Abstract
Despite extensive research, the rewarding effects of cannabinoids are still debated. Here, we used a newly established animal procedure called optogenetic intracranial self-stimulation (ICSS) (oICSS) to re-examine the abuse potential of cannabinoids in mice. A specific adeno-associated viral vector carrying a channelrhodopsin gene was microinjected into the ventral tegmental area (VTA) to express light-sensitive channelrhodopsin in dopamine (DA) neurons of transgenic dopamine transporter (DAT)-Cre mice. Optogenetic stimulation of VTA DA neurons was highly reinforcing and produced a classical “sigmoidal”-shaped stimulation–response curve dependent upon the laser pulse frequency. Systemic administration of cocaine dose-dependently enhanced oICSS and shifted stimulation–response curves upward, in a way similar to previously observed effects of cocaine on electrical ICSS. In contrast, Δ9-tetrahydrocannabinol (Δ9-THC), but not cannabidiol, dose-dependently decreased oICSS responding and shifted oICSS curves downward. WIN55,212-2 and ACEA, two synthetic cannabinoids often used in laboratory settings, also produced dose-dependent reductions in oICSS. We then examined several new synthetic cannabinoids, which are used recreationally. XLR-11 produced a cocaine-like increase, AM-2201 produced a Δ9-THC-like reduction, while 5F-AMB had no effect on oICSS responding. Immunohistochemistry and RNAscope in situ hybridization assays indicated that CB1Rs are expressed mainly in VTA GABA and glutamate neurons, while CB2Rs are expressed mainly in VTA DA neurons. Together, these findings suggest that most cannabinoids are not reward enhancing, but rather reward attenuating or aversive in mice. Activation of CB1R and/or CB2R in different populations of neurons in the brain may underlie the observed actions.
Keywords: brain-stimulation reward, cannabinoids, cocaine, dopamine, intracranial self-stimulation, optogenetics, ventral tegmental area, WIN55, 212-2, Δ9-THC
1 |. INTRODUCTION
Cannabis is the second most commonly used psychotropic substance, following alcohol.1,2 Cannabis is also the most commonly used illicit substance in the United States.2 There are around 37.6 million people who use cannabis in the United States, and of these, 11.8 million are young adults.2,3 About 30% of those who use cannabis develop cannabis use disorder, indicating that for many individuals, cannabis can be addictive.3,4 However, people report both positive and negative experiences following cannabis use. Some individuals report a sense of euphoria, relaxation, or perceptual distortions,5–7 but not all of those who try cannabis enjoy it. Others experience dysphoria, delusions, anxiety, fear, panic, or depression after cannabis use.8,9
Similar paradoxical effects of cannabinoids have been observed in experimental laboratory animals. Intravenous drug self-administration, conditioned place preference (CPP), or aversion (CPA), and electrical intracranial self-stimulation (ICSS) (eICSS) are the most commonly used behavioral procedures to evaluate the rewarding or aversive effects of drugs of abuse. Δ9-Tetrahydrocannabinol (Δ9-THC) is reportedly self-administered by squirrel monkeys,10,11 but not by rhesus monkeys.12,13 As with rhesus monkeys, it is challenging to train rodents to self-administer Δ9-THC.14,15 In the CPP procedure, most reports show aversion to Δ9-THC when it is on board, although a few reports show CPP.15–19 In eICSS, acute Δ9-THC administration is reported to facilitate brain-stimulation reward (BSR),20,21 inhibit BSR,22–25 or produce biphasic effects such that low doses facilitate whereas high doses inhibit BSR.26,27 Thus, it appears that cannabis or cannabinoids can be either rewarding or aversive in animals, depending upon the species, doses employed, or experimental conditions.
Given the essential role of dopamine (DA) in drug reward28,29 and recent progress in optogenetic techniques,30,31 we successfully established a new procedure called optogenetic ICSS (oICSS) in transgenic mice to re-evaluate the rewarding or reward-enhancing versus aversive or reward-attenuating effects of cannabinoids.32–34 In this procedure, an adeno-associated viral (AAV) vector carrying a Cre-dependent channelrhodopsin gene is microinjected into the ventral tegmental area (VTA) to express light-sensitive channelrhodopsin 2 (ChR2) protein selectively in DA neurons of transgenic DA transporter (DAT)-Cre mice. In contrast to classical eICSS, which nonspecifically stimulates multiple types of neurons or nerve fibers in the vicinity of an implanted electrode,27,35,36 oICSS is cell type-specific and mediated selectively by stimulation of VTA DA neurons33,34 or glutamate neurons.32 However, what remains unknown is whether and how cannabinoids alter such DA-dependent oICSS behavior.
In the present study, we examined the effects of multiple cannabinoids on DA-dependent oICSS, including the phytocannabinoids Δ9-THC and cannabidiol (CBD), the synthetic cannabinoids WIN-55,212-2 and arachidonyl-2′-chloroethylamide (ACEA) used in research studies, and several new synthetic cannabinoids (XLR-11, AM-2201, and 5F-AMB) that are sold in recreational drug markets and used by humans.37–39 Cocaine was included in the study as a positive reinforcer control, to replicate the validity of this procedure. Given that the majority of the cannabinoid compounds exhibit high binding affinities to CB1R and CB2R (Table 1), we used RNAscope in situ hybridization (ISH) and immunohistochemistry (IHC) assays to examine cellular distributions of CB1R and CB2R in different types of neurons in the VTA, including DA, glutamate, and GABA cells, to elucidate the possible cellular targets underlying cannabinoid action. We found that most of the tested cannabinoids produce reward attenuation or aversive effects on oICSS, likely due to the activation of CB1R and CB2R mainly on VTA glutamate and DA neurons.
TABLE 1.
Cannabinoid receptor binding affinities of cannabinoids tested in this study
| Compound | CB1R Ki (nM) | CB2R Ki (nM) | CB1R/CB2R | Reference | Half life (T1/2) | oICSS effecta |
|---|---|---|---|---|---|---|
| Δ9-THC | 35.3 | 3.9 | 9.05 | 40 | ~92–108 min67 | ↓ |
| 39.5 | 40 | 0.99 | 41 | |||
|
| ||||||
| Cannabidiol | 4,350 | 2,860 | 1.52 | 42 | ~120 min 68 | No change |
|
| ||||||
| ACEA | 1.4 | >2,000 | >0.0007 | 43 | N/A | ↓ |
|
| ||||||
| WIN55,212-2 | 9.94 | 16.2 | 0.613 | 40 | 24-36 h 69 | ↓ |
|
| ||||||
| XLR-11 | 24 | 2.1 | 11.4 | 44 | N/A | ↑ |
|
| ||||||
| 5F-AMB | 8.71 | 7.99 | 1.09 | 45 | N/A | No change |
|
| ||||||
| AM-2201 | 1.0 | 2.6 | 0.38 | 46 | 4-6 h 70 | ↓ |
Abbreviations: N/A, not available; oICSS, optogenetic intracranial self-stimulation.
The findings in the present study.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Adult male (n = 18) and female (n = 6) heterozygous DAT-Cre mice, aged 4–24 weeks (~30 g in weight), were used in the oICSS experiments, whereas wildtype (WT), GABA-CB1-KO (Vgat-Cre+/− × CB1flox/flox) and their WT littermates (Vgat-Cre+/−), glutamate-CB1-KO (VgluT2-Cre+/− × CB1flox/flox) and their WT littermates (VgluT2-Cre+/−) with C57BL/6 genetic background were used in the IHC and RNAscope ISH assays.32 Heterozygous DAT-Cre (B6.SJL-Slc6a3tm1.1(Cre)Bkmn/J; stock # 006660), VgluT2-Cre (Slc17a6tm2(cre) Lowl/J, stock # 016963) and Vgat-Cre (B6J.129S6(FVB)-Slc32a1tm2(cre) Lowl/MwarJ, stock# 028862-) knock-in mice were purchased from the Jackson Laboratory. CB1flox/flox mice were provided by Dr Qing-Rong Liu at the National Institute on Aging (NIA), as we reported previously.32 All of the transgenic mice used in this study were bred at the National Institute on Drug Abuse (NIDA) and maintained on a reverse 12 h light–dark cycle (lights off 7:00 a.m./lights on 7:00 p.m.) with food and water available ad libitum. The mutant lines were bred for >10 generations on the background of C57BL/6 mice from Charles River Laboratories (Frederick, MD, USA). Mouse genotyping was performed by Transnetyx, Inc. (Cordova, TN) using reverse transcription quantitative polymerase chain reaction (RT-qPCR) on tail snips. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Research Council and were approved by the NIDA Animal Care and Use Committee.
2.2 |. Surgeries
Mice were anesthetized with ketamine (90 mg/kg, intraperitoneal [ip]) and xylazine (10 mg/kg, ip) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). For intra-VTA microinjection of virus, a custom-made 30-gauge stainless injector was used to infuse Cre-inducible recombinant AAV that encodes ChR2 and enhanced yellow fluorescent protein (EYFP; AAV- EF1α-DIO-ChR2-EYFP; 150 nl, ~2 × 1012 genomes/ml, University of North Carolina Gene Therapy Center) unilaterally into the VTA (AP −3.28; ML 0.43; DV −4.41 mm relative to Bregma) using a micropump (WPI 2000 Ultra-MicroPump, Sarasota, FL, USA) with a speed of 50 nl/min. For oICSS, a custom-built optrode (200-μm multimode optical fiber, Thorlabs, Newton, NJ, USA) tethered to an intracranial ceramic ferrule (MM-FER2007C-2300, Precision Fiber Products, Inc., Milpitas, CA, USA) was implanted into the VTA approximately 1 mm above the AAV injection site. Figure 2A shows a graphic of the approach. To fix the optrode assembly to the skull, two screws were placed toward the front of the skull followed by a small layer of superglue. While the glue was still wet, dental cement was used to fix and seal the assembly together. Once the AAV vector injection and optrode implantation were finished, mice were given at least 4 weeks to recover from the surgery and to enable full AAV expression and ChR2 trafficking (as shown in Figure 1C,D) before oICSS experiments began.34
FIGURE 2.
