ABSTRACT
Background
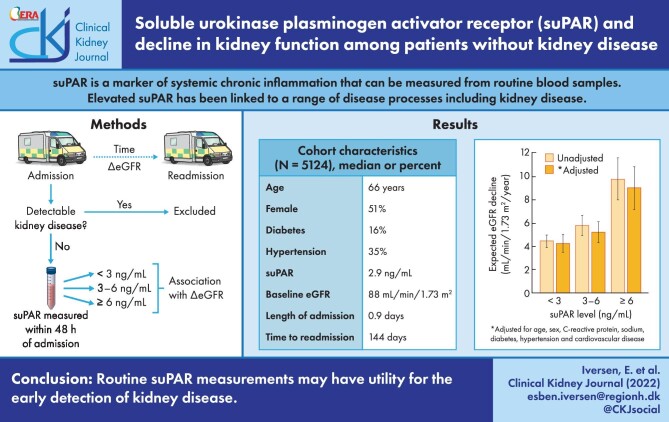
Hospitalized patients are at an increased risk of developing kidney disease after discharge, often despite the absence of any clinical indicators during hospitalization. Soluble urokinase plasminogen activator receptor (suPAR) is a marker of systemic chronic inflammation that can be measured from routine blood samples. We determined whether elevated suPAR during hospitalization is associated with a decline in estimated glomerular filtration rate (eGFR) after discharge.
Methods
This was a retrospective longitudinal cohort study of patients without detectable kidney disease presenting to the emergency department on two separate occasions during a 3-year period. The association between suPAR and a decline in eGFR was assessed by linear mixed models for repeated measures adjusting for age, sex, C-reactive protein, sodium, diabetes, hypertension and cardiovascular disease.
Results
In total, 5124 patients (median age 65.9 years, 51.0% female) were included. The median suPAR was 2.9 ng/mL, the median time to readmission was 144 days and the expected rate of eGFR decline over this period was 5.1 mL/min/1.73 m2/year. Adjusting for other risk factors, patients with suPAR <3, 3–6 or ≥6 ng/mL had an expected eGFR decline of 4.3, 5.2 or 9.0 mL/min/1.73 m2/year, respectively. Similarly, patients with suPAR in the lowest (<2.4 ng/mL), middle (2.4–3.6 ng/mL) or highest (≥3.6 ng/mL) tertile had an expected eGFR decline of 4.2, 4.6 or 6.5 mL/min/1.73 m2/year, respectively. In both cases, a higher suPAR level was significantly and independently associated with a higher rate of eGFR decline (P < .001).
Conclusions
A higher suPAR level was associated with accelerated eGFR decline among patients presenting to the emergency department, suggesting that routine suPAR measurements may have utility for the early detection of kidney disease.
Keywords: acute, emergency department, estimated glomerular filtration rate, kidney disease, soluble urokinase plasminogen activator receptor
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Patients admitted to the hospital are at an increased risk of developing kidney disease. An undetected decline in kidney function after hospitalization can lead to adverse drug reactions, which is a leading cause of readmission and disease progression [1, 2]. The incidence of chronic kidney disease (CKD) among the general population is approximately two new diagnoses per 1000 people (0.2%) per year [3, 4], whereas we have previously reported that as many as 2% of patients presenting to the emergency department (ED) are diagnosed with CKD within 2 years of discharge [5]. Despite the high rate of post-discharge renal complications, there are often no clinical indicators of kidney disease during hospitalization. Estimated glomerular filtration rate (eGFR) is considered the standard for assessing kidney function in the hospital, but eGFR alone is a poor predictor of future kidney disease and an insensitive marker of disease progression [6, 7]. A screening tool that predicts future eGFR decline may help clinicians detect patients at risk of developing renal complications.
Soluble urokinase plasminogen activator receptor (suPAR) is a marker of systemic chronic inflammation that can be measured from routine blood samples [8]. Earlier studies showed an association between suPAR and CKD [9] and more recent literature has focussed on the association between suPAR and acute kidney injury (AKI) [10, 11]. The mechanisms of these associations are not completely understood but may involve an indirect inflammatory pathway [12] or direct damage to renal podocytes [13] and tubules [10]. In either case, suPAR-related kidney damage will manifest as a decrease in eGFR. Therefore we investigated the association between suPAR and the change in eGFR over time. If a higher suPAR level is associated with accelerated eGFR decline, then it may offer a tool for quickly identifying patients at risk of developing renal complications after discharge.
