target 9–11 g/dL) % reaching target Hb at 16 weeks Dose range required to maintain Hb Mean change in IQ |
6.7 ± 0.7 → 9.6 ± 1.9 g/dL (P < .001) 90% 45–125 U/kg/week 92 ± 16.1 → 97.5 ± 17 (P = .007) |
| Van Damme-Lombaerts et al. [34] | Prospective case series | 115 | Multicentre France, Belgium, Switzerland |
Age 0.5–20 years (median 11.6) HD ESA naïve |
rHuEPO
iv 2–3/week Initial 75 U/kg/week, increased 75 U/kg/week each month if required |
12 months | Mean ∆Hb (target 9.6–11.2 g/dL) % reaching target Hb Median dose required to maintain Hb Quality of life Adverse effects |
6.7 → 9.7 g/dL 81% At target: 150 U/kg/week At 12 months: 200 U/kg/week Mean score reflecting questionnaire assessing sleep/rest, alertness, feeling and daily activities: 10.79 → 11.84 [+10% (P < .05)] 20 new or worsened hypertension 15 thrombotic events |
| Morris et al. [47] | Prospective case–control | 13 | Single centre UK |
Age 4.3–11 years 1/13 NIPD 1/13 HD 1/13 CKD CMT Group A: ESA naive Group B: stable Hb on RHuEPO |
rHuEPO
sc 3/week |
12 months | Echocardiography | Group A: reduction in mean indices of LVH [left ventricular mass index (P = .02) and cardiothoracic ratio (P = .005)] |
| Ongkingco et al. [33] | Prospective case series | 7 | Single centre USA |
Age 6.5–18.7 years (median 12.6) CCPD ESA naïve |
rHuEPO
sc 1–3/week Induction: 150 U/kg/week Maintenance: 8-week fixed dose 3/week 8-week adjusted dose 1/week |
24 weeks | Mean ∆HCT Mean rHuEPO dose |
0.20 → 0.32 (baseline → target achieved) 0.20 → 0.35 (baseline → end of 1/week maintenance period) (P = not significant) 3/week: 85.7 ± 40.4 U/kg/week 1/week: 87.0 ± 34.1 U/kg/week |
| Scharer et al. [65] | Prospective case series | 11 | Single centre Germany |
Age 0.6–17 years CKD CMT ESA naïve |
rHuEPO
sc 3/week Initial 150 U/kg/week |
13 months (mean) | Mean time to Hb target (11.5 g/dL) Mean EPO maintenance dose |
72 days (18–203) 135 U/kg/week |
| Aufricht et al. [92] | Prospective case series | 12 | Single centre Austria |
Age 0.8–12.5 years (mean 7.4) CAPD ESA naïve |
rHuEPO
sc 3/week Initial 100–120 U/kg/week |
40 weeks | Mean ∆HCT (target 0.35–0.40) % on single dose/week therapy |
0.24 (0.14–0.29)→0.40 (0.33–0.48) (P < .01) 80% |
| Montini et al. [93] | Prospective case series | 24 | Multiple centres Brazil, Italy |
Age 0.3–18 years (mean 10.3) PD ESA naïve |
rHuEPO
sc 3/week Initial 50 U/kg/week Up to 300 U/kg/week |
24 weeks | Mean ∆Hb Adverse effects |
6.5 ± 1.4 → 9.4 ± 1.7 g/dL One severe worsening HTN |
| Martin et al. [71] | Prospective case series | 18 | Single centre USA |
Age 3–20 years (mean 10.9) 15/18 CAPD 3/18 CKD CMT ESA naïve |
rHuEPO
iv/sc 150 U/kg/week then decreased to 75 U/kg/week once target HCT achieved |
6 months | Mean ∆HCT (target 0.33) Echocardiography Exercise capacity (modified Bruce) |
0.22 ± 0.03 → 0.33 ± 0.02 (P = .001) Unchanged during period, normal parameters See Table 4 |
| Suppiej et al. [75] | Prospective case series with case control | 14 | Single centre Italy |
Age 9–19 years (median 12.3) HD ESA naïve 10 healthy matched controls |
rHuEPO
iv 3/week |
13 weeks (mean) | Evoked potentials (BAEP and MN-SSEP) Mean ∆Hb |
Peripheral:
BAEP: Mean wave I latency reduced in ESKD versus controls (P < .01), unaffected by anaemia correction. MN-SSEP: mean PCV wrist → Erbs point, N9 and N20 amplitude reduced in ESKD versus controls (P < .05), unaffected by anaemia correction. Central: BAEP: no difference versus controls 6.6 ± 0.9 → 10.9 ± 1.2 g/dL (P < .0001) |
| Sallay et al. [94] | Prospective case series | 8 | Single centre Hungary |
Age 5.5–18 years (mean 12.2) 7/8 HD 1/8 CAPD ESA naïve |
rHuEPO
iv/sc Initial 160 U/kg/week up to maximum 400 U/kg/week |
28 weeks | Mean ∆HCT (target 0.33) Dose range required to maintain Hb |
0.18 → 0.33 250–300 U/kg/week |
| Hisano et al. [95] | Prospective multiple-dose case series |
12 | Single centre Japan |
Age 2–18 years CAPD ESA naïve |
rHuEPO
iv 1–3/week Group A (8/12): 89 U/kg/week Group B (4/12): 260 U/kg/week for 8 weeks then 88 U/kg/week |
24 weeks | Mean ∆HCT Adverse effects |
Group A: HCT 0.19 ± 0.02 → 0.29 ± 0.02 Group B: HCT 0.18 ± 0.03 → 0.30 ± 0.04 Two in Group B new HTNs |
| Goldraich and Goldraich [32] | Prospective case series | 6 | Single centre Brazil |
Age 0.5–15.8 years (mean 6) CAPD ESA naïve |
rHuEPO
sc 1/week 150 U/kg/week |
12 weeks | Mean ∆Hb Adverse effects |
6.6 ± 0.47 → 10.1 ± 1.2 g/dL 1 transient pain 1 pruritis |
| Campos and Garin [96] | Prospective case series | 11 | Single centre USA |
Age 0.5–20 years (median 14) HD ESA naïve |
rHuEPO
IV 3/week Initial 150 U/kg/week Adjusted based on HCT |
12 weeks | Mean ∆Hb Mean maintenance dose Adverse effects |
6.2 g/dL ± 0.4 → 10 g/dL ± 0.3 142.5 ± 13.5 U/kg/week Two worsening HTNs Two new HTNs One clotted graft |
| Stefanidis et al. [67] | Prospective case series | 10 | Single centre Greece |
Age 1.5–17 years (mean 9.1) CAPD ESA naïve |
rHuEPO
sc/iv 3/week 90–220 U/kg/week until target 9.5–10 g/dL achieved |
1 year | Mean ∆ growth velocity Mean ∆ anthropometric measures (weight, mid arm circumference, triceps skin fold thickness) |
No significant change after anaemia correction No significant change after anaemia correction |
| Reddingius et al. [41] | Prospective case series | 16 | Single centre Netherlands |
Age 0.8–16.5 years (median 4.1) PD ESA naïve |
rHuEPO
ip 3/week Initial 300 U/kg/week |
3–12 months | Mean ∆Hb (target 10.5–11.3 g/dL) Transfusion burden Mean final EPO dose |
7.9 → 10.8 g/dL 22 transfusions in 6 months prior to study → no further transfusions 279 U/kg/week |
| Warady et al. [70] | Prospective case series/case–control: exercise capacity |
9 | Single centre USA |
Age 7.8–17 years (mean 12.4) 8/9 APD 1/9 CAPD ESA naïve Five healthy age matched controls |
rHuEPO
sc 2/week Initial 100 U/kg/week Adjusted based on HCT |
16 weeks | Mean ∆HCT Transfusion burden Exercise capacity Adverse effects |
21.9 + 3.5% → 33.2 L/L + 3.1% 0.5 transfusions per patient-month → 0.05 transfusions per patient (P < .01). See Table 4 Six reports of pain at injection sites |