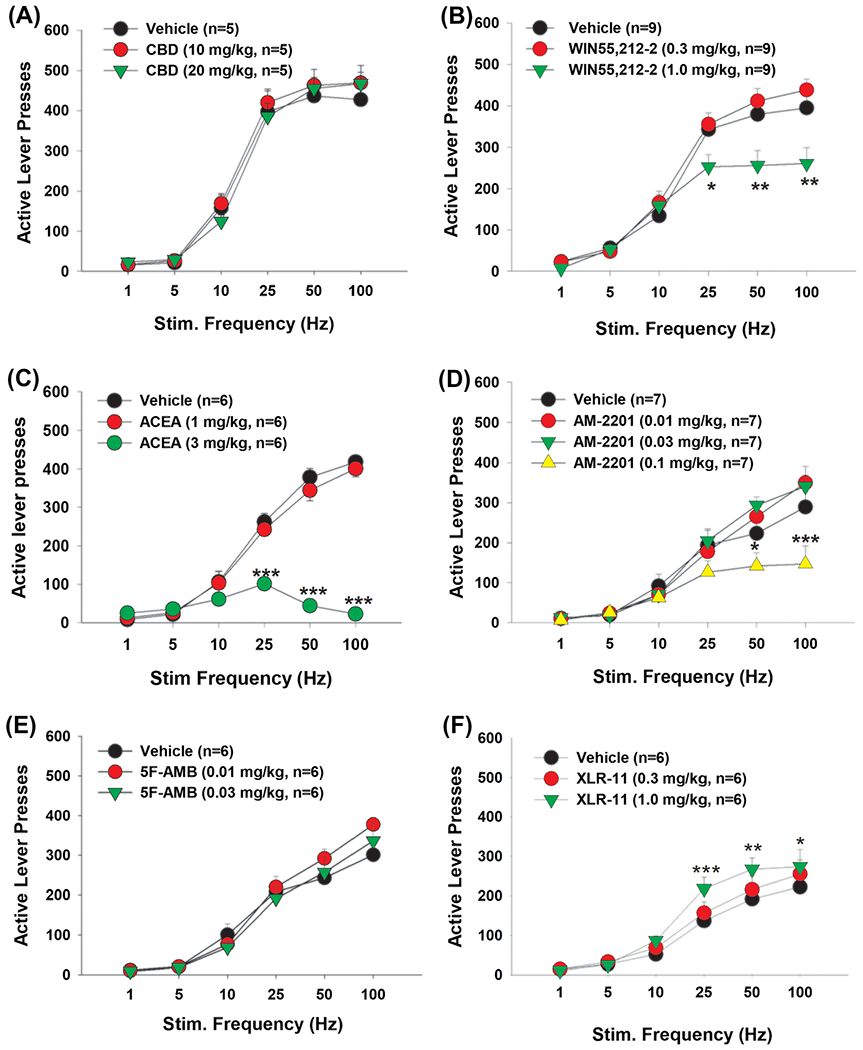
Effects of cannabinoids on optogenetic intracranial self-stimulation (oICSS) in dopamine transporter (DAT)-Cre mice. A, Cannabidiol (CBD) did not show significant effects on oICSS responding. B–D, WIN55,212-2, ACEA, and AM-2201 (respectively) dose-dependently shifted the oICSS curve downward. E, 5F-AMB did not show any significant effects at the current doses. F, XLR-11, at 1.0 mg/kg, significantly shifted the oICSS curve upward. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the vehicle control group
FIGURE 1.
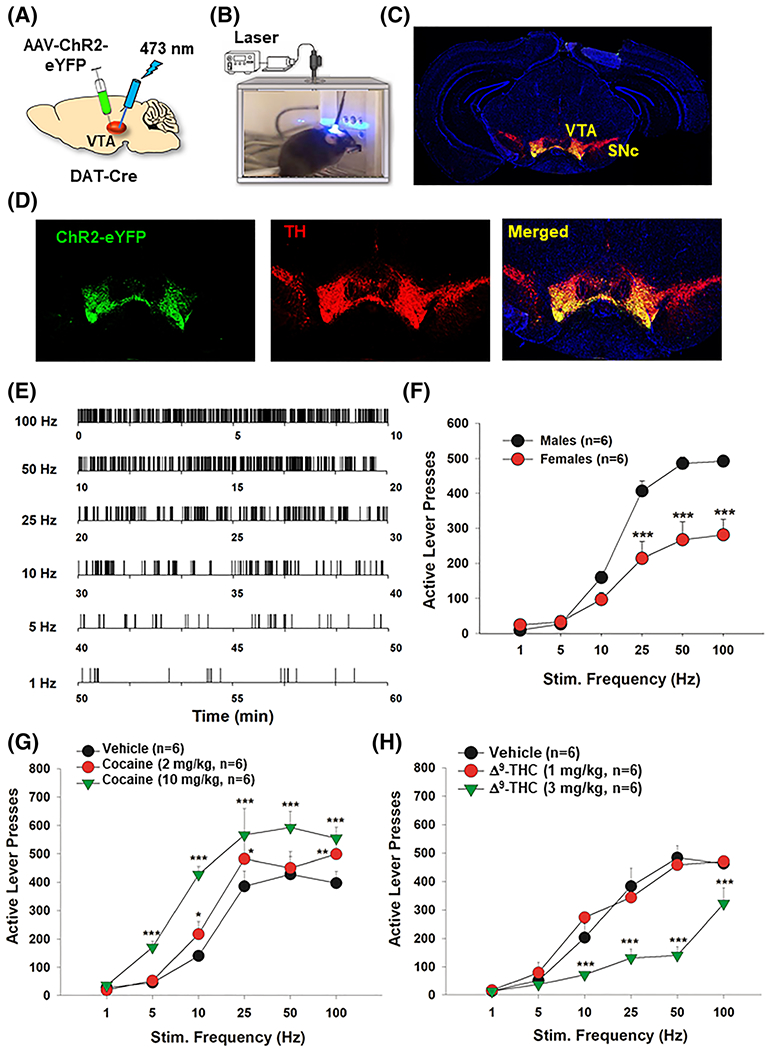
Optogenetic intracranial self-stimulation (oICSS) experiment and the effects of cocaine and Δ9-tetrahydrocannabinol (Δ9-THC) on oICSS in DAT-Cre mice. A, A schematic diagram of the AAV-ChR2-eYFP microinjection and intracranial optical fiber implantation within the ventral tegmental area (VTA) in dopamine (DA) transporter (DAT)-Cre mice. B, Image of the setup of the oICSS experiment. C, Immunostaining of whole brain slice indicating the placement of the AAV-ChR2-EYFP expression in the VTA. D, 20× magnification of the VTA showing ChR2-EYFP expression in VTA TH-positive DA neurons. E, Representative lever responding to different frequencies of laser stimulation in a single session from a single mouse. F, Graph of the lever responding over different frequencies of laser stimulation illustrating the stimulation–response curve in male and female mice. G, Cocaine (10 mg/kg, intraperitoneal [ip]) dose-dependently shifted the oICSS curve upward when compared with vehicle control. H, Δ9-THC dose-dependently shifted the oICSS curve downward. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the vehicle control group
2.3 |. oICSS apparatus
Standard mouse operant conditioning chambers (Med Associates, Fairfax, VT, USA) were used for the oICSS experiments (Figure 1B). Each operant chamber had two wall-mounted levers, two cue lights mounted above the levers, and a house light. Prior to oICSS sessions, each mouse was gently wrapped with a small piece of fabric, and the head mount was connected to a cable linked to an optical swivel (Doric Lenses Inc, Quebec, Canada). The optical swivel was connected to a 473-nm laser (OEM Laser Systems, Inc., Draper, UT, USA) which delivered light pulses in a controlled manner. To control the parameters of the laser stimulation, a customer-programed computer software was used to generate different pulse frequencies.
2.4 |. oICSS procedure
The general procedures for oICSS were modified based on those reported previously32,34 and on eICSS experiments.27 After recovery from surgery, mice were placed into operant chambers for oICSS training. Animals were initially trained on a fixed-ratio 1 (FR1) reinforcement schedule; each active lever response led to delivery of a 1-s pulse train of light stimulation (473 nm, 20 mW, 25 Hz) accompanied by a 1-s illumination of the cue light above the lever. While inactive lever presses were counted, they had no programmed consequence. Each daily training session lasted 60 min.
2.5 |. Rate-frequency oICSS procedure
Following establishment of lever pressing for oICSS, animals were presented with a series of six different stimulation frequencies (100, 50, 25, 10, 5, and 1 Hz) in descending order to obtain rate–frequency response curves (Figures 1E to 2F). Animals were allowed to respond for 10 min per stimulation frequency. We used a within-subjects design, which included three cohorts of mice, each randomly assigned to treatment with the drugs described in Table 2. The ip doses administered and pretreatment times were as follows: cocaine (0, 2, and 10 mg/kg, 5 min prior to testing), Δ9-THC (0, 1, and 3 mg/kg, 30 min prior to testing), CBD (10 and 20 mg/kg, 30 min prior to testing), WIN55,212-2 (0.3 and 1 mg/kg, 30 min prior to testing), ACEA (1 and 3 mg/kg, 30 min prior to testing), XLR-11 (0.3 and 1.0 mg/kg, 30 min prior to testing), AM-2201 (0.01, 0.03, and 0.1 mg/kg, 30 min prior to testing), or 5F-AMB (0.01 and 0.03 mg/kg, 30 min prior to testing). We chose to give cocaine 5 min, not 30 min, prior to the ICSS test because cocaine is a fast-acting DAT inhibitor, which produces an increase in extracellular DA in the nucleus accumbens within a minute after systemic cocaine administration.47 The doses of each compound were chosen based on literature reports or pilot experiments, to ensure that drug treatments did not produce significant sedation or locomotor inhibition. After each test, animals received 2 to 5 days of oICSS re-stabilization until baseline lever responding was re-established. The order of testing for the various doses of the drugs was counterbalanced. Each group of animals received 3–10 drug injections throughout the whole experiment (Table 2).