MATERIALS AND METHODS
Study design and setting
This was a retrospective longitudinal cohort study performed at Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark. This study was a subgroup analysis of a previously described cohort of patients presenting to the ED between November 2013 and March 2017 with suPAR and creatinine measured from routine blood samples [5]. The current study included all patients with two consecutive ED admissions (‘index admission’ and ‘readmission’) during the study period (>3 years). Exclusion criteria were: missing suPAR measurement, missing creatinine measurement, prior diagnosis of kidney disease defined by International Classification of Disease, Tenth Revision (ICD-10) codes (Supplementary data, Table S1), baseline eGFR <60 mL/min/1.73 m2 and suspected AKI defined as an increase in creatinine of 0.3 mg/dL within 2 days of admission or 1.5 times above baseline within 2 weeks of admission according to Kidney Disease: Improving Global Outcomes criteria [14]. The decision to exclude patients with suspected AKI was based on the assumption that rapid fluctuations in eGFR may represent acute illness or dehydration without true renal pathology [15]. In cases where a patient had multiple pairs of consecutive ED admissions meeting study criteria, the earliest pair was used for analysis. The study was approved by the Danish Data Protection Agency (HVH-2014-018, 02767) and the Danish Health and Medicines Authority 3-3013-1061/1).
Measurements
All measurements were recorded at index admission and creatinine was recorded at both index admission and readmission. Demographic information, including age and sex, was obtained from the Civil Registration System. Disease status, including diabetes, hypertension and cardiovascular disease, was determined by ICD-10 diagnosis codes (Supplementary data, Table S2) from the Danish National Patient Registry dating back to 1977 [16]. Blood samples were collected upon admission to the Acute Medical Unit and a standard panel of markers including creatinine, suPAR, C-reactive protein (CRP) and sodium was measured at the Department of Clinical Biochemistry using standardized assays according to the manufacturers’ instructions (Supplementary data, Table S3).
Exposures and outcomes
The primary explanatory variable was suPAR level in predefined categories of <3, 3–6 and ≥6 ng/mL, which have previously been associated with clinical outcomes [17–19]. Secondary explanatory variables were age, sex, CRP, sodium, diabetes, hypertension and cardiovascular disease. The primary endpoint was eGFR change from index admission to readmission. At both time points, eGFR was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration formula [20].
Statistics
Patient characteristics are presented with basic statistics: median with interquartile range (IQR) for continuous variables and number with percent of patients for discrete variables. Differences between patients with and without readmission were assessed by Wilcoxon rank sum test for continuous variables and chi-squared test for discrete variables. Differences between patients with different suPAR levels at index admission were assessed by one-way analysis of variance for continuous variables and chi-squared test for discrete variables. A graphical representation of the change in eGFR over time by suPAR level was generated using a B-spline non-parametric quantile regression, constrained by fixing the initial eGFR change to zero. The expected eGFR decline was assessed by a series of linear mixed-effects models for repeated measures, with random effects for individual patients (Supplementary data, Table S4). A ‘basic’ model including time to readmission as the only independent variable was used to determine the expected eGFR decline for the study population. Subsequently, an ‘unadjusted’ model including the suPAR level was used to determine the expected eGFR decline within each suPAR level. Finally, an ‘adjusted’ model including all secondary explanatory variables was used to determine the expected eGFR decline within each suPAR level independent of these potential confounders. In the adjusted model, the expected eGFR decline in the lowest suPAR level is reported for a ‘healthy’ patient with the following values: male, age 50 years, CRP 1 ng/mL, sodium 140 mEq/L and no comorbidities. Sensitivity analysis defining suPAR levels as tertiles within the study population (rather than predefined categories) was also performed. Due to variance heterogeneity and deviation from normality of the model residuals, the data were bootstrapped with 10 000 iterations to generate 95% confidence intervals (CIs) and P-values (P < .05 was considered statistically significant). All statistical analyses was performed in R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) [21]; the lme4 package was used for generation of linear mixed-effects models, the pbkr test package was used for bootstrapping and the ggplot2 package was used for generation of some figures.
