Abstract
Background:
Aging is a complex biological process and associated with a progressive decline in functions of most organs including the gastrointestinal (GI) tract. Age-related GI motor disorders/dysfunctions include esophageal reflux, dysphagia, constipation, fecal incontinence, reduced compliance, and accommodation. Although the incidence and severity of these diseases and conditions increase with age, they are often underestimated due in part to nonspecific and variable symptoms and lack of sufficient medical attention. They negatively affect quality of life and predispose the elderly to other diseases, sarcopenia, and frailty. The mechanisms underlying aging-associated GI dysfunctions remain unclear, and there is limited data examining the effect of aging on GI motor functions. Many studies on aging-associated changes to cells within the tunica muscularis including enteric neurons, smooth muscles, and interstitial cells have proposed that cell loss and/or molecular changes may be involved in the pathogenesis of age-related GI motor disorders/dysfunctions. There is also evidence that the aging contributes to phenotypic changes in innate immune cells, which are physically and functionally linked to other cells in the tunica muscularis and can alter GI (patho) physiology. However, various patterns of changes have been reported, some of which are contradictory, indicating a need for additional work in this area.
Purpose:
Although GI infection due to intestinal bacterial overgrowth, bleeding, and cancers are also important and common problems in the elderly patients, this mini-review focuses on data obtained from enteric neuromuscular aging research with the goal of better understanding the cellular and molecular mechanisms of enteric neuromuscular aging to enhance future therapy.
Keywords: aging, enteric neuron, gastrointestinal motility, interstitial cells of Cajal, muscularis propria macrophage, senescence
1 |. INTRODUCTION
Advancing age is one of major risk factors for most disease and disorders.1 The proportion of elderly individuals is rapidly increasing worldwide, especially in developed countries.2 Therefore, age-related diseases and disorders represent a major healthcare burden.1,2 Aging leads to decline in biological functions of most organs including the GI tract.3 Age-related GI motor dysfunctions/disorders include esophageal reflux, dysphagia, irritable bowel syndrome, chronic constipation, diverticulosis, rectal prolapse, fecal incontinence and compaction, and reduced compliance and accommodation that follows ingestion of a meal.2,4,5 These disorders are not specific to the elderly, but more common and frequent with age. They may contribute to early satiety, increase satiation, and loss of appetite leading to bodyweight decreases termed “anorexia of aging.” Although these diseases and conditions are not themselves fatal, they negatively affect quality of life and contribute to the development of subsequent undernutrition, immunosuppression, sarcopenia, and frailty.6,7 These dysfunctions and disorders ultimately lead to adverse outcomes with higher rates of morbidity and mortality.6 Importantly, recent reports have linked reduced food intake to increased overall mortality in elderly individuals and aged mice.8,9 Taken together, these findings suggest that reduced food intake due to GI motor dysfunctions may be linked to sarcopenia and frailty leading to increased overall mortality (Figure 1). In other words, it is important to study GI motor dysfunction to enhance lifespan and/or to improve quality of life. However, our understanding of the pathophysiology of age-related GI motor dysfunctions is still limited due in part to nonspecific symptoms, complicated mechanisms, and lack of sufficient medical attention.2,4,5 In this mini-review, we will discuss the role of aging-associated cellular and molecular changes and external factors in GI disorders/dysfunctions to understand and potentially reverse GI neuromuscular degeneration with age.
FIGURE 1.