TABLE 2.
Experimental groups and the drug treatments in each group of mice
| Group # | Treatment | Drug dose (mg/kg) |
|---|---|---|
| 1 (n = 6) | Cocaine (n = 6, males) | 0, 2, 10 |
| Δ9-THC (n = 6, males) | 0.3, 1 | |
| ACEA (n = 6, males) | 1, 3 | |
|
| ||
| 2 (n = 9) | WIN55,212-2 (n = 9, 6 males + 3 females) | 0, 0.3, 1 |
| CBD (n = 5, 3 males + 2 females) | 10, 20 | |
| AM-2201 (n = 7, 5 males + 2 females) | 0.01, 0.03, 0.1 | |
| 5F-AMB (n = 6, 4 males + 2 females) | 0.01, 0.03 | |
|
| ||
| 3 (n = 6) | XLR-11 (n = 6, males) | 0, 0.3, 1 |
Abbreviations: CBD, cannabidiol; Δ9-THC, Δ9-tetrahydrocannabinol.
2.6 |. Sucrose self-administration
To determine whether the reduction in oICSS could be due to nonspecific sedation or locomotor impairment caused by cannabinoids, we examined the effects of Δ9-THC or WIN55,212-2 on operant sucrose self-administration in mice. The procedures for oral sucrose self-administration were the same as we reported previously,34 except that active lever presses under a FR1 reinforcement schedule led to a delivery of 0.08 ml of 5% sucrose solution into a liquid food tray located on the operant chamber wall. An additional group (n = 12) of mice was used for sucrose self-administration training and testing. Sucrose deliveries were capped at 100 per session to prevent food satiation and a reduction in motivation for sucrose-taking. After training, the mice with stable self-administration behavior were selected for testing with vehicle treatment or one of two doses of Δ9-THC (1 or 3 mg/kg, ip, 30 min prior to test) or WIN55,212-2 (1 mg/kg, ip, 30 min prior to test). The treatment was counterbalanced in each mouse, and each test was separated by two additional training sessions on two consecutive days. The total number of sucrose deliveries during the 3-h self-administration session was used to evaluate the effects of Δ1-THC or WIN55,212-2 on sucrose self-administration.
2.7 |. IHC assays
We have previously reported that CB2Rs are expressed in VTA DA neurons,47 but it is unknown whether CB2Rs are also expressed in VTA GABA neurons and glutamate neurons. To further address this issue, we used IHC to examine CB2-immunostaining in these three phenotypes of neurons in mice. The IHC procedures were performed as reported previously.48 Briefly, mice were deeply anesthetized with 100 mg/kg pentobarbital and transcardially perfused with cold saline followed by 4% paraformaldehyde in 0.1-M phosphate buffer. Brain tissue was transferred to 20% sucrose in phosphate buffer at 4°C overnight. Coronal sections were cut at 25 μm on a cryostat (CM3050S, Leica Microsystems Nussloch GmbH, Nussloch, Germany). Tissue sections containing the VTA were blocked and floated in 5% bovine serum albumin and 0.5% Triton X-100 phosphate buffer for 2 h at room temperature. Dual-labeling IHC was performed using a CB2R antibody (Alomone, #ACR-002, 1:250) and an anti-tyrosine hydroxylase (anti-TH) monoclonal antibody (1:500; Millipore, Billerica, MA, USA). Sections were washed and incubated with a mixture of secondary antibodies, goat anti-rabbit Alexa 488 for CB2R, and goat anti-mouse Alexa 568 for TH (Millipore, #MAB318; 1:500), vGluT2 (Abcam, #ab79157, 1:500), or GAD67 (Abcam, #ab26116,1:500) in 5% bovine serum albumin and 0.5% Triton X-100 phosphate buffer for 2 h at room temperature. Sections were washed, mounted, and cover slipped. Fluorescent images were captured using a fluorescence microscope (Nikon Eclipse 80i) equipped with a digital camera (Nikon Instruments Inc., Melville, NY, USA). All images were captured under identical optical conditions.
2.8 |. RNAscope ISH
Using IHC methods, we have previously reported that CB1R-immunostaining was undetectable in the cell bodies of VTA DA neurons.32 To further determine the cellular distributions of CB1 receptor within the VTA, in this study, we used a highly sensitive RNAscope ISH assay to examine CB1R mRNA expression in VTA DA neurons, glutamate neurons, and GABA neurons. Mice were deeply anesthetized, and the whole brain was removed and rapidly frozen on dry ice. Fresh-frozen tissue sections (14 μm thick) were mounted on positively charged microscopic glass slides (Fisher Scientific) and stored at −80°C until RNAscope ISH assays could be performed. Multiple target gene-specific RNAscope probes were designed and provided by Advanced Cell Diagnostics (Newark, CA, USA). The riboprobes were used to observe the cellular distributions of cannabinoid receptor mRNA in VgluT2-expressing glutamate neurons, TH-expressing DA neurons, and GAD1-expressing GABAergic neurons, including the CB1R RNAscope probe (Cat #: 420721, targeting 530–1,458 bp of the mouse Cnr1 mRNA sequence, NM_007726.3), the VgluT2 RNAscope probe (Cat #: 319171-C3, targeting 1,986–2,998 bp of the Mus musculus VgluT2 mRNA sequence, NM_080853.3), the TH-specific RNAscope probe (Cat #: 317621-C2, targeting 483–1,603 bp of the Mus musculus TH mRNA sequence, NM_009377.1), and the GAD1-specific RNAscope probe (Cat#: 400951-C3, targeting 62–3,113 bp of NM_008077.4). The RNAscope mRNA-staining steps were performed following the manufacturer’s protocols. Stained slides were cover-slipped with fluorescent mounting medium (ProLong Gold Anti-fade Reagent P36930; Life Technologies) and scanned into digital images with an Olympus FluoView FV1000 confocal microscope at 40× or 60× magnification using manufacturer-provided software.
2.9 |. Drugs
Cocaine HCl was provided by the NIDA IRP Pharmacy. Δ9-Tetrahy-drocannabinol (Δ9-THC) and CBD were provided by NIDA Drug Supply Program. WIN55,212-2 and ACEA (arachidonyl-2′-chloroethylamide) were purchased from Sigma-Aldrich. AM-2201 [1-(5-fluoropentyl)-1H-indol-3-yl](1-naphthyl)methanone, 5F-AMB (methyl 2-(1-(5-fluoropentyl)-1H-indazole-3-carboxamido)-3-methylbutanoate), and XLR-11 (5″-fluoro-UR-144 or 5F-UR-144) were provided by Dr Michael H. Baumann at the NIDA IRP. The stock solution of Δ9-THC was 50 mg/ml (w/v) diluted in 100% ethanol, which was evaporated prior to dilution for injections. The vehicle used to prepare these cannabinoids was 5% Cremophore (C5135, Sigma-Aldrich) in sterile saline.
2.10 |. Data analysis
Data are available on request due to privacy/ethical restrictions. All data are presented as mean ± SEM. Data analysis was performed with SigmaPlot 12.0 (Systat Software, Inc. San Jose, CA, USA). Two-way ANOVA with repeated measures for drug dose and stimulation frequency were used to analyze the significance of the effects after each drug treatment. Post-hoc individual group comparisons were made using the Student–Newman–Keuls method. A value of p < 0.05 was chosen as the minimum criterion for statistical significance.
3 |. RESULTS
3.1 |. Cocaine enhances oICSS
Figure 1 shows the general experimental procedures (Figure 1A,B), representative AAV-ChR2-eYFP expression in the midbrain (Figure 1C) and in DA neurons in the VTA (Figure 1D). Examples of lever response patterns to different frequencies of laser stimulation are shown in Figure 1E, indicating that mice readily responded for optogenetic stimulation of VTA DA neurons in a frequency-dependent manner (Figure 1E,F). We first compared oICSS responses between males and females. We found that male mice showed more robust responses than females (Figure 1F). Two-way ANOVA revealed a significant sex main effect (F1,10 = 14.54, p < 0.01), stimulation frequency main effect (F5,50 = 140.09, p < 0.001), and a sex × frequency interaction (F5,50 = 14.60, p < 0.001). Based on this finding, we mainly used male mice (n = 18) in the following pharmacological experiments, only three females showing the same robust oICSS responses as males were included in one group of mice (Table 2).
We have previously reported that cocaine dose-dependently enhances BSR using eICSS.27,49 Therefore, in the present study, we determined whether cocaine produces a similar effect in oICSS. Consistent with our previous finding with eICSS,27,49 systemic administration of cocaine produced a significant dose-dependent increase in the number of lever responses for oICSS and upward shifted the stimulation–response curve compared with vehicle (Figure 1G). Two-way ANOVA with repeated measures for cocaine dose and stimulation frequency revealed significant main effects for cocaine treatment (F2,10 = 64.13, p < 0.001) and frequency (F5,25 = 21.2, p < 0.001), and a dose × frequency interaction (F10,50 = 6.7, p < 0.001). Post-hoc testing revealed that 2 and 10 mg/kg cocaine significantly increased active lever responding at the 10-, 25-, and 100-Hz frequencies (p < 0.05). The 10 mg/kg cocaine dose also significantly increased oICSS responding at 5 and 50 Hz (p < 0.001).
3.2 |. Δ9-THC decreases oICSS
To determine whether Δ9-THC, the major psychoactive component in cannabis, is rewarding or aversive, we pretreated mice with one of two doses of Δ9-THC prior to oICSS sessions. Contrary to the effects of cocaine, Δ9-THC significantly inhibited DA-dependent oICSS responding and dose-dependently shifted the oICSS curve downward (Figure 1H). Two-way ANOVA with repeated measures for Δ9-THC dose and stimulation frequency revealed a significant main effect of dose (F2,10 = 36.23, p < 0.001), frequency (F5,25 = 112.09, p < 0.001), and a dose × frequency interaction (F10,50 = 7.12, p < 0.001). Post-hoc testing indicated that compared with vehicle treatment, 3 mg/kg Δ9-THC significantly reduced oICSS responding at the 10-, 25-, 50-, and 100-Hz frequencies (p < 0.001).