RESULTS
Study population
In total, 29 265 unique patients presented to the ED during the study period. Of these, 8252 patients had at least one readmission during the study period and 5124 patients had at least one pair of admissions meeting study criteria (Supplementary data, Figure S1). Compared with patients without readmission, patients with readmission had lower median eGFR, higher median age, suPAR and CRP and higher prevalence of chronic diseases (all P < .001) (Supplementary data, Table S4). Among patients meeting the study criteria (N = 5124), the median age was 65.9 years, 51.0% were female, median eGFR was 88.0 mL/min/1.73 m2, median CRP was 6.0 mg/L and median suPAR was 2.9 ng/mL at baseline (Table 1). The prevalence of chronic diseases was 15.5% for diabetes, 34.8% for hypertension and 29.9% for cardiovascular disease. The median length of stay was 0.9 days and median time to readmission was 144 days. A higher suPAR level was significantly associated with older age and higher CRP and prevalence of chronic diseases (all P < .001). A higher suPAR level was also significantly associated with a longer length of stay and shorter time to readmission (both P < .001). Among patients with suPAR ≥6 ng/mL (n = 441), the median age was 71.4 years, median eGFR was 80.9 mL/min/1.73 m2, median CRP was 31 mg/L, median length of stay was 2.6 days and median time to readmission was 84 days.
Table 1.
Patient characteristics at index admission according to suPAR level
| Characterisitcs | All patients (N = 5124) | suPAR <3 ng/mL [n = 2738 (53.4%)] | suPAR 3–6 ng/mL [n = 1945 (38.0%)] | suPAR ≥6 ng/mL [n = 441 (8.6%)] |
|---|---|---|---|---|
| Demographics | ||||
| Female, n (%) | 2612 (51.0) | 1365 (49.9) | 1031 (53.0) | 216 (49.0) |
| Age (years), median (IQR) | 65.9 (50.5–76.9) | 59.1 (44.4–72.0) | 71.1 (58.6–80.4) | 71.4 (60.5–81.7) |
| Biomarkers | ||||
| suPAR (ng/mL), median (IQR) | 2.9 (2.2–4.1) | 2.2 (1.8–2.6) | 3.9 (3.4–4.6) | 7.6 (6.7–9.6) |
| eGFR (mL/min/1.73 m2), median (IQR) | 88.0 (75.3–102) | 92.2 (80.0–106) | 83.1 (71.3–96.8) | 80.9 (68.9–97.1) |
| CRP (mg/L), median (IQR) | 6.0 (2.0–28) | 3.0 (1.0–10) | 11 (3.0–49) | 31 (7.0–81) |
| Sodium (mEq/L), median (IQR) | 139 (136–141) | 139 (137–141) | 138 (135–140) | 136 (132–139) |
| Chronic disease status, n (%) | ||||
| Diabetes | 796 (15.5) | 326 (11.9) | 381 (19.6) | 89 (20.2) |
| Hypertension | 1782 (34.8) | 804 (29.4) | 792 (40.7) | 186 (42.2) |
| Cardiovascular disease | 1533 (29.9) | 726 (26.5) | 657 (33.8) | 150 (34.0) |
| Outcomes | ||||
| Length of stay (days), median (IQR) | 0.9 (0.4–3.9) | 0.7 (0.3–2.1) | 1.4 (0.5–5.1) | 2.6 (0.8–7.8) |
| Time to readmission (days), median (IQR) | 144 (35–378) | 170 (41–417) | 128 (32–338) | 84 (23–262) |
Association between suPAR level and decline in eGFR