| Ongkingco et al. [97] | Prospective case series | 10 | Single centre USA |
Age 13 days–18.6 years (mean 10.5) CCPD ESA naïve |
rHuEPO
sc 3/week Initial 150 U/kg/week Adjusted based on HCT |
11 weeks | % responsiveness to initial dose regimen (HCT increase of 0.05/week) Adverse effects |
91% Two worsening HTNs |
| Hisano et al. [98] | Prospective case series | 10 | Single centre Japan |
Age 2–18 years (mean 11.6) CAPD ESA naïve |
rHuEPO
sc/iv weekly 60–150 U/kg/week (mean 93 U/kg/week) |
24 weeks | Mean ∆Hb | 6.9 ± 0.8 → 9.4 ± 1.5 g/dL |
| Navarro et al. [99] | Prospective case series | 23 | Single centre Spain |
Age 0.1–19 years 11/23 CKD CMT 7/23 CAPD 5/23 HD ESA naive |
rHuEPO
sc/iv Initial 50 U/kg/week |
4.3 months (mean) | Mean ∆Hb (target 10–12 g/dL) Mean EPO dose Adverse effects |
7.4 ± 1.3 → 10.7 ± 1.4 g/dL (P < .001) 289 ± 86 U/kg/week Four worsening HTNs |
| Scigalla et al. [35, 36] | Prospective case series | 120 | Multicentre Germany, France, Switzerland |
Age 2–21 years (mean 13) 108/120 HD 12/120 CAPD ESA naïve |
rHuEPO
sc/iv Initial 120–300 U/kg/week |
41 weeks (mean) |
Mean ∆HCT Transfusion burden Median rHuEPO dose at 12 months Mean ∆SD score for height |
0.19 → 0.30 L/L (start → last value) 103 transfusion dependent → 0 transfusion dependent 138 U/kg/week No change (start → last value) |
| Bianchetti et al. [100] | Prospective case series | 18 | Single centre Switzerland |
Age 5–18 years (mean 12) HD ESA naïve |
rHuEPO
Epoetin alpha iv 2–3/week 75–300 U/kg/week (median 150 U/kg/week) Adjusted based on HCT |
13–78 weeks | Median ∆HCT Adverse effects |
0.17 ± 0.05 → 0.27 ± 0.02 Five worsening HTNs Three new HTNs One venous thrombosis |
| Rigden et al. [64, 66] | Prospective case series | 6 | Single centre UK |
Age 3.9–15.8 years HD ESA naïve |
rHuEPO
iv 3/week Initial 30 U/kg/week Increased 2 weekly 75, 150, 300, 450 U/kg/week |
24 weeks | Mean ∆Hb Mean time to target Hb (10–13 g/dL) % Responsiveness (increase in Hb NOS) Exercise tolerance Mean ∆ growth velocity Adverse effects |
7.1 → 10.5 g/dL 11 weeks 100% See Table 4 Small improvement in pre-pubertal children (unquantified) One vascular thrombosis |
| Offner et al. [37] | Prospective case series | 14 | Single centre Germany |
Age 5.9–22.1 years 4/14 CAPD 10/14 CCPD ESA naïve |
rHuEPO
intraperitoneal iv weekly 300 U/kg/week until HCT 0.3 then 100 U/kg/week |
7.8 months (mean) |
Mean ∆HCT Mean time to target HCT 0.3 Adverse effects |
0.19 → 0.28 3.1 ± 1.7 months One worsening HTN Intraperitoneal administration stopped due to three incidents of peritonitis |
| Montini et al. [69, 72, 75] | Prospective case series with case–control: exercise capacity NS function | 10 | Single centre Italy |
Age 2.5–18.75 years (median 11.8) HD ESA naïve |
rHuEPO
iv 3 weekly 75–150 U/kg/week |
18 weeks | Mean ∆Hb Exercise tolerance Evoked potentials (BAEP and MN-SSEP) Mean platelet count Mean bleeding time |
6.4 ± 0.9 → 11.5 ±1 g/dL See Table 4 Peripheral: significantly longer in patients versus controls (P < .0001). Anaemia correction produced no modification. Central: prolonged interpeak latency 1/10–returned to normal with correction of anaemia. 236 ± 84 → 391 ± 157 × 109/L (P < .05) |
| Sinai-Trieman et al. [31] | Prospective case series | 5 | Single centre USA |
Age 12–18 years (mean 16.2) CCPD ESA naïve |
rHuEPO
sc 3 weekly 450 U/kg/week |
5–8 months | Mean ∆HCT % Responsiveness (increase in HCT NOS) Transfusion burden |
12.8 ± 3.1 → 8.2 ±3.2 s (P < .01) 0.22 ± 3 → 0.33 ± 1.9% (P < .001) 100% 5–18 transfusions → 0 transfusions |
APD: automated peritoneal dialysis; CAPD: continuous ambulatory peritoneal dialysis; CCPD: continuous cycling peritoneal dialysis; CERA: continuous erythropoietin receptor activator; CMT: conservative management; DA: darbepoetin; EPO: erythropoietin; ESA: erythropoietin-stimulating agent; HCT: haematocrit; HD: haemodialysis; HTN: hypertension; iv: intravenous; NIPD: nightly intermittent peritoneal dialysis; NOS: not otherwise specified; PD: peritoneal dialysis; PCV: peripheral conduction velocity; rHuEPO: recombinant human erythropoietin; sc: subcutaneous; RR: respiratory rate; BAEP: brainstem auditory evoked potential; MNSEP: median nerve somatosensory evoked potentials.
Primary outcome measure—efficacy
rHuEPO
A total of 34 studies evaluated rHuEPO efficacy in 673 children. Three were randomized trials, with the majority (n = 31) being prospective observational case series. A total of 16 studies included children on peritoneal dialysis (PD), 6 included children on haemodialysis (HD), 1 investigated conservatively managed CKD and 8 were mixed. A total of 28 observational studies evaluated efficacy, of which 22 evaluated subcutaneous or intravenous administration. All of these confirmed improvements in indices of anaemia with rHuEPO administration.
The first paediatric observational study in 1989 highlighted that Hb could be successfully maintained in five paediatric dialysis patients on subcutaneous treatment, reducing the requirement for transfusion and subsequent development of anti-human leucocyte antigen (HLA) antibodies [31]. At 450 U/kg/week, the dose used was three times the upper limit of current KDIGO recommendations, and three of the five patients developed worsening hypertension.
Two studies used fixed dose regimens, with the remainder titrating dosing [31, 32]. Initial doses ranged from 30 to 450 U/kg/week, with target haematocrit (HCT) ranging from 0.33 to 0.40 L/L and Hb from 9 to 13 g/dL. Dose frequency was usually three per week, although two studies explored weekly dosing. Goldraich and Goldraich [32] demonstrated the efficacy of once weekly 150 U/kg dosing in six children on continuous ambulatory PD (CAPD). Ongkingco et al. [33] found no significant decrease in HCT after 8 weeks, decreasing from thrice to once weekly maintenance dosing (with associated cost-benefit), although the study suffered from significant dropouts resulting in only seven recruits.