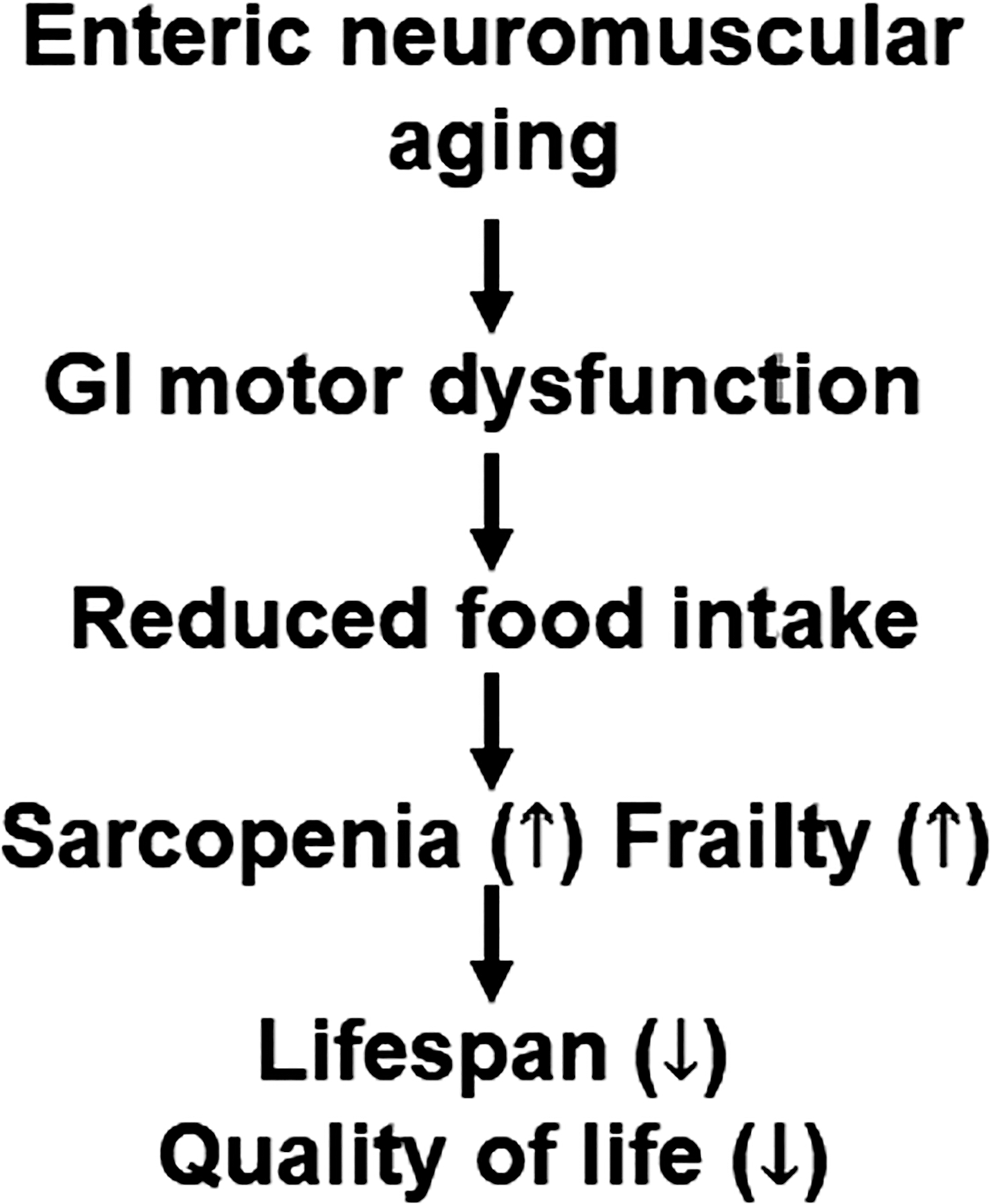
Proposed link between age-related GI motor dysfunction and reduced food intake, sarcopenia, frailty leading to shorten lifespan and reduced quality of life
2 |. OVERVIEW OF THE CELLULAR AND FUNCTIONAL CHANGES WITH AGE
Gastrointestinal motor functions are regulated by the enteric neuromuscular system, the intrinsic nervous system of the GI tract.10 The enteric neuromuscular system is the largest of the peripheral nervous systems and is a sophisticated tissues complex containing smooth muscles, a diversity of neurons including excitatory and inhibitory enteric neurons, glia, interstitial cells including interstitial cells of Cajal (ICC) and “fibroblast” like cells (FLCs) also known as telocytes or platelet-derived growth factor receptor alpha (Pdgfra)-positive cells.10 These cells are closely associated and provide important GI motor functions including ingestion, processing, absorption of nutrients, and disposing of waste.10 Therefore, declines or dysfunctions in any of these cells with age affect GI motor functions. Changes in number, size, and morphology of these cells with age have been reported, but there are conflicting results, especially in human studies probably due to many factors that can affect the maintenance and physiology of GI muscles.