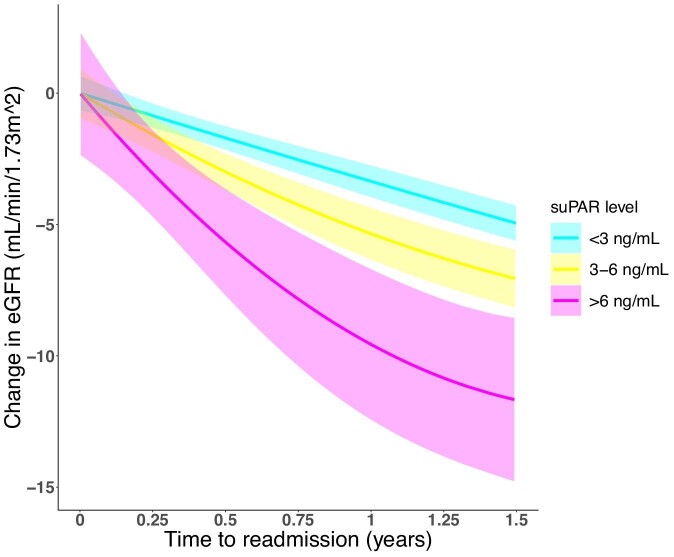
The expected rate of eGFR decline in the study cohort was 5.1 mL/min/1.73 m2/year (95% CI 4.7–5.5) (Supplementary data, Figure S2). A higher suPAR level at index admission was associated with lower baseline eGFR (Supplementary data, Figure S3) and a higher rate of eGFR decline (Figure 1). The expected rate of eGFR decline for patients with suPAR <3 ng/mL was 4.4 mL/min/1.73 m2/year (95% CI 3.9–5.0). Patients with suPAR 3–6 ng/mL had an additional eGFR decline of 1.4 mL/min/1.73 m2/year (95% CI 0.5–2.3; P = .002), and those with suPAR ≥6 ng/mL had an additional eGFR decline of 5.4 mL/min/1.73 m2/year (95% CI 3.5–7.2; P < .001). Adjusting for potential confounders, the expected rate of eGFR decline for a ‘healthy’ patient with suPAR <3 ng/mL was 4.3 mL/min/1.73 m2/year (95% CI 3.5–5.0). Patients with suPAR 3–6 ng/mL had an additional eGFR decline of 1.0 mL/min/1.73 m2/year (95% CI 0.1–1.9; P = .03), and those with suPAR ≥6 ng/mL had an additional eGFR decline of 4.7 mL/min/1.73 m2/year (95% CI 2.9–6.6; P < .001). Overall, a higher suPAR level was significantly associated with a higher rate of eGFR decline (P < .001).
FIGURE 1:
Change in eGFR according to suPAR level. Non-parametric quantile regression of change in eGFR over time according to suPAR level at index admission.
Sensitivity analysis based on suPAR tertiles
Defining suPAR levels as tertiles, the expected rate of eGFR decline for patients with suPAR in the lowest tertile (<2.4 ng/mL) was 4.2 mL/min/1.73 m2/year (95% CI 3.5–4.9). Patients with suPAR in the middle tertile (2.4–3.6 ng/mL) or highest tertile (≥3.6 ng/mL) had an additional eGFR decline of 0.8 mL/min/1.73 m2year (95% CI –0.2–1.7; P = .11) or 2.9 mL/min/1.73 m2year (95% CI 1.8–3.9; P < .001), respectively. Adjusting for potential confounders, the expected rate of eGFR decline for a ‘healthy’ patient with suPAR in the lowest tertile was 4.2 mL/min/1.73 m2/year (95% CI 3.4–5.0) and patients with suPAR in the middle or highest tertile had an additional eGFR decline of 0.4 mL/min/1.73 m2/year (95% CI –0.5–1.4; P = .39) or 2.3 mL/min/1.73 m2/year (95% CI 1.2–3.4; P < .001), respectively. Overall, a higher suPAR tertile was significantly associated with a higher rate of eGFR decline (P < .001).
Association between age, sex and decline in eGFR
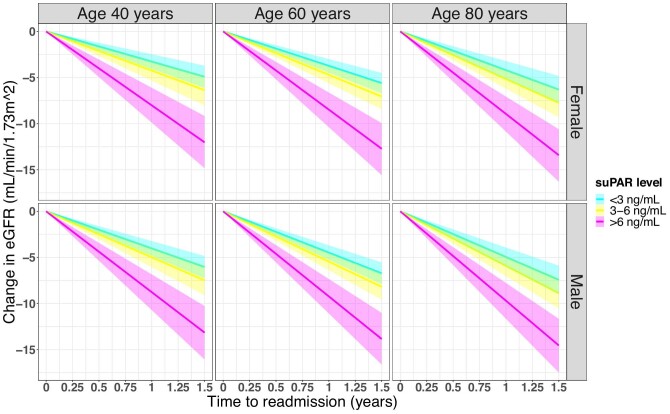
Male sex and older age were also associated with a higher rate of eGFR decline (Figure 2), although these associations were not significant. Each increase in age of 1 year corresponded to an additional eGFR decline of 0.02 mL/min/1.73 m2/year (95% CI 0.00–0.05; P = .07) and males compared with females had an additional eGFR decline of 0.75 mL/min/1.73 m2/year (95% CI –0.04–1.54; P = 1.0) (Supplementary data, Table S5).