The majority of observational studies investigated small cohorts of between 5 and 24 children (mean 15), although two larger multicentre studies were conducted in 1991 and 1994 [34–36]. The earlier of these included 120 children across multiple European centres [35, 36], reporting a mean final dose requirement of 138 U/kg/week. The second recruited 115 ESA-naïve children treated with rHuEPO for up to 1 year [34]. A total of 81% achieved a target Hb of 9.6–11.2 g/dL, although 68% of ‘non-responders’ were transplanted earlier. The median maintenance dose for children <30 kg was 225 U/kg/week and 107 U/kg/week for children >30 kg.
Six observational studies investigated intraperitoneal administration [37–42]. The first trial by Offner et al. [37] was halted early due to a high rate of peritonitis. Subsequently, Reddingius et al. [41] trained parents to inject rHuEPO into overnight 20 mL/kg bags, demonstrating a reduced requirement for transfusion without an increased peritonitis incidence. Reddingius et al. [42] and Kausz et al. [38] demonstrated in small cohorts of 10 and 14 patients, respectively, that intraperitoneal administration could maintain Hb when switched from subcutaneous rHuEPO without a significant dose increase. Administration was via a 50-mL intraperitoneal daytime dwell and Reddingius et al. [42] also demonstrated a mean dose reduction with this method against a 250-mL prolonged dwell (266 → 234 U/kg/week). Kausz et al. [38] demonstrated a possible increased risk of peritonitis versus historical controls {respiratory rate versus centre rate: 3.1 [95% confidence interval (CI) 0.92–6.3]}.
The largest study was conducted by Rusthoven et al. [39], who followed 20 ESA-naïve children for up to 1 year after starting rHuEPO in three divided doses delivered in 50-mL bags. They were able to maintain target Hb levels with a modest dose of 179 U/kg/week and with a low peritonitis incidence of 1 per 11.2 patient-months.
Three studies were randomized trials [30, 43–45]. Morris et al. [44] undertook a single-blinded placebo-controlled randomized crossover trial in 11 ESA-naïve children, demonstrating a significant increase in the median Hb from 7.3 to 11.2 g/dL (P < .001). Yalçınkaya et al. [43] randomized 20 ESA-naïve children on CAPD to receive low- (50 U/kg/week) or high-dose (150 U/kg/week) rHuEPO for 6 months and found that while both doses were efficacious, the higher dose led to a statistically significant increase in the mean arterial BP from 85 to 101 mmHg. Four participants in the high-dose arm had to temporarily discontinue therapy due to uncontrolled hypertension, with two instances of hypertensive encephalopathy. Brandt et al. [30] randomized 44 children to low (150 U/kg/week) and high (450 U/kg/week) dosing for 12 weeks or until a 10 g/dL target Hb was reached. Attainment of the Hb target in the higher dose cohort was more rapid, though with a non-significant higher incidence of hypertension [high dose 38%, low dose 21% (P = .17)].
A further three studies examined secondary outcomes only and are outlined below [18, 46, 47].
DA
A total of 11 studies investigated DA efficacy in 411 children. There were two randomized trials and nine observational studies (five prospective case series, one retrospective case series, one pro- and retrospective case series, one prospective case–control, one retrospective case–control). Two included children on PD, one included children on HD, three included conservatively managed CKD and three were mixed. Two analyzed DA in ESA-naïve children, three included children established on an ESA and the remaining four included a mixture of naïve and ESA-treated children. All demonstrated that DA was efficacious in reaching a specified Hb target. Targets were varied and generally aimed for 11–13 g/dL, although only two studies matched their target to the KDIGO recommendation of 11–12 g/dL [48, 49]. Cohorts within the observational studies varied between 3 and 39 (mean 19) participants.
Dosing regimens and adjustment strategies varied in the observational studies. Initial dosing was reported between 0.27 and 1.59 µg/kg/week, with both weekly and fortnightly dosing trialled, although most starting doses were close to the KDIGO recommendation of 0.45 µg/kg/week. All studies titrated dosing.
The first observational study, conducted by De Palo et al. [50], recruited seven children titrated to intravenous DA from rHuEPO using a conversion factor (weekly epoetin alfa dose/200 = weekly DA dose). An initial mean dose of 1.59 ± 1.19 µg/kg coincided with two cases of hypertension with a rapid increase in Hb to >13 g/dL, necessitating intermittent discontinuation of treatment. The mean dosage at 3 months was 0.51 ± 0.18 µg/kg/week and the authors subsequently recommended a long-term dose of 0.25–0.75 µg/kg/week.
In a French multicentre study of 39 children, Andre et al. [51] reported an almost 2-fold higher mean dose requirement in patients switched to DA from rHuEPO as compared with ESA-naïve children [0.73 versus 0.34 µg/kg/week (P = .015)]. This was not replicated in other studies involving both ESA-naïve children and children on rHuEPO [52–54].
A prospective case–control study compared the efficacy of rHuEPO to DA [48]. Can et al. [48] split 34 children equally to receive rHuEPO 2–3/week or DA weekly and found no differences in the efficacy or adverse effects profile between either group.
Durkan et al. [55] and Libudzic-Nowak et al. [56] specifically investigated infants <1 year of age. Durkan et al. [55] found that only 50% of the six patients recruited reached target Hb levels of 10–11 g/dL despite a high mean administered dose of 1.2 µg/kg/week and normal iron studies. Libudzic-Nowak et al. [56] achieved target Hb concentrations of 10.7–12 g/dL in three infants ages 1, 4 and 7 months, but requiring doses of 0.3–0.7 µg/kg/week, generally higher than in older children.
One retrospective case–control study appraised intraperitoneal administration. Rijk et al. [54] evaluated 19 children, 8 of whom were previously on intraperitoneal rHuEPO. A high median dose of 0.79 µg/kg/week was required to sustain Hb levels at a mean of 11.5 ± 1.2 g/dL. Six cases dropped out due to transplantation, with a relatively low peritonitis incidence of one episode every 25.1 months.
Two randomized trials investigated DA efficacy [28, 29]. Warady et al. [28] conducted an open-label non-inferiority trial in 124 children randomized (1:2) to ongoing rHuEPO therapy or DA, with results demonstrating an equivalent mean change in Hb over 28 weeks. The same team performed a prospective, multicentre double-blind randomized controlled trial of 114 ESA-naïve children comparing weekly versus fortnightly titrated dosing [29]. This showed that the mean time to target Hb of 10–12 g/dL was equivalent (22 days and 24 days, respectively), although a greater proportion of patients on weekly dosing reached the target Hb at 24 weeks (98% versus 84%).
A further three studies evaluated secondary outcomes only and are discussed below [57–59].