3.3 |. CBD has no effects on oICSS
Next, we examined the impact of CBD, a well-characterized non-psychomimetic component of cannabis with no reported abuse potential,50 on oICSS responding. We found that neither 10 nor 20 mg/kg CBD significantly shifted the oICSS curve compared with vehicle treatment (Figure 2A). Two-way ANOVA did not reveal a significant main effect of CBD treatment (F2,8 = 3.75, p > 0.05) or a dose × frequency interaction (F10,40 = 1.075, p > 0.05), suggesting that CBD is neither rewarding/reinforcing nor aversive/reward attenuating.
3.4 |. WIN55,212-2 inhibits oICSS
We then tested the effects of two synthetic cannabinoids (WIN55,212-2 and ACEA) that are used as tools in preclinical laboratory research. Consistent with Δ9-THC’s effects, systemic administration of WIN55,212-2 significantly shifted the oICSS curve downward in a dose-dependent manner (Figure 2B). Two-way ANOVA with repeated measures for dose and stimulation frequency revealed a significant WIN55,212-2 treatment main effect (F2,14 = 4.32, p < 0.05), a significant frequency main effect of (F5,40 = 198.05.1, p < 0.001) and a significant treatment × frequency interaction (F10,80 = 6.62, p < 0.001). Post-hoc testing indicated that the 1.0 mg/kg dose significantly reduced responding for the 25-, 50-, and 100-Hz frequencies compared with vehicle (p < 0.01).
3.5 |. ACEA inhibits oICSS
ACEA is a highly selective CB1R agonist with >1,400-fold selectivity for CB1R (Ki = 1.4 nM) over CB2R (Ki ≥ 2,000 nM).43 Unexpectedly, systemic administration of ACEA also inhibited oICSS in DAT-Cre mice in a dose-dependent manner (Figure 2C). Two-way ANOVA with repeated measures for dose and frequency revealed significant main effects of ACEA dose (F2,8 = 135.7, p < 0.001), frequency (F5,20 = 70.2, p < 0.001), and a dose × frequency interaction (F10,40 = 40.3, p < 0.001). Post-hoc analysis indicated a significant reduction in oICSS after 3 mg/kg ACEA when the stimulation frequency was 25, 50, or 100 Hz (p < 0.001).
3.6 |. AM-2201 inhibits oICSS
AM-2201 is a naphthoylindole synthetic cannabinoid that binds with high affinity to both CB1R (Ki = 1.0 nM) and CB2R (Ki = 2.6 nM)51 and produces cannabimimetic effects similar to Δ9-THC.52 In the present oICSS assays, systemic administration of AM-2201 also produced a reduction in oICSS and shifted the stimulation–response curve downward (Figure 2D). Two-way ANOVA with repeated measures for dose and frequency revealed significant main effects of AM-2201 treatment (F3,18 = 4.13, p < 0.05), frequency (F5,30 = 78.33, p < 0.001), and a dose × frequency interaction (F15,90 = 5.86, p < 0.001). Post-hoc testing indicated that, compared with vehicle, the 0.1 mg/kg dose of AM-2201 suppressed oICSS responding at the 50- and 100-Hz frequencies (p < 0.05).
3.7 |. 5F-AMB fails to alter oICSS
5F-AMB is a newer indazole-based synthetic cannabinoid that is abused in the United States and elsewhere.53 The agonistic activity of 5F-AMB at CB1R is reportedly 90 times more potent than Δ9-THC.37 Compared with other cannabinoids, 5F-AMB appeared very toxic. At the doses of 0.1 mg/kg or above, it produced significant sedation or locomotor impairment. Therefore, a very lower dose range of 5F-AMB was used in this experiment. Systemic administration of 5F-AMB, at the doses of 0.01–0.03 mg/kg, did not significantly alter oICSS responding compared with vehicle (Figure 2E). Two-way repeated measures ANOVA did not reveal significant main effects of treatment (F2,10 = 0.13, p > 0.05) or a dose × frequency interaction (F10,50 = 0.36, p > 0.05).
3.8 |. XLR-11 potentiates oICSS
XLR-11 is another new synthetic cannabinoid with higher affinity for CB2R (Ki = 2.1 nM) over CB1R (Ki = 24 nM).44 Unexpectedly, pretreatment with XLR-11 produced a modest cocaine-like enhancement of oICSS responding and shifted the oICSS curves upward (Figure 2F). Two-way repeated measures ANOVA revealed a significant dose × frequency interaction (F10,50 = 3.1, p < 0.01). Post-hoc testing revealed that, compared with vehicle, the 1 mg/kg dose of XLR-11 increased oICSS responding for the 25-, 50-, and 100-Hz frequencies (p < 0.05).
3.9 |. Δ9-THC or WIN55,212-2 does not alter oral sucrose self-administration
To determine whether the reduction in oICSS was due to non-specific sedation or locomotor inhibition after cannabinoid administration, we evaluated the effects of the same doses of Δ9-THC and WIN55,212-2 on operant lever responding for sucrose self-administration. We found that Δ9-THC, at 1 or 3 mg/kg, failed to alter sucrose self-administration as assessed by the total number of sucrose deliveries per session (Figure 3A, F2,10 = 2.34, p = 0.124) or by percentage changes in lever response over baseline (Figure 3B, F2,10 = 2.23, p = 0.156). Similarly, pretreatment with WIN55,212-2, at the dose (1 mg/kg) that inhibits oICSS, also failed to alter sucrose self-administration (Figure 3C, F2,12 = 1.44, p = 0.274; Figure 3D, F2,12 = 1.95, p = 0.185).
FIGURE 3.
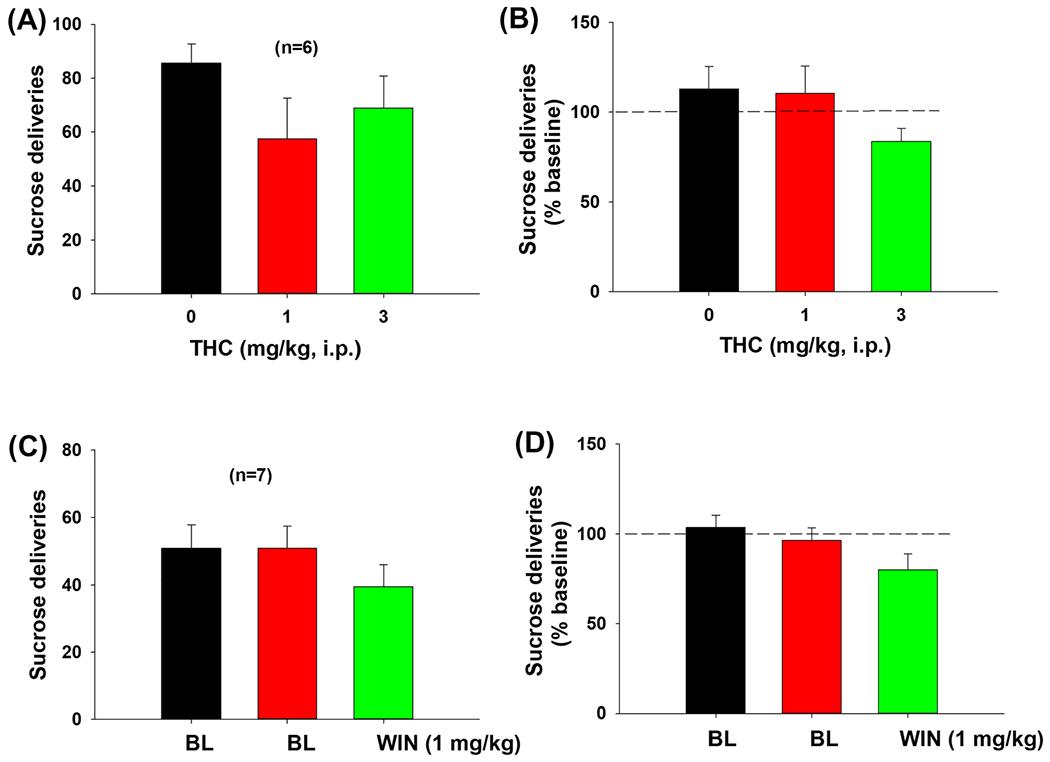
Impact of Δ9-tetrahydrocannabinol (Δ9-THC) and WIN55,212-2 on oral sucrose self-administration in mice. Pretreatment with Δ9-THC A,B, or WIN55,212-2 C,D, did not alter oral sucrose self-administration
3.10 |. CB1R mRNA is found in VTA GABA and glutamate neurons
We have previously reported that CB1R-immunostaining is detected mainly in cell membranes or never fibers but not in the cell bodies of VTA DA neurons.32 To further determine which cell types in the VTA express CB1R, here we used RNAscope ISH assays to examine the cellular distributions of CB1R mRNA in the VTA. In contrast to the findings in the IHC assays,32 CB1R mRNA (green) was detected the cell bodies of neurons in the VTA (Figure 4). Triple-staining RNAscope assays for labeling CB1R, TH, and GAD1 mRNA indicate that CB1R mRNA is not co-localized with TH mRNA (Figure 4A), but co-localized with GAD1 mRNA in VTA GABA neurons (Figure 4A). Selective deletion of CB1R from GABA neurons in GABA-CB1-KO mice almost completely abolished CB1 mRNA-staining (Figure 4B), suggesting that the detected mRNA signal is CB1-specific. Quantitative assays show that ~60% (57.78 ± 6.26%, from 251 GABA neurons in three mice) GABA neurons in the VTA express CB1 mRNA.
FIGURE 4.