FIGURE 2:
Impact of age and sex on suPAR-related eGFR decline. Graphical representation of combined model estimates for age, sex and suPAR level.
DISCUSSION
Summary of findings
In a cohort of 5124 patients presenting to the ED without detectable kidney disease, the expected rate of eGFR decline from index admission to readmission was 5.1 mL/min/1.73 m2/year and a higher suPAR level was significantly associated with an accelerated eGFR decline after discharge. This association was independent of baseline eGFR, age, sex, CRP, sodium, diabetes, hypertension and cardiovascular disease. Adjusting for these factors, patients with suPAR <3, 3–6 or ≥6 ng/mL had an expected eGFR decline of 4.3, 5.2 or 9.0 mL/min/1.73 m2/year, respectively. Defining suPAR levels as tertiles, patients with suPAR <2.4, 2.4–3.6 or ≥3.6 ng/mL had an expected eGFR decline of 4.2, 4.6 or 6.5 mL/min/1.73 m2/year, respectively.
Comparison to other studies
Normal eGFR decline in healthy adults is reported at 0.5–1.0 mL/min/1.73 m2/year [22, 23]. We observed an expected eGFR decline of 5.1 mL/min/1.73 m2/year, which is higher than in healthy adults and likely reflects the generally older and sicker patient population within the ED. We found that older age was associated with a slightly higher rate of eGFR decline, consistent with functional changes known to occur with ageing [24, 25]. Age-related eGFR decline is also affected by comorbidities such as diabetes and hypertension [26]. In a cohort of patients with type 1 diabetes but otherwise normal kidney function, de Boer et al. [27] reported an eGFR decline of 1.27–1.56 mL/min/1.73 m2/year. The expected eGFR decline for a ‘healthy’ patient in our cohort is still higher than expected based on these risk factors alone and may be due to selection for patients with readmission during the study period.
Compared with ‘healthy’ patients, we observed that patients with suPAR ≥6 ng/mL had an additional expected eGFR decline of 4.7 mL/min/1.73 m2/year. This is similar to previous findings by Hayek et al. [9] among patients undergoing cardiac catheterization. In that study, participants with suPAR <2.4 ng/mL had an eGFR decline of 0.9 mL/min/1.73 m2/year, whereas those with suPAR >4 ng/mL had an eGFR decline of 4.2 mL/min/1.73 m2/year. The authors note that suPAR-related eGFR decline was lower in patients with eGFR <60 mL/min/1.73 m2. Therefore the higher rate of eGFR decline observed in our cohort can be partly explained by the exclusion of patients with eGFR <60 mL/min/1.73 m2.Discrepancies between studies may also be related to the use of different suPAR levels.
Potential molecular mechanisms
We recently reported in the full patient population for this cohort (n = 29 265) that elevated suPAR at ED admission was associated with future kidney disease diagnosis [5]. The mechanism of this association was not explored, but we hypothesize that eGFR decline mediates the association. In the short term, rapid lowering of eGFR may lead to renal complications such as AKI or adverse drug reactions. In the long term, declining eGFR may represent chronic damage to renal podocytes. It remains unclear whether suPAR is responsible for this decline or simply an indicator of another underlying disease process, such as systemic chronic inflammation.