CERA
No randomized trials were identified regarding CERA use in children. Three observational studies evaluated CERA in 92 children [49, 60, 61]. Cano et al. [61] studied 16 children over 6 months converted from rHuEPO to fortnightly subcutaneous CERA. They found Hb was maintained, although dosing varied significantly (0.5–2.9 µg/kg/dose). Wedekin et al. [49] conducted a prospective case series on 12 children after renal transplant using a monthly intravenous dosing regimen. After 6 months of follow-up, they demonstrated an increase in mean Hb in ESA-naïve patients and maintained Hb levels in patients switched from DA (although only 75% achieved a target of 11–12 g/dL). Fischbach et al. [60] conducted an open-label multicentre study on 64 children on stable ESA regimens. An intermediate conversion factor (4 mg every 4 weeks for each weekly dose of 250 IU epoetin alfa/beta or 1.1 mg DA) derived from adult studies was tested against a twice higher conversion factor over 40 weeks. The intermediate factor proved less adequate at maintaining stable Hb, with mean Hb dropping below the lower target threshold of 10 g/dL on several occasions, whereas the higher factor was associated with more stable target Hb levels.
Secondary outcome measures
Safety
Most observational studies included a discussion of adverse effects, the most common being hypertension. Three studies specifically focussed on safety in large cohorts [18, 62, 63].
Borzych-Duzalka et al. [63] prospectively appraised the anaemia management of 1394 children on PD across 30 countries between 2007 and 2011 for up to 48 months. Of 1147 patients where the ESA dose was available, 2.1% with lower dose regimens (<6000 IU/m2/week) versus 5.3% with higher dose regimens (not specified) died (P = .02). Regression analysis demonstrated an independent increased risk of death on PD with higher ESA doses [hazard ratio (HR) per 1000 IU/m2/week 1.33; P < .01]. Children were more likely to be ESA sensitive with higher albumin levels, low serum parathyroid hormone and persisting diuresis.
Lestz et al. [18] conducted a retrospective cohort study using 12- to 18-month follow-up of mortality records linked to a US 2005 ESKD registry in 820 children on dialysis and ESA therapy who had not undergone transplantation during 12–18 months of follow-up. Over the observation period, 60 children (7%) died, primarily attributed to cardiovascular causes. ESAs were prescribed to 95% of survivors and 93% of those who died. Average ESA doses were significantly higher in those who died versus survivors [rHuEPO 502 versus 290 units/kg/week (P < .001), DA 0.59 versus 2.6 µg/kg/week (P < .001)] and multivariate analysis demonstrated an HR of death of 3.37 in a high-dose group (EPO ≥350 units/kg/week or DA ≥1.5 µg/kg/week) when compared with a lower reference range (EPO 100–<200 units/kg/week or DA 0.49–1.0 µg/kg/week). This finding was independent of a wide range of factors, including cause of ESKD, dialysis modality, access and achievement of a minimum target Hb level of 11 g/dL.
Schaefer et al. [62] conducted an observational registry study of 319 children across 37 centres, the most comprehensive study of the safety of DA in children. Children were followed for up to 2 years, although 176 children withdrew earlier. A total of 162 patients, 50.8% of the cohort, reported a total of 434 serious adverse events (SAEs), the most common of which were peritonitis (n = 32), gastroenteritis (n = 19) and hypertension (n = 13). The authors state that this is comparable with a general cohort of children with CKD.
Four patients (1.3%) suffered six documented serious adverse drug reactions (SADRs): arteriovenous fistula thrombosis, priapism, thrombocytopenia, haemolysis, haemolytic anaemia and partial blindness. The authors suggest the latter four SADRs had more plausible explanations than related to ESA administration. Six fatal adverse events occurred, but none were considered to be related to ESA administration. No new safety issues were identified.
Two studies primarily focussed on efficacy also included safety extensions to their trials. Warady et al.'s [28] open-label non-inferiority study of DA versus rHuEPO included documentation of adverse events deemed by the investigator to be treatment related, affecting 14% (n = 6) of the rHuEPO cohort and 20% (n = 16) of the DA cohort. Injection site pain was the most common adverse event [12% (n = 5) rHuEPO, 11% (n = 9) DA], with hypertension in three of the DA cohort, one instance of vascular access thrombosis in both cohorts and access stenosis in one in the DA cohort.
Fischbach et al. [57] included a 1-year safety extension to their trial of CERA, including 37 children. It found no additional safety signals, with two SAEs, both vascular access thromboses. Hypertension was reported as an adverse event in 13% [60].
Quality of life (QoL)
Three studies assessed QoL [29, 34, 45]. Two studies used a non-validated self-designed questionnaire in children on rHuEPO. Small patient numbers for analysis in the first study (n = 7) prevented meaningful conclusions, while the second lacked any control arm but did demonstrate an improved QoL from baseline [34, 45]. Warady et al. [29] used the Pediatric Quality of Life Inventory (PedsQL) score to assess changes in QoL in their RCT cohort of 114 children starting DA. The authors noted a statistically significant increase in the PedsQL score from baseline to 6 months (QW: 61.1 → 68.1, Q2W: 62.6 → 67.2).
Growth and nutrition
Five papers studied aspects of growth. Scigalla et al. [35] employed a height score (see Figure 3), finding no significant changes. Two studies assessed small cohorts of six participants [64–66]. Rees et al. [66], analysing Rigden et al.'s [64] 1990 cohort of six children on HD, described small improvements in growth velocity in the three youngest children over 1 year, with no appreciable effect in older participants. Scharer et al. [65] noted improvements in height standard deviation scores in the two youngest children in their cohort over ∼1 year of rHuEPO therapy [−1.8 → −1.0 and −3.7 → −2.5 standard deviation score], with minimal changes in four older children.
Figure 3.

Height score (Scigalla 1991 [35]).
Stefanidis et al. [67] found no significant change in growth in 10 children 1 year after anaemia correction. These papers were summarized as the subject of a 1996 review [68]. Subsequently, Boehm et al. [46] conducted a retrospective cohort study in 47 children followed from initial referral to pre-dialysis care and after the initiation of dialysis. They reported that rHuEPO therapy initiation at referral was the only modifiable factor independently associated with a catch-up growth velocity once dialysis was initiated {odds ratio (OR) 6.67 [95% confidence interval (CI) 1.00–44.10], P < .05}.
Exercise capacity
Five studies investigated exercise capacity using treadmill tests (see Table 4). Baraldi et al. [69] demonstrated improvements in several domains using an unspecified treadmill protocol 2–4 weeks following anaemia correction in seven children. Rigden et al. [64] demonstrated an improved treadmill time using the modified Bruce protocol in four children on HD. Warady et al. [70] assessed nine children undergoing PD, demonstrating improvements in all parameters using the Balke protocol 1 month following achievement of the target HCT of 30%. It was the only study that used controls, comparing results with five age-matched children without renal disease and confirming a significant improvement in children with renal disease. Martin et al. [71] found mild sustained improvements in treadmill time in 12 children. Morris et al. [45] included exercise testing, although the results were unpublished.
Table 4.
Assessments of exercise capacity
| Martin et al. [71] | Warady et al. [70] | Baraldi et al. [69] | Rigden et al. [64] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Modified Bruce | CMH Max/Balke | Not specified | Modified Bruce | ||||||||
| Protocol | A | B | A | Controls A | B | Controls B | A | B | Controls | A | B |
| VO2 (mL/kg/min) | 26.4 ± 4.1 | 25.1 ± 5.4 | 17.8 ± 5.2 | 40.8 ± 12.3 | 24.0 ± 7.6* | 42.0 ± 12.4 | 24.1 ± 7.1 | 32.6 ±12.7** | 44.7 ± 7.1 | ||
| VO2AT (mL/kg/min) | 13.1 ± 3.9 | 29.4 ± 6.3 | 17.1 ± 3.5* | 28.2 ± 8.4 | 17.6 ± 6.3 | 25.9 ± 8.1** | 31.4 ± 3.1 | ||||
| Treadmill time (min) | 10.3 | 12.1**** | 5.5 ± 1.3 | 8.7 ± 2.8 | 7.9 ± 1.5* | 9.4 ± 3.0 | 13.4 | 16.8*** | |||
A: initial evaluation; B: second evaluation; C: VO2AT, oxygen consumption at anaerobic threshold; VO2: peak oxygen consumption. *P < .05% patient versus control; **P < .05 B versus A; ***P < .02 B versus A; ****P = 0.001 B versus A.