While most studies on enteric neuromuscular aging focus on changes in enteric neurons, there are controversial reports. An important issue in enteric neuromuscular aging is that the age-related neuronal declines are variable between lower and upper GI tract. Although the extent of neuronal loss varies, there is solid evidence that excitatory neurons decline with age in the lower GI tract of humans, rats, and mice.4,11,12 In contrast, age-related loss of enteric neurons is less evident in the stomach of human, rats, and mice.4,13 While some reports showed a reduction in enteric neurons in the stomach, the extent of reductions are smaller than that reported in lower GI tract.4 Importantly, in the klotho mouse strain, which exhibits symptoms of accelerated aging due to genetically determined loss of expression of the Klotho protein,13–15 we found an age-related neuronal loss in the lower GI tract (jejunum, ileum, proximal, and distal colon), but not in stomach of the same mice.13 The cause of differential neuronal losses between lower and upper GI tracts is still unclear, but investigations of the cause may lead to a better understanding about the factors that contribute to neuronal aging in the enteric neuromuscular apparatus. Another important issue is that different subpopulations of enteric neurons may be more sensitive to age-related changes. Cholinergic excitatory neurons, identified by expression of choline acetyltransferase-like immunoreactivity, have been reported to consistently decline with age whereas nitric oxide synthase-1 (NOS1 or nNOS)-positive inhibitory neurons seem to be preserved with age.4,11,12 However, we cannot exclude the possibility of NOS1 dysfunction or more subtle functional abnormalities of inhibitory neurons in aging.16 In contrast, NOS1-positive neurons are reported to decline in stomachs of patients with diabetic gastroparesis.17 The mechanisms underlying age-related neuronal loss may be different from the NOS1-positive neuronal loss in diabetic gastroparesis. Possible explanations may be the severity of GI inflammation or/and different susceptibility of neurons to loss of growth factors.
Enteric glia, non-myelinating peripheral glial cells derived from neural crest stem cell, is another important cell type of enteric neuromuscular system.18 Recent findings indicate enteric glia represents novel druggable targets for several GI diseases and disorders including inflammatory bowel disease and postoperative ileus.18–21 Delayed colonic transit was associated with reduced glial Ca2+ responses and changes in connexin-43, a member of gap junction proteins, in middle-aged (12 months old) mice.22 Although reduced enteric glia is also observed in humans,23,24 detailed mechanisms remain unclear. Surprisingly, a recent report suggests that a subpopulation of enteric neurons, but not glia, originate from mesoderm and during aging these mesoderm-derived neurons become dominant form of all neurons in the enteric nervous system.25 However, detailed signaling mechanisms between the two lineages remain unclear and further investigations are needed.
Another key cell type involved in age-related GI motor dysfunctions is ICC. ICC are mesoderm-derived, mesenchymal cells that drive pacemaking through generation of electrical slow-wave activity.26 ICC also mediates neuromuscular neurotransmission between neurons, smooth muscles, and FLCs. In contrast to age-related neuronal loss, there are consistent reports that demonstrate ICC decline in the stomach but not small intestines and colon of mice, rats, and humans. Although it remains unclear why different cell types are affected in different GI locations, the differential changes may be due to environmental factors such as microbiota in GI systems. This intriguing possibility requires further investigation. There are no published studies investigating the effect of age on FLCs and so this remains to be investigated.
3 |. THE MECHANISMS UNDERLYING DEGENERATION OF ENTERIC NEUROMUSCULAR SYSTEM DURING AGING
The mechanisms underlying degeneration of the enteric nervous system in aging remain complicated, multi-factorial, and unclear given controversial and conflicting reports. We review basic aspects of aging-related degeneration of the enteric neuromuscular system with emphasis on the cellular and molecular mechanisms. There are relatively few reports about the effect of aging on stem cells in the enteric neuromuscular system, although this is likely to be fundamental to an understanding of age-related GI motor dysfunctions. The aging of tissue-specific stem cells has been proposed to play a major role in the decline of tissue and organ integrity and function in elderly people.27 Previous work has identified a subset of cells immunopositive for CD34 and CD44 with low Kit expression as ICC stem/precursor cells in murine stomach.28 Unlike ICC, KitlowCD34+CD44+ ICC stem cells (ICC-SC) do proliferate and serve to continually replenish ICC.29,30 Recent studies found ICC-SC decline precedes ICC decline with age, suggesting ICC-SC decline may play a major role in age-related ICC loss and gastric motor dysfunctions.31 Upregulated transformation-related protein 53 (Trp53; a tumor suppressor protein also known as an aging-related transcription factor) induced persistent ICC-SC cell cycle arrest leading to ICC loss without an increase in senescence or apoptosis (Figure 2).31 These ICC/ICC-SC declines were associated with gastric motor dysfunction in both klotho mice and aged mice.31 Furthermore, Trp53 inhibited ICC-SC proliferation via suppression of the extracellular signaling-regulated kinase (ERK) signaling pathway,31 suggesting age-related ICC-SC/ICC depletion could potentially be countered by the stimulation of ERK-mediated signaling pathways (Figure 2). Importantly, recent findings suggest that polycomb H3K27 methyl-transferase Ezh2 regulates age-related ICC/ICC-SC decline and this decline can be reversed by Ezh2 inhibition, indicating a potential utility of Ezh2 inhibitors for age-related GI dysmotility.32
FIGURE 2.