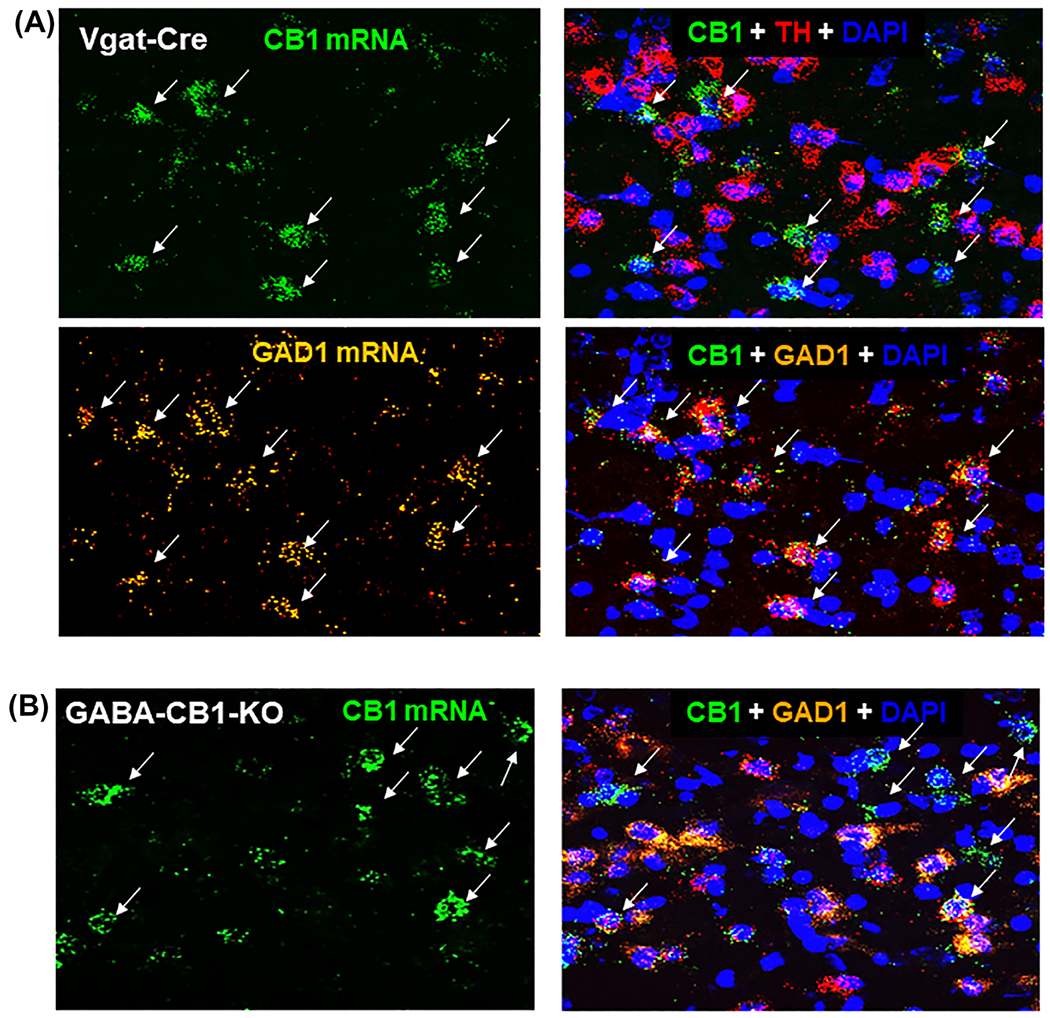
The cellular distributions of CB1 mRNA in the ventral tegmental area (VTA) by RNAscope in situ hybridization (ISH) assays. Triple-staining for CB1, TH, and GAD1 mRNA indicates that high densities of CB1R mRNA (green, arrows) were not co-localized with TH mRNA in VTA DA neurons (red), but co-localized with GAD1 mRNA in GABA neuron (orange, arrows) in Vgat-Cre mice A. Selective deletion of CB1 receptors from GABA neurons abolished CB1 mRNA-staining in the VTA of GABA-CB1-KO mice B. TH, Tyrosine hydroxylase; GAD1, glutamic acid decarboxylase 1; DAPI, 4′,6-diamidino-2-phenylindole, a fluorescent dye that binds to DNA as a marker of cell nuclei
We also examined CB1 mRNA in Vglut2-positive glutamate neurons in the midbrain, which were found mainly in the medial VTA (close to the midline). We found that CB1 mRNA is also co-localized with VgluT2 mRNA in VgluT2-Cre mice, but not in glutamate-CB1-KO mice (Figure 5), a finding similar as we reported previously.32
FIGURE 5.
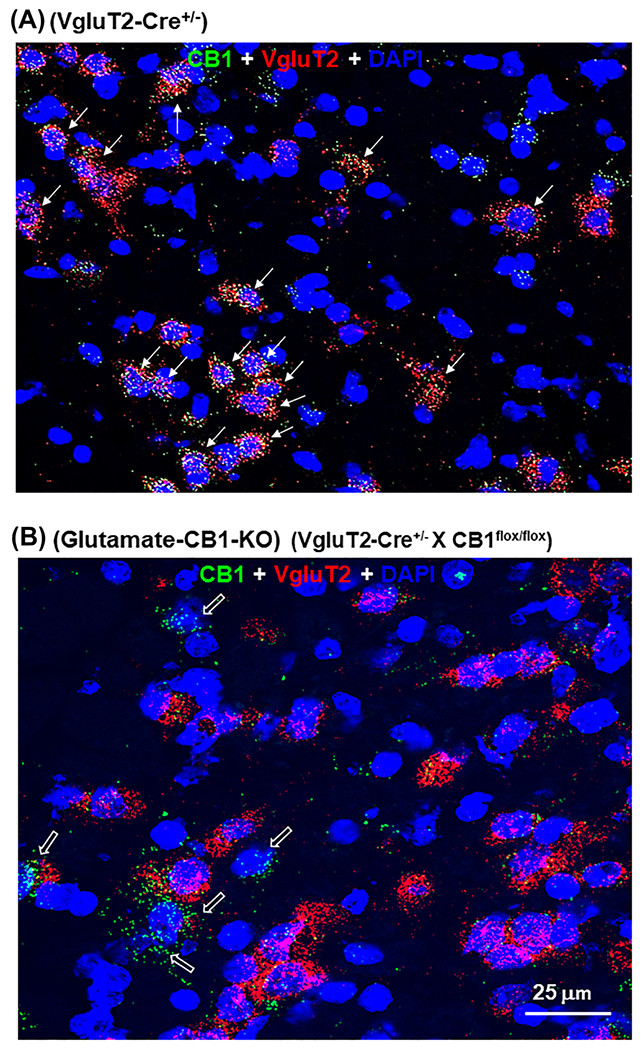
CB1 mRNA expression in glutamate neurons in the ventral tegmental area (VTA) by RNAscope in situ hybridization (ISH) assays. CB1R mRNA (green, arrows) was co-localized with VgluT2 mRNA (red) in glutamate neurons (red, arrows) in VgluT2-Cre mice A, but not in glutamate-CB1-KO mice B. CB1 mRNA was still detectable in other non-glutamate (VgluT2-negative, open arrows) neurons in the VTA in glutamate-CB1-KO mice B
3.11 |. CB2R is found mainly in VTA DA neurons, not in VTA GABA or glutamate neurons
Lastly, we used the IHC assays as we reported previously to examine the cellular distributions of CB2R in VTA DA neurons, GABA neurons, and glutamate neurons.47 We found that CB2R-immunostaining was expressed in ~90% (88.69 ± 2.65%, from 1,401 DA neurons in four mice; Figure 6A) of DA neurons, but not in VgluT2-positive glutamatergic neurons (Figure 6B) or GAD67-labeled GABAergic neurons (Figure 6C).
FIGURE 6.
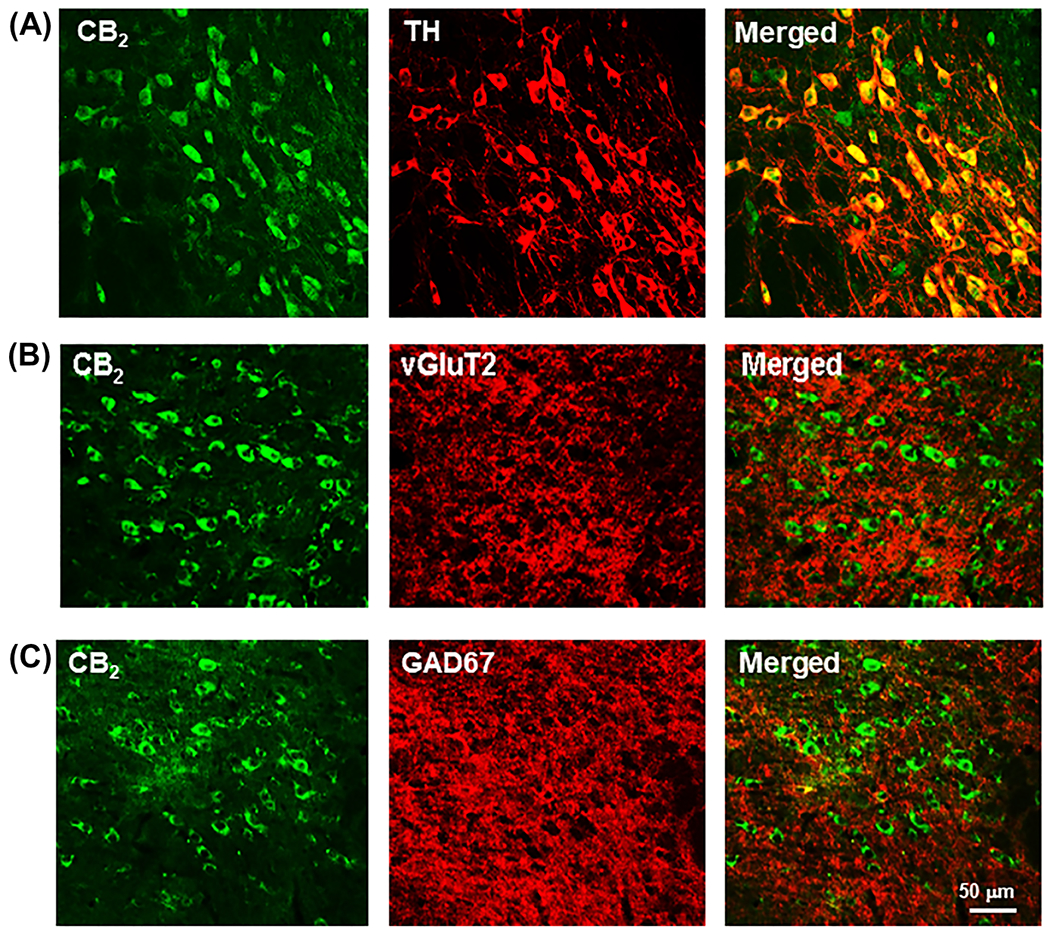
CB2R-immunostaining in different phenotypes of neurons in the ventral tegmental area (VTA), illustrating CB2R-immunostaining in VTA TH-positive DA neurons A, but not in VTA vGluT2-positive glutamate neurons B or GAD67-positive GABA neurons C. BL, baseline responding in the absence of drug treatment
4 |. DISCUSSION
The major findings from this study include (1) multiple cannabinoids (Δ;9-THC, WIN55-212,2, ACEA, and AM-2201) induce suppression of oICSS responding maintained by VTA DA neuron activation, suggesting reward-attenuating or aversive effects; (2) XLR-11 is the only cannabinoid that produced a cocaine-like enhancement of oICSS responding, suggesting possible rewarding or reward-enhancing effects; (3) CBD and 5F-AMB did not significantly impact oICSS, suggesting that these compounds lack rewarding or reinforcing effects at the drug doses tested; and (4) CB1R and CB2R exhibit different cellular distributions. CB1R mRNA is highly expressed in VTA GABA and glutamate neurons, not in DA neurons, while selective deletion of CB1 receptor from GABA neurons or glutamate neurons abolished CB1 mRNA in VTA GABA or glutamate neurons, respectively. In contrast, CB2Rs are expressed mainly in VTA DA neurons, not in VTA GABA or glutamate neurons. Together, these findings suggest that most cannabinoids are not rewarding or reward enhancing, but aversive or reward attenuating, and activation of CB1 and CB2 receptors in multiple types of neurons may underlie cannabinoid action on BSR.