Injection site pain
Schmitt et al. [58] conducted a double-blinded RCT with 13 children assigned to receive DA injections followed by rHuEPO or vice versa, demonstrating a statistically significant increase in subjective pain with DA.
Cardiovascular function
Four studies assessed cardiovascular function. Montini et al. [72] and Martin et al. [71] assessed echocardiographic changes after anaemia correction, finding no significant changes. Morris et al. [44] demonstrated a reduction in cardiac index with rHuEPO versus placebo. Morris et al. [47] also compared seven ESA-naïve children to those established on ESAs, demonstrating improvements in cardiothoracic ratio and left ventricular mass.
Other secondary outcome measures
Infrequently considered outcome measures included intelligence quotient (n = 1) [73], platelet function (n = 2) [71, 73] and evoked potentials (n = 1) [71, 74].
DISCUSSION
ESAs have transformed the management of renal anaemia, reducing transfusion burden and HLA sensitization. They are widely used in the USA and European Union, where up to 94% of children on HD are prescribed a regular ESA [76, 77]. Yet challenges remain—the European Dialysis Transplant Association registry reported in 2012 that 33.4% of children on dialysis <2 years of age and 31.2% >2 years had Hb levels below target [77].
This systematic review identifies a highly heterogeneous collection of studies assessing the use of ESAs in children. The challenges of recruiting within a paediatric cohort were apparent, with larger datasets requiring the involvement of multiple centres across countries.
Early studies of rHuEPO were characterized by small prospective observational cohorts demonstrating efficacy whether given subcutaneously or intravenously, while identifying that higher doses were associated with adverse events such as hypertension and vascular thrombosis. ESAs were shown to be less effective in the presence of iron deficiency and most subsequent studies ensured adequate iron stores.
A number of other secondary measures were explored using rHuEPO, varying from patient-relevant measures such as exercise tolerance and quality of life to physiological parameters, including cardiac function, evoked potentials, growth and nutrition, and platelet function. These were conducted on small cohorts.
The randomized placebo–controlled crossover trial and case–control studies conducted by Morris et al. [44, 47] suggest improvements in cardiac function following anaemia correction. Transplant recipients established on ESAs demonstrate comparably more minor cardiovascular improvements following transplantation when compared with CKD patients. This suggests that anaemia rather than uraemia correction plays a greater role in improving cardiac health or that other factors may be more important post-transplantation [47].
Studies on DA generally featured larger cohorts demonstrating non-inferiority against rHuEPO and established a similar safety profile [28, 62]. Weekly and fortnightly dosing both appear feasible treatment options [29]. QoL was explored in one study [29]. A modest increase in the PedsQL score was noted after 6 months of treatment, a finding supported by larger cross-sectional studies that demonstrate improved QoL in children with CKD without anaemia compared with those with persistent anaemia [78]. Further interrogation of outcomes relevant to patients has not been forthcoming. This review concurs with a Cochrane review of 2014 that noted that ‘formulations based on patient centred outcomes … are sparse and poorly reported’ [22]. More studies incorporating patient-centred outcomes are required to strengthen the rationale for intervention and choice of agent.
Early studies on CERA demonstrated efficacy in small paediatric cohorts and that Hb could be maintained when switching from other ESA preparations [60]. A higher conversion factor than that used in adults when changing from other ESA preparations may be required, and the safety profile appears similar to other ESAs [18]. Randomized trials comparing dosing regimens, comparing CERA with other ESAs and comparing patient preferences are lacking. A further dosing study is currently under way [79].
Intraperitoneal administration was predominantly evaluated using rHuEPO. It appears feasible and safe and is supported by pharmacokinetic studies demonstrating comparable bioavailability to other routes [80–82]. Nevertheless, it remains an uncommon route of administration. Intraperitoneal DA appears non-inferior to intraperitoneal rHuEPO, although only one study was identified.
Small studies on infants demonstrate that particularly high ESA doses may be required [55, 56]. Larger observational studies have also demonstrated higher ESA dose requirements in younger cohorts that appear consistent across ESA types [16, 34, 63]. One suggested reason for this apparent ESA resistance is a greater prevalence of iron deficiency: a study of anaemia in 2899 children on dialysis enrolled in the United States Renal Data System between 1996 and 2000 found that children ages 0–4 years were least likely to achieve target Hb, correlating with the lowest use of intravenous iron (33.9% versus 71%, ages 15–19) [83]. In contrast to this, Borzych-Duzalka et al.’s [63] study of 1394 children enrolled in the International Paediatric Peritoneal Dialysis Network registry between 2007 and 2011 found no relationship between Hb levels and iron supplementation, with an inverse association between Hb and ferritin levels (although transferrin saturation data were not available).
This suggests other mechanisms may contribute to an apparent ESA resistance in younger children. Speculated causes include higher numbers of EPO receptors that do not contribute to erythropoiesis, potentially ‘mopping up’ ESAs and reducing their haematopoietic potential [84]. Borzych-Duzalka et al. [63] also found reduced dose discrepancies in younger children when weight was substituted for body surface area (BSA) as a metric, suggesting requirements may be more proportional to metabolic rate than weight-based data suggest. Further studies that compared body weight with BSA dosing may help confirm this finding. Other studies have identified markers of dialysis adequacy, indices of nutritional intake, inflammatory status and hyperparathyroidism as primary factors in determining ESA resistance rather than iron deficiency [85, 86].
Nevertheless, the consistent finding of an independent relationship between higher ESA doses and mortality is of concern [18, 63]. High doses of ESAs can directly cause endothelial damage, vasoconstriction and platelet activation, all of which could plausibly increase the risk of cardiovascular mortality in children [87, 88]. Although the observational nature of the studies in question prevents the establishment of a definitive causal link, caution should clearly be applied when titrating ESAs in clinical practice, with careful consideration of all available interventions to maximize haemoglobin.
The most common reported adverse effect was hypertension. While some individual cases were clearly attributable to very high doses of ESAs [50, 74], in general the rate of hypertension in observational studies was noted to be comparable with other CKD cohorts.
Overall, there is no evidence to recommend one ESA as more efficacious or safe than any other. Factors influencing the decision of which ESA to choose will depend on considering the most convenient means of administration, taking into account age, mode of renal replacement therapy (if any) and patient preference. The morbidity and mortality risks associated with greater dosages of ESAs mandate thorough assessment of children with apparent ESA insensitivity.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the University of Glasgow Library for their help in formulating a search strategy and support in navigating the relevant databases.
Contributor Information
Gordon Bruce, Royal Hospital for Children Glasgow, Paediatric Nephrology, Glasgow, UK.
Peter Schulga, Royal Hospital for Children Glasgow, Paediatric Nephrology, Glasgow, UK.
Ben C Reynolds, Royal Hospital for Children Glasgow, Paediatric Nephrology, Glasgow, UK.
FUNDING
No funding was received for this article.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or in part.