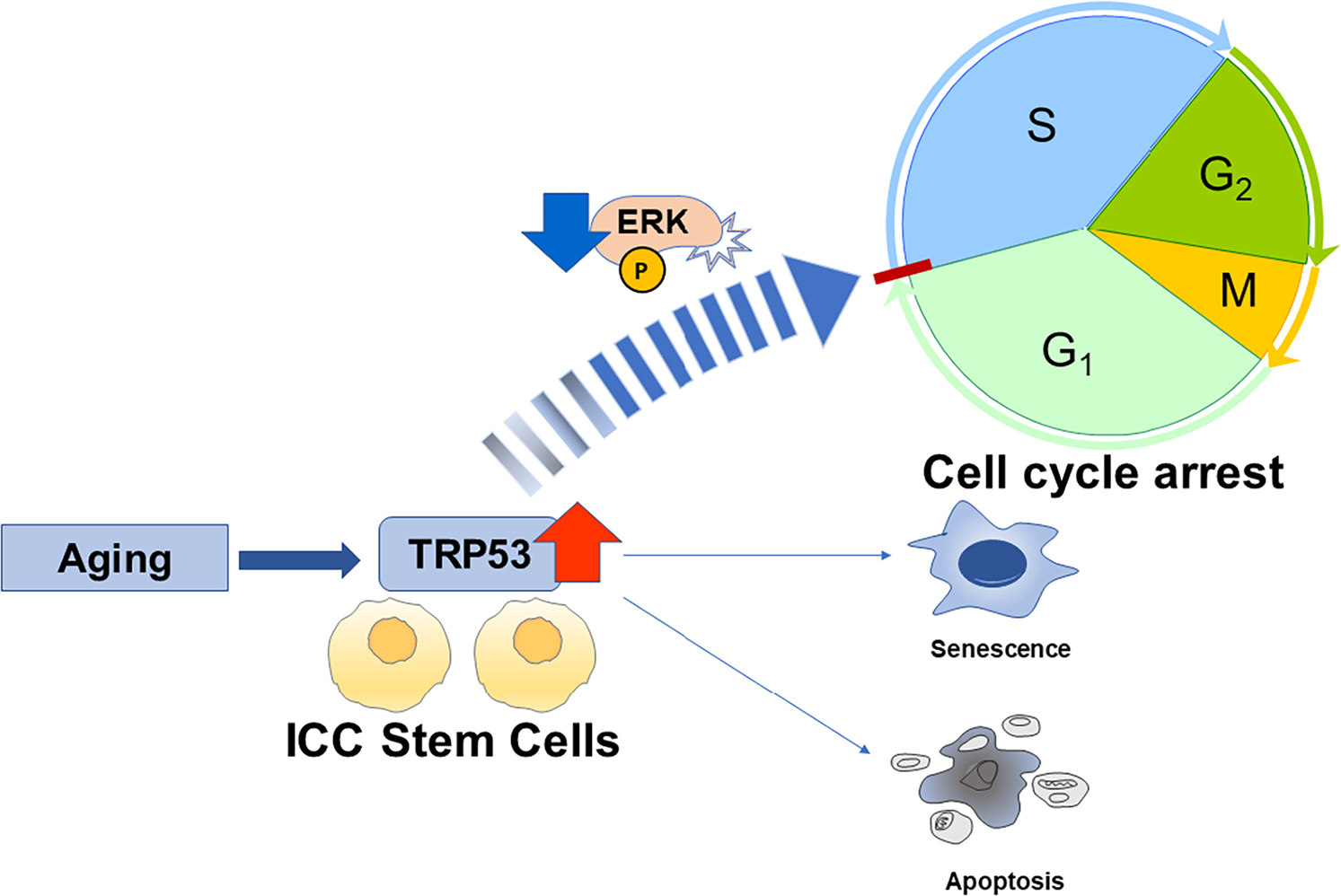
Proposed mechanisms underlying interstitial cells of Cajal (ICC) stem cell decline with age. Aging-associated deletion of ICC are due to TRP53-mediated cell cycle arrest of ICC stem cells without senescence and apoptosis
Enteric neural stem cells (ENSCs) are multipotent stem cells which can give rise to enteric neurons, glia, and myofibroblasts in GI tunica muscularis both under homeostatic and pathologic conditions.10 ENSCs can be identified by several markers including Sox2, p75, CD49b, and nestin. Patient-derived ENSCs can be readily obtained by endoscopic techniques, suggesting this approach as being appropriate for stem cell therapy to treat enteric neuropathies, degenerative neuromuscular conditions including Hirschsprung disease, esophageal achalasia, gastroparesis, chronic intestinal obstruction, and neuropathic constipation.33 ENSCs were reported to decline with age and concomitant with increased expression of p16Ink4a, an effective biomarker of aging.34 These in vivo findings corresponded to ex vivo organotypic culture systems demonstrating an age-related decline in 5-ethynyl-2’-deoxyuridine (EdU)-positive proliferating ENSCs.35 The ENSC decline were mediated by cytotoxic effect of interleukin-6 (IL-6), an inflammatory cytokine increased with age.35 These changes may be induced by age-dependent phenotypic changes of muscularis propria macrophage (MPMs).35
Muscularis propria macrophages are tissue -resident macrophages that are closely located to enteric neurons in the GI tunica muscularis and communicate with enteric neurons in a bidirectional manner.36,37 They are distributed across the wall of muscularis propria in all regions of the GI tract.38,39 These cells exhibit diverse morphologies and form close spatial contact of variable distance and morphologies with not just myenteric neurons but also ICC, FLCs, and smooth muscle cells.38 Immunohistochemical evidence indicates that, in mice, the gastric MPMs consist of at least 3 phenotypes: MPMs that are not activated and express few biological active cytokines and interleukins, MPMs that express indicators of a tissue-protective, anti-inflammatory phenotype including CD206, IL-10, and heme oxygenase-1 (HMOX1), and MPMs that express indicators of an injurious or pro-inflammatory phenotype including interleukin-1 beta (IL-1β), IL-6, and NOS2 also known as inducible nitric oxide synthase (iNOS).40 Single cell profiling finds that this diversity is even more complex including cells with similarities to central nervous system (CNS) microglia,41 which are increasingly linked to maintaining healthy function in the aged brain.42 These observations are consistent with the current understanding of the diversity of macrophage populations in other tissues including the cardiovascular system, lungs, and adipose tissues.43 Further detailed study is ongoing, but whether the MPMs are derived from long-lived, self-sustaining cells of embryological origin41 or adult circulating monocytes44 and the niche that the cells occupy likely determines the phenotype of the MPMs in any given tissue and in health or disease. In respect to aging, the potential decline in long-lived self-sustaining cells and their replacement with monocyte-derived MPMs likely contributes to the capacity of the MPMs to contribute to maintenance of healthy neuromuscular function and motility in the GI tract.41 From the perspective of alterations of the cellular and biochemical niche occupied by MPMs then the microbiome likely plays a significant role since not only the host but also the microbiome ages and this has been proposed to modify populations of MPMs, especially in the large intestine.45,46 Indeed, a major driver of diversity between MPMs in different regions of the gut is likely to be the proximity and connectivity to lumenal contents and signaling across the mucosa and submucosa via humoral factors or neurally mediated sensory signaling.47 The number and proliferation of ENSCs, a key component in maintaining enteric neuromuscular apparatus as reviewed in above, also decline with age via the age-dependent shift in MPM polarization to a pro-inflammatory phenotype (Figure 3), indicating age-related neurodegeneration is due in part to the decline in proliferation of ENSCs. However, the causes of age-related shift in macrophage polarization remains unclear and multiple factors have been proposed as the cause including cellular senescence.
FIGURE 3.