Furman et al. [28] described mechanisms involved in systemic chronic inflammation and suggested that this inflammation may be responsible for chronic disease processes such as cardiovascular disease, autoimmune disorders, cancer and kidney disease. There is promising evidence to support the role of suPAR as a mediator or by-product of systemic chronic inflammation, which would help explain its associations with a wide range of chronic diseases [29]. Alternatively, Hayek et al. [13] proposed that suPAR may contribute directly to kidney damage. In a mouse model, they showed that suPAR leads to proteinuria by activating β3-integrin on renal podocytes. This mechanism was also observed in renal tissues of human patients with primary nephrotic syndromes [30]. In a separate mouse model, Hayek et al. [14] demonstrated that elevated suPAR sensitizes mouse kidneys to contrast-induced nephropathy by increasing enzymatic oxidation within renal tubular cells, but that this effect could be attenuated by pretreatment with an anti-uPAR antibody. This finding was replicated in cell cultures obtained from human patients with focal segmental glomerular sclerosis [31]. Together, these findings provide direct, though separate, mechanisms for suPAR in both chronic and acute kidney disease.
Clinical applications
Regardless of its precise molecular mechanisms, we believe that routine suPAR measurements may have utility in the early detection of kidney disease. The ED offers a unique opportunity for this because nearly all patients admitted to a medical floor will come from the ED, whereas fewer than half of patients presenting to the ED are ultimately admitted [32, 33]. Given the significant association between suPAR and eGFR decline suPAR should be considered in predictive models for kidney disease. For example, Zacharias et al. [34] recently developed a predictive model based on six routine biomarkers and demonstrated its high predictive performance among a large cohort of patients with CKD. Among our cohort, we found that patients with suPAR ≥6 ng/mL at index admission (8.6% of patients) had a particularly high rate of eGFR decline, but risk stratification models are needed to determine the most appropriate suPAR cutoff values. Potential interventions for high-risk patients include closer monitoring of kidney function, medication review to identify and discontinue nephrotoxic medications [35] or preventive treatments to slow disease progression [36, 37]. If suPAR is directly involved in the pathogenesis of kidney disease, then interventions to decrease suPAR levels may also have a therapeutic benefit.
Limitations
This study has several limitations. First, to identify patients with two separate creatinine measurements, the study cohort was selected for patients with readmission during the study period. These patients were sicker at baseline than patients without readmission and therefore are not entirely representative of a typical patient in the ED. There was also high variability in time to readmission among the study cohort and the data does not include admissions to other hospitals. Ideally the study would have included all patients presenting to the ED with eGFR measured at a fixed time point after discharge. Second, calculating a change in eGFR between index admission and readmission assumes a steady state during each admission, but eGFR is susceptible to fluctuations in the ED [38]. We excluded patients with AKI and adjusted for volume status in our analysis, but it is not possible to fully account for eGFR fluctuations. This simply emphasizes the drawback of relying on eGFR in an acute setting and our findings should be validated using measured GFR. Finally, our analysis did not account for smoking status, which is known to affect suPAR concentration [39], or proteinuria, which is a risk factor for kidney disease independent of eGFR [40]. Similar studies have shown significant associations between suPAR and kidney-related outcomes independent of these factors [9, 41], but they should be considered in future studies.
CONCLUSIONS
In this retrospective longitudinal cohort study of hospitalized patients without detectable kidney disease, a higher suPAR level was significantly and independently associated with accelerated eGFR decline after discharge. These findings suggest that suPAR may have utility for the early detection of kidney disease.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the Department of Clinical Biochemistry at Amager and Hvidovre Hospital for all analyses used in this study, including suPAR measurements for 47 631 admissions.
Contributor Information
Esben Iversen, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark; Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Thomas Kallemose, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark.
Mads Hornum, Department of Nephrology, Rigshospitalet, Copenhagen, Denmark; Department of Clinical Medicine, Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Anne Kathrine Bengaard, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark; Department of Clinical Medicine, Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Capital Region Pharmacy, Herlev, Denmark.
Jan Olof Nehlin, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark.
Line Jee Hartmann Rasmussen, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark; Department of Psychology and Neuroscience, Duke University, Durham, NC, USA.
Haakon Sandholdt, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark.
Juliette Tavenier, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark.
Bo Feldt-Rasmussen, Department of Nephrology, Rigshospitalet, Copenhagen, Denmark; Department of Clinical Medicine, Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Ove Andersen, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark; Emergency Department, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark.
Jesper Eugen-Olsen, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark.
Morten Baltzer Houlind, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark; Capital Region Pharmacy, Herlev, Denmark; Department of Drug Design and Pharmacology, University of Copenhagen, Copenhagen, Denmark.