REFERENCES
- 1. Chesnaye N, Bonthuis M, Schaefer Fet al. Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatr Nephrol 2014; 29: 2403–2410 [DOI] [PubMed] [Google Scholar]
- 2. Atkinson MA, Martz K, Warady BAet al. Risk for anemia in pediatric chronic kidney disease patients: a report of NAPRTCS. Pediatr Nephrol 2010; 25: 1699–1706 [DOI] [PubMed] [Google Scholar]
- 3. Wong H, Mylrea K, Feber Jet al. Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 2006; 70: 585–590 [DOI] [PubMed] [Google Scholar]
- 4. Koshy SM, Geary DF. Anemia in children with chronic kidney disease. Pediatr Nephrol 2008; 23: 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jelkmann W. Physiology and pharmacology of erythropoietin. Transfus Med Hemother 2013; 40: 302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med 2003; 228: 1–14 [DOI] [PubMed] [Google Scholar]
- 7. Shih H-M, Wu C-J, Lin S-L. Physiology and pathophysiology of renal erythropoietin-producing cells. J Formos Med Assoc 2018; 117: 955–963 [DOI] [PubMed] [Google Scholar]
- 8. Lin FK, Suggs S, Lin CHet al. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA 1985; 82: 7580–7584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eschbach JW, Egrie JC, Downing MRet al. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. N Engl J Med 1987; 316: 73–78 [DOI] [PubMed] [Google Scholar]
- 10. Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer 2001; 84: 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elliott S, Lorenzini T, Asher Set al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol 2003; 21: 414–421 [DOI] [PubMed] [Google Scholar]
- 12. Curran MP, McCormack PL. Methoxy polyethylene glycol-epoetin beta. Drugs 2008; 68: 1139–1156 [DOI] [PubMed] [Google Scholar]
- 13. Palmer SC, Saglimbene V, Mavridis Det al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev 2014; 12: CD010590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phrommintikul A, Haas SJ, Elsik Met al. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet 2007; 369: 381–388 [DOI] [PubMed] [Google Scholar]
- 15. Rheault MN, Molony JT, Nevins Tet al. Hemoglobin of 12 g/dL and above is not associated with increased cardiovascular morbidity in children on hemodialysis. Kidney Int 2017; 91: 177–182 [DOI] [PubMed] [Google Scholar]
- 16. Kidney Disease: Improving Global Outcomes Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 17. Koulouridis I, Alfayez M, Trikalinos TAet al. Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: a metaregression analysis. Am J Kidney Dis 2013; 61: 44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lestz RM, Fivush BA, Atkinson MA. Association of higher erythropoiesis stimulating agent dose and mortality in children on dialysis. Pediatr Nephrol 2014; 29: 2021–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drüeke TB, Locatelli F, Clyne Net al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 20. Singh AK, Szczech L, Tang KLet al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 21. Pfeffer MA, Burdmann EA, Chen C-Yet al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 22. Palmer SC, Saglimbene V, Craig JCet al. Darbepoetin for the anaemia of chronic kidney disease. Cochrane Database Syst Rev 2014; 3: CD009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saglimbene VM, Palmer SC, Ruospo Met al. Continuous erythropoiesis receptor activator (CERA) for the anaemia of chronic kidney disease. Cochrane Database Syst Rev 2017; 8: CD009904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer SC, Navaneethan SD, Craig JCet al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med 2010; 153: 23–33 [DOI] [PubMed] [Google Scholar]
- 25. Jabs K, Harmon WE. Recombinant human erythropoietin therapy in children on dialysis. Adv Ren Replace Ther 1996; 3: 24–36 [DOI] [PubMed] [Google Scholar]
- 26. Warady BA, Silverstein DM. Management of anemia with erythropoietic-stimulating agents in children with chronic kidney disease. Pediatr Nephrol. 2014; 29: 1493–1505 [DOI] [PubMed] [Google Scholar]
- 27. Müller-Wiefel DE, Amon O. Use of recombinant human erythropoietin in children undergoing dialysis. Semin Dial 1994; 7: 413–420 [Google Scholar]
- 28. Warady BA, Arar MY, Lerner Get al. Darbepoetin alfa for the treatment of anemia in pediatric patients with chronic kidney disease. Pediatr Nephrol 2006; 21: 1144–1152 [DOI] [PubMed] [Google Scholar]
- 29. Warady BA, Barcia J, Benador Net al. De novo weekly and biweekly darbepoetin alfa dosing in pediatric patients with chronic kidney disease. Pediatr Nephrol 2018; 33: 125–137 [DOI] [PubMed] [Google Scholar]
- 30. Brandt JR, Avner ED, Hickman ROet al. Safety and efficacy of erythropoietin in children with chronic renal failure. Pediatr Nephrol 1999; 13: 143–147 [DOI] [PubMed] [Google Scholar]
- 31. Sinai-Trieman L, Salusky IB, Fine RN. Use of subcutaneous recombinant human erythropoietin in children undergoing continuous cycling peritoneal dialysis. J Pediatr 1989; 114: 550–554 [DOI] [PubMed] [Google Scholar]
- 32. Goldraich I, Goldraich N. Once weekly subcutaneous administration of recombinant erythropoietin in children treated with CAPD. Adv Perit Dial 1992; 8: 440–443 [PubMed] [Google Scholar]
- 33. Ongkingco JR, Ruley EJ, Turner MEet al. Efficacy of once- versus thrice-weekly subcutaneous recombinant human erythropoietin in children receiving continuous cycling peritoneal dialysis. Am J Nephrol 1994; 14: 14–18 [DOI] [PubMed] [Google Scholar]
- 34. van Damme-Lombaerts R, Broyer M, Businger Jet al. A study of recombinant human erythropoietin in the treatment of anaemia of chronic renal failure in children on haemodialysis. Pediatr Nephrol 1994; 8: 338–342 [DOI] [PubMed] [Google Scholar]
- 35. Scigalla P. Effect of recombinant human erythropoietin treatment on renal anemia and body growth of children with end-stage renal disease. The European Multicenter Study Group. Contrib Nephrol 1991; 88: 201–204 [DOI] [PubMed] [Google Scholar]
- 36. Scigalla P, Bonzel KE, Bulla Met al. Therapy of renal anemia with recombinant human erythropoietin in children with end-stage renal disease. Contrib Nephrol 1989; 76: 221–227 [DOI] [PubMed] [Google Scholar]
- 37. Offner G, Hoyer PF, Latta Ket al. One year's experience with recombinant erythropoietin in children undergoing continuous ambulatory or cycling peritoneal dialysis. Pediatr Nephrol 1990; 4: 498–500 [DOI] [PubMed] [Google Scholar]
- 38. Kausz AT, Watkins SL, Hansen Cet al. Intraperitoneal erythropoietin in children on peritoneal dialysis: a study of pharmacokinetics and efficacy. Am J Kidney Dis 1999; 34: 651–656 [DOI] [PubMed] [Google Scholar]