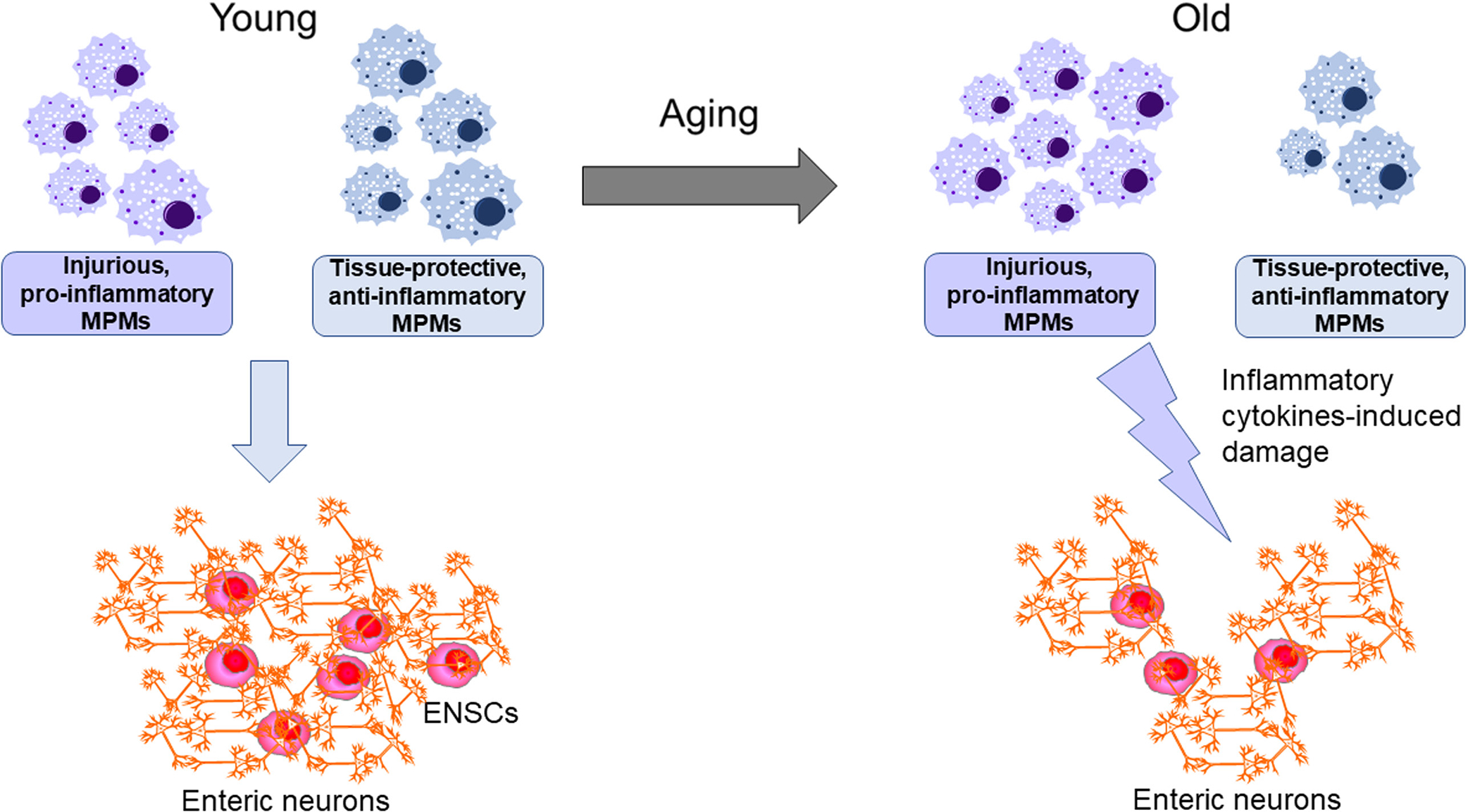
Proposed model for age-related loss of enteric neurons. Aging changes muscularis propria macrophages (MPMs)’s functional phenotype from tissue-protective, anti-inflammatory to injurious, pro-inflammatory. Enteric neuron and enteric neural stem cells (ENSCs) decline with age by this age-dependent shift in MPMs
Cellular senescence is a process that imposes a permanent growth arrest state on cells in response to various stressors including oxidative stress, DNA damage, and telomere attrition, resulting in organismal aging.3,48 Some other hallmarks of cellular senescence include resistance to apoptosis following chromatin damage, genomic instability, epigenetic alterations, loss of proteostasis, and mitochondrial dysfunction. Senescent cells become flat, enlarged and vacuolized during senescence.48 Senescent cells trigger profound phenotypic changes such as the production of inflammatory cytokines, chemokines, and proteases termed as the senescence-associated secretory phenotype (SASP).3,48 Senescent cells can induce tissues dysfunction and inflammation, immune evasion, paracrine senescence by secreting SASP.48 A growing body of evidence indicates cellular senescence is an important driver of aging and aging-related diseases through various mechanisms.48 The question of whether the adult ENS undergoes senescence during aging has been controversial. p21Waf1/Cip1, a cyclin-dependent kinase inhibitor and a marker used to detect senescent cells, was shown to increase in myenteric neurons of small intestine during aging, and this increase is concomitant with DNA damage response and production of reactive oxygen species (ROS).49 These senescent cells and consequent inflammatory processes during aging may be key to age-related shifts in macrophage polarization. Consistently, senescence-associated morphological changes including swollen nerve fibers and neurons in colons during aging have been observed.50 In contrast, a recent report revealed increased cellular senescence determined by senescence-associated β-galactosidase (SA-β-gal) activity, the most widely used marker to detect senescent cells, in the gastric mucosa but not in the tunica muscularis of aged mice.31 Similar mucosa-restricted increase in SA-β-gal activity was observe in progeric klotho mice.31 These findings suggests no significant involvement of cellular senescence in gastric ICC-SC/ICC depletion with age.31 Although we cannot explain these findings, the susceptibility of GI mucosa and muscle cells to the senescence process during aging may also be fundamentally and region- and cell-specifically different.