Electrical ICSS (eICSS) is a commonly used behavioral procedure to study brain reward function both in rats27,35,36,49,54 and mice.55 In this procedure, animals respond for brief electrical pulses to the medial forebrain bundle via an implanted electrode. Drugs of abuse, such as cocaine and amphetamine, cause a decrease in the stimulation threshold for electrical brain-stimulation reward (BSR) and shift the stimulation–response curve leftward or upward immediately after acute administration, indicating enhanced BSR and a summation between BSR and drug reward.27,35,36,55–57 Similarly, systemic administration of GBR12935 (a selective DAT inhibitor) or SKF82958 (a DA D1R-like agonist) also produced a dose-dependent decrease in BSR threshold and a leftward or upward shift of the eICSS curve.55,58 This effect was potent immediately after acute drug administration. Furthermore, a subthreshold effective dose of SKF-82958 potentiated the rewarding effects of low doses of cocaine. Repeated administration of cocaine or SKF82958 did not cause progressive changes in their ability to decrease BSR thresholds.55 These findings suggest that cocaine, DAT inhibitors, or D1R agonists each potentiate the rewarding effects of eICSS and imply that these drugs have rewarding effects of their own. In contrast, withdrawal from chronic cocaine or amphetamine administration is associated with depression-like effects and deficits in brain reward function, as assessed by BSR threshold elevation or a rightward/downward shift of eICSS.56–59 Based on these findings, if a test drug produces a cocaine-like leftward or upward shift in ICSS curve, we interpret the drug to be rewarding or reward enhancing. In contrast, if a drug, such as Δ9-THC, produces an opposite rightward or downward shift in ICSS curve, it is often interpreted as having reward attenuation or aversive effects.27,32
We note that we didn’t measure oICSS threshold (θ0) in a way similar as to that used previously in eICSS. However, the lack of θ0 does not affect the conclusions based on the ICSS curve shift assays in this study because both θ0 and ICSS curve shift describe the same drug effects. In addition to θ0, many other measures such as M25, M50, and M75 are also used in eICSS assays. We did not apply θ0 analysis in this assay because of a technical reason. In eICSS, we used 16 different electrical pulse frequencies ranging from 141 to 25 Hz to generate a stimulation–response curve, which allowed us to accurately calculate θ0 using best-fit mathematical algorithms as reported previously. 27 In the present oICSS study, we can only generate six different laser pulse frequencies ranging from 1 to 100 Hz using the currently available laser stimulators to establish a stimulation–response curve. Thus, more efforts are needed to optimize the oICSS procedure, particularly, by increasing the range of laser stimulation frequencies.
As stated above, the effects of cannabinoids on eICSS behavior are mixed. In some studies, Δ9-THC produced enhanced BSR,20,21 while in others, Δ9-THC and other cannabinoid receptor agonists reduced electrical BSR,26,60,61 or had no effect.24 In a number of investigations which examined dose–response relationships, cannabinoids produced biphasic effects such that lower doses were rewarding, while higher doses were aversive.27 The reasons underlying these disparate findings are unclear. One possible explanation is that electrical pulses into the brain may nonspecifically stimulate multiple types of neurons or nerve fibers and therefore complicate data interpretations. To overcome this limitation, we recently established a new animal procedure called oICSS to re-evaluate cannabinoid-induced rewarding versus aversive effects. In this procedure, animals press a lever to earn laser pulses that selectively stimulate VTA DA neurons33,34 or glutamate neurons32 via an optrode implanted. Consistent with findings using eICSS,27,36,49 we found that cocaine produced a significant increase in oICSS responding and shifted the stimulation–response curve leftward or upward. It is also in accordance with our previous finding that cocaine enhances oICSS maintained by photostimulation of VTA glutamate neurons.32 In contrast to cocaine, opioids such as oxycodone produce dose-dependent biphasic effects — low doses enhance and high doses reduce oICSS in DAT-Cre mice.34 These findings suggest that this new oICSS procedure is reliable in its ability to predict the rewarding versus aversive effects and abuse potential of psychotropic drugs as we discussed above in detail.
Compared with eICSS, the oICSS procedure has several advantages. First, the neurobiological basis of the behavior is clear, that is, it is DA neuron-specific or glutamate neuron-specific, depending upon the transfected cell type.32–34 Second, laser stimulation is safer than electrical stimulation for in vivo experiments. Little evidence indicates that laser stimulation (10–20 mW) of ChR2-expressing neurons leads to cell death.30 Third, oICSS responding that is driven by optogenetic stimulation of VTA DA neurons is more robust and stable over time than eICSS. Mice quickly learn to lever press for oICSS, and once they have acquired the behavior, responding may last up to 5 months, whereas eICSS behavior in rats usually lasts 1–2 months, based on our many years of experience.27,32–34,49,68 Lastly, oICSS appears to be more sensitive than eICSS in detecting subtle changes in BSR and enables the testing of multiple drugs in the same subjects (with appropriate washout periods), with the added benefit of reducing animal numbers. Therefore, oICSS could be especially suitable for screening a large number of compounds for abuse potential.
Using this oICSS procedure, we found, unexpectedly, that males responded more robustly than females, suggesting that males may be more sensitive to laser stimulation of VTA DA neurons. The mechanisms underlying such a sex difference are not fully understood. It is likely that midbrain DA neurons in females are less sensitive to laser stimulation. It is well documented that female rats acquired cocaine (heroin or nicotine) self-administration more rapidly than males and consumed significantly greater amounts of cocaine, heroin, or nicotine than did males under the same experimental conditions.62–64 The neural mechanisms underlying sex differences in drug intake are not fully understood. A prevailing hypothesis is that estrogen effects on the mesolimbic DA systems may underlie such sex differences in drug-taking behavior.64–66 Accordingly, such an estrogen-related mechanism may also explain why females respond less for VTA DA neuron stimulation. Whatever the mechanisms, the present finding suggests that increased drug intake observed in females might be a compensatory response to blunted DA neuron responses to drugs of abuse.
We used this new oICSS to re-evaluate the rewarding versus aversive effects of various cannabinoids in DAT-Cre mice. The most commonly used cannabinoids, such as Δ9-THC and WIN55,212-2, as well as synthetic cannabinoids such as AM-2201 and ACEA, all produced significant and dose-dependent reductions in oICSS maintained by stimulation of VTA DA neurons. This finding is consistent with our recent report that Δ9-THC also dose-dependently inhibits oICSS maintained by stimulation of VTA glutamate neurons in VgluT2-Cre mice.32 Similarly, beta-caryophyllene (BCP), a dietary terpenoid with CB2R agonist profile,67 inhibits oICSS maintained by optical stimulation of VTA DA neurons.68 The reduction in oICSS is unlikely due to non-specific locomotor impairment because the same drug doses did not produce sedation or locomotor inhibition based on pilot observations and previous reports.27 In addition, Δ9-THC or WIN55,212-2, at doses equal to or higher than those affecting oICSS, failed to alter lever responding for sucrose reward. Together, these findings suggest that the most commonly used cannabinoids (Δ9-THC and WIN55,212-2) and the synthetic cannabinoids ACEA and AM-2201 are not rewarding, but reward attenuating or aversive in experimental animals.
In contrast to the above findings, XLR-11 is the only cannabinoid tested herein that caused cocaine-like reward-enhancing effects in the oICSS procedure. While the effects of XLR-11 on oICSS were modest, they suggest that this new synthetic cannabinoid may have higher abuse potential than other cannabinoid compounds. XLR-11 was first identified as a constituent in herbal smoking mixtures that were sold under a variety of brand names.69 Previous studies show that XLR-11 displays similar or greater potency than Δ9-THC in rats and mice.38,44 In vivo, XLR-11 also produces Δ9-THC-like effects in rodents that were attenuated by rimonabant, a CB1R antagonist.44 The available epidemiological evidence also suggests that XLR-11 displays abuse liability in humans.69 As such, XLR-11 has been banned in many countries and has been listed as a controlled substance (Schedule I) in the United States since 2013.