- 39. Rusthoven E, van de Kar NC, Monnens LAet al. Long-term effectiveness of intraperitoneal erythropoietin in children on NIPD by administration in small bags. Perit Dial Int 2001; 21: 196–197 [PubMed] [Google Scholar]
- 40. Steele BT, Vigneux A.. Intraperitoneal erythropoietin treatment of children with chronic renal failure. Pediatr Nephrol 1995; 9: 359–360 [DOI] [PubMed] [Google Scholar]
- 41. Reddingius RE, Schroder CH, Monnens LA. Intraperitoneal administration of recombinant human erythropoietin in children on continuous ambulatory peritoneal dialysis. Eur J Pediatr 1992; 151: 540–542 [DOI] [PubMed] [Google Scholar]
- 42. Reddingius RE, de Boer AW, Schroder CHet al. Increase of the bioavailability of intraperitoneal erythropoietin in children on peritoneal dialysis by administration in small dialysis bags. Perit Dial Int 1997; 17: 467–470 [PubMed] [Google Scholar]
- 43. Yalçınkaya F, Tümer N, Çakar Net al. Low-dose erythropoietin is effective and safe in children on continuous ambulatory peritoneal dialysis. Pediatr Nephrol 1997; 11: 350–352 [DOI] [PubMed] [Google Scholar]
- 44. Morris KP, Skinner JR, Hunter Set al. Short term correction of anaemia with recombinant human erythropoietin and reduction of cardiac output in end stage renal failure. Arch Dis Child 1993; 68: 644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morris KP, Sharp J, Watson Set al. Non-cardiac benefits of human recombinant erythropoietin in end stage renal failure and anaemia. Arch Dis Child 1993; 69: 580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boehm M, Riesenhuber A, Winkelmayer WCet al. Early erythropoietin therapy is associated with improved growth in children with chronic kidney disease. Pediatr Nephrol 2007; 22: 1189–1193 [DOI] [PubMed] [Google Scholar]
- 47. Morris KP, Skinner JR, Hunter Set al. Cardiovascular abnormalities in end stage renal failure: the effect of anaemia or uraemia? Arch Dis Child 1994; 71: 119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Can C, Emre S, Bilge Iet al. Comparison of recombinant human erythropoietin and darbepoetin alpha in children. Pediatr Int 2013; 55: 296–299 [DOI] [PubMed] [Google Scholar]
- 49. Wedekin M, Ehrich JHH, Pape L.. Effective treatment of anemia in pediatric kidney transplant recipients with methoxy polyethylene glycol-epoetin beta. Pediatr Transplant 2011; 15: 329–333 [DOI] [PubMed] [Google Scholar]
- 50. de Palo T, Giordano M, Palumbo Fet al. Clinical experience with darbepoietin alfa (NESP) in children undergoing hemodialysis. Pediatr Nephrol 2004; 19: 337–340 [DOI] [PubMed] [Google Scholar]
- 51. Andre J-L, Deschenes G, Boudailliez Bet al. Darbepoetin, effective treatment of anaemia in paediatric patients with chronic renal failure. Pediatr Nephrol 2007; 22: 708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gaydarova M, Boueva A, Marinova Set al. Use of darbepoetin alfa (Aranesp®) for anemia treatment in children with chronic kidney disease—clinical experience of Bulgarian pediatric group. J IMAB 2017; 23: 1421–1426 [Google Scholar]
- 53. Geary DF, Keating LE, Vigneux Aet al. Darbepoetin alfa (Aranesp) in children with chronic renal failure. Kidney Int 2005; 68: 1759–1765 [DOI] [PubMed] [Google Scholar]
- 54. Rijk Y, Raaijmakers R, van de Kar Net al. Intraperitoneal treatment with darbepoetin for children on peritoneal dialysis. Pediatr Nephrol 2007; 22: 436–440 [DOI] [PubMed] [Google Scholar]
- 55. Durkan AM, Keating LE, Vigneux Aet al. The use of darbepoetin in infants with chronic renal impairment. Pediatr Nephrol 2006; 21: 694–697 [DOI] [PubMed] [Google Scholar]
- 56. Libudzic-Nowak AM, Cachat F, Pascual Met al. Darbepoetin alfa in young infants with renal failure: single center experience, a case series and review of the literature. Front Pediatr 2018; 6: 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kulzer P, Schaefer RM, Krahn Ret al. Effectiveness and safety of recombinant human erythropoietin (r-HuEPO) in the treatment of anemia of chronic renal failure in non dialysis patients. European Multicentre Study Group. Int J Artif Organs 1994; 17: 195–202 [PubMed] [Google Scholar]
- 58. Schmitt CP, Nau B, Brummer Cet al. Increased injection pain with darbepoetin-alpha compared to epoetin-beta in paediatric dialysis patients. Nephrol Dial Transplant 2006; 21: 3520–3524 [DOI] [PubMed] [Google Scholar]
- 59. Jander A, Wiercinski R, Balasz-Chmielewska Iet al. Anaemia treatment in chronically dialysed children: a multicentre nationwide observational study. Scand J Urol Nephrol 2012; 46: 375–380 [DOI] [PubMed] [Google Scholar]
- 60. Fischbach M, Wuhl E, Reigner SCMet al. Efficacy and long-term safety of C.E.R.A. maintenance in pediatric hemodialysis patients with anemia of CKD. Clin J Am Soc Nephrol 2018; 13: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cano F, Alarcon C, Azocar Met al. Continuous EPO receptor activator therapy of anemia in children under peritoneal dialysis. Pediatr Nephrol 2011; 26: 1303–1310 [DOI] [PubMed] [Google Scholar]
- 62. Schaefer F, Hoppe B, Jungraithmayr Tet al. Safety and usage of darbepoetin alfa in children with chronic kidney disease: prospective registry study. Pediatr Nephrol 2016; 31: 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Borzych-Duzalka D, Bilginer Y, Ha ISet al. Management of anemia in children receiving chronic peritoneal dialysis. J Am Soc Nephrol 2013; 24: 665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rigden SP, Montini G, Morris Met al. Recombinant human erythropoietin therapy in children maintained by haemodialysis. Pediatr Nephrol 1990; 4: 618–622 [DOI] [PubMed] [Google Scholar]
- 65. Scharer K, Klare B, Braun Aet al. Treatment of renal anemia by subcutaneous erythropoietin in children with preterminal chronic renal failure. Acta Paediatr 1993; 82: 953–958 [DOI] [PubMed] [Google Scholar]
- 66. Rees L, Rigden SP, Chantler C.. The influence of steroid therapy and recombinant human erythropoietin on the growth of children with renal disease. Pediatr Nephrol 1991; 5: 556–558 [DOI] [PubMed] [Google Scholar]
- 67. Stefanidis CJ, Koulieri A, Siapera Det al. Effect of the correction of anemia with recombinant human erythropoietin on growth of children treated with CAPD. Adv Perit Dial 1992; 8: 460–463 [PubMed] [Google Scholar]
- 68. Jabs K. The effects of recombinant human erythropoietin on growth and nutritional status. Pediatr Nephrol 1996; 10: 324–327 [DOI] [PubMed] [Google Scholar]
- 69. Baraldi E, Montini G, Zanconato Set al. Exercise tolerance after anaemia correction with recombinant human erythropoietin in end-stage renal disease. Pediatr Nephrol 1990; 4: 623–626 [DOI] [PubMed] [Google Scholar]