4 |. CONCLUSIONS AND FUTURE DIRECTIONS
Despite the recent advances in enteric neuromuscular research, the study of enteric neuromuscular aging is still in its early stage and our understanding of the mechanisms remains limited. Due to an exponential rise in the elderly population, enteric neuromuscular aging research is an area gaining more interest, and data obtained from these studies will provide rationale for discovering new therapeutic targets to potentially delay or reverse age-associated GI motility dysfunction leading to improved quality of life.
Key Points.
Mechanisms of aging-related GI motor dysfunctions remain complicated and unclear.
Age-related cellular and molecular changes are key to understand the mechanisms.
ACKNOWLEDGEMENTS
The authors thank Dr. Simon J. Gibbons (Mayo Clinic) for critical suggestions and discussions.
Funding information
This work was supported in part by the National Institutes of Health grants R01 DK121766 (Y.H.), Mayo Clinic Center for Biomedical Discovery Pilot Award (Y.H.), and American Gastroenterology Association-Allergan Foundation Pilot Research Award in Gastroparesis (Y.H.), P30 DK084567 (Mayo Clinic Center for Cell Signaling in Gastroenterology). The funding agencies had no role in the study analysis or writing of the manuscript. Its contents are solely the responsibility of the authors.
Abbreviations:
- CNS
central nervous system
- EdU
5-ethynyl-2’-deoxyuridine
- ENSCs
enteric neural stem cells
- ERK
extracellular signaling-regulated kinase
- FLCs
“fibroblast” like cells
- GI
gastrointestinal
- HMOX1
heme oxygenase-1
- ICC
interstitial cells of Cajal
- ICC-SC
interstitial cell of Cajal stem cells
- IL-10
interleukin-10
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- iNOS
inducible nitric oxide synthase
- Kit
v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog
- MPMs
muscularis propria macrophages
- NOS1
nitric oxide synthase-1
- Pdgfra
platelet-derived growth factor receptor alpha
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- SA-β-gal
senescence-associated β-galactosidase
- Trp53
transformation-related protein 53
Footnotes
CONFLICT OF INTEREST
The authors disclose no conflicts.
REFERENCES
- 1.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008;20:185–196. [DOI] [PubMed] [Google Scholar]
- 3.Aging Campisi J., cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saffrey MJ. Cellular changes in the enteric nervous system during ageing. Dev Biol. 2013;382:344–355. [DOI] [PubMed] [Google Scholar]
- 5.Saffrey MJ. Aging of the mammalian gastrointestinal tract: a complex organ system. Age (Dordr). 2014;36:9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox N, Ibrahim K, Sayer A, Robinson S, Roberts H. Assessment and Treatment of the anorexia of aging: a systematic review. Nutrients. 2019;11(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker BA, Chapman IM. Food intake and ageing–The role of the gut. Mech Ageing Dev. 2004;125:859–866. [DOI] [PubMed] [Google Scholar]
- 8.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couzin-Frankel J Diet studies challenge thinking on proteins versus carbs. Science. 2014;343:1068. [DOI] [PubMed] [Google Scholar]
- 10.Schneider S, Wright CM, Heuckeroth RO. Unexpected roles for the second brain: enteric nervous system as master regulator of bowel function. Annu Rev Physiol. 2019;81:235–259. [DOI] [PubMed] [Google Scholar]
- 11.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21:746–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broad J, Kung VWS, Palmer A, et al. Changes in neuromuscular structure and functions of human colon during ageing are region-dependent.Gut. 2019;68:1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izbeki F, Asuzu DT, Lorincz A, et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J Physiol. 2010;588:3101–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. [DOI] [PubMed] [Google Scholar]
- 15.Asuzu DT, Hayashi Y, Izbeki F, et al. Generalized neuromuscular hypoplasia, reduced smooth muscle myosin and altered gut motility in the klotho model of premature aging. Neurogastroenterol Motil. 2011;23:e309–e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725–G733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–1585.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest. 2015;125:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulbransen BD, Bashashati M, Hirota SA, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider R, Leven P, Glowka T, et al. Anovel P2X2-dependent purinergic mechanism of enteric gliosis in intestinal inflammation. EMBO Mol Med. 2021;13:e12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales-Soto W, Gulbransen BD. Enteric glia: a new player in abdominal pain. Cell Mol Gastroenterol Hepatol. 