Notably, CBD and 5F-AMB did not show significant effects on oICSS responding. These findings support the increasingly accepted view that CBD is neither rewarding/reward enhancing nor aversive or reward attenuating in both humans and experimental animals.41,70 5F-AMB is a designer CB1R agonist sold recently for recreational use in humans.71 5F-AMB has a much higher potency for CB1R (Ki = 8.71 ± 0.04 nM) and CB2R (7.99 ± 0.13 nM) than Δ9-THC.37,45 In humans, inhalation of 5F-AMB causes adverse effects such as impaired memory, loss of consciousness, and catalepsy with muscle rigidity.72 In the present study, we found that 5F-AMB is neither reward enhancing nor reward attenuating at the doses of 0.01–0.03 mg/kg, though higher doses could not be tested because they caused significant locomotor impairment.
The neural mechanisms through which cannabinoids enhance or inhibit DA-dependent oICSS are not fully understood. There are several possible explanations. First, the rewarding versus aversive effects of cannabinoids may depend on drug doses, such that low doses are rewarding while high doses are aversive, as sometimes reported for eICSS.27 However, the present findings do not support this hypothesis since we did not observe such biphasic effects on oICSS for any compound tested herein. A second possibility is that the reward-enhancing versus reward-attenuating effects of cannabinoids may be related to their binding affinities or efficacies at CB1R and CB2R. This possibility seems unlikely, because there appears to be no correlation between the in vivo effects in oICSS and in vitro binding and efficacy data (Table 1). For example, Δ9-THC is a partial agonist at CB1R and CB2R with Ki values of 35.3–39.5 nM for rat CB1R and 3.9–40 nM for rat CB2R,40,73 while WIN55,212-2 is a full agonist at CB1R (Ki = 9.94 nM) and CB2R (16.2 nM).40 Yet, Δ9-THC appears to be more effective than WIN55,212-2 in attenuating oICSS. Similarly, AM-2201 is a potent full agonist at CB1R and CB2R (with Ki = 1.0 nM at CB1R and Ki = 2.6 nM at CB2R),46,74 while ACEA is a potent and selective CB1R agonist (with Ki = 1.4 nM for CB1R and Ki ≥ 2,000 nM for CB2R; see Table 1). AM-2201 is not more potent than ACEA in suppressing oICSS responding.
A third possible explanation is that the rewarding or reward-enhancing versus aversive or reward-attenuating effects of cannabinoids could depend on cannabinoid actions at multiple neuronal subtypes or in different neural circuits. We have previously reported that CB1Rs expressed in glutamate neurons are involved in Δ9-THC-induced aversion,32 and CB2Rs are mainly expressed in VTA DA neurons.48,75 In the present study, we did not detect obvious CB1 mRNA expression in VTA DA neurons but detected significant CB1 mRNA expression in ~60% VTA GABA, while CB2Rs are detected in ~90% VTA DA neurons, but not in VTA GABA or glutamate neurons. Given that VTA DA neurons receive both excitatory glutamatergic and inhibitory GABAergic inputs,34,41 we hypothesized that cannabinoid binding to CB1R and CB2R on midbrain DA, glutamate, and GABA neurons may together underlie cannabinoid action observed in the present study (Figure 7). More specifically, CB1R activation on VTA GABA neurons could mediate cannabinoid reward via GABA-mediated DA neuronal disinhibition.5,6 By contrast, activation of CB1R on VTA glutamate neurons and CB2R on VTA DA neurons could produce reward attenuation (or aversion) by decreased glutamatergic inputs to DA neurons and inhibition of DA neurons, respectively.32,41,48,76 Thus, the unique behavioral effects produced by each cannabinoid may depend on the balance of both opposite actions mediated by distinct cell types (Figure 7). The discovery of CB1R expression in glutamate neurons and CB2R expression in VTA DA neurons may help explain why most of the cannabinoids we tested are not rewarding, but aversive. Namely, activation of both receptors in DA and glutamate neurons should theoretically inhibit VTA DA neurons and therefore suppress DA-mediated BSR. We note that this hypothesis may not explain well why XLR-11 was oICSS enhancing, because XLR-11 displays higher affinity to CB2Rs. One possible explanation is that XLR-11 may have other off-targets that regulate the mesolimbic DA system.
FIGURE 7.
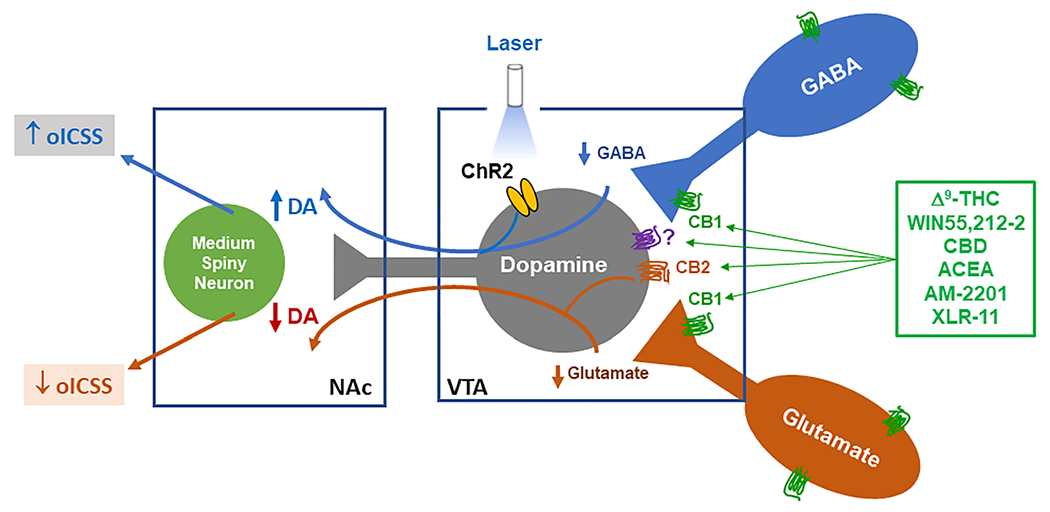
Diagram summarizing CB1R and CB2R expression in VTA dopaminergic (DA), glutamatergic, and GABAergic neurons. Cannabinoids may bind to CB1R in GABAergic neurons, producing rewarding or reward-enhancing effects. Conversely, cannabinoids may also bind to CB1R on VTA glutamate neurons or glutamatergic afferents, or to CB2R on DA neurons, producing aversive or reward-attenuating effects. The final subjective effect depends on the balance of both opposite actions. NAc, nucleus accumbens; oICSS, optogenetic intracranial self-stimulation; VTA, ventral tegmental area
In conclusion, we used a new animal procedure of oICSS to systematically re-evaluate the rewarding versus aversive effects of multiple phytocannabinoids and synthetic cannabinoids on responding maintained by stimulation of VTA DA neurons. We found that most cannabinoids (including Δ9-THC, WIN55212-2, AM-2201, and ACEA) are not reward enhancing, but aversive or reward attenuating in experimental animals. Although the receptor mechanisms underlying this effect require further study, it is likely that cannabinoid reward versus aversion is mediated by combined actions at CB1R and CB2R in distinct neuronal populations with different phenotypes, including DA, glutamate, and GABA neurons in the VTA. Importantly, oICSS reliably predicts the rewarding effects of cocaine. As such, the oICSS procedure may be a valuable tool for screening novel compounds for their abuse potential, to address the current surge in new synthetic psychostimulants, opioids, and cannabinoids in recreational drug markets worldwide.
ACKNOWLEDGEMENTS
This research was supported by the NIDA IRP (Z1A DA000633-01). All rights are reserved by the NIH.
Funding information
NIDA IRP, Grant/Award Number: DA000633-01
Footnotes
CONFLICT OF INTEREST
None of the authors have any conflicts of interest.
REFERENCES
- 1.Grigsby TM, Hoffmann LM, Moss MJ. Marijuana use and potential implications of marijuana legalization. Pediatr Rev. 2020;41(2):61–72. [DOI] [PubMed] [Google Scholar]
- 2.National Institute on Drug Abuse. What is the scope of marijuana use in the United States? https://www.drugabuse.gov/publications/research-reports/marijuana/what-scope-marijuana-use-in-united-states. Published 2020. Accessed May 30, 2020.
- 3.Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiat. 2015;72(12):1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute on Drug Abuse. Is marijuana addictive? https://www.drugabuse.gov/publications/research-reports/marijuana/marijuana-addictive. Published 2020. Accessed May 30, 2020.