CONFLICT OF INTEREST STATEMENT
J.E.O. is a cofounder, shareholder and Chief Scientific Officer of ViroGates A/S. J.E.O. and O.A. are named inventors on patents covering suPAR owned by Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark and licensed to ViroGates A/S. All remaining authors report no conflicts of interest. The results presented in this article have not been published previously in whole or part, except in abstract format.
AUTHORS’ CONTRIBUTIONS
O.A., J.E.O. and M.B.H. were responsible for the research idea. E.I., T.K., L.J.H.R., H.S., O.A., J.E.O. and M.B.H. were responsible for the study design. E.I., T.K. and H.S. were responsible for the statistical analysis. E.I., M.H., L.J.H.R., B.F.R., J.E.O. and M.B.H. were responsible for the interpretation of results. All authors contributed important intellectual content during manuscript drafting or revision, accept personal accountability for their own contributions and agree to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
FUNDING
M.H. was supported by a research grant from the Lundbeck Foundation (R187-2015-2148). L.J.H.R. was supported by a postdoctoral fellowship from the Lundbeck Foundation (R288-2018-380). M.B.H. was supported by a postdoctoral fellowship from the Capital Region's Research Foundation for Health Research in Denmark (A6882). All remaining authors report no external funding.
DATA AVAILABILITY STATEMENT
The data underlying this article are not publicly available due to privacy or ethical restrictions. The data will be shared upon reasonable request to the corresponding author.
REFERENCES
- 1. Tuttle KR, Alicic RZ, Short RAet al. Medication therapy management after hospitalization in CKD: a randomized clinical trial. Clin J Am Soc Nephrol 2018; 13: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedrós C, Quintana B, Rebolledo Met al. Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur J Clin Pharmacol 2014; 70: 361–367 [DOI] [PubMed] [Google Scholar]
- 3. Drey N, Roderick P, Mullee Met al. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 2003; 42: 677–684 [DOI] [PubMed] [Google Scholar]
- 4. Vestergaard SV, Christiansen CF, Thomsen RWet al. Identification of patients with CKD in medical databases: a comparison of different algorithms. Clin J Am Soc Nephrol 2021; 16: 543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iversen E, Houlind MB, Kallemose Tet al. Elevated suPAR is an independent risk marker for incident kidney disease in acute medical patients. Front Cell Dev Biol 2020; 8: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunsicker LG, Adler S, Caggiula Aet al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 1997; 51: 1908–1919 [DOI] [PubMed] [Google Scholar]
- 7. Levin A, Djurdjev O, Beaulieu Met al. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 2008; 52: 661–671 [DOI] [PubMed] [Google Scholar]
- 8. Rasmussen LJH, Petersen JEV, Eugen-Olsen J. Soluble urokinase plasminogen activator receptor (suPAR) as a biomarker of systemic chronic inflammation. Front Immunol 2021; 12: 5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayek SS, Sever S, Ko Y-Aet al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med 2015; 373: 1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayek SS, Leaf DE, Tahhan ASet al. Soluble urokinase receptor and acute kidney injury. N Engl J Med 2020; 382: 416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walls AB, Bengaard AK, Iversen Eet al. Utility of suPAR and NGAL for AKI risk stratification and early optimization of renal risk medications among older patients in the emergency department. Pharmaceuticals 2021; 14: 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furman D, Campisi J, Verdin Eet al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019; 25: 1822–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayek SS, Koh KH, Grams MEet al. A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med 2017; 23: 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179–c184 [DOI] [PubMed] [Google Scholar]
- 15. Kao SS, Kim SW, Horwood CMet al. Variability in inpatient serum creatinine: its impact upon short- and long-term mortality. QJM 2015; 108: 781–787 [DOI] [PubMed] [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M.. The Danish National Patient Register. Scand J Public Health 2011; 39: 30–33 [DOI] [PubMed] [Google Scholar]