- 70. Warady BA, Sabath RJ, Smith CAet al. Recombinant human erythropoietin therapy in pediatric patients receiving long-term peritoneal dialysis. Pediatr Nephrol 1991; 5: 718–723 [DOI] [PubMed] [Google Scholar]
- 71. Martin GR, Ongkingo JR, Turner MEet al. Recombinant erythropoietin (Epogen) improves cardiac exercise performance in children with end-stage renal disease. Pediatr Nephrol 1993; 7: 276–280 [DOI] [PubMed] [Google Scholar]
- 72. Montini G, Zacchello G, Baraldi Eet al. Benefits and risks of anemia correction with recombinant human erythropoietin in children maintained by hemodialysis. J Pediatr 1990; 117: 556–560 [DOI] [PubMed] [Google Scholar]
- 73. Burke JR. Low-dose subcutaneous recombinant erythropoietin in children with chronic renal failure. Australian and New Zealand Paediatric Nephrology Association. Pediatr Nephrol 1995; 9: 558–561 [DOI] [PubMed] [Google Scholar]
- 74. Fabris F, Cordiano I, Randi MLet al. Effect of human recombinant erythropoietin on bleeding time, platelet number and function in children with end-stage renal disease maintained by haemodialysis. Pediatr Nephrol 1991; 5: 225–228 [DOI] [PubMed] [Google Scholar]
- 75. Suppiej A, Montini G, Casara Get al. Evoked potentials before and after anemia correction with recombinant human erythropoietin in end-stage renal disease. Child Nephrol Urol 1992; 12: 197–201 [PubMed] [Google Scholar]
- 76. North American Pediatric Renal Trials and Collaborative Studies . 2011. NAPRTCS 2011 Annual Dialysis Report. https://naprtcs.org/system/files/2011_Annual_Dialysis_Report.pdf (8 April 2022, date last accessed) [Google Scholar]
- 77. van Stralen KJ, Krischock L, Schaefer Fet al. Prevalence and predictors of the sub-target Hb level in children on dialysis. Nephrol Dial Transplant 2012; 27: 3950–3957 [DOI] [PubMed] [Google Scholar]
- 78. Gerson A, Hwang W, Fiorenza Jet al. Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis 2004; 44: 1017–1023 [DOI] [PubMed] [Google Scholar]
- 79. Roche H-L. Ascertain the Optimal Starting Dose of Mircera Given Subcutaneously for Maintenance Treatment of Anemia in Pediatric Patients with Chronic Kidney Disease on Dialysis or Not Yet on Dialysis. https://clinicaltrials.gov/ct2/show/NCT03552393 (30 November 2021, date last accessed) [Google Scholar]
- 80. Reddingius RE, Schroder CH, Koster AMet al. Pharmacokinetics of recombinant human erythropoietin in children treated with continuous ambulatory peritoneal dialysis. Eur J Pediatr. 1994; 153: 850–854 [DOI] [PubMed] [Google Scholar]
- 81. Bargman JM, Jones JE, Petro JM. The pharmacokinetics of intraperitoneal erythropoietin administered undiluted or diluted in dialysate. Perit Dial Int 1992; 12: 369–372 [PubMed] [Google Scholar]
- 82. Macdougall IC, Roberts DE, Neubert Pet al. Pharmacokinetics of recombinant human erythropoietin in patients on continuous ambulatory peritoneal dialysis. Lancet 1989; 333: 425–427 [DOI] [PubMed] [Google Scholar]
- 83. Chavers BM, Roberts TL, Herzog CAet al. Prevalence of anemia in erythropoietin-treated pediatric as compared to adult chronic dialysis patients. Kidney Int 2004; 65: 266–273 [DOI] [PubMed] [Google Scholar]
- 84. Palis J, Segel GB. Developmental biology of erythropoiesis. Blood Rev 1998; 12: 106–114 [DOI] [PubMed] [Google Scholar]
- 85. Bamgbola OF, Kaskel FJ, Coco M. Analyses of age, gender and other risk factors of erythropoietin resistance in pediatric and adult dialysis cohorts. Pediatr Nephrol 2009; 24: 571–579 [DOI] [PubMed] [Google Scholar]
- 86. Seeherunvong W, Rubio L, Abitbol CLet al. Identification of poor responders to erythropoietin among children undergoing hemodialysis. J Pediatr 2001; 138: 710–714 [DOI] [PubMed] [Google Scholar]
- 87. Smith KJ, Bleyer AJ, Little WCet al. The cardiovascular effects of erythropoietin. Cardiovasc Res 2003; 59: 538–548 [DOI] [PubMed] [Google Scholar]
- 88. Vaziri ND, Zhou XJ. Potential mechanisms of adverse outcomes in trials of anemia correction with erythropoietin in chronic kidney disease. Nephrol Dial Transplant 2009; 24: 1082–1088 [DOI] [PubMed] [Google Scholar]
- 89. Hattori M, Matsunaga A, Akioka Yet al. Darbepoetin alfa for the treatment of anemia in children undergoing peritoneal dialysis: a multicenter prospective study in Japan. Clin Exp Nephrol 2013; 17: 582–588 [DOI] [PubMed] [Google Scholar]
- 90. Port RE, Ding RW, Fies Tet al. Predicting the time course of haemoglobin in children treated with erythropoietin for renal anaemia. Br J Clin Pharmacol 1998; 46: 461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sieniawska M, Roszkowska-Blaim M.. Recombinant human erythropoietin dosage in children undergoing hemodialysis and continuous ambulatory peritoneal dialysis. Pediatr Nephrol 1997; 11: 628–630 [DOI] [PubMed] [Google Scholar]
- 92. Aufricht C, Balzar E, Steger Het al. Subcutaneous recombinant human erythropoietin in children with renal anemia on continuous ambulatory peritoneal dialysis. Acta Paediatr 1993; 82: 959–962 [DOI] [PubMed] [Google Scholar]
- 93. Montini G, Zacchello G, Perfumo Fet al. Pharmacokinetics and hematologic response to subcutaneous administration of recombinant human erythropoietin in children undergoing long-term peritoneal dialysis: a multicenter study. J Pediatr 1993; 122: 297–302 [DOI] [PubMed] [Google Scholar]
- 94. Sallay P, Reusz G.. The use of human recombinant erythropoietin in children on chronic dialysis. Acta Paediatr Hung 1992; 32: 333–345 [PubMed] [Google Scholar]
- 95. Hisano S, Kaku Y, Ueda Ket al. Human erythropoietin in children undergoing continuous ambulatory peritoneal dialysis. Pediatr Int 1992; 34: 36–41 [DOI] [PubMed] [Google Scholar]
- 96. Campos A, Garin EH.. Therapy of renal anemia in children and adolescents with recombinant human erythropoietin (rHuEPO). Clin Pediatr (Phila) 1992; 31: 94–99 [DOI] [PubMed] [Google Scholar]
- 97. Ongkingco JR, Ruley EJ, Turner ME.. Use of low-dose subcutaneous recombinant human erythropoietin in end-stage renal disease: experience with children receiving continuous cycling peritoneal dialysis. Am J Kidney Dis 1991; 18: 446–450 [DOI] [PubMed] [Google Scholar]
- 98. Hisano S, Kaku Y, Ueda Ket al. Efficacy of once weekly erythropoietin therapy in children on continuous ambulatory peritoneal dialysis. Pediatr Int 1991; 33: 450–454 [DOI] [PubMed] [Google Scholar]
- 99. Navarro M, Alonso A, Avilla JMet al. Anemia of chronic renal failure: treatment with erythropoietin. Child Nephrol Urol 1991; 11: 146–151 [PubMed] [Google Scholar]
- 100. Bianchetti MG, Hammerli I, Roduit Cet al. Epoetin alfa in anaemic children or adolescents on regular dialysis. Eur J Pediatr 1991; 150: 509–512 [DOI] [PubMed] [Google Scholar]