2019;7:433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClain J, Grubisic V, Fried D, et al. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology. 2014;146(2):497–507.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun T, Li D, Hu S, et al. Aging-dependent decrease in the numbers of enteric neurons, interstitial cells of Cajal and expression of connexin43 in various regions of gastrointestinal tract. Aging. 2018;10:3851–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandic P, Lestarevic S, Filipovic T, Savic S, Stevic S, Kostic M. The effects of quercetin on liver regeneration after liver resection in rats. Folia Morphol (Warsz). 2016;75:188–195. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni SSM, Becker L, Wang Z, et al. Neural crest-derived neurons are replaced by a newly identified mesodermal lineage in the post-natal and aging enteric nervous system. BioRxiv. 2020. doi: 10.1101/2020.08.25.262832 Online ahead of print. [DOI] [Google Scholar]
- 26.Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology. 2016;31:316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. [DOI] [PubMed] [Google Scholar]
- 28.Lorincz A, Redelman D, Horvath VJ, Bardsley MR, Chen H, Ördög T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardsley MR, Horvath VJ, Asuzu DT, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi Y, Nguyen VTT. A narrative review of imatinib-resistant gastrointestinal stromal tumors. Gastrointestinal Stromal Tumor. 2021;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi Y, Asuzu DT, Bardsley MR, et al. Wnt-induced, TRP53-mediated cell cycle arrest of precursors underlies interstitial cell of cajal depletion during aging. Cell Mol Gastroenterol Hepatol. 2021;11:117–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syed SA, Hayashi Y, Lee JH, et al. Ezh2-Dependent Epigenetic Reprogramming Controls a Developmental Switch Between Modes of Gastric Neuromuscular Regulation BioRxiv. vol. 2018. Cold Spring Harbor Laboratory; 2018. [Google Scholar]
- 33.Burns AJ, Goldstein AM, Newgreen DF, et al. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev Biol. 2016;417:229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker L, Nguyen L, Gill J, Kulkarni S, Pasricha PJ, Habtezion A. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulkarni S, Ganz J, Bayrer J, Becker L, Bogunovic M, Rao M. Advances in enteric neurobiology: the "brain" in the gut in health and disease. J Neurosci. 2018;38:9346–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller PA, Koscsó B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji S, Traini C, Mischopoulou M, et al. Muscularis macrophages establish cell-to-cell contacts with telocytes/PDGFRalpha-positive cells and smooth muscle cells in the human and mouse gastrointestinal tract. Neurogastroenterol Motil. 2021;33:e13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikkelsen HB, Thuneberg L, Rumessen JJ, Thorball N. Macrophage-like cells in the muscularis externa of mouse small intestine. Anat Rec. 1985;213:77–86. [DOI] [PubMed] [Google Scholar]
- 40.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135(6):2055–2064.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Schepper S, Verheijden S, Aguilera-Lizarraga J, et al. Selfmaintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175(2):400–415.e13. [DOI] [PubMed] [Google Scholar]
- 42.Schuitemaker A, van der Doef TF, Boellaard R, et al. Microglial activation in healthy aging. Neurobiol Aging. 2012;33:1067–1072. [DOI] [PubMed] [Google Scholar]
- 43.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cipriani G, Gibbons SJ, Miller KE, et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology. 2018;154(8):2122–2136.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker L, Spear ET, Sinha SR, et al. Age-related changes in gut microbiota alter phenotype of muscularis macrophages and disrupt gastrointestinal motility. Cell Mol Gastroenterol Hepatol. 2019;7:243–245.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawano Y, Nakae J, Watanabe N, et al. Colonic Pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-dependent manner. Cell Metab. 2016;24:295–310. [DOI] [PubMed] [Google Scholar]
- 47.DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28:180–189. [DOI] [PubMed] [Google Scholar]
- 48.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jurk D, Wang C, Miwa S, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69–83. [DOI] [PubMed] [Google Scholar]