- 17. Rovina N, Akinosoglou K, Eugen-Olsen Jet al. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care 2020; 24: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasmussen LJH, Ladelund S, Haupt THet al. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: a strong marker of disease presence and severity, readmission and mortality. A retrospective cohort study. Emerg Med J 2016; 33: 769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bengaard AK, Iversen E, Kallemose Tet al. Using soluble urokinase plasminogen activator receptor to stratify patients for medication review in the emergency department. Br J Clin Pharmacol 2021; doi: 10.1111/bcp.14982 [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team . R: A language and environment for statistical computing. https://www.r-project.org/ (10 October 2020, date last accessed) [Google Scholar]
- 22. Rule AD, Gussak HM, Pond GRet al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 2004; 43: 112–119 [DOI] [PubMed] [Google Scholar]
- 23. Baba M, Shimbo T, Horio Met al. Longitudinal study of the decline in renal function in healthy subjects. PLoS One 2015; 10: e0129036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33: 278–285 [DOI] [PubMed] [Google Scholar]
- 25. Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol 2017; 28: 2838–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yokoyama H, Kanno S, Takahashi Set al. Determinants of decline in glomerular filtration rate in nonproteinuric subjects with or without diabetes and hypertension. Clin J Am Soc Nephrol 2009; 4: 1432–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Boer IH, Sun W, Cleary PAet al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011; 365: 2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eugen-Olsen J, Andersen O, Linneberg Aet al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med 2010; 268: 296–308 [DOI] [PubMed] [Google Scholar]
- 29. Marsland AL. suPAR: a newer biomarker of systemic chronic inflammation. Brain Behav Immun 2021; 98: 263–264 [DOI] [PubMed] [Google Scholar]
- 30. Fujimoto K, Kagaya Y, Fujii Aet al. Soluble urokinase receptor (suPAR) is a predictor of disease state and renal prognosis in primary nephrotic syndrome. Nephrol Dial Transplant 2020; 35(Suppl 3). https://academic.oup.com/ndt/article/35/Supplement_3/gfaa142.P0221/5853881 (3 February 2020, date last accessed) [Google Scholar]
- 31. Vincenti F, Rashmi P, Da Silva AAet al. Neutralization of uPAR with an anti-uPAR antibody ameliorates recurrent FSGS sera induced podocyte injury. Nephrol Dial Transplant 2020; 35(Suppl 3). https://academic.oup.com/ndt/article/35/Supplement_3/gfaa140.MO009/5852948 (3 February 2020, date last accessed) [Google Scholar]
- 32. Juul-Larsen HG, Petersen J, Sivertsen DMet al. Prevalence and overlap of disease management program diseases in older hospitalized patients. Eur J Ageing 2017; 14: 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abualenain J, Frohna WJ, Shesser Ret al. Emergency department physician-level and hospital-level variation in admission rates. Ann Emerg Med 2013; 61: 638–643 [DOI] [PubMed] [Google Scholar]
- 34. Zacharias HU, Altenbuchinger M, Schultheiss UTet al. A predictive model for progression of CKD to kidney failure based on routine laboratory tests. Am J Kidney Dis 2022; 79: 217–230 [DOI] [PubMed] [Google Scholar]
- 35. Houlind MB, Andersen AL, Treldal Cet al. A collaborative medication review including deprescribing for older patients in an emergency department: a longitudinal feasibility study. J Clin Med 2020; 9: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Larmour K, Levin A. Slowing progression in CKD: DAPA CKD and beyond. Clin J Am Soc Nephrol 2021; 16: 1117–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cherney D, Cosentino F, Dagogo-Jack Set al. Ertugliflozin and slope of chronic eGFR. Clin J Am Soc Nephrol 2021; 16: 1345–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siriwardane D, Woodman R, Hakendorf Pet al. Stability of plasma creatinine concentrations in acute complex long-stay admissions to a general medical service. Clin Med 2010; 10: 540–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eugen-Olsen J, Ladelund S, Sørensen LT. Plasma suPAR is lowered by smoking cessation: a randomized controlled study. Eur J Clin Invest 2016; 46: 305–311 [DOI] [PubMed] [Google Scholar]
- 40. Gansevoort RT, Matsushita K, van der Velde Met al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes: a collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80: 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Curovic VR, Theilade S, Winther SAet al. Soluble urokinase plasminogen activator receptor predicts cardiovascular events, kidney function decline, and mortality in patients with type 1 diabetes. Diabetes Care 2019; 42: 1112–1119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are not publicly available due to privacy or ethical restrictions. The data will be shared upon reasonable request to the corresponding author.