- 5.Fattore L, Fadda P, Spano MS, Pistis M, Fratta W. Neurobiological mechanisms of cannabinoid addiction. Mol Cell Endocrinol. 2008;286(1–2 Suppl 1):S97–S107. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29(4):225–232. [DOI] [PubMed] [Google Scholar]
- 7.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16(10):579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–1572. [DOI] [PubMed] [Google Scholar]
- 9.Raft D, Gregg J, Ghia J, Harris L. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain. Psychological correlates of the analgesic response. Clin Pharmacol Ther. 1977;21(1):26–33. [DOI] [PubMed] [Google Scholar]
- 10.Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of Δ9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl). 2003;169(2):135–140. [DOI] [PubMed] [Google Scholar]
- 11.Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3(11):1073–1074. [DOI] [PubMed] [Google Scholar]
- 12.John WS, Martin TJ, Solingapuram Sai KK, et al. Chronic Δ9-THC in rhesus monkeys: effects on cognitive performance and dopamine D2/D3 receptor availability. J Pharmacol Exp Ther. 2018;364(2):300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansbach RS, Nicholson KL, Martin BR, Balster RL. Failure of Δ9-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol. 1994;5(2):219–225. [DOI] [PubMed] [Google Scholar]
- 14.Wakeford AGP, Wetzell BB, Pomfrey RL, et al. The effects of cannabidiol (CBD) on Δ9-tetrahydrocannabinol (THC) self-administration in male and female long-Evans rats. Exp Clin Psychopharmacol. 2017;25(4):242–248. [DOI] [PubMed] [Google Scholar]
- 15.Barrus DG, Lefever TW, Wiley JL. Evaluation of reinforcing and aversive effects of voluntary Δ9-tetrahydrocannabinol ingestion in rats. Neuropharmacology. 2018;137:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubilius RA, Kaplick PM, Wotjak CT. Highway to hell or magic smoke? The dose-dependence of Δ9-THC in place conditioning paradigms. Learn Mem. 2018;25(9):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyatt WS, Fantegrossi WE. Δ9-THC exposure attenuates aversive effects and reveals appetitive effects of K2/“Spice” constituent JWH-018 in mice. Behav Pharmacol. 2014;25(3):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panagis G, Vlachou S, Nomikos GG. Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr Drug Abuse Rev. 2008;1(3):350–374. [DOI] [PubMed] [Google Scholar]
- 19.Vlachou S, Panagis G. Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm des. 2014;20(13):2072–2088. [DOI] [PubMed] [Google Scholar]
- 20.Gardner EL, Paredes W, Smith D, et al. Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol. Psychopharmacology (Berl). 1988;96(1):142–144. [DOI] [PubMed] [Google Scholar]
- 21.Lepore M, Liu X, Savage V, Matalon D, Gardner EL. Genetic differences in Δ9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci. 1996;58(25):PL365–PL372. [DOI] [PubMed] [Google Scholar]
- 22.Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther. 2012;343(2):389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66(3):869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlachou S, Nomikos GG, Stephens DN, Panagis G. Lack of evidence for appetitive effects of Δ9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol. 2007;18(4):311–319. [DOI] [PubMed] [Google Scholar]
- 25.Wiebelhaus JM, Grim TW, Owens RA, et al. Δ9-tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. J Pharmacol Exp Ther. 2015;352(2):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol. 2013;16(10):2273–2284. [DOI] [PubMed] [Google Scholar]
- 27.Spiller KJ, Bi GH, He Y, Galaj E, Gardner EL, Xi ZX. Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br J Pharmacol. 2019;176(9):1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14(2–3):169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez G, Trujillo-Pisanty I, Cossette MP, Conover K, Shizgal P. Role of dopamine tone in the pursuit of brain stimulation reward. J Neurosci. 2012;32(32):11032–11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry. 2012;71(12):1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jing MY, Han X, Zhao TY, et al. Re-examining the role of ventral tegmental area dopaminergic neurons in motor activity and reinforcement by chemogenetic and optogenetic manipulation in mice. Metab Brain Dis. 2019;34(5):1421–1430. [DOI] [PubMed] [Google Scholar]
- 32.Han X, He Y, Bi GH, et al. CB1 receptor activation on VgluT2-expressing glutamatergic neurons underlies Δ9-tetrahydrocannabinol (Δ9-THC)-induced aversive effects in mice. Sci Rep. 2017;7(1):12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman AH, Cao J, Keighron JD, et al. Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology. 2019;44(8):1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan CJ, Humburg B, Rice M, et al. The highly selective dopamine D3R antagonist, R-VK4-40 attenuates oxycodone reward and augments analgesia in rodents. Neuropharmacology. 2019;158:107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauco P, Wise RA. Synergistic effects of cocaine with lateral hypothalamic brain stimulation reward: lack of tolerance or sensitization. J Pharmacol Exp Ther. 1997;283(3):1160–1167. [PubMed] [Google Scholar]
- 36.Peng XQ, Li J, Gardner EL, et al. Oral administration of the NAALADase inhibitor GPI-5693 attenuates cocaine-induced reinstatement of drug-seeking behavior in rats. Eur J Pharmacol. 2010;627(1-3):156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banister SD, Longworth M, Kevin R, et al. Pharmacology of valinate and tert-leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem Nerosci. 2016;7(9):1241–1254. [DOI] [PubMed] [Google Scholar]
- 38.Schindler CW, Gramling BR, Justinova Z, Thorndike EB, Baumann MH. Synthetic cannabinoids found in “spice” products alter body temperature and cardiovascular parameters in conscious male rats. Drug Alcohol Depend. 2017;179:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hruba L, McMahon LR. Apparent affinity estimates and reversal of the effects of synthetic cannabinoids AM-2201, CP-47,497, JWH-122, and JWH-250 by rimonabant in rhesus monkeys. J Pharmacol Exp Ther. 2017;362(2):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinaldi-Carmona M, Barth F, Heaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350(2-3):240–244. [DOI] [PubMed] [Google Scholar]
- 41.Galaj E, Xi ZX. Potential of cannabinoid receptor ligands as treatment for substance use disorders. CNS Drugs. 2019;33(10):1001–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278(3):989–999. [PubMed] [Google Scholar]
- 43.Hillard CJ, Manna S, Greenberg MJ, et al. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J Pharmacol Exp Ther. 1999;289(3):1427–1433. [PubMed] [Google Scholar]
- 44.Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banister SD, Stuart J, Kevin RC, et al. Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem Nerosci. 2015;6(8):1445–1458. [DOI] [PubMed] [Google Scholar]
- 46.Wiley JL, Lefever TW, Marusich JA, et al. Evaluation of first generation synthetic cannabinoids on binding at non-cannabinoid receptors and in a battery of in vivo assays in mice. Neuropharmacology. 2016;110(Pt A):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dreyer JK, Vander Weele CM, Lovic V, Aragona BJ. Functionally distinct dopamine signals in nucleus accumbens core and shell in the freely moving rat. J Neurosci. 2016;36(1):98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang HY, Gao M, Liu QR, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A. 2014;111(46):E5007–E5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xi ZX, Song R, Li X, et al. CTDP-32476: a promising agonist therapy for treatment of cocaine addiction. Neuropsychopharmacology. 2017;42(3):682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elsaid S, Kloiber S, Le Foll B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog Mol Biol Transl Sci. 2019;167:25–75. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson SM, Banister SD, Kassiou M. Bioisosteric fluorine in the clandestine design of synthetic cannabinoids. Aust J Chem. 2015;68(1):4–8. [Google Scholar]
- 52.World Health Organization. AM-2201 Critical Review Report, Agenda item 4.7. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 53.Shanks KG, Behonick GS. Death after use of the synthetic cannabinoid 5F-AMB. Forensic Sci Int. 2016;262:e21–e24. [DOI] [PubMed] [Google Scholar]
- 54.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19(1):319–340. [DOI] [PubMed] [Google Scholar]
- 55.Gilliss B, Malanga CJ, Pieper JO, Carlezon WA Jr. Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss-Webster mice. Psychopharmacology (Berl). 2002;163(2):238–248. [DOI] [PubMed] [Google Scholar]
- 56.Lin D, Koob GF, Markou A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat—interactions between the two drugs. Psychopharmacology (Berl). 1999;145(3):283–294. [DOI] [PubMed] [Google Scholar]
- 57.Lin D, Koob GF, Markou A. Time-dependent alterations in ICSS thresholds associated with repeated amphetamine administrations. Pharmacol Biochem Behav. 2000;65(3):407–417. [DOI] [PubMed] [Google Scholar]
- 58.Moerke MJ, Ananthan S, Banks ML, et al. Interactions between cocaine and the putative allosteric dopamine transporter ligand SRI-31142. J Pharmacol Exp Ther. 2018;367(2):222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stoker AK, Markou A. Withdrawal from chronic cocaine administration induces deficits in brain reward function in C57BL/6J mice. Behav Brain Res. 2011;223(1):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vlachou S, Nomikos GG, Panagis G. CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology (Berl). 2005;179(2):498–508. [DOI] [PubMed] [Google Scholar]
- 61.Vlachou S, Nomikos GG, Panagis G. Effects of endocannabinoid neurotransmission modulators on brain stimulation reward. Psychopharmacology (Berl). 2006;188(3):293–305. [DOI] [PubMed] [Google Scholar]
- 62.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl). 1999;144(1):77–82. [DOI] [PubMed] [Google Scholar]
- 63.Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74(3):541–549. [DOI] [PubMed] [Google Scholar]
- 64.Flores RJ, Uribe KP, Swalve N, O’Dell LE. Sex differences in nicotine intravenous self-administration: a meta-analytic review. Physiol Behav. 2019;203:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bobzean SA, DeNobrega AK, Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014;259:64–74. [DOI] [PubMed] [Google Scholar]
- 66.Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009;34(Suppl 1):S227–S236. [DOI] [PubMed] [Google Scholar]
- 67.Gertsch J, Leonti M, Raduner S, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105(26):9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He Y, Galaj E, Bi GH, Wang XF, Gardner E, Xi ZX. β-Caryophyllene, a dietary terpenoid, inhibits nicotine taking and nicotine seeking in rodents. Br J Pharmacol. 2020;177(9):2058–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Health Organization. XLR-11 critical review report—agenda item 4.12. Geneva, Switzerland: 2016. [Google Scholar]
- 70.Calpe-Lopez C, Garcia-Pardo MP, Aguilar MA. Cannabidiol treatment might promote resilience to cocaine and methamphetamine use disorders: a review of possible mechanisms. Molecules. 2019;24(14):2583–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uchiyama NSY, Kawamura M, Kikura-Hanajiri R, Hakamatsuka T. Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl) benzofuran(2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol. 2014;32(2):266–281. [Google Scholar]
- 72.Kaneko S Motor vehicle collisions caused by the ‘super-strength’ synthetic cannabinoids, MAM-2201, 5F-PB-22, 5F-AB-PINACA, 5F-AMB and 5F-ADB in Japan experienced from 2012 to 2014. Forensic Toxicol. 2017;35(2):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chimalakonda KC, Seely KA, Bratton SM, et al. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40(11):2174–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xi ZX, Peng XQ, Li X, et al. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14(9):1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jordan CJ, Xi ZX. Progress in brain cannabinoid CB2 receptor research: from genes to behavior. Neurosci Biobehav Rev. 2019;98:208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]


